Abstract
PCR procedures amplifying portions of the 16S rRNA and NADH oxidase genes of Brachyspira aalborgi and Serpulina pilosicoli were applied to DNA extracted from paraffin-embedded human colonic or rectal tissues from 30 Norwegian, Australian, and U.S. patients, 16 of whom had histologic evidence of intestinal spirochetosis (IS). B. aalborgi-specific sequences were identified by PCR in 10 of the IS patients (62.5%) but none of the others, while S. pilosicoli sequences were not detected in tissues from any patient. Direct sequencing of products from three of the positive samples provided further confirmation of the presence of B. aalborgi. B. aalborgi may be a more common cause of intestinal spirochetosis than has been previously thought.
Intestinal spirochetosis (IS) is a condition of humans (4) and many animal species (8, 35, 37) in which there is a densely packed attachment of spirochetes by one end to the colonic epithelium, forming a “false brush border” (15). Generally, intestinal spirochetes associated with the condition in humans have not been well characterized (3, 14, 15, 23, 38, 47), although two species, Brachyspira aalborgi (18) and Serpulina pilosicoli (formerly Anguillina coli) (25, 39, 45, 46), have been identified. These species are distinct in a number of morphologic, biochemical, and molecular characteristics. Recently, it has been proposed that S. pilosicoli be renamed Brachyspira pilosicoli (28), but it is still a matter of contention whether or not the two organisms belong to the same genus (17, 34).
The pathogenicity of these organisms is unresolved. There are a number of reports in which IS, caused by as-then-uncharacterized spirochetes, has been associated with a variety of gastrointestinal disorders, including chronic diarrhea and rectal bleeding (5–7, 12, 13, 15, 21, 24, 31). The one detailed study in which B. aalborgi was isolated from humans with histologic evidence of IS, however, suggested that this spirochete is commensal (16). In contrast, there are a number of detailed studies on S. pilosicoli, including pathogenicity studies, in which strains isolated from humans have been used to infect and cause intestinal disease in both chicks and pigs (27, 42, 44). Unlike S. pilosicoli, B. aalborgi has failed to colonize experimentally infected chicks (41). Thus, it appears that human strains of S. pilosicoli have pathogenic potential, as do animal strains (35, 37), and this has been reinforced by the recovery of S. pilosicoli from the bloodstream of a series of critically ill human patients (43). The potential invasive properties of such organisms have been highlighted by a series of studies in which unidentified spirochetes were observed invading human intestinal epithelium (1, 14, 29) and liver parenchyma (23).
While B. aalborgi has been isolated on only one occasion from a human, a patient in Denmark (18), and this is the only strain available for study (513AT/ATCC 43994T), S. pilosicoli has been isolated from humans in the United States (20), Germany (22), Oman (3), the United Kingdom (25), France (10), Papua New Guinea (40), and Australia (24, 39). S. pilosicoli also naturally infects pigs (36), dogs (40), and chickens (26).
Although the condition known as IS has been defined from both a histologic and a microbiologic viewpoint, only two studies have concurrently examined both aspects, including full identification of the spirochetes involved. In one, B. aalborgi was isolated from 1 of 5 patients showing evidence of IS (18); in the other, S. pilosicoli was isolated from 22 of 41 rectal biopsies (53.7%) showing evidence of IS (39). An inherent problem in epidemiologic studies applied to intestinal spirochetes has been the selective culturing techniques used to isolate these fastidious anaerobes before they can be characterized (19). In particular, S. pilosicoli grows more rapidly in culture than does the single available strain of B. aalborgi, and the possibility remains that an S. pilosicoli isolate obtained from a culture may be masking other species or strains of spirochetes also present in the sample. The study in which S. pilosicoli was isolated from only 53.7% of human rectal biopsy specimens showing histologic evidence of IS could be interpreted to suggest that other, more fastidious spirochetes, including B. aalborgi, may have been present in the culture-negative specimens (39). Methods of molecular detection and characterization that can be applied directly to infected tissue without the need for culture are therefore required in order to resolve this possibility. The approach taken in the present study was to apply PCR to DNA extracted from human intestinal biopsies showing evidence of IS, so as to gain further insight into which spirochete species are predominantly involved in human IS. This information would allow the use of more appropriate diagnostic techniques for the condition. Tissues from patients without histologic evidence of IS were also tested, to confirm the specificity of the tests.
Thirty-one biopsy samples from the large intestines of 30 patients were analyzed by PCR; these included one sample each from 12 elderly patients in Norway, three samples from 2 human immunodeficiency virus (HIV)-positive patients in Washington, D.C., one sample each from 2 patients in Melbourne, Australia, and one sample each from 14 negative control patients in Perth, Australia (Table 1). All patients had symptoms of gastrointestinal disease, which prompted biopsies being taken to assist with diagnosis (Table 1). Only the two samples from the patients in Melbourne were cultured, with the medium and culture conditions recommended for Serpulina spp. (19), and both failed to yield spirochetes. The 17 samples from 16 patients in Norway, the United States, and Melbourne, Australia, were all selected for testing because they showed histologic evidence of IS, with a basophilic fringe of spirochetes attached by one cell end to the intact surface epithelium (Fig. 1). In the cases of all of the Norwegian and Australian IS specimens, the epithelial surface was intact and there was no evidence of inflammation in the lamina propria. As previously described, however (14), the lamina propria of the samples from the two U.S. patients both showed inflammatory infiltrates, and electron microscopic examination revealed the presence of spirochetes within foamy macrophages in the lamina propria and in mucosal epithelial cells. The 14 samples from Perth were selected for testing as negative controls because they did not show histologic evidence of IS. These patients had a variety of other intestinal disorders (Table 1). Three other cecal samples from chicks which had been experimentally infected with strains of S. pilosicoli isolated from humans (strains Karlos or WesB [41]) or Serpulina intermedia (porcine strain PWS/AT [32]) were also included as controls (Table 1). The former two were positive controls for S. pilosicoli, while the latter was a further negative control. No positive tissue controls from infected chicks were available for B. aalborgi, because this organism fails to colonize chicks (41). All samples had been routinely fixed in 10% phosphate-buffered formalin, dehydrated, and embedded in paraffin wax. The samples from the two chicks infected with S. pilosicoli showed histologic evidence of IS, with a false brush border of spirochetes being evident, as previously described (41).
TABLE 1.
Sources of intestinal samples from 16 patients diagnosed with IS, 14 patients negative for IS, and 3 experimentally infected chicks and subsequent PCR results with primers specific for the B. aalborgi and S. pilosicoli 16S rRNA and nox genes
| Source | Sex/agea | Biopsy location | PCR result
|
Histologic findings and/or clinical histology | |||
|---|---|---|---|---|---|---|---|
|
B. aalborgi
|
S. pilosicoli
|
||||||
| 16S rRNA | nox | 16S rRNA | nox | ||||
| Chick experimentally infected with S. pilosicoli Karlos | Cecum | − | − | + | + | ||
| Chick experimentally infected with S. pilosicoli WesB | Cecum | − | − | + | + | ||
| Chick experimentally infected with S. intermedia PWS/A | Cecum | − | − | − | − | ||
| South Norway | F/37 | Colorectal | − | − | − | − | Spirochetosis; ulcer of the colon |
| South Norway | F/55 | Colorectal | − | − | − | − | Spirochetosis; adenocarcinoma |
| South Norway | F/76 | Colorectal | − | − | − | − | Spirochetosis; adenocarcinoma |
| South Norway | M/67 | Colorectal | − | − | − | − | Spirochetosis; tubulovillous adenoma; diverticulitis |
| South Norway | M/74 | Colorectal | − | − | − | − | Spirochetosis; tubulovillous adenoma |
| South Norway | M/55 | Colorectal | + | + | − | − | Spirochetosis; adenocarcinoma; carcinoid tumor of appendix |
| South Norway | M/60 | Colorectal | + | + | − | − | Spirochetosis; squamous cell carcinoma of the transverse colon |
| South Norway | M/70 | Colorectal | + | + | − | − | Spirochetosis; adenocarcinoma; diverticulosis |
| South Norway | M/73 | Colorectal | + | + | − | − | Spirochetosis; adenocarcinoma |
| South Norway | M/75 | Colorectal | + | + | − | − | Spirochetosis; adenocarcinoma |
| South Norway | M/76 | Colorectal | + | + | − | − | Spirochetosis; adenocarcinoma |
| South Norway | M/80 | Colorectal | + | + | − | − | Spirochetosis; diverticulitis |
| United States | M/26 | Ascending colon | + | + | − | − | Spirochetosis; HIV+; 171 CD4 cells/μl; fever; anorexia; weight loss; cramping abdominal pain; bloody diarrhea |
| United States | M/26 | Rectum | + | + | − | − | Same patient as above |
| United States | M/48 | Descending colon | + | + | − | − | Spirochetosis; HIV+; 20 CD4 cells/μl; Kaposi’s sarcoma; weight loss; alternating watery diarrhea and constipation without melana; hyperplastic polyps; tubulovillous adenoma |
| Melbourne, Australia | F/16 | Colorectal | + | + | − | − | Spirochetosis; abdominal pain; otherwise normal |
| Melbourne, Australia | F/9 | Rectum | − | − | − | − | Spirochetosis; PR bleeding; spirochetosis in the past; mild nonspecific inflammation of the esophagus and stomach; otherwise normal |
| Perth, Australia | F/45 | Random colon | − | − | − | − | Negative for spirochetosis; iron deficiency |
| Perth, Australia | F/45 | Transverse colon | − | − | − | − | Negative for spirochetosis; diarrhea |
| Perth, Australia | F/47 | Rectum | − | − | − | − | Negative for spirochetosis; polyps; family history of carcinoma |
| Perth, Australia | F/54 | Cecum | − | − | − | − | Negative for spirochetosis; abdominal pain |
| Perth, Australia | F/65 | Descending colon | − | − | − | − | Negative for spirochetosis; diarrhea |
| Perth, Australia | F/78 | Sigmoid | − | − | − | − | Negative for spirochetosis; adenoma, diverticuli |
| Perth, Australia | M/36 | Left colon | − | − | − | − | Negative for spirochetosis; change in bowel habit |
| Perth, Australia | M/39 | Sigmoid colon | − | − | − | − | Negative for spirochetosis; diarrhea?; polyp |
| Perth, Australia | M/41 | Right colon | − | − | − | − | Negative for spirochetosis; change in bowel habit |
| Perth, Australia | M/43 | Middle colon | − | − | − | − | Negative for spirochetosis; diarrhea |
| Perth, Australia | M/69 | Rectum | − | − | − | − | Negative for spirochetosis; polyp |
| Perth, Australia | M/74 | Sigmoid colon | − | − | − | − | Negative for spirochetosis; minor inflammatory changes (history of Crohn’s disease) |
| Perth, Australia | M/75 | Rectum | − | − | − | − | Negative for spirochetosis; polyp |
| Perth, Australia | M/77 | Rectum | − | − | − | − | Negative for spirochetosis; polyp |
F, female; M, male. Ages are given in years.
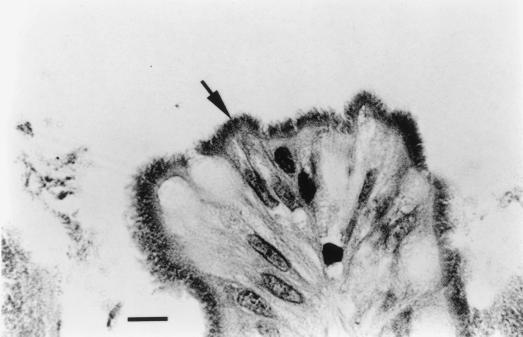
FIG. 1.
Photomicrograph of paraffin-embedded rectal biopsy tissue from a Norwegian patient (male, 60 years old) showing a false brush border of spirochetes subsequently shown by PCR to be B. aalborgi, attached by one end to the mucosa. Bar, 10 μm; stain, hematoxylin and eosin.
DNA from paraffin-embedded tissue (PET) samples was extracted by a modification of a previously described method (11). PET samples were sliced into 15- to 20-μm sections with a microtome blade, which was thoroughly cleaned between samples. Negative control chick samples were cut between sequential human samples. Each section was dewaxed by placing it in 200 μl of xylene and then on a rocker for 5 min at room temperature. The samples were then centrifuged at 10,000 × g for 10 min, and the supernatant was discarded. A second xylene incubation was performed, and 200 μl of 100% ethanol was added, incubated, and centrifuged, as for xylene. A second ethanol incubation and centrifugation was performed, the supernatant was discarded, and the samples were dried at 50°C in an oven for 30 min. Twenty micrograms of proteinase K (Boehringer GmbH, Mannheim, Germany) in 100 μl of 50 mM Tris-HCl, pH 8.3, was added and incubated overnight at 37°C. The samples were then boiled for 8 min, and 1 μl of each resultant extract was used as template DNA for PCR analysis.
The 16S rRNA gene sequences of B. aalborgi 513T and S. pilosicoli P43/6/78T, HRM-7A, and WesB (accession no. Z22781, U23032, Y10314, and unsubmitted, respectively), as well as the NADH oxidase (nox) gene sequences for these strains (accession no. AF060816, AF060807, AF060806, and AF060808, respectively), were obtained from the GenBank sequence database. Pairs of primers to specifically detect the presence of the B. aalborgi 16S rRNA gene, the S. pilosicoli 16S rRNA gene, the B. aalborgi nox gene, and the S. pilosicoli nox gene were designed from these sequences by using SeqEd (version 1.0.3; Applied Biosystems, Foster City, Calif.) and Amplify (version 1.2; University of Wisconsin, Madison, Wis.). The only exception was the previously described forward primer Acoli 1, used in detection of the S. pilosicoli 16S rRNA gene, which had been designed in our laboratory (30). Four separate PCR protocols were utilized with the primers, thermocycling conditions, and predicted product sizes outlined in Table 2.
TABLE 2.
Primers and thermocycling conditions for B. aalborgi- and S. pilosicoli-specific reactionsa
| PCR test | Primer sequence (name) | Predicted product size (bp) | Thermocycling program |
|---|---|---|---|
| B. aalborgi 16S rRNA | 5′-TAC CGC ATA TAC TCT TGA C-3′ (F Ba 16S) | 472 | 94°C for 4.5 min; (94°C for 30 s, 46°C for 30 s, 72°C for 30 s) × 33 cycles |
| 5′-CCT ACA ATA TCC AAG AAC C-3′ (R Ba 16S) | |||
| B. aalborgi nox | 5′-GGT TGA CTC AAG CAC TAC-3′ (F Ba nox) | 334 | 94°C for 4.5 min; (94°C for 30 s, 46°C for 30 s, 72°C for 30 s) × 30 cycles |
| 5′-AAA CCG TAT TTT GTT CCA GG-3′ (R Ba nox) | |||
| S. pilosicoli 16S rRNA | 5′-AGA GGA AAG TTT TTT CGC TTC-3′ (Acoli 1) | 196 | 94°C for 4.5 min; (94°C for 30 s, 51°C for 30 s, 72°C for 30 s) × 33 cycles |
| 5′-GTC GCT CCA TCA GAC TTT-3′ (R Sp 16S) | |||
| S. pilosicoli nox | 5′-GTA ACT CCT CCT ATT GAG-3′ (F Sp nox) | 465 | 94°C for 4.5 min; (94°C for 30 s, 45°C for 30 s, 72°C for 30 s) × 30 cycles |
| 5′-GCA CCA TTA GGT AAA GTC-3′ (R Sp nox) |
All reactions were performed on an Applied Biosystems 2400 thermocycler.
All amplification mixtures consisted of a 25-μl reaction mix of 1× PCR buffer, 0.55 U of Tth Plus DNA polymerase, 1.5 mM of MgCl2 (Biotech International Ltd, Bentley, Western Australia, Australia), 5 nmol of each deoxynucleoside triphosphate (Amersham Pharmacia Biotech AB, Uppsala, Sweden), and 12.5 pmol of each primer (Table 2). Three percent (vol/vol) dimethyl sulfoxide (Sigma-Aldrich Co., St. Louis, Mo.) was also added, and the reaction was separated into two phases by the addition of 20 μl of Chill-out 14 liquid wax (MJ Research Inc, Watertown, Mass.) phase separator to facilitate a hot start PCR, as previously described (2). The PCR products were subjected to electrophoresis in 1.5% (wt/vol) agarose gels in 1× Tris-acetate buffer, stained with ethidium bromide, and viewed over UV light.
The specificities of the PCRs were confirmed by testing each on DNA extracted from pure cell cultures of intestinal spirochetes obtained from the collection at the Reference Centre for Intestinal Spirochetes, Murdoch University. These included B. aalborgi 513AT; S. pilosicoli P43/6/78T, HRM-2B, “S. jonesii,” Karlos, Oman-26, and WesB; S. intermedia PWS/AT; Serpulina hyodysenteriae B78T; and Serpulina innocens B256T (25). Further confirmation of the specificities of the reactions when applied to biopsies was obtained by sequencing of PCR products. 16S rRNA and nox PCR products to be sequenced (Table 2) were purified with a commercially available kit (QIAquick PCR purification kit; QIAGEN GmbH, Hilden, Germany), according to the manufacturer’s specifications. One amplified product from biopsies taken from each of the three geographic regions (Australia, Norway, and the United States) was sequenced with the previously described primer pairs (Table 2) with a commercially available cycle sequencing kit (ABI PRISM dye terminator cycle sequencing ready reaction kit; Applied Biosystems), according to the manufacturer’s specifications. The sequence data obtained were aligned and compared with previously published sequences of the B. aalborgi and S. pilosicoli 16S rRNA and nox genes obtained from GenBank with SeqEd.
Both PCRs designed to amplify DNA from B. aalborgi generated a product, which was consistent with the predicted size (Table 2), from 11 of 17 PET DNA extracts (65%) from human biopsy material that had histologic evidence of IS. The S. pilosicoli-specific PCRs generated products only from the two positive control chick samples (Table 1), again of the predicted size (Table 2). No amplification was obtained from tissues from the chick infected with S. intermedia, nor from colonic tissues obtained from the 14 Australian patients who had no histologic evidence of IS. Of the 11 positive human samples, two belonged to the same patient, a 26-year-old HIV positive male from the United States. Therefore, 10 of 16 IS patients (62.5%) showed evidence of colonization by B. aalborgi. Seven of the 12 patients (58%) from South Norway, both patients (100%) from the United States, and 1 of 2 Australian patients from Melbourne (50%) were positive for B. aalborgi. The results for the four PCRs correlated completely, although the amount of PCR product generated varied between samples. Negative results from the 14 non-IS patients helped to demonstrate the specificity of the reactions and showed that B. aalborgi is not present at detectable levels in patients who lack histologic evidence of IS.
The PCR products sequenced with the B. aalborgi primers for both the 16S rRNA and nox genes were identical or closely related to the corresponding B. aalborgi sequences (Table 3). The 16S rRNA PCR products from the three PET samples that were sequenced were identical to the corresponding B. aalborgi (513T) sequence over the 449 bp of sequence that was compared. In contrast, they differed from the corresponding S. pilosicoli (P43/6/78T) sequence in 41 of 449 bp (90.9% similarity). An even greater divergence was found when the corresponding nox sequences were compared over 325 bp, with the three PET samples having 98.8 to 99.4% similarity to B. aalborgi and 80.0 to 80.3% similarity to S. pilosicoli (Table 3).
TABLE 3.
Similarities between PET PCR product sequences obtained from three samples and B. aalborgi 513T and S. pilosicoli P43/6/78T
| Sample (patient sex/age) or organism | % Similarity of 16S rRNA PCR products (449 bp)
|
% Similarity of nox PCR products (325 bp)
|
||
|---|---|---|---|---|
| B. aalborgi | S. pilosicoli | B. aalborgi | S. pilosicoli | |
| Australia (F/16) | 100 | 90.9 | 98.8 | 80.3 |
| Norway (M/60) | 100 | 90.9 | 98.8 | 80.0 |
| United States (M/26) | 100 | 90.9 | 99.4 | 80.0 |
| B. aalborgi | 90.9 | 80.0 | ||
The PCR techniques utilized in this study were designed to specifically detect the presence of either B. aalborgi or S. pilosicoli DNA in extracts from PET. Amplification of the nox gene was selected to confirm the results of 16S rRNA PCR, as the nox gene is less conserved than the 16S rRNA gene and is known to be present in strains throughout the genus Serpulina (33). Care was taken to ensure that the PCR products did not exceed 500 bp, due to the likelihood of the DNA in the PET being highly fragmented. A problem faced when developing primers for B. aalborgi is that only one strain of B. aalborgi is available, and so only the sequences from this strain could be used and only one cultured strain could be tested. The primers appeared to be specific, however, as they did not amplify DNA extracted directly from cultured S. pilosicoli strains. In comparison, the S. pilosicoli-specific primers were designed and tested with a relatively large number of positive control strains, as well as with positive control intestinal tissues from chicks.
From the results of the present study, it appears that B. aalborgi may be more commonly involved and more significant in IS than has been suggested. A recent study in Sydney based on rectal biopsies from male homosexual volunteers showing histologic evidence of IS pointed to S. pilosicoli as being the predominant causative agent of the condition (39). However, selective culturing was used to obtain the spirochetes, and as S. pilosicoli grows much more readily than the single available strain of B. aalborgi on the selective agar which is routinely used for isolation of intestinal spirochetes, failure to detect B. aalborgi may simply be the result of unsuitable diagnostic methods. For example, the medium originally used to obtain the B. aalborgi isolate in Denmark contained the antibiotics spectinomycin and polymyxin (18), while more recent culture studies have used a combination of spectinomycin, colistin, and vancomycin (40) or spectinomycin alone (39). Secondly, a PCR specific for B. aalborgi was not applied in the Sydney study; thirdly, it focused on rectal biopsies, and it may be that S. pilosicoli more commonly colonizes this site. Interestingly, the results from the study based in Sydney, where S. pilosicoli was isolated by culture from only 53.8% of biopsy specimens showing evidence of IS, suggested that the true prevalence of infection with intestinal spirochetes is underestimated by culture alone. In the present study, five of the Norwegian IS patients, as well as one of the Australian IS patients, failed to return a positive PCR result for any of the primers (Table 1) despite clear histologic evidence of IS in the sections (Fig. 1). This could have been because there was insufficient specific DNA in the piece of tissue that was processed for PCR, because the target DNA was too damaged and/or fragmented to be amplified, or because the patients were infected by other, as-yet-uncharacterized spirochetes.
The only other study that has used B. aalborgi- and S. pilosicoli-specific PCRs applied them to PET colonic samples from nonhuman primates (9). Both B. aalborgi and S. pilosicoli DNA was detected in some samples, and in some cases concurrently. The PCRs for S. pilosicoli used in the present study have been shown to work on animal tissues heavily colonized by the spirochete, but no amplification was obtained from the human material. Again, it may be that S. pilosicoli was present but in numbers too low to be detected by the PCR techniques used in this study. The negative PCR results for both S. pilosicoli and B. aalborgi in the 14 patients without histologic evidence of IS suggest that these organisms are uncommon in the general population.
Where colonization with B. aalborgi did occur, it generally failed to cause pathologic changes, although in the two U.S. HIV patients there was invasion of the colonic mucosa by the spirochetes, together with an inflammatory response (14). In this case, IS was considered to be at least a contributing factor to the diarrhea and bloody stools experienced by one of the patients (14). This is similar to the situation reported for S. pilosicoli in rectal biopsies (39), although under certain circumstances the organism seems to be invasive (42–44). It may be that predisposing factors such as immunosuppression are required before the spirochetes become invasive.
This study is the first since B. aalborgi was recognized in 1982 that has confirmed it as an etiologic agent of human IS. Furthermore, evidence of infection with this organism has been obtained from patients in Norway, Australia, and the United States, suggesting that B. aalborgi is widespread in human populations and that the original isolate was typical of the species. It will be important to extend these studies with further samples and in particular to determine whether, and at what prevalence, the organism colonizes individuals in developing regions of the world, where S. pilosicoli is ubiquitous. The present results also suggest that significant limitations are present in the selective culturing techniques currently employed in the diagnosis of IS and that these techniques require refinement.
Acknowledgments
We thank Gerard Spoelstra, Division of Veterinary and Biomedical Sciences, Murdoch University, for preparing PET for extraction and Frances Brigg, State Agricultural Biotechnology Centre, Murdoch University, for assistance with sequencing.
This study was funded by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Antonakopoulos G, Newman J, Wilkinson M. Intestinal spirochaetosis: an electron microscopic study of an unusual case. Histopathology. 1982;6:477–488. doi: 10.1111/j.1365-2559.1982.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 2.Atyeo, R. F., T. B. Stanton, N. S. Jensen, S. D. Suriyaarachichi, and D. J. Hampson. Differentiation of Serpulina species by NADH oxidase gene (nox) sequence comparisons and nox-based polymerase chain reaction tests. Vet. Microbiol., in press. [DOI] [PubMed]
- 3.Barrett S P. Intestinal spirochaetes in a Gulf Arab population. Epidemiol Infect. 1990;104:261–266. doi: 10.1017/s0950268800059434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett S P. Human intestinal spirochaetosis. In: Hampson D J, Stanton T B, editors. Intestinal spirochaetes in domestic animals and humans. Wallingford, England: CAB International; 1997. pp. 243–266. [Google Scholar]
- 5.Cotton D W K, Kirkham N, Hicks D A. Rectal spirochaetosis. Br J Vener Dis. 1984;60:106–109. doi: 10.1136/sti.60.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crucioli V, Busuttil A. Human intestinal spirochaetosis. Scand J Gastroenterol. 1981;16:177–179. [PubMed] [Google Scholar]
- 7.Douglas J G, Crucioli V. Spirochaetosis: a remediable cause of diarrhoea and rectal bleeding? Br Med J. 1981;283:1362. doi: 10.1136/bmj.283.6303.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhamel G E. Intestinal spirochaetes in non-production animals. In: Hampson D J, Stanton T B, editors. Intestinal spirochaetes in domestic animals and humans. Wallingford, England: CAB International; 1997. pp. 301–320. [Google Scholar]
- 9.Duhamel G E, Elder R O, Muniappa N, Mathiesen M R, Wong V J, Tarara R P. Colonic spirochetal infections in nonhuman primates that were associated with Brachyspira aalborgi, Serpulina pilosicoli, and unclassified flagellated bacteria. Clin Infect Dis. 1997;25:S186–S188. doi: 10.1086/516245. [DOI] [PubMed] [Google Scholar]
- 10.Fournié-Amazouz E, Baranton G, Carlier J P, Chambreuil G, Cohadon F, Collin P, Jolivet A G, Hermes I, Lemarie C, Saint Girons I. Isolations of intestinal spirochaetes from the blood of human patients. J Hosp Infect. 1995;30:160–162. doi: 10.1016/0195-6701(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 11.Frank T S, Svoboda-Newman S M, Hsi E D. Comparison of methods for extracting DNA from formalin-fixed paraffin sections for nonisotopic PCR. Diagn Mol Pathol. 1996;5:220–224. doi: 10.1097/00019606-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gad A, Willén R, Furugård K, Fors B, Hradsky M. Intestinal spirochaetosis as a cause of longstanding diarrhoea. Uppsala J Med Sci. 1977;82:49–54. doi: 10.3109/03009737709179059. [DOI] [PubMed] [Google Scholar]
- 13.Gebbers J-O, Ferguson D J P, Mason C, Kelly P, Jewell D P. Spirochaetosis of the human rectum associated with an intraepithelial mast cell and IgE plasma cell response. Gut. 1987;28:588–593. doi: 10.1136/gut.28.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guccion J G, Benator D A, Zeller J, Termanini B, Saini N. Intestinal spirochetosis and acquired immunodeficiency syndrome: ultrastructural studies of two cases. Ultrastruct Pathol. 1995;19:15–22. doi: 10.3109/01913129509014599. [DOI] [PubMed] [Google Scholar]
- 15.Harland W A, Lee F D. Intestinal spirochaetosis. Br Med J. 1967;3:718–719. doi: 10.1136/bmj.3.5567.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henrik-Nielsen R, Orholm M, Pedersen J O, Hovind-Hougen K, Teglbjærg P S, Thaysen E H. Colorectal spirochetosis: clinical significance of the infestation. Gastroenterology. 1983;85:62–67. [PubMed] [Google Scholar]
- 17.Hookey J V, Barrett S P, Reed C S, Barber T. Phylogeny of human intestinal spirochaetes inferred from 16S rDNA sequence comparisons. FEMS Microbiol Lett. 1994;117:345–350. doi: 10.1111/j.1574-6968.1994.tb06790.x. [DOI] [PubMed] [Google Scholar]
- 18.Hovind-Hougen K, Birch-Andersen A, Henrik-Nielsen R, Orholm M, Pedersen J O, Teglbjærg P S, Thaysen E H. Intestinal spirochetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi gen. nov., sp. nov. J Clin Microbiol. 1982;16:1127–1136. doi: 10.1128/jcm.16.6.1127-1136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson S R, Wingar C R. Selective medium for the isolation of Treponema hyodysenteriae. Vet Rec. 1981;109:384–385. doi: 10.1136/vr.109.17.384. [DOI] [PubMed] [Google Scholar]
- 20.Jones M J, Miller J N, George W L. Microbiological and biochemical characterization of spirochetes isolated from the feces of homosexual males. J Clin Microbiol. 1986;24:1071–1074. doi: 10.1128/jcm.24.6.1071-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan L R, Takeuchi A. Purulent rectal discharge associated with a nontreponemal spirochete. JAMA. 1979;241:52–53. [PubMed] [Google Scholar]
- 22.Käsbohrer A, Gelderblom H R, Arasteh K, Heise W, Grosse G, L’Age M, Schönberg A, Koch M A, Pauli G. Intestinale spirochätose bei HIV-Infektion. Dtsch Med Wochenschr. 1990;115:1499–1506. doi: 10.1055/s-2008-1065183. [DOI] [PubMed] [Google Scholar]
- 23.Kostman J R, Patel M, Catalano E, Camacho J, Hoffpauir J, DiNubile M J. Invasive colitis and hepatitis due to previously uncharacterized spirochetes in patients with advanced human immunodeficiency virus infection. Clin Infect Dis. 1995;21:1159–1165. doi: 10.1093/clinids/21.5.1159. [DOI] [PubMed] [Google Scholar]
- 24.Lee J I, Hampson D J. Intestinal spirochaetes colonizing Aborigines from communities in the remote north of Western Australia. Epidemiol Infect. 1992;109:133–141. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J I, Hampson D J. Genetic characterisation of intestinal spirochaetes and their association with disease. J Med Microbiol. 1994;40:365–371. doi: 10.1099/00222615-40-5-365. [DOI] [PubMed] [Google Scholar]
- 26.McLaren A J, Trott D J, Swayne D E, Oxberry S L, Hampson D J. Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens and allocation of known pathogenic isolates to three distinct genetic groups. J Clin Microbiol. 1997;35:412–417. doi: 10.1128/jcm.35.2.412-417.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muniappa N, Duhamel G E, Mathiesen M R, Bargar T W. Light microscopic and ultrastructural changes in the ceca of chicks inoculated with human and canine Serpulina pilosicoli. Vet Pathol. 1996;35:542–550. doi: 10.1177/030098589603300509. [DOI] [PubMed] [Google Scholar]
- 28.Ochiai S, Mori K, Adachi Y. Unification of the genera Serpulina and Brachyspira, and proposals of Brachyspira hyodysenteriae comb nov, Brachyspira innocens comb nov and Brachyspira pilosicoli comb nov. Microbiol Immunol. 1997;41:445–452. doi: 10.1111/j.1348-0421.1997.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 29.Padmanabhan V, Dahlstrom J, Maxwell L, Kaye G, Clarke A, Barrett P J. Invasive intestinal spirochaetosis: a report of three cases. Pathology. 1996;28:283–296. doi: 10.1080/00313029600169174. [DOI] [PubMed] [Google Scholar]
- 30.Park N Y, Chung C Y, McLaren A J, Atyeo R F, Hampson D J. Polymerase chain reaction for identification of human and porcine spirochaetes recovered from cases of intestinal spirochaetosis. FEMS Microbiol Lett. 1995;125:225–230. doi: 10.1111/j.1574-6968.1995.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 31.Sanna A, Dettori G, Aglianò A M, Branca G, Grillo R, Leone F, Rossi A, Parisi G. Studies of treponemes isolated from human gastrointestinal tract. Ig Mod. 1984;81:959–973. [Google Scholar]
- 32.Stanton T B, Fournié-Amazouz E, Postic D, Trott D J, Grimont P A D, Baranton G, Hampson D J, Saint Girons I. Recognition of two new species of intestinal spirochetes: Serpulina intermedia sp. nov. and Serpulina murdochii sp. nov. Int J Syst Bacteriol. 1997;47:1007–1012. doi: 10.1099/00207713-47-4-1007. [DOI] [PubMed] [Google Scholar]
- 33.Stanton T B, Hanzelka B L, Jensen N S. Survey of intestinal spirochaetes for NADH oxidase by gene probe and by enzyme assay. Microb Ecol Health Dis. 1995;8:93–100. [Google Scholar]
- 34.Stanton T B, Trott D J, Lee J I, McLaren A J, Hampson D J, Paster B J, Jensen N S. Differentiation of intestinal spirochetes by multilocus enzyme electrophoresis analysis and 16S rRNA sequence comparisons. FEMS Microbiol Lett. 1996;136:181–186. doi: 10.1111/j.1574-6968.1996.tb08046.x. [DOI] [PubMed] [Google Scholar]
- 35.Swayne D E, McLaren A J. Avian intestinal spirochaetes and avian intestinal spirochaetosis. In: Hampson D J, Stanton T B, editors. Intestinal spirochaetes in domestic animals and humans. Wallingford, England: CAB International; 1997. pp. 267–300. [Google Scholar]
- 36.Taylor D J, Simmons J R, Laird H M. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec. 1980;106:324–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- 37.Taylor D J, Trott D J. Porcine intestinal spirochaetosis and spirochaetal colitis. In: Hampson D J, Stanton T B, editors. Intestinal spirochaetes in domestic animals and humans. Wallingford, England: CAB International; 1997. pp. 211–242. [Google Scholar]
- 38.Tompkins D S, Foulkes S J, Godwin P G R, West A P. Isolation and characterization of intestinal spirochaetes. J Clin Pathol. 1986;39:535–541. doi: 10.1136/jcp.39.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trivett-Moore N L, Gilbert G L, Law C L H, Trott D J, Hampson D J. Isolation of Serpulina pilosicoli from rectal biopsy specimens showing evidence of intestinal spirochetosis. J Clin Microbiol. 1998;36:261–265. doi: 10.1128/jcm.36.1.261-265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trott D J, Combs B G, Mikosza A S J, Oxberry S L, Robertson I D, Passey M, Taime J, Sehuko R, Alpers M P, Hampson D J. The prevalence of Serpulina pilosicoli in humans and domestic animals living in the Eastern Highlands of Papua New Guinea. Epidemiol Infect. 1997;119:369–379. doi: 10.1017/s0950268897008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trott D J, Hampson D J. Evaluation of day-old specific pathogen-free chicks as an experimental model for pathogenicity testing of intestinal spirochaete species. J Comp Pathol. 1998;118:365–381. doi: 10.1016/s0021-9975(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 42.Trott D J, Huxtable C R, Hampson D J. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect Immun. 1996;64:4648–4654. doi: 10.1128/iai.64.11.4648-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trott D J, Jensen N S, Saint Girons I, Oxberry S L, Stanton T B, Hampson D J. Identification and characterization of Serpulina pilosicoli isolates from the blood of critically ill patients. J Clin Microbiol. 1997;35:482–485. doi: 10.1128/jcm.35.2.482-485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trott D J, McLaren A J, Hampson D J. Pathogenicity of human and porcine intestinal spirochetes in 1-day-old specific-pathogen-free chicks: an animal model of intestinal spirochetosis. Infect Immun. 1995;63:3705–3710. doi: 10.1128/iai.63.9.3705-3710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trott D J, Stanton T B, Jensen N S, Duhamel G E, Johnson J L, Hampson D J. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int J Syst Bacteriol. 1996;46:206–215. doi: 10.1099/00207713-46-1-206. [DOI] [PubMed] [Google Scholar]
- 46.Trott D J, Stanton T B, Jensen N S, Hampson D J. Phenotypic characteristics of Serpulina pilosicoli, the agent of intestinal spirochaetosis. FEMS Microbiol Lett. 1996;142:209–214. doi: 10.1111/j.1574-6968.1996.tb08432.x. [DOI] [PubMed] [Google Scholar]
- 47.Willen R, Carlen B, Cronstedt J, Willen H. Intestinal spirochaetosis of the colon diagnosed with colono-ileoscopy and multiple biopsies. Endoscopy. 1985;17:86–88. doi: 10.1055/s-2007-1018467. [DOI] [PubMed] [Google Scholar]