Abstract
Coronavirus disease-2019 (COVID-19) is associated with systemic inflammation, endothelial activation, and multiorgan manifestations. Lipid-modulating agents may be useful in treating patients with COVID-19. These agents may inhibit viral entry by lipid raft disruption or ameliorate the inflammatory response and endothelial activation. In addition, dyslipidemia with lower high-density lipoprotein cholesterol and higher triglyceride levels portend worse outcomes in patients with COVID-19. Upon a systematic search, 40 randomized controlled trials (RCTs) with lipid-modulating agents were identified, including 17 statin trials, 14 omega-3 fatty acids RCTs, 3 fibrate RCTs, 5 niacin RCTs, and 1 dalcetrapib RCT for the management or prevention of COVID-19. From these 40 RCTs, only 2 have reported preliminary results, and most others are ongoing. This paper summarizes the ongoing or completed RCTs of lipid-modulating agents in COVID-19 and the implications of these trials for patient management.
Key Words: COVID-19, fibrate, lipid-modulating agent, niacin, omega-3, statin
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; CETP, cholesterol ester transfer protein; COVID-19, coronavirus disease-2019; CRP, C-reactive protein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL, high-density lipoprotein; ICU, intensive care unit; NAD, nicotinamide adenine dinucleotide; PCR, polymerase chain reaction; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOC, standard of care
Central Illustration
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) cellular entry is mediated by attachment to angiotensin-converting enzyme 2 (ACE2). Lipid rafts are plasma membrane microdomains, mainly composed of cholesterol, glycosphingolipids, and phospholipids, capable of changing their composition in response to stimuli that may play a critical role in this process (1). SARS-CoV-2 can trigger an uncontrolled innate inflammatory response (cytokine storm) leading to local and systemic tissue damage commonly seen in advanced coronavirus disease-2019 (COVID-19) (2). Inflammation and resultant endothelial injury might lead to a hypercoagulable state and predispose patients to microthrombosis and macrothrombosis (3,4).
Lipid-modulating agents may limit inflammation and thromboinflammation in COVID-19 by exerting antiviral, anti-inflammatory, immunomodulatory, and antithrombotic effects (5). Moreover, lower high-density lipoprotein (HDL) cholesterol and higher triglyceride levels are associated with worse outcomes in patients with COVID-19 (6). Through lipid raft disruption (7), lipid profile improvement, and other effects, lipid-modulating agents may affect the outcomes of patients with COVID-19. Moreover, as previously seen in other SARS infections, SARS-CoV-2 infection could lead to the MYD88 gene being highly induced, with resultant activation of the nuclear factor kappa-light-chain-enhancer of the activated B-cell pathway (8,9). Statins have inhibitory effects on this pathway (and a reduction in type 1 interferon) and hyperinflammation (10,11).
The current paper systematically summarizes the randomized controlled trials (RCTs) evaluating lipid-modulating therapies for the prevention or treatment of COVID-19. The presumed mechanisms of action and existing knowledge of RCTs, as well as knowledge gaps that may influence the design of future trials, are highlighted.
Methods
Data source and search strategy
ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were searched to identify RCTs investigating trials of lipid-modulating agents in COVID-19 (date of last search, March 31, 2021). We used key words for COVID-19 or SARS-CoV-2 or coronavirus disease-2019 and statins (including atorvastatin, rosuvastatin, simvastatin, fluvastatin, lovastatin, pitavastatin, and pravastatin), fibrates (including fenofibrate, clofibrate, bezafibrate, gemfibrozil, and pemafibrate), ezetimibe, bile acid sequestrants (colesevelam, cholestyramine, and colestipol), proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (including alirocumab, evolocumab, and inclisiran), omega-3 fatty acids (including icosapent ethyl, eicosapentaenoic acid [EPA], and docosahexaenoic acid [DHA]), niacin, nicotinic acid, nicotinamide, vitamin B3, evinacumab, mipomersen, lomitapide, bempedoic acid, and cholesteryl ester transfer protein (CETP) inhibitors (anacetrapib, dalcetrapib, evacetrapib, torcetrapib, and TA-8995).
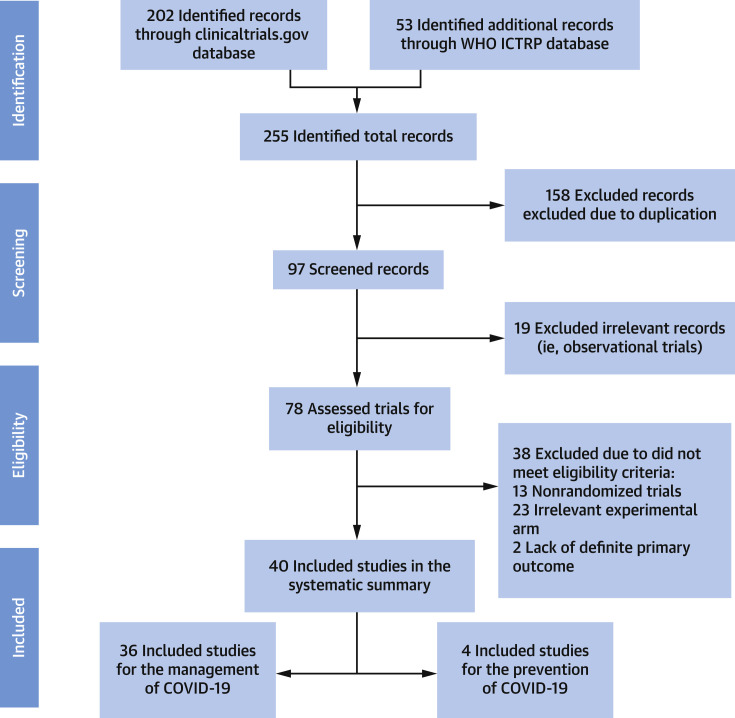
We separated the RCTs between those with agents used for the treatment of patients with COVID-19 versus those used for preventing the development (or severity) of COVID-19. Study eligibility criteria for inclusion in this review were RCT design with a lipid-modifying agent and description of inclusion and exclusion criteria and the primary outcome at ClinicalTrials.gov or the World Health Organization International Clinical Trials Registry Platform. Figure 1 describes the search strategy and screening of the studies. For RCTs that met the aforementioned eligibility criteria, we searched MEDLINE with PubMed Interface, Google Scholar, and pre-print servers for published design papers or final result manuscripts.
Figure 1.
PRISMA Flow Diagram
Key words used for the literature search included: COVID-19 or SARS-CoV-2 with statin, atorvastatin, rosuvastatin, simvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, fibrate, fenofibrate, bezafibrate, gemfibrozil, pemafibrate, ezetimibe, bile acid sequestrant, colesevelam, cholestyramine, colestipol, omega-3, icosapent ethyl, eicosapentaenoic acid, docosahexaenoic acid, PCSK9 inhibitors, Proprotein Convertase Subtilisin/Kexin type 9, alirocumab, evolocumab, inclisiran, niacin, nicotinic acid, nicotinamide, evinacumab, bempedoic acid, mipomersen, lomitapide, cholesterol ester transfer protein inhibitors, CETP inhibitors, anacetrapib, dalcetrapib, evacetrapib, and torcetrapib. COVID-19 = coronavirus disease-2019; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses; WHO ICTRP = World Health Organization International Clinical Trials Registry Platform.
Summary of the search results
A total of 255 records were screened; 97 required further manual review. Ultimately, 40 RCTs met the eligibility criteria, of which 36 were related to the management of COVID-19: 17 for statins, 10 for omega-3 fatty acids, 3 for fibrates, 5 for niacin, and 1 for dalcetrapib. In addition, 4 RCTs of omega-3 fatty acids were identified for the prevention of COVID-19. No RCTs were identified for ezetimibe, bile acid sequestrants, proprotein convertase subtilisin/kexin type 9 inhibitors, evinacumab, mipomersen, lomitapide, bempedoic acid, or CETP inhibitors other than dalcetrapib for the management or prevention of COVID-19.
A summary of the methodological features of the ongoing RCTs categorized according to drug class is provided in Table 1 . Factors such as number of enrollees, comparator types, blinding, type of primary outcomes (clinical or surrogate outcomes), blinded outcome adjudication, and the existence of published design paper are included. For 7 studies, a design paper or study protocol was available (12, 13, 14, 15, 16, 17, 18). Of all RCTs, only 1 has reported the results (at the National Lipid Association Virtual Scientific Sessions) (19).
Table 1.
Methodological Features of the Ongoing RCTs Categorized According to Drug Class
| Total No. of Patients | Total No. of RCTs | Sample Size |
Enrolling Sites |
Comparator Types |
Blinding |
Primary Clinical Outcome | Primary Surrogate Outcome | Blinded Outcome Adjudication | Design Paper Published | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <150 Participants | >150 Participants | Single-Center | Multicenter | Placebo | SOC/No Intervention | Open Label | Single | Double | |||||||
| Statins | 18,215 | 17 | 7 | 10 | 10 | 7 | 5 | 12 | 11 | 1 | 5 | 14 | 3 | 2 | 4 |
| Fibrates | 1,050 | 3 | 1 | 2 | 1 | 2 | 3 | – | – | – | 3 | 3 | 0 | 1 | – |
| Niacin | 1,200 | 5 | 4 | 1 | 4 | 1 | 5 | – | – | – | 5 | 4 | 1 | – | – |
| Omega-3 fatty acids | 21,898 | 14 | 8 | 6 | 9 | 5 | 7 | 7 | 2 | 5 | 7 | 8 | 6 | – | 3 |
| Dalcetrapib | 227 | 1 | – | 1 | – | 1 | 1 | – | – | – | 1 | 1 | – | – | – |
SOC = standard of care; RCTs = randomized controlled trials.
Potential mechanisms of action of lipid-modulating agents in patients with COVID-19
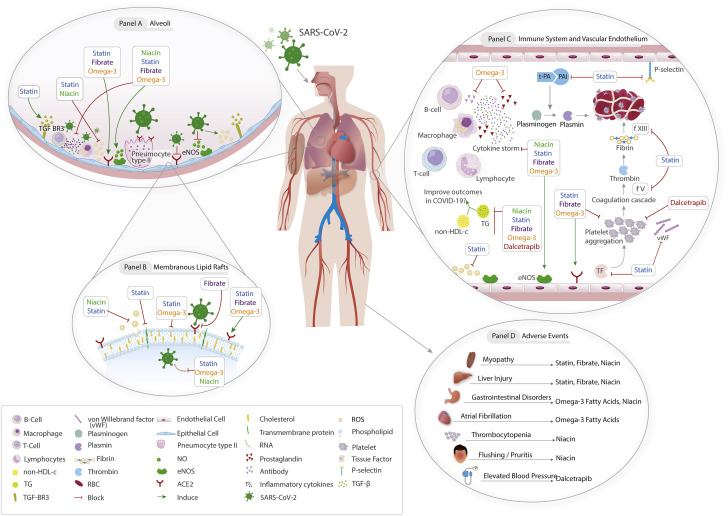
Figure 2 illustrates the potential pathways through which lipid-modulating drugs that have ongoing RCTs may affect outcomes in COVID-19. These agents include statins, omega-3 fatty acids, fibrates, niacin, and dalcetrapib.
Figure 2.
Posited Mechanisms of Action of Lipid-Modulating Agents in COVID-19
(A) Binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to pneumocytes results in cytokine release. Statins, fibrates, and omega-3 fatty acids can maintain the alveolar epithelial integrity. Statins may alleviate pulmonary fibrosis. (B) Fibrates, statins, and omega-3 fatty acids can inhibit viral entry. (C) Omega-3 fatty acids can inhibit the excessive antibody release. Statins, fibrates, omega-3 fatty acid, and dalcetrapib may have antiplatelet activities. Statins may also have antithrombotic properties. (D) Possible adverse effects of lipid-modulating agents. ACE = angiotensin-converting enzyme; COVID-19 = coronavirus disease-2019; CYP = cytochrome P450; RBD = receptor-binding domain; TG = triglyceride.
Statins inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (20) and the production of isoprenoid intermediates that are critical for viral entry, immune signaling, and the inflammatory cascade (21). These agents also induce transcription factors such as Krüppel-like factor-2, limiting inflammation and prothrombotic functions of activated endothelial cells (22). Statins exert antioxidant and antiapoptotic effects, potentiate the production of nitric oxide (3,23), and up-regulate transforming growth factor beta receptor III, thereby reducing collagen deposition and pulmonary fibrosis (24).
Data in observational studies, RCTs, and meta-analyses in patients with sepsis are controversial. Two RCTs showed no improvement in acute respiratory distress syndrome (ARDS) versus placebo (25,26). However, some studies suggest a benefit associated with statin use (27). Secondary analysis of the HARP-2 (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction) trial suggested potential improved survival in patients with a high inflammatory status (28). In a meta-analysis of cohort studies and RCTs of patients with ARDS, statin use was not associated with reduced mortality in patients with acute lung injury but correlated with increased ventilator-free days and reduced Sequential Organ Failure Assessment scores (29). In COVID-19, a recent single-center retrospective study suggested lower adjusted mortality rates in patients with antecedent statin use compared with nonusers (30).
Omega-3 polyunsaturated fatty acids act as a precursor to lipid mediators that reduce inflammation and may prove beneficial in the COVID-19 inflammatory response (14). Icosapent ethyl, an ethyl ester of EPA, has exhibited anti-inflammatory properties (15). Multiple RCTs have evaluated omega-3 polyunsaturated fatty acids in ARDS. Although individual trial results have been mixed, a meta-analysis found favorable outcomes with regard to ventilator-free days, length of stay in the intensive care unit (ICU), organ failure, and mortality in patients receiving a diet enriched with EPA and gamma-linolenic acid (31,32).
In vitro studies suggest that fenofibrate, a fibric acid derivative, destabilizes the receptor-binding domain of the SARS-CoV-2 spike protein and inhibits the receptor-binding domain that binds to ACE2. This may reduce viral infectivity by up to 70% (33).
Niacin (nicotinic acid, nicotinamide) increases HDL cholesterol levels and may reduce inflammatory mediators. Niacin may also possess antiviral activity through increasing nicotinamide adenine dinucleotide (NAD), as nicotinamide restores poly-adenosine diphosphate–ribose polymerase functions, which inhibit the viral replication and support innate immunity to SARS-CoV-2 (34).
CETP inhibitors (eg, dalcetrapib) raise HDL cholesterol levels, which may have anti-inflammatory properties and inhibit platelet activation (35). However, off-target effects should be considered. In the ILLUMINATE (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events) trial, torcetrapib (another CETP inhibitor) had favorable effects on lipids but showed an increase in death due to sepsis and increased systolic blood pressure (36).
Summary of the Ongoing RCTS
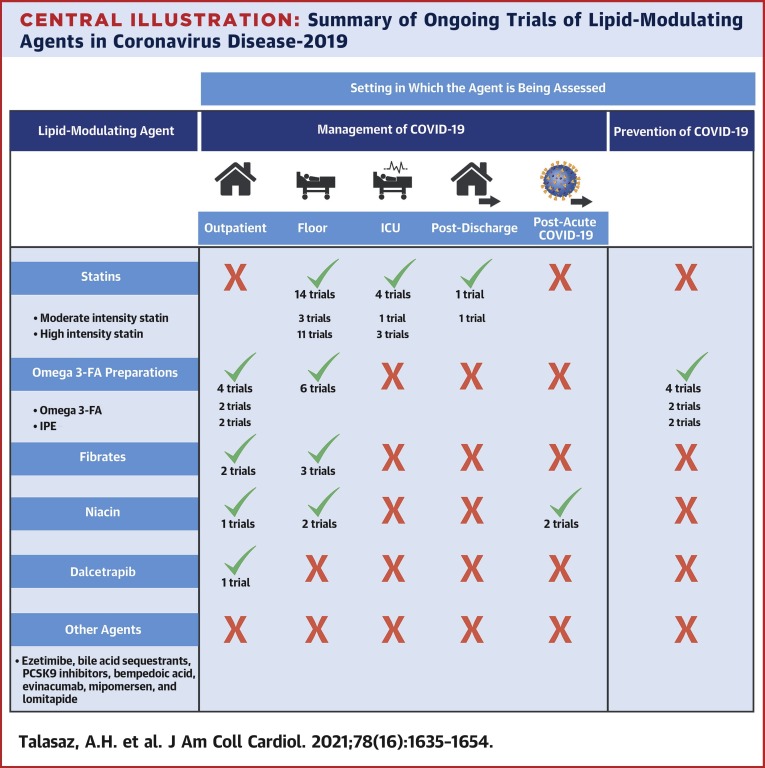
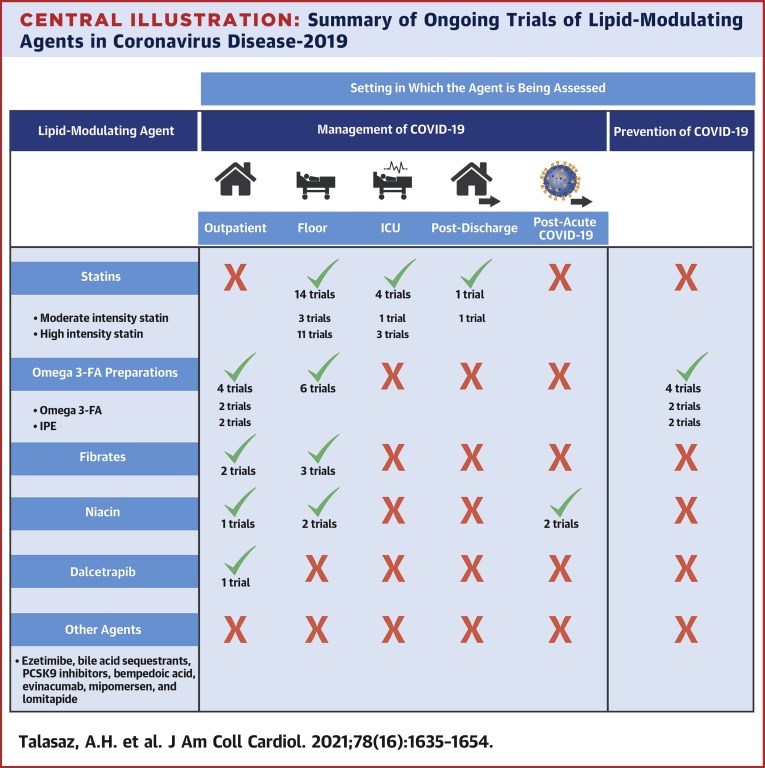
A graphical summary of design features of all RCTs for statins, omega-3 fatty acid preparations, fibrates, and niacin for the management of COVID-19 is illustrated in Figures 3, 4, 5, and 6 , respectively. Also, the section on RCTs for the management of patients with diagnosed COVID-19 begins with statin therapy RCTs, followed by RCTs of omega-3 fatty acid preparations, fibrates, niacin, and dalcetrapib. This sequence does not describe treatment preference. Figure 7 presents a graphical summary of design features of RCTs for the prevention of COVID-19 (only omega-3 fatty acid had such RCTs). The Central Illustration summarizes all ongoing trials per each class of drug. In each section, discussion of these trials is provided according to the clinical setting.
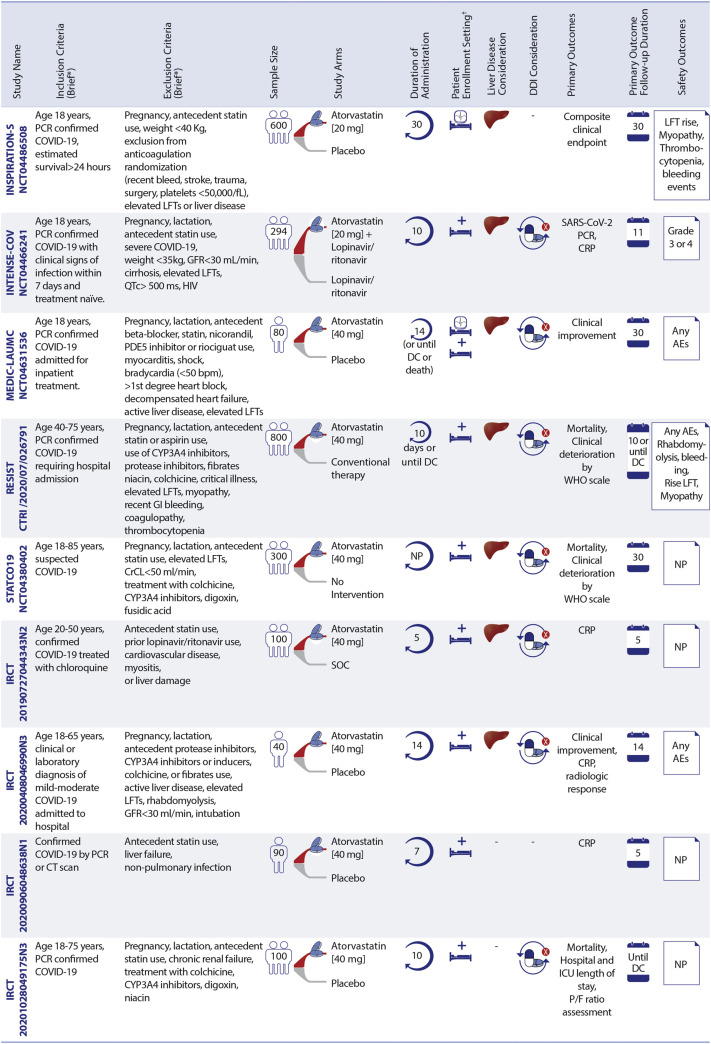
Figure 3.
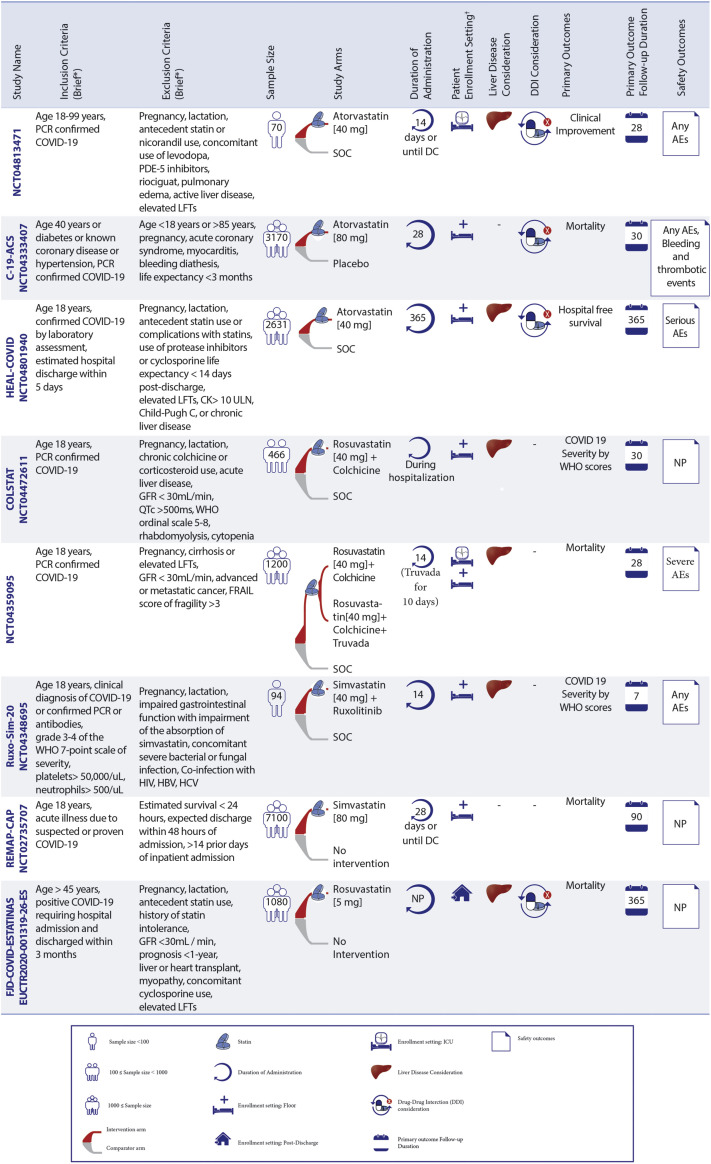
Ongoing Statin Therapy RCTs in Patients With COVID-19
Statin trials are evaluating patients in different settings. ∗See full list of inclusion/exclusion criteria in the original trial records. †Enrollment setting includes hospitalized and post-discharge. AEs = adverse events; CK = creatine kinase; CrCl = creatinine clearance; CRP = C-reactive protein; CT = computed tomography; DC = discharge; DDI = drug–drug interaction; FRAIL = Fatigue Resistance, Ambulation, Illnesses, and Loss of weight questionnaire; GFR = glomerular filtration rate; HBV = hepatitis B virus; HCV = hepatitis C virus; LFT = liver function tests; MI = myocardial ischemia; NP = not pointed; P/F ratio = arterial oxygen partial pressure/fractional inspired oxygen; PDE5 = phosphodiesterase type 5; SOC = standard of care; RCTs = randomized controlled trials; C-19-ACS = Preventing cardiac complications of COVID-19 disease with early acute coronary syndrome therapy: a randomized controlled trial; COLSTAT = Colchicine/Statins for the prevention of COVID-19 complications; RESIST = A randomized control trial of statin and aspirin as adjuvant therapy in patients with SARS-CoV-2 infection; STATCO19 = Atorvastatin as adjuvant therapy in COVID-19. All other full study names are provided in the text.
Figure 4.
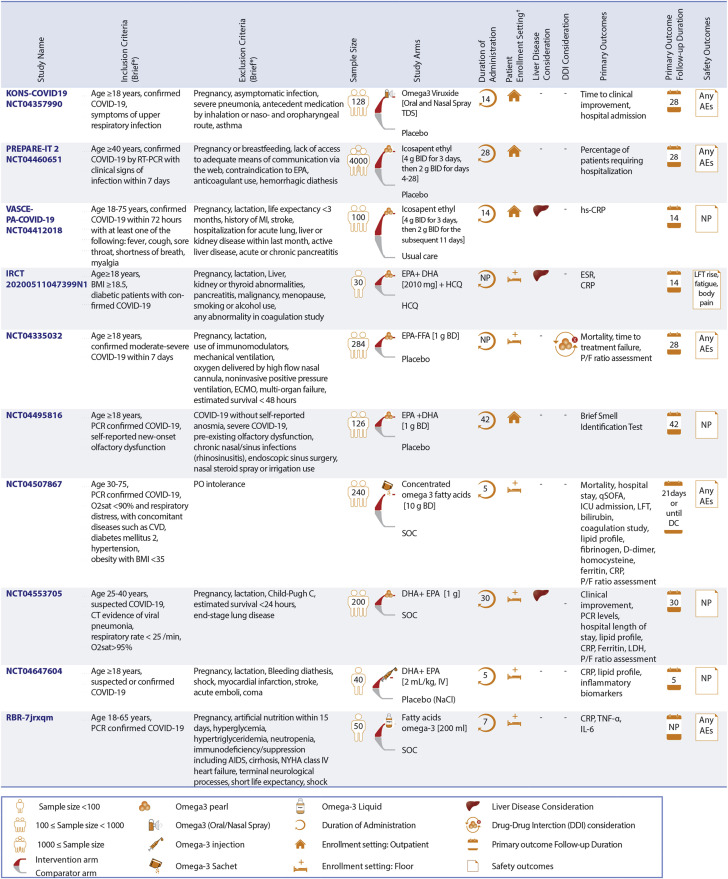
Ongoing RCTs of Omega-3 Fatty Acids in Patients With COVID-19
Omega-3 fatty acid preparations are being assessed among non-intensive care unit (ICU) inpatients, and outpatients. ∗See full list of inclusion/exclusion criteria in the original trial records. †Enrollment setting includes hospitalized non-ICU patients and outpatient settings. BMI = body mass index; CVD = cardiovascular disease; DHA = docosahexaenoic acid; ESR = erythrocyte sedimentation rate; EPA = eicosapentaenoic acid; FFA = free fatty acid; HCQ = hydroxychloroquine; hs-CRP = high-sensitivity C-reactive protein; IL = interleukin; IV = intravenous; LDH = lactate dehydrogenase; NYHA = New York Heart Association; O2sat = oxygen saturation; qSOFA = quick Sequential Organ Failure Assessment; TNF = tumor necrosis factor; other abbreviations as in Figure 3. Full study names are provided in the text.
Figure 5.
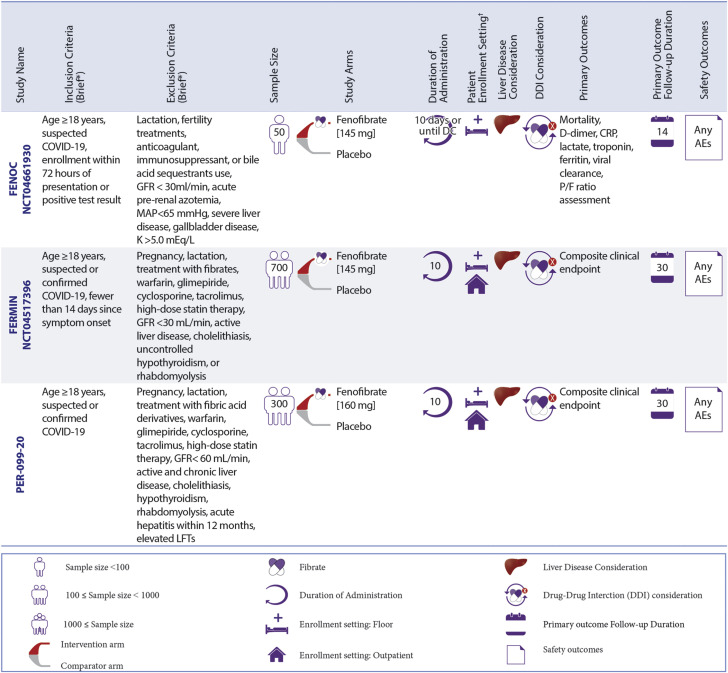
Ongoing RCTs of Fibrates in Patients With COVID-19
Fenofibrate is being evaluated in different settings, including inpatient non-ICU, and outpatient settings. ∗The full list of inclusion and exclusion criteria should be found in the original trial records. †Patient enrollment setting includes hospitalized non-ICU patients and outpatient settings. MAP = mean arterial pressure; other abbreviations as in Figures 3 and 4. Full study names are provided in the text.
Figure 6.
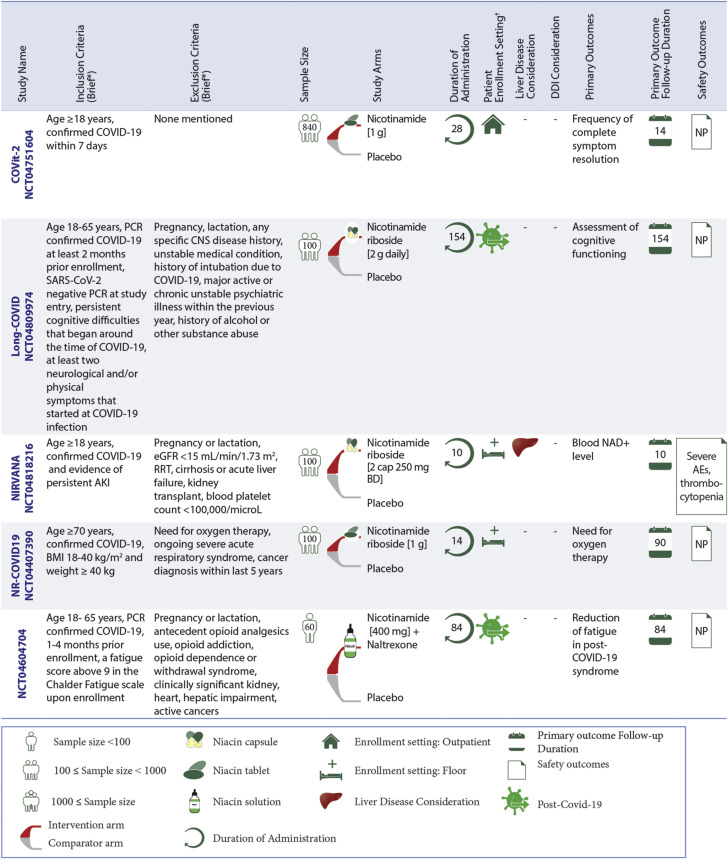
Ongoing RCTs of Nicotinamide in Patients With COVID-19
Niacin is being evaluated in different settings, including inpatient non-ICU, outpatient, and post-acute COVID-19 settings. ∗The full list of inclusion and exclusion criteria should be found in the original trial records. †Patient enrollment setting includes hospitalized non-ICU patients, outpatient, and post-acute COVID-19 settings. AKI = acute kidney injury; CNS = central nervous system; eGFR = estimated glomerular filtration rate; NAD = nicotinamide-adenine dinucleotide; RRT = renal replacement therapy; other abbreviations as in Figures 3 and 4. Full study names are provided in the text.
Figure 7.
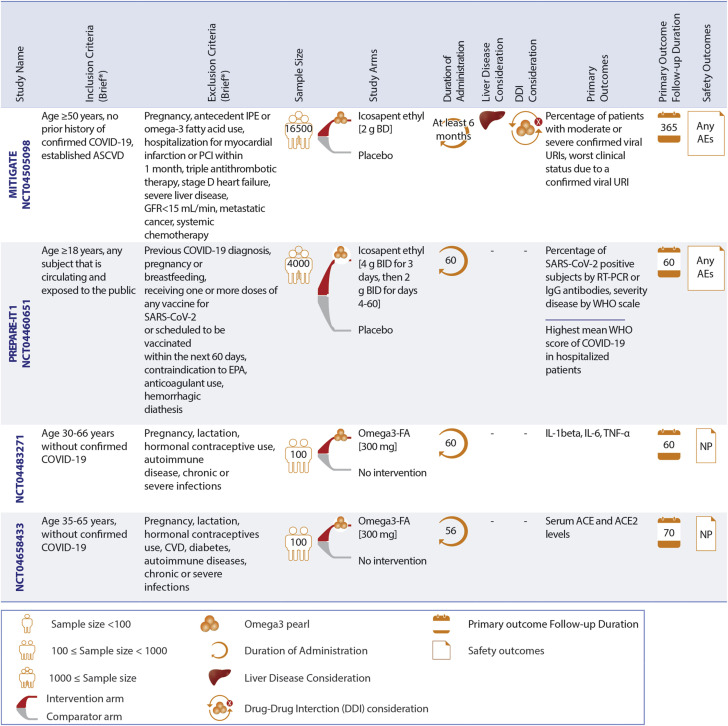
Ongoing RCTs of Omega-3 Fatty Acids for Prevention of COVID-19
Omega-3 fatty acid preparations are being evaluated in those with moderate to high risk of COVID-19. ∗The full list of inclusion and exclusion criteria should be found in the original trial records. AF = atrial fibrillation; ASCVD = atherosclerotic cardiovascular disease; IgG = immunoglobulin G; IL = interleukin; IPE = icosapent ethyl; PCI = percutaneous coronary intervention; RT-PCR = reverse transcription polymerase chain reaction; TNF = tumor necrosis factor; URI = upper respiratory tract infection; other abbreviations as in Figures 23 and 4.
Central Illustration.
Summary of Ongoing Trials of Lipid-Modulating Agents in Coronavirus Disease-2019
Statins are the most frequently studied lipid-modulating agents in randomized controlled trials of patients with coronavirus disease 2019 (COVID-19). Omega-3 fatty acid preparations are the only studied lipid-modulating agents in the prevention of COVID-19. Niacin is the only lipid-modulating agent being studied for post-acute COVID-19. Additional details are provided in Figures 3, 4, and 5.
RCTs for management of patients with diagnosed COVID-19
Ongoing RCTs of statin therapy
Seventeen RCTs of statin therapy have been registered: 16 in the hospitalized setting and 1 in a post-discharge setting. These trials assess either moderate-intensity statin therapy (with simvastatin 40 to 80 mg daily or atorvastatin 20 mg daily or rosuvastatin 5 mg daily) or high-intensity statin therapy (with atorvastatin 40 to 80 mg daily or rosuvastatin 40 mg daily) (37).
Ongoing RCTs of statin therapy in hospitalized non-ICU patients
Statins are being evaluated in 14 RCTs, with the number of participants ranging from 40 to 7,100 patients in the non-ICU hospital settings. These RCTs include 12 for hospitalized non-ICU patients and 2 that enroll both ICU and non-ICU patients (MEDIC-LAUMC [Managing Endothelial Dysfunction in COVID-19: A Randomized Controlled Trial at LAUMC] and Effectiveness and Safety of Medical Treatment for SARS-CoV-2 [COVID-19] in Colombia; NCT04359095).
Moderate-intensity statin therapy is being tested in 3 of these 14 RCTs for hospitalized non-ICU patients. The primary outcomes include mortality within 90 days in the REMAP-CAP (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia) study for 7,100 hospitalized non-ICU patients (17) and assessment of COVID-19 severity according to World Health Organization scores within 7 days in the Ruxo-Sim-20 (Study of Ruxolitinib Plus Simvastatin in the Prevention and Treatment of Respiratory Failure of COVID-19) trial for 94 participants. The comparators of these trials are no intervention and standard of care (SOC), respectively. The third RCT of moderate-intensity statin therapy, INTENSE-COV (Combination therapies to reduce carriage of SARS-Cov-2 and improve outcome of COVID-19 in Ivory Coast: a phase randomized IIb trial), plans to randomize 294 patients to atorvastatin plus lopinavir/ritonavir versus lopinavir/ritonavir for the co-primary outcomes of the proportions of patients with undetectable SARS-CoV-2 polymerase chain reaction (PCR) and C-reactive protein (CRP) <27 mg/L at day 11.
High-intensity statin therapy is being assessed in 11 of 14 ongoing RCTs, with a total of 8,977 hospitalized non-ICU patients with COVID-19. These 11 RCTs are studying high-intensity statin therapy compared with no treatment (2 of 11) or SOC (5 of 11) or placebo (4 of 11). Mortality during hospitalization or within 10 to 30 days is the most common primary outcome in 5 of 11 RCTs with high-intensity statins for hospitalized non-ICU patients (13,18). The HEAL-COVID (Helping Alleviate the Longer-term Consequences of COVID-19) trial plans to enroll 2,631 participants to explore the effect of high-intensity statin therapy on hospital-free survival within 12 months from enrollment.
Patients with liver disease are excluded in 2 of 3 RCTs with moderate-intensity statins and 8 of 11 trials with high-intensity statins. Considerations of drug–drug interactions at the time of enrollment were evaluated in 1 of 3 RCTs and 8 of 11 RCTs with moderate- and high-intensity statins, respectively.
Ongoing RCTs of statin therapy in critically ill patients
Statins are being assessed in 4 RCTs in ICU patients, of which 2 enroll both ICU and non-ICU patients (MEDIC-LAUMC [Managing Endothelial Dysfunction in COVID-19: A Randomized Controlled Trial at LAUMC] and NCT04359095). Two other RCTs include ICU patients only (INSPIRATION-S [The Intermediate versus Standard-dose Prophylactic Anticoagulation and Statin In Critically-ill Patients with COVID-19: An Open Label Randomized Controlled Trial] with 600 participants [12] and Managing Endothelial Dysfunction in Critically Ill COVID-19 Patients at LAUMCRH [NCT04813471]) with 70 patients with COVID-19.
INSPIRATION-S is the only trial of moderate-intensity statin therapy versus placebo in ICU patients (12). A composite of all-cause mortality, venous or arterial thrombotic events, and treatment with extracorporeal membrane oxygenation within 30 days is the study’s primary outcome. The INSPIRATION-S trial completed patient enrollment in April 2021. Thirty-day preliminary results were presented at the 2021 American College of Cardiology Annual Scientific Sessions. Among 587 randomized patients, atorvastatin 20 mg once daily compared with placebo did not result in a significant reduction in the primary composite outcome of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause death with an odds ratio of 0.84 (95% confidence interval [CI]: 0.58-1.21; P = 0.35). In the prespecified analysis of patients hospitalized within 7 days of symptom onset, there was a hypothesis-generating reduction in the odds of the primary outcome (odds ratio: 0.60; 95% CI: 0.37-0.99; P interaction = 0.05) (38).
The remaining RCTs in critically ill patients (3 of 4 trials) are investigating high-intensity statin therapy; 2 of these trials (NCT04813471 and NCT04359095) are studying high-intensity statin therapy compared with SOC. The MEDIC-LAUMC is assessing the effects of high-intensity atorvastatin versus placebo among 80 participants. In addition, 2 of 3 trials of high-intensity statin therapy (MEDIC-LAUMC and NCT04813471) consider drug–drug interactions before enrollment, and clinical improvement within 1 month is being assessed as the primary outcome in these 2 trials. The other trial, NCT04359095, is assessing the influence of high-intensity statin therapy on mortality within 28 days as the primary outcome among 1,200 ICU or non-ICU patients. Patients with liver disease are excluded in all of the RCTs with moderate- or high-intensity statins for critically ill patients.
Ongoing RCTs of statin therapy in post-discharge patients
Rosuvastatin (5 mg daily) is the only statin-based intervention under investigation in the post-discharge setting. The FJD-COVID-ESTATINAS RCT (Multicenter, randomized, controlled, open-label clinical trial to assess the prognostic implications of rosuvastatin treatment in patients discharged after hospitalization for COVID-19) is evaluating rosuvastatin compared with no treatment in 1,080 patients discharged from hospitalization for COVID-19. The primary outcome is a composite of mortality, myocardial infarction, or ischemic stroke within 12 months. Patients with liver disease or concomitant treatment with cyclosporine are excluded. Additional details about ongoing clinical trials of statin therapy are provided in Figure 3.
Ongoing RCTs of omega-3 fatty acid preparations
There are 10 ongoing RCTs evaluating the role of omega-3 fatty acid preparations for the management of COVID-19: 6 RCTs in hospitalized non-ICU patients and 4 ongoing RCTs in the outpatient setting.
Ongoing RCTs of omega-3 fatty acid preparations in hospitalized non-ICU patients
Omega-3 fatty acids are being evaluated in 6 RCTs, with the number of participants ranging from 30 to 284 patients in the non-ICU hospital settings. Most RCTs (5 of 6) assess the oral use of omega-3 fatty acid preparations compared with SOC (3 of 5) or with placebo (1 of 5). The oral use of omega-3 fatty acid preparations plus hydroxychloroquine compared with hydroxychloroquine alone is assessed in the Comparison of the Effectiveness of Omega-3 and Hydroxychloroquine on Inflammatory Factors, Liver Enzymes, and Clinical symptoms in Diabetic COVID-19 Patients (IRCT20200511047399N1) study. Only one RCT (Resolving Inflammatory Storm in COVID-19 Patients by Omega-3 Polyunsaturated Fatty Acids; NCT04647604) explores intravenous administration of omega-3 fatty acid preparations versus placebo (14). Moreover, 3 of 6 RCTs of hospitalized non-ICU patients have co-primary outcomes, including inflammatory markers and lipid levels. Elevated liver enzymes are being evaluated as the safety outcome only in the IRCT20200511047399N1 trial. Patients with liver disease are excluded in 2 of the 6 RCTs of omega-3 fatty acid in hospitalized non-ICU patients.
Ongoing RCTs of omega-3 fatty acid preparations in outpatient setting
Omega-3 fatty acid preparations are being evaluated in 4 ongoing RCTs for the treatment of COVID-19: KONS-COVID19 (Viruxal Oral and Nasal Spray for Treating the Symptoms of COVID-19), VASCEPA-COVID-19 (An Investigation on the Effects of Icosapent Ethyl [Vascepa] on Inflammatory Biomarkers in Individuals With COVID-1), PREPARE-IT 2 (Prevention of COVID19 With EPA in Healthcare Providers at Risk–Intervention Trial 2), and the COVID-19 Anosmia Study (NCT04495816) (16).
The KONS-COVID19 trial tests omega-3 inhaled use versus placebo among 128 outpatient participants. The primary outcome is time to clinical improvement within 28 days. The VASCEPA-COVID-19 trial assesses the oral use of icosapent ethyl compared with usual care in a total of 100 outpatient participants. High-sensitivity CRP level is the primary outcome in VASCEPA-COVID-19. Patients with active severe liver disease are excluded in this trial. Initial findings from the VASCEPA-COVID-19 were presented at the National Lipid Association Scientific Sessions (19). The investigators found that the use of icosapent ethyl for 14 days reduced high-sensitivity CRP (3.2 vs 1.6 mg/L; P = 0.011) and led to symptom improvement, assessed by using the InFLUenza Patient-Reported Outcome score in outpatients with COVID-19 after 14 days. The PREPARE-IT 2 trial is assessing the effects of icosapent ethyl versus placebo in 2,000 outpatient participants. The impact of oral use of omega-3 fatty acid preparations versus placebo on olfactory performance within 6 weeks among outpatients is under evaluation in the NCT04495816 trial with 126 participants. Additional details about ongoing RCTs of omega-3 fatty acid preparations for treatment of COVID-19 are described in Figure 4.
Ongoing RCTs of fibrates
Three RCTs are investigating fibrates in patients with COVID-19. These RCTs are testing fenofibrate versus placebo in hospitalized non-ICU patients: FENOC (Fenofibrate for Patients With COVID-19 Requiring Hospitalization), FERMIN (Fenofibrate as a Metabolic Intervention for COVID-19), and Fenofibrate as a Metabolic Intervention for Coronavirus Disease 2019 [COVID-19]: A Randomized Controlled Trial [PER-099-20]). FERMIN and PER-099-20 also enrolled outpatients. The sample size of these studies ranges from 50 to 700 patients.
The main outcomes in the FENOC study include improvement in laboratory markers, the ratio of arterial oxygen partial pressure to fractional inspired oxygen, and mortality. The composite endpoint as the primary outcome for the FERMIN and PER-099-20 trials will be a global rank score that grades patients based on survival, need for respiratory/mechanical support, the fraction of inspired oxygen/percent oxygen saturation, the number of days out of the hospital for outpatient participants who are hospitalized after enrollment, and the modified Borg dyspnea scale for the outpatient subset not hospitalized. All these trials consider drug–drug interactions before the enrollment. Patients with active liver disease are excluded in all 3 of these trials. Additional information about these RCTs is summarized in Figure 5.
Ongoing RCTs of niacin
Five RCTs of niacin therapy were identified: 2 in hospitalized non-ICU patients, 1 in the outpatient setting, and 2 ongoing RCTs in post-acute COVID-19. All of these trials are studying niacin compared with placebo.
Ongoing RCTs of niacin in hospitalized non-ICU patients
Niacin is being assessed in 2 ongoing RCTs for 100 hospitalized non-ICU patients in each trial: NIRVANA (Nicotinamide Riboside in SARS-CoV-2 [COVID-19] Patients for Renal Protection) and NR-COVID19 (Effects of Nicotinamide Riboside on the Clinical Outcome of Covid-19 in the Elderly). The primary outcomes of these trials are the alteration of blood NAD+ level within 10 days and the need for oxygen therapy with a follow-up duration of 90 days, respectively. In NIRVANA, thrombocytopenia is being evaluated as the safety outcome, and patients with liver disease are being excluded.
Ongoing RCTs of niacin in outpatient setting
The COVit-2 (Improvement of the Nutritional Status Regarding Nicotinamide [Vitamin B3] and the Disease Course of COVID-19) trial is the only study of niacin therapy versus placebo in an outpatient setting; 840 patients plan to be enrolled. The frequency of complete symptom resolution within 2 weeks is the primary outcome.
Ongoing RCTs of niacin in post–COVID-19 setting
Niacin is being evaluated in 2 RCTs in the post–COVID-19 setting: Long-COVID (Clinical Trial of Niagen to Examine Recovery in People with Persistent Cognitive and Physical Symptoms After COVID-19 Illness) and Pilot Study Into Low Dose Naltrexone (LDN) and Nicotinamide Adenine Dinucleotide (NAD+) for Treatment of Patients With Post-COVID-19 Syndrome (NCT04604704). These RCTs assess the oral use of niacin in 100 participants and iontophoresis patches for 60 patients with COVID-19, respectively.
The Long-COVID trial is studying the impact of niacin on the cognitive function of patients with a positive PCR at least 2 months before enrollment. The primary outcome follow-up duration is 22 weeks. NCT04604704 plans to enroll 60 patients with PCR-confirmed COVID-19 1 to 4 months before enrollment. This trial assesses the reduction in fatigue in post–COVID-19 syndrome within 12 weeks as the primary outcome. Patients with liver disease are excluded in the NCT04604704 trial. Additional details about the RCTs of niacin are illustrated in Figure 6.
Ongoing RCT of CETP inhibitors
The dal-COVID (Effect of Dalcetrapib in Patients with Confirmed Mild to Moderate COVID-19) trial is the only study of dalcetrapib (900 mg, 1,800 mg, and 3,600 mg daily) versus placebo and includes 208 outpatients with mild to moderate COVID-19. The primary outcome is time to sustained symptom resolution within 28 days. Patients with liver disease are excluded from the NCT04676867 trial. Drug–drug interactions are being considered before enrollment.
RCTs for prevention of contracting COVID-19
Use of omega-3 fatty acid preparations as a preventive measure against COVID-19 is being investigated in 4 RCTs: MITIGATE (A Pragmatic Randomized Trial of Icosapent Ethyl for High-Cardiovascular Risk Adults) (15), PREPARE-IT 1 (Prevention of COVID19 With EPA in Healthcare Providers at Risk–Intervention Trial 1), the Effect of Omega-3 on Selected Cytokines Involved in Cytokine Storm (NCT04483271), and the Effect of Omega-3 Supplements on the Serum Levels of ACE/ACE2 Ratio as a Potential Key in Cardiovascular Disease and COVID-19 (NCT04658433).
The MITIGATE and PREPARE-IT 1 trials are studying the effects of icosapent ethyl versus SOC and placebo, respectively. NCT04483271 and NCT04658433 assess omega-3 fatty acid supplements compared with no treatment for 100 patients in each trial. The number of confirmed viral infections and worst clinical status due to a viral upper respiratory infection are the co-primary outcomes for MITIGATE with 16,500 participants. The number of confirmed viral infections is the primary outcome in PREPARE-IT 1 with 2,000 participants. The primary outcomes for the NCT04483271 and NCT04658433 trials are inflammatory markers such as interleukin-1beta, interleukin-6, tumor necrosis factor-alpha, and serum ACE and ACE2. Patients with severe liver disease are excluded only in MITIGATE. Additional details about these RCTs are summarized in Figure 7.
Discussion
The perspective of the COVID-19 disease state has broadened from pneumonia to a systemic multiorgan disease, with systemic inflammation and thrombosis as key features (2,39). The current review identified 34 RCTs that evaluate the role of lipid-modulating agents in the management of acute COVID-19, 2 RCTs in patients with post-acute COVID-19, and 4 RCTs for prevention of contracting (or severity of) COVID-19. Results from these trials may expand the armamentarium for management of COVID-19. The neutral results of recent RCTs of escalated-dose anticoagulation in critically ill patients with COVID-19 (40,41) may indicate the significance of the maladaptive immune response in severe COVID-19 (42). It is in this context that lipid-modulating agents with pleotropic effects offer possible therapeutic potential (43). The moderate immunomodulating effect of these agents lessens the chance of excessive immunosuppression and superinfection, commonly seen with other anti-inflammatory agents.
Despite such hope, certain methodological limitations of some of the ongoing RCTs deserve attention. These include small sample size, use of primary surrogate outcomes, and lack of blinded outcome adjudication, which hamper the rigor of the trials. More than 1 year into the pandemic, 40 RCTs of lipid-modifying therapies were identified, with only 21 (with total estimated sample size of 7,675) having a double-blind design. The results from only 2 trials have been communicated in preliminary form and none in the peer-reviewed published form. In addition, despite the scientific rationale summarized in this paper, translation to clinical benefit is not assured. Among the immune-modulating therapies, only steroids have shown consistent efficacy in patients with COVID-19 (44, 45, 46, 47, 48). The neutral results with ivermectin (49) and hydroxychloroquine (50), and the mixed results with colchicine (51,52) and tocilizumab (53,54), remind us that biological plausibility may not translate into meaningful treatment. Hence, the ongoing lipid-modulating therapy RCTs are of particular interest.
Statins as multipurpose drugs?
Despite initial concern that statins might increase expression of ACE2 and facilitate SARS-CoV-2 entry with potential deleterious effects, observational studies suggest an association between antecedent statin use and improved survival. In a population-based propensity-matched study of 10,448 patients with COVID-19, Lee et al (55) reported a significant reduction in hazard of death in statin users compared with nonusers (hazard ratio: 0.64; 95% CI: 0.43-0.95; P = 0.02). In a retrospective propensity matching analysis of 1,296 patients with COVID-19, Gupta et al (30) reported similar reduction in the odds of death with antecedent statin use (odds ratio: 0.47; 95% CI: 0.36-0.62; P < 0.001). Such nonrandomized observational studies are subject to confounding, including confounding by indication, and require confirmation in ongoing RCTs. There are several limitations to the ongoing statin investigations. Several RCTs do not include thrombotic events among their prespecified outcomes. Quality of life is evaluated in only 3 RCTs. The role of statin therapy in the post-discharge setting for patients with COVID-19 is being studied in only 1 RCT with 1,080 patients but not in the outpatient setting.
Omega-3 polyunsaturated fatty acids: hope or hype?
Anti-inflammatory effects (56) and potential impact on ARDS progression (57) make omega-3 fatty acids worthwhile agents for investigation. Functional limitations and quality of life are evaluated exclusively in outpatient trials, whereas mortality and clinical improvement are evaluated in hospitalized patients. Two trials with considerable sample size evaluate the role of icosapent ethyl in the prevention of contracting COVID-19.
Several limitations apply to the omega-3 fatty acid RCTs. There are relatively few total numbers of patients enrolled in trials of omega-3 fatty acids for the management of COVID-19 (Figure 4). Heterogeneity in use of various formulations (EPA, DHA, or EPA-DHA; ethyl esters vs free fatty acids) and impurities and/or oxidative alterations in unregulated supplement preparations make it more difficult to determine the specific effects of each. Icosapent ethyl will be studied in 2 large outpatient trials (PREPARE-IT 1 and MITIGATE) as a preventive agent but not in the inpatient setting.
Fibrates: new spark for a dying candle?
Despite the declining use for cardiovascular risk reduction (58), fibrates may decrease viral entry and SARS-CoV-2 infectivity by increasing sulfatide levels (59) and inhibiting the receptor-binding domain to ACE2 (33). Strengths of the ongoing fibrate trials include endpoint selection; death, ARDS-related outcomes, inflammatory markers, and invasive mechanical support are the primary outcomes under evaluation. Drug interactions were generally considered with fenofibrate initiation. However, fenofibrate is the only fibrate under investigation in RCTs including patients with COVID-19, and its lack of benefit in cardiovascular disease prevention may raise skepticism regarding its utility in COVID-19.
Niacin: time for a comeback?
Anti-inflammatory effects (34) and potential protection against lung injury (60) have made niacin a target for investigation in COVID-19. Niacin is under evaluation in both acute and post-acute settings. Clinical improvement and symptom resolution are primary outcomes in the majority of the RCTs. RCTs in the post–COVID-19 setting will evaluate fatigue and cognitive function for 3 to 6 months.
As with fenofibrate, lack of benefit and presence of adverse effects with nicotinic acid seen in prior cardiovascular RCTs (61,62) may dampen enthusiasm for use in COVID-19. The ongoing trials of niacin in COVID-19 are evaluating the role of nicotinamide, another form of niacin that is not typically used as a lipid-modifying agent. Trials in the inpatient and post-acute setting are limited by small sample size, and variability in niacin dosing may lead to heterogeneous results.
CETP inhibitors: room for potential benefit?
Low HDL levels are associated with increased severity of COVID-19 and correlate with approved biomarkers such as ferritin and D-dimer levels (6). Dalcetrapib will be the first CETP inhibitor to be tested in patients with mild to moderate COVID-19, with time to symptom resolution as the primary outcome. Of note, dalcetrapib did not improve clinical outcomes in those with acute coronary syndromes (63). Among CETP inhibitors, only anacetrapib showed a modest cardiovascular benefit (9% relative risk reduction) (64), whereas torcetrapib (36,65) led to increases in systolic blood pressure and worse cardiovascular outcomes. Findings from dal-COVID will help clarify whether dalcetrapib merits further testing in COVID-19.
Expecting the unexpected: possible adverse effects for lipid-modulating agent trials
Based on the knowledge from RCTs in cardiovascular diseases, important adverse effects should be monitored when the results of these RCTs accrue. Myopathy is the most common adverse effect of statin use and is managed by conversion to other statin formulations. Severe muscle complications (ie, rhabdomyolysis) are exceedingly rare (66). Rise in liver enzyme levels is another potential but infrequent adverse effect. Fibrates may also increase the risk of myopathy and hepatocyte injury (67). Drug–drug interactions play an important role in this increased risk and are considered in the ongoing COVID-19 RCTs (68). Other risks associated with statin use, including hemorrhagic stroke in those with previous stroke, are not of clinically important magnitude (69, 70, 71). New-onset or worsening of atrial fibrillation is a concern with omega-3 fatty acids (72, 73, 74), and increased bleeding due to the effects on platelet aggregation should be monitored in the RCT results.
The adverse effects of niacin, specifically nicotinic acid, include flushing, pruritus, gastrointestinal disorder, thrombocytopenia, hyperuricemia, hyperglycemia, myopathy, and hepatotoxicity (75). However, only nicotinamide is used in COVID-19 studies. The NIRVANA trial assesses thrombocytopenia as a safety outcome and excluded patients with liver disease.
These potential risks are limited by the short duration of treatment in most trials. Of note, the adverse events in massive event-driven cardiovascular trials result from multiple months to years of treatment, whereas the current COVID-19 trials generally have much shorter treatment duration. The possible adverse effects of lipid-modulating agents are illustrated in Figure 2.
Conclusions
Lipid-modulating agents may mitigate the multiorgan damage associated with COVID-19 through anti-inflammatory, antiviral, and pleiotropic effects. Findings from ongoing rigorously conducted and adequately powered RCTs can assess the possible efficacy of lipid-modulating agents in the prevention or treatment of various stages of COVID-19 and may open new horizons for research and clinical practice.
Funding Support and Author Disclosures
Dr Gupta received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation and from the Ben C. Martin Law Firm for work related to an inferior vena cava filter litigation; received consulting fees from Edward Lifesciences; and holds equity in the health care telecardiology startup Heartbeat Health. Dr Madhavan was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). Dr Van Tassell has received research support from Novartis, Swedish Orphan Biovitrum, Olatec Therapeutics, and Serpin Pharma; and is a consultant of R-Pharm and Serpin Pharma. Dr Jimenez has served as an advisor or consultant for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Leo Pharma, Pfizer, ROVI, and Sanofi; has served as a speaker or a member of a speaker bureau for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Leo Pharma, ROVI, and Sanofi; and has received grants for clinical research from Daiichi-Sankyo, Sanofi, and ROVI. Dr Monreal has served as an advisor or consultant for Sanofi, Leo Pharma, and Daiichi-Sankyo; and has received a nonrestricted educational grant by Sanofi and Bayer to sponsor the Computerized Registry of Patients with Venous Thromboembolism. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; participates in speaker engagements with Novartis and Roche Diagnostics; and participates on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the National Institutes of Health. Dr Fanikos has served as a consultant for Portola, Inc and Pfizer, Inc. Dr Piazza has received research grant support from BSC, Bayer, Bristol Myers Squibb/Pfizer Alliance, Portola, Amgen, and Janssen; and has received consulting fees from Amgen, Pfizer, and BSC. Dr Parikh has served as a noncompensated advisor to Abbott Vascular, Boston Scientific, Cardiovascular Systems Inc, Cordis, Janssen, Medtronic, and Philips; receives institutional research support from Abbott Vascular, Boston Scientific, SurModics, TriReme, and Veryan Medical; and is a consultant to Abiomed, Inari Medical, Penumbra, and Terumo. Dr Bhatt has served on the advisory board for Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; has served on the Board of Directors for the Boston VA Research Institute, Society of Cardiovascular Patient Care, and ToBeSoft; serves as Chair of the American Heart Association Quality Oversight Committee; has served on the data monitoring committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo), Population Health Research Institute; has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other, Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has been a site co-investigator, Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; has been a trustee for the American College of Cardiology; and has received unfunded research from FlowCo, Merck, and Takeda. Dr Lip is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo (no fees are received personally). Dr Stone has received speaker or other honoraria from Cook, Terumo, and Orchestra Biomed; has been a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, and CardioMech; and has equity/options from Ancora, Cagent, Applied Therapeutics, BioStar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Med Focus family of funds, and Valfix. Dr Krumholz has received personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, Siegfried & Jensen Law Firm, Arnold & Porter Law Firm, Ben C. Martin Law Firm, and the National Center for Cardiovascular Diseases (Beijing, China); has ownership in Hugo Health and Refactor Health; has contracts from the U.S. Centers for Medicare & Medicaid Services; and has received grants from Medtronic, the U.S. Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information, outside the submitted work. Dr Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Beren, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc; and is a member of the scientific advisory board for Amgen, Corvidia Therapeutics, CSL Behring, DalCor Pharmaceuticals, Genentech, Kowa Pharmaceuticals, Olatec Therapeutics, PlaqueTec, Kancera, Medimmune, Novartis, Novo Nordisk, and XBiotech, Inc. Dr Libby is on the Board of Directors of XBiotech, Inc; and has a financial interest in XBiotech, a company developing therapeutic human antibodies. Dr Libby's interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr Goldhaber has received research support from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Boston Scientific, Daiichi-Sankyo, Janssen, the National Heart, Lung, and Blood Institute, and the Thrombosis Research Institute; and has received consulting fees from Bayer, Agile, Boston Scientific, and Boehringer Ingelheim. Dr Bikdeli is a consulting expert, on behalf of the plaintiff, for litigation related to 2 specific brand models of inferior vena cava filters. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors express their sincere gratitude to Fatemeh Esmaeili, MS, for her kind assistance in the preparation of the figures.
Footnotes
Alan Morrison, MD, PhD, served as Guest Associate Editor for this paper. Athena Poppas, MD, FACC, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Guo H., Huang M., Yuan Q., et al. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oesterle A., Laufs U., Liao J.K. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masana L., Correig E., Ibarretxe D., et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sviridov D., Miller Y.I., Ballout R.A., Remaley A.T., Bukrinsky M. Targeting lipid rafts—a potential therapy for COVID-19. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.574508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheahan T., Morrison T.E., Funkhouser W., et al. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4(12) doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88(2):913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgendorff A., Muth H., Parviz B., et al. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int J Clin Pharmacol Ther. 2003;41(9):397–401. doi: 10.5414/cpp41397. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. MBio. 2015;6(4) doi: 10.1128/mBio.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikdeli B., Talasaz A.H., Rashidi F., et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb Res. 2020;196:382–394. doi: 10.1016/j.thromres.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghati N., Roy A., Bhatnagar S., et al. Atorvastatin and aspirin as adjuvant therapy in patients with SARS-CoV-2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnardottir H., Pawelzik S.-C., Öhlund Wistbacka U., et al. Stimulating the resolution of inflammation through omega-3 polyunsaturated fatty acids in COVID-19: rationale for the COVID-Omega-F trial. Front Physiol. 2021;11:1748. doi: 10.3389/fphys.2020.624657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosy A.P., Malik U.I., Thomas R.C., et al. Rationale and design of the pragmatic randomized trial of icosapent ethyl for high cardiovascular risk adults (MITIGATE) Am Heart J. 2021;235:54–64. doi: 10.1016/j.ahj.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner D., Garvey K., Arrighi-Allisan A., et al. Letter to the editor: study summary—randomized control trial of omega-3 fatty acid supplementation for the treatment of COVID-19 related olfactory dysfunction. Trials. 2020;21(1):942. doi: 10.1186/s13063-020-04905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.REMAP-CAP . July 2020. Randomized, Embedded, Multifactorial Adaptive Platform trial for Community-Acquired Pneumonia, Domain-Specific Appendix: Statin Therapy REMAP-CAP: REMAP-CAP.https://static1.squarespace.com/static/5cde3c7d9a69340001d79ffe/t/5f1bbb351849fd6f694bb8b5/1595652928010/REMAP-CAP+Domain-Specific+Appendix+-+Simvastatin+Domain+V1.1+-+23+July+2020_WM.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanagarantam P., Francis D.P., Cole G., et al. Preventing cardiac complication of COVID-19 disease with early acute coronary syndrome therapy: a randomised controlled trial. Joint Research Compliance Office: Joint Research Compliance Office; 2020. https://storage.googleapis.com/found-by-me/documents/trials/c-19-acs/Protocol%20C-19-ACS.pdf
- 19.Bhatt D.L., Kumbhani D.J., Eagle K.A. December 12, 2020. Effects of icosapent ethyl on inflammatory biomarkers in individuals with COVID-19—VASCEPA COVID-19 CardioLink-9. Paper presented at: National Lipid Association Virtual Scientific Sessions. [Google Scholar]
- 20.Krisko T.I., Armstrong E.J., Cohen D.E. 2016. Pharmacology of cholesterol and lipoprotein metabolism. In: Principles of Pharmacology: The Pathophysiologic Basics of Drug Therapy: Fourth Edition. Wolters Kluwer Health. [Google Scholar]
- 21.Mehrbod P., Omar A.R., Hair-Bejo M., Haghani A., Ideris A. Mechanisms of action and efficacy of statins against influenza. BioMed Res Int. 2014;2014 doi: 10.1155/2014/872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SenBanerjee S., Lin Z., Atkins G.B., et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Experiment Med. 2004;199(10):1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merx M.W., Weber C. Statins in the intensive care unit. Curr Opin Crit Care. 2006;12(4):309–314. doi: 10.1097/01.ccx.0000235207.00322.96. [DOI] [PubMed] [Google Scholar]
- 24.Sun F., Duan W., Zhang Y., et al. Simvastatin alleviates cardiac fibrosis induced by infarction via up-regulation of TGF-β receptor III expression. Br J Pharmacol. 2015;172(15):3779–3792. doi: 10.1111/bph.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAuley D.F., Laffey J.G., O’Kane C.M., et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 26.Truwit J.D., Bernard G.R., Matthay M.A., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parihar S.P., Guler R., Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol. 2019;19(2):104–117. doi: 10.1038/s41577-018-0094-3. [DOI] [PubMed] [Google Scholar]
- 28.Calfee C.S., Delucchi K.L., Sinha P., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y. Efficacy of statin therapy in patients with acute respiratory distress syndrome/acute lung injury: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22(10):3190–3198. doi: 10.26355/eurrev_201805_15080. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A., Madhavan M.V., Poterucha T.J., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontes-Arruda A., DeMichele S., Seth A., Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. J Parenter Enter Nutr. 2008;32(6):596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 32.Rogero M.M., de C Leão M., Santana T.M., et al. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies S.P., Mycroft-West C.J., Pagani I., et al. The hyperlipidaemic drug fenofibrate significantly reduces infection by SARS-CoV-2 in cell culture models. bioRxiv. January 11, 2021 doi: 10.1101/2021.01.10.426114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehmel M., Jovanović N., Spitz U. Nicotinamide riboside—the current state of research and therapeutic uses. Nutrients. 2020;12(6):1616. doi: 10.3390/nu12061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorokin A.V., Karathanasis S.K., Yang Z.H., Freeman L., Kotani K., Remaley A.T. COVID-19—associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34(8):9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barter P.J., Caulfield M., Eriksson M., et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 37.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Bikdeli B., Talasaz A.H., Sharif-Kashani B., et al. American College of Cardiology Scientific Sessions; May 2021. INSPIRATION-S Investigators. Atorvastatin vs placebo in patients with COVID-19 admitted to the ICU: the INSPIRATION-S Trial.https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=9DF17809F35C4CA6B149C64C9D700AC6 Slides available at: [Google Scholar]
- 39.Mechanick J.I., Rosenson R.S., Pinney S.P., Mancini D.M., Narula J., Fuster V. Coronavirus and cardiometabolic syndrome: JACC Focus Seminar. J Am Coll Cardiol. 2020;76(17):2024–2035. doi: 10.1016/j.jacc.2020.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeghipour P., Talasaz A.H., Rashidi F., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation in critically ill patients with Covid-19—preliminary report. medRxiv. 2021 [Google Scholar]
- 42.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C., Choi W.J. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch Pharm Res. 2021:1–18. doi: 10.1007/s12272-020-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wootton D. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomazini B.M., Maia I.S., Cavalcanti A.B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dequin P.-F., Heming N., Meziani F., et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterne J.A., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramakrishnan S., Nicolau D.V., Langford B., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López-Medina E., López P., Hurtado I.C., et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Self W.H., Semler M.W., Leither L.M., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deftereos S.G., Giannopoulos G., Vrachatis D.A., et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Network Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.13136. e2013136-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes M.I., Bonjorno L.P., Giannini M.C., et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1) doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lan S.-H., Lai C.-C., Huang H.-T., Chang S.-P., Lu L.-C., Hsueh P.-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H.-Y., Ahn J., Park J., et al. Beneficial effect of statins in COVID-19–related outcomes—brief report: a national population-based cohort study. Arterioscler Thromb Vasc Biol. 2021;41(3):e175–e182. doi: 10.1161/ATVBAHA.120.315551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duvall M.G., Levy B.D. DHA-and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darwesh A.M., Bassiouni W., Sosnowski D.K., Seubert J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol Ther. 2020:107703. doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Everhart A., Desai N.R., Dowd B., et al. Physician variation in the de-adoption of ineffective statin and fibrate therapy. Health Serv Res. Published online February 10, 2021 doi: 10.1111/1475-6773.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buschard K. Fenofibrate increases the amount of sulfatide which seems beneficial against Covid-19. Med Hypotheses. 2020;143:110127. doi: 10.1016/j.mehy.2020.110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagai A., Matsumiya H., Hayashi M., Yasui S., Okamoto H., Konno K. Effects of nicotinamide and niacin on bleomycin-induced acute injury and subsequent fibrosis in hamster lungs. Experiment Lung Res. 1994;20(4):263–281. doi: 10.3109/01902149409064387. [DOI] [PubMed] [Google Scholar]
- 61.AIM-HIGH Investigators. Boden W.E., Probstfield J.L., et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 62.HPS2-THRIVE Collaborative Group. Landray M.J., Haynes R., et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz G.G., Olsson A.G., Abt M., et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 64.HPS3/TIMI55–REVEAL Collaborative Group. Bowman L., Hopewell J.C., et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 65.Bikdeli B., Barreto-Filho J.A. Reducing the cardiovascular disease burden: justified means for getting to the end. Circ Cardiovasc Qual Outcomes. 2012;5(4):580–586. doi: 10.1161/CIRCOUTCOMES.111.964072. [DOI] [PubMed] [Google Scholar]
- 66.Wood F.A., Howard J.P., Finegold J.A., et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med. 2020;383(22):2182–2184. doi: 10.1056/NEJMc2031173. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y.-Q., Zhao S-p, Ye H.-J. Efficacy and safety of coenzyme A versus fenofibrate in patients with hyperlipidemia: a multicenter, double-blind, double-mimic, randomized clinical trial. Curr Med Res Opin. 2020;36(6):941–945. doi: 10.1080/03007995.2020.1747416. [DOI] [PubMed] [Google Scholar]
- 68.Talasaz A.H., Kakavand H., Van Tassell B., et al. Cardiovascular complications of COVID-19: pharmacotherapy perspective. Cardiovasc Drugs Ther. 2020:1–11. doi: 10.1007/s10557-020-07037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adhyaru B.B., Jacobson T.A. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–769. doi: 10.1038/s41569-018-0098-5. [DOI] [PubMed] [Google Scholar]
- 70.Pandit A., Kumar P., Kumar A., Chakravarty K., Misra S., Prasad K. High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand. 2016;134(1):22–28. doi: 10.1111/ane.12540. [DOI] [PubMed] [Google Scholar]
- 71.Mach F., Ray K.K., Wiklund O., et al. Adverse effects of statin therapy: perception vs. the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526–2539. doi: 10.1093/eurheartj/ehy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhatt D.L., Steg P.G., Miller M., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 73.Kalstad A.A., Myhre P.L., Laake K., et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation. 2021;143(6):528–539. doi: 10.1161/CIRCULATIONAHA.120.052209. [DOI] [PubMed] [Google Scholar]
- 74.Lombardi M., Carbone S., Del Buono M.G., et al. Omega-3 fatty acids supplementation and risk of atrial fibrillation: an updated meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):e69–e70. doi: 10.1093/ehjcvp/pvab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huber R., Wong A. Nicotinamide: an update and review of safety & differences from niacin. Skin Therapy Lett. 2020;25(5):7–11. [PubMed] [Google Scholar]