Abstract
Background:
The addition of PD-L1 inhibitors to platinum-based chemotherapy (CT) has newly received United States Food and Drug Administration (FDA) approval in extensive stage-small cell lung cancer (ES-SCLC). PD-1 agents similarly improved survival rates, even if not yet supported by international regulatory agencies. The current work aims to assess different efficacy and safety profiles among chemoimmunotherapy plus immuno-oncology (CT+IO) approaches according to different immune checkpoint inhibitor (ICI) subtypes.
Material & Methods:
We included in our meta-analysis six first-line randomised controlled trials (RCTs) comparing the association of single-agent ICI with CT versus CT alone in ES-SCLC. Pooled hazard ratios (HRs) and risk ratios (RRs) for progression-free survival (PFS), overall survival (OS), objective response rates (ORR), 12-month duration of response rate (DORR), disease control rate (DCR), treatment-related adverse events (TRAEs) and discontinuation rates (DRs) were obtained. Moreover, we performed indirect comparisons according to ICI subtypes, also among subgroups and landmark survival analyses.
Results:
Although no ORR benefit was observed, our results showed how CT+IO significantly improved DORR, resulting in improved PFS and OS with no differences in TRAEs; however, CT+IO led to a significant increase in DR. Interestingly, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1, the use of cisplatin, and the absence of brain metastases seem to be associated with a survival gain using CT+IO in ES-SCLC. Indirect comparisons suggested a slight advantage in favour of programmed cell death-1 (PD-1) and programmed death ligand 1 (PD-L1) over anti-CTLA-4 agents in terms of efficacy with no additional safety concerns. No further differences were observed between PD-1 and PD-L1 inhibitors among subgroups and landmark survival analyses with benefit trends towards anti-PD-1 in terms of DORR and DR.
Conclusion:
While confirming a survival advantage of CT+IO in selected patients, these results suggested the association of PD-1 inhibitors with CT as a viable option for novel therapeutic approaches in the frontline management of ES-SCLC. Further trials evaluating anti-CTLA-4 agents should be carefully studied in biomarker-selected patients.
Keywords: chemo-immunotherapy, ES-SCLC, indirect comparison, meta-analysis, PD-L1/PD-1 inhibitors
Introduction
Small cell lung cancer (SCLC) accounts for approximately 10–15% of new lung cancer diagnoses with most patients being diagnosed at an advanced stage and harboring an exceptionally lethal behavior.1,2 According to the latest International Association for the Study of Lung Cancer (IASLC) staging system, Extensive-Stage SCLC (ES-SCLC) traditionally refers to the disease extending beyond one hemithorax at the initial diagnosis. 3 For decades, platinum-doublet chemotherapy (CT) has represented the standard of care for ES-SCLC patients, resulting in only transient radiographic response and clinical improvement together with limited survival rates.4,5 Unfortunately, after poor outcomes and decades of failed clinical research, the overall survival (OS) rate has not increased significantly. 6 Despite rapid United States (US) Food and Drug Administration (FDA) approval of immuno-oncology (IO) agents as monotherapy in later settings, 7 the standard first-line treatment approach has been relatively unchanged for three decades.8–10 More recently, the addition to CT of a single-agent immune checkpoint inhibitor (ICI) targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) receptors has been revealed to be safer and more effective than CT alone, whereas, on the other hand, the association of a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitor did not show any impact on efficacy in patients with treatment-naïve ES-SCLC.11,12 Adding both the PD-L1 agents atezolizumab and durvalumab to CT led to the first OS improvement in the first-line setting of ES-SCLC. 13 Conversely, although being associated with improved landmark survival rates at 12 and 24 months that consistently mirrored the favorable trends of PD-L1 agents, the median OS of the PD-1 inhibitor pembrolizumab in association with CT did not cross the pre-specified threshold for a survival benefit. 13 Furthermore, nivolumab in combination with CT significantly improved survival rates; however, only immature data was presented in the EA5161 phase II study. Moreover, unfortunately, no accurate predictive biomarkers that can precisely guide the use of ICIs in such patients have been identified. 14 Thus, no wide consensus on the role of chemo-immunotherapy (CT+IO) in the first-line treatment of ES-SCLC has been established, 15 and, in the absence of direct comparisons among these ICIs, it remains crucial to identify any differences in both efficacy and toxicity profiles that may help clinicians select the best drug for each patient. Therefore, we performed a systematic review and meta-analysis of all phase II/III randomised clinical trials comparing the association of single-agent CTLA-4/PD-1/PD-L1 inhibitors with CT versus CT alone in untreated ES-SCLC patients. Finally, the current work aimed to assess indirect comparisons among different ICIs in combination with platinum-based CT in ES-SCLC patients, focusing on differences among subgroups and landmark survival analyses according to different ICIs subtypes.
Methods
Search strategy and study selection
We searched for results of phase II and III randomised controlled trials (RCTs) comparing first-line standard CT+IO versus standard CT alone in patients with histological diagnosis of unresectable or advanced ES-SCLC (stage IVA/IVB according to the 8th TNM classification and clinical staging system).16,17 We excluded non-randomised, cohort, cross-sectional, retrospective and case-control studies. Furthermore, we also excluded other reviews (systematic or not) and meta-analyses. Moreover, we excluded duplicates and trials whose results for relevant outcomes were not available or ongoing trials or trials with fewer than 10 patients. Studies were included if they compared standard platinum-based CT plus single-agent IO regimens [containing anti-PD-1 (nivolumab or pembrolizumab) or anti-PD-L1 (atezolizumab or durvalumab) or anti-CTLA-4 (ipilimumab)] to CT alone (including cisplatin or carboplatin in association with etoposide or paclitaxel). The research was performed using specific Mesh terms such as ‘Small Cell Lung Carcinoma’ and free text terms such as ‘immunotherapy’ or ‘IO’ or ‘immune-checkpoint’ and ‘survival’ using Boolean operators (Supplemental Figure S1). Data collected on Medline (PubMed), Scopus, and Cochrane-Library databases were collected until 20 March 2021, limiting the search to English-only articles; for potential abstracts, we also explored the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) abstracts repositories, as well as the National Institute of Health (NIH) website (www.clinicaltrials.gov) for as yet unpublished ongoing studies, considering these as a source of grey literature.
We registered our systematic strategy on the PROSPERO database (code: CRD42020204916). The selected outcomes were: objective response rate (ORR), defined as the proportion of patients with reduced disease burden; duration of response rate (DORR), defined as the time from the first evidence of response to disease progression or death, whichever occurred first; disease control rate (DCR), defined as the proportion of patients in whom the best overall response is determined as complete response, partial response or stable disease; progression-free survival (PFS), defined as the time interval from randomisation to disease progression or death; OS, defined as the time interval between randomisation and death from any cause; treatment-related adverse events (TRAEs), defined as the proportion of patients experiencing treatment-related toxicity; and discontinuation rate (DR), defined as the proportion of patients that discontinued treatment due to toxicity.
The data collected for these outcomes were stratified according to a pre-specified analysis based on the indirect comparison of different IO strategies [anti-PD-1 versus anti-CTLA4 versus anti-PD-L1 monoclonal antibodies (moAbs)]. Only data from studies that investigated patients aged ⩾18 years, with no sex restrictions were collected. Two authors (AG and VG) independently selected trials according to the previously established inclusion and exclusion criteria. Subsequently, articles considering the pre-specified relevant outcomes were included in the final analysis. Disagreements were debated and solved by consulting a senior author (AR).
Data extraction and assessment of quality of included studies
Data were gathered in a predefined file in which we reported trial name, drug protocol, sample size and the results of the selected outcomes (ORR, DORR, DCR, PFS, OS, TRAEs and DR). Moreover, among subgroup analyses in each eligible trial, the following data were collected, if available: sex, age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), use of platinum salt, presence of brain and liver metastases and smoking status. For the calculation of the pooled landmark survival analyses at pre-specified timepoints (6, 12 and 18 months for PFS; 12, 18 and 24 months for OS), the number of patients at risk was extracted from Kaplan–Meier survival curves. Articles with different follow up were identified, while the more updated and methodologically robust was included in our final analysis. Six RCTs (CA184-041, 18 CA184-056, 19 IMpower133,20,21 EA5161, 22 KEYNOTE-604, 23 CASPIAN24,25) were included in the final analysis. For the IMpower133 and CASPIAN trials, two full texts were selected because they contain outcomes of interest, providing a total of seven full texts and one abstract in the final analysis.
Statistical analysis
Statistical analysis was performed using RevManver 5.3, 26 and Comprehensive Meta-analysis version 3.0. 27 As already described, the outcomes selected to perform a standard meta-analysis and indirect comparisons were ORR, DORR, DCR, PFS, OS, TRAEs and DR. We considered hazard ratios (HRs) to evaluate the association for PFS and OS, with the relative 95% confidence intervals (CI). Furthermore, we considered risk ratios (RRs) as an association measure for ORR (computed as the ratio of the total number of events to the total of patients randomised in experimental and control groups), DORR (computed as the ratio of the number of patients experiencing a 12-month response to the total number of patients), DCR (computed as the ratio of the number of patients achieving a complete response, partial response or stable disease to the total number of patients), TRAEs (computed as the ratio of the number of grade 3–5 treatment-related toxicities to the total number of toxicities), DR (computed as the ratio of the number of treatments discontinued due to toxicities to the total number of treated patients, according to intention-to-treat analysis). This meta-analysis was performed in two different stages. In the first phase, we used the standard meta-analytical technique to compare IO performance in addition to platinum-based CT versus platinum-based CT alone in first-line ES-SCLC according to each pre-specified outcome (ORR, DORR, DCR, PFS, OS, TRAEs and DR), computing the logarithm of the HR (logHRs) or the RR (logRR) and their standard error (logSE) for all the studies included in the analysis. Thus, we obtained pooled data for each comparison. In the second stage, we used the methodology described by Bucher and Glenny to perform indirect comparisons to maintain the trial randomisation advantage producing a robust pooled estimate for treatment outcomes.28–30 As an example, suppose that anti-CTLA-4st is the estimate of the pooled comparison between IO+CT versus standard CT for the anti-CTLA-4 trials and anti-PD-1st is the estimate of the pooled comparison IO+CT versus standard CT for the anti-PD-1 trials, then the estimate of the indirect comparison between anti-CTLA-4/anti-PD-1 low can be calculated as follows: anti-CTLA-4/anti-PD-1 low_indirect: (logHR or logRR) = anti-CTLA-4st (logHR or logRR) – anti-PD-1 lowst (logHR or logRR). The variance (standard error; SE) can be obtained with the following computation: Var (log anti-CTLA-4/anti-PD-1_indirect) = Var (log anti-CTLA-4st) + Var (anti-PD-1st). The same strategy was used to obtain indirect comparisons for anti-CTLA-4 versus anti-PD-L1 moAbs and for anti-PD-1 versus anti-PD-L1 comparisons. Heterogeneity between studies was explored through the Cochrane Q test and the inconsistency test (I 2 ). In particular, a high degree of heterogeneity was diagnosed if the I 2 test was greater than 50% or the p value was statistically significant. 31 Then, the meta-analysis was computed using the random effect-based model by Der Simonian and Laird; otherwise, the fixed effect-based model by Mantel–Haenszel was performed. Moreover, we explored publication bias risk using Egger’s test and produced the relative funnel plot for asymmetry. The manuscript was realised and drafted according to the preferred reporting items for systematic reviews and meta-analyzes (PRISMA) guidelines (Supplemental Figure S2). 32 The p values were considered significant if p ⩽ 0.05.
Results
Selected studies
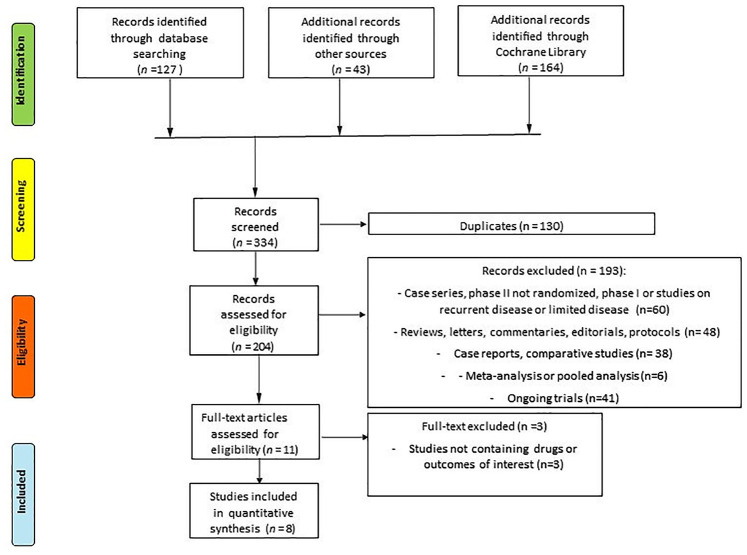
The search for relevant articles identified a total of 334 records; 130 duplicated records were excluded. A total of 204 trials were assessed for eligibility and eventually 3 trials were excluded because no drugs of interest or no data about the principal outcomes of our indirect comparison (ORR, DORR, DCR, PFS, OS, TRAEs and DR) were reported. Finally, six RCTs for a total of seven full-text studies and one abstract met our inclusion/exclusion criteria and were included in the standard meta-analysis and indirect comparisons (Figure 1).
Figure 1.
PRISMA flow diagram showing the selection algorithm of retrieved papers according to the inclusion/exclusion criteria.
PRISMA, preferred reporting items for systematic reviews and meta-analyzes.
Study characteristics
The baseline characteristics and the outcomes measures of each included trial are reported in Tables 1 and 2, respectively. The main patient characteristics and the available subgroup analyses of OS are described in Tables 3 and 4, respectively. As suggested by the Cochrane Handbook for Systematic Reviews of Interventions, we used the modified Jadad’s score to investigate the potential risk of bias of selected trials. 33 Briefly, we declared as ‘Yes’ or ‘No’ the potential presence or absence of bias respectively, considering a total of six domains: allocation concealment, sequence generation, personnel and outcome assessors, blinding of participants, incomplete outcome data and selective outcome reporting. We defined ‘Unclear’ studies with high difficulties in risk of bias definition. Accordingly, two different authors (AG and VG) assessed the risk of bias, and disagreements were debated and solved consulting a senior author (AR).
Table 1.
Main baseline characteristics of each included trial considered in this meta-analysis.
| Study | Treatment arm | Number of patients | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|
| CA184-041 | ipilimumab + CP versus CP | 43 versus 45 | 3.9 versus 5.2 | 9.1 versus 9.9 |
| CA184-156 | ipilimumab + EP versus EP | 478 versus 476 | 4.6 versus 4.4 | 11.0 versus 10.9 |
| IMpower133 | atezolizumab + EP versus EP | 201 versus 202 | 5.2 versus 4.3 | 12.3 versus 10.3 |
| EA5161 | nivolumab + EP versus EP | 80 versus 80 | 5.5 versus 4.6 | 11.3 versus 8.5 |
| KEYNOTE-604 | pembrolizumab + EP versus EP | 228 versus 225 | 4.5 versus 4.3 | 10.8 versus 9.7 |
| CASPIAN | durvalumab + EP versus EP | 268 versus 269 | 5.1 versus 5.4 | 12.9 versus 10.5 |
CP, carboplatin plus paclitaxel; EP, etoposide plus platinum compound; OS, overall survival; PFS, progression-free survival.
Table 2.
Stratification of clinical outcomes measures considered in this pooled analysis.
| Study | ORR, (n) | DORR, (n) | DCR, (n) | PFS, HR (95%CI) | OS, HR (95% CI) | TRAEs G3–5 (n) | DR, (n) |
|---|---|---|---|---|---|---|---|
| CA184-041 | 14/43 versus 22/45 | 2/43 versus 1/45 | 30/43 versus 42/45 | 0.93 (0.59–1.48) | 0.95 (0.59–1.54) | 19/42 versus 19/44 | 3/43 versus 4/45 |
| CA184-156 | 297/478 versus 296/476 | 14/478 versus 10/476 | 422/478 versus 422/476 | 0.85 (0.75–0.97) | 0.94 (0.81–1.09) | 231/478 versus 214/476 | 86/478 versus 9/450 |
| IMpower133 | 121/201 versus 130/202 | 18/121 versus 7/130 | 163/201 versus 173/202 | 0.77 (0.63–0.95) | 0.76 (0.60–0.95) | 116/198 versus 113/196 | 32/198 versus 13/196 |
| EA5161 | 39/75 versus 33/70 | NA | NA | 0.65 (0.46–0.91) | 0.67 (0.46–0.98) | 59/75 versus 44/70 | NA |
| KEYNOTE-604 | 161/228 versus 139/225 | 20/224 versus 3/222 | 201/228 versus 195/225 | 0.73 (0.60–0.88) | 0.80 (0.64–0.98) | 185/223 versus 179/223 | 33/223 versus 14/223 |
| CASPIAN 2020 | 182/268 versus 155/269 | 62/268 versus 16/269 | 202/268 versus 197/269 | 0.80 (0.66–0.96) | 0.75 (0.62–0.91) | 163/265 versus 166/266 | 27/265 versus 25/266 |
CI, confidence interval; DCR, disease control rate; DORR, duration of response rate; DR, discontinuation rate; g., grade; HR, hazard ratio; NA, not available; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TRAEs, treatment-related adverse events.
Table 3.
Patient characteristics across subgroups of the trials included in this meta-analysis.
| Patient characteristics | CA184-041 | CA184-156 | IMpower133 | EA5161 | KEYNOTE-604 | CASPIAN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT+IO n = 43 (%) | CT n = 45 (%) | CT+IO n = 478 (%) | CT n = 476 (%) | CT+IO n = 201 (%) | CT n = 202 (%) | CT+IO n = 80 (%) | CT n = 80 (%) | CT+IO n = 228 (%) | CT n = 225 (%) | CT+IO n = 268 (%) | CT n = 269 (%) | |
| Sex | ||||||||||||
| Male | 33 (7) | 33 (73) | 317 (66) | 326 (68) | 129 (64.2) | 132 (65.3) | 35 (43.7) | 36 (45) | 152 (66.7) | 142 (63.1) | 190 (70.9) | 184 (68.4) |
| Female | 10 (23) | 12 (27) | 161 (34) | 150 (32) | 72 (35.8) | 70 (34.7) | 45 (56.3) | 44 (55) | 76 (33.3) | 83 (36.9) | 78 (29.1) | 85 (31.6) |
| Age | ||||||||||||
| <65 years | 35 (81) | 36 (80) | 299 (63) | 277 (58) | 111 (55.2) | 106 (52.5) | NA | NA | 115 (50.4) | 101 (44.9) | 167 (62.3) | 157 (58.4) |
| ⩾65 years | 8 (19) | 9 (20) | 179 (37) | 199 (42) | 90 (44.8) | 96 (47.5) | NA | NA | 113 (50.6) | 124 (55.1) | 101 (37.7) | 112 (41.6) |
| ECOG PS | ||||||||||||
| 0 | 8 (19) | 12 (27) | 137 (29) | 147 (31) | 73 (36.3) | 67 (33.2) | 23 (28.7) | 24 (30) | 60 (26.3) | 56 (24.9) | 99 (36.9) | 90 (33.5) |
| 1 | 34 (79) | 33 (73) | 340 (71) | 328 (69) | 128 (63.7) | 135 (66.8) | 57 (71.3) | 56 (70) | 168 (73.7) | 169 (75.1) | 169 (63.1) | 179 (66.5) |
| Platinum salt | ||||||||||||
| Carboplatin | 43 (100) | 45 (100) | 314 (66) | 317 (67) | 201 (100) | 202 (100) | NA | NA | 161 (70.6) | 156 (69.3) | 201 (75) | 201 (74.7) |
| Cisplatin | 0 (0) | 0 (0) | 164 (34) | 159 (33) | 0 (0) | 0 (0) | NA | NA | 67 (29.4) | 69 (30.7) | 67 (25) | 68 (25.3) |
| Brain mts | ||||||||||||
| Yes | 0 (0) | 0 (0) | 55 (12) | 45 (10) | 17 (8.5) | 18 (8.9) | NA | NA | 33 (14.5) | 22 (9.8) | 28 (10.4) | 27 (10) |
| No | 43 (100) | 45 (100) | 423 (88) | 431 (90) | 184 (91.5) | 184 (91.1) | NA | NA | 195 (85.5) | 203 (90.2) | 240 (89.6) | 242 (90) |
| Liver mts | ||||||||||||
| Yes | NA | NA | NA | NA | 77 (38.3) | 72 (35.6) | NA | NA | 95 (41.7) | 92 (40.9) | 108 (40.3) | 104 (38.7) |
| No | NA | NA | NA | NA | 124 (61.7) | 130 (63.4) | NA | NA | 133 (58.3) | 133 (59.1) | 160 (59.7) | 165 (61.3) |
| Smoking status | ||||||||||||
| Smoker | 38 (88) | 41 (91) | 268 (56) | 271 (57) | 192 (95.5) | 199 (98.5) | NA | NA | 220 (96.5) | 217 (96.4) | 246 (91.8) | 254 (94.4) |
| Non-smoker | 5 (12) | 4 (10) | 172 (36) | 167 (35) | 9 (4.5) | 3 (1.5) | NA | NA | 8 (3.5) | 8 (3.6) | 22 (8.2) | 15 (5.6) |
CT, platinum-based chemotherapy; ECOG, Eastern Cooperative Oncology Group; IO, immune-oncology; mts, metastases; NA, not available; PS, performance status.
Table 4.
Subgroup analyses of overall survival across the trials included in this meta-analysis.
| Patients’ characteristics | CA184-041 HR (95% CI) | CA184-156 HR (95% CI) | IMpower133 HR (95% CI) | EA5161 | KEYNOTE-604 HR (95% CI) | CASPIAN HR (95%CI) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | NA | 1.07 (0.89–1.28) | 0.83 (0.63–1.10) | NA | 0.76 (0.59–0.98) | 0.79 (0.63–0.99) |
| Female | NA | 1.06 (0.81–1.37) | 0.64 (0.43–0.94) | NA | 0.88 (0.61–1.26) | 0.65 (0.45–0.93) |
| Age | ||||||
| <65 years | N.A | 1.08 (0.90–1.31) | 0.94 (0.68–1.28) | NA | 0.83 (0.61–1.12) | 0.72 (0.56–0.91) |
| ⩾65 years | NA | 1.14 (0.87– 1.49) | 0.59 (0.42–0.82) | NA | 0.78 (0.59–1.05) | 0.84 (0.62–1.12) |
| PS | ||||||
| 0 | NA | 1.28 (0.98–1.69) | 0.73 (0.48–1.10) | NA | 0.68 (0.44–1.05) | 0.77 (0.56–1.06) |
| 1 | NA | 0.99 (0.83–1.18) | 0.78 (0.60–1.03) | NA | 0.86 (0.68–1.09) | 0.76 (0.60–0.96) |
| Platinum salt | ||||||
| Carboplatin | NA | 1.14 (0.96–1.37) | 0.76 (0.60–0.95) | NA | 0.83 (0.65–1.07) | 0.79 (0.63–0.98) |
| Cisplatin | NA | 0.93 (0.71–1.21) | NA | NA | 0.73 (0.49–1.08) | 0.67 (0.46–0.97) |
| Brain mts | ||||||
| Yes | NA | 1.58 (1.02–2.44) | 0.96 (0.46–2.01) | NA | 1.32 (0.72–2.42) | 0.79 (0.44–1.41) |
| No | NA | 1.03 (0.88–1.20) | 0.74 (0.58–0.94) | NA | 0.75 (0.60–0.96) | 0.76 (0.62–0.92) |
| Liver mts | ||||||
| Yes | NA | NA | 0.75 (0.52–1.07) | NA | 0.75 (0.55–1.02) | 0.87 (0.66–1.16) |
| No | NA | NA | 0.76 (0.56–1.01) | NA | 0.82 (0.62–1.08) | 0.68 (0.53–0.88) |
| Smoking status | ||||||
| Smoker | NA | 1.09 (0.89–1.32) | NA | NA | 0.86 (0.66–1.11) | 0.75 (0.62–0.91) |
| Non-smoker | NA | 1.02 (0.80–1.30) | NA | NA | 0.71 (0.49–1.02) | 0.83 (0.41–1.71) |
CI, confidence interval; HR, hazard ratio; mts, metastases; NA, not available.
Meta-analysis results
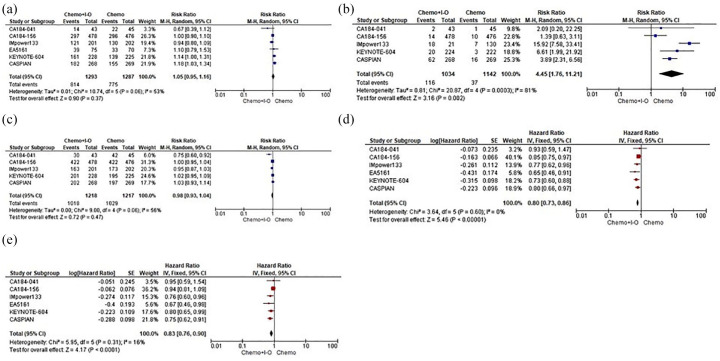
Seven full-text studies and one abstract for a total of six RCTs (2595 patients) evaluated the addition of a single-agent anti-PD-1 (nivolumab or pembrolizumab), anti-PD-L1 (atezolizumab or durvalumab) or anti-CTLA-4 (ipilimumab) to standard CT in comparison with standard CT alone in ES-SCLC patients. In particular, although no clear advantages in terms of ORR and DCR were underlined, our pooled results showed how single-agent IO addition to CT was able to significantly improve DORR (RR 4.45, 95% CI 1.76–11.21), resulting in long-term benefits in PFS (HR 0.80, 95% CI 0.73–0.86) and OS (HR 0.83, 95% CI 0.76–0.90) when compared with CT alone (Figure 2).
Figure 2.
Forest plots of efficacy endpoints including RRs of ORR. (a) DORR. (b) DCR. (c) Along with HR of PFS. (d) OS. (e) ES-SCLC patients assigned to receive first-line CT+IO regimens versus CT alone.
CI, confidence interval; CT, platinum-based chemotherapy; DCR, disease control rate; DORR, duration of response rate; ES-SCLC, extensive stage-small cell lung cancer; HR, hazard ratio; IO, immuno-oncology; IV, inverse variance; M–H, Mantel–Haenszel; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RR, risk ratio; SE, standard error.
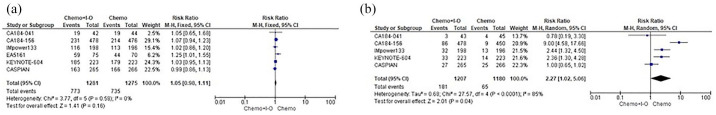
As regards safety, the IO addition did not seem to produce a statistically significant overload in terms of TRAEs between the different strategies; however, IO led to a significant increase in treatment discontinuation (RR 2.27, 95% CI 1.02–5.06) compared with CT alone (Figure 3).
Figure 3.
Forest plots of safety endpoints including RRs of TRAEs. (a) DR. (b) ES-SCLC patients assigned to receive first-line CT+IO regimens versus CT alone.
CI, confidence interval; CT, platinum-based chemotherapy; DR, discontinuation rate; ES-SCLC, extensive stage-small cell lung cancer; HR, hazard ratio; IO, immune-oncology; M–H, Mantel–Haenszel; RR, risk ratio; SE, standard error; TRAEs, treatment-related adverse events.
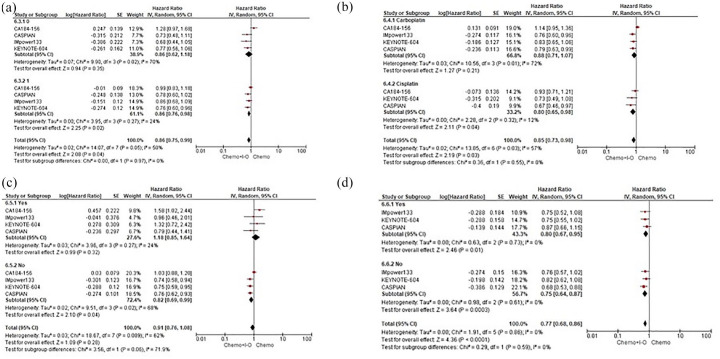
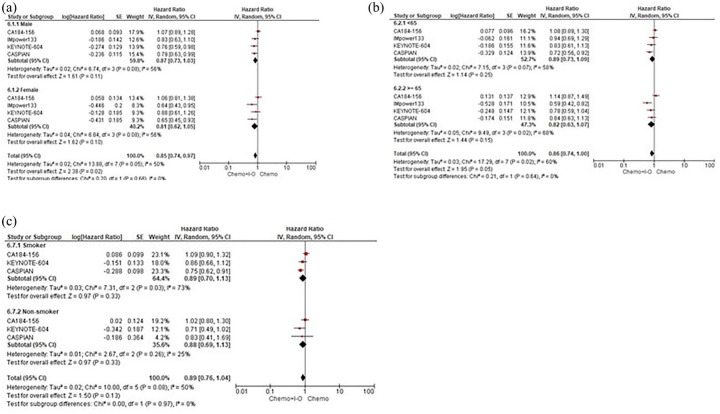
When specifically evaluating the OS according to subgroup analyses, based on the available outcomes of four trials (CA184-156, IMpower133, KEYNOTE-604 and CASPIAN), our pooled results showed that the IO addition comparing with CT alone led to a statistically significant improvement in survival in those patients with ECOG PS of 1 (HR 0.86, 95% CI 0.76–0.98), receiving cisplatin (HR 0.80, 95% CI 0.65–0.98) and presenting without brain metastases (HR 0.82, 95% CI 0.69–0.99). Patients both with and without liver metastases (HR 0.80, 95% CI 0.67–0.95 and HR 0.75, 95% CI 0.64–0.87, respectively) seemed to experience a survival benefit when adding IO agents to the CT backbone. No significant differences between the two treatment strategies were observed in terms of sex, age and smoking status with an ECOG PS of 0, the use of carboplatin and the presence of brain metastases not eventually predicting OS with the IO addition to CT regimens (Figures 4 and 5).
Figure 4.
Subgroup analyses for OS according to ECOG PS. (a) Platinum salt. (b) Brain mts. (c) Liver mts. (d) ES-SCLC patients assigned to receive first-line CT+IO regimens versus CT alone.
CI, confidence interval; CT, platinum-based chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; ES-SCLC, extensive stage-small cell lung cancer; IO, immuno-oncology; IV, inverse-variance; mts, metastases; OS, overall survival; PS, performance status; SE, standard error.
Figure 5.
Subgroup analyses for OS according to sex. (a) Age. (b) Smoking status. (c) ES-SCLC patients assigned to receive first-line CT+IO regimens versus CT alone.
CI, confidence interval; CT, platinum-based chemotherapy; IO, immuno-oncology; IV, inverse-variance; OS, overall survival; SE, standard error.
Indirect comparison results
After meta-analysis to obtain pooled data, we used the Bucher and Glenny technique to perform an indirect comparison according to the different IO strategies (anti-CTLA-4, anti-PD-1 and anti-PD-L1). Subgroup and landmark survival analyses were available only for four trials (one for the anti-CTLA-4 ipilimumab, one for the anti-PD-1 pembrolizumab, and two for the anti-PD-L1 atezolizumab and durvalumab).
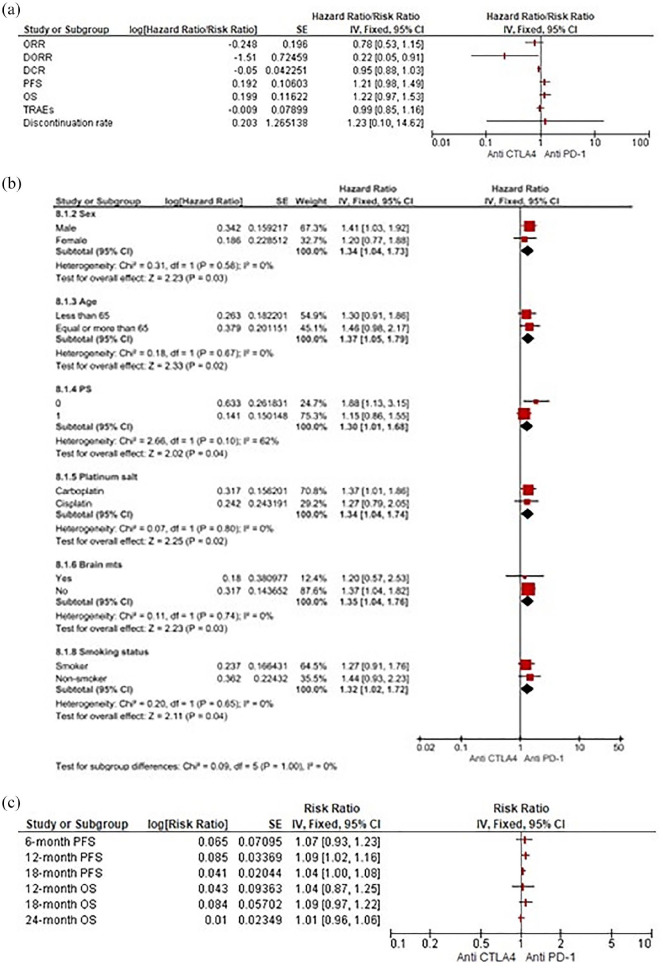
Anti-CTLA-4 versus anti-PD-1 agents
Comparing CTLA-4 and PD-1 inhibitors, our pooled results strongly suggest a potential survival benefit in terms of PFS (HR 1.21, 95% CI 0.98–1.49) and OS (HR 1.22, 95% CI 0.97–1.53) for the anti-PD-1 class, estimated at around 20% in reducing the risk of disease progression and death. These results did seem to be due to the prolonged and significant DORR (RR 0.22, 95% CI 0.05–0.91) contributed by anti-PD-1. Notably, no significant differences in terms of DCR, TRAEs and DR were found (Figure 6a). According to the subgroup analyses for OS, anti-CTLA-4 agents compared with PD-1 inhibition were associated with a higher risk of death in male patients (HR 1.41, 95% CI 1.03– 1.92) presenting with an ECOG PS of 0 (HR 1.88, 95% CI 1.13–3.15), without brain metastases (HR 1.37, 95% CI 1.04–1.82) and receiving carboplatin (HR 1.37, 95% CI 1.01–1.86) (Figure 6b). As regards pooled landmark survival analyses, the pembrolizumab-CT arm, when compared with the ipilimumab addition, confirmed a statistically significant improvement in PFS at 12 months (HR 1.09, 95% CI 1.02–1.16) with only a benefit trend at 18 months (HR 1.04, 95% CI 1.00–1.08). No differences in PFS at 6 months and in OS at any pre-specified time points were observed (Figure 6c).
Figure 6.
(a) Forest plots for indirect comparisons between anti-CTLA-4 and anti-PD-1 agents according to efficacy and safety outcomes. (b) Along with subgroup. (c) Landmark survival analyses.
CI, confidence interval; PD-1, programmed death 1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; IV, inverse-variance; SE, standard error.
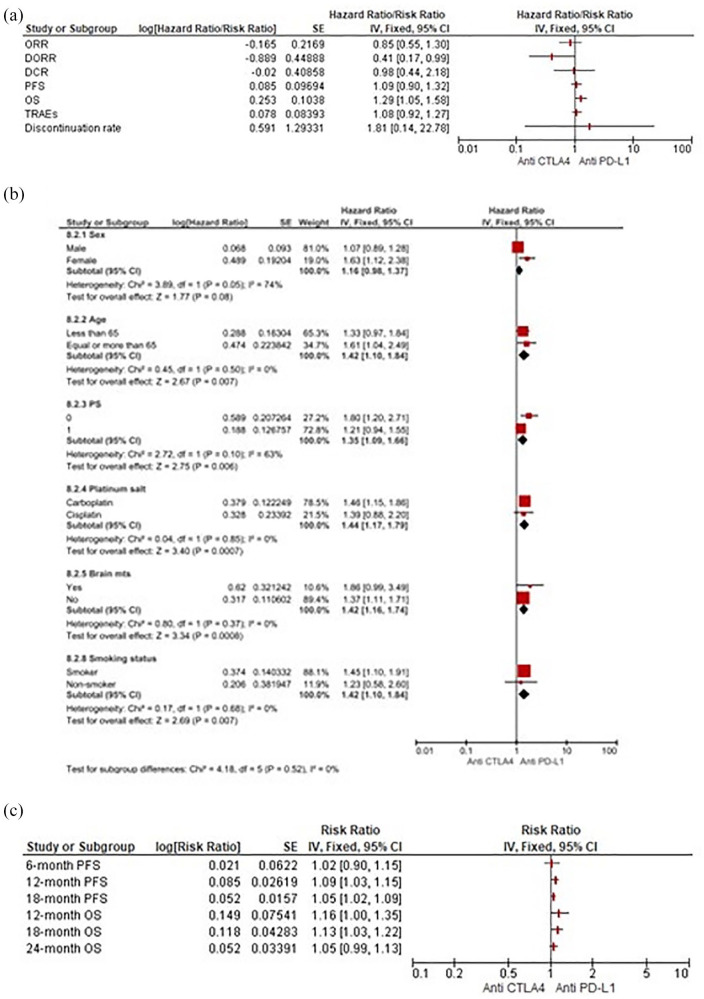
Anti-CTLA-4 versus anti-PD-L1 agents
Similarly to anti-PD-1, our pooled results pointed out the same overall benefit in favour of anti-PD-L1 agents for PFS (HR 1.09, 95% CI 0.90–1.32), OS (HR 1.29, 95% CI 1.05–1.58) and DORR (RR 0.41, 95% CI 0.17–0.99). No relevant differences for DCR, TRAEs and DR between classes were highlighted (Figure 7a). Dealing with OS, PD-L1 inhibitors significantly outperformed the CTLA-4 inhibition strategy in all available outcomes in subgroup analyses (Figure 7b). While showing no differences in PFS at 6 months, when compared with ipilimumab the anti-PD-L1 agents did produce a significant reduction in risk of progression at 12 and 18 months (HR 1.09, 95% CI 1.03–1.15 and HR 1.05, 95% CI 1.02–1.09, respectively), resulting in improved OS at 18 months (HR 1.13, 95% CI 1.03–1.22) and presenting only a survival benefit trend at 12 and 24 months (Figure 7c).
Figure 7.
(a) Forest plots for indirect comparisons between anti-CTLA-4 and anti-PD-L1 agents according to efficacy and safety outcomes. (b) Along with subgroup. (c) Landmark survival analyses.
CI, confidence interval; PD-L1, programmed death ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; IV, inverse-variance; SE, standard error.
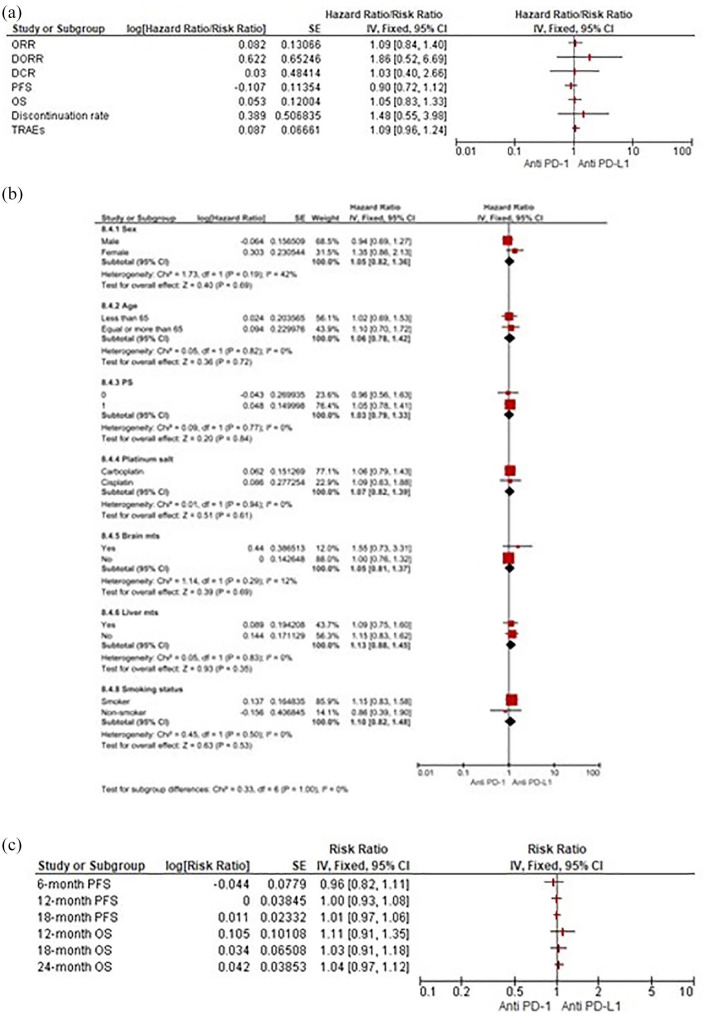
Anti-PD-1 versus anti-PD-L1 agents
Our pooled results did not show any relevant significant difference regarding both efficacy and safety endpoints between these two classes. Only benefit trends for DORR (RR 1.86, 95% CI 0.52–6.69) and DR (RR 0.49, 95% CI 0.15–1.61) using anti-PD-1 moAbs over anti-PD-L1 were observed (Figure 8a). Likewise, no survival differences according to subgroups or discrepancies in PFS and OS were observed at any pre-specified timepoints between PD-1 and PD-L1 inhibitors (Figure 8b–c).
Figure 8.
(a) Forest plots for indirect comparisons between anti-PD-1 and anti-PD-L1 agents according to efficacy and safety outcomes. (b) Along with subgroup. (c) Landmark survival analyses.
CI, confidence interval; PD-1, programmed death 1; PD-L1, programmed death ligand 1; IV, inverse-variance; SE, standard error.
Risk of bias assessment
In our analysis, publication bias Egger’s test was calculated for every outcome showing no statistical significance (Supplemental Figure S3). The overall quality assessment was evaluated according to the CONSORT checklist statement. We reported an average good quality of all trials. Some problems related to ‘Blinding of participants and personnel’ (performance bias) and ‘Blinding of outcome assessment’ (Detection bias) domains were observed because many of the studies were open-label (Supplemental Figure S4).
Discussion
So far, ES-SCLC has been considered a challenging disease with only a dismal prognosis. With the FDA approval of PD-L1 inhibitors atezolizumab and durvalumab in combination with platinum-based CT, ICIs have finally entered the therapeutic paradigm of the first-line setting for ES-SCLC.34,35 More recently, the clinical world of oncologists has again been excited by the results of the randomised KEYNOTE-604 and EA5161 trials, showing that the PD-1 inhibitors pembrolizumab and nivolumab led to improved survival rates when concurrently combined with CT, highlighting the importance of concurrent administration of CT and IO agents.23–25 However, although a consistent and reproducible pattern of efficacy improvement based on the reduction of both death and disease progression risk has been noted when adding ICIs to CT, additional studies to provide clarity on the benefit of CT+IO in this setting are warranted. A combination approach based on the association of CT with the PD-L1 inhibitors atezolizumab and durvalumab could now be considered as an emerging standard for newly diagnosed ES-SCLC patients. In this scenario, the majority of oncologists have long considered the different ICIs targeting PD-1 or PD-L1 as equally effective and clinically interchangeable options. However, establishing optimal therapeutic options still addresses an unmet clinical need in the first-line setting. Accordingly, although it is very reassuring to see similar data between these two pivotal clinical trials, several differences influencing the choice of these two approved medications in clinical practice need to be considered (differing study designs, divergent use and duration of platinumregimens, various implementation of prophylactic cranial irradiation and dissimilar inclusion of patients with brain metastases). Furthermore, although finally resulting in an OS improvement, the addition of atezolizumab to CT did result in a numerically lower ORR when compared with placebo. 36 Moreover, the interim results of the trials investigating PD-1 agents at this time reveal them to have limited immediate impact on daily practice and not yet homogeneously supported by international regulatory agencies.
Hence, since it will be unlikely to see head-to-head comparison studies, this work represents an attempt to indirectly compare these combination approaches to identify any potential differences in both activity and toxicity profiles. We encompassed publicly available results from randomised phase II/III studies testing CT+IO strategies in the first-line setting, including six RCTs that compared the association of a single-agent ICI with CT versus CT alone in treatment-naïve ES-SCLC patients. Although cross-trial comparisons are always misleading in this context, all these trials do present CT plus single-agent IO and CT as common experimental and control arms, respectively, enabling us to have a reasonable comparison of outcomes. Although no clear advantage in terms of activity was directly underlined, our pooled results showed how the addition of ICIs to CT significantly improved the duration of response, resulting in statistically significant long-term survival benefits and no additional differences in terms of adverse events. However, patients receiving CT+IO had a higher risk of discontinuing treatment comparing with the sole administration of CT. Intriguingly, an ECOG PS of 1 together with the use of cisplatin and the absence of brain metastases resulted in clinical characteristics positively predicting the OS of patients undergoing CT+IO compared with CT alone. This result notwithstanding, OS did not seem to be dramatically affected by the presence of hepatic disease. Of note, indirect comparisons according to the different IO subtypes suggested a slight advantage in favour of both PD-1 and PD-L1 over anti-CTLA-4 agents in terms of efficacy outcomes along with no additionally significant differences in the safety profile. Specifically, when indirectly comparing PD-1 with PD-L1 inhibitors, no relevant significant differences regarding both efficacy and safety endpoints were observed, with unprecedented benefit trends in terms of duration of response and treatment tolerability in favour of anti-PD-1 over anti-PD-L1. Based on the pooled results of our meta-analysis, the association of a single-agent IO with CT was confirmed to provide a survival benefit when compared with CT alone, providing a tolerable and effective therapeutic option in the upfront management of ES-SCLC, especially in selected patients.
Additionally, to further assess which patients would most benefit from ICIs treatment, we performed a separate subgroup analysis evaluating both the efficacy and safety profiles of different IO agents. Namely, as regards indirect comparisons according to different ICIs subtypes, PD-1 inhibitors were found to be not inferior to PD-L1 agents in terms of both efficacy and safety outcomes, additionally demonstrating a more durable response and less treatment discontinuation, with no significant differences in survival according to subgroups. Conversely, concurrent administration of the CTLA-4 inhibitor ipilimumab with CT was significantly associated with heightened toxicity risks and reduced efficacy outcomes. In this context, in light of the negative results from the earlier CA184-156 study and the updated CASPIAN trial, which showed not insignificant toxicities when using ipilimumab or tremelimumab, further studies evaluating the anti-CTLA-4 strategy are unwarranted outside of a biomarker-selected population. Nonetheless, as the phased introduction of ipilimumab after two cycles of induction CT appeared to yield better efficacy compared with the concurrent administration in the CA184 trials, a therapeutic approach investigating the role of CTLA-4 inhibitors as part of a maintenance rather than an induction strategy is worth exploring in the future research landscape.
In terms of landmark efficacy, the outcomes for median PFS, median OS, 12-month OS and 24-month OS turned out to be very similar for most of the CT+IO studies conducted in the first-line setting. When focussing on pooled landmark survival comparisons among CT+IO approaches, the calculation of patients at risk was feasible only for four trials (ipilimumab, atezolizumab, pembrolizumab and durvalumab), given the absence of data regarding nivolumab that are still not published in extenso. With this limitation, the PD-1 inhibitor pembrolizumab resulted to be not inferior to PD-L1 agents in reducing the risk of disease progression and/or death at any predetermined timepoint, while demonstrating significantly improved PFS at 12 months when compared indirectly with ipilimumab addition. In this vein, PD-L1 inhibitors were broadly confirmed to enhance survival rates compared with the CTLA-4 inhibition strategy.
Although considering the latest available ES-SCLC data to perform multiple indirect comparisons of first-line association of an IO agent with CT, this study had some limitations. First, these results should always be interpreted with caution since they are based on indirect comparisons among only a few studies. Secondly, we considered the concurrent contribution of a single-agent IO addition and did not take into account the role of a dual ICI blockade (the anti-PD-L1 durvalumab + the anti-CTLA-4 tremelimumab, recently emerging from updates to the CASPIAN trial) that did not improve survival rates when compared with CT alone, in order to evaluate only homogeneous data that would not affect the final analysis. Thirdly, important differences among the included trials (unselected patients’ population, sample size, low incidence, and different treatment of brain metastases, use and duration of platinum compounds and differing median OS in the CT-based control arms) must be considered; in this context, the use of carboplatin plus paclitaxel as common platinum-based CT backbone in the CA184-041 trial in contrast to platinum plus etoposide of the remaining trials could be assumed as a source of bias. Finally, there were some limitations to the analysis of toxicity data since we only reported chemo-immunotherapy TRAEs and did not focus on immune-related adverse events (irAEs), which, however, did not appear a cause for concern; indeed, in the RCTs investigating PD-1 and PD-L1 inhibitors, toxicity profiles were very similar to the CT-based control arms, with most of the grade 3/4 TRAEs being haematologic (thus, attributable to CT backbones) and most of the irAEs being primarily low grade.
Conclusions
Whilst broadly confirming a clear survival advantage with the use of CT+IO over CT alone in the frontline management of ES-SCLC patients, the results of this indirect meta-analysis proved that specific patient clinical characteristics (such as ECOG PS of 1, the use of cisplatin and the absence of brain metastases) seem to be associated with a survival gain using CT+IO in ES-SCLC patients. Namely, although longer follow up with robust prospective data is needed, in this setting, according to subgroup analyses, pembrolizumab combined with CT indirectly resulted in the same survival benefit and tolerability of FDA-approved PD-L1 inhibitors. Although some interesting differences in both activity and safety profiles among these ICIs subtypes were revealed, these findings should not be considered as a decisional tool to establish the superiority of one therapeutic approach over another. Considering the limitations and the potential bias related to indirect comparisons, these could serve only as scientific support to help oncologists in their future clinical and research decisions. These compelling results seem to suggest the association of PD-1 inhibitors with CT as an additional viable option for novel treatment approaches and development in the near future, mostly considering that currently available data regarding pembrolizumab and nivolumab appear insufficient to change practice standards. Finally, even though potential biomarkers such as immunohistochemical evaluation of PD-L1 and tumor mutational burden failed to be predictive for first-line ICIsin ES-SCLC,20,25 further trials based on anti-CTLA-4 strategies should eventually be carefully studied in biomarker-selected patients, given the lack of efficacy and non-negligible toxicities in this setting.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-4-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Acknowledgments
Valerio Gristina contributed to the current work under the Doctoral Programme in Experimental Oncology and Surgery, University of Palermo. All the authors thank Chiara Drago for English language revision.
Footnotes
Author contributions: All authors contributed to conception and design of the study, data collection and analysis and interpretation of data; drafting and revising the article critically for content; and final approval of the version to be submitted.
Valerio Gristina: Conceptualization, data curation, investigation, software, visualization, roles/writing - original draft.
Antonio Galvano: Data curation, formal analysis, investigation, methodology, software, resources, roles/writing - original draft.
Luisa Castellana: Data curation, roles/writing - original draft, investigation.
Lavinia Insalaco: Data curation, roles/writing - original draft, investigation.
Stefania Cusenza: Data curation, roles/writing - original draft, investigation.
Giuseppa Graceffa: Supervision, validation, resources, writing – review & editing.
Federica Iacono: Visualization, validation, roles/writing - original draft.
Nadia Barraco: Visualization, validation, roles/Writing - original draft.
Marta Castiglia: Visualization, validation, roles/Writing - original draft.
Alessandro Perez: Visualization, validation, roles/writing - original draft.
Sergio Rizzo: Supervision, validation, writing - review and editing.
Antonio Russo: Project administration, resources, supervision, validation, visualization, writing - review & editing.
Viviana Bazan: Conceptualization, project administration, resources, supervision, validation, visualization, writing - review and editing.
Conflict of interest statement: A. Russo reports personal fees from Bristol, Pfizer, Bayer, Kyowa Kirin, Ambrosetti for advisory board activity; speaker honorarium from Roche Diagnostics. The remaining authors declare no potential conflicts of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Valerio Gristina, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Antonio Galvano, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Luisa Castellana, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Lavinia Insalaco, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Stefania Cusenza, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Giuseppa Graceffa, Division of General and Oncological Surgery, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Federica Iacono, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Nadia Barraco, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Marta Castiglia, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Alessandro Perez, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Sergio Rizzo, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy.
Antonio Russo, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, via del vespro, n. 129, Palermo, 90127, Italy.
Viviana Bazan, Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, University of Palermo, Palermo, Italy.
References
- 1. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24: 4539–4544. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Nicholson AG, Chansky K, Crowley J, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 300–311. [DOI] [PubMed] [Google Scholar]
- 4. Kauffmann-Guerrero D, Kahnert K, Syunyaeva Z, et al. Pretherapeutic inflammation predicts febrile neutropenia and reduced progression-free survival after first-line chemotherapy in SCLC. Oncol Res Treat 2018; 41: 506–512. [DOI] [PubMed] [Google Scholar]
- 5. Sabari JK, Lok BH, Laird JH, et al. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 2017; 14: 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmerman S, Das A, Wang S, et al. 2017–2018 scientific advances in thoracic oncology: small cell lung cancer. J Thorac Oncol 2019; 14: 768–783. [DOI] [PubMed] [Google Scholar]
- 7. Gill J, Prasad V. A reality check of the accelerated approval of immune-checkpoint inhibitors. Nat Rev Clin Oncol 2019; 16: 656–658. [DOI] [PubMed] [Google Scholar]
- 8. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999; 17: 1794–1801. [DOI] [PubMed] [Google Scholar]
- 9. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012; 30: 1692–1698. [DOI] [PubMed] [Google Scholar]
- 10. Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta- analysis. Lung Cancer 2000; 30: 23–36. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong SA, Liu SV. Immune checkpoint inhibitors in small cell lung cancer: a partially realized potential. Adv Ther 2019; 36: 1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puri S, Shafique M. Combination checkpoint inhibitors for treatment of non-small-cell lung cancer: an update on dual anti-CTLA-4 and anti-PD-1/PD-L1 therapies. Drugs Context 2020; 9: 2019-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farid S, Liu SV. Chemo-immunotherapy as first-line treatment for small-cell lung cancer. Ther Adv Med Oncol 2020; 12: 1758835920980365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 2020; 17: 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of Small Cell Lung Cancer (SCLC). Front Oncol 2020; 10: 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng SH, Yang S-T. The new 8th TNM staging system of lung cancer and its potential imaging interpretation pitfalls and limitations with CT image demonstrations. Diagn Interv Radiol 2019; 25: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Schil PE, Rami-Porta R, Asamura H. The 8th TNM edition for lung cancer: a critical analysis. Ann Transl Med 2018; 6: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensivedisease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013; 24: 75–83. [DOI] [PubMed] [Google Scholar]
- 19. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016; 34: 3740–3748. [DOI] [PubMed] [Google Scholar]
- 20. Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 21. Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021; 39: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol 2020; 38: 9000. [Google Scholar]
- 23. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020; 38: 2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394: 1929–1939. [DOI] [PubMed] [Google Scholar]
- 25. Paz-Ares LG, Dvorkin M, Chen Y, et al. Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): updated results from the phase III CASPIAN study. Lancet Oncol 2021; 22: 51–65. [DOI] [PubMed] [Google Scholar]
- 26. Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020. [Google Scholar]
- 27. Meta Analysis Workshop. Comprehensive Meta Analysis Version 3.0. Computer Software, 2013. [Google Scholar]
- 28. Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 29. Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005; 9: 1–134, iii–iv. [DOI] [PubMed] [Google Scholar]
- 30. Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001; 323: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019; 10: ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 34. U.S. Food and Drug Administration. FDA approves atezolizumab for extensive-stage small cell lung cancer. Silver Spring, MD: FDA, 2019. [Google Scholar]
- 35. U.S. Food and Drug Administration. FDA approves durvalumab for extensive-stage small cell lung cancer. Silver Spring, MD: FDA, 2020. [Google Scholar]
- 36. Gill J, Cetnar JP, Prasad V. A timeline of immune checkpoint inhibitor approvals in small cell lung cancer. Trends Cancer 2020; 6: 736–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-4-tam-10.1177_17588359211018018 for Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses by Valerio Gristina, Antonio Galvano, Luisa Castellana, Lavinia Insalaco, Stefania Cusenza, Giuseppa Graceffa, Federica Iacono, Nadia Barraco, Marta Castiglia, Alessandro Perez, Sergio Rizzo, Antonio Russo and Viviana Bazan in Therapeutic Advances in Medical Oncology