SUMMARY
BACKGROUND:
In 2018, the WHO Member States committed to providing TB preventive treatment (TPT) to at least 30 million people by 2022. However, only 6.3 million people had initiated TPT by the end of 2019. Major knowledge gaps and research needs in diagnosis, treatment and the programmatic management of TPT (PMTPT) require to be addressed urgently.
METHODS:
In September 2019, a group of stakeholders involved in PMTPT in high TB burden countries met to develop an action agenda to support the global expansion of PMTPT.
RESULTS:
Barriers at the health system level, and priorities for research to overcome these, were identified for each step of the PMTPT cascade. The need for data on TPT financing, gaps and coverage under national health insurance schemes, as well as the need for mathematical and cost-effectiveness modelling of the impact of TPT on TB incidence and mortality were highlighted. Specific research needs were identified for high-risk populations such as household contacts of any age and people living with HIV, as well as other people at risk.
CONCLUSIONS:
The meeting facilitated agreement on a set of actions needed to ensure that PMTPT continues to expand to achieve the End TB Strategy targets.
Keywords: End TB Strategy, diagnosis, treatment
Abstract
CONTEXTE :
En 2018, les états membres de l’OMS se sont engagés à fournir un traitement préventif antituberculeux (TPT) à au moins 30 millions de personnes d’ici 2022. Cependant, seuls 6,3 millions de personnes avaient été mises sous TPT à la fin de l’année 2019. Le manque considérable de connaissances et les besoins importants en recherche en matière de diagnostic, de traitement et de prise en charge programmatique du TPT (PMTPT) doiventêtre comblés sans attendre.
MÉTHODES :
En septembre 2019, un groupe de parties prenantes engagées dans la PMTPT dans les pays à forte incidence de TB s’est réuni pour formuler un plan d’action afin de soutenir l’expansion mondiale de la PMTPT.
RÉSULTATS :
Des obstacles au niveau des systèmes de santé, ainsi que des priorités de recherche afin de surmonter ces obstacles, ont été identifiés à chaque étape de la cascade de la PMTPT. Le besoin de données relatives au financement du TPT, les lacunes et la couverture des soins par les régimes d’assurance maladie nationaux, ainsi que les besoins en modélisations mathématiques et coût-efficacité de l’impact du TPT sur l’incidence et la mortalité de la TB ontété mis en évidence. Des besoins particuliers en matière de recherche ont été identifiés pour les groupes de population à haut risque tels que les contacts domestiques de toutâge et les personnes vivant avec le VIH, ainsi que pour d’autres personnes à risque.
CONCLUSIONS :
La réunion a permis de faciliter la prise de décisions relative à un ensemble d’actions nécessaires afin de garantir l’expansion de la PMTPT dans le but d’atteindre les objectifs de la Stratégie de l’OMS pour mettre fin à la TB.
TB infection (TBI), previously referred to as latent TB infection, is a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of TB disease.1 It is estimated that about a quarter of the global population has been infected with M. tuberculosis.2 By preventing incident TB cases, TB preventive treatment (TPT) constitutes one of the principal interventions recommended by the WHO to achieve the targets of the End TB Strategy.3 TPT fits within a larger framework of preventive actions envisaged by Pillars 1 and 2 of the End TB Strategy, ranging from screening for TB disease, infection control, prevention and care of HIV and other comorbidities and health risks, access to universal health care, social protection and poverty alleviation. At the UN High-Level Meeting (UNHLM) on TB in 2018,4 Member States committed to providing TPT to at least 30 million people by 2022, including 6 million people living with HIV (PLHIV), 4 million household contacts aged <5 years and 20 million household contacts aged ≥5 years.
The WHO issued guidelines for the programmatic management of TB preventive treatment (PMTPT)5 that provide recommendations on the implementation of PMTPT, including selection of target populations, diagnostic and treatment standards, as well as tools for implementation and monitoring and evaluation.6 However, the number of people provided with TPT has increased very slowly in recent years, from 1.0 million in 2015 to 2.2 million in 2018 and 4.1 million in 2019. The combined total of 6.3 million by the end of 2019 was only 21% of the UNHLM 5-year target of 30 million.7 In 2019, 433,156 recipients of TPT were children under 5 and only 105,240 household contacts ≥5 years. The remaining 3.5 million who initiated TPT were PLHIV.7 There are many reasons for the slow uptake of TPT in high and intermediate incidence countries. A recently published global survey identified fragmented implementation of global TPT guidelines and limited access to necessary diagnostic tools as major barriers.8 Studies from several countries in Asia have reported current challenges with PMTPT, and described many issues related to diagnosis and treatment.9,10
Here, we report the results of an expert consensus meeting convened by the WHO and the McGill International TB Centre, McGill University, Montreal, QC, Canada. Full details of the agenda, presentations, findings and attendees can be found in the official meeting report.11
METHODS
In September 2019, a meeting was convened by the WHO and the McGill TB Centre to discuss the major knowledge gaps and research needs to support the roll out of PMTPT in high and intermediate incidence countries. The meeting also aimed to formulate recommendations for the most urgent action items for governments and donors. Stakeholders from major funding agencies, international organisations, national TB programmes (NTPs) and academic institutions involved in TB prevention were invited. Industry representatives were also invited to provide input from the manufacturing perspective. All attendees completed conflict of interest forms as per WHO protocol. Full details of participants are provided in the appendix. Ethical approval was not required for this activity.
The discussion was structured around the cascade of care in the management of TBI, defined as a multi-step process of individuals moving through the health system, to receive care for TBI.12 For each step of a four-step cascade model (Figure 1), the participants identified known barriers, knowledge gaps and future research needs. The discussion focused on three at-risk populations: PLHIV, household contacts of TB patients aged <5 years and household contacts aged ≥5 years, as these were the three risk groups specifically identified in the UN General Assembly Declaration.7 Other people at risk were considered briefly. During the workshop draft recommendations were crafted, and finalised through multiple revisions until final consensus was achieved among all workshop participants who did not have identified conflicts of interest.
Figure 1.
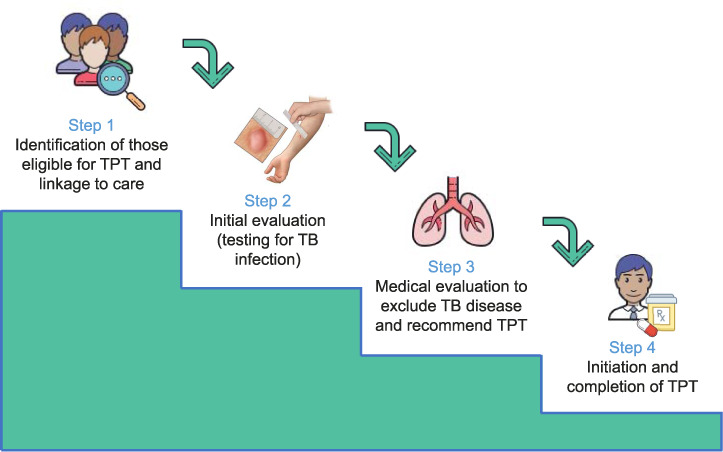
Cascade of prevention for the programmatic management of TB infection. TPT = TB preventive treatment.
Implementation barriers and research gaps for TPT expansion
We first identified general barriers to TPT expansion, including the lack of priority for national TB programmes and the lack of awareness among healthcare workers, as well as barriers to access to diagnostics and preventive treatment, challenges with financing and understanding the perspective of the person who is infected with M. tuberculosis (Table 1 and Figure 2). We then identified barriers and research gaps related to specific steps of the TPT care cascade. Issues that relate specifically to PLHIV, household contacts under 5 years, household contacts over 5 years, and other people at risk are described in detail in the following section.
Table 1.
General barriers and research gaps
| General barriers | Research gaps |
|---|---|
| Lack of priority |
|
| Access to diagnostics and drugs |
|
| Financing |
|
| Understanding the patient perspective |
|
PMTPT = programmatic management of TPT; TPT = TB preventive therapy.
Figure 2.
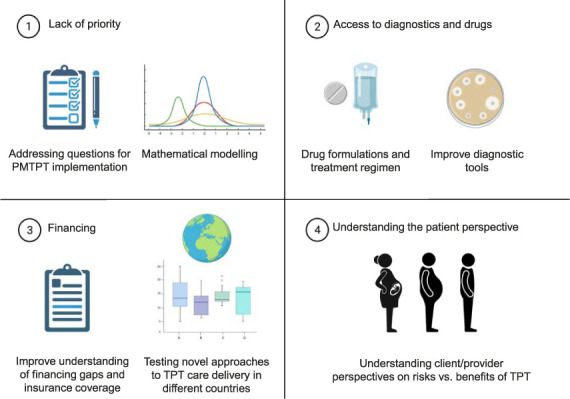
Barriers to the implementation of the PMTPTand research priorities (created using BioRender.com). PMTPT=programmatic management of TPT; TPT = TB preventive therapy.
Lack of priority
Barriers
For decades, PMTPT has been a low priority for NTPs due to limited funding and other competing priorities. Another important barrier to the implementation of PMTPT is the lack of priority among healthcare workers, and lack of awareness of WHO and/or national recommendations on PMTPT. Health workers may also be hesitant to implement PMTPT because of the fear of development of drug resistance or other adverse events from treatment in otherwise healthy individuals.9
Research gaps
Modelling studies have shown that PMTPT can significantly contribute to reducing TB incidence at the population level,13 but further mathematical modelling is needed to show the impact of potential contribution of PMTPT to reducing TB incidence and mortality in different epidemiological situations. Such research should demonstrate the population benefits of PMTPT in reducing the global TB burden for NTPs, implementers, and donors, and the individual benefits of TPT for healthcare workers. Mathematical modelling can also address questions about which tools (e.g., diagnostics, treatment) would have the greatest impact, at what cost, and what additional tools would support implementation and scale-up of PMTPT.
Access to diagnostics and treatment
Barriers
The tuberculin skin test (TST) based on purified protein derivative (PPD), and interferon-gamma release assays (IGRAs) are currently used to diagnose TBI. Although TST has lower specificity for infection in areas with high bacilli Calmette-Guérin (BCG) vaccination coverage (especially if given after infancy), the two tests perform equally well for prediction of future disease.5,14 Reduced access to both tests delays the roll-out of PMTPT, particularly in household contacts aged ≥5 years, where testing for TBI is desirable to maximise benefit over risk. The availability of many products and formulations fragment the market and affect supply channels and costs. At the meeting, a Global Drug Facility representative explained that the consolidation of standards or recommendations for preferred formulations and tools would facilitate competition, lower prices and optimise access to high-quality products.
Research gaps
Improved procurement and life cycle management of new and old tools, and financial support for procurement and technical assistance will expedite the introduction of new products. The goal would be to simplify the set of elements that are needed to deliver TPT under field conditions, such as screening tests, diagnostics, data collection systems (especially for household contact investigations) and medicines (including consolidation of regimens and formulations for TPT).
Financing
Barriers
The ongoing shortfall of funds for PMTPT is anticipated to persist in the coming years in middle-and high-burden countries. This need was noted at the expert meeting, prior to the onset of the COVID-19 pandemic in March 2020. The global pandemic of COVID-19 has resulted in major reallocation of budgets, equipment and staff from TB frontline work to pandemic mitigation, exacerbating the problem of limited funding for TPT (see section below on “Reflections after the meeting”). The financing landscape for TPT is gradually shifting from donor-funded to government-funded. Going forward, TB prevention should continue to be financed through domestic resource mobilisation, and increasingly as part of reforms for universal health coverage or innovative schemes for market shaping and social impact bonds. In countries adopting universal health-care systems, there is an opportunity to expand TPT provision through these new systems by allowing diagnosis and treatment by providers who are not part of TB programmes. This would be feasible only if TPT is very safe and easy to complete, and if the diagnosis of TBI (including the exclusion of TB disease) is simplified.
Research gaps
Data on PMTPT financing and coverage under national health services or health insurance schemes are needed. A priority for research is the development and testing of incentivised models of TPT delivery in different countries and the identification of effective models of care provision and payment.
The perspective of persons infected with M. tuberculosis
Barriers
The voices of the persons infected with M tuberculosis has not been systematically integrated into research. Information is lacking on how people perceive risk, how they understand a diagnosis of TBI and how much value they place on preventing TB disease.
Research gaps
Qualitative research such as focus group discussions and in-depth interviews with key informants is needed to understand acceptability of testing, use of different algorithms of varying complexity for investigation, and the trade-offs of convenience, opportunity cost, duration and safety of preventive treatment. It is also important to understand perspectives of patients in different settings and from different target populations regarding these issues.
Barriers and research priorities related to the cascade of care Step 1: Identification and linkage to care
Barriers
From an operational perspective, the initial step of identifying people who would benefit from TPT is an important barrier. Evidence on finding target populations and linking them to care is contradictory: in some studies, home visits were essential in identifying and linking persons to care,15,16 but other studies reported that home visits had little impact, as the greater number of persons identified did not result in greater numbers successfully taking treatment.17
Research gaps
Epidemiological research should concentrate on refining estimates of risk to identify persons and groups at highest risk of progression to active TB disease. Modelling studies and qualitative research could be used to increase understanding of the impact of different strategies for linkage to care in different contexts.18,19 More information on research priorities is provided in Table 2.
Table 2.
Research gaps organised by steps in the PMTPT cascade of care
| Cascade step | Research gaps |
|---|---|
| Step 1: Identification and linkage to care |
|
| Step 2: Initial evaluation (testing for TB infection) |
|
| Step 3: Medical evaluation to exclude TB disease and recommend TPT |
|
| Step 4: TPT |
|
PMTPT = programmatic management of TPT; PLHIV = people living with HIV; ART =antiretroviral therapy; IGRA = interferon-gamma release assay; CXR = chest X-ray; TPT = TB preventive therapy.
Step 2: Testing for TB infection
Barriers
There is a worldwide shortage of quality-assured PPD reagents for TSTs for TBI and very few countries have a local manufacturer of PPD. Only a limited number of middle- and high-burden countries currently use IGRAs due to the high cost of the test, and the need for associated systems for sample transportation, equipped laboratories and skilled human resources in these laboratories. The major limitation of current tests for TBI is their poor predictive value for future TB disease; the resultant need to treat large numbers of persons to prevent relatively few cases of TB critically impacts the cost-effectiveness profile of the entire cascade, as well as individual risk-benefit considerations. New TSTs and IGRAs are expected to come on the market soon.20,21
Research gaps
Market shaping research is essential to improve availability of tests, including demand creation. In the short term, research should focus on re-evaluating testing for TBI using currently available tests in at-risk populations. Studies that can quantify the balance of benefit to harms of giving TPT with and without testing for TBI in contacts aged ≥5 years would be helpful to inform local policies. The development and evaluation of biomarkers for disease progression is a high priority, as is implementation research to identify the most efficient pathways for use of the new diagnostic tests for TBI. More information on research priorities is provided in Table 2.
Step 3: Medical evaluation to exclude TB disease and recommend TPT
Barriers
The optimal approach to exclude TB disease prior to initiating TPT remains a challenge in most high and intermediate incidence countries. Many TB programmes rely on symptom screening; however, this has adequate sensitivity only in people living with HIV infection who are not receiving antiretroviral therapy (ART), but has unacceptably low sensitivity in all other groups. Any abnormality on chest X-ray (CXR) has a 94% sensitivity, making it a useful tool for the exclusion of TB disease in asymptomatic candidates for TPT.22 Among the barriers to broader use of CXR are a shortage of trained technicians to perform the X-ray and qualified personnel to interpret them. The high cost for equipment and materials result in high costs per test, which are usually paid by the patient, constituting a major barrier. Digital radiography has lower operating costs, no film processing and decreased radiation, and can be interpreted remotely by experts, but has high initial capital costs. Use of computer-aided detection (CAD) for pulmonary TB screening could replace human readers, and there are several commercially available CAD software programmes for this. However, to date these are costly, due to high licensing and per-use fees. Moreover, more evidence is required regarding which algorithms to use, whether to use machine learning or deep learning and how to optimise the use of human interpretation to improve specificity.23
Research gaps
Given the utility of CXRs for diagnosis of a wide range of health problems, it is evident that CXR equipment and personnel should not be restricted to detect TB alone. The utility and cost-effectiveness of implementation and use of CXR as part of general health services should be studied further. Improving diagnostic accuracy of CXR when assessing individuals for eligibility for TPT will require additional research on the best mechanisms to increase access to expert interpretation—ideally through CAD. Future research should focus on cost-effectiveness and where to place CAD in screening algorithms (i.e., to use it as a rule-out test or to increase the pre-test probability when used as part of stepwise diagnostic testing). More information on research priorities is provided in Table 2.
Step 4: TB preventive treatment (initiation and completion)
Barriers
The long duration and limited tolerability of isoniazid-based treatment regimens substantially reduces the acceptability and compliance to treatment, thereby affecting effectiveness and epidemiological impact of TPT. Authoritative agencies have recently recommended rifamycin-based regimens that can reduce treatment duration to 1–4 months.5,24 However, rifapentine, included in many of the novel shorter regimens, is only available in a few high-burden countries, hindering the implementation of regimens that rely on this drug. On the other hand, while the TBI treatment choices are largely decided at the national or regional level, individual treatment choices and perception of side effects should receive greater attention. Carefully weighing the public health benefit against the potential risk or benefit to the individual is necessary in different settings and populations. This will become even more important as TPT eligibility is expanded to populations that may be at lower risk of developing TB.
Research gaps
The evaluation of short regimens of 1–2 months’ duration for efficacy, safety, tolerability and acceptability is needed immediately, and in the longer term, research should evaluate even shorter regimens and/or single-dose regimens such as ‘depot’ injections of a long-acting agent,25 as the 3–4 months’ duration of the current “short regimens” is seen as a major challenge for patients and health systems. Furthermore, attitudes of providers and at-risk individuals towards TPT should be explored to help overcome the barriers to the acceptance of treatment, adapted to the local setting. Studies of safety, adverse events and medication adherence in programmatic settings should also be prioritised in all populations. More information on research priorities is provided in Table 2 (Figure 3).
Figure 3.
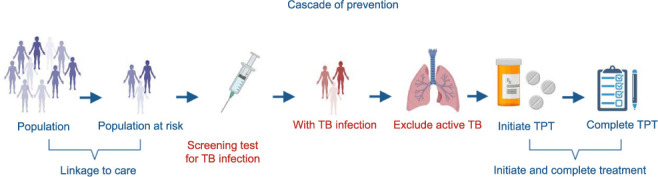
Barriers and research priorities related to the cascade of prevention (created using BioRender.com). TPT = TB preventive therapy.
Research gaps specific to certain high-risk populations
People living with HIV
Considering the massive expansion of ART for PLHIV, research to re-evaluate the benefits and risks of TPT with or without testing for TBI in PLHIV of different ages and in diverse epidemiological settings should be prioritised. In addition, pharmacokinetic studies of new TPT regimens in PLHIV on ART are important to evaluate drug-drug interactions. This is particularly crucial for the new options for TPT, which increase the possibility for drug-drug interactions. Safety in pregnant and postpartum women with HIV who are on ART is also a concern.26 A further priority in settings with high TB transmission is to assess the need, and benefits of repeated courses of short TPT regimens.
Household contacts aged ≥5 years
Research needs include the advantages and disadvantages, from individual and public health perspectives, of testing for TBI using current or new tools, the epidemiological impact of treating household contacts of varying age ranges, the public health benefits in terms of active case-finding, methods for excluding TB disease, questions related to linkage to care and models of care for community delivery of TPT, including digital adherence technologies. The evaluation of alternative mechanisms for linkage to care, such as involving primary care providers or community health workers is also a research need for this high-risk population. An important challenge is acceptance and safety of TPT, particularly among adult contacts. Finally, research is needed to assess if other close contacts (e.g., workplace or social) should also be considered for TPT, as is done in some low-prevalence countries, particularly if active case-finding is done among these populations.
Household contacts aged <5 years
Generally, the research priorities for household contacts under 5 years, are the same as those identified for household contacts aged ≥5 years, with a few additional areas. These include access to care, particularly overcoming stigma and beliefs of parents who may be hesitant to initiate their children on TPT, and the best methods to rule out TB disease in view of the well-known difficulties of CXR interpretation in young children. Priority should also be given to the currently available shorter HP (isoniazid + rifapentine) regimens, including the development of child-friendly formulations, safety and tolerability, and potential drug-drug interactions between these regimens and paediatric ART or malaria medications.
Other people at risk
The WHO does not currently recommend systematic testing for TBI and TPT for people with diabetes, people who engage in the harmful use of alcohol, tobacco smokers and underweight people, as there is a paucity of data from clinical trials on the benefits and harms of TPT. Evidence on the likelihood of progression from infection to TB disease is needed for these populations, as well as those exposed to silica, those on steroid treatment, those with rheumatological disease or cancer and indigenous populations. In addition, studies of efficacy and adverse events of shorter, better-tolerated treatment regimens are needed for certain risk groups (e.g., people who use drugs, people who engage in harmful use of alcohol and older persons). Finally, research should evaluate service delivery models to improve the management of TPT, including the provision of additional interventions to smokers, harm reduction services for people who use drugs or who engage in the harmful use of alcohol and those who are incarcerated (Table 3).
Table 3.
Research gaps for high-risk populations
| Population | Research gaps |
|---|---|
| PLHIV |
|
| Household contacts aged ≥5 years |
|
| Household contacts under 5 years |
|
| Other people at risk |
|
PMTPT = programmatic management of TPT; PLHIV = people living with HIV; ART =antiretroviral therapy; HP = isoniazid þ rifapentine; TPT = TB preventive therapy.
REFLECTIONS AFTER THE MEETING
In March 2020, the world changed with the spread of SARS CoV-2 and the declaration by the WHO of a global health emergency. As COVID-19 spread, health infrastructure and resources have been reattributed, leaving already underfunded TB programmes vulnerable.27–29 The impact on individuals with TB and available services has been immediate, as TB notifications fell by 88% in the Global Fund-implementing countries, and the number of individuals initiating TB treatment were substantially reduced.30 The longer-term epidemiological impact on TB is not yet clear; however, preliminary modelling studies suggest an anticipated 6.3 million added cases and 1.4 million additional deaths between 2020 and 2025.31 In September 2020, the UN Secretary General’s report of progress toward meeting the 2018 UNHLM targets32 made two recommendations for TB prevention: 1) dramatically scale up provision of TPT, and 2) ensure that TB prevention and care are safeguarded in the context of COVID-19 and other emerging threats.
CONCLUSIONS
TPT is an essential intervention towards achieving the goals of the End TB Strategy. Given the absence of a scalable, effective vaccine, it is of paramount importance in the short and medium term. Conceptualisation of the cascade of care for TB prevention provides a framework to systematically analyse the barriers and enablers of the approach. Since 2018, pivotal achievements include the development and assessment of shorter and safer treatment regimens, and the adoption of a full package of TB care and prevention interventions for PLHIV. Guidelines for the roll out of TB prevention activities have been developed by the WHO.
In order to reach the End TB goals, research to support scale up of PMTPT must be strengthened, with priority given to several areas identified in this meeting. Strengthened biomedical research is needed to develop better diagnostics and treatment regimens, research into models of service delivery, particularly for adult contacts, is critical to expand TPT, and strengthened behavioural science is needed to understand attitudes and beliefs of persons who could benefit from TPT. Stimulating and investing in research will be pivotal to support PMTPT scale-up to reach the UNHLM targets.
Although COVID-19 has ravaged health systems across the world, it has also presented several opportunities for TB programmes. Advances in digital technologies may be leveraged by TB programmes to support TPT. There may be greater opportunities for systematic screening for TB and TBI of individuals presenting with symptoms suggestive of COVID. Looking for these and other synergies may be helpful to rebuild programmes that have been derailed by the COVID pandemic. A major effort to redirect human and financial resources back into TB programmes is needed to ensure that PMTPT can achieve the UNHLM targets.
Acknowledgments
The authors thank A-G Märtson, Department of Clinical Pharmacy and Pharmacology, Groningen, The Netherlands, for creating Figures 2 and 3.
References
- 1.Getahun H, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 2.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uplekar M, et al. for WHO’s Global TB Programme WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 4.United Nations General Assembly. New York, NY, USA: UN; 2018. 2018/Resolution A/RES/73. 3. Political declaration of the high-level meeting of the General Assembly on the fight against tuberculosis.http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/73/3 [Google Scholar]
- 5.World Health Organization. Geneva, Switzerland: WHO; 2020. WHO consolidated TB guidelines. Module 1: Prevention. Tuberculosis Preventive Treatment.https://www.who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-module-1-prevention-tuberculosis-preventive-treatment [PubMed] [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: WHO; 2020. WHO operational handbook on tuberculosis. Module 1: prevention - tuberculosis preventive treatment. [Google Scholar]
- 7.World Health Organization. Geneva, Switzerland: WHO; 2020. Global tuberculosis report, 2020. [Google Scholar]
- 8.Faust L, et al. How are high burden countries implementing policies and tools for latent tuberculosis infection? A survey of current practices and barriers. Health Sci Rep. 2020;3:e158. doi: 10.1002/hsr2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paton NA, et al. Diagnosis and management of latent tuberculosis infection in Asia: Review of current status and challenges. Int J Infect Dis. 2019;87:21–29. doi: 10.1016/j.ijid.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020;35:269–275. doi: 10.3904/kjim.2020.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland: WHO; 2019. Meeting Report of the Technical Consultation on latent TB infection management: research in support of scale-up.https://www.who.int/publications/m/item/technical-consultation-on-latent-tb-infection-management-research-in-support-of-scale-up [Google Scholar]
- 12.Alsdurf H, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 13.Dye C, et al. Prospects for tuberculosis elimination. Ann Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 14.Rangaka MX, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen CM, et al. Tuberculosis household accompaniment to improve the contact management cascade: a prospective cohort study. PLoS ONE. 2019;14(5):e0217104. doi: 10.1371/journal.pone.0217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duarte R, et al. Improving tuberculosis contact tracing: the role of evaluations in the home and workplace. Int J Tuberc Lung Dis. 2012;16(1):55–59. doi: 10.5588/ijtld.10.0511. [DOI] [PubMed] [Google Scholar]
- 17.Zachariah R, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7(11):1033–1039. [PubMed] [Google Scholar]
- 18.Miller AP, et al. Healthcare facility-based strategies to improve tuberculosis testing and linkage to care in non-U.S.-born population in the United States: a systematic review. PLoS One. 2019;14(9):e0223077. doi: 10.1371/journal.pone.0223077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prater C, Holzman S, Shah M. Programmatic effectiveness of latent tuberculosis care cascade in a community health center. J Immigr Minor Health. 2021;23(3):566–573. doi: 10.1007/s10903-020-01047-w. [DOI] [PubMed] [Google Scholar]
- 20.Hamada Y, et al. Framework for the evaluation of new tests for tuberculosis infection. Eur Respir J. 2021 Jan 21; doi: 10.1183/13993003.04078-2020. doi. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kik SV, et al. An evaluation framework for new tests that predict progression from tuberculosis infection to clinical disease. Eur Respir J. 2018;52(4):1800946. doi: 10.1183/13993003.00946-2018. [DOI] [PubMed] [Google Scholar]
- 22.Assefa Y, et al. Screening tools to exclude active pulmonary TB in high TB burden countries: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(6):728–734. doi: 10.5588/ijtld.18.0547. [DOI] [PubMed] [Google Scholar]
- 23.Harris M, et al. A systematic review of the diagnostic accuracy of artificial intelligence-based computer programs to analyze chest x-rays for pulmonary tuberculosis. PLoS One. 2019;14(9):e0221339. doi: 10.1371/journal.pone.0221339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterling TR, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(RR-# 1):1–8. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Geneva, Switzerland: WHO; 2020. Target product profiles for tuberculosis preventive treatment.https://www.who.int/publications/i/item/target-product-profiles-for-tuberculosis-preventive-treatment [Google Scholar]
- 26.Hamada Y, et al. The safety of isoniazid tuberculosis preventive treatment in pregnant and postpartum women: systematic review and meta-analysis. Eur Respir J. 2020;55:1901967. doi: 10.1183/13993003.01967-2019. [DOI] [PubMed] [Google Scholar]
- 27.Migliori GB, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis. 2020;26(11):2709–2712. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQuaid CF, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020;56(2):2001718. doi: 10.1183/13993003.01718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQuaid CF, et al. The impact of COVID-19 on tuberculosis burden: a review of the data available to evaluate and respond. Int J Tuberc Lung Dis. 2021;25(6):436–446. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stop TB Partnership. One year on, new data shows the global impact of COVID-19 on TB epidemic is worse than expected. Press Briefing on 18 March 2021. http://www.stoptb.org/covid19.asp.
- 31.Cilloni L, et al. The potential impact of the COVID-19 response on tuberculosis in high burden countries: a modelling analysis. EClinicalMedicine. 2020;28:100603. doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Geneva, Switzerland: WHO; 2020. Progress towards achieving global tuberculosis targets and implementation of the UN Political Declaration on Tuberculosis.https://cdn.who.int/media/docs/default-source/documents/tuberculosis/overview-progress-towards-achievingglobal-tuberculosis-targets-and-implementation-of-the-un-political-declaration-on-tuberculosis0e0390d4-087a-418e-8035-8238f7b8793d.pdf?sfvrsn=e8ad804d_1&download=true [Google Scholar]


