Abstract
Objective
Radiation therapy is a cornerstone of brain metastasis (BrM) management but carries the risk of radiation necrosis (RN), which can require resection for palliation or diagnosis. We sought to determine the relationship between extent of resection (EOR) of pathologically-confirmed RN and postoperative radiographic and symptomatic outcomes.
Methods
A single-center retrospective review was performed at an NCI-designated Comprehensive Cancer Center to identify all surgically-resected, previously-irradiated necrotic BrM without admixed recurrent malignancy from 2003-2018. Clinical, pathologic and radiographic parameters were collected. Volumetric analysis determined EOR and longitudinally evaluated perilesional T2-FLAIR signal preoperatively, postoperatively, and at 3-, 6-, 12-, and 24-months postoperatively when available. Rates of time to 50% T2-FLAIR reduction was calculated using cumulative incidence in the competing risks setting with last follow-up and death as competing events. The Spearman method was used to calculate correlation coefficients, and continuous variables for T2-FLAIR signal change, including EOR, were compared across groups.
Results
Forty-six patients were included. Most underwent prior stereotactic radiosurgery with or without whole-brain irradiation (n=42, 91%). Twenty-seven operations resulted in gross-total resection (59%; GTR). For the full cohort, T2-FLAIR edema decreased by a mean of 78% by 6 months postoperatively that was durable to last follow-up (p<0.05). EOR correlated with edema reduction at last follow-up, with significantly greater T2-FLAIR reduction with GTR versus subtotal resection (p<0.05). Among surviving patients, a significant proportion were able to decrease their steroid use: steroid-dependency decreased from 54% preoperatively to 15% at 12 months postoperatively (p=0.001).
Conclusions
RN resection conferred both durable T2-FLAIR reduction, which correlated with EOR; and reduced steroid dependency.
Keywords: Brain metastasis, radiation necrosis, radiation therapy, surgical resection
Introduction
Radiation therapy (RT) is a cornerstone of brain metastasis (BrM) treatment, with or without surgical resection or CNS-active systemic therapy. However, radiation necrosis (RN) is a common complication of intracranial radiation characterized by cerebral inflammation and perilesional edema, occurring in some 5-40% of treated lesions.1,2,3-8 RN incidence is dependent on radiation dose, tumor size, and in some cases adjuvant treatments (e.g. immune checkpoint inhibition), and is challenging to quantify due to inconsistent radiographic appearance and varying diagnostic criteria, with some definitions including only the symptomatic proportion. Furthermore, histopathological confirmation is rarely available and admixed recurrent tumor often confounds the radiographic picture, making study of these lesions difficult.9 Initial management is conservative with observation for asymptomatic lesions or, for symptomatic lesions, oral corticosteroids, VEGF inhibitor bevacizumab, oral pentoxifylline and vitamin E, laser interstitial thermal therapy (LITT), or hyperbaric oxygen.10-19 Once palliative conservative measures have failed, surgical resection is considered. Additionally, in the setting of noninvasive diagnostic uncertainty using standard magnetic resonance imaging (MRI), perfusion mapping, positron emission tomography (PET) scans and other imaging modalities, surgical resection also serves to provide tissue diagnosis when clinically warranted.
Palliative RN resection results in reduced perilesional edema and patient symptoms.8,22-26 However, the role of extent of resection (EOR) on edema reduction and symptomatic control and durability have not been established, which is a significant knowledge gap given that complete removal of necrotic, previously-irradiated tumors can be precluded based on eloquent location. Furthermore, the margins of a necrotic tumor can be difficult to define intraoperatively as compared to generally encapsulated untreated BrM and hypercellular gliomas, for example, and the unclear clinical significance of surgical aggressiveness towards this goal can obscure surgical decision-making. Therefore, we retrospectively reviewed a large NCI-designated Comprehensive Cancer Center experience of resected, pathologically-confirmed RN free of admixed viable metastatic disease and tracked long-term radiographic and symptomatic changes with volumetrically-ascertained EOR to inform the relevant relationships.
Methods
Design
This is a retrospective, single-center observational study. The institutional review board approved this study (IRB#16-1531) and granted a waiver of consent.
Patient Selection
Consecutive patients who underwent open surgical resection of previously-irradiated BrMs between 2003-2018 were screened, generating 312 cases. Of these, 241 were excluded for pathologic diagnosis of viable tumor admixed with RN as determined by a neuropathologist. Cases were excluded if there was any identifiable viable metastatic disease given the indeterminate clinical significance of these deposits. Of the 71 remaining specimens with pure RN, 20 were excluded due to imaging follow-up of <1 year given the a priori hypothesis that surgical effects would be durable to that timepoint; purely cystic lesion on imaging; or pathologic description of a predominantly-hemorrhagic lesion confounding the area of pathologically pure RN. At our institution, patients with suspected RN are treated conservatively with close radiographic surveillance when asymptomatic, and medically when symptomatic. Resection is generally offered with palliative intent in the setting of refractory symptoms (e.g. steroid dependency/toxicity or bevacizumab intolerance/contraindication including thromboembolism or hemorrhage history), or when pathologic confirmation is indicated (e.g. in the setting of suspected recurrence or infection). Three patients underwent same-site reoperation for recurrent RN, and two patients each underwent additional distant-site necrosis resections; only these patients’ first surgeries were included for analysis. This resulted in a study population of 46 distinct patients with 1 resected necrotic lesion each.
Pathologic Criteria for Radiation Necrosis
RN was independently diagnosed by the institution’s neuropathologists, and was defined by the presence of coagulative and fibrinoid necrosis, hyalinized vasculature, focal areas of perivascular lymphocytes, and associated inflammation.27 Multiple sites from the specimens were examined as per institutional protocol to evaluate for any viable recurrent metastatic disease, which was grounds for exclusion from this study.
Surgical Treatment and Follow-up
Included patients had immediate pre- and post-operative (within 48 hours) contrasted magnetic resonance imaging per institutional practice. Postoperative MR imaging at 3-, 6-, 12-, and 24-months were also reviewed where available; not all patients had imaging at each timepoint. Patients who did not have ≥12 months follow-up were excluded given the a priori hypothesis above to ensure appropriate classification of patients with transient versus durable symptomatic changes. The contrast-enhancing volume was contoured on the preoperative and immediately post-operative 3D T1 pre- and post-contrast images using the Brainlab SmartBrush segmentation tool (Munich, Germany) to determine EOR. Gross total resection (GTR) was defined as 100% removal of the enhancing tumor and as subtotal resection (STR) as <100% (i.e. including near-total resections). T2-FLAIR signal was also segmented using the SmartBrush tool. Symptoms, steroid use, and antiepileptic use were obtained at each of these timepoints when available. Symptoms were extracted from the inpatient and outpatient visit notes, which included review of systems and narrative descriptions. More detailed descriptors such as Karnofsky Performance Scale and Eastern Cooperative Oncology Group performance status were not consistently available and were therefore not included. In the immediate postoperative period, patients typically received an increased steroid dose that was then weaned as tolerated by symptoms and irrespective of EOR.
Statistical Analysis
The study population was characterized using descriptive statistics, including frequencies, medians, and interquartile ranges. Overall survival (OS) was calculated from date of surgery to death or last follow-up and presented using Kaplan-Meier curves. Rates of time to 50% T2-FLAIR signal reduction were calculated using cumulative incidence in the competing risks setting, where follow-up time was calculated from date of surgery to MRI date where 50% T2-FLAIR signal reduction was achieved, death, or last follow-up, whichever occurred first. The Spearman method was used to calculate correlation coefficients. Continuous variables for T2-FLAIR signal percentage change were compared across groups using the Wilcoxon rank sum test and Kruskal-Wallis test where appropriate. McNemar’s test was used to assess the difference in proportions of patients using steroids. The Wilcoxon signed rank test was performed to assess steroid use reduction over time. Fisher’s exact test was used to evaluate the association between EOR and postoperative symptom changes. All tests were two-sided with a statistical significance level of <0.05. All analyses were performed using R statistical software.
Results
Descriptive Characteristics
Forty-six unique patients were included (Table 1). Three patients ultimately underwent same-site reoperation for recurrent RN, and 2 underwent RN resection at a different intracranial site. Only unique patients at the time of their first surgery were included in the analysis. Median age at surgery was 60 years (range 35-83). Lesions were most commonly parietal (N=14, 30%), frontal (N=13, 28%) and occipital (N=8, 17%). Twelve had a prior craniotomy plus adjuvant or neoadjuvant index-site irradiation (26%). Histologies included non-small cell lung cancer, (N=20, 43%) breast cancer (N=9, 20%) and melanoma (N=9 each, 19%), in line with the incidence of brain metastases. The most common dominant referable symptoms were headache (N=13, 27%) and seizures (N=11, 23%). Twenty-five patients (54%) required corticosteroids immediately preoperatively (additional patients had been previously treated with steroid courses) and 4 (8%) had received bevacizumab at any point for RN. Preoperatively, patients were generally followed with serial MRI, perfusion sequences and/or FDG-PET in cases of diagnostic uncertainty; all resections were performed with palliative intent. Twenty-seven lesions underwent GTR (59%) and the remainder STR. The EOR interquartile range (IQR) was 80-100%. Mean time between preoperative MRI and surgery was 5.8 days. Median radiographic follow-up length was 22 months; 72% had a 24-month scan.
Table 1:
Patient characteristics.
| Variable | N = 46 (%) |
|---|---|
| Gender | |
| Female | 26 (57%) |
| Male | 20 (43%) |
| Age at Surgery | 60 (35, 83) |
| Preoperative Radiation Therapy | |
| SRS – hypofractionated | 11 (24%) |
| SRS – single-fraction | 25 (54%) |
| WBRT | 4 (9%) |
| WBRT + SRS | 6 (13%) |
| Surgical Lobe | |
| Parietal | 14 (30%) |
| Frontal | 13 (28%) |
| Occipital | 8 (18%) |
| Cerebellum | 5 (11%) |
| Temporal | 5 (11%) |
| Insula | 1 (2%) |
| Laterality | |
| Left | 21 (46%) |
| Right | 25 (54%) |
| Prior Craniotomy at Index Site | |
| No | 34 (74%) |
| Yes | 12 (26%) |
| Pathologic Diagnosis | |
| NSCLC | 20 (44%) |
| Breast Cancer | 9 (20%) |
| Melanoma | 8 (17%) |
| Small Cell Lung Cancer | 4 (9%) |
| Renal Cell Carcinoma | 2 (4%) |
| Sarcoma | 1 (2%) |
| Testicular Cancer | 1 (2%) |
| Thyroid Cancer | 1 (2%) |
| Dominant Preoperative Symptom | |
| Gait Disturbance | 2 (4%) |
| Headache | 13 (28%) |
| Seizure | 11 (24%) |
| Sensory Disturbance | 1 (2%) |
| Speech Disturbance | 2 (4%) |
| Vision Disturbance | 2 (4%) |
| Weakness | 3 (7%) |
| Other | 9 (20%) |
| Unknown | 3 (7%) |
| Extent of Resection | |
| Gross Total Resection | 27 (59%) |
| Subtotal Resection | 19 (41%) |
| Percent Resection | 100 (33, 100) |
Statistics presented: N (%); median (minimum, maximum)
NSCLC = Non-small Cell Lung Cancer, SRS = Stereotactic Radiosurgery, WBRT = Whole Brain Radiation Therapy
Radiation History
All lesions had been previously irradiated. Thirty-six patients (78%) had undergone stereotactic radiosurgery (SRS) prior to RN development (n=25 single-fraction, 11 hypofractionated), 6 (13%) received a combination of SRS plus whole-brain radiation therapy (WBRT), and 4 (9%) WBRT alone. The median time from most recent radiation treatment to RN surgical resection was 11 months (IQR 6-19).
Postoperative Changes in T2-FLAIR Signal Volume
Among all resected lesions, there was a statistically significant mean T2-FLAIR signal decrease versus baseline preoperative volume of 63% at 3 months, 78% at 6 months, 78% at 12 months, and 80% at last follow-up up to 24 months (p<0.05 for all) (Figure 1). GTR resulted in a significantly greater T2-FLAIR reduction versus STR by last follow-up (p<0.05). Patients with GTR experienced more rapid cumulative incidence of 50% T2-FLAIR volume reduction compared versus those with STR (p=0.05) (Figure 1B).
Figure 1:
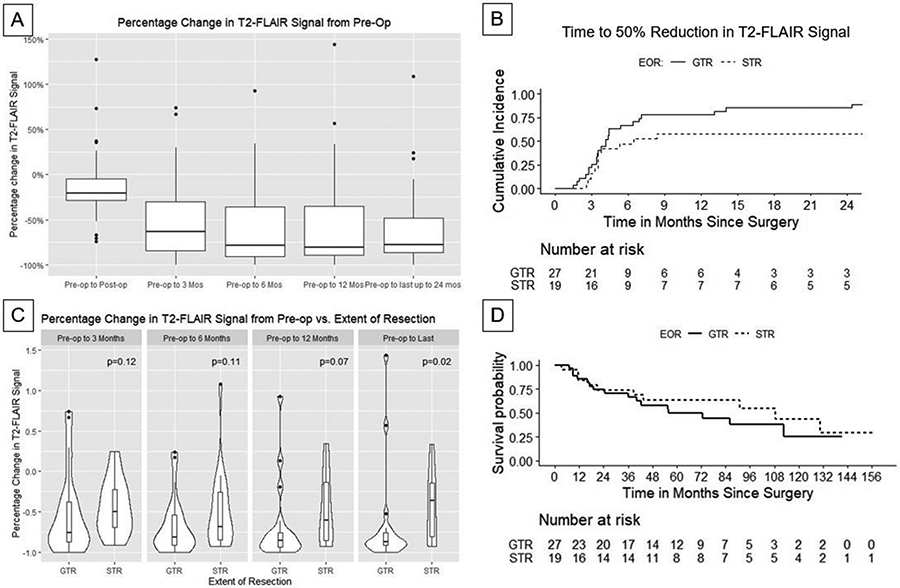
A) Percent change in T2-FLAIR signal over time relative to pre-operative T2-FLAIR volume. B) Kaplan-Meier curves demonstrating observed reduction in T2-FLAIR signal stratified by extent of resection (EOR). C) Percent change in T2-FLAIR signal over time as a function of extent of resection (gross-total vs. subtotal resection). D) Kaplan-Meier curves for overall survival for patients with gross total resection (GTR) and subtotal resection (STR).
There was no significant correlation between the ratio of preoperative contrast-enhancing tumor volume-to-T2-FLAIR volume with postoperative T2-FLAIR volume reduction on an absolute basis, however T2-FLAIR reduction was proportional to preoperative T2-FLAIR volume. Similarly, while preoperative contrast-enhancing tumor volume was not associated with T2-FLAIR volume reduction, T2-FLAIR reduction was proportional to preoperative enhancing RN volume. Figure 2 demonstrates representative T2-FLAIR changes in with STR and GTR. Exploratory analyses identified no differences in time to 50% T2-FLAIR reduction by histology (non-small cell lung cancer vs. other), brain location (parietal lobe vs. other), or form of preoperative radiation (single-fraction vs. hypofractionated SRS; Gray’s test p-values 0.37-0.91).
Figure 2:
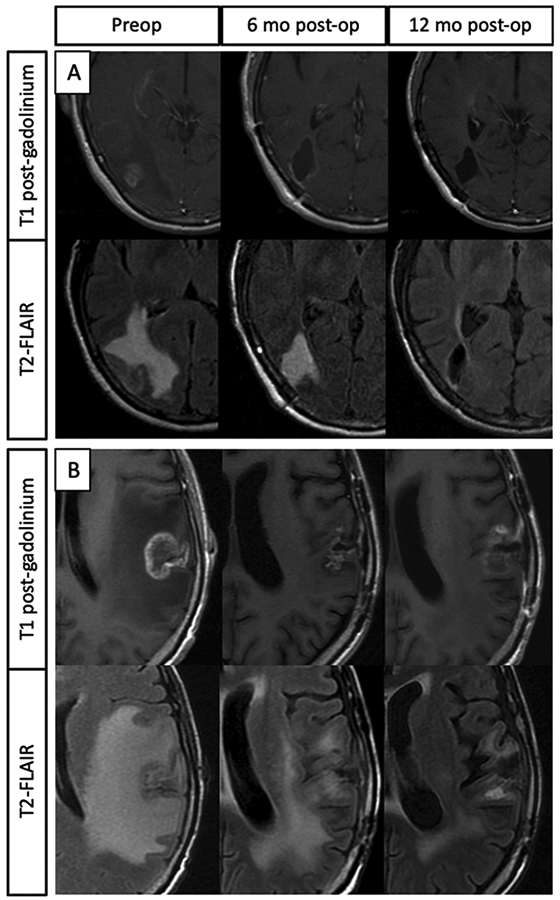
A) Representative images of a gross-totally resected RN lesion. The T1 post-contrast sequences demonstrate gross total resection of enhancing lesional tissue. The T2-FLAIR sequences demonstrate significant preoperative perilesional signal which progressively decreases post-operatively. B) Representative images of a sub-totally resected RN lesion. T1 post contrast images demonstrate a small amount of residual contrast enhancement at the 6- and 12-month post-operative time points. The T2-FLAIR sequences reveal significant perilesional signal preoperatively, which progressively decreases post-operatively.
Postoperative Symptom Changes and Steroid Use
Of the 42 patients with symptom data recorded, 23 (55%) experienced symptomatic improvement at 3 months postoperatively that remained stable thereafter, and 15 (36%) had stable symptoms postoperatively (Table 2). The remaining 4 (9%) experienced worsened symptoms. Of these, 1 with headaches suffered progressive ICP-related symptoms owing to multiple progressive lesions, another suffered from increased seizure frequency requiring anticonvulsant titration, another suffered from worsening sensory symptoms following resection from primary sensory cortex, and the fourth suffered from progressive neurologic decline referable to the subtotally-resected index necrotic occipital necrotic metastasis with progressive edema and other progressive lesions. There was no statistically-significant association between EOR and postoperative symptom changes or difference in symptom reduction when stratifying by focal deficits versus ICP-related symptoms (p>0.05). There was no statistically-significant correlation between T2-FLAIR volume percent change and postoperative symptom improvement at any timepoint (p>0.05). Across the cohort, the proportion of patients not requiring steroids among those who survived at least 12 months (N=39) increased from 18/39 preoperatively (46%) to 33/39 (85%) at 12 months (p=0.001). Among those patients remaining on steroids, their use decreased from 8mg daily dexamethasone equivalents preoperatively (range 2-36) to 3mg at 12-months postoperatively (range 1-8, p=0.063).
Table 2.
Change in patient symptoms by 3 months postoperatively; and steroid dependency
| Change in dominant RN-referable symptom | |||||
|---|---|---|---|---|---|
| Change in symptom | Total, N=46 | GTR, N=27 | STR, N=19 | Focal deficits, N=33 |
ICP-related symptoms, N=13 |
| Improved | 23 (50%) | 14 (52%) | 9 (47%) | 15 (46%) | 8 (61%) |
| Unchanged | 15 (32%) | 10 (37%) | 5 (26%) | 11 (33%) | 4 (31%) |
| Worsened | 4 (9%) | 2 (7%) | 2 (11%) | 3 (9%) | 1 (8%) |
| Unknown | 4 (9%) | 1 (4%) | 3 (16%) | 4 (12%) | 0 (0%) |
| Steroid dependency | |||||
| Time point (N) | Patients alive not taking steroids, N (%) | ||||
| Preoperative | 21/46 (46%) | ||||
| 3 months postoperatively | 35/46 (76%) | ||||
| 6 months postoperatively | 41/45 (91%) | ||||
| 12 months postoperatively | 33/39 (85%)* | ||||
p=0.001 versus preoperatively in patients alive at both timepoints.
Patient Survival and Follow-up
Median radiographic follow-up was 22 months (Table 1). Median OS was 86 months, with no significant difference in OS among those undergoing GTR versus STR (p=0.46, Figure 1). Among those with known cause of death, 9 were CNS-related deaths and 7 deaths non-CNS related.
Patients Requiring Repeat Same-Site RN Resection
Of the 3 patients undergoing repeat surgery for recurrent RN, 2 were melanoma metastases and 1 non-small cell lung cancer.All 3 suffered from seizures as their dominant referable complaint. One had initially undergone GTR with subsequent partial resection of the recurrent necrotic (again pathologically confirmed to harbor no viable tumor), with 53% reduction in T2-FLAIR at final follow-up. The other 2 underwent 79% and 96% EOR at the first RN operation with GTR of the recurrent lesion, with pathology also confirming persistent metastasis-free necrosis. Those patients experienced 47% and 73% reductions in T2-FLAIR by last follow-up, respectively. There was no difference in preoperative contrast-enhancing volume or ratio of T2-FLAIR to enhancement among these cases as compared to the remainder of the cohort studied.
Postoperative Complications
No patients experienced new post-operative deficits that were persistent at 3-month follow-up, death from surgical complications, wound complications requiring operative intervention, or other surgical-site infections. One patient did suffer from worsening of preexisting sensory symptoms following a postcentral gyrus metastasis resection.
Discussion
This series represents the largest and longest-followed reported cohort of surgically-removed and pathologically-confirmed RN, and the first analysis of EOR on long-term outcome of this condition (Table 3).8,22,24,28 Additionally, this cohort only includes patients with brain metastases, a population which unlike gliomas harbors discrete tumors without admixed or infiltrative neoplastic disease confounding outcome study, and additionally provides important information on outcomes with subtotal resection. Our findings are in line with those of case reports and heterogeneous retrospective studies which demonstrate that resection of the contrast-enhancing RN leads to radiographic and symptomatic improvement with low morbidity.8,22,24
Table 3:
Literature review on the surgical management of brain metastasis radiation necrosis
| Authors (year) |
Diagnoses included | Treatment | Preoperative tumor volume |
Pathologic confirmation of RN |
Extent of resection |
Follow up duration |
Outcomes | Periprocedural complications |
|---|---|---|---|---|---|---|---|---|
| Kimura T, et al. (2003) | Brain metastases | Resection (N=4), medical therapy (steroids, heparin, warfarin; N=2) | Not reported | Yes (surgical cases only) | 3/4 GTR (75%); 1/4 STR (25%) | Not specified | 2/4 surgical patients with qualitative improvement; 2/4 with subsequent worsening. Both medically managed patients with progressive neurologic decline. | Not specified |
| Mcpherson CM et al (2004) | Brain metastases (N=3) plus anaplastic astrocytoma, anaplastic oligodendroglioma and glioblastoma (N=8) | Resection (N=11) | Not reported | No | 9/11 GTR (82%); 2/11 STR (18%) | 13 months (mean; range 3-26) | Significant reduction in steroid use in 9/9 steroid-dependent patients; 8/11 patients with subjectively stable to improved neurologic function; 3/11 with worsened or new referable symptoms | Wound infection, carotid dissection, pulmonary embolism (N=1 each) |
| Telera S, et al. (2013) | Brain metastases | Resection (N=15) | Not reported | No: mixed group of pure RN (N=7), admixed RN+viable tumor (N=8) | 15/15 GTR (100%) | 14 months (median; range 6-38) | Post hospitalization outcomes not reported; brain edema resolved in all cases by 4 weeks, allowing “substantial” reduction or suspension of corticosteroid therapy | Transient dysphasia (N=2); seizure, CSF fistula, cerebellar ataxia, extremity weakness (N=1 each) |
| Shah AH, et al. (2020) | Brain metastases (N=14), high-grade glioma (6), meningioma (3), esthesioneuroblastoma (1) | Resection (N=24) | Not reported | Yes | 24/24 GTR (100%) | 13.3 months (mean; range 0.4-42.4) | 9/14 metastasis patients with symptomatic improvement by last follow up (64%). Among metastases, median time to steroid freedom of 3.4 weeks. | None |
| Rahmathulla G, et al. (2012) | Brain metastases | LITT (N=1) | 20mm maximal diameter | Yes (needle biopsy) | N/A | 7 weeks | Complete steroid wean with near resolution of symptoms | None |
| Rao MS, et al. (2014) | Brain metastases | LITT (N=15) | 3.7 cm3 (mean; range 0.5-25.5) | No: mixed group of pure RN and admixed RN+viable tumor, proportion not reported | N/A | 24 weeks (median; range: 4-84) | 5/8 symptomatic patients with improvement (63%), 1 with worsening (13%). 2/16 suffered subsequent recurrence requiring craniotomy, of which both were pathologically-confirmed RN (13%). | Asymptomatic hemorrhage, new hemiparesis (N=1 each) |
| Sujijantarat N, et al. (2020) | Brain metastases | LITT (N=25), bevacizumab (N=13) | 2.2 cm3(median; range 0.3-12.6) | Yes (LITT cases only) | N/A | 110 weeks (median; range 23-370) | LITT cohort: 9/13 symptomatic patients with improvement by 6 months (69%). Steroid dependency decreased from 56% to 32% at last follow up. Transient lesion enlargement through month 3 followed by reduction (enhancement evaluated only). N=5 required salvage bevacizumab therapy. | LITT cohort: Confusion and headache, worsening left-sided paresis, seizure, deep venous thrombosis (N=1 each) |
| Current study | Brain metastases | Resection (N=46) | 10.5 cm3(median; range 0.4-31.1) | Yes | 27/46 GTR (59%); 19/46 STR (41%) | 23 months (median; range 6-31) | 23/42 with improved symptoms at 3 months (55%); 15/42 stable (36%). Steroid dependency reduction from 54% to 15% at 1 year. Radiographic edema reduction was greater in the GTR cohort. | Worsened preexisting focal deficit (N=1). 3 patients required repeat craniotomy for refractory RN. |
When our study is combined with the existing literature, a clearer picture of the role of surgical resection of refractory, symptomatic RN arises. Several small series have shown that RN resection is safe and provides symptomatic relief.8,22,24,28 Shah et al. further detailed the positive impact of surgical resection on Karnofsky Performance Score in BrM patients with symptomatic RN with limited follow-up of 13 months.22 McPherson et al.28 and Shah et al.22 both demonstrated the role of resection in reducing postoperative steroid dependency. Our data support these findings, and also add several key insights into the effects of surgical resection on necrotic irradiated BrM which inform surgical candidacy and prognosis.
First, our data demonstrate that the majority of the T2-FLAIR reduction surrounding RN occurs by 6 months and is durable thereafter, which is important for the growing population of patients with brain metastases requiring irradiation, with inherently limited if growing life expectancy29. To this point, we identify a median survival of 86 months in this cohort which is substantially longer than the at-large BrM population and those described in other large surgical BrM series (Figure 1D).30 Acknowledging surgical selection bias for patients who survived long enough to develop RN and who had follow-up of ≥1 year, this finding likely reflects a population with treatable, well-controlled CNS and primary/extracranial disease and highlights the value of such palliative and diagnostic treatments for this patient group, even those with presumed RN; indeed some half of patients who died in this cohort suffered non-CNS cause of death. In the modern era of aggressive local brain-directed therapies for patients with CNS-centric metastatic disease, a growing body of literature suggests both increasing survival and, in some cases, an increasing proportion of non-neurologic death, making control of brain metastases and their complications ever more important.29,31
Importantly, we show that EOR is indeed related to radiographic outcome of volumetric T2-FLAIR signal, a critical finding not previously documented in the literature and which may guide clinical practice. In those patients in whom GTR was achieved, a significantly larger reduction in T2-FLAIR volume was seen by last follow-up compared to those who underwent STR. Additionally, time to 50% reduction in T2-FLAIR volume was accomplished more quickly in patients with GTR than in those with STR. The importance of this finding is underlined by our GTR rate of just 59%, which we posit is related to (1) infiltrative margins that are more microsurgically difficult to distinguish from normal brain than encapsulated viable metastases, (2) eloquent tract involvement/abutment in some cases, and (3) surgeon hesitancy to pursue aggressive removal in the face of intraoperative assessments of necrosis (including gross appearance and frozen section readouts) and a lack of prior EOR data in this space.
While alternative non-invasive, and minimally-invasive strategies including LITT have some evidence of efficacy, these also carry associated toxicity profiles. Furthermore, LITT is typically reserved for smaller necrotic lesions (on the order of 2.2cc described in a recent series) than those described in this series of symptomatic tumors given the potential for short-term swelling with the former approach, and long-term data have yet to mature.17
Steroid use was significantly reduced at all evaluated time points, which is a meaningful clinical endpoint for patients with metastatic disease including patients who may require immunotherapy or cell-based therapeutics for extracranial disease control. Multiple studies have suggested that steroid use blunts the CNS- and systemic effects of immunotherapies including checkpoint inhibitors, which are a mainstay in several cancers including melanoma. Thus, rapid steroid reduction is increasingly important.29,32-35 We did not identify a statistically meaningful correlation between EOR and symptom amelioration, which we hypothesize may be related to a combination of partial masking by steroid use (which was indeed reduced with surgical treatment), underpowering due to the small number of patients who met inclusion criteria, heterogeneity of referable symptoms, and the rarity of this indication for RN resection. Due to the significant association of EOR with reduction in T2-FLAIR volume, we believe it is reasonable to infer that symptomatic improvement would follow a similar pattern if the patient population were larger or more homogeneous. For patients with symptomatic RN, the significant EOR association with FLAIR reduction can be used as a potential surrogate to guide surgical decision-making. However, the comparable symptomatic relief seen in both groups also supports the conclusion that subtotal resection of the contrast enhancing lesion is a reasonable palliative approach when complete removal is not feasible.
It is important to note that while surgical resection of RN yielded significant and durable radiographic and symptomatic improvements, there were cases with refractory RN requiring repeat intervention, which were all re-proven histologically to represent pure RN without evidence of admixed viable metastatic disease. The only point of commonality between in these 3 patients was a preoperative contrast-enhancing lesion volume <7cc with T2-FLAIR volume ≥6 times that of the enhancing lesion volume. There were no other common factors with respect to tumor histology, location, or type of pre-operative RT. None received additional postoperative RT. These cases highlight that further basic investigation to understand the physiology of RN including clinico-pathologic correlatives (histology, location, prior radiation type), and outlier analysis of medically- and surgically-refractory cases, are both warranted and underway.
Though our study is the largest analysis of the relationship of EOR on RN resection outcomes, there are several important limitations. First, our study is retrospective and thus subject to selection bias and incomplete data. We attempted to limit the impact of the former by utilizing cross-sectional databases to identify patients who met inclusion criteria. Retrospective and subjective symptomatology documentation convey a degree of heterogeneity to the data. As a result, while we confirmed previous findings of symptomatic improvement with RN resection, we were not able to establish a statistically meaningful correlation between EOR and these improvements which we hypothesize is due limited powering. Third, while the pathologists independently evaluated multiple areas of each specimen prior to determining that there was no evidence of viable tumor, given the importance of this distinction to patients’ subsequent care, these findings are limited by the potential of sampling error which may partially explain the difference seen in the non-GTR vs GTR groups. However, this is not thought to play a significant role given that durable responses were seen in all but 3 cases, which included both GTR and non-GTR cases; each tumor had several cubic centimeters of resected tissue specimen available to the neuropathologists as these were open resections, and institutional protocol for such cases includes multifocal sampling given the importance of accurate diagnosis in these cases; and long survival was identified in both cohorts relative to the brain-metastatic population consistent with well-controlled disease, together adding to the confidence that all cases indeed represented pure RN. Fourth, our study represents the experience of a single center and may not reflect the surgical and cancer-directed outcomes experienced elsewhere. Prospective and multi-center investigations are needed both into RN treatment and risk factors (tumor-intrinsic and -extrinsic). Finally, the separate-but-related clinical entity of post-irradiation RN plus admixed recurrent malignancy is also worthy of future attention.
Nonetheless, our data showing consistent, durable efficacy of surgical resection of pathologically-confirmed radiation necrosis over a 15-year study period, in a range of brain metastasis diagnoses, make these findings broadly generalizable, and identify the key role of resection in conveying palliative benefit to this growing population.
Conclusions
Surgical resection of RN conferred durable radiographic improvement, and reduced steroid dependency, with EOR correlating with durable radiographic improvement. GTR conferred better improvements in perilesional edema versus STR.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations:
- BrM
Brain Metastasis
- EOR
Extent of Resection
- GTR
Gross Total Resection
- LITT
Laser Interstitial Thermal Therapy
- RN
Radiation Necrosis
- RT
Radiation Therapy
- SRS
Stereotactic Radiosurgery
- STR
Subtotal Resection
- WBRT
Whole Brain Radiation Therapy
Footnotes
Conflicts of Interest: NSM has consulted for AstraZeneca and received grant support from GT Medical Technologies. RJY has consulted for Agios, Puma, NordicNeuroLab and ICON plc, and received grant support from Agios, unrelated to this report.
Availability of data and material: The datasets generated during and/or analyzed during the current study are not publicly available to decrease the risk of breach of patient privacy but available from the corresponding author on reasonable request.
Ethics approval: This study was approved by the institutional review board (IRB# 16-1531) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate: The institutional review board (IRB# 16-1531) granted this study a wavier of consent.
Consent for publication: The institutional review board (IRB# 16-1531) granted this study a wavier of consent. No patient identifiers are presented in this study.
References
- 1.Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–1359. doi: 10.1148/rg.325125002 [DOI] [PubMed] [Google Scholar]
- 2.Loganadane G, Dhermain F, Louvel G, et al. Brain Radiation Necrosis: Current Management With a Focus on Non-small Cell Lung Cancer Patients. Front Oncol. 2018;8:336. doi: 10.3389/fonc.2018.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. doi: 10.1007/s11060-015-1881-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94(6):899–904. doi: 10.3171/jns.2001.94.6.0899 [DOI] [PubMed] [Google Scholar]
- 6.Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar AK, Kondziolka D, Lunsford LD. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57(2):452–464. doi: 10.1016/s0360-3016(03)00568-6 [DOI] [PubMed] [Google Scholar]
- 7.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. doi: 10.1016/j.ijrobp.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Telera S, Fabi A, Pace A, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol. 2013;113(2):313–325. doi: 10.1007/s11060-013-1120-8 [DOI] [PubMed] [Google Scholar]
- 9.Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457. doi: 10.1016/j.ijrobp.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 10.Vellayappan B, Tan CL, Yong C, et al. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel U, Patel A, Cobb C, Benkers T, Vermeulen S. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Translational Cancer Research. 2014;3(4):373–382-382. doi: 10.21037/2950 [DOI] [Google Scholar]
- 12.Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209(1-2):115–117. doi: 10.1016/s0022-510x(03)00007-8 [DOI] [PubMed] [Google Scholar]
- 13.Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–366. doi: 10.1159/000163557 [DOI] [PubMed] [Google Scholar]
- 14.Tye K, Engelhard HH, Slavin KV, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. 2014;117(2):321–327. doi: 10.1007/s11060-014-1391-8 [DOI] [PubMed] [Google Scholar]
- 15.Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67(6):1799–1815; discussion 1815. doi: 10.1227/NEU.0b013e3181fa775b [DOI] [PubMed] [Google Scholar]
- 16.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-oncology. 2013;15(9):1257–1263. doi: 10.1093/neuonc/not085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sujijantarat N, Hong CS, Owusu KA, et al. Laser interstitial thermal therapy (LITT) vs. bevacizumab for radiation necrosis in previously irradiated brain metastases. J Neurooncol. 2020;148(3):641–649. doi: 10.1007/s11060-020-03570-0 [DOI] [PubMed] [Google Scholar]
- 18.Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74(6):658–667; discussion 667. doi: 10.1227/NEU.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 19.Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200. doi: 10.1159/000338251 [DOI] [PubMed] [Google Scholar]
- 20.Grkovski M, Kohutek ZA, Schöder H, et al. 18F-Fluorocholine PET uptake correlates with pathologic evidence of recurrent tumor after stereotactic radiosurgery for brain metastases. Eur J Nucl Med Mol Imaging. 2020;47(6):1446–1457. doi: 10.1007/s00259-019-04628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatzoglou V, Yang TJ, Omuro A, et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016;18(6):873–880. doi: 10.1093/neuonc/nov301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AH, Mahavadi AK, Morell A, et al. Salvage craniotomy for treatment-refractory symptomatic cerebral radiation necrosis. Neurooncol Pract. 2020;7(1):94–102. doi: 10.1093/nop/npz028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Sako K, Tohyama Y, et al. Diagnosis and treatment of progressive space-occupying radiation necrosis following stereotactic radiosurgery for brain metastasis: value of proton magnetic resonance spectroscopy. Acta Neurochir (Wien). 2003;145(7):557–564; discussion 564. doi: 10.1007/s00701-003-0051-0 [DOI] [PubMed] [Google Scholar]
- 24.Wong S-T, Loo K-T, Yam K-Y, et al. Results of excision of cerebral radionecrosis: experience in patients treated with radiation therapy for nasopharyngeal carcinoma: Clinical article. Journal of Neurosurgery. 2010;113(2):293–300. doi: 10.3171/2010.1.JNS091039 [DOI] [PubMed] [Google Scholar]
- 25.Grossman R, Shimony N, Hadelsberg U, et al. Impact of Resecting Radiation Necrosis and Pseudoprogression on Survival of Patients with Glioblastoma. World Neurosurg. 2016;89:37–41. doi: 10.1016/j.wneu.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 26.Mou Y, Sai K, Wang Z, et al. Surgical management of radiation-induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: report of 14 cases. Head Neck. 2011;33(10):1493–1500. doi: 10.1002/hed.21639 [DOI] [PubMed] [Google Scholar]
- 27.Miyatake S-I, Nonoguchi N, Furuse M, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(1):50–59. doi: 10.2176/nmc.ra.2014-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol. 2004;68(1):41–47. doi: 10.1023/b:neon.0000024744.16031.e9 [DOI] [PubMed] [Google Scholar]
- 29.Bander ED, Yuan M, Carnevale JA, et al. Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer. Published online March 2, 2021. doi: 10.1002/cncr.33459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bander ED, Yuan M, Reiner AS, et al. Durable 5-year local control for resected brain metastases with early adjuvant SRS: the effect of timing on intended-field control. Neuro-Oncology Practice. 2021;(npab005). doi: 10.1093/nop/npab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niwińska A, Tacikowska M, Murawska M. The Effect of Early Detection of Occult Brain Metastases in HER2-Positive Breast Cancer Patients on Survival and Cause of Death. International Journal of Radiation Oncology*Biology*Physics. 2010;77(4):1134–1139. doi: 10.1016/j.ijrobp.2009.06.030 [DOI] [PubMed] [Google Scholar]
- 32.Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6. doi: 10.1186/s40425-018-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrelli F, Signorelli D, Ghidini M, et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers. 2020;12(3):546. doi: 10.3390/cancers12030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(8):722–730. doi: 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iorgulescu JB, Gokhale PC, Speranza MC, et al. Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in Glioblastoma. Clin Cancer Res. 2021;27(1):276–287. doi: 10.1158/1078-0432.CCR-20-2291 [DOI] [PMC free article] [PubMed] [Google Scholar]


