Abstract
Background:
The number of human influenza A (H7N9) infections has escalated since 2013 with high resultant mortality. We conducted a phase II, randomized, partially-blinded trial to evaluate the safety and immunogenicity of an MF59-adjuvanted inactivated, split virion, H7N9 influenza vaccine (H7N9 IIV) administered at various dose levels and schedules in older adults.
Methods:
479 adults ≥65 years of age in stable health were randomized to one of six groups to receive either 3.75, 7.5 or 15 μg of influenza A/Shanghai/02/2013 (H7N9) IIV adjuvanted with MF59 given as a 3-dose series either on days 1, 28 and 168 or on days 1, 57 and 168. Immunogenicity was assessed using both hemagglutination inhibition (HAI) and microneutralization (MN) assays prior to and 28 days following each dose. Safety was assessed through 1 year following the last dose.
Results:
Subjects in all groups had only modest immune responses, with the HAI GMT <20 after the second vaccine dose and <29 after the third vaccine dose. HAI titers ≥40 were seen in <37% of subjects after the second dose and <49% after the third dose. There were no significant differences seen between the two dose schedules. MN titers followed similar patterns, although the titers were approximately two-fold higher than the HAI titers. Logistic regression modeling demonstrated no statistically significant associations between the immune responses and age, sex or body mass index whereas recent prior receipt of seasonal influenza vaccine significantly reduced the HAI response [OR 0.13 (95% CI 0.05, 0.33); p<0.001]. Overall, the vaccine was well tolerated. Two mild potentially immune mediated adverse events occurred, lichen planus and guttate psoriasis.
Conclusions:
MF59-adjuvanted H7N9 IIV was only modestly immunogenic in the older adult population following three doses. There were no significant differences in antibody responses noted among the various antigen doses or the two dose schedules.
In 2013, health officials in China documented human infections with a novel avian influenza A (H7N9) strain [1]. Since that time, annual influenza A (H7N9) virus epidemics have been reported from China, with the fifth wave (Oct 2016-Sept 2017) demonstrating the largest number of human infections compared with prior and subsequent waves. One thousand five hundred and sixty eight laboratory confirmed cases of human infection with influenza A ( H7N9) were reported through October 2017, with a 39% mortality rate [2]. During these outbreaks, two divergent lineages of H7N9 viruses have emerged: the Pearl River Delta and the Yangtze River Delta lineages. Using the CDC Influenza Risk Assessment Tool, developed to assess potential pandemic risk for emerging influenza viruses, the A(H7N9) viruses A/Shanghai/02/2013 and A/Hong Kong/125/2017have the highest risk scores among the 14 novel influenza strains evaluated to date. These viruses are characterized as posing a moderate to high potential pandemic risk [3].
Laboratory studies for the H5N1 strain of avian influenza showed that mutations of only 2 or 3 amino acids within the receptor-binding pocket of the HA molecule enhanced the affinity of avian viruses for binding to the mammalian type receptor [4]. Surveillance for H7N9 mutations identified strains that contain similar HA amino acid mutations [5, 6]. Furthermore, studies in ferrets have shown that strains of H7N9 have acquired the potential to spread by respiratory droplet transmission [7, 8]. The fact that the fifth epidemic of H7N9 influenza demonstrated the largest number of human cases increases the need to understand the potential efficacy of H7N9 influenza vaccines in various populations.
One key strategy for influenza pandemic preparedness depends on creating stockpiles of appropriate influenza vaccines and, in some cases, adjuvants that can be used for point-of-care mixing. These stockpiles allow for rapid deployment as cases escalate. Unadjuvanted H7N9 IIV candidates have shown poor immunogenicity even in young healthy populations, though the addition of the oil-in-water adjuvants MF59 and AS03 improved immunogenicity. Mulligan et al. showed that 62% of individuals ages 19–64 who received two doses of 3.75 ug of influenza A (H7N9) hemagglutinin (HA) mixed with MF59 administered three weeks apart seroconverted 4 weeks after their second dose, while doses of HA protein higher than 3.75 ug of HA showed no improvement (5). A second study evaluated healthy adults ages 19–64, given two 15 μg doses of H7N9 hemagglutination inhibition (HAI) administered three weeks apart with either MF59 or AS03 (6). Fifty seven percent of subjects in the MF59 group achieved seroprotective antibody levels (HAI titer ≥ 40 ) whereas the addition of AS03 led to 84% of recipients who achieved seroprotection [9].
Advanced age has been associated with a higher risk of infleunza A/H7N9 mortality [10] and in general theelderly typically show lower immune responses to seasonal influenza vaccines. A number of strategies have been used to enhance immune responses including use ofhigher HA doses [11–14] and the addition of potent adjuvants [15]. In previous unadjuvanted avian H5N1 influenza virus vaccine studies longer intervals between the first and second dose of vaccine impacted the peak immune response [16] and alate third dose of unadjuvanted H5N1 vaccine enhanced the microneutralization (MN) GMT [17]. Longer intervals between prime and boost doses of an AS03-adjuvanted H5N1 vaccine also impacted the cross reactive immune responses to heterologous influenza A strains [18].
In this study, elderly individuals were evaluated to assess the antigen dose-response and effect of extending the dose interval between the first and second doses of an MF59-adjuvanted A (H7N9) influenza IIV. In addition, a late third dose of vaccine was administered to determine whether additional doses improved the GMT of HAI and MN antibodies.
Methods
Study Design and Participants
This was a Phase II, randomized, partially-blinded, controlled trial conducted in healthy adults ages ≥65 at 10 centers in the United States from October 2014 – July 2016. Participants were allocated to one of six study groups (Fig 1) to evaluate the safety, reactogenicity and immunogenicity of 3 injections of an influenza A/Shanghai/02/2013 (H7N9) split virion, IIV given with MF59. Eligible participants were in stable health; controlled chronic illnesses were not exclusionary though individuals with autoimmune disorders were excluded. The study is registered at www.ClinicalTrials.govhttp://www.clinicaltrials.gov/(NCT02213354) where the full eligibility criteria are outlined.
Figure 1:
CONSORT Diagram
Individuals were assigned randomly with equal probability to 1 of 6 dose groups that evaluated three different HA antigen doses (3.75, 7.5 or 15 μg) and two different vaccination schedules (Days 1, 29 and 169 or Days 1, 57 and 169). Randomization was performed using a computerized program, stratified by site and with random block sizes of 6 or 12 within each site. The randomization table was generated with SAS version 9.3. The study dose assignment was known only by the study pharmacist and the vaccine administrator; however, the vaccine visit schedule was not masked to participants or staff.
Individuals maintained a Memory Aid for 8 days following each dose of study vaccine and telephone visits were conducted approximately 3 and 9 days after each vaccination to review the Memory Aid, evaluate for adverse events (AEs) and assess changes to concomitant medications. Participants had serum collected for immunological evaluations before each vaccine dose and 28 days after each vaccination. Dose groups 1, 3 and 5 also had serum collected on day 85. Telephone visits were performed to evaluate for new onset chronic medical conditions and serious adverse events (SAEs) on days 171, 197, 349 and 534.
The study protocol and informed consent were approved by the National Institute of Allergy and Infectious Diseases (NIAID) Division of Microbiology and Infectious Diseases, and each local institutional review board. Participants provided written informed consent prior to any study activities. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
Study Vaccine
The formulation contained 3.75, 7.5 or 15 μg HA of the monovalent, inactivated, split influenza virus preservative-free preparation of the influenza A/Shanghai/02/2013 (H7N9) strain (H7N9 IIV; a Pearl River Delta lineage strain) (manufactured by Sanofi Pasteur using a manufacturing process similar to the manufacturing process used to produce the licensed Influenza Virus Vaccine Fluzone family of products), mixed with 0.25 mL MF59C.1 adjuvant (manufactured by Novartis Vaccines and Diagnostics). MF59C.1 is an oil-in-water emulsion composed of squalene stabilized by the addition of polysorbate 80 and sorbitan trioleate.
Study Objectives
Co-primary objectives were the descriptive assessment of the HAI antibody responses following the second study vaccination, and SAEs and solicited reactogenicity events experienced in each dose group. Secondary objectives assessed the HAI titers following the third study vaccination, MN antibody responses following the second and third vaccinations, and the evaluation of unsolicited non-serious AEs as well as new onset chronic medical conditions. Exploratory objectives were included to assess the effects of age, sex, body mass index and prior receipt of seasonal influenza vaccine on HAI antibody responses and to assess the durability of HAI titers.
Immunogenicity assessments
HAI and MN antibody titers were measured at Southern Research, (Birmingham, Alabama) using previously described methods [19]. Serum samples were tested in duplicate against the homologous influenza A/Shanghai/02/2013 (H7N9) strain. The GMT of replicate results was used for analysis. The initial dilution was defined as 1:10 per US Food and Drug Administration recommendations; serum samples without detectable HAI or MN activity were assigned a titer of 5.
Statistics
Sample size. The study was designed as a descriptive study with a planned enrollment of at least 360 subjects (60 subjects per group) with the allowance for additional enrollment up to 600 subjects. The study was not designed to test any specific hypothesis. This minimum group size allowed for 80% power to detect a 20% difference in seroconversion rates between two groups. Solicited AEs and reactogenicity events were summarized by severity for each day after each study vaccination and as the maximum severity over all 8 days. SAEs and new onset of chronic medical conditions were recorded for the duration of the study period.
Immunologic assessment
The immunological endpoints for the primary and secondary objectives included the proportion of participants who had a HAI or MN titer of ≥40 and the proportion of participants who met the definition of seroconversion (4 fold or greater increase in HAI titer from a baseline titer of ≥10 or a titer after vaccination of ≥40 if the baseline titer was <10) 28 days after each vaccination.
Differences between study groups were evaluated using a X2 test to compare the percentage of participants with a titer of 40 or higher and a non-parametric Kruskal-Wallis test for comparisons of titer magnitude. Statistical significance was considered at a level of α=0.05 and all tests were 2-sided. Analysis was performed using SAS (SAS Institute), version 9.3. Analyses were not adjusted for multiple comparisons and missing data were minimal so imputation was not performed.
A logistic regression model was fitted to evaluate the association between covariates including study group, age (65–74, 75–84 and 85 and over), sex, body mass index (<30 and ≥ 30), HA antigen dosage (3.75, 7 or 15 ug), dosing interval (second dose at day 29 or 57) and receipt of seasonal influenza vaccine in the two years prior to enrollment (no receipt in either the 2012–2013 or the 2013–2014 vaccine season vs prior receipt of either vaccine) with the HAI titer ≥40 28 days after the second vaccination. A forward stepwise selection algorithm was used to determine which of the covariates to include in the final model. At each step, independent variables were considered for addition or removal from the model at a significant level of α=.10. Model fit was assessed using residual X2 and Hosmer-Lemeshow tests [20]. A second logistic regression model was fit controlling for age, sex and prior receipt of seasonal influenza vaccine. The intention to treat (ITT) analysis subset included participants who received at least 1 dose of study vaccine and had valid HAI results prior to vaccination and for at least 1 visit after vaccination. The per protocol (PP) analysis subset included all participants in the ITT subset except those who did not receive all three doses of study vaccine within the specified window or who had major protocol deviations. Results of analyses of the two subsets were similar and only the ITT subset analyses are presented.
Safety Analyses
Safety data including SAEs, unsolicited AEs and solicited AEs were summarized. Solicited AEs were summarized for the 8 days following each vaccination, by severity for each day after study vaccination and the maximum severity over 8 days and by the proportion of subjects reporting each symptom.
Results
A total of 479 subjects were enrolled between Oct 31, 2014-January 30, 2015 and 477 received the first vaccination, 428 (89%) received the second and 379 (79%) received the third vaccination (CONSORT Figure 1). The mean age was 72.2 years (age range 65–89 years) and was similar across treatment groups. Fifty-four percent of enrolled subjects were male; 92% were white, 4% Black, 2% Asian; 68% had a body mass index (BMI) less than 30. The majority of subjects (94%) had received an influenza vaccine during at least one of the previous two influenza seasons (2013/14 or 2014/15) and 86% of subjects had received influenza vaccines during both previous influenza seasons (Supplemental Table 1).
Immune Response
Nearly all subjects had an HAI titer <40 pre-vaccination. HAI GMTs 28 days after the second dose showed only modest immune responses, with the HAI GMT <20 for all groups (Table 1). Given the modest changes in these groups, logistic regression modelling was used to evaluate statistical significance. When logistic regression models for HAI seroconversion were evaluated comparing HA antigen dosage using 3.75 ug as the reference, there were no statistically significant changes seen with increasing dose (p=0.726 for 7.5 ug and p=0.069 for the 15 ug dose). There were only modest increases in HAI titers following the third vaccination with larger differences seen with increasing antigen dosage.
Table 1.
Hemagglutinin Inhibition Antibody Against A/H7N9
| Group 1 3.75mcg+MF59 (D1-D29-D169) | Group 2 3.75mcg+MF59 (D1-D57-D169) | Group 3 7.5mcg+MF59 (D1-D29-D169) | Group 4 7.5mcg+MF59 (D1-D57-D169) | Group 5 15mcg+MF59 (D1-D29-D169) | Group 6 15mcg+MF59 (D1-D57-D169) | |
|---|---|---|---|---|---|---|
| Day 1 (Pre Vac 1, All Subjects) | ||||||
| n | 74 | 72 | 81 | 70 | 71 | 74 |
| GMT (95% CI) | 5.3 (5.1 – 5.4) | 5.7 (5.2 – 6.2) | 5.3 (5.1 – 5.5) | 5.6 (5.3 – 5.9) | 5.6 (5.1 – 6.1) | 5.8 (5.3 – 6.4) |
| Titer≥1:40 - %(95% CI) | 0.0 (0.0–4.9) | 1.4 (0.0–7.5) | 0.0 (0.0–4.5) | 0.0 (0.0–5.1) | 1.4 (0.0–7.6) | 1.4 (0.0–7.3) |
| Day 57 (28 Days Post Vac 2, Groups 1,3,5) a | ||||||
| n | 73 | - | 80 | - | 70 | - |
| GMT (95% CI) | 12.1 (9.1 – 16.2) | - | 11.5 (9.2 – 14.4) | - | 14.2 (10.6 – 19.1) | - |
| Titer≥1:40 - %(95% CI) | 20.5 (12.0–31.6) | - | 20.0 (11.9–30.4) | - | 22.9 (13.7–34.4) | - |
| Seroconversion - %(95% CI) | 20.5 (12.0–31.6) | - | 20.0 (11.9–30.4) | - | 21.4 (12.5–32.9) | - |
| Day 85 (56 Days Post Vac 2, Groups 1,3,5; 28 days Post Vac 2, Groups 2,4,6) | ||||||
| n | 73 | 72 | 81 | 69 | 71 | 74 |
| GMT (95% CI) | 10.3 (8.0 – 13.3) | 13.3 (10.2 – 17.5) | 9.7 (7.9 – 11.9) | 12.3 (9.2 – 16.3) | 12.0 (9.4 – 15.3) | 19.3 (14.0 – 26.6) |
| Titer≥1:40 - %(95% CI) | 16.4 (8.8–27.0) | 20.8 (12.2–32.0) | 16.0 (8.8–25.9) | 18.8 (10.4–30.1) | 19.7 (11.2–30.9) | 36.5 (25.6–48.5) |
| Seroconversion - %(95% CI) | 16.4 (8.8–27.0) | 19.4 (11.1–30.5) | 16.0 (8.8–25.9) | 18.8 (10.4–30.1) | 18.3 (10.1–29.3) | 33.8 (23.2–45.7) |
| Day 169 (Pre Vac 3, All Subjects) | ||||||
| n | 69 | 67 | 72 | 63 | 67 | 69 |
| GMT (95% CI) | 7.0 (6.0 – 8.1) | 8.1 (6.9 – 9.6) | 6.9 (6.1 – 7.8) | 8.3 (6.6 – 10.5) | 8.5 (7.1 – 10.1) | 10.8 (8.5 – 13.6) |
| Titer≥1:40 - %(95% CI) | 4.3 (0.9–12.2) | 4.5 (0.9–12.5) | 4.2 (0.9–11.7) | 9.5 (3.6–19.6) | 7.5 (2.5–16.6) | 17.4 (9.3–28.4) |
| Seroconversion - %(95% CI) | 4.3 (0.9–12.2) | 3.0 (0.4–10.4) | 4.2 (0.9–11.7) | 9.5 (3.6–19.6) | 6.0 (1.7–14.6) | 14.5 (7.2–25.0) |
| Day 197 (28 Days Post Vac 3, All Subjects) | ||||||
| n | 66 | 66 | 65 | 59 | 61 | 66 |
| GMT (95% CI) | 18.9 (13.3 – 26.8) | 18.6 (14.1 – 24.5) | 24.6 (18.0 – 33.7) | 20.0 (14.4 – 27.9) | 27.2 (19.4 – 38.2) | 28.6 (20.6 – 39.6) |
| Titer≥1:40 - %(95% CI) | 37.9 (26.2–50.7) | 33.3 (22.2–46.0) | 44.6 (32.3–57.5) | 35.6 (23.6–49.1) | 47.5 (34.6–60.7) | 48.5 (36.0–61.1) |
| Seroconversion - %(95% CI) | 37.9 (26.2–50.7) | 31.8 (20.9–44.4) | 44.6 (32.3–57.5) | 35.6 (23.6–49.1) | 45.9 (33.1–59.2) | 45.5 (33.1–58.2) |
n = number of subjects in the intention-to-treat population with available results; GMT = Geometric mean titer
Serum samples for antibody testing were not collected from subjects in Groups 2,4,6 at Day 57.
When comparing the two dose schedules, there were no consistent trends except Group 6 (15 μg on Days 1, 57, 169) showed a slight increase in immune response (37% achieved seroprotection following the second dose) compared to Group 5 (15 μg on Days 1, 28, 169) which achieved seroprotection in only 20%. These changes were statistically insignificant (p=0.382).
Durability of HAI titers was evaluated using geometric mean fold ratio (GMFR) of HAI antibody titers from 28 days post-second vaccination (Day 57 for groups 1, 3 and 5 or Day 85 for groups 2, 4 and 6) to just prior to the third vaccination (Day 169). GMFR ranged from 0.50 to 0.65. All groups showed GMTs of less than 11 and only 4 to 17% of subjects had HAI titers of ≥40. MN antibody titers followed similar trends for all dose groups and interval schedules, although the GMTs were higher than those seen with HAI assays with MN GMT ranging between 21–39 approximately 4 weeks after dose 2 and HAI GMT ranging between 12–19 at the same time point (Table 2 and Figure 2b). Scatter plots of HAI response against MN response by study day and treatment group demonstrated good correlation between the two assays for 28 days post-dose 2 and 3 time points (r=0.74; p<.001 and r=0.80; p<.001, respectively) (Supplemental Figure 1).
Table 2.
Neutralizing Antibody Against A/H7N9
| Group 1 3.75mcg+MF59 (D1-D29-D169) | Group 2 3.75mcg+MF59 (D1-D57-D169) | Group 3 7.5mcg+MF59 (D1-D29D169) | Group 4 7.5mcg+MF59 (D1-D57D169) | Group 5 15mcg+MF59 (D1-D29D169) | Group 6 15mcg+MF59 (D1-D57D169) | |
|---|---|---|---|---|---|---|
| Day 1 (Pre Vac 1, All Subjects) | ||||||
| n | 74 | 72 | 81 | 70 | 71 | 74 |
| GMT (95% CI) | 6.3 (5.7 – 6.8) | 6.7 (6.0 – 7.4) | 7.0 (6.4 – 7.7) | 6.0 (5.6 – 6.5) | 6.6 (6.0 – 7.2) | 6.8 (6.0 – 7.7) |
| Titer≥1:40 - %(95% CI) | 0.0 (0.0–4.9) | 0.0 (0.0–5.0) | 0.0 (0.0–4.5) | 0.0 (0.0–5.1) | 0.0 (0.0–5.1) | 1.4 (0.0–7.3) |
| Day 57 (28 Days Post Vac 2, Groups 1,3,5) a | ||||||
| n | 73 | - | 80 | - | 70 | - |
| GMT (95% CI) | 26.6 (20.1 – 35.2) | - | 20.7 (16.6 – 25.8) | - | 21.9 (17.1 – 28.0) | - |
| Titer≥1:40 - %(95% CI) | 46.6 (34.8–58.6) | - | 25.0 (16.0–35.9) | - | 25.7 (16.0–37.6) | - |
| Seroconversion - %(95% CI) | 42.5 (31.0–54.6) | - | 22.5 (13.9–33.2) | - | 25.7 (16.0–37.6) | - |
| Day 85 (56 Days Post Vac 2, Groups 1,3,5; 28 days Post Vac 2, Groups 2,4,6) | ||||||
| n | 73 | 72 | 81 | 70 | 71 | 74 |
| GMT (95% CI) | 21.3 (16.7 – 27.1) | 33.3 (25.1 – 44.2) | 18.5 (15.3 – 22.3) | 23.7 (18.0 – 31.1) | 19.3 (15.4 – 24.2) | 38.5 (29.2 – 50.8) |
| Titer≥1:40 - %(95% CI) | 37.0 (26.0–49.1) | 50.0 (38.0–62.0) | 23.5 (14.8–34.2) | 34.3 (23.3–46.6) | 25.4 (15.8–37.1) | 55.4 (43.4–67.0) |
| Seroconversion - %(95% CI) | 31.5 (21.1–43.4) | 47.2 (35.3–59.3) | 21.0 (12.7–31.5) | 34.3 (23.3–46.6) | 25.4 (15.8–37.1) | 47.3 (35.6–59.3) |
| Day 169 (Pre Vac 3, All Subjects) | ||||||
| n | 69 | 67 | 72 | 63 | 67 | 69 |
| GMT (95% CI) | 14.7 (12.2 – 17.8) | 18.9 (15.4 – 23.2) | 15.2 (12.6 – 18.4) | 14.1 (11.1 – 17.8) | 14.1 (11.6 – 17.3) | 22.0 (17.3 – 28.0) |
| Titer≥1:40 - %(95% CI) | 15.9 (8.2–26.7) | 19.4 (10.8–30.9) | 18.1 (10.0–28.9) | 14.3 (6.7–25.4) | 16.4 (8.5–27.5) | 36.2 (25.0–48.7) |
| Seroconversion - %(95% CI) | 13.0 (6.1–23.3) | 19.4 (10.8–30.9) | 15.3 (7.9–25.7) | 14.3 (6.7–25.4) | 16.4 (8.5–27.5) | 31.9 (21.2–44.2) |
| Day 197 (28 Days Post Vac 3, All Subjects) | ||||||
| n | 66 | 66 | 65 | 59 | 61 | 66 |
| GMT (95% CI) | 59.0 (41.8 – 83.3) | 49.3 (37.4 – 65.1) | 57.5 (42.1 – 78.6) | 45.5 (33.7 – 61.4) | 44.6 (32.6 – 60.9) | 70.5 (51.7 – 96.1) |
| Titer≥1:40 - %(95% CI) | 60.6 (47.8–72.4) | 65.2 (52.4–76.5) | 69.2 (56.6–80.1) | 61.0 (47.4–73.5) | 60.7 (47.3–72.9) | 78.8 (67.0–87.9) |
| Seroconversion - %(95% CI) | 59.1 (46.3–71.0) | 62.1 (49.3–73.8) | 64.6 (51.8–76.1) | 61.0 (47.4–73.5) | 59.0 (45.7–71.4) | 71.2 (58.7–81.7) |
n = number of subjects in the intention-to-treat population with available results; GMT = Geometric mean titer
Serum samples for antibody testing were not collected from subjects in Groups 2,4,6 at Day 57.
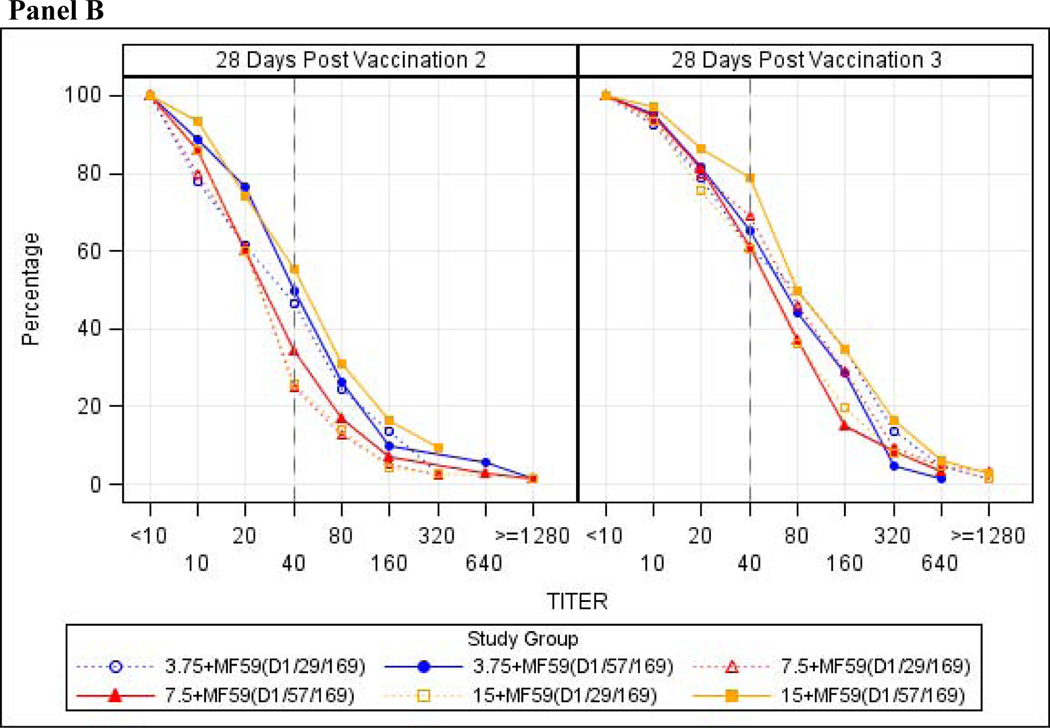
Figure 2:
Reverse Cumulative Distribution of Hemagglutination Inhibition and Neutralization Antibody Titers Against influenza A/H7N9 antigen. Reverse Cumulative Distribution of Hemagglutination Inhibition Antibody Titers (Panel A) and Neutralization Antibody Titers (Panel B) against the A/H7N9 influenza vaccine strain examining the intent to treat population
Analysis of age cohorts, sex, BMI and prior receipt of seasonal influenza vaccine
As mentioned above, there were no statistically significant relationships between HA antigen dosage and dosing interval with seroconversion. A second logistic regression model was fit controlling additionally for age (65–74, ≥75), sex, BMI, and prior receipt of seasonal influenza vaccine (none in the past two seasons vs receipt in at least one of the past two seasons). The vast majority of subjects in this study received seasonal influenza vaccines in one or both of the two prior years (94%). However, despite the fact that the study had few subjects who were unvaccinated in prior seasonal influenza vaccines, the impact of prior receipt of seasonal influenza vaccine was strong enough to show a statistically significant reduction in seroconversion rate [OR 0.13 (95% CI 0.05, 0.33); p<0.001].
Safety and reactogenicity
Solicited systemic and injection site reactions were common with 37–42% of subjects in each group reporting any solicited systemic symptoms and 63%−70% of subjects in each group reporting any injection site event (Table 3). Of the 477 subjects who received at least one dose of vaccine, only 1 subject in group 3 experienced severe solicited systemic events; this person reported headache, feverishness, fatigue, malaise and myalgia. All other systemic reactogenicity events were mild to moderate with the most commonly reported events being headache and fatigue (data not shown). Fourteen subjects experienced one or more severe injection site reactions following any dose of vaccine. These individuals reported one or more signs of bruising, erythema or induration. Mild injection site reactogenicity events occurred in 53–61% of subjects in each dose group after any study vaccination; 4–9% of subjects reported moderate grade local events (data not shown). There were no significant differences in the frequency or nature of solicited systemic or injection site events between antigen dose levels, the two dosing schedules or following repeated vaccination. Overall, 50% of subjects reported at least one unsolicited, non-serious AE, 7% (n=33) of which were considered related to the study product. The most common classification of unsolicited AEs included infections and infestations, musculoskeletal, respiratory and gastrointestinal disorders. There were no trends seen that suggested higher reactogenicity rates in any particular dose group (data not shown). A total of 75 SAEs were reported in this elderly population, but none were deemed related to study product. Cardiovascular events, infections, fractures or joint replacements and cerebrovascular events were among the more common events. Only two events occurred within the 8 days following any vaccination (chest pain with coronary artery disease 2 days after vaccination and myocardial infarction 3 days after vaccination).
Table 3.
Number and Percentage of Subjects Experiencing Any Solicited Events Following Any Vaccination
| Post Any Vaccination | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 3.75mcg+MF59 (D1-D29-D169) (N=79) | Group 2 3.75mcg+MF59 (D1-D57-D169) (N=79) | Group 3 7.5mcg+MF59 (D1-D29-D169) (N=82) | ||||||||||
| Symptom | N* | n | % | 95% CI | N* | n | % | 95% CI | N* | n | % | 95% CI |
| Any Symptom | 79 | 59 | 75 | 63.6, 83.8 | 79 | 60 | 76 | 65.0, 84.9 | 82 | 65 | 79 | 68.9, 87.4 |
| Any Systemic Symptom | 79 | 33 | 42 | 30.8, 53.4 | 79 | 34 | 43 | 31.9, 54.7 | 82 | 30 | 37 | 26.2, 48.0 |
| Elevated Oral Temperature | 79 | 3 | 4 | 0.8, 10.7 | 79 | 4 | 5 | 1.4, 12.5 | 82 | 3 | 4 | 0.8, 10.3 |
| Feverishness | 79 | 10 | 13 | 6.2, 22.0 | 79 | 6 | 8 | 2.8, 15.8 | 82 | 8 | 10 | 4.3, 18.3 |
| Fatigue | 79 | 20 | 25 | 16.2, 36.4 | 79 | 17 | 22 | 13.1, 32.2 | 82 | 16 | 20 | 11.6, 29.7 |
| Malaise | 79 | 8 | 10 | 4.5, 19.0 | 79 | 11 | 14 | 7.2, 23.5 | 82 | 18 | 22 | 13.6, 32.5 |
| Myalgia | 79 | 16 | 20 | 12.0, 30.8 | 79 | 21 | 27 | 17.3, 37.7 | 82 | 14 | 17 | 9.7, 27.0 |
| Arthralgia | 79 | 8 | 10 | 4.5, 19.0 | 79 | 8 | 10 | 4.5, 19.0 | 82 | 6 | 7 | 2.7, 15.2 |
| Headache | 79 | 12 | 15 | 8.1, 25.0 | 79 | 17 | 22 | 13.1, 32.2 | 82 | 14 | 17 | 9.7, 27.0 |
| Nausea | 79 | 3 | 4 | 0.8, 10.7 | 79 | 3 | 4 | 0.8, 10.7 | 82 | 6 | 7 | 2.7, 15.2 |
| Any Local Symptom | 79 | 52 | 66 | 54.3, 76.1 | 79 | 51 | 65 | 53.0, 75.0 | 82 | 57 | 70 | 58.4, 79.2 |
| Pain | 79 | 18 | 23 | 14.1, 33.6 | 79 | 30 | 38 | 27.3, 49.6 | 82 | 24 | 29 | 19.7, 40.4 |
| Tenderness | 79 | 42 | 53 | 41.6, 64.5 | 79 | 42 | 53 | 41.6, 64.5 | 82 | 49 | 60 | 48.3, 70.4 |
| Itching/Pruritus | 79 | 4 | 5 | 1.4, 12.5 | 79 | 3 | 4 | 0.8, 10.7 | 82 | 1 | 1 | 0.0, 6.6 |
| Ecchymosis/Bruising (functional grade) | 79 | 7 | 9 | 3.6, 17.4 | 79 | 8 | 10 | 4.5, 19.0 | 82 | 10 | 12 | 6.0, 21.3 |
| Ecchymosis/Bruising (measurement grade) | 79 | 7 | 9 | 3.6, 17.4 | 79 | 8 | 10 | 4.5, 19.0 | 82 | 10 | 12 | 6.0, 21.3 |
| Erythema/Redness (functional grade) | 79 | 19 | 24 | 15.1, 35.0 | 79 | 15 | 19 | 11.0, 29.4 | 82 | 24 | 29 | 19.7, 40.4 |
| Erythema/Redness (measurement grade) | 79 | 19 | 24 | 15.1, 35.0 | 79 | 15 | 19 | 11.0, 29.4 | 82 | 24 | 29 | 19.7, 40.4 |
| Induration/Swelling (functional grade) | 79 | 11 | 14 | 7.2, 23.5 | 79 | 16 | 20 | 12.0, 30.8 | 82 | 14 | 17 | 9.7, 27.0 |
| Induration/Swelling (measurement grade) | 79 | 11 | 14 | 7.2, 23.5 | 79 | 16 | 20 | 12.0, 30.8 | 82 | 14 | 17 | 9.7, 27.0 |
| Post Any Vaccination | ||||||||||||
| Group 4 7.5mcg+MF59 (D1-D57-D169) (N=79) | Group 5 15mcg+MF59 (D1-D29-D169) (N=79) | Group 6 15mcg+MF59 (D1-D57-D169) (N=79) | ||||||||||
| Symptom | N* | n | % | 95% CI | N* | n | % | 95% CI | N* | n | % | 95% CI |
| Any Symptom | 79 | 59 | 75 | 63.6, 83.8 | 79 | 59 | 75 | 63.6, 83.8 | 79 | 63 | 80 | 69.2, 88.0 |
| Any Systemic Symptom | 79 | 33 | 42 | 30.8, 53.4 | 79 | 34 | 43 | 31.9, 54.7 | 79 | 29 | 37 | 26.1, 48.3 |
| Elevated Oral Temperature | 79 | 1 | 1 | 0.0, 6.9 | 79 | 2 | 3 | 0.3, 8.8 | 79 | 5 | 6 | 2.1, 14.2 |
| Feverishness | 79 | 4 | 5 | 1.4, 12.5 | 79 | 8 | 10 | 4.5, 19.0 | 79 | 8 | 10 | 4.5, 19.0 |
| Post Any Vaccination | ||||||||||||
| Group 1 3.75mcg+MF59 (D1-D29-D169) (N=79) | Group 2 3.75mcg+MF59 (D1-D57-D169) (N=79) | Group 3 7.5mcg+MF59 (D1-D29-D169) (N=82) | ||||||||||
| Symptom | N* | n | % | 95% CI | N* | n | % | 95% CI | N* | n | % | 95% CI |
| Fatigue | 79 | 18 | 23 | 14.1, 33.6 | 79 | 16 | 20 | 12.0, 30.8 | 79 | 23 | 29 | 19.4, 40.4 |
| Malaise | 79 | 11 | 14 | 7.2, 23.5 | 79 | 13 | 16 | 9.1, 26.5 | 79 | 13 | 16 | 9.1, 26.5 |
| Myalgia | 79 | 11 | 14 | 7.2, 23.5 | 79 | 7 | 9 | 3.6, 17.4 | 79 | 13 | 16 | 9.1, 26.5 |
| Arthralgia | 79 | 10 | 13 | 6.2, 22.0 | 79 | 2 | 3 | 0.3, 8.8 | 79 | 4 | 5 | 1.4, 12.5 |
| Headache | 79 | 17 | 22 | 13.1, 32.2 | 79 | 8 | 10 | 4.5, 19.0 | 79 | 14 | 18 | 10.0, 27.9 |
| Nausea | 79 | 3 | 4 | 0.8, 10.7 | 79 | 3 | 4 | 0.8, 10.7 | 79 | 2 | 3 | 0.3, 8.8 |
| Any Local Symptom | 79 | 54 | 68 | 56.9, 78.4 | 79 | 50 | 63 | 51.7, 73.9 | 79 | 55 | 70 | 58.2, 79.5 |
| Pain | 79 | 22 | 28 | 18.3, 39.1 | 79 | 21 | 27 | 17.3, 37.7 | 79 | 22 | 28 | 18.3, 39.1 |
| Tenderness | 79 | 46 | 58 | 46.6, 69.2 | 79 | 42 | 53 | 41.6, 64.5 | 79 | 48 | 61 | 49.1, 71.6 |
| Itching/Pruritus | 79 | 3 | 4 | 0.8, 10.7 | 79 | 4 | 5 | 1.4, 12.5 | 79 | 3 | 4 | 0.8, 10.7 |
| Ecchymosis/Bruising (functional grade) | 79 | 6 | 8 | 2.8, 15.8 | 79 | 10 | 13 | 6.2, 22.0 | 79 | 9 | 11 | 5.3, 20.5 |
| Ecchymosis/Bruising (measurement grade) | 79 | 6 | 8 | 2.8, 15.8 | 79 | 10 | 13 | 6.2, 22.0 | 79 | 8 | 10 | 4.5, 19.0 |
| Erythema/Redness (functional grade) | 79 | 16 | 20 | 12.0, 30.8 | 79 | 17 | 22 | 13.1, 32.2 | 79 | 16 | 20 | 12.0, 30.8 |
| Erythema/Redness (measurement grade) | 79 | 16 | 20 | 12.0, 30.8 | 79 | 17 | 22 | 13.1, 32.2 | 79 | 16 | 20 | 12.0, 30.8 |
| Induration/Swelling (functional grade) | 79 | 15 | 19 | 11.0, 29.4 | 79 | 11 | 14 | 7.2, 23.5 | 79 | 17 | 22 | 13.1, 32.2 |
| Induration/Swelling (measurement grade) | 79 | 14 | 18 | 10.0, 27.9 | 79 | 11 | 14 | 7.2, 23.5 | 79 | 17 | 22 | 13.1, 32.2 |
N = Number of subjects in the Safety Analysis population. n = number of subjects reporting the specified symptom.
N* = Number of subjects with non-missing data for the specified symptom for at least one post-vaccination assessment; used as denominator for percentages and 95% CI calculations.
95% Confidence Intervals are from an exact binomial distribution (Clopper-Pearson).
one subject was randomized to Group 2, but followed the Group 1 schedule, so is counted under Group 1 for all safety and immunogenicity analysis populations
Two AEs of special interest were reported. One individual developed skin biopsy-proven lichen planus involving both lower extremities approximately two weeks after receipt of the second dose of vaccine. A second subject developed mild patches of dry skin on his face approximately 3–4 weeks following his first vaccination. The second vaccine was administered and approximately 3 weeks later red, scaly skin patches developed on both upper and lower extremities. A skin biopsy was consistent with guttate psoriasis vulgaris. Both were considered related to the study product due to timing of onset and absence of a previous compatible medical history.
Discussion
A number of different H7N9 vaccine candidates have been evaluated in Phase I and II studies [9, 21–25], but most of these have been evaluated only in young healthy adults. Of particular note are the studies from Mulligan et al. and Jackson et al. that evaluated adults 19–64 years of age using vaccine preparations that were identical to the MF59-adjuvanted H7N9 vaccine product that was used in this study [9, 23]. Overall, the study presented here showed immune responses in subjects ages 65 and older that were lower than those observed in the younger populations. The HAI GMTs 28 days post-vaccine dose 2 in the elderly ranged from 12 to 14 compared to 25 to 34 in the 19–64 year old populations. Similarly, seroprotection rates were higher in the younger populations.
In general, elderly individuals have less robust immune responses to many vaccines including influenza vaccines. The addition of adjuvants, increased antigen content and changes to the vaccine dose or dose schedule have been used to try to improve immune responses. The addition of MF59 to various influenza virus vaccines usually leads to higher rates of seroprotection compared to the rates achieved in this study [26–29]. Unfortunately, influenza A/H7N9 IIVs consistently appear to be less immunogenic than other vaccines targeting other influenza strains, including other avian viruses such as H5N1. MF59-adjuvanted H5N1 vaccines, like H7N9 vaccine, require a two-dose series to generate a significant immune response, but the GMT, seroconversion and seroprotection rates have been higher in both the younger and older populations when compared to MF59-adjuvanted H7N9 vaccine [9, 23, 26, 27]. Additionally, H5N1 vaccine have shown that a third dose of influenza vaccine can improve HAI and MN titers and/or increase the durability of MN titers [17, 30]. The study presented here did show that the addition of a third dose of vaccine improved the HAI and MN titers, but the GMT and seroprotection rates were still low. The reasons behind these differences in immunogenicity are not known.
One advantage of adjuvanted avian influenza vaccines has been the ability to reduce the antigen dose required to generate seroprotective levels of antibody. Mulligan et al. showed that 3.75 μg of HA protein when combined with MF59 generated a similar immune response compared to 7.5 and 15 μg of antigen combined with MF59 [23]. We evaluated various doses of the influenza A/H7N9 IIV, and no statistically significant differences in seroconversion rates were seen between the three doses of HA antigen after two doses of vaccine, but given the low immunogenicity in general the concept of dose sparing is of little consequence.
Our study, demonstrated that MN titers were approximately two times higher than HAI titers, a trend seen in other H7N9 influenza vaccine studies (9, 23). However, both HAI and MN assays suffer from lack of standardization and intra and inter lab variability ([31]). At this time, there is a general consensus that an HAI titer of 40 or greater correlates with a 50% reduction in the risk of contracting influenza whereas there is no clearly established correlate of protection for the MN titers. The more important concept is that the absolute concentration of antibody as demonstrated by either assay is less critical than the trends in seroconversion rates and peaks following various doses with or without adjuvant. The MN assay does have the advantage that it measures functional antibodies that can inhibit various life stages of productive influenza virus replication from binding of the influenza virus HA head to the cell surface receptor to HA stem antibodies that block membrane fusion and ultimately reduce viral egress from the cell into the cytoplasm. Better standardization of existing immunoassays is needed and new assays are being developed to help understand the roles of HA stem and neuraminidase antibodies play in protection ([32].
These findings suggest that other strategies or adjuvants will be needed to boost the seroprotection rates in the elderly. A number of influenza vaccine studies have evaluated the adjuvant AS03. Jackson et al evaluated young healthy volunteers who received H7N9 vaccine adjuvanted with either AS03 and MF59 and demonstrated that AS03 elicited higher HAI and MN titers for H7N9 vaccine in younger adults ([9]). Additionally, indirect comparison’s using meta-analysis have shown AS03 to have higher Geometric Mean Fold Ratios (GMFR) when compared to MF59 for influenza A(H1N1)pdm09 vaccines ([33]). Madan et al. evaluated AS03-adjuvanted H7N1 vaccines in adults 65 and older and demonstrated that two doses of 3.75 μg of HA was equivalent to 7.5 μg of HA [34]. Their study showed HAI and MN titers that were significantly higher than titers seen in the study presented here. The AS03 adjuvanted H7N1 vaccine generated HAI GMTs ranging from 46–75 and seroconversion rates ranged from 47–82% of subjects in the different study groups and showed cross-protection for heterologous H7N9 influenza strains. We did not evaluate whether recipients generated cross-reactive antibodies to other H7 strains of influenza or between the Yangtze River and Pearl River lineages of H7N9 influenza strains. In fact, there is relatively little information regarding the cross protection generated from any of the H7N9 vaccines or natural infection. Mouse studies have demonstrated that there is a four-fold lower antibody titer against heterologous group viruses than against homologous group viruses [35]. Similarly, MF59- and AS03-adjuvanted H5N1 vaccines have shown that the vaccines induced cross-reactive antibodies to antigenically drifted H5N1 strains of influenza virus, and that receipt of a third dose enhanced the generation of cross protective antibody [18, 36, 37]. Though the study presented here did save serum samples for future use, the low GMTs generated to the homologous influenza strain suggest that even if there is some cross protection, there will be even lower seroconversion rates seen against heterologous influenza strains. Both MF39 and AS03 are oil-in-water emulsions. MF59 is composed of squalene, polysorbate 80 and sorbitan trioleate. AS03 contains squalene, DL-α-tocopherol and polysorbate 80. The exact mechanism of the adjuvants MF59 and AS03 are not known. Both adjuvants induce enhance antigen uptake by dendritic cells and generate a local immunostimulatory environment (reviewed in [38]. However, DL-α-tocopherol on its own has been associated with increased uptake of antigen by monotcytes as well as increased expression of cytokines and chemokines associated with the innate immune system, which are important in antibody responses [39]. The immune responses for both adjuvants are complex and have many similarities, but it has been postulated that immunologic potencies of the compounds may reside in differences that are more quantitative than qualititative or related to different kinetics (Del Giudice, Rappuoli Seminars in Immunology).
Receipt of prior seasonal influenza vaccine was associated with reduced probability of seroconversion. This phenomenon has been seen in previous H5N1, H7N9, and seasonal influenza vaccine studies [40–42]. It has been suggested that previous vaccine antigens create memory clones that cross-recognize the antigens in subsequent vaccines. Given the cross-reactivity of some of the stem epitopes between H3 and H7 influenza strains, it is possible that the H7 vaccine stimulates preexisting H3 stem specific memory responses rather than a new response to the H7 HA head [43].
The MF59-adjuvanted H7N9 vaccine was well tolerated with most solicited injection site and systemic reactogenicity events being only mild to moderate in nature. The use of adjuvants has raised concerns over the potential enhancement or new onset of autoimmune diseases. In this study, two individuals developed skin disorders that have been associated with an autoimmune origin, lichen planus and guttate psoriasis. Both events developed 2–4 weeks after receiving vaccine and were considered related to study product. However, it is important to note that new onset psoriasis has been seen in individuals who received 2009 H1N1 influenza vaccines and most of the cases occurred in individuals who received unadjuvanted vaccines [44–46]. Gunes et al. characterized 43 patients who developed psoriasis or had exacerbations of psoriasis following unadjuvanted influenza vaccine in 2009 [44]. Similar to the two subjects presented in this study, most individuals developed symptoms approximately 2–3 weeks following vaccination. MF59-adjuvanted influenza vaccines have been licensed in various countries for over two decades and in the U.S since 2015. FLUAD™, an MF59-adjuvanted seasonal influenza vaccine has been licensed for adults 65 years of age and older. More than 65 million people have received MF59-adjuvanted influenza vaccines and a meta-analysis evaluating safety data from over 10,000 elderly individuals determined that injection site reactogenicity was more frequent with the adjuvanted vaccine, but the severity of signs and symptoms were mild to moderate in nature and short-lived [47]. Additionally, no differences in unsolicited AEs, SAEs or new onset of chronic medical conditions were identified (reviewed in [48]). Given the timing of the two cases seen in this study, the vaccine in this study may have been a triggering factor for the skin disorders. However, the unadjuvanted portions of the vaccine could have triggered the onset of these two autoimmune-mediated skin disorders or they may have occurred coincidentally and were unrelated to vaccination.
The elderly remains a vulnerable population for H7N9 infection, and epidemiological data associate older age with higher mortality [6, 49]. The results from this study do not demonstrate strong immunological responses to this MF59-adjuvanted H7N9 IIV in older adults. The study presented here did show some improvement in HAI and MN titers following a third dose, although this is not an ideal vaccine schedule for a pandemic situation and a third dose is relatively impractical. Fortunately, pandemic modeling has shown that vaccines that show even modest efficacy can impact the spread of pandemic strains of influenza [50, 51]. Additional studies are needed to understand the biological mechanisms that underlie the poor immunological responses seen with H7N9 vaccines, and new strategies are needed to identify more effective vaccines and vaccine strategies for this avian strain of influenza virus.
Supplementary Material
Acknowledgements
We are grateful to the 13-0034 Study Team Group who participated in the implementation of this study including Jack Stapleton, Dilek Ince from the University of Iowa; Nadine Rouphael, Jumi Yi, Andres Camacho-Gonzalez, Inci Yildirim and Larry Anderson from Emory University, Irene Graham and Edwin Anderson from Saint Louis University, Rebecca Brady from Cincinnati Children’s Hospital Medical Center, Clarence Buddy Creech at Vanderbilt University, Robert Atmar, Wendy Keitel and Shital Patel from Baylor College of Medicine, and Ashley Wegel and Karineh Tarpinian from Emmes. Additionally, we thank the Vaccine Treatment and Evaluation Unit study teams at each institution and the exceptional contributions provided by the scientific and professional staff from the National Institutes of Health, Division of Microbiology and Infectious Diseases. We are grateful for the manuscript review expertise provided by the following colleagues at BARDA: Corrina Pavetto, MS, Bai Yeh, MBA, Vittoria Cioce, PhD, Christine Oshansky, PhD, and James King, MD. These individuals were not specifically compensated for these contributions.
Funding
This work was funded by the National Institutes of Health, National Institute of Allergy and Infectious Diseases Contract Numbers Vaccine and Treatment Evaluation Unit Contracts HHSN2722013000201 (University of Iowa), HHSN27220080000C (Vanderbilt University), HHSN272200800006C (Cincinnati Children’s Hospital), HHSN272200800005C (Emory University), HHSN272201300019I (Kaiser), HHHSN272201300018I (Emory University), HHSN272200800002C and HHSN272201300015I (Baylor College of Medicine), Emory University (HHSN272200800005C, HHSN272200800001C (University of Maryland), HHSN272200800003C (Saint Louis University), 272201300171 (Duke University), HHSN272200800013C and HHSN272201500002C (Emmes), and the Institute for Clinical and Translational Science at the University of Iowa (UL1TR002537) andthe Clinical and Translational Science Award funds from NCATS at Vanderbilt University (2UL1TR000445), and the Atlanta Clinical and Translational Science Award from NCATS (UL1TR000454). The 2013 H7N9 vaccine and MF59 adjuvant were provided by the US Department of Health and Human Services (HHS) Biomedical Advanced Research and Development Authority (BARDA) from the National Prepandemic Influenza Vaccine Stockpile and were manufactured by Sanofi Pasteur and Seqirus, respectively.
Footnotes
Conflict of Interest
PLW, LAJ, KE, DIB, MJM, HES, RER, HH, SEF, KS have no conflicts of interest. WHC has consulted for Sequirus, FluGen and Pharmaron on influenza related projects.
All authors fulfill the criteria for authorship. No writing assistance was provided in the preparation of this manuscript. Each author has provided all relevant conflicts of interest as outlined in the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–97. [DOI] [PubMed] [Google Scholar]
- [2].WHO. Avian and other zoonotic influenza. 2018. [Google Scholar]
- [3].Centers for Disease Control and Prevention. 2016, November 3. [Google Scholar]
- [4].Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–10. [DOI] [PubMed] [Google Scholar]
- [5].Wang D, Yang L, Zhu W, Zhang Y, Zou S, Bo H, et al. Two Outbreak Sources of Influenza A (H7N9) Viruses Have Been Established in China. J Virol. 2016;90:5561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xiang N, Li X, Ren R, Wang D, Zhou S, Greene CM, et al. Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic - China, September 2015-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1390–4. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013;341:410–4. [DOI] [PubMed] [Google Scholar]
- [8].Zhu H, Wang D, Kelvin DJ, Li L, Zheng Z, Yoon SW, et al. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science. 2013;341:183–6. [DOI] [PubMed] [Google Scholar]
- [9].Jackson LA, Campbell JD, Frey SE, Edwards KM, Keitel WA, Kotloff KL, et al. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. Jama. 2015;314:237–46. [DOI] [PubMed] [Google Scholar]
- [10].Yang Y, Zhong H, Song T, He J, Guo L, Tan X, et al. Epidemiological and clinical characteristics of humans with avian influenza A (H7N9) infection in Guangdong, China, 2013–2017. Int J Infect Dis. 2017;65:148–55. [DOI] [PubMed] [Google Scholar]
- [11].Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. [DOI] [PubMed] [Google Scholar]
- [13].Palache AM, Beyer WE, Sprenger MJ, Masurel N, de Jonge S, Vardy A, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11:3–9. [DOI] [PubMed] [Google Scholar]
- [14].Ruben FL, Potter CW, Stuart-Harris CH. Humoral and secretory antibody responses to immunization with low and high dosage split influenza virus vaccine. Arch Virol. 1975;47:157–66. [DOI] [PubMed] [Google Scholar]
- [15].Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21:4234–7. [DOI] [PubMed] [Google Scholar]
- [16].Belshe RB, Frey SE, Graham I, Mulligan MJ, Edupuganti S, Jackson LA, et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008;197:580–3. [DOI] [PubMed] [Google Scholar]
- [18].Gillard P, Caplanusi A, Knuf M, Roman F, Walravens K, Moris P, et al. An assessment of prime-boost vaccination schedules with AS03A -adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults. Influenza Other Respir Viruses. 2013;7:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hosmer DW, Hosmer T. and Lemeshow S A Goodness-of-Fit Tests for the Multiple Logistic Regression Model. Communications in Statistics. 1980:1043–69. [Google Scholar]
- [21].Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Sci Transl Med. 2014;6:234ra55. [DOI] [PubMed] [Google Scholar]
- [22].Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med. 2013;369:2564–6. [DOI] [PubMed] [Google Scholar]
- [23].Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. 2014;312:1409–19. [DOI] [PubMed] [Google Scholar]
- [24].Rudenko L, Isakova-Sivak I, Naykhin A, Kiseleva I, Stukova M, Erofeeva M, et al. H7N9 live attenuated influenza vaccine in healthy adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2016;16:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu UI, Hsieh SM, Lee WS, Wang NC, Kung HC, Ou TY, et al. Safety and immunogenicity of an inactivated cell culture-derived H7N9 influenza vaccine in healthy adults: A phase I/II, prospective, randomized, open-label trial. Vaccine. 2017;35:4099–104. [DOI] [PubMed] [Google Scholar]
- [26].Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Czajka H, Unal S, Ulusoy S, Usluer G, Strus A, Sennaroglu E, et al. A phase II, randomised clinical trial to demonstrate the non-inferiority of low-dose MF59-adjuvanted pre-pandemic A/H5N1 influenza vaccine in adult and elderly subjects. J Prev Med Hyg. 2012;53:136–42. [PubMed] [Google Scholar]
- [28].Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, et al. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17:99–104. [DOI] [PubMed] [Google Scholar]
- [29].Reisinger KS, Holmes SJ, Pedotti P, Arora AK, Lattanzi M. A dose-ranging study of MF59((R))-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum Vaccin Immunother. 2014;10:2395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Beigel JH, Voell J, Huang CY, Burbelo PD, Lane HC. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J Infect Dis. 2009;200:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Domnich A, Manini I, Panatto D, Calabro GE, Montomoli E. Immunogenicity Measures of Influenza Vaccines: A Study of 1164 Registered Clinical Trials. Vaccines (Basel). 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pavlova S, D’Alessio F, Houard S, Remarque EJ, Stockhofe N, Engelhardt OG. Workshop report: Immunoassay standardisation for “universal” influenza vaccines. Influenza Other Respir Viruses. 2017;11:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hauser MI, Muscatello DJ, Soh ACY, Dwyer DE, Turner RM. An indirect comparison meta-analysis of AS03 and MF59 adjuvants in pandemic influenza A(H1N1)pdm09 vaccines. Vaccine. 2019;37:4246–55. [DOI] [PubMed] [Google Scholar]
- [34].Madan A, Ferguson M, Rheault P, Seiden D, Toma A, Friel D, et al. Immunogenicity and safety of an AS03-adjuvanted H7N1 vaccine in adults 65years of age and older: A phase II, observer-blind, randomized, controlled trial. Vaccine. 2017;35:1865–72. [DOI] [PubMed] [Google Scholar]
- [35].Kwon HI, Kim YI, Park SJ, Song MS, Kim EH, Kim SM, et al. Evaluation of the Immune Responses to and Cross-Protective Efficacy of Eurasian H7 Avian Influenza Viruses. J Virol. 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106:7962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, et al. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Giuseppe Del Giudice RR, Arnaud Didierlaurent. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Seminars in Immunology. 2018. [DOI] [PubMed] [Google Scholar]
- [39].Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. [DOI] [PubMed] [Google Scholar]
- [40].Nolan T, Richmond PC, Formica NT, Hoschler K, Skeljo MV, Stoney T, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. [DOI] [PubMed] [Google Scholar]
- [41].Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Winokur PL, Patel SM, Brady R, Chen WH, El-Kamary SS, Edwards K, et al. Safety and Immunogenicity of a Single Low Dose or High Dose of Clade 2 Influenza A(H5N1) Inactivated Vaccine in Adults Previously Primed With Clade 1 Influenza A(H5N1) Vaccine. J Infect Dis. 2015;212:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible Triggering Effect of Influenza Vaccination on Psoriasis. J Immunol Res. 2015;2015:258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sbidian E, Eftekahri P, Viguier M, Laroche L, Chosidow O, Gosselin P, et al. National survey of psoriasis flares after 2009 monovalent H1N1/seasonal vaccines. Dermatology. 2014;229:130–5. [DOI] [PubMed] [Google Scholar]
- [46].Shin MS, Kim SJ, Kim SH, Kwak YG, Park HJ. New Onset Guttate Psoriasis Following Pandemic H1N1 Influenza Vaccination. Ann Dermatol. 2013;25:489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- [48].Tsai TF. Fluad(R)-MF59(R)-Adjuvanted Influenza Vaccine in Older Adults. Infect Chemother. 2013;45:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu ZQ, Zhang Y, Zhao N, Yu Z, Pan H, Chan TC, et al. Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. Int J Environ Res Public Health. 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Germann TC, Kadau K, Longini IM Jr., Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103:5935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.