Abstract
Purpose
Serum carcinoembryonic antigen (SCEA) level is often measured in patients with CRC but suffers from poor sensitivity and specificity as a diagnostic biomarker. CEA is more abundant in stool than in serum, but it has not been widely studied. This study aimed to elucidate the efficacy of fecal CEA (FCEA) as a potential non-invasive biomarker for early diagnosis of CRC.
Materials and Methods
We retrospectively analyzed the determination of FCEA and SCEA levels by electrochemiluminescence. We evaluated the diagnostic accuracy of FCEA and SCEA levels in early-stage CRC patients and healthy controls using ROC curve.
Results
A total of 298 people were included: 115 patients with CRC, 35 patients with adenomatous polyp (APC), 46 patients with non-gastrointestinal cancer (NGC), and 102 healthy controls (HC). The FCEA concentrations in CRC and APC patients were significantly higher than that of NGC and HC, and this is different from SCEA expression in APC and NGC. As a diagnostic biomarker of CRC, FCEA had significantly larger AUC compared with SCEA (.802 vs .735, P < .001). For identifying early-stage colorectal cancer, FCEA showed better diagnostic efficacy (AUC: .831) than SCEA (AUC: .750), and the combination of the 2 biomarkers was even higher (AUC: .896). The sensitivity of FCEA was higher than that of SCEA (78.7% vs 29.8%). When SCEA was negative, 80.3% of CRC and 54.6% of APC cases could be identified by FCEA.
Conclusion
Compared with SCEA, FCEA has more advantages in the diagnosis of the early stage of colorectal cancer and adenomatous polyps.
Keywords: colorectal cancer, fecal CEA, serum CEA, early-stage, adenomatous polyps
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal malignancies across the globe. In 2030, the global burden of CRC is expected to increase by 60% to over 2.2 million new cases and 1.1 million deaths. 1 CRC is the fifth most common malignant tumor in China, with the fourth highest mortality rate and rising morbidity rates. 2 Patients’ survival from CRC is highly dependent on the stage of cancer at diagnosis. The 5-year relative survival rates are as follows: 65% for patients with CRC, 91% for patients diagnosed with stage I, and 82% for patients diagnosed with stage II. However, the 5-year survival rate declines to 12% for CRC patients at stage IV. 3
Colonoscopy is considered the current gold standard for CRC detection, but it is an invasive surgery that requires advanced preparation. In a population that has low compliance, its use for screening purposes is limited. 4 Fecal immunochemistry test is a widely used non-invasive test; its sensitivity for CRC screening is 79% (95% CI, 69% to 86%) 5 ; while its sensitivity for detection of advanced adenomas ranges from 6% to 56%. Screening studies involving cohort sizes over 8000 showed sensitivities of less than 28%. 6
Most gastrointestinal cancers contain tumor markers such as CEA. Some years after the discovery of CEA, the same research group found that serum CEA can be detected by radioimmunoassay in patients with CRC. CEA levels are low in healthy individuals and patients with other diseases 7 ; but may rise between 4 to 8 months before the development of cancer-related symptoms. CEA is considered the most important biomarker for detecting CRC. 8 However, serum CEA does not have sufficient sensitivity and specificity to diagnose CRC, so it is commonly used to monitor the recurrence of tumors instead. 9
CEA is formed in the cytoplasm and can be detected in the serum, cerebrospinal fluid, urine, and feces. Feces, which are composed of undigested food, endogenous secretions, microbiota, and exfoliated host cellular components, can be used to assess the entire intestinal environment including the occurrence of CRC and its biological effects on epithelial cells. 10 The levels of CEA in feces are higher than serum CEA, especially at early stages, prompting researchers to advocate using FCEA for CRC screening. 11 To add to the body of research in this field, we still need to address these 2 questions: (1) how to use a simple way to quantify FCEA; (2) whether the presence of nonspecific cross-reacting antigen 2 (NCA-2) affects the diagnostic efficacy of CEA in CRC.11-14
In this study, we used electrochemical luminescence to quantitatively detect the levels of FCEA and SCEA, and analyzed the differences of CEA concentrations in CRC, adenomatous polyps and healthy controls. We hope to highlight the advantages of FCEA in early diagnosis of CRC.
Materials and Methods
Patient Recruitment and Sample Collection
A total of 298 cases of the following conditions were retrospectively analyzed: 115 patients with CRC, 35 patients with adenomatous polyps, 46 patients with non-gastrointestinal cancer, and 102 healthy controls. The cases data enrolled in this study were from patients who visited our hospital from April 1, 2019 to December 31, 2019. This study was approved by the Clinical Research Ethics Committee of the Sun Yat-sen University Cancer Center (Approval no. GZR2020-118, Approval date: March 8, 2020), and all patients provided written informed consent at their first visit to our center. The patients included in the analysis met the following criteria: (1) Diagnosis of cancer is confirmed by histopathology and the tumors are classified according to the TNM staging system by the American Joint Committee on Cancer. 15 (2) Patient’s without chemoradiotherapy or surgical treatment. (3) Absence of other cancers. Healthy controls were assessed by clinicians from the Department of Physical Examination in the same period.
Sample Preparation
Blood and fecal samples were collected from patients within 7 days prior to surgery or treatment, while samples from healthy controls were taken on the day of the physical examination. Approximately 3–5 mL venous blood was extracted, allowed to clot at room temperature, and then centrifuged at 3500 r/min for 10 minutes. Subsequently, the supernatant was divided into 200-μl aliquots and stored at −80°C until testing. We used a quantitative stool collection tube containing 4 mL buffer to collect .1 mg of stool from 3 different fresh stools. Loose stools, watery stools, blood stools, or hard stools can cause inaccurate sampling, so they are excluded. The samples were homogenized for 2 minutes and centrifuged for 10 minutes at 10 000 r/min. The supernatant fluid was retained, filtered if necessary, and stored in the refrigerator at minus −80°C until further use.
Analysis of Tumor Markers
Electrochemiluminescence immunoassay (ECLIA) kit (Roche Diagnostics, Germany) was used for CEA quantitative detection according to the manufacturer’s instruction. Serum concentrations of the tested tumor markers were assayed by the Cobas 8000 automated immunoassay system with supporting reagents (Roche Diagnostics, Germany). Normal reference values of CEA were < 5.0 ng/mL.
After verification by our laboratory, this reagent can be used to quantitatively detect the CEA content of the fecal supernatant. The performance verification data and conclusions are in Supplemental Additional Material 1.
Statistical Analysis
Statistical analysis was performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA), and the figures were drawn in GraphPad Prism 7.0 (San Diego, CA, USA). Data that conform to a normal distribution are represented by mean ± SEM, while the non-normally distributed data were represented by median and IQR. Multi-group data were compared using the Kruskal–Wallis test, and data between 2 groups were compared by the Mann–Whitney test. We evaluated the diagnostic efficacy of SCEA and FCEA to detect CRC by computing the sensitivity and specificity (with 95% confidence intervals) and plotting a receiver operating characteristic (ROC) curve. We chose P < .05 (two-tailed) as a cutoff point for statistical significance.
Results
Demographic and Clinical Features of the Subjects
Table 1 presents the basic demographics of the participants. There were 298 participants in the study; 115 CRC patients (64 males, 51 females, average age = 59.1 ± 11.4 years) and 183 cases in the control group (94 males, 89 females, average age = 46.2 ± 12.1 years). There were no significant differences in gender and age between the case group and the control group (P > .05). Non-gastrointestinal cancers include lung cancer, liver cancer, cervical cancer, ovarian cancer, thyroid cancer, etc. (for more details, see Supplemental Table 1). The diagnosis of CRC was supported by pathologists; and all tumors were classified histologically as adenocarcinoma.
Table 1.
Demographic and Clinical Features of the Subjects.
| Characteristic | CRC cases (n = 115), n (%) | Control cases (n = 183), n (%) | P value |
|---|---|---|---|
| Age | 59.08 ± 11.40 | 46.22 ± 12.12 | .464 |
| Male | 64 (55.65%) | 94 (51.37%) | |
| Female | 51 (44.35%) | 89 (48.63%) | .470 |
| Tumor location n (%) | |||
| Colon | 58 (50.43) | — | — |
| Ascending colon | 8 (6.96) | — | — |
| Drop B Junction | 4 (3.48) | — | — |
| Sigmoid colon | 32 (27.82) | — | — |
| Hepatic flexure colon | 4 (3.48) | — | — |
| Ileocecal colon | 2 (1.74) | — | — |
| Transverse colon | 6 (5.21) | — | — |
| Multiple primary colon cancers (hepatic curvature, sigmoid colon) | 2 (1.74) | — | — |
| Rectosigmoid junction | 7 (6.09) | — | — |
| Rectum | 50 (43.48) | — | — |
| Tumor staging n (%) | |||
| I | 22 (19.13) | — | — |
| II | 25 (21.74) | — | — |
| III | 52 (45.22) | — | — |
| IV | 13 (11.30) | — | — |
| Unknown | 3 (2.61) | — | — |
| Gross classification n (%) | |||
| Eminence type | 44 (38.26) | — | — |
| Ulcerative type | 55 (47.83) | — | — |
| Infiltration type | 3 (2.61) | — | — |
| Unknown | 13 (11.30) | — | — |
| Histological grade n (%) | |||
| Well | 5 (4.35) | — | — |
| Moderate | 88 (76.52) | — | — |
| Poor | 9 (7.83) | — | — |
| Unknown | 13 (11.30) | — | — |
| Tumor size n (%) | |||
| < 5 cm | 39 (33.91) | — | — |
| ≥ 5 cm | 72 (62.61) | — | — |
| Unknown | 4 (3.48) | — | — |
FCEA and SCEA Levels in Colorectal Cancer and Three Controls
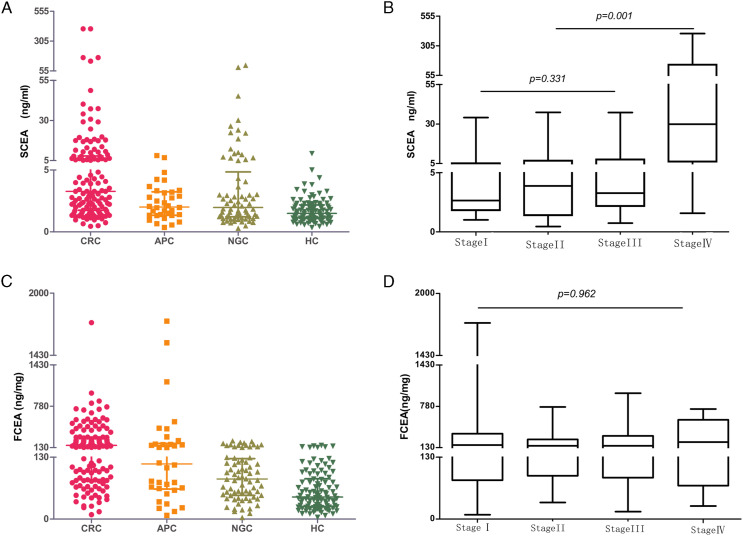
We found that the median FCEA in the CRC group (149.76 ng/mg) was significantly higher than that of the non-gastrointestinal cancer group (83.58 ng/mg) and healthy controls (46.19 ng/mg), but there was no significant difference compared with APC (P = .167). In contrast, serum CEA levels in CRC and APC were significantly different (P < .001) (Figures 1A, 1C and Table2).
Figure 1.
The distribution of SCEA and FCEA in each CRC stage and three control groups.
Table 2.
Quantitative Detection of CEA in CRC Patients and 3 Control Groups.
| Different group(n) | FCEA (ng/mg) | P value | SCEA (ng/ml) | P value |
|---|---|---|---|---|
| CRC (115) | 149.76 (81.0–240.9) | Ref | 3.28 (1.75–9.92) | Ref |
| APC (35) | 113.58 (63.9–182.97) | .167 | 1.97 (1.34–3.21) | < .001 |
| NGC (46) | 83.58 (53.42–135.29) | < .001 | 1.84 (1.17–4.67) | .033 |
| HC (102) | 46.19 (26.17–84.72) | < .001 | 1.50 (1.09–2.17) | < .001 |
| < .001 | .014 |
Besides, we observed that the levels of SCEA, the extent of lymph node metastasis and distant metastasis in CRC patients at stage IV were significantly higher than those at stage I–III. However, the FCEA level was not correlated with tumor stage (Figures 1B, 1D and Table 3).
Table 3.
The Relationship Between Clinical Features and CEA.
| Variable | FCEA | P value | SCEA | P value |
|---|---|---|---|---|
| AJCC stage | .962 | < .001* | ||
| Stage I(22) | 149.70 (72.82–234.20) | 2.67 (1.99–6.36) | < .001* | |
| Stage II(25) | 152.30 (82.22–223.80) | 3.98 (1.40–6.82) | < .001* | |
| Stage III(52) | 148.02 (83.98–288.18) | 3.18 (1.82–7.66) | < .001* | |
| Stage IV(13) | 131.90 (66.54–500.00) | 17.39 (5.58–44.44) | Ref | |
| Depth of the tumor | .747 | <.001* | ||
| T1(9) | 102.40 (65.33–213.30) | 2.15 (1.40–2.48) | .028* | |
| T2(19) | 143.50 (86.61–241.00) | 3.27 (1.82–9.92) | .017 | |
| T3(67) | 142.40 (80.64–218.10) | 4.25 (1.68–8.65) | .002* | |
| T4(15) | 194.00 (70.05–468.30) | 10.31 (2.49–29.09) | Ref | |
| Lymph node metastasis | .608 | < .001* | ||
| NO (49) | 149.80 (81.78–231.50) | 2.74 (1.56–6.82) | ||
| YES (62) | 142.50 (79.58–275.20) | 4.17 (.74–14.42) | ||
| Distant metastases | .540 | < .001* | ||
| No (99) | 149.80 (82.47–241.00) | 3.05 (1.68–7.46) | ||
| Yes (13) | 120.50 (64.79–377.20) | 23.24 (7.00–46.57) | ||
| Gross classification(n) | .029* | .019* | ||
| Eminence type (44) | 104.0 (64.13–197.30) | Ref | 2.445 (1.505–6.413) | Ref |
| Ulcerative type (55) | 181.3 (80.64–376.20) | .032* | 4.250 (2.590–17.530) | .006* |
| Infiltration type (3) | 541.8 (109.30–986.40) | .064 | 2.310 (1.280–18.030) | .845 |
| Tumor size (cm) (n) | < .001* | .023* | ||
| ≥ 5 (39) | 201.3 (109.3–510.3) | 5.490 (2.490–10.450) | ||
| < 5 (72) | 115.1 (67.68–209.9) | 2.780 (1.480–6.040) | ||
*P < .05 was considered statistically significant.
We further analyzed the relationship between CEA concentrations and the pathological characteristics of CRC. We found that both FCEA and SCEA concentrations showed significant differences according to the tumor’s gross classification. CEA levels were the highest in the infiltrating type; higher in the ulcer type than the uplift type; higher in tumors of diameter greater than or equal to 5 cm than that of tumors of diameter < 5 cm. Besides, there was no significant difference between the concentrations of FCEA and SCEA, and tumor location, and differentiation degree. Although FCEA and SCEA were consistent in pathological characteristics, we found no significant correlation between SCEA and FCEA concentrations in CRC group and control group (P = .363).
ROC Analyses of the Diagnostic Efficacies of FCEA and SCEA in Colorectal Cancer
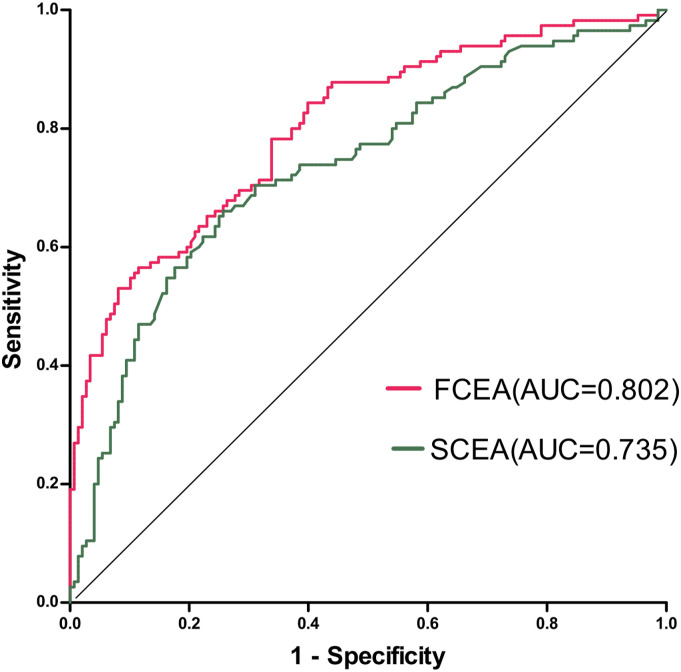
Next, we plotted ROC curves based on the quantitative results of CEA in electrochemiluminescence detection to determine the diagnostic effect of CEA in stool and serum on distinguishing CRC from non-gastrointestinal cancer and healthy controls (Figure 2). The AUC of FCEA expression was .802, which was significantly higher than that of SCEA (AUC = .735, P < .001). The cutoff value of FCEA expression was identified according to the Youden index at > 130 ng/mg for the diagnosis of CRC. At this cutoff point, sensitivity was 76.5%, and specificity was 73.0% while according to the standard cutoff value of SCEA (> 5 ng/mL), sensitivity was 38.3%, and specificity was 91.0%.
Figure 2.
SCEA and FCEA compared the ROC curves of CRC versus non-gastrointestinal cancer and healthy controls.
Early Diagnosis Value of FCEA and SCEA in CRC Patients
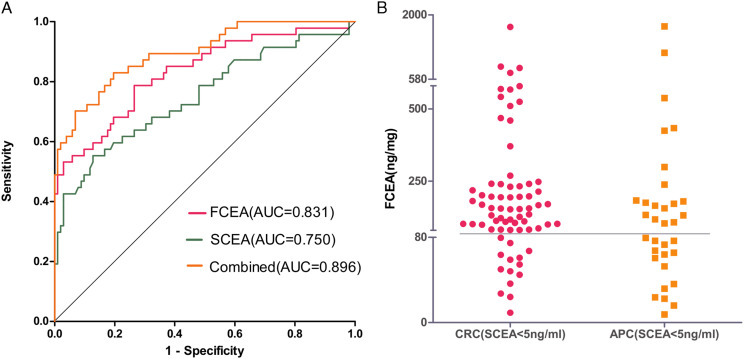
As shown in Table 4 and Figure 3A, ROC curves show diagnostic strength to identify early (stage I+II) CRC from healthy controls with an indicator of SCEA, FCEA, and combined (SCEA + FCEA). ROC curve analysis was performed to evaluate the accuracy of SCEA and FCEA levels in diagnosing CRC. The AUC of FCEA expression was .831, which was higher than that of SCEA (AUC = .750, P < .001), and the AUC of combined SCEA and FCEA reached the even more significant value of .896.
Table 4.
The Diagnostic Efficacy of SCEA, FCEA and the Combination of the 2 in Early CRC (stage I+II).
| CRC (stage I+II) vs HC | AUC (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| SCEA | 0.750 (0.659–0.840) | 29.80% (17.34–44.89%) | 98.04 % (93.10–99.76%) |
| FCEA | 0.831 (0.757–0.906) | 78.70% (64.34–89.30%) | 73.50% (63.87–81.78%) |
| SCEA + FCEA | 0.896 (0.841–0.951) | 66.10% (55.63–74.81%) | 83.33% (74.66–89.98%) |
Figure 3.
(A) ROC comparisons of SCEA, FCEA, and combined (SCEA+ FCEA) to identify the early (Stage I + II) CRC from healthy controls. (B) The distribution of CRC and APC of FCEA in SCEA negative.
Further analysis on CRC patients showed that, among the CRC patients, 71 patients had SCEA levels below 5 ng/mL (the standard cutoff value), and 80.3% (57 of 71) were positive in the fecal CEA screening (according to the maximum Youden index, FCEA > 80 ng/mg). Out of the 35 patients with adenomatous polyps, 33 had serum CEA levels below 5 ng/mL, but when screening with FCEA, 54.6% (18 of 33) of the patients were positive. (Figure 3B)
Discussion
After its first discovery in 1965, CEA was subsequently recommended by the American Society of Clinical Oncology and the European Group on Tumor Markers as a marker for colorectal cancer. 16 Often, CEA is chosen as a biomarker because they are easily accessible and inexpensive. Serum CEA measurement is a routine measurement performed in the CRC precursor and is used to distinguish between cancer patients and healthy individuals. Elevated SCEA concentration exceeding 5.00 ng/mL signifies possible cancer. However, screening for CEA by blood is more invasive than screening by fecal sampling, as drawing blood requires trained personnel. Furthermore, serum CEA is not organ-specific and abnormal values may be found in a wide range of carcinomas, such as colorectal, breast, lung and pancreatic cancer, and so on.17-19 The clinical application of SCEA is also not widespread due to its lack of sensitivity and specificity. 9
In this study, 46 cases of non-gastrointestinal cancer were included as controls, including endometrial, liver, kidney, nasopharyngeal, breast, testicular, and lung cancers. Out of the 46 cases, 12 (26.09%) patients had SCEA concentration exceeding 5 ng/mL, among which 8 (66.67%) patients had concentration exceeding 10 ng/mL—showing that SCEA was highly expressed in non-gastrointestinal cancer. Fecal CEA showed a different pattern; only 10 of 46 (21.74%) patients had FCEA concentration above the threshold (130 ng/mg), among which, 9 of 10 (90%) patients had FCEA concentration below 260 ng/mg. The expression of FCEA in non-gastrointestinal cancer was significantly lower than that in CRC. Therefore, SCEA is not specific for CRC and as it is expressed in a variety of cancers. Using FCEA as a tumor marker alleviates these shortcomings; as it differentiates CRC and non-gastrointestinal tumors better than SCEA. Furthermore, to the best of our knowledge, this is the first study evaluating the diagnostic accuracy of FCEA in patients with non-gastrointestinal cancer.20,21
By studying CEA level and clinicopathological characteristics, our data showed that SCEA was highly expressed in patients with distant metastasis, consistent with previous studies. 18 Although FCEA concentration was not related to the stage of the disease, it was related to tumor gross type: FCEA concentration in patients with ulcerative type was higher than that in patients with uplifted type, and the highest in patients with infiltrating type. On the other hand, FCEA concentration is associated with tumor size: FCEA concentration in CRC patients with tumor diameter greater than or equal to 5 cm was significantly higher than that of CRC patients with tumor diameter less than 5 cm. One study explored the direct relationship between tumor volume in rectal cancer and overall survival and posited that the relationship between FCEA and the prognosis of colorectal cancer patients should be further studied. 22
In this study, we aimed to evaluate the efficacy of FCEA as a non-invasive cancer biomarker through evaluating its role in the diagnosis of early stage CRC (stage I, II) patients. In early-stage detection of CRC, FCEA achieved overall sensitivity of 78.7% (95% CI; 64.34–89.30%), with an AUC value of .831 (95% CI; .757–.906)—results that were better than SCEA ([sensitivity, 29.79%; 95% CI, 17.34–44.89%], [AUC, .750; 95% CI, .659–.840]). The combination of the higher sensitivity of FCEA and the higher specificity of SCEA results in higher diagnostic efficiency (AUC = .896). FCEA is a promising biomarker in CRC detection, with the advantage of convenience and non-invasiveness. In addition, colonoscopy provides high sensitivity and specificity in detecting adenomas and polyps. Therefore, FCEA screening can be chosen first, and then SCEA or endoscopy can be chosen next.
Most colorectal neoplasia are adenocarcinomas originating from epithelial cells of the colorectal mucosa. They usually develop from focal changes in benign precancerous polyps, and fully progress from polyps to cancer in several years.23,24 According to the latest update to the American Cancer Society guideline, screening with any one of multiple options is associated with a significant reduction in CRC incidence through the detection and removal of adenomatous polyps and other precancerous lesions and with a reduction in mortality through incidence reduction and early detection of CRC. 25 The most common and clinically important polyps are adenomatous polyps, which represent approximately one-half to two-thirds of all colorectal polyps and are associated with a higher risk of CRC. 26 Therefore, 35 patients with adenomatous polyps were included in this study, among them, 33 (94.29%) had SCEA concentration within the normal reference range—indicating that SCEA was not suitable for screening adenomatous polyps. Thus, we looked for positive markers for the adenomatous polyp stage. In the adenomatous polyp group, 94.29% (33 of 35) had SCEA concentration less than 5 ng/mL, which was significantly different from that of CRC (P = .001). At the same time, there was no statistical difference in FCEA between the CRC group and the adenomatous polyp group (P = .167), and 60.61% (20 of 33) were positive. Although we lack the means to distinguish between adenomatous polyps and CRC, these results are welcome because we were only aiming to screen the population for precancerous lesions.
FCEA showed the following characteristics in the screening of CRC: (1) Low expression in non-gastrointestinal cancer and high expression in adenomatous polyps. (2) An expression that is independent of tumor stage, tumor location, and tumor differentiation. However, ulcerative type tumor was bigger than eminence type, increased with tumor diameter, and produced enough FCEA even for small tumors.
Summing up the arguments of previous studies, researchers believe that FCEA is more sensitive than SCEA in CRC screening.20,27 The reasons may be as follows: (1) Cancer tissue is prone to necrosis and shedding, thus can be continuously renewed and released. It enters the intestinal cavity easily and is discharged with feces. (2) After growth of CRC cells, CEA is transported from the portal vein to the liver and then decomposed, decreasing CEA concentration in the blood. (3) Through systematic quantitative collection of fecal samples according to the standards, CEA can be released from the exfoliated cancer cells completely, and the probability of CEA detection increases.
Our study presents several limitations. Because of the convenience sampling method used in healthy controls, we cannot ascertain how representative our sample is. We welcome multi-center collaborations to get a more representative sample in the future. Nevertheless, the diagnostic sensitivity of FCEA in CRC detection varies in the current literature. In the past, 28 the variation was due to the difficulty in fecal quantification, and having a single the control group. In this study, we used quantitative fecal sampling tube to simplify quantification, and advanced electrochemiluminescence to produce high-quality get results. Meanwhile, the antibody used in the Roche ECLIA system may react with CEA and meconium antigen, which is a nonspecific cross-reacting antigen (NCA). Normal feces contain both CEA and NCA antigens. 29 Most CRC cells synthesize NCA more actively than normal colon mucosa. Our results showed that CEA concentration in feces was greater than that in serum, which may be the result of NCA antigen reaction. If we can better discriminate CRC patients from healthy individuals, it is unimportant which antigens the antibodies bind. The family of the CEA is complex where it comprises 29 genes; out of which, 18 are expressed: 7 belonging to the CEA subgroup and 11 to the pregnancy-specific glycoprotein subgroup. 29 At this stage, we do not wish to discuss the CEA family at the molecular level, but we can confirm the advantages of FCEA in early CRC screening from our experiments.
Conclusion
Fecal CEA is more sensitive and accurate as a biomarker for early-stage CRC, even in the precancerous stage, and can be used for CRC screening.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211048292 for Fecal CEA Has an Advantage in the Diagnosis of Colorectal Cancer at Early Stage by Linfang Li, Shan Xing, Miantao Wu, Yufeng Ao, Xin Zheng, Rongzeng Cai, Runkun Han, Jingcong Li, Xiaohui Li and Qiuyao Zeng in Cancer Control
Acknowledgments
We thank the staff at the Director of Clinical Laboratories, Sun Yat-sen University Cancer Center for providing support on research conditions in this study. The author is particularly grateful to Liu Yuhui of the Humoral chamber for his assistance in collecting fecal specimens.
Appendix
Abbreviations
- APC
adenomatous polyps
- AUC
area under the curve
- CRC
colorectal cancer
- FCEA
fecal carcinoembryonic antigen
- HC
healthy controls
- NGC
non-gastrointestinal cancer
- ROC
receiver-operating characteristic
- SCEA
serum carcinoembryonic antigen
Author Contributions: Linfang Li, Qiuyao Zeng, Shan Xing and Xiaohui Li contributed equally to this work. Linfang Li and Qiuyao Zeng: Conceptualization, investigation, and writing—original draft preparation, Writing—review and editing; Miantao Wu and Yufeng Ao: Formal analysis and software; Xin Zheng, Runkun Han and Jingcong Li: Review and editing; Xiaohui Li and Shan Xing: Resources, supervision, and funding acquisition.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants No. 2019A1515010798 from the Natural Science Foundation of Guangdong Province, China.
Ethics Statement: This study was approved by the Clinical Research Ethics Committee of the Sun Yat-sen University Cancer Center, and all patients provided written informed consent at the first visit to our center. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (www.researchdata.org.cn) with the approval RDD number as RDDB2020000892.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Linfang Li https://orcid.org/0000-0002-2336-9885
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro MS, Wallace MB. Endoscopic treatment of early cancer of the colon. Gastroenterol Hepatol. 2015;11:445-452. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer. Ann Intern Med. 2014;171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US multi-society task force on colorectal cancer. Gastroenterology. 2017;152:1217-1237. [DOI] [PubMed] [Google Scholar]
- 7.Thomson DMP, Krupey J, Freedman SO, Gold P. The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci Unit States Am. 1969;64:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338-351. [DOI] [PubMed] [Google Scholar]
- 9.Wang YR, Yan JX, Wang LN. The diagnostic value of serum carcino-embryonic antigen, alpha fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J Cancer Res Ther. 2014;10 Suppl:307-309. [DOI] [PubMed] [Google Scholar]
- 10.Kato I, Startup J, Ram JL. Fecal biomarkers for research on dietary and lifestyle risk factors in colorectal cancer etiology. Curr Colorectal Cancer Rep. 2014;10:114-131. [Google Scholar]
- 11.Fujimoto S, Kitsukawa Y, Itoh K. Carcinoembryonic antigen (CEA) in gastric juice or feces as an aid in the diagnosis of gastrointestinal cancer. Ann Surg. 1979;189:34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed DLJ, Taylor G. Carcinoembryonic antigen in faeces. Br Med J. 1972;1:85-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias GE, Holyoke DE, Chu MT. Carcinoembryonic antigen (CEA) in feces and plasma of normal subjects and patients with colorectal carcinoma. Dis Colon Rectum. 1974;17:38-41. [DOI] [PubMed] [Google Scholar]
- 14.Kitsukawa Y. Immunoreactive carcinoembryonic antigen [CEA] levels in feces from colorectal cancer patients. Jpn J Surg. 1979;9:102-109. [DOI] [PubMed] [Google Scholar]
- 15.Hari DM, Leung AM, Lee J-H, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg. 2013;217:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [DOI] [PubMed] [Google Scholar]
- 17.Huang S-C, Lin J-K, Lin T-C, et al. Concordance of carcinoembryonic antigen ratio and response evaluation criteria in solid tumors as prognostic surrogate indicators of metastatic colorectal cancer patients treated with chemotherapy. Ann Surg Oncol. 2015;22:2262-2268. [DOI] [PubMed] [Google Scholar]
- 18.Accordino MK, Wright JD, Vasan S, et al. Serum tumor marker use in patients with advanced solid tumors. J Oncol Pract. 2016;12(1):65-66, e36-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao C, Zhang G, Zhang L. Serum CEA levels in 49 different types of cancer and noncancer diseases. Prog Mol Biol Transl Sci. 2019;162:213-227. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Lee S, Park S, et al. Gastrointestinal tract cancer screening using fecal carcinoembryonic antigen. Ann Clin Lab Sci. 2003;33:32-38. [PubMed] [Google Scholar]
- 21.Matsuoka Y, Matsuo Y, Sugano K, Ohkura H, Kuroki M, Kuroki M. Characterization of carcinoembryonic antigen-related antigens in normal adult feces. Jpn J Canc Res. 1990;81:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tayyab M, Razack A, Sharma A, Gunn J, Hartley JE. Correlation of rectal tumor volumes with oncological outcomes for low rectal cancers: does tumor size matter? Surg Today. 2015;45:826-833. [DOI] [PubMed] [Google Scholar]
- 23.Schreuders EH, Grobbee EJ, Spaander MCW, Kuipers EJ. Advances in fecal tests for colorectal cancer screening. Curr Treat Options Gastroenterol. 2016;14:152-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [DOI] [PubMed] [Google Scholar]
- 26.Bond J. Colon polyps and cancer. Endoscopy. 2005;37:208-212. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto S, Kitsukawa Y, Itoh K. Carcinoembryonic antigen (CEA) in gastric juice or feces as an aid in the diagnosis of gastrointestinal cancer. Ann Surg. 1979;189:34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Kim H-J, Lee IK, Oh S-T, Han K. Fecal occult blood test/fecal carcinoembriogenic antigen dual rapid test as a useful tool for colorectal cancer screening. Eur Surg. 2017;49:127-131. [Google Scholar]
- 29.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Canc Biol. 1999;9:67-81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211048292 for Fecal CEA Has an Advantage in the Diagnosis of Colorectal Cancer at Early Stage by Linfang Li, Shan Xing, Miantao Wu, Yufeng Ao, Xin Zheng, Rongzeng Cai, Runkun Han, Jingcong Li, Xiaohui Li and Qiuyao Zeng in Cancer Control