Abstract
Gibberellin (GA) plays a key role in the release of bud dormancy and the GA receptor GID1 (GIBBERELLIN INSENSITIVE DWARF1) and DELLA protein are the GA signaling parts, but the molecular mechanism of GA-GID1-DELLA module regulating leaf bud dormancy in peach (Prunus persica) is still not very clear. In this study, we isolated and characterized the GID1 gene PpGID1c from the peach cultivar “Zhong you No.4.” Overexpressing PpGID1c in Arabidopsis promoted seed germination, which indicated that PpGID1c has an important function in dormancy. The expression level of PpGID1c in peach leaf buds during endodormancy release was higher than that during ecodormancy and was positively correlated with GA4 levels. Our study also found that GA4 had the most obvious effect on promoting the bud break, indicating that GA4 may be the key gibberellin to promoting peach leaf bud endodormancy release. Moreover, a quantitative real-time PCR (qRT-PCR) found that GA4 could increase the expression of the gibberellin signaling gene PpDELLA2. A yeast two-hybrid (Y2H) assay suggested that the PpGID1c interaction with the PpDELLA1 protein was not dependent on gibberellin, while the PpGID1c interaction with PpDELLA2 required GA4 or another gibberellin. These findings suggested that the GA4-GID1c-DELLA2 module regulates peach leaf bud endodormancy release, with this finding significantly enhancing our comprehensive understanding of bud endodormancy release and revealing a new mechanism for regulating leaf bud endodormancy release in peach.
Keywords: Prunus persica, leaf bud endodormancy release, GA INSENSITIVE DWARF1 GID1c, DELLA protein, GA4
Introduction
In temperate and boreal regions, perennial plants have seasonal cycles of growth and dormancy to survive during the winter cold (Tylewicz et al., 2018). Accordingly, a study by Lang (1987) divided the stages of bud dormancy into paradormancy, endodormancy, and ecodormancy. This classification has been widely accepted by researchers in the study of bud dormancy. Paradormancy is the dormancy caused by the structure of the plant itself. It is affected by neighboring organs or tissues, which inhibit the growth of the plant. Endodormancy, also known as internal dormancy, is a dormant phenomenon controlled by its own internal factors. In this stage, only when dormant buds meet a certain low-temperature accumulation can they burst under appropriate conditions. Ecodormancy is a phenomenon in which plants cannot grow due to external environmental factors, such as growth stagnation caused by natural environmental stresses like low temperature and drought (Horvath et al., 2003). The bud dormancy associated with winter is generally called endodormancy (Wang et al., 2015). Bud endodormancy is essential for woody plants to resist the cold environment of winter. Recently, deciduous fruit trees have been incompletely flowering due to global warming and higher temperatures, thereby reducing yields and ultimately affecting the economic returns of fruit farmers. Therefore, understanding the regulatory mechanism of bud endodormancy is important for a better grasp of the flowering and fruiting of agricultural production and the improving of the yield and quality of fruits (Tuan et al., 2017).
Gibberellins are believed to play a key role in regulating bud dormancy. Gibberellin content determines the timing of the endodormancy release of pear buds (Yang et al., 2019). During Japanese apricot bud endodormancy, GA4 treatment can promote bud burst (Zhuang et al., 2015). Gibberellic acid (GA) also regulates plant growth and development, including seed germination and bud dormancy (Ogawa et al., 2003; Zheng et al., 2018b). It is believed that GA plays a key role in the release of dormancy in buds, but the molecular mechanism of gibberellin regulation is undefined. Gibberellic acid signaling consists of three important parts, including GA, the GA receptor GID1 (GIBBERELLIN INSENSITIVE DWARF1), and DELLA protein (Fukazawa et al., 2015). Gibberellin signal transduction responds to a series of signals through the GA-GID1-DELLA pathway (Hirano et al., 2008). The GID1 receptor belongs to the Hormone Sensitive Lipase (HSL) family and contains motifs for HGG and GDSSG which can bind to GA (Gazara et al., 2018). Without GA binding, the N-terminal extension (N-Ex) of GID1 has a flexible structure that is highly sensitive to protease treatment. The binding of GA to the thC-terminal domain of GID1 induces a conformational switch of its N-Ex to cover the GA-binding pocket (like closing the lid). This binding also creates hydrophobic surfaces for DELLA binding and changes the N-terminal domain, which promotes binding to DELLA, and thus inhibits GA signaling (Sun, 2010). The identification of GID1 in rice was the first elucidation of a protein responsive to GA signaling (Ueguchi-Tanaka et al., 2005). There are three GID1 (AtGID1a, AtGID1b, and AtGID1c) genes in Arabidopsis as GA receptors (Nakajima et al., 2006), and GID1b also interacts with DELLAs under low GA levels. A rice GID1 suppressor mutant reveals that GA is not always required for the interaction between its receptor, GID1, and DELLA proteins. This suggests that GA-dependent or GA-independent pathways induce GA stimulation in growth and development (Yamamoto et al., 2010).
According to the phenotype of the gibberellin insensitive dwarf1mutant, the GID1 family genes are likely partially redundant in Arabidopsis (Gallego-Giraldo et al., 2014a). Gibberellin insensitive dwarf1 genes generally control fruit set and fruit growth in Arabidopsis (Gallego-Giraldo et al., 2014b), while GID1a primarily regulates growth and GID1b and GID1c play an important role in Arabidopsis seed development (Gallego-Giraldo et al., 2014a). Gibberellin insensitive dwarf1ac and GID1b play distinct roles in Brassicaceae seed germination (Voegele et al., 2011). The rice GID1 mutant exhibits a dwarf phenotype (Ueguchi-Tanaka et al., 2005). In cucumber (Cucumis sativus), CsGID1a is essential for fruit locule formation (Liu et al., 2016). There are two GID1 genes in peach that are similar to GID1b and GID1c in Arabidopsis (Hollender et al., 2016). The silencing of the GID1c gene in peach results in dwarfing (Hollender et al., 2016; Cantín et al., 2018; Cheng et al., 2019). Moreover, a recent study indicated that the GID1 gene might serve a role in the release of peach vegetative bud dormancy (Hollender et al., 2016).
The peach originated in China and has been cultivated for about 3,000 years (Zheng et al., 2014). Today, peaches (Prunus persica) are widely cultivated and recognized as economically important deciduous fruit across the world (Cao et al., 2016). The production of peaches is dependent on the breakage of bud endodormancy, but the mechanism of endodormancy release in peaches is still unclear. Thus, understanding the mechanism of bud endodormancy is of vital importance to control the breaking of buds. In this study, we aimed to characterize the expression patterns of GA-GID1-DELLA genes in peach bud endormancy. Together, this study will better inform how we can use the mechanisms of peach bud endormancy to regulate peach production and management.
Materials and Methods
Plant Materials and Dormancy Treatments
Experiments were conducted at the Shandong Agricultural University from 2018 to 2019. The annual branches of the peach buds of 10-year-old trees (P. persica var. nectarina cv. Zhongyou 4) were used as test materials. Peach bud samples were collected every 15 days or so, immediately frozen in liquid nitrogen (LN), and then stored at −80°C. On December 25, 2018, different types of hormones [GA3, GA4, GA5, abscisic acid (ABA), and FLU: ABA synthesis inhibitor] were used to treat peach branches for 48 h. After 25 days, we observed the burst rates of the peach leaf buds. Gibberellins, FLU, and ABA were purchased from Thermo Fisher (Shanghai, China). The use of hydroponics to determine the burst rates is the most common method to define the endodormancy process (Li et al., 2011). First, we selected robust annual peach branches every 15 days, inserted them into the water, and placed them in a light incubator. The culture conditions were 25°C, light for 16 h, and dark for 8 h with a 200-μmol·m−2·s−1 light intensity treatment. Every 2 to 3 days, the base was cut off, and the bud burst rate was measured after 25 days. When the burst rate reached more than 50%, the endodormancy was considered released (Wang et al., 2015).
RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from 0.5 g of bud tissue using an RNAprep Pure Plant Kit (Tian Gen, Beijing, China) according to the instructions of the manufacturer. A NanoPhotometer P360 (Implen, Munich, Germany) was used to assess the quality and quantity of the RNA. First-strand cDNA was generated using a HiScript Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) according to the instructions of the manufacturer. The product was used either immediately in the next reaction or stored at −20°C. Quantitative real-time PCR (qRT-PCR) was performed using SYBR Premix Ex Taq (Takara) on a CFX96 real-time PCR detection system (Bio-Rad). The PpUBQ gene was used as the internal control (Supplementary 1). Three biological replicates were used for each analysis. The PCR protocol was as follows: pre-denaturation at 95°C for 2 min, 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°Cfor 40 s. When the reaction was complete, we proceeded to the dissociation curve reaction. The 2−ΔΔCT method was used to estimate the relative expression level (Livak and Schmittgen, 2001). The Statistical Analysis GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) was used to construct charts. The statistical analysis was performed using the IBM SPSS Statistics 19 software (IBM Corporation, New York, USA) to analyze the significance of the differences among data, with a significance level of p < 0.05 under Duncan's test.
Gene Isolation and Bioinformatic Analysis
Based on the previous results, there were two gibberellin insensitive dwarf1 genes identified in the peach genome. Prupe.6G332800 (Ppa018174) was referred to as PpGID1c in peaches, which shared 95.3% similarity with AtGID1c (Hollender et al., 2016). The structure of the PpGID1 genes was analyzed using TBtools (Chen C. et al., 2018). According to previous research reports, GID1 protein sequences from a total of eight species were obtained, and all protein sequences were downloaded from the TAIR (http://arabidopsis.org/), Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html), and RGAP websites (http://rice.plantbiology.msu.edu/index.shtml). The sequence alignment was performed using the DNAMAN software (Lynnon Biosoft, Foster City, CA, USA). The phylogenetic tree was built with MEGA6 by employing the neighbor-joining method (Tamura et al., 2013).
Liquid Chromatography-Tandem Mass Spectrometry
Extraction steps: Grind the peach bud sample in LN until it is crushed, accurately weigh all the samples into a test tube, add 10 ml of acetonitrile solution add 2 μl of internal standard mother liquor, extract overnight at 4°C, centrifuge at 12,000 g for 5 min, and take the supernatant. Then, add five times the volume of acetonitrile solution to the precipitate again, extract two times, and combine the supernatants. Afterward, add 35 mg of C18 filler, shake vigorously for 30 s, centrifuge at 10,000 g for 5 min, and take the supernatant. Blow-dry with N and reconstitute with 200 μl of methanol dissolve, pass through a 0.22-μm organic phase filter membrane, and put it in a refrigerator at −20°C to be tested on the machine.
Liquid phase conditions: column: poroshell 120 SB-C18 reversed-phase chromatography (2.1 × 150, 2.7 um); column temperature: 30°C; mobile phase: A:B = (methanol/0.1% formic acid) (water/0.1% formic acid); injection volume: 2 μl; mass spectrometry parameters: ionization mode: electrospray ionization (ESI) positive and negative ion mode monitoring separately; scan type: multiple reaction monitoring (MRM); curtain gas: 15 psi; spray voltage: +4,500 V, −4,000 V; atomizing gas pressure: 65 psi; auxiliary gas pressure: 70 psi; atomization temperature: 400°C. Selected reaction monitoring conditions for deprotonated GA4 [(M-H)-]: substance name: GA4; parent ion: 331.1 (m/z); product ion: 243.2*/213.1(m/z); declustering voltage: −131 (V); collision energy: −24/−39 (V).
Construction of the PpGID1c Overexpression Vector and Plant Transformation
We cloned the full-length Open Reading Frame sequence of PpGID1c (Supplementary 2). A homologous recombination technique (Puchta, 2002) was used to connect it to the PRI-GFP (35S: GFP) vector. The expression vector was then transformed into Agrobacterium tumefaciens strain GV3101, while selected monoclonal Agrobacterium colonies were used to screen for positive clones by PCR. Transgenic Arabidopsis seeds were obtained by the floral dip method (Clough and Bent, 2010). The harvested seeds were screened with 50 mg·L−1 of kanamycin (Kan). Resistant seedlings grew normally without chlorosis, while we used PCR to screen positive plants. We selected plants with high target gene expression in Arabidopsis as candidates in the T1 generation, while T2 generation seeds were segregated for further selection. Seeds that did not appear to segregate in the T3 generation were selected as homozygous strains; after qRT-PCR identification, at least three high expression homozygous strains were obtained, which were named PpGID1c-ox1, PpGID1c-ox3, and PpGID1c-ox5, and the phenotypes were observed and recorded.
Yeast Two-Hybrid Assay
The CDS (coding sequence) sequences of the PpGID1c, PpDELLA1, and PpDELLA2 genes were ligated to the pGBKT7 and pGADT7 plasmid backbones by homologous recombination technology, respectively, to generate the BD-PpGID1c, AD-PpDELLA1, and AD-PpDELLA2 fusion plasmid vectors (restriction enzyme sites: NdeI and EcoRI). Co-transform Yeast Two-Hybrid yeast competent cells with different combinations were spread on SD/-Leu/-Trp medium (synthetic defined minimal medium without leucine and tryptophan), inverted at 30°C for 2–3 days, had positive clones picked then verified by PCR and diluted to an Optical Density equal to about 0.02, and then inoculated to SD/-Ade/-His/-Leu/-Trp (synthetic defined minimal medium without adenine, histidine, leucine and tryptophan) solid culture plates for interaction detection between PpGID1c and PpDELLA.
Results
Isolation and Characterization of PpGID1
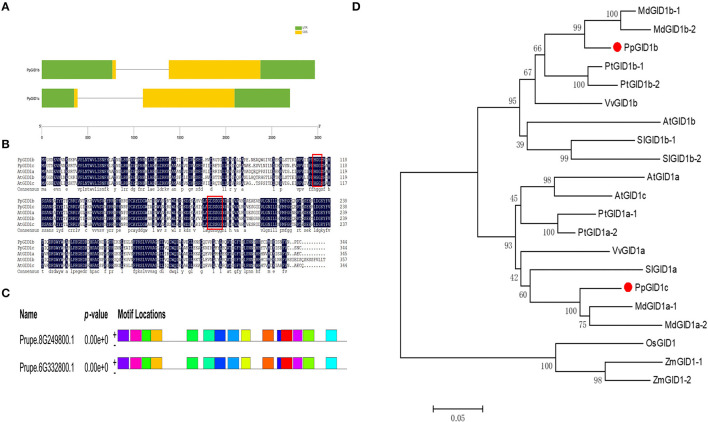
To further explore the function of the peach gibberellin insensitive dwarf1 gene, a BLAST comparison analysis of the GID1 protein sequence in Arabidopsis was performed, which revealed two similar GID1 genes in the peach gene database. Based on the similarity to the GID1 protein sequence in Arabidopsis, we named Prupe.6G332800 and Prupe.8G249800 as PpGID1c and PpGID1b, respectively (Supplementary 3). PpGID1c consists of a 2,698-bp genomic region and encodes 344 amino acids. A gene structure analysis found that both the PpGID1b and PpGID1c genes contain two exons and one intron (Figure 1A). Sequence alignments were carried out with the GID1 genes between peach and Arabidopsis. The PpGID1 protein had high similarity with other Arabidopsis GID1 proteins, and it also has conserved HGG and GDSSG domains. This conserved domain is the key site for gibberellin receptors to sense gibberellin (Figure 1B). MEME (http://meme.nbcr.net/meme/cgi-bin/meme.cgi) and Pfam (http://pfam.sanger.ac.uk/) were used to analyze the conservative structure of PpGID1, which found that PpGID1c and PpGID1b had similar motif gene structures, indicating that the GID1 gene is highly conserved in peaches (Figure 1C). To analyze the phylogenetic relationship among the GID1 genes, we collected GID1 genes from eight species according to a study by Yoshida et al. (2018) (Supplementary 4). Phylogenetic analysis also revealed the evolutionary relationship of GID1 homologs in different species and that the peach GID1c is highly similar to the apple GID1 protein, which all belong to the Rosaceae species (Figure 1D).
Figure 1.
Structure and protein sequence analyses of PpGID1b and PpGID1c. (A) PpGID1b and PpGID1c gene structure. The yellow parts indicate exons, the green parts indicate the CDS sequences, and the lines indicate introns. (B) Alignment of the amino acid sequences of PpGID1b and PpGID1c with AtGID1a, AtGID1b, and AtGID1c. The red boxes are marked with HGG and GDSSG domains. (C) Analysis of PpGID1 conserved elements. (D) Phylogenetic tree of gibberellin insensitive dwarf1 (GID1) proteins from different species. Prunus persica: Prupe.8G249800 (PpGID1b), Prupe.6G332800 (PpGID1c). Populus trichocarpa: Potri.005G040600 (PtGID1a-1), Potri.013G028700-(PtGID1a-2), Potri.002G213100 (PtGID1b-1), Potri.014G135900 (PtGID1b-2). Vitis vinifera: GSVIVG01022014001 (VvGID1a), GSVIVG01011037001 (VvGID1b). Arabidopsis thaliana: AT3G05120 (AtGID1a), AT3G63010(AtGID1b), AT5G27320 (AtGID1c). Solanum lycopersicum: Solyc01g098390 (SlGID1a), Solyc06g008870 (SlGID1b-1), Solyc09g074270 (SlGID1b-2). Oryza sativa: LOC_Os05g33730 (OsGID1). Zeamays: GRMZM2G173630_T01 (ZmGID1-1), GRMZM2G016605_T02 (ZmGID1-2). Malusdomestica: MDP0000319301(MdGID1a-1), MDP0000445131 (MdGID1a-2), MDP0000319522 (MdGID1b-1), MDP0000929994(MdGID1b-2). PpGID1b and PpGID1c are marked with red dots.
GA4 Levels and the Expression of PpGID1 During Peach Leaf bud Dormancy
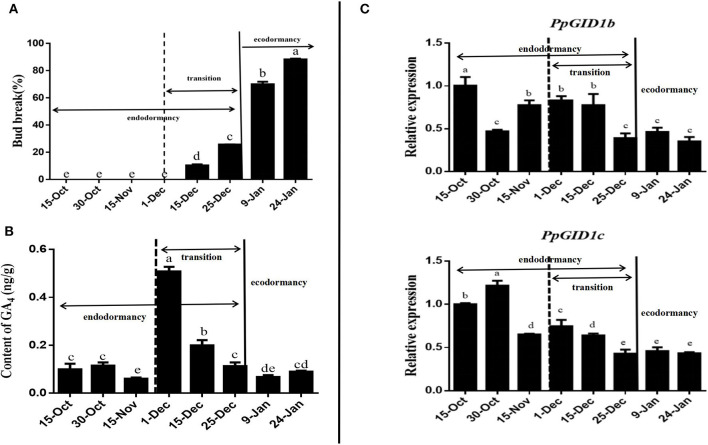
To determine whether the expression of PpGID1 is related to peach leaf bud endodormancy, we first defined the dormancy status of the peach leaf buds from 2018 to 2019 (Supplementary 5). As shown in Figure 2A, from October 15 to December 1, the peach leaf buds did not burst after 25 days of in vitro culture and were in endodormancy. From December 1, the peach leaf buds began to burst. On December 25, the leaf bud burst rate was 25.7%, which was still lower than 50%, indicating that the buds were still in endodormancy. Thus, it was in endodormancy from October 15 to December 1, with the endodormancy transition occurring from December 1 to 25. This transition stage is a critical period for relieving endodormancy. The burst rate of leaf buds exceeded 50% from January 9 to 24 of the following year. The main factor limiting leaf budburst at this stage was the low outer temperature, which was the period of ecodormancy.
Figure 2.
Determination of dormancy progress, GA4 levels, and the expression of PpGID1. (A) Bud-break percentage of the Zhongyou 4 variety from October 15, 2018, to January 24, 2019. Annual shoots were collected from peach trees in the field on the dates indicated and placed in water for 25 d before assessment. Shoots with <50% bud-break were considered dormant, and the values are means of 20 shoots. (B) GA4 levels in leaf bud determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). (C) Relative expression of PpGID1b and PpGID1c during peach leaf bud dormancy. Data are means (± SD) of the three biological replicates, and expression is relative to that of PpUBQ. Different letters indicate significant differences between means as determined by an ANOVA followed by Duncan's multiple range test (P < 0.05).
Next, we determined GA4 levels and the PpGID1 expression pattern during peach leaf bud dormancy (supplementaries 6–8). As shown in Figure 2B, throughout the dormant period, the GA4 content of the peach leaf buds showed the tendency to increase first before decreasing, which reached the highest on December 1st. The GA4 content of the endodormancy release period was significantly higher than that in the ecodormancy period, which was similar to observations in poplar (Karlberg et al., 2010; Rinne et al., 2011). In addition, the expression level of PpGID1b and PpGID1c during ecodormancy was significantly lower than endodormancy. Furthermore, during the period of endodormancy release (December 1st to 25th), the expression of PpGID1c was positively correlated with changes in GA4 levels (Supplementary 9), suggesting that PpGID1c may have a promoting effect on the endodormancy release of peach leaf bud (Figure 2C).
Heterologous Overexpression of PpGID1c in Arabidopsis
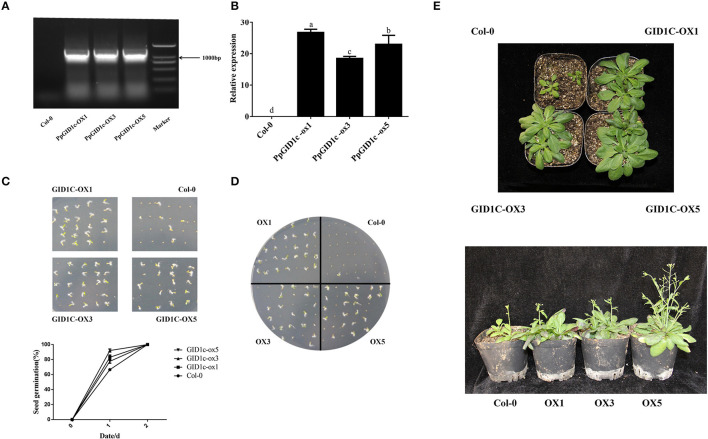
Due to the difficulty in obtaining transgenic peach plants, to gain further insight into the function of PpGID1c, the 35S::PpGID1c fusion plasmid was heterologously transformed into Arabidopsis. The transgenic Arabidopsis lines were identified by PCR and qRT-PCR (Figures 3A,B). To elucidate the roles of PpGID1c in endodormancy, the homozygous Arabidopsis seeds were plated on an MS medium and the germination rate of PpGID1c and Col-0 seeds was observed. Compared with Col-0, PpGID1c transgenic seeds showed a higher germination rate on day 1 and all were germinated on day 2 (Figure 3C). When GA biosynthesis inhibitor PAC was added to the MS medium, the Col-0 seeds could not germinate, but PpGID1c transgenic Arabidopsis seeds could relieve this inhibition, which showed the phenomenon of promoting seed germination (Figure 3D). It can be seen that PpGID1c transgenic Arabidopsis can receive a stronger GA signal, which is beneficial to the promotion of seed germination.
Figure 3.
PpGID1c transgenic Arabidopsis line overexpression identification. (A) PCR identification of T3 generation positive transgenic lines. (B) Quantitative real-time PCR (qRT-PCR) identification of T3 generation positive transgenic lines. (C) Determination of germination rate of Col-0 and PpGID1c transgenic Arabidopsis in an MS medium. (D) Determination of the germination rates of Col-0 and PpGID1c transgenic Arabidopsis seeds in MS + PAC. (E) Phenotyping of rosette leaves and flowering in Col-0 and PpGID1c transgenic Arabidopsis.
Next, we observed the effect of PpGID1c overexpression on the growth and development of Arabidopsis. As shown in Figure 3E, the PpGID1c-overexpression Arabidopsis rosette leaves became longer and wider, with the growth vigor being obviously stronger than Col-0. As the plant grew, the PpGID1c-overexpression Arabidopsis height became higher compared with Col-0, while the number of inflorescences and branches increased significantly. It also bloomed earlier (Figure 3E).
In summary, the PpGID1c promotes Arabidopsis seed germination and growth, suggesting that the PpGID1c may have the effect of accelerating the release of endodormancy.
Effects of Exogenous Hormones on the Release of Endodormancy in Peach Leaf Buds
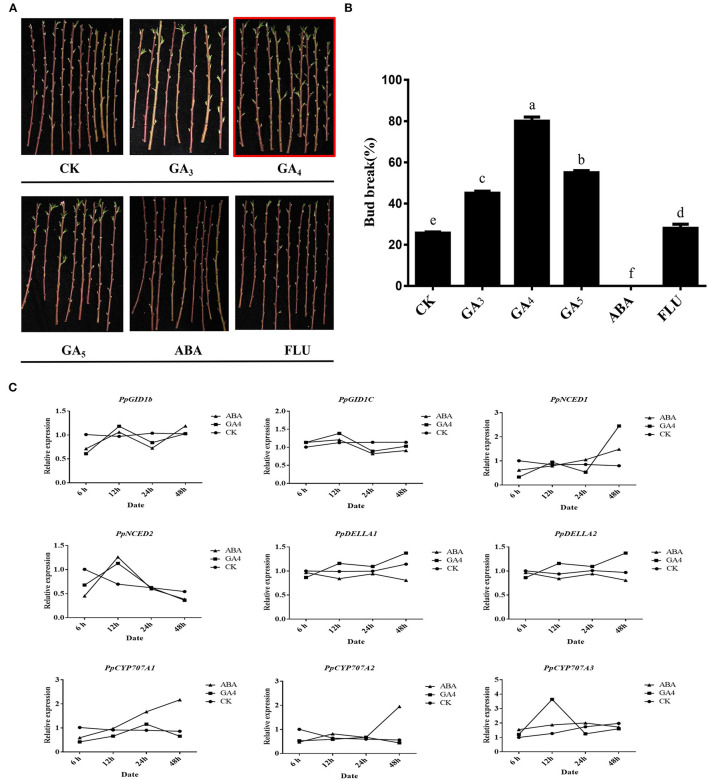
Phytohormones play an important role in the dormancy–growth cycle (Horvath et al., 2003; Ruttink et al., 2007). Among them, ABA and GA are the two most important hormones that antagonistically regulate bud endodormancy induction, maintenance, and release (Wang et al., 2015). A high level of endogenous ABA is the primary factor in maintaining bud endodormancy (Zheng et al., 2018a), while GA is responsible for endodormancy release (Zhuang et al., 2013). To analyze the effects of different hormones on peach leaf buds during endodormancy, we used a water treatment as CK, while different types of hormones were used to treat the annual peach branches (Figure 4A). The break rate of the peach leaf buds cultured in water was 25.6%, which was <50%, indicating that it was still in endodormancy. The application of GA could make the break rate reach more than 50%, indicating that GA can promote the release of endodormancy. It also indicated that GA4 treatment had the most obvious effect on promoting the bud break, indicating that GA4 may be the key gibberellin in promoting the release of peach leaf bud endodormancy (Figure 4B). In addition, ABA could significantly inhibit the bud break of peach leaf buds. Under treatment with fluridone, an ABA biosynthesis inhibitor promoted the endodormancy release in peach leaf buds, suggesting that ABA plays a crucial role in endodormancy maintenance, which is consistent with studies on poplar (Azeez et al., 2021).
Figure 4.
Effects of different hormone treatments on the burst of peach leaf buds. (A) Effects of different hormone treatments (GA3, GA4, GA5, ABA, and FLU) on the burst of leaf buds. (B) Calculation of peach leaf buds breaks rate. (C) Effects of GA4 and ABA treatments on GA and ABA signaling pathway gene expression.
The dynamic changes in gibberellin and abscisic acid synthesis and decomposition jointly regulate the process of plant dormancy. As the GA4 and ABA treatments had significant effects on peach leaf bud breakage, we further explored the expression of gibberellin signaling- and ABA metabolism pathway-related genes (Supplementary 10). DELLA family proteins play a key role in plant GA signal transmission (Zheng et al., 2015). CYP707A family genes are the coding genes of ABA 8'-hydroxylase, while 9-cis-epoxy carotenoid dioxygenase (NCED) is the key rate-limiting enzyme for ABA biosynthesis in plants; they are the key genes in the process of ABA synthesis and catabolism and have been shown to be involved in peach bud endodormancy (Li et al., 2018). As shown in Figure 4C, the PpDELLAs showed opposite expression patterns during the GA4 and ABA treatment. GA4 treatment promoted the expression of PpDELLAs and PpNCED1. Abscisic acid treatment downregulated the expression of PpDELLAs and upregulated the expression of PpCYP707A1/2. It is worth noting that the PpDELLA1 expression trend was consistent with CK after 48 h of GA4 treatment. However, not only did the expression of PpDELLA2 not decrease but it increased significantly. These results indicate that GA4 mainly affects the expression of PpDELLA2 and then promotes endodormancy release in peach leaf buds, although this is unreported.
The Interaction Between PpGID1c and PpDELLAs
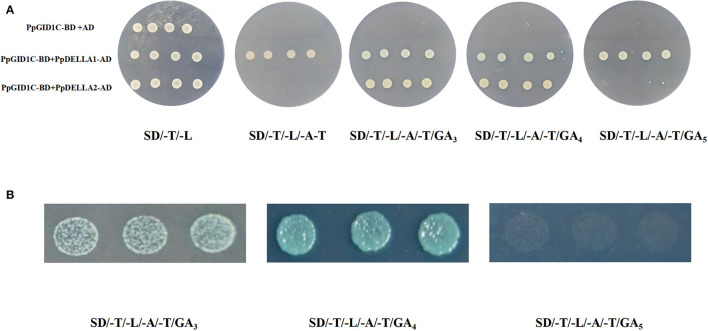
Two DELLA proteins were identified in the peach genome, which all contain the DELLA and GRAS domains. We named them PpDELLA1 and PpDELLA2. Next, we tested whether the PpGID1c interaction with PpDELLA proteins requires gibberellin. We first tested whether there was an autoactivation of PpGID1c. As shown in Figure 5A, PpGID1c-BD and AD empty vectors can grow normally on SD/-L/-T solid medium but cannot grow on SD/-A/-H/-L/-T solid medium, indicating there was no autoactivation of PpGID1c. Then, we performed an interaction detection with different combinations. Regardless of the presence of GA, PpGID1c-BD and PpDELLA1-AD can grow normally on SD/-A/-H/-L/-T solid medium. In contrast, PpGID1c-BD and PpDELLA2-AD cannot grow on SD/-A/-H/-L/-T solid medium without GA. However, when GA3 or GA4 is present, PpGID1c can interact with PpDELLA2 and grow normally. It is worth noting that the SD/-A/-H/-L/-T solid medium added with GA4, the yeast with the combination of PpGID1c-BD and PpDELLA2-AD, had the best growth rate and morphology in the first 48 h (Figure 5B). These results indicate that the GA4-PpGID1c-PpDELLA2 model plays an important role in the endodormancy release period of peach leaf buds.
Figure 5.
Interaction between PpGID1c and PpDELLA1/2. (A) The Y2H-Gold yeast strain was co-transformed with the bait and prey to SD/-Leu/-Trp and SD/-Leu/-Trp/-His/-Ade/GA medium. Yeast cells transformed with AD + BD-PpGID1c were included as negative controls. (B) Co-transformed the PpGID1c-BD and PpDELLA2-AD yeast strain in the first 48 h of SD/-Leu/-Trp/-His/-Ade/GA medium.
Discussion
The seasonal dormancy in deciduous fruit trees is a complex physiological state that is regulated by many plant hormones and genes (Cooke et al., 2012). Gibberellic acid is known to be particularly important in controlling dormancy (Zhuang et al., 2013; Wen et al., 2016). A study by debeaujoni (2000) reported that GA-deficient Arabidopsis mutants have increased dormancy and require exogenous GA to germinate, with the removal of the seed coat possibly relieving this need because the seed coat germination barrier and the embryo dormancy caused by ABA need GA to overcome. Gibberellic acid can also improve bud breakage depending on the status of bud dormancy (Zheng et al., 2018a). A previous study in peach found that different GA can promote the breakage of floral bud dormancy (Reinoso et al., 2002). Moreover, GA3 and GA4 play different roles in Populus, as GA4 can induced bud breakage while GA3 cannot (Rinne et al., 2011). In this study, the different GA that we tested all could promoted leaf bud endodormancy release during the treatments. We also found that, compared with other GA treatments, GA3 treatment will cause the severe shedding of peach leaf buds, with the unshed leaf buds becoming <50% (Figure 4). Gibberelic acid4 treatment has a significant effect on promoting the burst of leaf buds in the endodormancy period of peaches. In addition, the GA4 content of peach leaf buds during the endodormancy release period was significantly higher than in the ecodormancy period. The content of GA4 was also positively correlated with the expression of PpGID1c (Supplementary 9), which leads us to believe that GA4 is the key gibberellin to promote the release of endodormancy.
Previous studies have found that PpGID1 may be related to peach bud burst (Hollender et al., 2016) and that the GID1 gene has the function of promoting the release of Arabidopsis seed dormancy (Amber et al., 2015). The ectopic expression of the Pinus tabulaeformis GID1 gene in Arabidopsis can promote the germination of seeds (Du et al., 2017). In rice, the overexpression of OsGID1-enhanced seed germination and plant growth and development; it also exhibited a GA-overdose phenotype (Chen L. et al., 2018). The overexpression of the GID1c of P. salicina can partially compensate for the dwarf phenotype of the Arabidopsis gid1a-gid1c double mutant (El-Sharkawy et al., 2014). The Arabidopsis gid1b-gid1c double mutant seeds show a phenotype of enhanced dormancy or a failure to germinate (Voegele et al., 2011). In this study, we also found that the PpGID1c-overexpression was observed to promote Arabidopsis seed germination (Figure 3C), and can relieve the inhibition of PAC compared with Col-0 seeds (Figure 3D). As the plant grows, PpGID1c-overexpressing promoted Arabidopsis growth and flowering (Figure 3E), suggesting that the GID1c gene might be functionally conserved. Moreover, the expression of PpGID1c was upregulated during endodormancy and lowered during ecodormancy (Figure 2C). Thus, we further analyzed the Pearson correlation between the content of GA4 and the relative expression of PpGID1c during endodormancy, endodormancy release, and ecodormancy (Supplemental 12), which indicated that they all had a consistent trend of change in the corresponding period, showing a significant correlation (p < 0.05). All results indicated that PpGID1c has a pivotal role in the release of endodormancy in peach leaf buds.
A study by Silverstone et al. (1998) reported five DELLA genes (RGA, GAI, RGL1, RGL2, and RGL) in Arabidopsis. The DELLA proteins are master components of GA signaling, are repressors of plant growth, and are degraded after binding to GAs (Sun, 2010). Gibberellin stimulates seed germination, stem elongation, and flowering by negatively regulating the DELLA repressors of GA responses (Hauvermale et al., 2012). The overexpression of PmRGL2 in poplar delayed the onset of bud dormancy and resulted in dwarf plants relative to wild-type trees.
The DELLA-dependent feedback regulation of GA biosynthesis has been verified in many GA-sensitive and -insensitive mutants (McGinnis et al., 2003; Dill et al., 2004). The gid1 mutant in rice and Arabidopsis showed excessive amounts of DELLA proteins and significantly higher concentrations of endogenous GAs compared with wild-type plants (Ueguchi-Tanaka et al., 2005; Griffiths et al., 2006). For example, GA-insensitive Arabidopsis mutants, for example, and the gain-of-function DELLA-mutants such as gai showed impaired germination (Willige et al., 2007). By contrast, loss-of-function DELLA-mutants such as gai-t6 showed enhanced germination (Kucera et al., 2005). In this study, GA4 treatment significantly increased the expression of the GA signaling pathway gene PpDELLA2. The Y2H assay showed that the interaction between PpGID1c and PpDELLA1 does not depend on GA signal, but the interaction between PpGID1c and PpDELLA2 requires a GA4 signal (Figure 5).
Bud endodormancy is a complex physiological process that is indispensable for the survival, growth, and development of deciduous perennial plants. The timely release of endodormancy is essential for the flowering and fruit production of deciduous fruit trees (Yang et al., 2021). It has been shown that GA4 can directly promote the endodormancy release of pear buds (Yang et al., 2019). During endodormancy release, GA4 binds to the GID1 receptor, while DELLA binding further stabilizes the GA4-GID1-DELLA complex. The complex weakens the inhibitory effect of the DELLA protein on plant growth (Du et al., 2017) where there is an accumulation of the GA4-GID1-DELLA complex, which induces the biosynthesis of GA4. When GA4 levels are high, GA4 subsequently mediates the expression of GID1b, GID1c, and DELLA and further regulates the release of endodormancy (Middleton et al., 2012). In short, our research showed that GA4 plays a key role in the endodormancy release of peach leaf buds and also provided new insights into the mechanism of action of the GA4-GID1c-DELLA2 pathway model in peach leaf bud endodormancy (Figure 6). A better understanding of the mechanism of endodormancy will be of great help in the artificial regulation of endodormancy to cope with climate change and in creating new cultivars with different chilling requirements.
Figure 6.
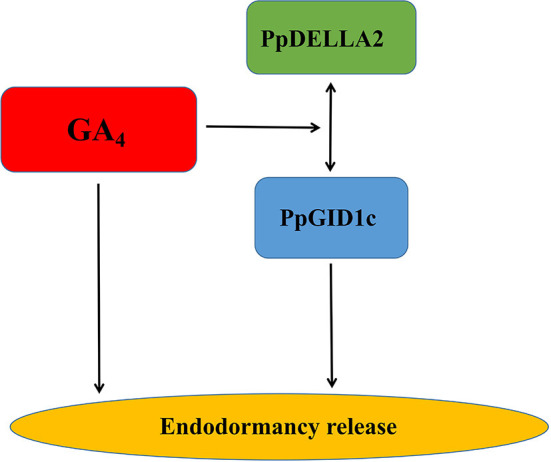
A simple model representing the role of GA4-PpGID1c-PpDELLA2 in the regulation of peach leaf bud endodormancy release.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
LL, QT, and SL designed the study. SL, QT, and LL performed the experiments and analyzed the data. SL wrote the paper. LL and QT are the co-correspondences. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by (1) the National Natural Science Foundation of China (No. 31872041); (2) National key research and development plan (No. 2018YFD1000104); (3) Shandong Province Agricultural Good Seed Project grant, Nos. 2020LZGC007 and 2020LZGC00702; (4) Funding for major agricultural application technology innovation projects in Shandong Province; (5) Provincial Natural Science Foundation of Shandong (No. ZR2018MC023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.713514/full#supplementary-material
References
- Amber L., Tuttle K. M., Takebayashi Y., Seo M., Steber C. M. (2015). Loss of Arabidopsis thaliana Seed Dormancy is Associated with Increased Accumulation of the GID1 GA Hormone Receptors.[J]. Plant Cell Physiol. 56, 1773–1785. 10.1093/pcp/pcv084 [DOI] [PubMed] [Google Scholar]
- Azeez A., Zhao Y. C., Singh R. K. (2021). EARLY BUD-BREAK 1 and EARLY BUD-BREAK 3 control resumption of poplar growth after winter dormancy. Nat. Commun. 12:1123. 10.1038/s41467-021-21449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantín C. M., Arús P., Eduardo I. (2018). Identification of a new allele of the Dw gene causing brachytic dwarfing in peach. BMC Res. Notes 11:386. 10.1186/s13104-018-3490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K., Zhou Z., Wang Q., Guo J., Zhao P., Zhu G., et al. (2016). Genome-wide association study of 12 agronomic traits in peach. Nat. Commun. 7:13246. 10.1038/ncomms13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Xia R., Chen H., He Y. (2018). TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. bioRxiv 289660. [Google Scholar]
- Chen L., Cao T., Zhang J., Lou Y. (2018). Overexpression of OsGID1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Int. J. Mol. Sci. 19, 2744. 10.3390/ijms19092744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Zhang M., Tan B., Jiang Y., Zheng X., Ye X., et al. (2019). A single nucleotide mutation in GID 1c disrupts its interaction with DELLA 1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotechnol. J. 13:94. 10.1111/pbi.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J., Bent A. F. (2010). Floral dip: a simplified method for Agrobacterium- mediated transformation of Arabidopsis thaliana. Plant Journal 16(6):735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Cooke J. E., Eriksson M. E., Junttila O. (2012). The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant CellEnviron. 35, 1707–1728. 10.1111/j.1365-3040.2012.02552.x [DOI] [PubMed] [Google Scholar]
- debeaujoni K. (2000). Gibberellin requirement for Ara bidops seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 122, 415–424. 10.1104/pp.122.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A., Thomas S. G., Hu J., Steber C. M., Sun T. P. (2004). The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellininduced degradation. Plant Cell 16, 1392–1405. 10.1105/tpc.020958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R., Niu S., Liu Y., Sun X., Porth I., El-Kassaby Y. A., et al. (2017). The gibberellin GID1-DELLA signalling module exists in evolutionarily ancient conifers. Sci. Rep. 7, 1–11. 10.1038/s41598-017-11859-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I., Sherif S., El Kayal W., Mahboob A., Abubaker K., Ravindran P., Jyothi-Prakash P. A., et al. (2014). Characterization of gibberellin-signalling elements during plum fruit ontogeny defines the essentiality of gibberellin in fruit development. Plant Mol. Biol. 84, 399–413. 10.1007/s11103-013-0139-8 [DOI] [PubMed] [Google Scholar]
- Frisby J. W., Seley S. D. (1993). Chilling of endodormant peach propagules: IV.Terminal shoot growth of cuttings, including gibberellic acid treatments. J.Am. Soc. Hortic. Sci. 118, 263–268. 10.21273/JASHS.118.2.263 [DOI] [Google Scholar]
- Fukazawa J., Ito T., Kamiya Y., Yamaguchi S., Takahashi Y. (2015). Binding of GID1 to DELLAs promotes dissociation of GAF1 from DELLA in GA dependent manner. Plant Signal. Behav. 10:e1052923. 10.1080/15592324.2015.1052923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo C., Hu J., Urbez C., Gomez M. D., Sun T.p, Perez-Amador M. A. (2014b). Role of the gibberellin receptors GID 1 during fruit-set in Arabidopsis. Plant J. 79, 1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo C., Hu J., Urbez C., Gomez M. D., Sun T. P., Perez-Amador M. A. (2014a). Role of the gibberellin receptors GID1 during fruit-set in Arabidopsis. Plant J. 79, 1020–1032. 10.1111/tpj.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazara R. K., Moharana K. C., Bellieny-Rabelo D., Venancio T. M. (2018). Expansion and diversification of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) family in land plants. Plant Mol. Biol. 97, 435–449. 10.1007/s11103-018-0750-9 [DOI] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z. L., Powers S. J., et al. (2006). Genetic characterization and functional analysis of the GID1gibberellin receptors in Arabidopsis. Plant Cell 18, 3399–3414. 10.1105/tpc.106.047415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauvermale A. L., Ariizumi T., Steber C. M. (2012). Gibberellin Signaling: A Theme and Variations on DELLA Repression. Plant Physiol. 160, 83–92. 10.1104/pp.112.200956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Ueguchi-Tanaka M., Matsuoka M. (2008). GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 13, 192–199. 10.1016/j.tplants.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Hollender C. A., Hadiarto T., Srinivasan C., Scorza R., Dardick C. (2016). A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol. 210, 227–239. 10.1111/nph.13772 [DOI] [PubMed] [Google Scholar]
- Horvath D. P., Anderson J. V., Chao W. S., Foley M. E. (2003). Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci. 8, 534–540. 10.1016/j.tplants.2003.09.013 [DOI] [PubMed] [Google Scholar]
- Karlberg A., Englund M., Petterle A., Molnar G., Sjödin A., Bako L., Bhalerao R. P. (2010). Analysis of global changes in gene expression during activity-dormancy cycle in hybrid aspen apex. Plant Biotechnol. 27, 1–16. 10.5511/plantbiotechnology.27.1 [DOI] [Google Scholar]
- Kucera B., Cohn M. A., Leubner-Metzger G. (2005). Plant hormone interactions during seed dormancy release and germination[J]. Seed Sci. Res. 15, 281–307. 10.1079/ssr2005218 [DOI] [Google Scholar]
- Lang G. (1987). Endo-, para-and ecodormancy: physiological terminology and classification for dormancy research. Hortic. Sci. 22, 271–277. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8246370 [Google Scholar]
- Li J., Xu Y., Niu Q., He L., Teng Y., Bai S. (2018). Abscisic Acid (ABA) Promotes the induction and maintenance of pear (Pyrus pyrifolia White Pear Group) flower bud endodormancy[J]. Int. J. Mol. Sci. 19:310. 10.3390/ijms19010310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Tan Y., Wang H., Leng C. Y., Li D. M., Chen X. D., Gao D. S. (2011). Transcriptional levels of AQPs genes in peach floral buds during dormancy. Chin. J. Appl. Ecol. 22, 2855–2860. [PubMed] [Google Scholar]
- Liu B., Liu X., Yang S., Chen C., Xue S., Cai Y., et al. (2016). Silencing of the gibberellin receptor homolog, CsGID1a, affects locule formation in cucumber (Cucumis sativus) fruit. New Phytol. 210, 551–563. 10.1111/nph.13801 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative pcr and the 2–11CT method. Methods 4, 402–408. [DOI] [PubMed] [Google Scholar]
- Lv L., Huo X., Wen L., Gao Z., Khalil-Ur-Rehman M. (2018). Isolation and role of pmrgl2 in ga-mediated floral bud dormancy release in japanese apricot (Prunus mume Siebold et Zucc.). Front Plant Sci. 9:27. 10.3389/fpls.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis K. M., Thomas S. G., Soule J. D., Strader L. C., Zale J. M., Sun T. P., et al. (2003). The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15, 1120–1130. 10.1105/tpc.010827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton A. M., Ubeda-Tomas S., Griffiths J., Holman T., Hedden P., Thomas S. G. (2012). Mathematical modeling elucidates the role of transcriptional feedback in gibberellin signaling. Proc. Natl. Acad. Sci. U.S.A. 19, 7571–7576. 10.1073/pnas.1113666109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Hirano Y., Sun T. P., Hakoshima T. (2008). Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456, 459–463 10.1038/nature07519 [DOI] [PubMed] [Google Scholar]
- Nakajima M., Shimada A., Takashi Y., Kim Y. C., Park S. H., Ueguchi-Tanaka M., et al. (2006). Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 46, 880–889. 10.1111/j.1365-313X.2006.02748.x [DOI] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15, 1591–1604. 10.1105/tpc.011650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H. (2002). Gene replacement by homologous recombination in plants. Plant Mol. Biol. 48,173. 10.1007/978-94-010-0448-0_12 [DOI] [PubMed] [Google Scholar]
- Reinoso H., Luna V., Dauría C., Pharis R. P., Bottini R. (2002). Dormancy in peach (Prunus persica) flower buds. VI. Effects of gibberellins and an acylcyclohexanedione (trinexapac-ethyl) on bud morphogenesis in field experiments with orchard trees and on cuttings. Canad. J. Bot. 80, 664–674. 10.1139/b02-051 [DOI] [Google Scholar]
- Rinne P. L., Welling A., Vahala J., Ripel L., Ruonala R., Kangasjärvi J., et al. (2011). Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1, 3-β-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23, 130–146. 10.1105/tpc.110.081307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttink T., Arend M., Morreel K., et al. (2007). A molecular timetable for apical bud formation and dormancy induction in poplar[J]. The Plant Cell, 19, 2370–2390. 10.1105/tpc.107.052811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. L., Ciampaglio C. N., Sun T. (1998). The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway[J]. The Plant Cell, 10, 155–169. 10.2307/3870695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.-,p. (2010). Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol. 154, 567–570. 10.1104/pp.110.161554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan P. A., Bai S., Saito T., Ito A., Moriguchi T. (2017). Dormancy-associated mads-box (dam) and the abscisic acid pathway regulate pear endodormancy through a feedback mechanism. Plant Cell Physiol. 58, 1378–1390. 10.1093/pcp/pcx074 [DOI] [PubMed] [Google Scholar]
- Tylewicz S., Petterle A., Marttila S., Miskolczi P., Azeez A., Singh R. K., et al. (2018). Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 360, 212–215. 10.1126/science.aan8576 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Ashikari M., Nakajima M., Itoh H., Katoh E., Kobayashi M., et al. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693. 10.1038/nature04028 [DOI] [PubMed] [Google Scholar]
- Voegele A., Linkies A., Müller K., Leubner-Metzger G. (2011). Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J. Exp. Bot. 62, 5131–5147. 10.1093/jxb/err214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Gao Z., Du P., Xiao W., Tan Q., Chen X., et al. (2015). Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of peach (Prunus persica). Front. Plant Sci. 6:1248. 10.3389/fpls.2015.01248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L. H., Zhong W. J., Huo X. M., et al. (2016). Expression analysis of ABA- and GA-related genes during four stages of bud dormancy in Japanese apricot (Prunus mume Sieb. et Zucc)[J]. J. Horticult. Sci. Biotechnol. 91, 1–8. 10.1080/14620316.2016.1160546 [DOI] [Google Scholar]
- Willige B. C., Ghosh S., Nill C., Zourelidou M., Dohmann E. M., Maier A., et al. (2007). The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19, 1209–1220. 10.1105/tpc.107.051441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Hirai T., Yamamoto E., Kawamura M., Sato T., Kitano H., et al. (2010). A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. Plant Cell 22, 3589–3602. 10.1105/tpc.110.074542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Gao Y., Wu X., et al. (2021). Bud endodormancy in deciduous fruit trees: advances and prospects. Hortic. Res. 8:139. 10.1038/s41438-021-00575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Niu Q., Tang Y., Ma Y., Yan X., Li J., et al. (2019). PpyGAST1 is potentially involved in bud dormancy release by integrating the GA biosynthesis and ABA signaling in ‘Suli' pear (Pyrus pyrifolia White Pear Group). Environ. Exp. Bot. 162, 302–312. 10.1016/j.envexpbot.2019.03.008 [DOI] [Google Scholar]
- Yoshida H., Tanimoto E., Hirai T., Miyanoiri Y., Mitani R., Kawamura M., et al. (2018). Evolution and diversification of the plant gibberellin receptor GID1. Proc. Nat. Acad. Sci. 115, E7844–E7853. 10.1073/pnas.1806040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Acheampong A. K., Shi Z. (2018a). Abscisic acid catabolism enhances dormancy release of grapevine buds. Plant Cell Environ. 41, 2490–2503. 10.1111/pce.13371 [DOI] [PubMed] [Google Scholar]
- Zheng C., Halaly T., Acheampong A. K., Takebayashi Y., Jikumaru Y., Kamiya Y., Or E. (2015). Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 66, 1527–1542. 10.1093/jxb/eru519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Kwame Acheampong A., Shi Z., Halaly T., Kamiya Y., Ophir R., et al. (2018b). Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 69, 1635–1648. 10.1093/jxb/ery022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Crawford G. W., Chen X. (2014). Archaeological evidence for peach (Prunus persica) cultivation and domestication in China. PLoS ONE 9:e106595. 10.1371/journal.pone.0106595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W., Gao Z., Wang L., Zhong W., Ni Z., Zhang Z. (2013). Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. J. Exp. Bot. 64, 4953–4966. 10.1093/jxb/ert284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W., Gao Z., Wen L., Huo X., Cai B., Zhang Z. (2015). Metabolic changes upon flower bud break in Japanese apricot are enhanced by exogenous GA4. Hortic Res. 2:15046. 10.1038/hortres.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.