Abstract
Background:
Leptomeningeal metastasis (LM) occurs in 3–5% of patients with solid metastatic tumors and often portends a severe prognosis including symptomatic hydrocephalus and intracranial hypertension. Cerebrospinal fluid (CSF) shunting can provide symptomatic relief in this patient subset; however, few studies have examined the role of shunting in the palliation, prognosis and overall oncologic care of these patients.
Objective:
To identify and evaluate risk factors associated with prognosis after CSF diversion and assess surgical, symptomatic and oncologic outcomes in this population.
Methods:
A retrospective study was conducted on patients with solid-malignancy LM treated with a shunt at an NCI-designated Comprehensive Cancer Center between 2010–2019.
Results:
One hundred and ninety patients with metastatic LM underwent CSF diversion. Overall survival was 4.14 months from LM diagnosis (95%CI:3.29–4.70) and 2.43 months (95%CI:2.01–3.09) from shunting. Karnofsky Performance Status (KPS) at time of shunting and brain metastases (BrM) number at LM diagnosis demonstrated significant associations with survival (HR=0.66; 95%CI[0.51–0.86], p=0.002; HR=1.40; 95%CI[1.01–1.93] per 10 BrM, p=0.04, respectively). Eighty-three percent of patients experienced symptomatic relief, and 79% were discharged home or to rehabilitation facilities post-shunting. Post-shunt, 56% of patients received additional systemic therapy or started or completed WBRT. Complications included infection (5%), symptomatic subdural hygroma/hematoma (6.3%), and shunt externalization/removal/repair (8%). Abdominal seeding was not identified.
Conclusions:
CSF diversion for LM with hydrocephalus and intracranial hypertension secondary to metastasis can achieve symptomatic relief, hospital discharge, and return to further oncologic therapy, with a complication profile unique to this pathophysiology. However, decision-making in this population must incorporate end-of-life goals of care given limited prognosis.
Keywords: Cerebrospinal fluid, intracranial hypertension, leptomeningeal metastasis, metastasis, shunt
Introduction
Metastatic spread to the leptomeningeal compartment and subarachnoid spaces of the brain, known as leptomeningeal metastasis (LM; also known as leptomeningeal disease or carcinomatous meningitis), occurs in 3–5% of patients with solid tumor malignancies1. LM incidence roughly mirrors that of the commonest CNS parenchymal sources including non-small cell lung cancer (NSCLC), breast cancer, and melanoma, and its development is associated with poor prognosis and shortened survival on the scale of months2–11. Treatments are limited for these patients, in part due to clinical trial exclusion. Existing treatment options primarily include whole brain radiation therapy (WBRT), craniospinal irradiation, systemic therapies, and intrathecal chemotherapy, none of which significantly controls the disease or improves survival2,7,8,12–16.
While LM portends poor survival, symptomatic intracranial hypertension or hydrocephalus secondary to LM can be palliated with cerebrospinal fluid (CSF) diversion via extracranial shunting17–21. This approach improves intracranial pressure (ICP)-related LM symptoms, but its role in the overall oncologic and medical care of this population remains poorly defined and studied only in limited, small case series22. In particular, defining the patients that may or may not benefit from LM shunting, its modern risk profile, and factors associated with meaningful palliation are vital, despite a lack of detailed outcome reporting of this intervention. This retrospective study at a large Comprehensive Cancer Center aims to fill these gaps and identify risk factors associated with post-shunting prognosis to clarify its potential utilization in oncologic care.
Methods:
Study Cohort
This retrospective study of patients treated at Memorial Sloan Kettering Cancer Center (MSK) between 2010–2019 was approved by the Institutional Review Board. Patient consent for retrospective data collection was waived by the IRB. Patients diagnosed with LM via magnetic resonance (MR) imaging or CSF cytopathology secondary to solid tumor malignancies and treated with CSF diversion involving placement of a ventricular shunt terminating extracranially were included (n=190). Patients were excluded if the LM source was unclear (multiple CNS-metastatic cancers, additional primary CNS malignancy without cytologic clarification), or if shunting indication was normal pressure hydrocephalus or noncommunicating/obstructive hydrocephalus. Patients with a diagnosis of LM, based on MRI or LP, were excluded if they had no symptoms associated with high pressure (i.e. cranial neuropathy alone) or had normal opening pressure on LP. Chart review identified demographics, treatment and clinical histories. Targetable alterations included estrogen or progesterone receptor positivity [ER+/PR+], HER2+ status, and tyrosine kinase mutations/amplifications with Food and Drug Administration-approved medications [i.e. EGFR, BRAF, ALK, and NTRK]).
Statistical Analysis
Descriptive statistics such as proportions, means, and standard deviations were used to characterize the cohort. The Wilcoxon two-sample test was used to compare length of stay for inpatient versus outpatient consultations, both for the whole cohort and specifically patients that survived the hospitalization. Cumulative shunt incidence was estimated in the competing risks setting from time of LM until shunt, with death as a competing event. Kaplan-Meier methodology was used to estimate overall survival separately from LM and from shunt. Follow-up time was calculated from LM (or shunt) until death for those who died or until last follow-up for those who were censored. Risk factors were associated with survival in univariable Cox proportional hazards models. Treatment modalities were entered into the model as time-dependent variables. Cause-specific regression models were used to explore the associations between variables of interest and complications following shunt. Variables which were statistically significantly associated with outcome in the univariable setting were used to build multivariable models. Recursive partitioning analysis (RPA) was used to identify the cut-points of Karnofsky Performance Status (KPS) and number of brain metastases (BrM) most statistically significantly associated with survival. Tests were two-sided with an alpha level of statistical significance <0.05. All analyses were performed in SAS v9.4 (The SAS Institute, Cary, NC) and R v4.0.4 (The R Foundation for Statistical Computing).
Results:
Demographics and survival
One hundred and ninety patients were included (Table 1). Mean age at LM diagnosis was 56.7+/− 12.2 years (+/−standard deviation), with a predominance of women (68%). The most common primary cancer histologies were non-small cell lung cancer (NSCLC; 41%) and breast cancer (34%). LM was initially diagnosed by lumbar puncture alone in 21% of cases (i.e. without radiographically-apparent disease burden), MRI in 19.5% without or with negative LP, and corroborated by both modalities in 59.5%. In 44% of radiographically-apparent cases, LM was present in both cranial and spinal locations. Thirty-three percent of patients (n=63) harbored LM without parenchymal brain metastases (BrM) at first diagnosis of CNS disease. For patients that initially presented with parenchymal BrM at CNS diagnosis, mean time from BrM diagnosis to LM diagnosis was 9.1+/−12.1 months. A mean of 3.9+/−7 BrM were present at time of LM diagnosis for all comers. Prior to LM diagnosis, 20% of patients had undergone craniotomy, 23% stereotactic radiosurgery (SRS), 20% WBRT, 78% chemotherapy, 40% targeted therapy (including 25% receiving tyrosine kinase inhibitor therapy), and 13% immunotherapy. Headache was the most common dominant complaint present at LM diagnosis (31%) followed by gait imbalance (16%) or altered mental status (15%). The majority of patients (73%) had active systemic disease at time of LM diagnosis. Following LM diagnosis, treatments included WBRT (48% of patients), chemotherapy (44%), targeted systemic therapy (30%), immunotherapy (12%), and intrathecal chemotherapy (2%).
Table 1.
Descriptive demographics.
| Variable | Level | N | %* | Median | Mean | St dev | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Age at LMD diagnosis | continuous, years | 190 | 100 | 57.6 | 56.7 | 12.2 | 25.9 | 90.3 |
| Sex | Male | 61 | 32 | |||||
| Female | 129 | 68 | ||||||
| Race | White | 143 | 75 | |||||
| African American | 16 | 8 | ||||||
| Asian | 25 | 13 | ||||||
| Other/Unknown | 6 | 3 | ||||||
| Histology | NSCLC | 77 | 41 | |||||
| Breast | 65 | 34 | ||||||
| Melanoma | 15 | 8 | ||||||
| RCC | 1 | 1 | ||||||
| GI | 5 | 3 | ||||||
| Ovarian | 8 | 4 | ||||||
| Endometrial | 1 | 1 | ||||||
| Sarcoma | 2 | 1 | ||||||
| Thyroid | 1 | 1 | ||||||
| Prostate | 3 | 2 | ||||||
| Urothelial | 2 | 1 | ||||||
| Squamous cell | 2 | 1 | ||||||
| SCLC | 3 | 2 | ||||||
| Other/unknown | 5 | 3 | ||||||
| Targetable mutation status | None | 84 | 44 | |||||
| Yes | 105 | 55 | ||||||
| Unknown | 1 | 1 | ||||||
| KPS at LMD Diagnosis | 30 | 3 | 2 | |||||
| 50 | 6 | 3 | ||||||
| 60 | 19 | 10 | ||||||
| 70 | 36 | 19 | ||||||
| 80 | 45 | 24 | ||||||
| 90 | 21 | 11 | ||||||
| 100 | 3 | 2 | ||||||
| Unknown | 57 | 30 | ||||||
| Time from CNS diagnosis to LMD diagnosis | continuous, months | 190 | 100 | 0.2 | 6.5 | 12.2 | 0 | 85.4 |
| Method of LMD diagnosis | MRI | 37 | 19 | |||||
| LP | 40 | 21 | ||||||
| Both | 113 | 59 | ||||||
| LP/Ventricular opening pressure | continuous | 160 | 84 | 27 | 29.9 | 13.8 | 6 | 73 |
| Protein count | continuous, mg/dL | 166 | 87 | 68 | 134.7 | 192.6 | 15 | 1390 |
| Location of LMD on MRI | Cranial | 50 | 26 | |||||
| Spine | 11 | 6 | ||||||
| Both | 83 | 44 | ||||||
| No LMD on MRI | 39 | 21 | ||||||
| No spinal imaging | 7 | 4 | ||||||
| Number of BrM at LMD Diagnosis | continuous | 190 | 100 | 1 | 3.9 | 7 | 0 | 40 |
| Presence of ventriculomegaly | No | 103 | 54 | |||||
| Yes | 87 | 46 | ||||||
| Active systemic disease at LMD diagnosis | No | 51 | 27 | |||||
| Yes | 139 | 73 | ||||||
| Symptoms | None | 7 | 4 | |||||
| Headache | 59 | 31 | ||||||
| Vomiting | 25 | 13 | ||||||
| Vision/hearing | 15 | 8 | ||||||
| AMS | 28 | 15 | ||||||
| Gait | 31 | 16 | ||||||
| Incontinence | 3 | 2 | ||||||
| Cranial neuropath | 7 | 4 | ||||||
| Pressure waves | 11 | 6 | ||||||
| Seizure | 4 | 2 | ||||||
| Prior to LMD, History of: | Craniotomy | 38 | 20 | |||||
| SRS | 43 | 23 | ||||||
| WBRT | 38 | 20 | ||||||
| Chemotherapy | 148 | 78 | ||||||
| Targeted therapy | 76 | 40 | ||||||
| Immunotherapy | 24 | 13 | ||||||
| TKI | 48 | 25 |
Percentages may not total to 100% due to rounding.
Abbreviations: LMD=Leptomeningeal disease; BrM=Brain metastases; LP=Lumbar Puncture; KPS=Karnofsky Performance Status; CNS=Central Nervous System
Median overall survival (OS) from time of LM diagnosis was 4.14 months (Figure 1A, 95%CI:3.29–4.70). Supplemental Figure 1 demonstrates univariable analysis for factors associated with OS, which identified sex, race, LP opening pressure, CSF protein count, KPS at LM diagnosis, altered mental status, and post-LM treatment with immunotherapy as significantly associated. Interestingly, year of LM diagnosis, targetable mutation status, treatment with radiation or targeted therapies after LM diagnosis and presence of systemic disease did not significantly associate with overall survival. Figure 1B demonstrates the multivariable hazard ratios for factors associated with OS. Black race (HR=2.69; 95%CI[1.12–6.46], p=0.03) and other/unknown race (HR=4.21; 95% CI [1.54–11.52], p=0.005) remained associated with shorter OS relative to white race. Higher protein count on LP also remained significantly associated with worse OS (HR=1.13 per 10 units 95%CI[1.01–1.27], p=0.04.
Figure 1.
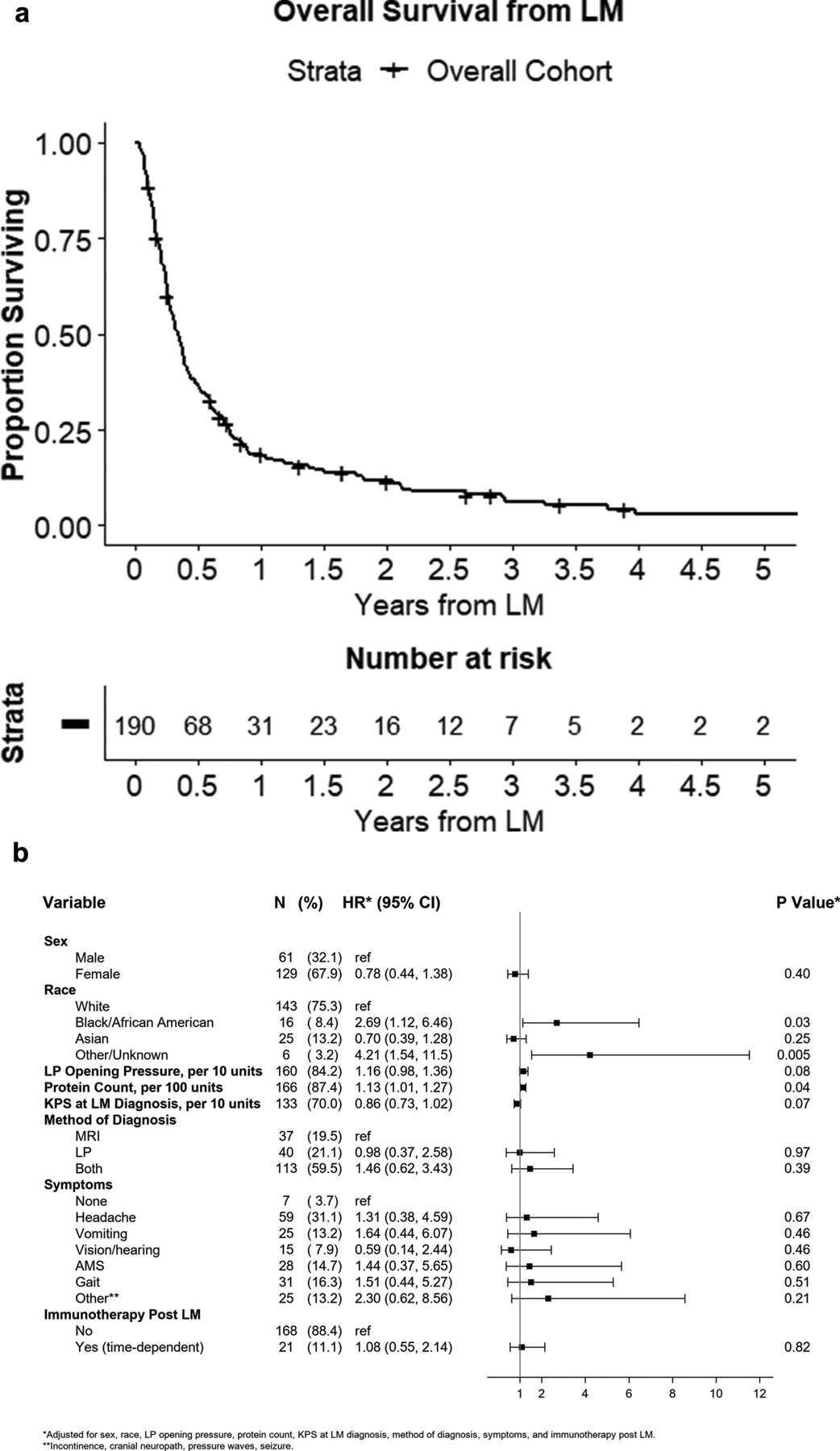
Overall survival from leptomeningeal disease (LM) diagnosis. (A) Kaplan-Meier curve demonstrating overall survival from time of LM diagnosis. (B) Forest plot demonstrating hazard ratios from multivariable analysis of factors associated with overall survival from time of LM diagnosis.
Shunting interventions
Cumulative incidence for shunt placement from time of LM diagnosis is shown in Figure 2A. Mean time from LM diagnosis to shunt was 78.8 +/−271.4 days (median 15.5 days; Table 2). A majority (76%) required hospitalization for LM-referable symptoms and presented to neurosurgical attention via inpatient consultation. At time of CSF diversion, KPS was ≥60 in 35% of patients, <60 in 12%, and not prospectively collected in 53%. CSF diversion was accomplished via ventriculo-peritoneal shunt (VPS) in 99% of patients and 1% underwent ventriculo-pleural (VPleural) shunt. There were no cases of upfront ventriculoatrial or -cystic shunts. A frontal approach was used in 91% of cases, with occipital entry in 9%, generally those in which pathology hindered a frontal approach. Intraoperative guidance (frameless stereotaxy, ultrasound, and/or fluoroscopy) was utilized in 16% of cases, generally for cases with smaller ventricular size. Programmable valves were placed in 82% of cases. Access surgeons (e.g. general/colorectal surgeon) were used for assistance with the abdominal portion of the case in 9% of patients, often due to prior extensive abdominal surgery or pathology. Sutures were used to close cranial incisions in 36% of patients, largely for patients with imminent WBRT, recent bevacizumab treatment, or re-operation. The remainder of cranial incisions underwent staple skin closure.
Figure 2.
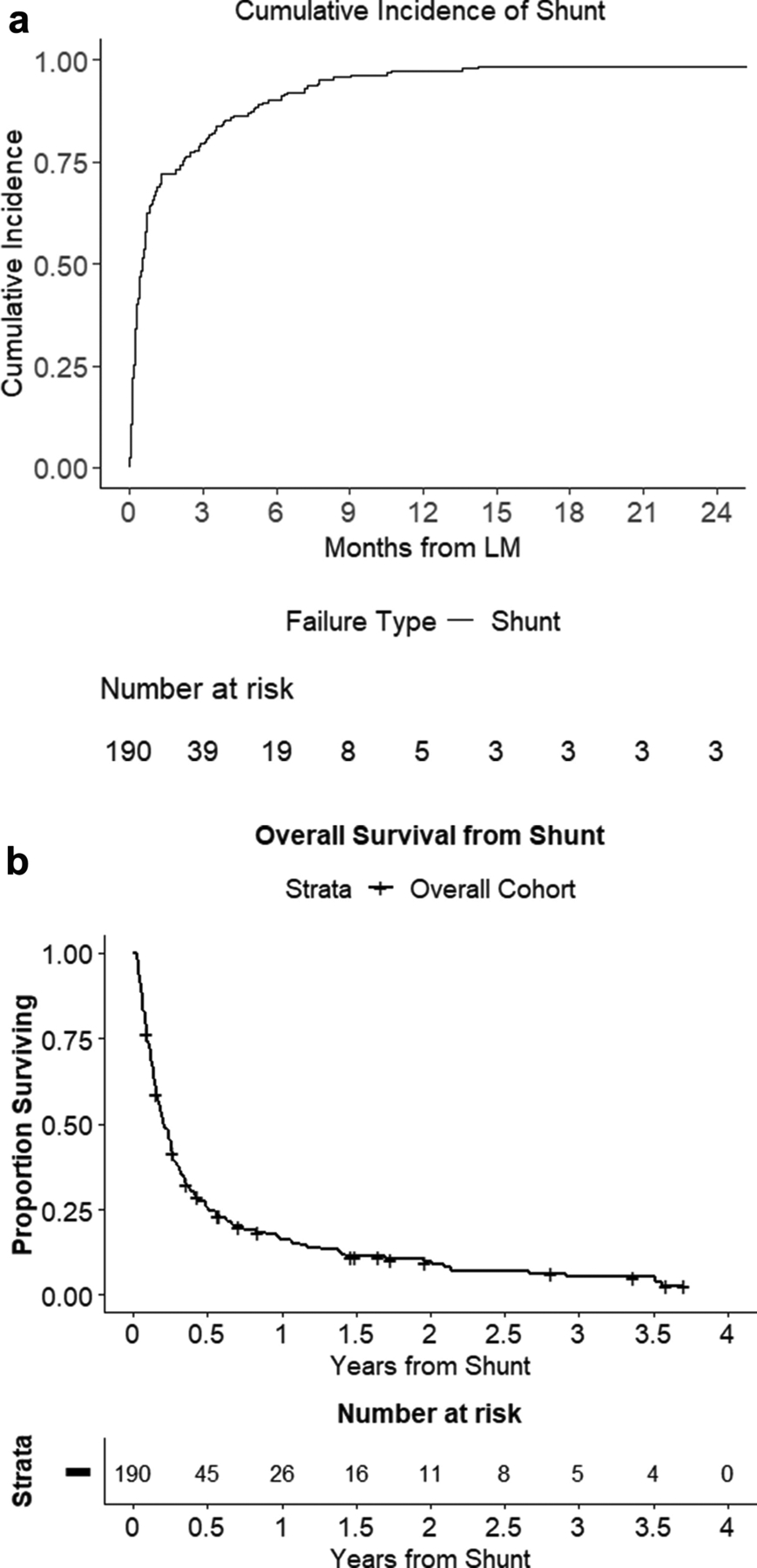
CSF diversion as an intervention for LM. (A) Cumulative incidence curve of shunt placement from time of LM diagnosis. (B) Kaplan-Meier curve demonstrating overall survival from time of shunt placement.
Table 2.
Shunt treatment summary
| Variable | Level | N | %* | Median | Mean | St dev | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Age at time of shunt | continuous, years | 190 | 100 | 57.6 | 56.9 | 12.2 | 25.9 | 90.3 |
| Time from LMD to shunt | continuous, days | 190 | 100 | 15.5 | 78.8 | 271.4 | 0 | 3234 |
| Length of Hospitalization | continuous, days | 190 | 100 | 4 | 7.9 | 11.4 | 1 | 74 |
| KPS at time of shunt | 30 | 2 | 1 | |||||
| 40 | 1 | 1 | ||||||
| 50 | 19 | 10 | ||||||
| 60 | 25 | 13 | ||||||
| 70 | 31 | 16 | ||||||
| 80 | 10 | 5 | ||||||
| 90 | 2 | 1 | ||||||
| Unknown | 100 | 53 | ||||||
| Shunt location | Frontal | 172 | 91 | |||||
| Occipital | 18 | 9 | ||||||
| Shunt type | VPS | 189 | 99 | |||||
| VPleural | 1 | 1 | ||||||
| Programmable Valve | Yes | 156 | 82 | |||||
| No | 34 | 18 | ||||||
| Intraoperative guidance | None | 161 | 85 | |||||
| Neuro-navigation | 11 | 6 | ||||||
| Neuro-navigation+Ultrasound | 1 | 1 | ||||||
| Ultrasound | 16 | 8 | ||||||
| Fluoroscopy | 1 | 1 | ||||||
| General surgical involvement | No | 172 | 91 | |||||
| Yes | 18 | 9 | ||||||
| Cranial closure | Sutures | 68 | 36 | |||||
| Staples | 122 | 64 | ||||||
| Inpatient presentation | No | 46 | 24 | |||||
| Yes | 144 | 76 | ||||||
| Discharge location | Home | 135 | 71 | |||||
| Acute/Subacute Rehabilitation | 16 | 8 | ||||||
| Hospice | 26 | 14 | ||||||
| Death in hospital | 13 | 7 | ||||||
Percentages may not total to 100% due to rounding.
Abbreviations: VPS=ventriculoperitoneal shunt; VPleural=ventriculopleural shunt; KPS=Karnofsky Performance Status
Outcomes after shunting
The median overall survival from shunting was 2.43 months (95%CI:2.01–3.09; Figure 2B). Postoperatively, 71% of patients were discharged to the home setting, 8% of patients were transferred to an acute/subacute rehabilitation center, and 14% to hospice; 7% died in the hospital. Subjective symptom improvement was seen immediately post-op or by first follow-up in 83% of patients. After shunting, 39% of patients received chemotherapy, targeted therapy, or immunotherapy. WBRT was newly started after shunting in 25% of patients. In 11% of patients, WBRT was previously started but halted due to symptoms referable to ICP, and subsequently resumed after shunt placement with completion of the remaining treatment fractions. The mean length of stay was 7.9+/−11.4 days post-shunt. Post-shunt hospitalization length was not significantly different for inpatient shunt consultations versus patients who were admitted electively for the procedure (8.5+/−12.2 days versus 6.1+/−8.2, respectively, p=0.17), and remains the case when excluding patients that died in the hospital (6.3+/−7.4 days versus 5.1+/–4.95, respectively, p=0.34). The long length of stay for these patients takes into account both additional treatments and social needs above simply surgical recovery and monitoring.
Univariable (Supplemental Figure 2) and multivariable analyses (Figure 3A) were conducted to identify factors associated with overall survival from time of CSF diversion. Univariable analysis identified time interval from LM-to-shunt, subjective improvement of symptoms, race, LP opening pressure, KPS, immunotherapy prior to shunt, and number of BrM at LM diagnosis to associate with OS. Targetable mutation status, presence of systemic disease, and treatment with targeted or radiation therapies before or after shunt and immunotherapy after shunt did not associate with overall survival on univariable analysis. On the multivariable analysis, KPS at time of shunting and BrM number at LM diagnosis maintained significant associations with survival (HR=0.66; 95%CI[0.51–0.86], p=0.002; HR= HR=1.40; 95%CI[1.01–1.93] per 10 BrM, p=0.04, respectively). Recursive partitioning analysis (RPA) identified a KPS cutoff of 60 as most significantly associated with survival after shunting (Figure 3B). A median survival of 1.95 months (95%CI:1.12–2.37) was identified for patients with KPS <60, and 3.63 months (95%CI:2.40–5.79) for KPS 60+. An RPA cutpoint for BrM at LM of <8 versus 8+ was identified with median overall survival of 2.89 months (95%CI:2.33–3.25) and 1.04 months (95%CI:0.79–1.45), respectively. Interestingly, race was statistically associated with OS from CSF diversion in univariable analysis; however, once KPS was added to the multivariable model, this association attenuated. Postoperatively, subjective symptomatic improvement also demonstrated a significant association with improved survival on multivariable analysis (HR=0.45, 95%CI[0.23–0.89], p=0.02).
Figure 3.
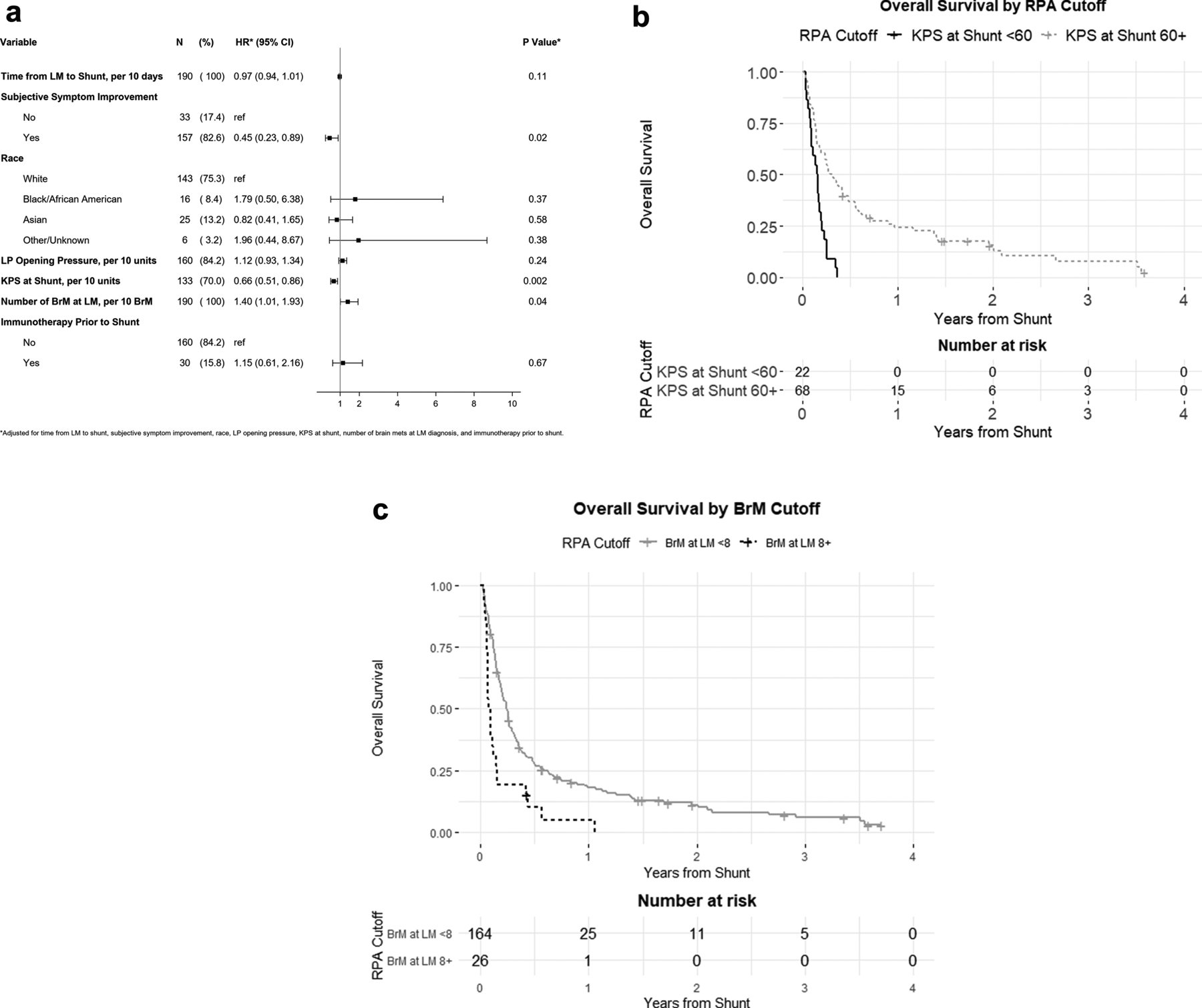
Factors associated with prognosis after shunt placement. (A) Forest plot demonstrating hazard ratios from multivariable analysis of survival from time of shunt placement. (B) Kaplan-Meier curves demonstrating longer overall survival for patients with KPS of 60+ compared with KPS<60 at time of shunting and (C) <8 BrM versus 8+ BrM at LM diagnosis.
Complications following CSF diversion
Table 3 describes shunting-referable or possibly-referable complications. Infection (cellulitis, meningoencephalitis), possibly-related infection (sepsis without meningitis) or wound breakdown occurred in 5% of cases. Radiographic subdural hygroma/hematoma was noted in 13% (n=25) of patients postoperatively. Of these, 48% experienced potentially-related symptoms including headache, altered mental status, gait decline, or seizure. Interventions for subdural collections included no intervention in 16% (n=4); shunt programming adjustment alone in 68% (n=17), operative evacuation of subdural after failed adjustment in 4% (n=1), and externalization for removal/ligation of shunt in 12% (n=3). Shunt externalization, removal, revision and/or repair was required in 8% of patients, with two patients undergoing both externalization and subsequent removal (n=15 patients/17 events). Indications for these procedures included infection (n=3), subdural collection (n=3), and some combination of proximal/distal failure or catheter migration in the remaining cases (n=9). No cases of metastatic abdominal seeding were identified.
Table 3.
Shunt Complications
| Variable | Level | N | %* |
|---|---|---|---|
| Shunt Complications (N=190) | None | 150 | 79 |
| Wound infection/Meningitis/Sepsis | 9 | 5 | |
| Subdural hygroma/hematoma | 25 | 13 | |
| Shunt malfunction | 9 | 5 | |
| Shunt Interventions (N=190) | Externalization | 3 | 2 |
| Removal | 5 | 3 | |
| Revision/Repair | 9 | 5 | |
| Number of Shunt Adjustments (N=190) | 0 | 130 | 68 |
| 1 | 40 | 21 | |
| 2 | 16 | 8 | |
| 3 | 3 | 2 | |
| 4 | 1 | 1 | |
| Any symptoms referrable to subdural collection (N=25) | No | 13 | 52 |
| Yes | 12 | 48 | |
| Specific symptoms potentially referrable to subdural (N=12): | Headache | 3 | 25 |
| AMS | 5 | 42 | |
| Gait | 2 | 17 | |
| Seizure | 2 | 17 | |
| Intervention for Subdural (N=25) | None | 4 | 16 |
| Shunt program adjustment + Burr-hole Drainage | 1 | 4 | |
| Shunt program adjustment alone | 17 | 68 | |
| Shunt program adjustment + Externalization + removal | 2 | 8 | |
| Externalization + removal | 1 | 4 | |
| Indication for Shunt Externalization/Removal/Revision (N=25) | Proximal valve failure alone | 1 | 7 |
| Proximal valve and Distal failure/migration | 3 | 20 | |
| Distal failure/migration of distal catheter alone | 5 | 33 | |
| Subdural hematoma | 3 | 20 | |
| Infection | 3 | 20 |
Percentages may not total to 100% due to rounding.
Abbreviations: AMS=Altered mental status
No variables assessed in this study significantly associated with risk of developing complications, in general, after shunting (Figure 4). However, when evaluating subdural hematoma/hygroma separately, multivariable analysis identified increased time from LM diagnosis to shunt placement (HR=1.02 per 30 days; 95% CI [1.01–1.04], p=0.008) and male sex (HR=2.61; 95%CI[1.12–6.07], p=0.03) as risk factors for developing post-operative subdural collections.
Figure 4.
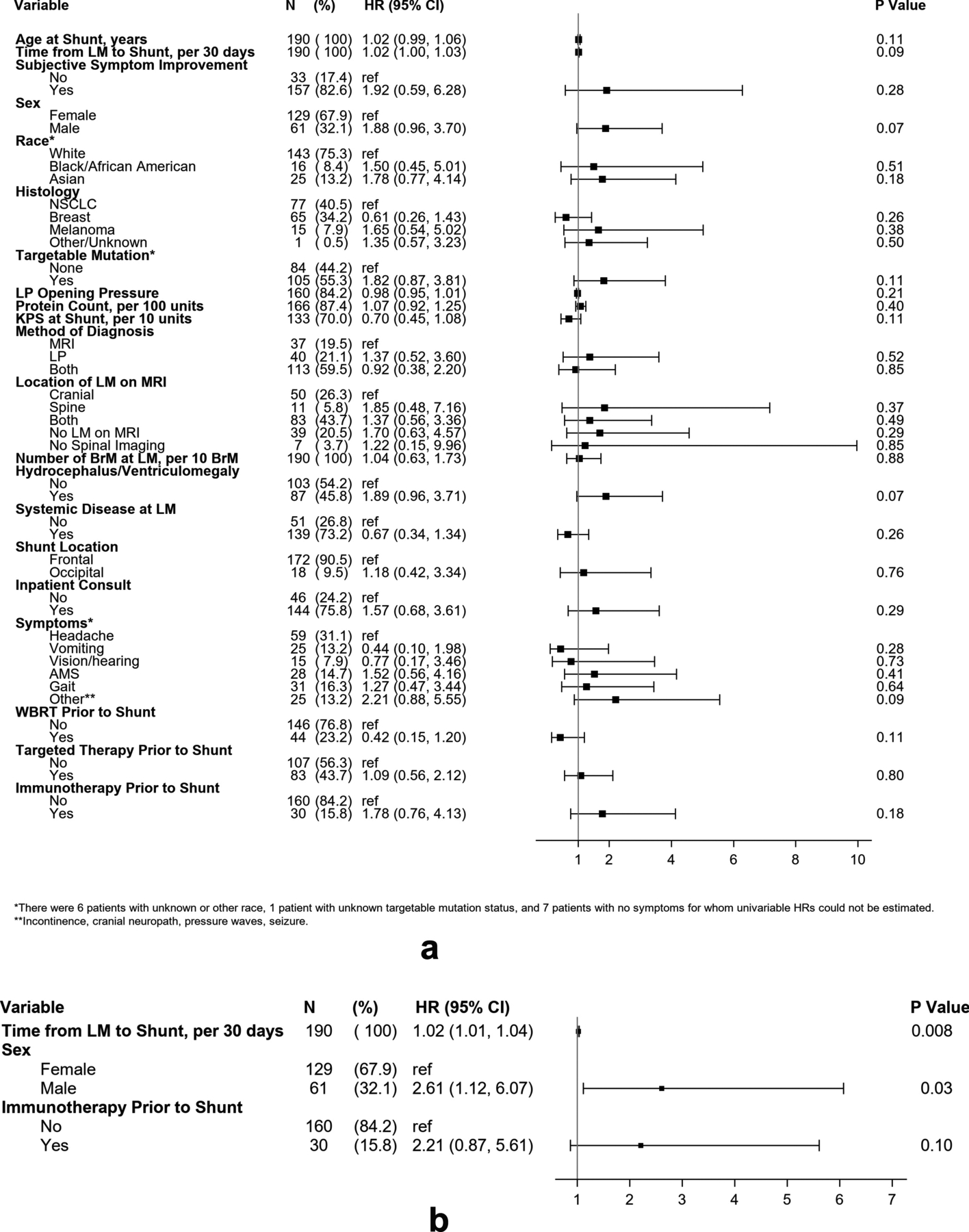
Factors associated with complications following shunt placement. (A) Forest plot demonstrating hazard ratios for factors assessed by univariable analysis with occurrence of any complication after shunt placement. (B) Forest plot demonstrating hazard ratios for multivariable analysis of subdural hygroma/hematoma occurrence after shunt placement.
Discussion
Patients diagnosed with LM secondary to metastatic tumors carry an extremely poor prognosis ranging from 2–5 months3,4,7. In this study of patients who ultimately underwent palliative CSF diversion for intracranial hypertension, which included a diversity of primary solid tumor types, a median overall survival of 4.14 months from LM diagnosis was identified. The survival of 3.63 months identified in even highly-symptomatic shunt-dependent patients with KPS>60 compares favorably to that described in previously published all-comer LM cohorts3,5–7,10. That year of LM diagnosis did not associate with differences in overall survival suggests that improving cancer outcomes generally2,11,23–25 have not yet translated into progress in LM survival, at least for the subpopulation requiring shunt placement. Procedures performed in this population must therefore have clear and defined outcomes that ethically qualify their potential harms and can be reviewed in goals of care discussions22. Palliative symptomatic relief has been the primary outcome focus of the CSF diversion/shunting case series literature to date17–21. However, other meaningful endpoints analyzed in this study included hospital discharge (e.g. to home or rehabilitation) or stabilization to allow for additional potentially active therapies.
At our institution, patients were generally referred by their neuro-oncologist and treated with a shunt if they presented with symptoms of elevated ICP (positional headache, nausea, vomiting, altered mental status, etc.) and new hydrocephalus, or if these symptoms were present with no frank hydrocephalus, but with known LM and high opening pressure on LP17. With this paradigm, patient selection allowed for 83% of patients to achieve subjective palliation of intracranial hypertension, with nearly an equivalent proportion discharged to a non-medical setting for end-of-life. Discharge from the hospital setting is a common, meaningful, and achievable goal for this population, which often present acutely and require hospital admission for their symptoms of hydrocephalic crisis or malignant intracranial hypertension, as also described herein. Notably, onset of these severe surgical symptoms occurred on the scale of days from LM diagnosis, suggesting rapid and unpredictable ICP deterioration is a feature of this disease, though not the rule. Ultimately, our conclusions regarding discharge outcomes are limited since no comparable control group of symptomatic patients involving medical management alone existed at our institution for review. The high rate of post-shunting symptomatic improvement did translate into a significant patient proportion motivated to continue with cancer- and LM-directed palliative therapies. 56% of patients received additional systemic therapy or started or completed WBRT. While these additional therapies are currently considered palliative, with no known survival benefits and no association with survival in our cohort analysis, the evidence for symptom stabilization and ability to tolerate further treatment after shunting is vital for promoting enrollment of LM patients into new or ongoing clinical trials. As new therapeutic options are identified and tested through well-designed clinical trials, the impact of shunting may expand from palliation to bridging patients to more impactful, therapeutic treatments. These potential outcomes must be clearly and thoroughly discussed and weighed by patients and providers to determine appropriate care on a patient-to-patient basis. The analysis presented herein provides data on which to base these goals of care discussions.
In the LM and benign normal pressure hydrocephalus populations, shunting carries reported complication rates ranging from 8–19%17,22,26–28. Our data corroborate this risk profile, with nontrivial risks of repeat procedures, infection and symptomatic subdural collections. The true rate of symptomatic hygroma/subdural hematoma is unclear given that many of the referable symptoms are also seen with the underlying LM given headaches (typically nonpositional) and altered mental status are a common feature of the disease. Nonetheless, most subdural complications were managed with shunt programming adjustment alone, demonstrating the utility of employing programmable valves, and the ability to manage non-operatively. Four patients did require shunt removal/externalization/ligation or operative subdural collection evacuation. Importantly, the potential complication of symptomatic metastatic abdominal seeding often discussed in the case report literature was not identified in any of the 190 patients in this series, although this may also be due to short survival of this patient population29,30. Our institutional practice does not include intracranial ventriculo-cisternostomy, despite recent publications27,31, given the low likelihood of sustained, long-term patency in this population and short survival mitigating the advantages of reduced long-term hardware implantation.
Ultimately, the complications we report establish that shunting is not an innocuous procedure especially in this population with survival of just 2.43 months postoperatively. Our multivariable analysis to define the patient population with the best post-shunting prognosis identified KPS of 60+, fewer (< 8) BrM, and improvement in subjective symptoms as factors associated with improved prognosis after shunting. While the latter cannot be fully predicted preoperatively, this behooves a multidisciplinary team to take special care in ascribing potential symptoms to ICP/hydrocephalus. Often a mixed symptomatic constellation is present, related to nodular disease (affecting the cranial nerves or spinal cord), seizures, toxic/metabolic derangements in the cancer population, parenchymal metastases, and the (typically non-positional) headaches sometimes seen in LM even without ICP elevation. These patients may not benefit significantly from shunt placement. The prospective factors of BrM burden and KPS corroborate reported survival correlates for patients with LM in general, with greater BrM number and worse performance status portending poor prognosis2,4,5,25. The significant survival drop-off with KPS<60 or with presence of numerous BrM at time of shunting may be reasonable bases to reconsider whether the benefits of the procedure outweigh the risks in that subset of patients.
Limitations
This study was conducted at a large Comprehensive Cancer Center allowing a large cohort compared to prior examinations of this topic, but also carries the selection biases of the patient population seen at a quaternary referral center and referred for neurosurgical intervention. This study is also limited by its retrospective design, which results in some limited data availability. In particular, the lack of recorded KPS at time of shunting for half of the study subjects may bias the analysis of this data point. While retrospective analysis allowed only for subjective assessment of symptom improvement, a more quantitative or objective assessment including quality of life metrics, prospectively collected, would be of great interest in future studies. As a retrospective study without a control group, the conclusions are also limited comparing the outcomes of shunting versus an alternative such as medical management in augmenting cancer-directed treatment strategies.
Conclusion
CSF diversion for leptomeningeal metastasis secondary to metastatic solid tumors can achieve symptomatic relief, hospital discharge, and return to further oncologic therapy, with a complication profile unique to this pathophysiology. Careful diagnostic evaluation, presurgical functional status, and surrogates of CNS burden of disease may assist with selection of candidates most likely to benefit and survive longest, however in all patients this must be balanced with palliative and end-of-life goals of care.
Supplementary Material
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Evan Bander is supported by the Leon Levy Foundation Fellowship in Neuroscience.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of Interest: At the time of research execution and publication, all authors are affiliated with Memorial Sloan Kettering Cancer Center. The authors of this research deny any conflicts of interest regarding this study and make the following disclosures: NSM: Consulting fees for advisory board participation from AstraZeneca and trial support from GT Medical Technologies (to institution) unrelated to the current work.
Consent to Participate: Informed consent was obtained from all individual participants included in the study.
Ethics approval: This study was approved by the IRB at Memorial Sloan Kettering Cancer Center
References
- 1.Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–452. doi: 10.1016/S1474-4422(06)70443-4 [DOI] [PubMed] [Google Scholar]
- 2.Ferguson SD, Bindal S, Bassett RL, et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). J Neurooncol. 2019;142(3):499–509. doi: 10.1007/s11060-019-03121-2 [DOI] [PubMed] [Google Scholar]
- 3.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro Oncol. 2008;10(6):1010–1018. doi: 10.1215/15228517-2008-062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Lee J-I, Nam D-H, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8(2):185–191. doi: 10.1097/JTO.0b013e3182773f21 [DOI] [PubMed] [Google Scholar]
- 5.Bruna J, González L, Miró J, et al. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009;115(2):381–389. doi: 10.1002/cncr.24041 [DOI] [PubMed] [Google Scholar]
- 6.Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. Journal of the Neurological Sciences. 2004;223(2):167–178. doi: 10.1016/j.jns.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. doi: [DOI] [PubMed] [Google Scholar]
- 8.Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726–2733. doi: 10.1016/j.ejca.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. The Lancet Oncology. 2018;19(1):e43–e55. doi: 10.1016/S1470-2045(17)30689-7 [DOI] [PubMed] [Google Scholar]
- 10.Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019;135:85–94. doi: 10.1016/j.critrevonc.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 11.Bander ED, Yuan M, Carnevale JA, et al. Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer. Published online March 2, 2021. doi: 10.1002/cncr.33459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82(9):1756–1763. [PubMed] [Google Scholar]
- 13.DeAngelis LM, Boutros D. Leptomeningeal metastasis. Cancer Invest. 2005;23(2):145–154. [PubMed] [Google Scholar]
- 14.Groves MD, Glantz MJ, Chamberlain MC, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10(2):208–215. doi: 10.1215/15228517-2007-059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A Randomized Controlled Trial Comparing Intrathecal Sustained-release Cytarabine (DepoCyt) to Intrathecal Methotrexate in Patients with Neoplastic Meningitis from Solid Tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 16.Buszek SM, Chung C. Radiotherapy in Leptomeningeal Disease: A Systematic Review of Randomized and Non-randomized Trials. Front Oncol. 2019;9. doi: 10.3389/fonc.2019.01224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omuro AMP, Lallana EC, Bilsky MH, DeAngelis LM. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64(9):1625–1627. doi: 10.1212/01.WNL.0000160396.69050.DC [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Park JB, Gwak H-S, Kwon J-W, Shin S-H, Yoo H. Clinical outcome of cerebrospinal fluid shunts in patients with leptomeningeal carcinomatosis. World J Surg Oncol. 2019;17. doi: 10.1186/s12957-019-1595-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nigim F, Critchlow JF, Kasper EM. Role of ventriculoperitoneal shunting in patients with neoplasms of the central nervous system: An analysis of 59 cases. Mol Clin Oncol. 2015;3(6):1381–1386. doi: 10.3892/mco.2015.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuya K, Nakasu Y, Hayashi N, et al. Palliative cerebrospinal fluid shunting for leptomeningeal metastasis-related hydrocephalus in patients with lung adenocarcinoma: A single-center retrospective study. PLoS One. 2019;14(1). doi: 10.1371/journal.pone.0210074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami Y, Ichikawa M, Bakhit M, et al. Palliative shunt surgery for patients with leptomeningeal metastasis. Clin Neurol Neurosurg. 2018;168:175–178. doi: 10.1016/j.clineuro.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Lamba N, Fick T, Nandoe Tewarie R, Broekman ML. Management of hydrocephalus in patients with leptomeningeal metastases: an ethical approach to decision-making. J Neurooncol. 2018;140(1):5–13. doi: 10.1007/s11060-018-2949-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schvartsman G, Ma J, Bassett RL, et al. Incidence, patterns of progression, and outcomes of preexisting and newly discovered brain metastases during treatment with anti-PD-1 in patients with metastatic melanoma. Cancer. 2019;125(23):4193–4202. doi: 10.1002/cncr.32454 [DOI] [PubMed] [Google Scholar]
- 24.Iorgulescu JB, Harary M, Zogg CK, et al. Improved Risk-Adjusted Survival for Melanoma Brain Metastases in the Era of Checkpoint Blockade Immunotherapies: Results from a National Cohort. Cancer Immunol Res. 2018;6(9):1039–1045. doi: 10.1158/2326-6066.CIR-18-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperduto PW, Mesko S, Li J, et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. JCO. 2020;38(32):3773–3784. doi: 10.1200/JCO.20.01255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Kong DS, Seol HJ, Nam D-H, Lee J-I. Ventriculoperitoneal shunt for hydrocephalus caused by central nervous system metastasis. J Neurooncol. 2011;104(2):545–551. doi: 10.1007/s11060-010-0512-2 [DOI] [PubMed] [Google Scholar]
- 27.Gonda DD, Kim TE, Warnke PC, Kasper EM, Carter BS, Chen CC. Ventriculoperitoneal shunting versus endoscopic third ventriculostomy in the treatment of patients with hydrocephalus related to metastasis. Surg Neurol Int. 2012;3:97. doi: 10.4103/2152-7806.100185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordan E, Palandri G, Lanzino G, Murad MH, Elder BD. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. Journal of Neurosurgery. 2018;131(4):1024–1036. doi: 10.3171/2018.5.JNS1875 [DOI] [PubMed] [Google Scholar]
- 29.Berger MS, Baumeister B, Geyer JR, Milstein J, Kanev PM, LeRoux PD. The risks of metastases from shunting in children with primary central nervous system tumors. Journal of Neurosurgery. 1991;74(6):872–877. doi: 10.3171/jns.1991.74.6.0872 [DOI] [PubMed] [Google Scholar]
- 30.Narayan A, Jallo G, Huisman TA. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: Case report and review of the literature. Neuroradiol J. 2015;28(5):536–539. doi: 10.1177/1971400915609348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CC, Kasper E, Warnke P. Palliative stereotactic-endoscopic third ventriculostomy for the treatment of obstructive hydrocephalus from cerebral metastasis. Surg Neurol Int. 2011;2:76. doi: 10.4103/2152-7806.82083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


