Abstract
Mutations in codon 12 of KRAS have been identified in 13% of non-small cell lung cancer patients. Developing targeted therapies against KRASG12C mutation has proven to be challenging due to the abundance of GTP in the cytoplasm, rapid hydrolysis of GTP, and difficulty designing small molecules to achieve sufficient concentration for KRAS inhibition. Based on promising results in both preclinical and clinical trials, sotorasib, a novel KRASG12C inhibitor, was given conditional approval by the FDA in May 2021. The Phase I portion of the clinical trial produced 32% confirmed response with 56% of patients with stable disease. About 91.2% of patients who received the highest dose of 960mg daily achieved disease control. The Phase II portion, which used 960mg daily dosing resulted in 37.1% of patients with confirmed response and 80.6% of patients with disease control. Both phase I and phase II had similar progression-free survival, in 6.3 months and 6.8 months, respectively. In both phases, grade 4 adverse events occurred in only one patient. The most common adverse events were elevations in LFTs, which down-trended upon dose reduction and steroid treatment. While the conditional approval of sotorasib was a major breakthrough for those patients harboring KRASG12C mutations, resistance mutations to sotorasib are increasingly common. Many proposals have been made to address this, such as the use of combination therapy for synthetic lethality, which are producing encouraging results. Here, we explore in further detail the development of sotorasib, its efficacy, mechanism of resistance, and strategies to overcome these resistances.
Keywords: sotorasib, AMG 510, Lumakras, non-small cell lung cancer, CodeBreak 100, KRASG12C
Introduction
Rat sarcoma viral oncogene (RAS) is the oldest driver mutation identified in 1982 and consists of three isoforms: KRAS, HRAS, and NRAS.1 Of these isoforms, KRAS is the most common, making up 85–90% of all RAS proteins. KRAS mutations exist in many cancers including pancreatic, colorectal and lung. While KRAS mutations are rare in certain lung cancers such as squamous cell carcinomas (SCC), they exist in 15–30% of all lung adenocarcinoma patients in the United States and are often associated with smoking, increased PD-L1 expression, and high tumor mutational burden.2 Higher expression of PD-L1 was discovered in tumor and immune cell lines in KRAS mutant lung adenocarcinoma (37%), most notably in KRASG12C and KRASG12V cells compared to wild-type cells (18%).3 Interestingly, KRAS mutations occur less frequently at less than ten percent of NSCLC in Asia due to a higher proportion of EGFR mutations. Different sub-types of mutations exist within the KRAS gene with most occurring in exon 2 or 3 (G12, G12, Q61).4 KRAS codon 12 mutations, which comprise 91% of KRAS mutations, are further divided into sub-types including G12C, V, D, A, S. The next most common KRAS mutation occurs on codon 13, which comprises 5% of KRAS mutations with sub-types C, D, and R. The remaining codon mutations in KRAS are infrequent (codon 61, 9, 14, 18, 19, 33, 88, 14, etc.).
Studies have been inconsistent regarding KRAS mutation’s effects on metastasis and survival. Yu et al found that mutations in KRAS codon 13 led to shorter survival compared to mutations in KRAS codon 12.5 Another study found that no significant difference in survival existed between patients with KRAS mutation versus wild type.6 Contrarily, Goulding et al performed a meta-analysis, which suggested that KRAS mutation may be a negative prognostic marker for survival in patients with advanced NSCLC.7
Unfortunately, development of targeted therapies against the KRAS mutation has been largely unsatisfactory. The first-line treatment for KRAS NSCLC commonly includes immune checkpoint inhibitors with or without chemotherapy. However, effective second-line options for KRAS NSCLC that progress beyond first-line therapies are limited and include pemetrexed if not already given as part of a first-line regimen, docetaxel with ramucirumab, or docetaxel alone. Combination therapy with ramucirumab, an anti-VEGF2 inhibitor, and docetaxel has been shown to produce a median progression-free survival (PFS) of just 4.5 months and response of 23% in the REVEL trial.8 Due to this reason as well as a lack of targeted therapies, KRAS NSCLC patients who fail first-line therapies often have poor outcomes.
Development of KRASG12C inhibitors has been deemed difficult given GTP abundance in the cytoplasm and its rapid hydrolysis as well as difficulty designing targets that have high bioavailability and potency to inhibit KRAS. After multiple trials of drug development, sotorasib, otherwise known as AMG 510 or LUMAKRAS, a novel KRASG12C inhibitor, was conditionally approved by the Food and Drug Administration (FDA) on May 28th 2021 and has shown promising results in both preclinical and clinical trials.
Three main molecular subgroups of KRASG12C have been of interest and these include KRASG12C with STK11 mutations, TP53 mutations, and Kelch-like ECH-associated protein 1 (KEAP1) mutations. The STK11 gene encodes a serine/threonine protein kinase that acts as a tumor suppressor. In a single-institution retrospective study, the objective response rates (ORR) to first-line systemic therapy in patients treated with PD-1 blockade were shown to be 7.4% in patients with KRAS and STK11 co-mutation, 35.7% in patients with TP53 and KRAS co-mutation, and 28.6% in patients with KRAS mutation alone.9 Patients with KRAS also exhibited shorter PFS if STK11 co-mutation was present. KEAP1 mutation has been found in approximately 20% of KRAS mutant NSCLC.10 KEAP1 negatively regulates nuclear factor erythroid 2-like 2 (NRF2) and regulates the antioxidant response. This system depends on the pentose phosphate pathway (PPP) and glutaminolysis, which suggests that targeting either path may serve as a potential therapeutic approach. The use of sotorasib should also be studied further in KRASG12C patients with STK11, TP53, or KEAP 1 co-mutation.
Structure of Sotorasib
In 2013, Shokat et al studied the use of a covalent inhibitor to target cysteine 12 of KRAS.11 Cysteine reactive libraries were screened for compounds that were modified to enhance oral bioavailability and maintain potency. ARS-1620 was the first covalent KRASG12C inhibitor that demonstrated in vivo efficacy. In order for the compound to access the binding pocket, chemical groups such as quinazoline nitrogen were substituted to improve linkage. Many resulting compounds were created, however encountered issues shortly due to poor bioavailability, poor potency, and/or inability to completely restrict bond rotations. Concentration of GTP within the cell is abundant, which creates a challenging environment with competition against GTP in the hydrolysis domain of KRAS. After multiple adjustments were made, the (R)-38 compound, which was renamed as AMG 510, was created. It successfully demonstrated good activity in cellular assays, dose proportional plasma exposure, moderate permeability, and excellent oral bioavailability, and ultimately was approved as sotorasib.
Sotorasib binds to the inactive guanosine diphosphate (GDP)-bound KRAS via a covalent bond between cysteine 12 (C12) and the acrylamide warhead and non-covalent bonds between isopropylpyridine substituent and a cryptic pocket, which includes H95 (histidine), Y96 (tyrosine), and Q99 (glutamine). The pocket on the switch II region (S-IIP) is only present in an inactive GDP bound form and binds the molecular core.
Pharmacodynamics
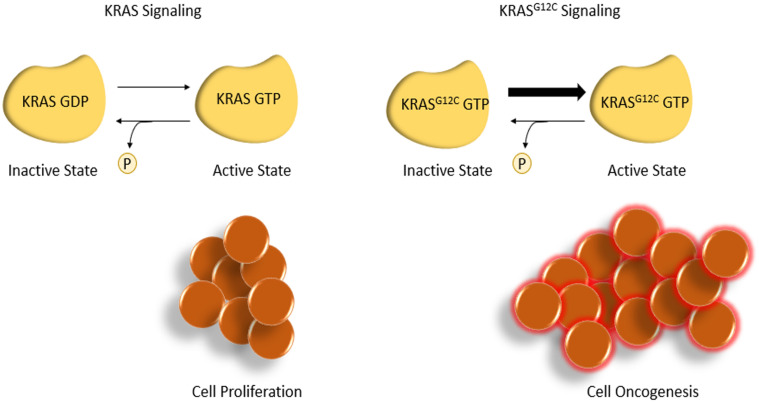
The KRAS protein alternates between a GDP-bound inactive form and a guanosine triphosphate (GTP)-bound active state, with downstream effects on cell signaling and proliferation. A mutation in the KRAS codon 12 mutation impairs GTP hydrolysis, leading to a constitutively active state promoting cell proliferation. Sotorasib binds to the KRASG12C cysteine residue to lock the protein in its inactive form, inhibiting cell proliferation and promoting apoptosis (Figure 1). This residue is not present in wild-type KRAS, limiting off-target effects.
Figure 1.
KRASG12C signaling produces cell mutagenesis.
Pharmacokinetics
The median time for sotorasib to reach peak plasma is one to two hours, with a mean elimination half-life of 5.5 hours (Table 1).12 Despite this half-life, sotorasib is approved as a once daily dosing, possibly due to high-dose administration. It is metabolized by conjugation or oxidative metabolism via the cytochrome P450 family 3 subfamily A (CYP3A) enzyme located primarily in the liver and intestines. Thus, it should be used with caution in patients who take CYP3A4 inducers such as rifampin, phenobarbital, and carbamazepine, or inhibitors such as itraconazole. Acid reducing agents such as proton pump inhibitors (PPIs) and H2 antagonists should also be used with caution with sotorasib, as they may decrease drug absorption. If co-administration of acid reducing agents cannot be avoided, four to ten hours should be given between the two drugs. It has been advised that women on sotorasib should not breastfeed children until one week after the final dose.
Table 1.
Sotorasib Steady State PK Parameters
| Dose (mg) | Sample Size (N) | Tmax (hr) | Cmax (ug/mL) | AUC |
|---|---|---|---|---|
| 180 | 6 | 0.73 | 31.7 | 5.13 |
| 360 | 24 | 1 | 38.9 | 5.53 |
| 720 | 11 | 1.1 | 42.1 | 4.75 |
| 960 | 24 | 1.1 | 32.4 | 5.07 |
Per Amgen, sotorasib does not accumulate with multiple doses and increase in dose is less than dose proportional. AUC and Cmax both increased when sotorasib was given with high fat meal by 1.38 and 1.03 fold respectively. As this was a minimal change, sotorasib can be given with or without food.
Preclinical Studies
In preclinical studies, Canon et al were able to demonstrate AMG 510 activity in both cell lines and murine models.13 In cell viability assays, AMG 510 effectively inhibited cell growth with an encouraging half maximal inhibitory concentration (IC50) of 0.004µm to 0.032 µm in almost all KRASG12C cell lines. Sotorasib favorably inhibited almost all phosphorylation of extracellular signal regulated kinase (ERK), located downstream of KRAS leading to tumor regression in mice with KRASG12C tumors in a dose responsive manner. In addition, AMG 510 improved antitumor effects of chemotherapy and led to durable cures in eight out of ten mice. Of note, the immune system appeared to play an important role in the mechanisms of action of AMG 510. Interestingly, AMG 510 did not produce effects in mice that lacked T cells, suggesting that only mice with intact immune systems would benefit from AMG 510 as a monotherapy. The addition of an anti-checkpoint inhibitor to AMG 510 successfully induced sustained regression of tumor growth in most mice. Furthermore, mice that were previously cured with AMG 510 did not grow tumors when they were re-challenged with KRASG12C cells, suggesting some level of adaptive immunity.
When evaluating toxicity, one preclinical study demonstrated that rats receiving 960mg sotorasib daily developed renal toxicity with necrosis and degeneration of kidney tubules, primarily at the proximal tubule.14 Partial recovery of tubular damage was noted after two months. Other adverse effects in rats included decreased weight gain, leukocytosis, thrombocytosis, and hypocalcemia. One rat receiving 750mg/kg sotorasib developed seminiferous tubule degeneration. Radioactive AMG 510 injected into rat tissue showed extension to liver, kidney, thyroid, pancreas, and adrenal gland but demonstrated poor brain distribution.12
Phase I Portion of the CodeBreak 100 Trial
The phase I portion of the CodeBreak 100 multicenter trial evaluated the use of sotorasib in 129 enrolled patients. Fifty-nine patients had NSCLC, 53 of whom were current or former smokers, 42 had colorectal cancer, and 28 patients had other tumor types. In the NSCLC group, patients who were 18 years or older with locally advanced or metastatic KRASG12C NSCLC were included.15 Patients with untreated brain metastasis, active systemic therapy within 28 days of sotorasib initiation, and/or radiation within two weeks of sotorasib initiation were excluded from the trial. The primary endpoint was safety, particularly dose limiting toxicities within 21 days of the first dose. Secondary end points included pharmacokinetics, objective response based on RECIST v1.1 criteria, duration of response (DOR), disease control, PFS, and duration of stable disease. Subjects received a median of three lines of therapies prior to entering the trial and were divided into dose escalation cohorts of 180mg, 360mg, 720mg, and 960mg. Treatment was discontinued in 107 patients largely due to disease progression. Seventy-four patients received at least three months of treatment and 38 patients received at least six months of treatment.
In patients with NSCLC, 19 of the 59 patients achieved confirmed response and 33 patients had stable disease. In the 960mg cohort (N=85), 12 patients (35.5%) exhibited confirmed response and 31 patients (91.2%) had disease control (Table 2). About 71.2% of subjects had shrinkage of their tumor at week six during the first evaluation. The median time to response was 1.4 months with a median DOR of 10.9 months and median PFS of 6.3 months. In patients who exhibited response, the duration of response was at least three months in 11 patients, at least six months in six patients, and at least nine months in five patients. The median duration of stable disease was four months. These results are superior to current therapies, which show a nine to 18% response to second- or third-line therapies and a median PFS of 2.5 to four months.16
Table 2.
Summary of Properties from Clinical Trials
| Preclinical Trial Properties |
| IC50: 0.004µm to 0.032 µm |
| Phase 1 Clinical Trial Properties |
| Half-life: 5.5 hours Tmax: 1.1 Confirmed response rate: 35.5% Disease control: 91.2% Median time to response: 1.4 months Median DOR: 10.9 months PFS: 6.3 months Most common side effects: Diarrhea, nausea Patients with grade ¾ events: 11.6% Grade 3 events: Elevated ALT and AST, diarrhea, anemia, hepatitis, lymphopenia, elevated GGT, hyponatremia Grade 4 event: Elevated ALT |
| Phase 2 Clinical Trial Properties |
| ORR: 37.1% Disease Control: 80.6% Stable disease: 43.5% Progressive disease: 16.1% Median DOR = 11.1 months Median time to response: 1.4 months Median duration of treatment: 5.5 months PFS: 6.8 months Most common side effects: diarrhea, nausea, fatigue, arthralgia, elevated ALT or AST |
Abbreviations: ORR, objective response rate; DOR, duration of response; PFS, progression-free survival; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Unfortunately, these results were not met in patients with other cancer types. Only three (7.1%) of the 42 colorectal cancer patients demonstrated confirmed response, with a median DOR of 5.4 months. In the “other” tumor types group, only four patients had confirmed response, all with different cancer types: pancreatic, endometrial, appendiceal, and melanoma. It would be of interest to explore these inconsistent results between cancer types and strategies to overcome this.
Overall, subjects experienced mostly grade one or two reversible toxicities. The most common adverse events were diarrhea and nausea. Fifteen patients (11.6%) had grade three or four treatment-related adverse events. Grade three events included elevation in alanine aminotransferase (ALT), diarrhea, anemia, elevated aspartate aminotransferase (AST), hepatitis, low lymphocyte counts, elevated gamma-glutamyl transferase (GGT), and hyponatremia. One patient experienced a grade four elevation in ALT, which returned to baseline after dose reduction and steroid treatment. There were no dose limiting toxicities or treatment-related deaths. From the phase I trial, sotorasib showed promising activity potentially superior to standard second- and third-line therapy in KRAS NSCLC with a fairly tolerable adverse event profile in patients with NSCLC harboring KRASG12C mutations.
Phase II Portion of the CodeBreak 100 Trial
The phase II portion of the CodeBreak 100 multicenter trial took place between the dates of August 2019 and February 2020.17 This phase included subjects 18 years or older with locally advanced or metastatic KRASG12C NSCLC who had disease progression after programmed death-1 (PD-1), programmed death ligand-1 (PDL-1), and/or a platinum-based chemotherapy. A total of 126 subjects, 117 of whom were current or former smokers, were included. Subjects were treated with 960mg sotorasib daily, based on the phase I results. Primary end points measured were complete and partial objective response. Secondary end points included the time to response, PFS, OS, and safety profile.
The median PFS was 6.8 months (95% CI: 5.1, 8.2), similar to the phase I PFS of 6.3 months (Table 2). Objective response was seen in 46 (37.1%) patients, and disease control was achieved in 100 (80.6%) patients. Of the 46 patients with objective response, four had complete response (3.2%) and 42 had partial response (33.9%). Fifty-four had stable disease (43.5%), and 20 had progressive disease (16.1%). The median DOR was 11.1 months, median time to respond was 1.4 months, and median duration of treatment was 5.5 months. Shrinkage of tumor was observed in 102 patients (82.3%) with median best percentage decrease of 60% in diameter. Response was observed across all PD-L1 expression levels. Eighty-eight patients (69.8%) received therapy for at least 3 months, 60 patients (47.6%) for at least 6 months, and 41 patients (32.5%) for at least 9 months.
Adverse events occurred in 125 patients (99.2%). The most common were diarrhea in 64 patients (50.8%), nausea in 39 patients (31%), fatigue in 32 patients (25.4%), arthralgia in 27 patients (21.4%), elevated AST in 27 patients (21.4%), and elevated ALT in 26 patients (20.6%). Treatment-related adverse events occurred in 88 (69.8%) patients with one grade four event (0.8%) and 25 grade three events (19.8%). Based on these results, it is recommended to monitor liver function tests (LFTs) every three weeks, then monthly or as clinically indicated. Similarly to phase I results, phase II results demonstrated sotorasib to be potentially superior to standard second- and third-line therapies in KRAS NSCLC, with tolerable side effects at the 960mg dose.
Phase 3 Registration Trial (Codebreak 200)
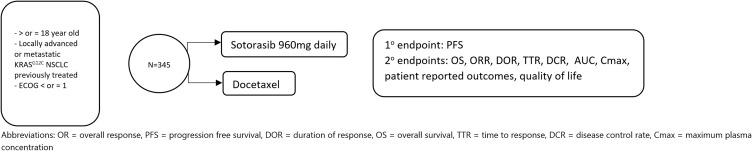
Currently, a confirmatory Phase III trial is undergoing investigation as CodeBreak 200 and will compare sotorasib to docetaxel in KRASG12C patients who had already received chemotherapy or immunotherapy (Figure 2). We eagerly await the results.
Figure 2.
CodeBreak 200 Phase III registration trial schema.
Mechanism of Resistance
Given preclinical and clinical trial results, sotorasib was given conditional approval by the FDA as the first KRASG12C inhibitor in adults with KRASG12C mutated NSCLC who had at least one prior systemic therapy. Unfortunately, the development of resistance to sotorasib has become increasingly common and can occur at both upstream sites (EGFR, HER2, FGFR) and downstream (MAPK/MEK pathway) sites. Resistance mechanisms may also occur by means of bypass mechanisms such as MET amplifications, loss of function mutations in NF1 and PTEN, and oncogenic fusions involving ALK, RET, BRAF, RAF1, and FGFR3 (Table 3).18 Although clinical attempts have been made to target these down-stream sites such as MAPK, therapeutic benefit has been limited, which may be due to alternate RAS dependent pathway activations.
Table 3.
Mechanisms of Resistance
| On Target Resistance Mechanisms | Off Target Resistance Mechanisms |
|---|---|
| Clinical findings | |
| G12D | BRAF mutations |
| G12R | BRAF fusions |
| G12V | MEK |
| G12W | MAP2K1 |
| G13D | NRAS |
| Q61H | ALK |
| R68S | RET |
| H95D | FGFR3 |
| H95Q | RAF1 |
| H95R | |
| Y96C | |
| In vitro findings | |
| Y96D | MEK |
| Y96S | |
| G13D | |
| R68M | |
| A59S | |
| A59T |
Some notable resistance mutations include r68, H85, and Y96. The Y96D mutation, in particular, disrupts the hydrogen bond between Y96 and the carboxyl group of AMG 510, affecting the switch pocket. This has been illustrated by researchers, who have expressed KRASG12C and KRAS G12C/Y96D double mutant proteins in cell lines. Cells that expressed KRASG12C/Y96D were resistant to AMG 510 with an IC50 of over 100 fold. Higher basal activation and proportion of active GTP bound KRAS were present in cells expressing the Y96D mutations.19
One study exposed 142 Ba/F3 cells, transduced with KRASG12C via retroviruses, to sotorasib or adagrasib, a similar novel KRASG12C inhibitor and looked for secondary resistance mutations.20 Twelve secondary mutations were identified in 124 clones. Y96D and Y96S mutations were found to be resistant to both inhibitors. The addition of BI-3406, a SOS1 inhibitor, plus trametinib was able to provide activity against these resistances. In this study, mutations G13D, R68M, A59S, and A59T were shown to be highly resistant to sotorasib. In order to overcome these resistance mutations, a combination therapy approach has been suggested.
Ongoing Studies and Future Directions
In newly diagnosed advanced metastatic NSCLC, it is critical to check for driver mutations such as EGFR, ALK, ROS1, ERBB2, BRAF, RET, and MET, as these driver mutations may serve as targets for therapies when positive.21 With the approval of sotorasib, KRASG12C has now joined the ranks as a targetable mutation and should continue to be examined in further studies. With the exciting conditional approval of sotorasib, many future directions remain to be explored including whether or not sotorasib can be used as an effective first-line therapy (monotherapy or in combination), postoperative adjuvant therapy, or neoadjuvant therapy.
As of now, there is an ongoing confirmational phase III study comparing sotorasib and docetaxel with pending results. The FDA is also mandating Amgen to evaluate sotorasib at the minimal 240mg daily. All doses ranging between 180mg and 960mg of sotorasib produced saturable absorption at similar levels in prior studies. This suggests that the dose of sotorasib is higher than what is needed to inhibit and phosphorylate ERK. At 240mg, sotorasib should be dosed as twice daily to maintain a level above IC90 of ERK phosphorylation. A higher dose may have been chosen as no drug limiting toxicities were noted at 960mg daily, it is more conveniently dosed than twice daily, and a higher dose level may overcome errors in the in vitro studies.
As both phase I and phase II portions of the CodeBreak trial excluded those with active brain metastases, possibly because sotorasib did not penetrate the brain in rats, it would be interesting to explore the intracranial activity of sotorasib as a monotherapy or in combination therapy in this patient population.
Combination therapy may be one approach to overcome resistance mechanisms.22 Combination may be superior to monotherapy by means of targeting other sites in the KRAS pathway. For example, the use of a SH2 containing protein tyrosine phosphatase-2 (SHP2) inhibitor, mammalian target of rapamycin (mTORC1) inhibitor, or checkpoint inhibitor along with sotorasib may overcome resistance mechanisms. SHP2 inhibitors increase RAS signaling, which may overcome resistance as GTPase activity is dependent on Son of Sevenless (SOS)1/2 activity downstream of RAS. This was demonstrated in vitro pancreatic ductal adenocarcinoma (PDAC) and NSCLC models by Fedele and colleagues.23 In this study, the combinatory use of SHP2 inhibitor and G12C inhibitor synergistically extended survival with no toxic effects. Inhibition of other targets including MET, SRC, GFGR, and PI3K along with G12C also improved growth inhibition in mutant cell lines.24 Based on the preclinical studies of sotorasib, which demonstrated that the immune system must be intact to obtain the benefits of sotorasib, combination therapy with immune checkpoint therapy such as PD-1 antibodies may also improve efficacy compared to monotherapy alone.
Based on these results, we may use what we learn for the development of other KRAS mutations such as G13C, which is less common although still accounts for 17% of KRAS mutation NSCLC patients and 56% in never smokers. On a grander scale, what we learn may guide us in perfecting therapies in KRAS positive colorectal cancers and other types of cancers beyond NSCLC.
Disclosure
Dr Misako Nagasaka reports personal fees from AstraZeneca, Caris Life Sciences, Daiichi-Sankyo, Takeda, Novartis, EMD Serono, Blueprint Medicine and grants from Tempus, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Cox AD, Der CJ. Ras history: the saga continues. Small GTPases. 2010;1(1):2–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14(18):5731–5734. doi: 10.1158/1078-0432.CCR-08-0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk AT, Yazbeck N, Thon L, et al. Impact of Kras mutant subtypes on PD-L1 expression in lung adenocarcinoma. Ann Oncol. 2016;27(6):vi28. doi: 10.1093/annonc/mdw363.40 [DOI] [Google Scholar]
- 4.Wood K, Hensing T, Malik R, Salgia R. Prognostic and predictive value in KRAS in non-small-cell lung cancer a review. JAMA Oncol. 2016;2(6):805–812. doi: 10.1001/jamaoncol.2016.0405 [DOI] [PubMed] [Google Scholar]
- 5.Yu HA, Sima CS, Shen R, et al. Comparison of the characteristics and clinical course of 677 patients with metastatic lung cancers with mutations in KRAS codons 12 and 13. J Clin Oncol. 2013;31(15):8025. doi: 10.1200/jco.2013.31.15_suppl.8025 [DOI] [Google Scholar]
- 6.Shepherd FA, Domerg C, Hainaut P, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31(17):2173–2181. doi: 10.1200/JCO.2012.48.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulding RE, Chenoweth M, Carter GC, et al. KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: a systematic literature review and meta-analysis. Cancer Treat Res Commun. 2020;24:100200. doi: 10.1016/j.ctarc.2020.100200 [DOI] [PubMed] [Google Scholar]
- 8.Garon EB, Ciuleanu T, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 9.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23:1362–1368. doi: 10.1038/nm.4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanman BA, Allen JR, Allen GJ, et al. Discovery of a covalent inhibitor of KRASG12C (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63:52–65. doi: 10.1021/acs.jmedchem.9b01180 [DOI] [PubMed] [Google Scholar]
- 12.Center for Drug Evaluation and Research Application Number 214665Orig1s000. Drug approval package: Lumakras; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214665Orig1s000TOC.cfm. Accessed August 16, 2021.
- 13.Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumor immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]
- 14.Werner JA, Davies R, Wahlstrom J, et al. Mercapturate pathway metabolites of sotorasib, a covalent inhibitor of KRASG12C, are associated with renal toxicity in the Sprague Dawley rat. Toxicol Appl Pharmacol. 2021;423:115578. doi: 10.1016/j.taap.2021.115578 [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. NEJM. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi H, Okamoto I, Taguri M, Morita S, Nakagawa K. Postprogression survival in patients with advanced non-small-cell lung cancer who receive second-line or third-line chemotherapy. Clin Lung Cancer. 2013;14(3):261–266. doi: 10.1016/j.cllc.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. NEJM. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awad MM, Liu S, Rybkin II, et al. Acquired resistance to KRASG12C inhibition in cancer. NEJM. 2021;384:2382–2393. doi: 10.1056/NEJMoa2105281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Lin JJ, Li C, et al. Clinical acquired resistance to KRASG12C inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Res Briefs. 2021. doi: 10.1158/2159-8290.CD-21-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga T, Suda K, Fujino T, et al. KRAS secondary mutations that confer acquired resistance to KRAS G12C inhibitors, sotorasib and adagrasib, and overcoming strategies: insights from in vitro experiments. J Thorac Oncol. 2021;16(8):1321–1332. doi: 10.1016/j.jtho.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Chu QS. Targeting non-small cell lung cancer: driver mutation beyond epidermal growth factor mutation and anaplastic lymphoma kinase fusion. Ther Adv Med Oncol. 2020;12:175883591989575. doi: 10.1177/1758835919895756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunnett-Kane V, Nicola P, Blackhall F, Lindsay C. Mechanisms of resistance to KRASG12C inhibitors. Cancers. 2021;13(1):151. doi: 10.3390/cancers13010151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedele C, Li S, Teng KW, et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J Exp Med. 2021;218(1). doi: 10.1084/jem.20201414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misale S, Fatherree JP, Cortez E, et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin Cancer Res. 2019;25(2):796–807. doi: 10.1158/1078-0432.CCR-18-0368 [DOI] [PubMed] [Google Scholar]