Abstract
Polyunsaturated fatty acids (PUFAs) such as docosahexaneoic acid (DHA) and eicosapentaneoic acid (EPA), play a critical role in a variety of neuronal functions, including facilitating neuronal growth and differentiation, increasing the density of the neuritic network, modulating cell membrane fluidity, regulating intracellular signaling and gene expression, and exhibiting antioxidant characteristics. Dietary DHA is selectively enriched and actively retained in the central nervous system, mainly in synaptic membranes, dendrites, and photoreceptors. In this review, we highlight the myriad roles of PUFAs in brain function and human health. Diets rich in DHA are inversely proportional to cognitive decline and incidence of neurodegenerative disorders. Conversely, diets deficient in DHA impair the proper development of brain and the visual system in children and increase risk of brain disorders in the elderly. Finally, DHA and EPA have been shown to reduce inflammation and may prove to be beneficial in reducing the severity of the SARS-COVID infection.
Introduction: Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are required for optimal human health and wellbeing. PUFAs are classified by the position of the first double bond in a fatty acid counting from the methyl end. There are two major classes of PUFAs: omega-6 fatty acids (first double bond is at the sixth carbon from methyl end) and omega-3 fatty acids (first double bond is at the third carbon from methyl end). PUFAs are derived from essential fatty acids (EFA) such as linoleic acid (LA) and alpha-linolenic acid (ALA), fatty acids that cannot be synthesized in the body and need to be ingested through diet. The parent omega-6 fatty acid, LA is desaturated in the body to form arachidonic acid while parent omega-3 fatty acid ALA is desaturated by a microsomal enzyme system to form DHA and EPA. However, there is a limited metabolic capability in humans for this conversion underscoring the need for their inclusion in diet.
PUFAs and Brain Health
EFAs and PUFAs and their metabolic products play a critical role in the maintenance of structural and functional integrity of the central nervous system and retina.1 Extensive research has shown their crucial role in maintaining neuronal homeostasis. PUFA’s exert their effects through modulating the fluidity of the neuronal membrane, regulating signal transduction, promoting neurite outgrowth, and impacting the function of key ion channels and receptors.2 DHA has also been shown to influence cell membrane fluidity by promoting signaling interactions between macromolecules to form lipid rafts: cholesterol and sphingolipid-enriched microdomains in the plasma membrane, known to serve as local sites for housing signaling proteins, and orchestrating key signaling processes.3 The most extensively studied PUFA is the omega-3 fatty acid called DHA.2 This is the most abundant fatty acid in the brain and is a structural component of the neuronal membrane. DHA plays a critical role in neuronal growth and differentiation, neuronal signaling, synthesis of bioactive mediators, synaptic plasticity, and remodeling of the neuritic network.4 Diets high in DHA are inversely proportional to cognitive decline and the onset of neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.
As mentioned above, DHA (22:6 n−3) is a product of ALA, an essential fatty acid that must be obtained from the diet. There is a significant body of evidence showing that a high (n−3):(n−6) dietary intake ratio, such as in the Mediterranean diet, has widespread positive health effects.5 Mediterranean diets have lower intakes of added sugar, saturated fat, trans-fat, lower glycemic index, and a lower ratio of (n−6):(n−3) fatty acids. This type of diet appears to reduce risk for Alzheimer’s disease and other forms of dementia. Additionally, a Mediterranean diet includes most of the nutritional components important for lowering risk for cardiovascular disease, hypertension, and type II diabetes.6
Western diets are low in omega-3 fatty acids, including ALA found mainly in plant oils, and DHA, which is found in fish. Epidemiological studies have linked low maternal DHA to increased risk of poor neural development in infants.7 Dietary deficiency of n−3 fatty acids during fetal development in utero and postnatal state has shown negative effects on cognitive abilities and a loss of discriminative learning ability.8 Intervention studies have shown that improving maternal DHA intake decreases the risk of poor visual and neural development in children. Sufficient evidence has concluded the occurrence of efficient transfer of DHA from mother to fetus before birth and during lactation after birth, with short and long-term implications for neural function. Most of the brain growth is completed by five to six years of age. It is recommended for pregnant and nursing women to take at least 2.6 g of omega-3 fatty acids and 100–300 mg of DHA daily to meet the needs of the fetus and suckling infant.1 Follow-up studies have shown that infants of mothers who took supplements of EFAs and DHA have higher mental processing scores, eye-hand coordination, psychomotor scores, and stereo acuity at four years of age. Intake of EFAs and DHA during preschool years may have a beneficial role in prevention of attention deficit hyperactivity disorder and enhancing capability and academic performance.1
Although a good natural source of PUFAs is seafood, especially oily fish, one can obtain them via consuming fish oil capsules. Very long chain omega-3 fatty acids are readily incorporated from capsules into transport (blood lipids), functional (cell and tissue), and storage (adipose) pools.9 At sufficient levels of incorporation, EPA and DHA influence the physical nature of cell membranes and membrane protein-mediated responses, increase the generation of bioactive lipids, and promote cell signaling and gene expression in many different cell types.9 Through these mechanisms, they impact overall cellular homeostasis and modulate the cellular response to external signals. In most cases the effects seen are associated with improvements in disease biomarker profiles and health-related outcomes.9 Given that the capacity of the human brain to synthesize DHA locally is appreciably low, the uptake of DHA from circulating lipid pools is essential to maintaining its homeostatic levels. Although, several plasma pools have been proposed to supply the brain with DHA, recent evidence suggests non-esterified-DHA and lysophosphatidycholine-DHA are the primary sources.10 The uptake of DHA and its enrichment in the brain appears to be regulated by several complementary pathways associated with its metabolism. Following entry into the brain, DHA is esterified into and recycled amongst membrane phospholipids and converted to bioactive mediators which regulate signaling pathway important to synaptogenesis, cell survival, and neuroinflammation, and may be relevant to treating neurological diseases.10
Role of DHA in Neurite Formation in Brain
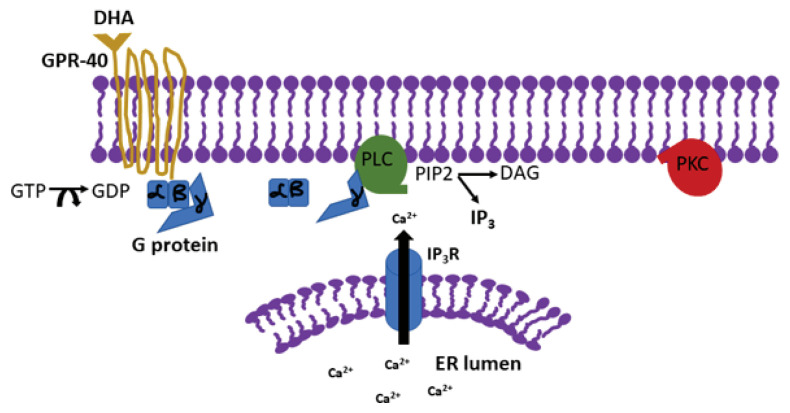
DHA is a critical component in the development of neurons and neurites in young mammals, since its peak concentration has been observed to be in the late gestational period.11 However, it has been shown that DHA plays a critical role in the adult brain as well. DHA accounts for roughly 15% of the fatty acid content in the mature human brain, and it is believed that decreased levels can lead to cognitive decline and the onset of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease.4 Various studies have been done to unravel the precise mechanisms underlying the protective and proliferative properties of DHA in the brain. One study found the DHA to be an endogenous ligand for the G protein coupled receptor called GPR40, which is expressed in the hippocampus of adult monkeys, and was shown to be associated with neurogenesis after ischemia.12 It is important to note that the hippocampus, an area of the brain critical for memory, is especially sensitive to changes in membrane fluidity, hence DHA levels are likely to have a more substantial impact in this region. Interaction of DHA with the GPR40 was found to be responsible for promoting neurogenesis and neuritogenesis. This effect was mediated through increase in intracellular Ca2+ which was released via activation of inositol tris phosphate receptor (IP3-R) (Figure 1). This finding implies that DHA, at least partially, acts through the IP3 receptor-mediated signaling.12
Figure 1.
Mechanism of action of docosahexaenoic acid. Docosahexaenoic acid (DHA) binds to the G protein coupled receptor GPR-40 which leads to the activation of phospholipiase C (PLC), conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to form diacylglycerol (DAG) and inositol tris phosphate (IP3), which activates the IP3 receptor leading to the release of calcium from ER stores. The increase of intracellular Ca2+ activates protein kinase C (PKC) which leads to synaptogenesis
Adapted from Ma et al., 2010, DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene, Brain Research, 2010, 1330, 1 – 8.
Role of DHA in Cognitive Decline
A significant body of literature supports the role of DHA in overall brain function, including neuronal growth and differentiation as well as optimizing neuronal signaling. Most data from human studies show its contribution to optimal visual acuity development, an assertion supported by the presence of high amounts of DHA in rod outer segment membranes.13 While DHA levels affect early brain development in infants, potential effects are also increasingly observed during adult life. Epidemiological studies support a link between low dietary intake of DHA and a higher risk of cognitive decline and onset of neurodegenerative and psychiatric disorders.14 The primary insult from loss of DHA from membrane glycerophospholipids, is on the flexibility and fluidity of membrane lipids which in turn affects the optimal function of critical integral membrane proteins (receptors, voltage-gated ion channels and enzymes).15 This leads to effects on second messenger systems, neurotransmitter release, and subsequent downstream cascade likely to have deleterious changes in brain and visual function. Many studies have also reported that dietary omega-3 deficiency results in changes in learning, coping with stress, behavioral changes, and responses in visual function.13 Circulating plasma DHA is significantly related to cognitive abilities during aging, and is inversely associated with cognitive decline. A diet characterized by higher intakes of foods containing high levels of n−3 fatty acids, and/or lower intake of n−6 fatty acids is strongly associated with a lower incidence of Alzheimer’s disease and other brain disorders. Supplementation of DHA improves some behaviors associated with attention deficit hyperactivity, bipolar disorder, schizophrenia, and impulsive behavior, as well as cognition.8
DHA, Inflammation, and COVID
Inflammation is a normal process that is a critical part of host defense and tissue healing. However, excessive and/or unresolved inflammation can lead to progressive tissue damage, disease, and pathology. Omega-3 fatty acids are generally considered anti-inflammatory, and different mechanisms that promote these effects have been elucidated. DHA exhibits antioxidant properties by decreasing the accumulation of reactive oxygen species (ROS) such as lipid peroxide in vivo, which may protect neurons from oxidative damage and hence prevent neuron loss. The omega-6 polyunsaturated fatty acid arachidonic acid (ARA) makes a significant contribution to the fatty acids present in the membrane phospholipids of cells involved in inflammation. ARA is a precursor to potent pro-inflammatory mediators including prostaglandins and leukotrienes. For this reason, many anti-inflammatory pharmaceuticals have been developed that target the ARA pathway to successfully control inflammation. There is also evidence that a high omega-6 fatty acid diet inhibits the anti-inflammatory and inflammation-resolving effect of omega-3 fatty acids.16
In the recent COVID-19 pandemic, a section of infected patients experienced a condition referred to as a “cytokine storm.”17 A full understanding of the cellular and molecular mechanisms underlying the “cytokine storm” has not yet been unraveled.18 Recent articles suggest that specific nutrients such as vitamin B6, B12, C, D, E, and folate; trace elements, including zinc, iron, selenium, magnesium, and copper may play a key role in the management of the cytokine storm.19,20 Among these micronutrients, long chain PUFAs such as EPA and DHA are worth mentioning because of their direct influence in the immunological response to viral infections.21,22 The possible beneficial effects of EPA and DHA supplementation in the modulation of immune response in SARS-CoV-2 infection is under investigation.23,24,25,26,27,28 Among these complex immunomodulatory effects, interleukin-6 (IL-6) and interleukin-1β (IL-1β) have been shown to be affected by dietary EPA and DHA intake. In addition, poly (ADP-ribose) polymerase enzymes with anti-inflammatory properties translatable to human COVID-19 infection were shown to improve tissue levels of DHA and EPA and their downstream anti-inflammatory metabolites,29,30 further underscoring the applicability of DHA and EPA in the management of COVID-19. Neutralization of IL-6 via a monoclonal antibody (Tocilizumab) has been identified as a feasible therapeutic target in SARS-CoV-infections,31 nevertheless, reducing the expression of additional proinflammatory cytokines (e.g., IL-1β, IL-38) may have additional therapeutic and protective effects.32
Acknowledgment
This project was funded by KCU’s Summer Student Research Fellowship. The author would like to thank all the contributors to the original work described in this review.
Footnotes
Natalie Powell is a medical student at Kansas City University College of Osteopathic Medicine (KCU-COM). Suman Chaudhary, MS, is a Research Associate at KCU-COM, and Asma Zaidi, PhD, (above), is Vice Chair of Basic Sciences and Professor of Biochemistry at KCU-COM, Kansas City Campus, Kansas City, Missouri.
Disclosure
None reported.
References
- 1.Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr. 2005;72(3):239–42. [PubMed] [Google Scholar]
- 2.Yehuda S, et al. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23(5):843–53. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 3.Marin R, Diaz M. Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front Neurosci. 2018;12:128. doi: 10.3389/fnins.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma D, et al. DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res. 2010;1330:1–8. doi: 10.1016/j.brainres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Kan I, et al. Docosahexaenoic acid and arachidonic acid are fundamental supplements for the induction of neuronal differentiation. J Lipid Res. 2007;48(3):513–7. doi: 10.1194/jlr.C600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68(Suppl 2):S102–11. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 8.Mallick R, Basak S, Duttaroy AK. Docosahexaenoic acid, 22:6n−3: Its roles in the structure and function of the brain. Int J Dev Neurosci. 2019;79:21–31. doi: 10.1016/j.ijdevneu.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Calder PC, Yaqoob P. Understanding omega-3 polyunsaturated fatty acids. Postgrad Med. 2009;121(6):148–57. doi: 10.3810/pgm.2009.11.2083. [DOI] [PubMed] [Google Scholar]
- 10.Lacombe RJS, Chouinard-Watkins R, Bazinet RP. Brain docosahexaenoic acid uptake and metabolism. Mol Aspects Med. 2018;64:109–134. doi: 10.1016/j.mam.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Uauy R, et al. Essential fatty acids in visual and brain development. Lipids. 2001;36(9):885–95. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- 12.Ma D, et al. Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus. Hippocampus. 2008;18(3):326–33. doi: 10.1002/hipo.20393. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair AJ. Docosahexaenoic acid and the brain- what is its role? Asia Pac J Clin Nutr. 2019;28(4):675–688. doi: 10.6133/apjcn.201912_28(4).0002. [DOI] [PubMed] [Google Scholar]
- 14.Lauritzen L, et al. DHA Effects in Brain Development and Function. Nutrients. 2016;8(1) doi: 10.3390/nu8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazan NG. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol Aspects Med. 2018;64:18–33. doi: 10.1016/j.mam.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisoncik JR, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscogiuri G, et al. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74(6):850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant WB, et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder PC, et al. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12(4) doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messina G, et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L. Oxidation of Polyunsaturated Fatty Acids and its Impact on Food Quality and Human Health. Advances in Food Technology and Nutritional Sciences - Open Journal. 2015;1(6):135–142. [Google Scholar]
- 24.Calder PC. n−3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72(3):326–36. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 25.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5℃6):327–35. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Allam-Ndoul B, et al. A Study of the Differential Effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on Gene Expression Profiles of Stimulated Thp-1 Macrophages. Nutrients. 2017;9(5) doi: 10.3390/nu9050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maskrey BH, et al. Emerging importance of omega-3 fatty acids in the innate immune response: molecular mechanisms and lipidomic strategies for their analysis. Mol Nutr Food Res. 2013;57(8):1390–400. doi: 10.1002/mnfr.201200723. [DOI] [PubMed] [Google Scholar]
- 28.Zivkovic AM, et al. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric (Berkeley) 2011;65(3):106–111. doi: 10.3733/ca.v065n03p106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtin N, et al. Repositioning PARP inhibitors for SARS-CoV-2 infection(COVID-19); a new multi-pronged therapy for acute respiratory distress syndrome? Br J Pharmacol. 2020;177(16):3635–3645. doi: 10.1111/bph.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiss B, et al. Poly(ADP) ribose polymerase-1 ablation alters eicosanoid and docosanoid signaling and metabolism in a murine model of contact hypersensitivity. Mol Med Rep. 2015;11(4):2861–7. doi: 10.3892/mmr.2014.3044. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti P, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]