Abstract
Summary
Single-cell DNA template strand sequencing (Strand-seq) enables chromosome length haplotype phasing, construction of phased assemblies, mapping sister-chromatid exchange events and structural variant discovery. The initial quality control of potentially thousands of single-cell libraries is still done manually by domain experts. ASHLEYS automates this tedious task, delivers near-expert performance and labels even large datasets in seconds.
Availability and implementation
github.com/friendsofstrandseq/ashleys-qc, MIT license.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Strand-seq is a single-cell short-read sequencing technique that assays sister chromatid inheritance patterns at the level of individual chromosomes (Falconer et al., 2012; Sanders et al., 2017). The Strand-seq protocol generates strand-specific sequencing libraries by labeling and later removing the non-template strand during DNA replication. The strand-of-origin information is recovered in silico as the read alignment either in Crick (C, forward) or in Watson (W, reverse) direction. The Strand-seq protocol affords unique insights in diverse applications, e.g. locating sister chromatid exchange events (Claussin et al., 2017; Falconer and Lansdorp, 2013), characterizing complex genomic variation (Sanders et al., 2016, 2020) or providing long-range phase information to assist genome assembly (Ghareghani et al., 2018; Porubský et al., 2017, 2020). Decreasing costs allow for studying larger cohorts, but the initial quality control (QC) step to discard low-quality libraries or control probes still requires human intervention. Domain experts manually evaluate and label each library in datasets comprising up to thousands of single cells. Replacing this laborious process with an automated tool for Strand-seq QC would be a prerequisite for further scaling of Strand-seq in the future. We developed a software for the Automatic Selection of High-quality Libraries for the Extensive analYsis of Strand-seq data (ASHLEYS). ASHLEYS is based on established machine learning technology and ships with ready-to-use classification models trained on a large cohort of Strand-seq libraries. ASHLEYS pretrained classifiers have been vetted on independent test data to ensure stable generalization performance on new Strand-seq data with similar feature characteristics. Next, we describe ASHLEYS’ feature model and summarize the performance of the default classifier recommended for QC of new Strand-seq libraries.
2 Materials and methods
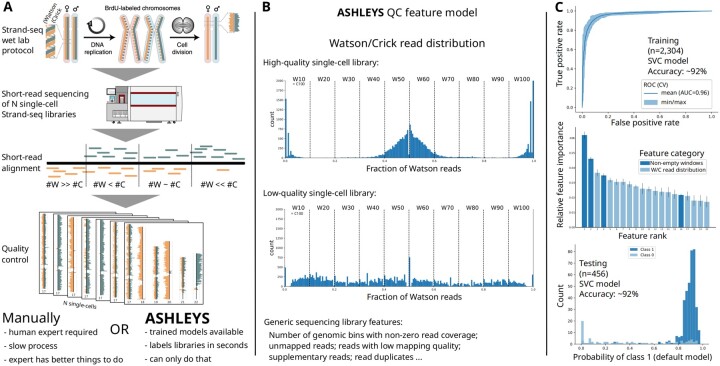
ASHLEYS’ main input is a set of BAM (Li et al., 2009) files, one per single-cell paired-end Strand-seq library aligned to a reference genome. We provide a supporting pipeline (Supplementary Information) for data preprocessing following established examples (Fig. 1A) (Sanders et al., 2017, 2020). ASHLEYS feature modeling uses statistics that describe either generic library QC characteristics, e.g. the number of unmapped reads or the number of low-quality alignments [default: , Sanders et al. (2016, 2020)], or the W/C read distribution, which is a feature unique to Strand-seq data. Generic count features are normalized by the total library size to account for varying sequencing depth. The W/C feature computation is implemented as a sliding window approach covering a range of window sizes in a single run to capture technical artifacts at various size ranges (Supplementary Information). The window is shifted by half of its size in each step and ASHLEYS counts W and C reads per window. Due to the complementarity, only the W fraction of reads is stored. The feature is then modeled by binning the W fractions in steps of 0.1 and counting the number of windows per bin. The expectation for a high-quality library is to observe a W fraction of for of the windows, and closer to zero or one for of the windows each, due to the random strand segregation during (diploid) cell division (Fig. 1B). Other common library issues lead to W/C signal ‘dropouts’ (Sanders et al., 2017), which are modeled as the number of windows with non-zero W/C read coverage. The aggregated feature table for all libraries can then be used to train a new classifier, provided that expert labeling is available, or to predict quality labels using one of ASHLEYS pretrained models.
Fig. 1.
Summary of Strand-seq QC with ASHLEYS: (A) the Strand-seq protocol generates a large number of single-cell libraries that need to pass QC before downstream analysis. Strand-seq panels reused from Sanders et al. (2020; Fig. 1a). (B) Example for the Strand-seq specific feature of the Watson/Crick read distribution for high- (top) and low-quality (middle) libraries. ASHLEYS also evaluates library quality based on generic sequencing library features (bottom text). (C) Performance summary for the SVC model shipped with ASHLEYS for training and testing stages of model building. Feature names omitted in feature importance plot for improved readability (cf. Supplementary Fig. S1)
ASHLEYS is implemented as a Python3 tool using standard libraries for BAM processing (Pysam, github.com/pysam-developers/pysam) and machine learning (Pedregosa et al., 2011), and includes a test dataset.
3 Models
ASHLEYS pretrained classifiers were tuned on a large dataset (n = 2304) generated as part of the Human Genome Structural Variation Consortium (HGSVC) (Chaisson et al., 2019 Ebert, 2021 ). Model training including hyperparamter tuning and training error estimation was performed with 50 iterations of nested class-balanced 5-fold cross-validation (Fig. 1C, Supplementary Information). Model generalization performance was assessed on an independent test dataset (n = 456) labeled by the same domain expert (Sanders et al., 2020). We recommend a linear support vector classifier (SVC) as default model for labeling new Strand-seq data. The SVC model shows consistently high performance on training (F1 score 93.9%, accuracy 91.6%) and on independent test data (F1 score 95.6%, accuracy 92.5%) (Fig. 1C), suggesting that the SVC is not overfitted to the training dataset.
In conclusion, ASHLEYS’ high performance and the resulting gains in efficiency facilitate scaling Strand-seq to even larger cohorts without burdening domain experts with an overwhelming amount of repetitive QC. This raises promising expectations for addressing further challenges such as extensive aneuploidy in cancer.
Supplementary Material
Acknowledgements
The authors thank the HGSVC for making the Strand-seq data publicly available. C.E. acknowledges the support by the Saarbrücken Graduate School of Computer Science.
Funding
Funding for this research project by the Human Genome Structural Variation Consortium (HGSVC) came from the following grants: National Institutes of Health (NIH) U24HG007497 (to J.O.K. and T.M.), the German Research Foundation [391137747 and 395192176 to T.M.] and the German Federal Ministry for Research and Education [BMBF 031L0184 to J.O.K. and T.M.].
Conflict of Interest: none declared.
Data availability statement
The Strand-seq data produced by the HGSVC are available under the accession PRJEB39750.
Contributor Information
Christina Gros, Center for Bioinformatics Saar, Saarland University, 66123 Saarbrücken, Germany.
Ashley D Sanders, European Molecular Biology Laboratory (EMBL), Genome Biology Unit, 69117 Heidelberg, Germany.
Jan O Korbel, European Molecular Biology Laboratory (EMBL), Genome Biology Unit, 69117 Heidelberg, Germany.
Tobias Marschall, Institute for Medical Biometry and Bioinformatics, Heinrich Heine University, 40225 Düsseldorf, Germany.
Peter Ebert, Institute for Medical Biometry and Bioinformatics, Heinrich Heine University, 40225 Düsseldorf, Germany.
References
- Chaisson M.J.P. et al. (2019) Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun., 10, 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussin C. et al. (2017) Genome-wide mapping of sister chromatid exchange events in single yeast cells using strand-seq. eLife, 6, e30560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert,P. et al. (2021) Haplotype-resolved diverse human genomes and integrated analysis of structural variation. Science, 372, eabf7117 10.1126/science.abf7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E., Lansdorp P.M. (2013) Strand-seq: a unifying tool for studies of chromosome segregation. Semin. Cell Dev. Biol., 24, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E. et al. (2012) DNA template strand sequencing of single-cells maps genomic rearrangements at high resolution. Nat. Methods, 9, 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareghani M. et al. (2018) Strand-seq enables reliable separation of long reads by chromosome via expectation maximization. Bioinformatics, 34, i115–i123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. ; 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics, 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F. et al. (2011) Scikit-learn: machine learning in python. J. Mach. Learn. Res., 12, 2825–2830. [Google Scholar]
- Porubský D. et al. (2017) Dense and accurate whole-chromosome haplotyping of individual genomes. Nat. Commun., 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubský D. et al. (2020) Fully phased human genome assembly without parental data using single-cell strand sequencing and long reads. Nat. Biotechnol., 39, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A.D. et al. (2016) Characterizing polymorphic inversions in human genomes by single-cell sequencing. Genome Res., 26, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A.D. et al. (2017) Single-cell template strand sequencing by Strand-seq enables the characterization of individual homologs. Nat. Protoc., 12, 1151–1176. [DOI] [PubMed] [Google Scholar]
- Sanders A.D. et al. (2020) Single-cell analysis of structural variations and complex rearrangements with tri-channel processing. Nat. Biotechnol., 38, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Strand-seq data produced by the HGSVC are available under the accession PRJEB39750.