Abstract
Objective
To investigate the clinical significance of human leukocyte antigen (HLA)-E levels in oesophageal squamous cell carcinoma (ESCC).
Methods
The levels of HLA-E immunostaining in ESCC lesions and 47 corresponding adjacent normal tissues were measured using immunohistochemistry. The correlation between the levels of immunostaining and clinical parameters was analysed.
Results
This study analysed 110 paraffin-embedded primary tumour lesions and 47 case–controlled paracancerous tissues that were surgically resected from 110 patients with ESCC. Positive immunostaining for HLA-E was observed in 88.2% (97 of 110) of ESCC lesions and 29.8% (14 of 47) of normal oesophageal tissues. There was no correlation between HLA-E immunostaining in ESCC lesions and clinicopathological characteristics such as lymph node metastasis, tumour–node–metastasis stage and differentiation grade. Kaplan–Meier survival analysis revealed a significantly better prognosis in patients with higher levels of HLA-E immunostaining than in those with lower levels of HLA-E immunostaining; overall survival was 28.6 months (95% confidence interval [CI], 23.2, 34.0) versus 15.3 months (95% CI, 11.5, 19.1), respectively. Furthermore, multivariate analysis showed that the HLA-E level was an independent prognostic factor in patients with ESCC.
Conclusion
A higher level of HLA-E immunostaining was associated with favourable survival in patients with ESCC.
Keywords: Human leukocyte antigen, HLA-E, oesophageal squamous cell carcinoma, immune, prognosis
Introduction
Oesophageal cancer is the sixth leading cause of cancer deaths around the world. 1 It has a very poor prognosis, with a 5-year overall survival of less than 30%, despite improvements in multimodal therapy. 2 Oesophageal squamous cell carcinoma (ESCC), known for being aggressive and having a poor prognosis, is one of the most common subtypes of oesophageal cancer. 3
Tumour progression depends on the interaction of malignant cells and the tumour microenvironment, which includes blood cells, endothelial cells, stromal cells, immune regulatory molecules and infiltration of various immune cells.4,5 Previous studies have shown that the tumour microenvironment influences the occurrence and development of ESCC.6,7 The non-classical human leukocyte antigen (HLA) class I molecules, including HLA-E, HLA-G and HLA-F, can interact with receptors on immunocytes, such as T-cells, natural killer (NK) cells, dendritic cell, and macrophages, thereby executing immunoregulatory effects.8–10 The role of HLA-E in cytotrophoblasts has been shown to be important in maintaining fetal–maternal immune tolerance. 11 Recently, studies have found that HLA-E is highly expressed in various tumours and is related to tumour size, metastasis and patient prognosis.12–14 In patients with ESCC, it was found that high levels of HLA-G and HLA-F play an important role in tumour progression.15,16 However, to date, the clinical significance of HLA-E levels in ESCC remains unknown.
In the present study, HLA-E levels in ESCC were assessed by immunohistochemistry and the relationship between HLA-E levels and clinicopathological factors, such as patient outcome, was analysed.
Patients and methods
Patients and specimens
This retrospective study analysed paraffin-embedded primary tumour lesions and case–controlled paracancerous tissues that were surgically resected from consecutive patients with ESCC at the Department of Hepatobiliary Surgery, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, Zhejiang Province, China between January 2005 and December 2010. Patients receiving preoperative anti-cancer treatment were excluded. Tumour staging was based on the tumour–node–metastasis (TNM) classification system in the staging manual established by the Union for International Cancer Control. 17 The patient charts provided information on sex, age, smoking, alcohol consumption, T stage, lymph node metastasis, TNM stage, differentiation grade, diagnosis date, death date caused by ESCC, or final follow-up date, and other clinicopathological data. Patients were followed-up for 60 months or until death.
The Ethical Committee of Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, Zhejiang Province, China provided guidelines for using the human tissue specimens in January 2020 (no. K20200113). All of the patients provided written informed consent to participate in this study.
Immunohistochemistry and staining evaluation
The levels of HLA-E in tumour cells were determined using the mouse monoclonal antibody MEM-E/02 (1:100 dilution, ab2216; Abcam®, Cambridge, MA, USA) that binds to the heavy-chain polypeptides of human HLA-E. Immunohistochemistry was conducted based on the MEM-E/02 protocol from the manufacturer. Sections (4 μm) were cut from formalin-fixed and paraffin-embedded tissue blocks. The tissue sections were then deparaffinized and rehydrated in a graded series of ethanol. This was followed by antigen retrieval in 0.01 M boiled citrate buffer (pH 6.0) for 20 min. Then, the slides were left to cool in 0.01 M citrate buffer (pH 6.0) for 2 h, washed twice using distilled water, washed twice with 10 mM phosphate-buffered saline (PBS; pH 7.0–7.3) and then incubated overnight at 4 °C with primary antibody that was diluted to the recommended concentration (1:100 dilution). This was followed by washing in 10 mM PBS (pH 7.0–7.3) three times. Diluted horseradish peroxidase-conjugated goat antimouse secondary antibody (1:1000 dilution; ab6789; Abcam®) was applied at room temperature for 30 min. Then the slides were washed in 10 mM PBS (pH 7.0–7.3) three times. Finally, the levels of HLA-E were observed using a 3,3'Diaminobenzidine Substrate Kit (ab64238; Abcam®) with haematoxylin counterstaining and a light microscope (DM2000; Leica, Solms, Germany). Placental tissues were used as the positive controls. Three pathologists that were blind to the clinical data and patient outcomes were responsible for measuring the levels of HLA-E staining. The three pathologists were responsible for evaluating the percentage of tumour cells that had positive immunostaining for HLA-E and recording the mean value of scores as the final result. HLA-E observed in the membrane and cytoplasm of ESCC cells was considered as positive immunostaining, with the percentage of positive cells determined based on whether HLA-E was present rather than the intensity of the staining. When the final mean score was >5%, HLA-E immunostaining was considered positive; when final mean score was ≤5%, HLA-E immunostaining was considered negative.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Pearson χ2-test was used to analyse the correlation between HLA-E immunostaining and clinical features. Kaplan–Meier survival curves and log-rank test were used to determine the overall survival of patients based on the period from diagnosis date to death date or final follow-up date. Cox regression analysis was conducted to determine whether positive HLA-E immunostaining was an independent predictor for patient survival. A P-value < 0.05 was considered statistically significant.
Results
This retrospective study analysed 110 paraffin-embedded primary tumour lesions and 47 case–controlled paracancerous tissues that were surgically resected from 110 patients with ESCC. Of all of the patients, 0.9% (one of 110 patients) were in TNM stage I, 48.2% (53 of 110 patients) were in TNM stage II, 49.1% (54 of 110 patients) were in TNM stage III and 1.8% (two of 110 patients) in TNM stage IV. Patients were followed-up for 60 months or until death, with a mean follow-up time of 21.6 months (range, 3.5–60 months) and there were 90 of 110 deaths (81.8%) from ESCC.
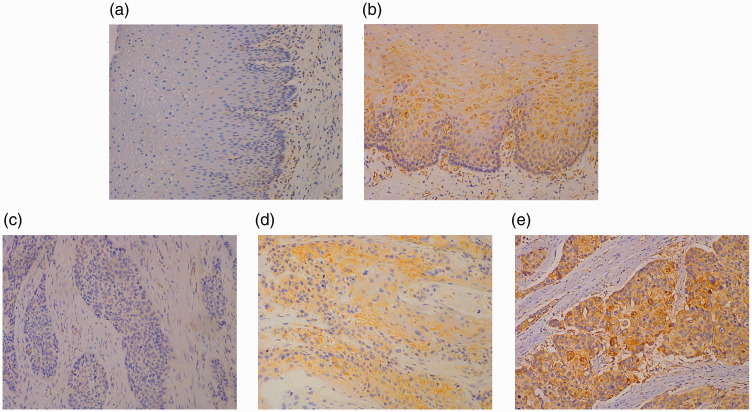
Figure 1 presents HLA-E immunostaining in both the membranes and cytoplasm of ESCC cells in 88.2% (97 of 110) of ESCC lesions and 29.8% (14 of 47) of normal oesophageal tissues (P < 0.05), with different staining intensities.
Figure 1.
Representative photomicrographs showing the immunohistochemical staining of human leukocyte antigen-E (HLA-E) in normal oesophageal tissues and primary oesophageal squamous cell carcinoma (ESCC) lesions. Negative (a) and positive (b) immunostaining for HLA-E in normal oesophageal tissues; negative (c) and positive (d) immunostaining for HLA-E ≤50% in ESCC lesions; (e) positive immunostaining for HLA-E >50% in ESCC lesions. Immunostaining for HLA-E was considered as negative when the percentage of stained cells was ≤5%. Scale bar 30 µm. The colour version of this figure is available at: http://imr.sagepub.com.
Assessment of the correlation between HLA-E immunostaining and clinicopathological parameters required the stratification of all patients into a group with low HLA-E levels (n = 55) and a group with high HLA-E levels (n = 55) on the basis of the median levels of HLA-E in ESCC. No significant differences were observed between the two groups based on the levels of HLA-E immunostaining for sex, age, smoking, alcohol consumer, T stage, lymph node metastasis, TNM stage and grade of differentiation (Table 1).
Table 1.
Correlation between the levels of immunostaining for human leukocyte antigen (HLA)-E in the oesophageal squamous cell carcinoma specimens and clinicopathological parameters.
| Clinical parameters | HLA-E immunostaining levels |
|
|---|---|---|
| Lown = 55 | Highn = 55 | |
| Age, years | ||
| ≤58 | 30 | 21 |
| >58 | 25 | 34 |
| Sex | ||
| Male | 36 | 40 |
| Female | 19 | 15 |
| Smoking | ||
| Yes | 34 | 31 |
| No | 21 | 24 |
| Alcohol consumer | ||
| Yes | 22 | 26 |
| No | 33 | 29 |
| T stage | ||
| T1/2 | 8 | 14 |
| T3/4 | 47 | 41 |
| Lymph node metastasis | ||
| Negative | 24 | 26 |
| Positive | 31 | 29 |
| TNM stage | ||
| I/II | 24 | 30 |
| III/IV | 31 | 25 |
| Grade of differentiation | ||
| Well + moderate | 36 | 43 |
| Poor | 19 | 12 |
Data presented as n of patients.
No significant between-group differences; P ≥ 0.05; Pearson χ2-test.
TNM, tumour–node–metastasis.
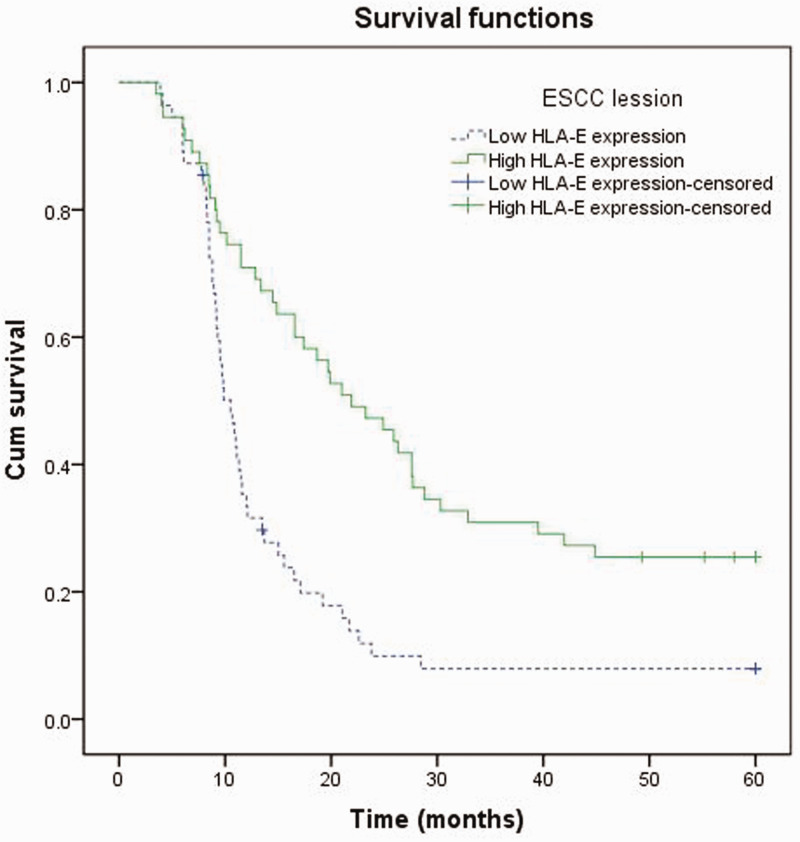
The duration from the date of diagnosis to the date of death caused by ESCC was defined as the patient survival time. A significantly better prognosis was shown in the group of patients with high levels of HLA-E immunostaining, which had a mean survival time of 28.6 months (95% CI, 23.2,34.0); compared with an extremely poor prognosis that was observed in the group with low levels of HLA-E immunostaining, which had a mean survival time of 15.3 months (95% CI, 11.5, 19.1) (P < 0.05) (Figure 2). Cox proportional hazards model analysis was used in the assessment of prognostic parameters in patients with ESCC. Univariate analysis showed a significantly lower hazard ratio (HR) in the group with high levels of HLA-E immunostaining compared with that in the group with low levels HLA-E immunostaining (hazard ratio [HR] 0.430; 95% confidence interval [CI], 0.280, 0.661; P < 0.001) (Table 2). Moreover, multivariate analysis revealed that high levels of HLA-E immunostaining served as an independent prognostic parameter (HR 0.398; 95% CI, 0.254, 0.623; P < 0.001).
Figure 2.
Kaplan–Meier analysis of overall survival (OS) for patients with oesophageal squamous cell carcinoma (ESCC). Comparison of OS between patients with high HLA-E immunostaining (n = 55) and those with low HLA-E immunostaining in ESCC lesions (n = 55) (P < 0.001).
Table 2.
Univariate and multivariate Cox regression analysis of the levels of immunostaining for human leukocyte antigen (HLA)-E and overall survival of patients with oesophageal squamous cell carcinoma (n = 110).
| Univariate analysis |
Multivariate analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | B | SE | Wald | df | HR (95% CI) | P-value | B | SE | Wald | df | HR (95% CI) | P-value |
| Age, years>58 versus ≤58 | –0.012 | 0.211 | 0.003 | 1 | 0.989 (0.653, 1.496) | NS | ||||||
| SexFemale versus male | –0.259 | 0.239 | 1.168 | 1 | 0.772 (0.483, 1.234) | NS | ||||||
| SmokingYes versus no | 0.101 | 0.214 | 0.221 | 1 | 1.106 (0.727, 1.682) | NS | ||||||
| Alcohol consumerYes versus no | 0.242 | 0.213 | 1.294 | 1 | 1.274 (0.840, 1.932) | NS | ||||||
| T stageT3/4 versus T1/2 | 0.875 | 0.313 | 7.815 | 1 | 2.398 (1.299, 4.428) | P = 0.005 | ||||||
| Lymph metastasisPositive versus negative | 0.870 | 0.220 | 15.699 | 1 | 2.386 (1.552, 3.670) | P < 0.001 | ||||||
| HLA-E immunostainingHigh versus low | –0.844 | 0.220 | 14.783 | 1 | 0.430 (0.280, 0.661) | P < 0.001 | –0.922 | 0.229 | 16.234 | 1 | 0.398 (0.254, 0.623) | P < 0.001 |
| TNM stageIII/IV versus I/II | 0.984 | 0.220 | 20.016 | 1 | 2.675 (1.738, 4.118) | P < 0.001 | 1.053 | 0.227 | 21.421 | 1 | 2.866 (1.835, 4.475) | P < 0.001 |
| Grade of differentiationWell + moderate versus poor | 0.071 | 0.230 | 0.095 | 1 | 1.074 (0.683, 1.686) | NS | ||||||
NS, no significant association; P ≥ 0.05.
HR, hazard ratio; CI, confidence interval; TNM, tumour–node–metastasis.
Discussion
Human leukocyte antigen-E is encoded by a gene on the short arm of chromosome 6 and it has limited polymorphism compared with the HLA-I molecules. 18 To date, only two main protein variants, HLA-E*01:01 and HLA-E*01:03, have been identified.19,20 The HLA-E protein is comprised of a heavy chain paired with a light chain (β2-microglobulin), with the former including extracellular α1-3 domains, a transmembrane region and intracellular domains. 21 HLA-E usually needs to be combined with oligopeptides (8–10 amino acids) to stabilize the cytomembrane expression and thus the level of HLA-E expression is mainly decided by the availability of these peptides. 22 HLA-E receptors (CD94/NKG2C, CD94/NKG2A and αβ TCR), expressed on different types of immune cells (NK cells, cytotoxic CD8 T-cells (CTLs) and NK-CTLs),23–26 can transfer activating or inhibiting signals. For example, when HLA-E binds to NKG2A, cytotoxicity of NK cells can be inhibited; when HLA-E interacts with CD94/NKG2C, NK cells can be activated. 26 The capability of HLA-E to transfer activating or inhibiting signals suggests that their key role may be complicated in regulating the immune response. The clinical significance of HLA-E in tumours is largely unknown. Some studies have linked the expression of HLA-E to the clinicopathological characteristics of tumours, but many contradicting results have been observed.14,27,28
In this current study, the level of HLA-E immunostaining was investigated and its predictive significance in ESCC was analysed. The current results showed that HLA-E immunostaining was upregulated in ESCC tissues compared with adjacent normal tissues, but there was no relationship with clinical parameters, such as lymph node metastasis, TNM stage and grade of differentiation. However, high levels of HLA-E immunostaining predicted a favourable survival outcome and it appeared to be an independent prognostic factor for patients with ESCC.
The clinical significance of HLA-E expression is different in other cancers. For example, a study that investigated the expression of HLA-E in 137 patients with colorectal carcinoma found that the overexpression of HLA-E was not an independent factor for overall survival, but there was still a correlation with tumour metastasis and poor prognosis. 14 In patients with early breast cancer, the expression of HLA-E resulted in a worse recurrence-free period, with an almost three-times higher risk of recurrence for HLA-E positive patients compared with HLA-E negative patients. 12 However, this current study found no correlation between HLA-E and TNM stage and found that high levels of HLA-E immunostaining predicted an improved prognosis in patients with ESCC. Similar to these current results, high expression of HLA-E led to longer survival in cervical adenocarcinomas 29 and glioblastoma, 28 although it was not an independent prognostic factor. In addition to membrane-bound HLA-E, soluble HLA-E (sHLA-E) expression detected in neuroblastoma was also found to be correlated with favourable overall survival, thus indicating a potential relationship between sHLA-E and immune surveillance. 30 Perhaps it is the complex regulatory function of HLA-E on cellular immune responses that leads to HLA-E showing different prognostic significance in different tumours. HLA-E can interact with both activating and inhibiting receptors on NK or CD8 T-cells, which may be related to the subtle balance between immune escape and surveillance in malignant tumours. For example, if the interaction between HLA-E and an activating receptor is dominant, it will play the role of immune surveillance to improve the prognosis of patients, which may be the underlying mechanism of the phenomenon that increased HLA-E immunostaining predicted a favourable prognosis for patients with ESCC in the present study. Conversely, if the interaction between HLA-E and an inhibiting receptor is dominant, the prognosis will be poor.
This current study had several limitations. First, these current results might have been affected by the limited number of patients that were studied. Secondly, the number of patients in TNM stage I and IV was small, so the patients were divided into two groups according to TNM stages I/II and III/IV. Thirdly, no in-depth molecular mechanism research was undertaken in this study.
In conclusion, this current study has shown for the first time the close relationship between the levels of HLA-E immunostaining in ESCC tumour specimens and the survival time of patients with ESCC. Higher levels of HLA-E immunostaining were a good prognostic factor for patients with ESCC. This finding might provide a novel target for the immunotherapy of ESCC. The role and underlying mechanism of HLA-E requires further functional studies.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The study was funded by the National Natural Science Foundation of China (Grant No. 81872237), the Science Technology Program of Zhejiang Province on Scientific Research Project (Grant No. GF19H160059, Y17H160289 and LQ18H160028) and Taizhou Science and Technology Planning Project (Grant No.1901ky06).
ORCID iD: Fa-Biao Zhang https://orcid.org/0000-0001-5612-414X
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. DOI: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herskovic A, Russell W, Liptay M, et al. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol 2012; 23: 1095–1103. DOI: 10.1093/annonc/mdr433. [DOI] [PubMed] [Google Scholar]
- 3.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6: 112–120. DOI: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose S, Panda AK, Mukherjee S, et al. Curcumin and tumor immune-editing: resurrecting the immune system. Cell Div 2015; 10: 6. DOI: 10.1186/s13008-015-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zhu B, Zhang M, et al. Roles of immune microenvironment heterogeneity in therapy-associated biomarkers in lung cancer. Semin Cell Dev Biol 2017; 64: 90–97. DOI: 10.1016/j.semcdb.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Jesinghaus M, Steiger K, Slotta-Huspenina J, et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget 2017; 8: 46756–46768. DOI: 10.18632/oncotarget.18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene 2016; 35: 5337–5349. DOI: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont C, Jacquier A, Verine J, et al. CD8(+)PD-1(−)ILT2(+) T Cells Are an Intratumoral Cytotoxic Population Selectively Inhibited by the Immune-Checkpoint HLA-G. Cancer Immunol Res 2019; 7: 1619–1632. DOI: 10.1158/2326-6066.cir-18-0764. [DOI] [PubMed] [Google Scholar]
- 9.Lo Monaco E, Tremante E, Cerboni C, et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia 2011; 13: 822–830. DOI: 10.1593/neo.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulberger CL, McMurtrey CP, Holzemer A, et al. Human Leukocyte Antigen F Presents Peptides and Regulates Immunity through Interactions with NK Cell Receptors. Immunity 2017; 46: 1018–1029. DOI: 10.1016/j.immuni.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King A, Allan DS, Bowen M, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol 2000; 30: 1623–1631. DOI: 10.1002/1521-4141(200006)30:6<1623::aid-immu1623>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.de Kruijf EM, Sajet A, van Nes JG, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol 2010; 185: 7452–7459. DOI: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti BR, Carvalho-Galano DF, Feitosa NL, et al. Differential expression of immune-modulatory molecule HLA-E in non-neoplastic and neoplastic lesions of the thyroid. Int J Immunopathol Pharmacol 2013; 26: 889–896. DOI: 10.1177/039463201302600407. [DOI] [PubMed] [Google Scholar]
- 14.Guo ZY, Lv YG, Wang L, et al. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell Immunol 2015; 293: 10–16. DOI: 10.1016/j.cellimm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lin A, Zhang X, Zhou WJ, et al. Human leukocyte antigen-G expression is associated with a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Cancer 2011; 129: 1382–1390. DOI: 10.1002/ijc.25807. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Lin A, Zhang JG, et al. Alteration of HLA-F and HLA I antigen expression in the tumor is associated with survival in patients with esophageal squamous cell carcinoma. Int J Cancer 2013; 132: 82–89. DOI: 10.1002/ijc.27621. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017; 12: 36–42. DOI: 10.1016/j.jtho.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyo CW, Williams LM, Moore Y, et al. HLA-E, HLA-F, and HLA-G polymorphism: genomic sequence defines haplotype structure and variation spanning the nonclassical class I genes. Immunogenetics 2006; 58: 241–251. DOI: 10.1007/s00251-005-0076-z. [DOI] [PubMed] [Google Scholar]
- 19.Strong RK, Holmes MA, Li P, et al. HLA-E allelic variants. Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J Biol Chem 2003; 278: 5082–5090. DOI: 10.1074/jbc.M208268200. [DOI] [PubMed] [Google Scholar]
- 20.Arnaiz-Villena A, Vargas-Alarcon G, Serrano-Vela JI, et al. HLA-E polymorphism in Amerindians from Mexico (Mazatecans), Colombia (Wayu) and Chile (Mapuches): evolution of MHC-E gene. Tissue Antigens 2007; 69: 132–135. DOI: 10.1111/j.1399-0039.2006.763_2.x. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan LC, Hoare HL, McCluskey J, et al. A structural perspective on MHC class Ib molecules in adaptive immunity. Trends Immunol 2006; 27: 413–420. DOI: 10.1016/j.it.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 22.O'Callaghan CA, Tormo J, Willcox BE, et al. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol Cell 1998; 1: 531–541. DOI: 10.1016/s1097-2765(00)80053-2. [DOI] [PubMed] [Google Scholar]
- 23.Wada H, Matsumoto N, Maenaka K, et al. The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur J Immunol 2004; 34: 81–90. DOI: 10.1002/eji.200324432. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser BK, Barahmand-Pour F, Paulsene W, et al. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J Immunol 2005; 174: 2878–2884. DOI: 10.4049/jimmunol.174.5.2878. [DOI] [PubMed] [Google Scholar]
- 25.Vales-Gomez M, Reyburn HT, Erskine RA, et al. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J 1999; 18: 4250–4260. DOI: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietra G, Romagnani C, Manzini C, et al. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J Biomed Biotechnol 2010; 2010: 907092. DOI: 10.1155/2010/907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva TG, Crispim JC, Miranda FA, et al. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol Histopathol 2011; 26: 1487–1497. DOI: 10.14670/hh-26.1487. [DOI] [PubMed] [Google Scholar]
- 28.Kren L, Slaby O, Muckova K, et al. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathology 2011; 31: 129–134. DOI: 10.1111/j.1440-1789.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 29.Spaans VM, Peters AA, Fleuren GJ, et al. HLA-E expression in cervical adenocarcinomas: association with improved long-term survival. J Transl Med 2012; 10: 184. DOI: 10.1186/1479-5876-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morandi F, Cangemi G, Barco S, et al. Plasma levels of soluble HLA-E and HLA-F at diagnosis may predict overall survival of neuroblastoma patients. Biomed Res Int 2013; 2013: 956878. DOI: 10.1155/2013/956878. [DOI] [PMC free article] [PubMed] [Google Scholar]