Abstract
Background
An. funestus is a major Afrotropical vector of human malaria. This study sought to investigate the larval ecology, sporozoite infection rates and blood meal sources of An. funestus in western Kenya.
Methods
Larval surveys were carried out in Bungoma (Highland) and Kombewa (lowland) of western Kenya. Aquatic habitats were identified, characterized, georeferenced and carefully examined for mosquito larvae and predators. Indoor resting mosquitoes were sampled using pyrethrum spray catches. Adults and larvae were morphologically and molecularly identified to species. Sporozoite infections and blood meal sources were detected using real-time PCR and ELISA respectively.
Results
Of the 151 aquatic habitats assessed, 62/80 (78%) in Bungoma and 58/71(82%) in Kombewa were positive for mosquito larvae. Of the 3,193 larvae sampled, An. funestus larvae constitute 38% (1224/3193). Bungoma recorded a higher number of An. funestus larvae (85%, 95%, CI, 8.722–17.15) than Kombewa (15%, 95%, CI, 1.33–3.91). Molecular identification of larvae showed that 89% (n = 80) were An. funestus. Approximately 59%, 35% and 5% of An. funestus larvae co-existed with An. gambiae s.l, Culex spp and An. coustani in the same habitats respectively. Of 1,221 An. funestus s.l adults sampled, molecular identifications revealed that An. funestus constituted 87% (n = 201) and 88% (n = 179) in Bungoma and Kombewa, respectively. The Plasmodium falciparum sporozoite rate of An. funestus in Bungoma and Kombewa was 2% (3/174) and 1% (2/157), respectively, and the human blood index of An. funestus was 84% (48/57) and 89% (39/44) and for Bungoma and Kombewa, respectively.
Conclusion
Man-made ponds had the highest abundance of An. funestus larvae. Multiple regression and principal component analyses identified the distance to the nearest house as the key environmental factor associated with the abundance of An. funestus larvae in aquatic habitats. This study serves as a guide for the control of An. funestus and other mosquito species to complement existing vector control strategies.
Introduction
Malaria is still the most devastating vector-borne disease in sub-Saharan Africa, contributing approximately 215 million cases in 2019, which accounted for about 94% of all global cases [1]. Anti-vectorial programmes consisting mainly of long-lasting insecticide-treated nets and indoor residual spraying have contributed immensely towards reducing malaria incidence and mortality in malaria-,endemic areas of sub-Saharan Africa, which are characterized with having high entomological inoculation rates (EIR, infective bites per person per year) [1–3]. Kenya is noted among 17 countries estimated to have attained a reduction in malaria incidence in 2020 compared to 2015 [1]. Despite this unprecedented success in the fight against malaria, the Global Technical Strategy (GTS) 2020 milestone for reducing mortality and morbidity has not been achieved globally [1].
Currently, there is no “silver bullet” to successfully achieve the elimination and eradication goal outlined by the GTS. An important component of malaria vector control that needs reconsideration in the malaria elimination and eradication agenda is larval source management. Historical records have shown that a key component of malaria eradication efforts in Israel, Italy, and the United States of America was source reduction through larval habitat modifications [4]. In malaria-endemic areas of Africa, the use of insecticide-treated net, when combined with larval control, has been predicted to reduce the number of adult mosquito emergence by 50% and subsequently decrease the entomological inoculation rate (EIR) up to 15 to 25-fold [5]. However, the use of larval control strategies requires adequate knowledge of the larval ecology of the vectors, as well as better characterization of their breeding habitats in different ecological settings [6]. Targeting the most productive breeding habitats for larval control can be cost-effective, depending on the anopheline species, heterogeneity of aquatic habitats, and proximity to human dwellings [7,8].
Anopheles funestus sensu stricto (s.s) (hereafter, An. funestus) is a major Afrotropical vector of human malaria, exhibiting anthropophilic, and endophilic behaviours [9,10]. In western Kenya, An. funestus is one of the principal vectors of human malaria, owing to its high resistance to pyrethroids used for bed net impregnation, high sporozoite rate, and persistence in indoor malaria transmission [11,12]. Historically, western Kenya witnessed a decline in An. funestus populations after the introduction of insecticide-based control tools [13] and this reduction was observed for some time until a resurgence was reported a decade ago [12,14]. While this resurgence might be influencing malaria transmission in western Kenya, very few studies have examined and characterized the larval habitats of An. funestus. Unlike reports on An. gambiae s.s. and An. arabiensis in this region, few studies have found An. funestus larvae in large permanent habitats with thick aquatic vegetation and algae [15,16].
Anopheles mosquitoes breed in a range of aquatic habitats and assessing findable habitats is key in controlling immature stages of vectors. However, locating breeding habitats of An. funestus is difficult, as it infrequently breeds in notable habitats with other malaria vectors. Hence, a better understanding and characterization of the breeding habitats and ovipositional behavioural patterns of An. funestus in endemic areas is crucial before larval source management can make malaria elimination and eradication feasible. Moreover, knowledge on Plasmodium falciparum infection rate and the feeding preference of An. funestus can help in predicting the intensity of malaria transmission. In this study, we characterized the aquatic habitats and the larval abundance of An. funestus. We also examined Plasmodium falciparum sporozoite infection rates and blood meal sources of indoor resting adults’ mosquitoes in highland and lowland areas.
Materials and methods
Study sites
This study was conducted in two distinct locations, a highland town (Bungoma) and a lowland town (Kombewa), situated about 55 km apart in western Kenya (Fig 1).
Fig 1. Map of the study areas in western Kenya.
The map was generated using ArcGIS Pro 2.6 software. Map source: ESRI, CGIAR, and USGS (available at: www.esri.com).
Bungoma [00.54057˚N, 034.56410˚E, 1386–1,545m above sea level (asl)] is characterized by a perennial malaria outbreak. The mean annual rainfall and temperatures are 150 mm and 22.5°C respectively [17]. The area previously had Plasmodium falciparum malaria episodes > 45% with a hospitalization rate up to 55% [18]. Agricultural production of crops (tobacco, cereals, sugar cane, onions and other vegetables) and raising of farm animals (cattle, sheep and goat) and poultry form the backbone of the rural economy of Bungoma. The principal vectors of human malaria in Bungoma are An. funestus, An. gambiae and An. arabiensis [19,20].
Kombewa (340 30’E, 00 07’N, 1150–1300 m asl) is located in Kisumu County, a lowland area with slow water drainage, in the Lake Victoria basin. This area experiences two rainy seasons: a long season from March to May characterized by peak malaria transmission and a short season between October and November. The mean annual rainfall and temperatures are 1200 mm-1300 mm and 20°C—35°C respectively [21]. There is, however, yearly variation in the rainfall pattern in the region. It is a hyperendemic malaria zone with a P. falciparum parasitemia rate of 57.5% [12] and EIR of 31.1 infective bites per person per year [11]. An. funestus has been reported to be the principal malaria vector predominating in this study area [11,22].
Larval sampling
Larval surveys were carried out monthly from November 2019 to November 2020. Breeding sites were identified within 2km of the study villages. Larvae were sampled using standard dippers (350 ml) and 10L bucket [23]. A maximum of 20 dips was taken at each habitat to identify the presence or absence of larvae and aquatic predators. Mosquito larvae were sampled along the edges of the habitats after waiting for 3–5 minutes for larvae to rise to the water surface if there were any. An. funestus sensu lato (s.l) larvae were preserved in absolute ethanol for subsequent identification using molecular analysis. Larvae were morphologically identified following referenced keys [24,25]. All breeding sites were georeferenced using a global positioning system installed in ODK software in Android Samsung tablet (Version SAM-T380). Larval habitats variables were characterized following a questionnaire that was imputed using ODK software.
Characterization of aquatic habitats
All potential breeding sites for mosquito larvae were identified and classified as: man-made pond, natural pond/rain pool, drainage ditch, swamp/marshes and tyre tracks. Man-made ponds/pits were made purposely for moulding clay pots in Bungoma, whereas in Kombewa, they were dug for sand winning. Aquatic habitats were classified as permanent if they could hold water for more than three weeks. Environmental variables including the presence of vegetation, category of vegetation, the height of vegetation, vegetation coverage, substrate type, and distance to a nearest house, habitat dimensions (length, width and depth), water physics (flow status and clarity) and present or absent of aquatic predators were recorded for each water body. In addition, the surrounding land use type (cultivated land/cropland, grassland/pasture, wetland/swamp and road) were also recorded.
The vegetation cover was classified as: emergent, freely floating, submerged, and no vegetation. The height of vegetation coverage was measured using a meter stick and grouped into < 5, 6–10, and > 10 m. The distance to the nearest dwellings was estimated and grouped as (A) < 100 m, (B) 100–200 m, and (C) 201–500 m. The types of plants and predators in the habitats were identified using picture charts. The dimensions (length, width and depth) of each habitat were measured using a meter stick, and the average depth was recorded. An Aquafluor™ meter (Model: 8000–010, Turner Design, San Jose, CA, USA) was used to measure cyanobacteria (blue-green algae) levels in water samples from the aquatic habitats.
The clarity of the water was observed and classified as (A) clear (transparent like a glass), (B) opaque (not transparent and impenetrable to light), (C) cloudy (normally appeared white in color) and (D) muddy/brownish (brown in color due to disturbance stirring its deposits). The substrate type was classified as (A) mud/dirty, (B) sand and (C) stone. Land-use types for the surrounding habitats were described as (A) cultivated land/cropland (farmland use for cultivation of food crops and other crops), (B) grassland/pasture (lands with suitable grasses for grazing animals) and (C) wetland/swamp (surroundings characterized by mostly aquatic plants species covered by water/ low-lying ground not cultivated and covered with water) and (D) road (land meant for passage of vehicles and people).
Adult sampling
To find out whether larval abundance correlated with the adult Anopheles density, adult mosquitoes were collected indoors using the pyrethrum spray catches [26] from randomly selected houses near the aquatic habitats from November 2019 to August 2020. The adult collection was carried out every two months during the sampling period. There was an equal distribution of houses in the study sites so sixty (60) houses were randomly selected near the larval habitats at each study site during each field visit. Houses were selected based on the presence of residents in the house, permission to get access into the indoor living rooms and proximity to nearby aquatic habitats. Collections were carried out in the morning hours between 06:30 to 10:00 h [27]. The physiological status of the anophelines was visually classified into blood-fed (abdomen is dilated and bright red), unfed (empty abdomen/no blood meal), half gravid (the abdomen is whitish posteriorly and dark reddish anteriorly) and gravid (dilated and whitish abdomen) [28]. Samples were stored in 1.5 ml Eppendorf tubes containing silica gel desiccant and cotton wool at -20° C in the Sub-Saharan Africa International Centre of Excellence for Malaria Research, Tom Mboya University College, Homa Bay, Kenya, for further analysis.
DNA extraction and molecular identification of species
Adult mosquitoes were cut into two parts to separate the head and thorax from the abdomen. DNA was extracted from the head and the thorax using the Chelex®-100 method [29], while the abdomen was preserved for blood meal analysis. DNA was extracted from the larvae of An. funestus s.l. using the ethanol precipitation method [30]. Molecular identification of sibling species of the An. funestus group was performed using multiplex polymerase chain reaction (PCR) by amplifying the polymorphic ITS2 region of ribosomal DNA using species-specific primers for An. funestus, An. rivulorum, An. vaneedeni, An. parensis, An. leesoni, An. rivulorum-like by following already developed protocols [31,32]. A sub-sample of 641 (551 adults and 90 larvae) were identified using PCR. Of this number, 20% (n = 110) of the adult mosquitoes and 11% (n = 10) of the larvae failed to amplify. Of the An. funestus s.l specimens that failed to amplify by PCR after three attempts, 65 (60 adults and 5 larvae) were randomly selected and sent to the University of California, Irvine, for sequencing using the Sanger sequencing method. The ITS2 region of nuclear ribosomal DNA was amplified using the forward primer ITS2A (TGTGAACTGCAGGACACAT) and the reverse primer ITS2B (TATGCTTAAATTCAGGGGGT); amplicons were sequenced using ABI Big Dye Terminator Cycle Sequencing Kits, as described by Daibin et al., [33].
Plasmodia species genotyping using multiplex RT-PCR
A modified TaqMan assay was used to detect Plasmodia species (P. falciparum, P. ovale and P. malarae) infections in the DNA samples as previously described [34,35]. Plasmodium falciparum species-specific 18S ribosomal RNA primers (P. falciparum forward primer 5-ATTGCTTTTGAGAGGTTTTGTTACTTT-3 and reverse primer 5-GCTGTAGTATTCAAACACAATGAACTCAA-3, P. malariae forward primer 5-AGTTAAGGGAGTGAAGACGATCAGA-3 and reverse primer 5-CAACCCAAAGACTTTGATTTCTCATAA-3 and P. ovale forward and reverse primer 5-AACCCAAAGACTTTGATTTCTCATAA-3 and 5-CCGACTAGGTTTTGGATGAAAGATTTTT-3) and probes (FAM- CATAACAGACGGGTAGTCAT-MGB for P. falciparum, VIC-ATGAGTGTTTCTTTTAGATAGC-MGB for P. malariae and (NED- CGAAAGGAATTTTCTTATT-MGB for P. ovale) were used. A final volume of 12μl containing 2 μl of sample DNA, 6 μl of PerfeCTa® qPCR ToughMix™, Low ROX™ Master mix (2X), 0.5 μl of each probe, 0.4 μl of each forward primers (10 μM), 0.4 μl of each reverse primers (10 μM) and 0.1 μl of double-distilled water was loaded in QuantStudio™ 3 Real-Time PCR System. The thermal profile used was set as follows; 50°C for 2 min, (95°C for 2 min, 95°C for 3 sec and 58°C for 30 sec) for 45 cycles. The real-time PCR was performed in the QuantStudio 3 real-PCR instrument (Applied Biosystems, Thermo Fisher Scientific). The Plasmodium infection was analyzed by comparing melt curves with the positive controls using QuantSudio TM Design and Analysis Desktop software v1.5.1.
Blood meal analysis
Direct enzyme-linked immunosorbent assays (ELISA) was used to detect the origin of blood meal in the abdomen of blood-fed Anopheles mosquitoes following existing protocol [36]. Briefly, 50μl of phosphate-buffered saline (PBS) was added to the abdomen of each mosquito specimen and was incubated overnight. Following grinding, 950μl of PBS was added to each sample for washing and 50μl of each sample was loaded into each of the wells of the ELISA plate and incubated for two hours. Hosts specific positive controls and negative control from unfed lab-raised mosquitoes were also added. Following incubation and washing, anti-host specific conjugates (antibodies) against human, goat, chicken and dog proteins were added to the wells. ABTS (2, 2’-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) peroxidase substrate for each human, goat, chicken and dog blood meal source was added to each of the wells. The samples were incubated for thirty minutes for the reaction to occur. The non-reacting samples were tested subsequently using bovine immunoglobulin G. All ELISA results were read visually [37].
Ethical statement
This study was approved by Maseno University’s Ethics Review Committee (MUERC/00778/19). Verbal consent was sought and granted from heads of households and owners of farmlands where the adult mosquitoes and larvae were sampled.
Data analysis
Data were analyzed using SPSS software (Version 21 for Windows, SPSS Inc., Chicago, IL) and Graph Pad Prism V.8.0.1. The relative abundance of each species was expressed as a percentage of the number of larvae per species divided by the total number of larvae collected for all species combined per habitat type. The relative abundance of An. funestus was calculated as the number of An. funestus larvae from a specific habitat divided by the total number of An. funestus larvae in all samples from a habitat type.
The types of breeding sites, the number of mosquito larvae sampled and species, and the number of aquatic predators were presented in tables and figures. The Kruskal–Wallis test was used to compare the number of larvae in different habitat types and for samples having more than two groups: habitat type (man-made pond, natural pond/rain pool, drainage ditch, swamp/marshes, tyre tracks), distance to the nearest house (<100, 100–200, 201–500), water clarity (clear, opaque, brownish/muddy, cloudy), aquatic plant species in a habitat [Pennisetum purpureum (elephant grass), Schoenoplectus californicus, others/unknown], and land-use type (cultivated land/cropland, grassland/pasture, wetland/swamp, road). The Mann–Whitney U test was used to compare samples with two variables: vegetation (present or absent), category of vegetation (emergent or non-emergent), water flow status (stagnant or flowing water), and predators (present or absent). Multiple regression and principal components analyses were used to identify environmental variables associated with the abundance of An. funestus in the aquatic habitats. The human blood index (HBI) was calculated as the percentage of Anopheles mosquitoes that fed on humans over the total number of blood-fed Anopheles for which the blood meal origins were determined. The sporozoite rate of P. falciparum was calculated as the proportion of Anopheles tested for sporozoites over the total genotyped. Spearman’s correlation (rs) was used to find the relationship between the adult An. funestus population and the larval density at each study site. The results were considered statistically significant at P <0.05. For the sequence data analysis of the An. funestus s.l. specimen that failed to amplify by PCR, the de novo assembly of reads was performed using geneious software [38]. Basic Local Alignment Search Tool (BLAST) was used to identify sequence similarities against sequences in GeneBank. Sequences with high identity scores or low E-value were retrieved and used in the construction of a phylogenetic tree to identify the unknown. Evolutionary analyses were conducted in MEGA X after basic alignment using ClustalW algorithm [39]. All sequences of ITS2 are available at GenBank under accession numbers MZ435355-MZ435414.
Results
Distribution of An. funestus larvae and other mosquito larvae in Bungoma and Kombewa
A total of 151 potential mosquito aquatic habitats were assessed. Of these, 62/80 (78%; 95% CI: 0.68–0.87) and 58/71 (82%; 95% CI: 0.73–0.91) in Bungoma and Kombewa were positive for mosquito larvae, respectively. The number of aquatic habitats with An. funestus larvae in Bungoma was 55/80 (69%; 95% CI; 0.58–0.79), whereas 23/71 (32%; 95% CI: 0.21–0.43) were in Kombewa. In all, An. funestus larval habitats constituted 65% (n = 78) of the mosquito-positive habitats, whereas An. gambiae s.l, An. coustani, and Culex spp positive habitats made up of 57% (n = 69), 14% (n = 17) and 36% (n = 43), respectively.
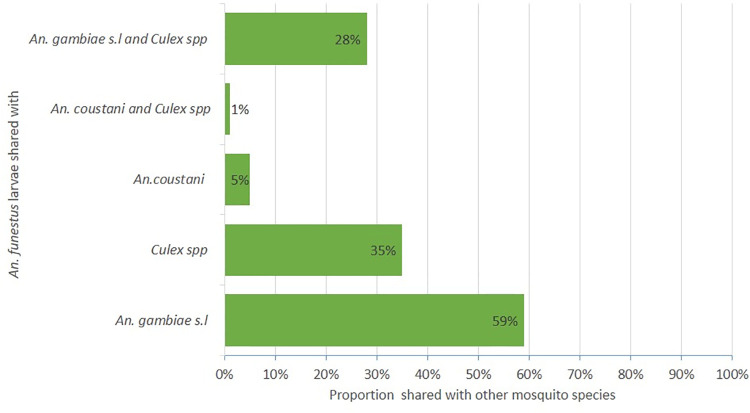
A total of 3,193 mosquito larvae (39% An. funestus, 30% An. gambiae s.l., 28% Culex spp. and 3% An. coustani) were collected from the various habitats in Bungoma and Kombewa. Bungoma had a higher number of An. funestus larvae (85% 95%, CI, 8.722–17.15) than Kombewa (15% 95%, CI, 1.33–3.91). Approximately 59% of An. funestus larvae were collected from breeding sites with co-existing An. gambiae s.l larvae in the same habitats (Fig 2). Similarly, 35% and 5% of An. funestus larvae were found co-existing with Culex spp., and An. coustani larvae, respectively (Fig 2). No pupae were identified during the sampling. Molecular results on the sibling species of the An. funestus group larvae revealed that 89% (n = 80) were An. funestus, 11% (n = 9) were An. rivulorum and 1% (n = 1) was An. sp.9.
Fig 2. Proportion of An. funestus larvae shared with other mosquitoes in the larval habitats.
Characteristics of mosquito larval habitats
An. funestus larvae were found in various habitats, with or without vegetation: man-made ponds, natural ponds/rain pools, drainage ditches, swamp/marshes, and tyre tracks. There were no significant differences in An. funestus larval density between the various habitat types of An. funestus (χ2 = 8.917, df = 4, P = 0.063). However, man-made ponds comprised the highest number of An. funestus positive habitats (36%, n = 28) and had the highest proportion of larvae with An. funestus in Bungoma (53%, n = 553) and in Kombewa (61%, n = 115) (Table 1). The larval abundance of An. funestus in man-made ponds, natural ponds/rain pools and drainage ditches was significantly different between the study sites (P< 0.05) (Table 1). Field observations showed that man-made ponds constituted the main permanent aquatic habitat type in the study areas. This was followed by swamp/marshes, natural ponds/rain pools, drainage ditches and tyre tracks, at frequencies of 27%, 19%, 15%, and 3%, respectively. There was no significant difference in the means among the various predators found in the larval habitats (P = 0.05). Fig 3 shows the mean distribution of the various predators.
Table 1. Number of different mosquito species collected from aquatic habitats in Bungoma and Kombewa in Kenya in the months of November 2019 to November 2020.
| Study site | Habitat | Larval Counts per Mosquito Species (n (%)) | ||||
|---|---|---|---|---|---|---|
| Type | N | An. funestus s.l | An. gambiae s.l | An. coustani | Culex spp | |
| Bungoma | Man-made ponds | 26 | 553 (53)a | 81(37) | 0(0) | 15(47)i |
| Natural ponds/rain pools | 12 | 100 (10) c | 2(1)g | 0(0) | 0(0) | |
| Drainage ditches | 19 | 374(36) e | 120(54) | 0(0) | 3(9)k | |
| Swamps/marshes | 21 | 8(1) | 18(8) | 0(0) | 14(44) | |
| Tyre tracks | 2 | 0(0) | 0(0) | 0(0) | 0(0) | |
| Kombewa | Man-made ponds | 20 | 115(61)b | 67(9) | 33(32) | 162(19)j |
| Natural ponds/rain pools | 11 | 25(13) d | 249(34)h | 24(23) | 188(21) | |
| Drainage ditches | 17 | 40(21)f | 286(39) | 47(45) | 489(56)l | |
| Swamp/marshes | 22 | 9(5) | 137(18) | 0(0) | 34(4) | |
| Tyre tracks | 1 | 0(0) | 0(0) | 0(0) | 0(0) | |
| Bungoma | Total | 80 | 1035 | 221 | 0 | 32 |
| Kombewa | Total | 71 | 189 | 739 | 104 | 873 |
N is the number of habitats and n is the number of mosquitoes of different species in each habitat type. a and b, c and d, e and f, g and h, i and j, k and l superscripts indicate statistical significance at P<0.05 between the number of mosquito larvae in each habitat type between the study sites.
Fig 3. Distribution of aquatic predators in the various habitats.
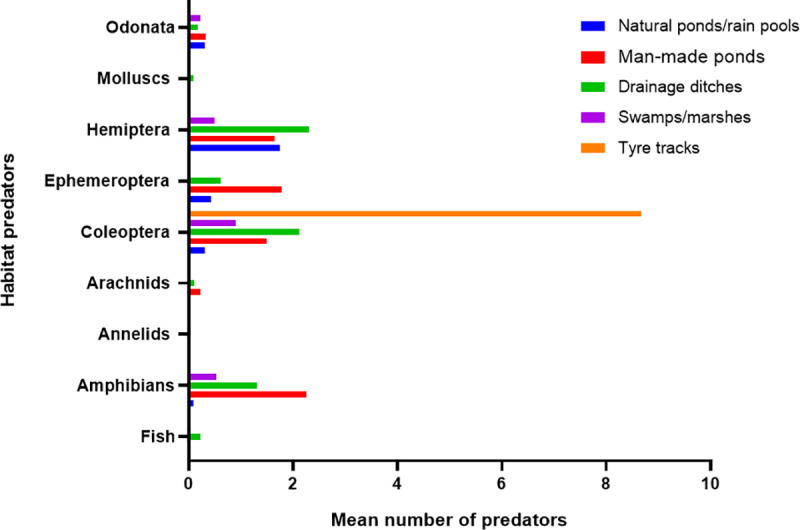
Odonata: damselfly/dragonfly, Molluscs: snails/slugs/mussels, Hemiptera: backswimmer/giant water bugs/cacidas, Ephemeroptera: mayfly, Coleoptera: water beetles/weevils, Arachnids: spiders/ticks/mites, Annelids: Segmented worms, Amphibians: frogs/toads/tadpoles, Fish: tilapia.
Association between environmental variables and An. funestus larval abundance
The various environmental variables in the larval habitats associated with the presence of An. funestus larvae and other mosquito species are summarized in Table 2.
Table 2. Characteristics of aquatic habitats of An. funestus and other mosquito species at Bungoma and Kombewa western Kenya (November 2019-November, 2020).
| Environmental variable | All aquatic breeding sites n (%) | Potential habitats without mosquito larvae n (%) | Aquatic habitats with An. funestus n (%) | Aquatic habitats with An. gambiae s.l n (%) | Aquatic habitats with An. coustani n (%) | Aquatic habitats with Culex spp n (%) |
|---|---|---|---|---|---|---|
| Habitat type | ||||||
| Man-made pond | 46 (31) | 6 (19) | 28(36) | 13(19) | 6(35) | 12(28) |
| Natural pond/rain pool | 23(15) | 3 (10) | 15(19) | 13(19) | 4(24) | 6(14) |
| Drainage ditch | 36(24) | 12(39) | 12(15) | 19(27) | 1(6) | 8(19) |
| Swamp/marshes | 43(28) | 10(32) | 21(27) | 22(32) | 6 (35) | 16(37) |
| Tyre tracks | 3(2) | 0(0) | 2(3) | 2(3) | 0(0) | 1(2) |
| Vegetation | ||||||
| Present | 133(88) | 28 (90) | 67(86) | 63(91) | 17(100) | 41(95) |
| Absent | 18(12) | 3 (10) | 11(14) | 6(9) | 0(0) | 2(5) |
| Category of vegetation | ||||||
| Emergent | 101(76) | 19(68) | 48(72) | 53(84) | 14(82) | 34(83) |
| Free floating | 29(22) | 9(32) | 16(24) | 8(13) | 3(18) | 5(12) |
| Submerged | 3(2) | 0(0) | 3(4) | 2(3) | 0(0) | 2(5) |
| Water Flow Status | ||||||
| Stagnant/standing water | 145(96) | 31(100) | 74(95) | 64(93) | 17(100) | 40(93) |
| Flowing/Disturbed Water | 6(4) | 0(0) | 4(5) | 5(7) | 0 | 3(7) |
| Predators | ||||||
| Present | 100(66) | 12(39) | 54(69) | 56(81) | 16(94) | 35(81) |
| Absent | 51(34) | 19(61) | 24(31) | 13(19) | 1(6) | 8(19) |
| Distance to Nearest House (m) | ||||||
| <100 | 39(26) | 17(55) | 12(15) | 11(16) | 4(24) | 10(23) |
| 100–200 | 76(50) | 13(42) | 38(49) | 34(49) | 8(47) | 25(58) |
| 201–500 | 36(24) | 1(3) | 28(36) | 24(35) | 5(29) | 8(19) |
| Water Clarity | ||||||
| Clear | 43(28) | 11(35) | 26(33) | 17(25) | 6(35) | 11(25) |
| Opaque | 69(46) | 13(42) | 39(50) | 27(39) | 9(53) | 18(42) |
| Cloudy | 30(20) | 4(13) | 10(13) | 21(30) | 2(12) | 12(28) |
| Muddy/Brownish | 9(6) | 3(10) | 3(4) | 4(6) | 0(0) | 2(5) |
| Land-use type | ||||||
| Cultivated land/cropland | 82(54) | 18(58) | 56(72) | 36(52) | 0(0) | 13(30) |
| Grassland/Pasture | 61(40) | 11(36) | 18(23) | 31(45) | 16(94) | 27(63) |
| Wetland/swamp | 4(3) | 1(3) | 2(2.5) | 2(3) | 0(0) | 0(0) |
| Road | 4(3) | 1(3) | 2(2.5) | 0(0) | 1(6) | 3(7) |
Multiple regression analysis models revealed that distance to the nearest house (P = 0.0122) was the best predictor of An. funestus larval density in the habitats (Table 3). The F-ratio in the ANOVA table revealed that, the An. funestus larval density was significantly associated statistically with key habitat variables (habitat type, land use type, vegetation coverage, vegetation height, distance to house, habitat size, water depth, water clarity, algae abundance and predator counts) at the time of larval sampling (F = 2.06, d.f = 10, 114, P = 0.033, R2 = 0.153). Furthermore, principal component analysis identified the distance to the nearest house as the key environmental factor associated with the abundance of An. funestus larvae (S1 Fig).
Table 3. Multiple regression analysis of factors associated with An. funestus larval density.
| Habitat variable | Beta | Std.Err. of Beta | B | Std.Err. of B | t(114) | P-level |
|---|---|---|---|---|---|---|
| Habitat Type | -0.0876 | 0.0984 | -0.0613 | 0.0688 | -0.8902 | 0.3752 |
| Land use type | -0.1816 | 0.1047 | -0.2464 | 0.1420 | -1.7348 | 0.0855 |
| Vegetation coverage | 0.0957 | 0.0997 | 0.0024 | 0.0025 | 0.9598 | 0.3392 |
| Vegetation Height | -0.0219 | 0.0890 | -0.0058 | 0.0236 | -0.2466 | 0.8056 |
| Distance to house | 0.2355 | 0.0925 | 0.0016 | 0.0006 | 2.5468 | *0.0122 |
| Habitat size | -0.0594 | 0.0929 | -0.0006 | 0.0009 | -0.6391 | 0.5240 |
| Water depth | 0.1088 | 0.0894 | 0.2402 | 0.1975 | 1.2163 | 0.2264 |
| Water clarity | 0.0171 | 0.1065 | 0.0033 | 0.0207 | 0.1605 | 0.8728 |
| Algae abundance | -0.1588 | 0.0980 | -0.0014 | 0.0008 | -1.6193 | 0.1081 |
| Predator counts | -0.0568 | 0.0909 | -0.0045 | 0.0072 | -0.6254 | 0.5329 |
Beta standardized coefficient, B the unstandardized coefficient value, * significant at P<0.05.
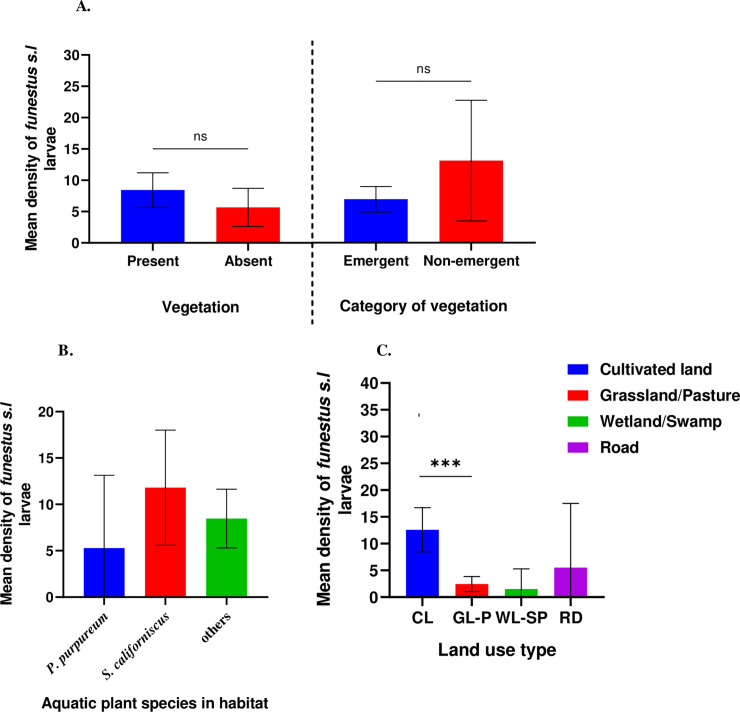
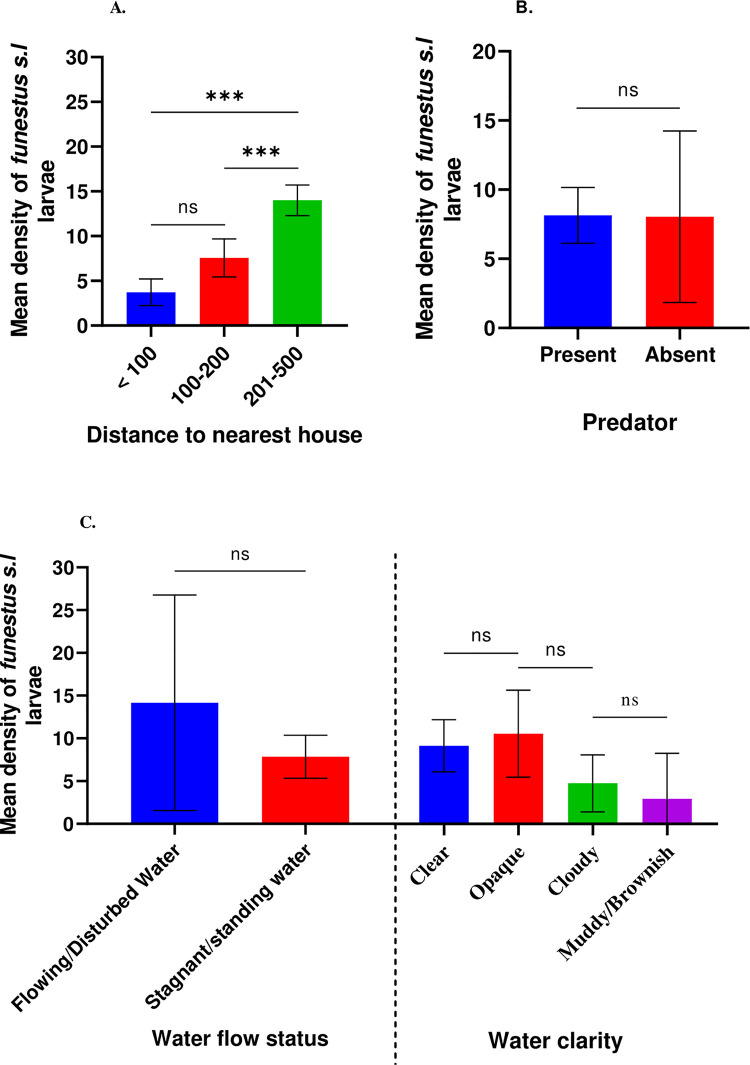
The Mann–Whitney U and Kruskal–Wallis tests showed that An. funestus larval density had no significant difference between present and absent of vegetation (U = 1192, P = 0.978) (Fig 4A), emergent and non-emergent vegetation (U = 1369, P = 0.166) (Fig 4A), stagnant and flowing water (U = 287, P = 0.136) (Fig 5C), present and absent of aquatic predators (U = 2215, P = 0.162) (Fig 5B), among clear, opaque, cloudy and muddy/brownish water (χ2 = 7.316, df = 3, P = 0.062) (Fig 4C), or with the presence of Pennisetum purpureum, Schoenoplectus californiscus and other aquatic plant species in the habitats (χ2 = 2.671, df = 2, P = 0.263) (Fig 4B) respectively. However, An. funestus larval density showed statistically significant differences associated with distances to the nearest house (<100, 100–200 and 201–500 m) (χ2 = 25.138, df = 2, P<0.0001) (Fig 5A) and land-use types (cultivated land/cropland, grassland/pasture, wetland/swamp and road) (χ2 = 29.197, df = 3, P = 0.000) (Fig 4C). Our field observation showed that An. funestus is most abundant in habitats surrounded by cultivated land compared to those in the grassland areas, wetlands, and roads.
Fig 4. Association of habitat variables with An. funestus density (A) vegetation (present and absent) and category of vegetation (emergent and non-emergent), (B) aquatic species in the habitats, (C) land use type.
Fig 5. Association of habitat variables with An. funestus larval density (A) distance to the nearest house, (B) predator (present and absent in habitats) and (C) water flow status and water clarity.
Relationship between larval abundance and adults mosquitoes sampled indoors
The results of the Pearson correlation test showed that there was a statistically significant but weakly positive linear relationship between An. funestus larval abundance and the presence of adult mosquitoes collected indoors in Bungoma (rs = 0.178, P = 0.026). However, there was no significant relationship between An. funestus larval abundance and adult mosquitoes sampled indoors in Kombewa (rs = 0.003, P = 0.972).
Composition of indoor resting Anopheles mosquitoes
A total of 1,221 An. funestus s.l and 195 An. gambiae s.l were collected indoors in Bungoma and Kombewa. Of the 1,221 An. funestus s.l sampled, 46% (n = 565) and 54% (n = 656) were collected from Bungoma and Kombewa respectively. For molecular identification, a sub-sample of 551 Anopheles comprising 380 An. funestus s.l and 171 An. gambiae s.l from the study sites were analysed. Of the An. funestus s.l. analysed, 201 and 179 were from Bungoma and Kombewa, respectively. Our results revealed that An. funestus predominated in the two study areas: An. funestus constituted 87% (n = 201) and 88% (n = 179) in Bungoma and Kombewa, respectively, while An. rivulorum constituted 13% (n = 201) and 12% (n = 179) for Bungoma and Kombewa, respectively.
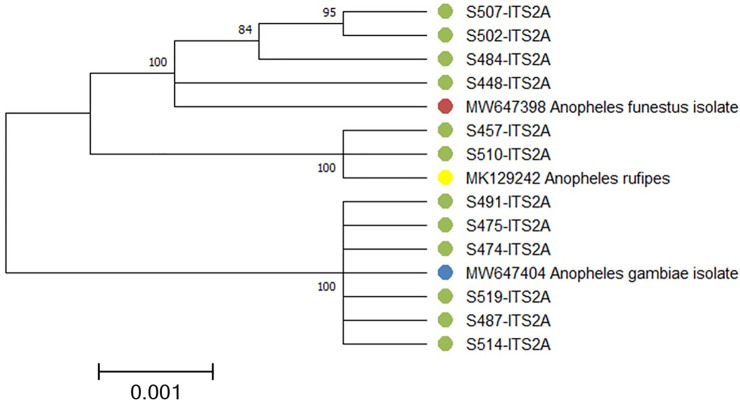
Of the 171 An. gambiae s.l. analysed, 76 and 95 were from Bungoma and Kombewa, respectively. An. gambiae was the main species identified in Bungoma (86%, n = 76) and Kombewa (81%, n = 95). An. arabiensis made up 15% (n = 76) and 19% (n = 95) in Bungoma and Kombewa, respectively. The evolutionary relationship of the unamplified sequenced data is shown in Fig 6.
Fig 6. Evolutionary relationships of taxa.
The evolutionary history was inferred using the Neighbor-Joining method [40]. The optimal tree with the sum of branch length = 3.05389016 is shown (Fig 6). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [41]. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modelled with a gamma distribution (shape parameter = 1). This analysis involved 15 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There was a total of 815 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [42].
Plasmodium falciparum (Pf) sporozoite infection rate
All the sub-samples (n = 551) subjected to molecular identification were genotyped to detect sporozoite. The Pf sporozoite rate of An. funestus in Bungoma and Kombewa was 2% (3/174) and 1% (2/157), respectively (Table 4). However, none of the An. rivulorum found in Bungoma and Kombewa was positive for Pf sporozoite (Table 4). Only one An. gambiae mosquito from Kombewa (1%, 1/77) tested positive for Pf sporozoite. However, sporozoites were not detected in An. arabiensis in the study sites.
Table 4. Sporozoite rate of Anopheles mosquitoes sampled from indoors in Bungoma and Kombewa, November 2019 to November 2020.
| Site | An. gambiae s.l | No. tested | Pf +ve (%) | An. funestus s.l | No. tested | Pf +ve (%) |
|---|---|---|---|---|---|---|
| Bungoma | An. gambiae | 65 | 0 (0) | An. funestus | 174 | 3 (2) |
| An. arabiensis | 11 | 0 (0) | An. rivulorum | 27 | 0 (0) | |
| Total | 76 | 201 | ||||
| Kombewa | An. gambiae | 77 | 1 (1) | An. funestus | 157 | 2 (1) |
| An. arabiensis | 18 | 0 (0) | An. rivulorum | 22 | 0 (0) | |
| Total | 95 | 179 |
Numbers in the bracket indicate sporozoite rate; Pf, Plasmodium falciparum, +ve, positive.
Anopheles blood meal origins
A total of 208 samples (Bungoma, n = 114 and Kombewa, n = 94) were analysed for the origin of the mosquito blood meals. The HBI of An. funestsus was 84% (48/57) and 89% (39/44) for Bungoma and Kombewa, respectively (Table 5). Table 5 shows the HBI for all species tested.
Table 5. Blood meal sources of Anopheles mosquitoes sampled from indoors in Bungoma and Kombewa, November 2019 to November 2020.
| Site | Species | No. tested | Blood meal sources | HBI | ||
|---|---|---|---|---|---|---|
| human | bovine | goat | ||||
| Bungoma | An. gambiae | 43 | 21 | 15 | 7 | 49 |
| An.arabiensis | 6 | 4 | 2 | 0 | 67 | |
| An. rivulorum | 8 | 6 | 2 | 0 | 75 | |
| An. funestus | 57 | 48 | 7 | 2 | 84 | |
| Total | 114 | 79 | 26 | 9 | ||
| Kombewa | An. gambiae | 34 | 20 | 10 | 4 | 59 |
| An. arabiensis | 3 | 3 | 0 | 0 | 100 | |
| An. rivulorum | 13 | 10 | 3 | 0 | 77 | |
| An. funestus | 44 | 39 | 3 | 2 | 89 | |
| Total | 94 | 72 | 16 | 6 | ||
HBI, human blood index.
Discussion
The great diversities of anopheline larval habitats in addition to their inaccessibility makes larval ecology studies of malaria mosquitoes methodologically cumbersome [15]. The presence of quality larval habitats is significant in determining the abundance and distribution of adult mosquitoes. This study was designed to add to the limited amount of information on the larval ecology of An. funestus in western Kenya. Two study areas were selected, a highland site (Bungoma) and a lowland site (Kombewa), and their aquatic habitats were examined and characterized to determine if there have been changes in the breeding habitats of An. funestus in the village sites. This study revealed that An. funestus is a major vector influencing malaria transmission in the region, confirming a previous report that An. funestus has re-emerged and could be responsible for malaria transmission in western Kenya [12].
Our findings revealed that An. funestus larvae thrive in a wide range of aquatic habitats and co-breeds with other malaria vectors in the same habitats. Although there were no significant differences observed in the various habitats types, man-made ponds had the highest proportion of An. funestus larvae. Man-made ponds, created mostly for making clay pots and sand winning, contributed remarkably to the proportion of An. funestus habitats and larvae abundance in the study areas. This corroborates previous findings in western Kenya where man-made habitats accounted for an increase in populations of An. gambiae [43,44]. Field observations have shown that man-made ponds are permanent habitats that hold water for a longer period compared to other habitat types. This suggests that malaria transmission in the study areas is partly man-made, and thus, proper environmental management specifically through habitat manipulation could curtail malaria transmission by major vectors of human malaria. This study confirms how anthropogenic modification of ecosystems can contribute greatly to the abundance and distribution of malaria vectors [45].
We found that more than 50% of An. funestus larvae co-existed in aquatic habitats with An. gambiae s.l larvae. Moreover, An. funestus shared the same habitats with Culex spp and An. coustani. Previous studies in neighbouring countries, Tanzania [46] and Uganda [47] have reported that An. funestus shared habitats with other Anopheles and Culex spp., indicating that any larval control programme targeting An. funestus would have a profound effect in controlling other equally important vectors of human malaria and other mosquito-borne diseases.
Hitherto, it has been reported that An. funestus prefers breeding in aquatic habitats with thick vegetation [15,16] but this study revealed that An. funestus can breed in habitats with aquatic vegetation or without vegetation. The presence of vegetation has been noted to be an important environmental variable associated with Anopheles mosquito larvae density [48]. For example, aquatic macrophytes play important role in the oviposition, larval survival and development of anophelines as they serve as a food source, protection for the larval stages and provide enabling environment for mosquito breeding [49–52]. However, this study revealed that An. funestus can breed in habitats with or without aquatic vegetation. Our data showed that there was no significant difference in the means between habitats with aquatic vegetation and habitats without aquatic vegetation.
Multiple linear regression analysis showed that the abundance of algae in the habitat was not a predicting factor for the density of An. funestus in this study. However, Gimnig et al [15] noted that algae abundance was positively correlated with An. gambiae density in western Kenya and also an important factor predicting the abundance of An. pseudopunctipennis in the Americas [53] and crucial for the development of An. pretoriensis in the Rift Valley province of Ethiopia [54].
While Aklilu et al [54] noted that algae abundance was an important factor, distance to the nearest house was not an important component associated with An. gambiae s.l and An. pretoriensis abundance in their study. However, the proximity of productive larval habitats to human or animal habitation to obtain a blood meal can determine the density of adult mosquitoes [55]. In western Kenya, a previous study reported that the distance to the nearest house was significantly associated with the abundance of An. gambiae [56]. Among the environmental variables assessed in our study, principal component and multiple linear regression analyses identified the distance to the nearest house as a major predictor of An. funestus abundance in habitats, in agreement with Minakawa et al [56] for An gambiae. Our findings suggest that larval source management targeting An. funestus aquatic habitats located near houses could reduce the adult mosquito population. The implementation of integrated vector management will also help to control both the aquatic stages and adults vectors in both study sites.
Aquatic predators are well known to influence the abundance of mosquito larvae in breeding environments and are considered beneficial biological control agents of mosquito larvae [57–60]. Notwithstanding, there was no significant difference in An. funestus larval density between aquatic habitats with predators and habitats without predators. A similar study by Ndenga et al [61] noted that the presence of predators was not significantly associated with the low density of An. gambiae s.l larvae. Conversely, a previous study in Tanzania [62] and central Sudan [63] reported that most predators were identified in habitats with fewer densities of mosquito larvae. However, we witnessed that there was no reduction in the density of An. funestus larvae in the presence of predators in shared habitats. This could be ascribed to the presence of other prey in larval habitats. Kumar et al [64] documented that in the presence of alternative prey, the habitats, the larval consumption-ability of predators was significantly reduced in the habitats.
This study revealed that An. funestus was the predominant indoor resting vector corroborating the findings of previous investigations in Bungoma [19,20] and Kombewa [12,22]. The relative abundance, high sporozoite rate and HBI of An. funestus suggest that it is the main vector mediating malaria transmission in the study areas. We speculate that the adaptation of An. funestus to breed in warmer, open sunlit habitats may significantly reduce the developmental time of larval stages and increase the adult population of this species.
We acknowledge, however, the following limitations of our study: first, this study did not integrate detailed water chemistry analysis as part of the variable in assessing the larval ecology of An. funestus. Hence, further studies should be undertaken to assess the physicochemical characteristics of aquatic habitats that allow the co-existence of An. funestus with other mosquito species. Second, we were unable to examine the productivity of An. funestus aquatic habitats and their ability to facilitate the development of larvae to emerged adults.
Conclusion
An. funestus was found breeding in a variety of aquatic habitats and co-existing with larvae of other mosquito species and aquatic predators. An. funestus was found in permanent and semi-permanent aquatic habitats, with or without aquatic vegetation, slow-moving/disturbed or stagnant water that was clear, opaque, cloudy and brownish. The only significant factor predicting the abundance of An. funestus in the aquatic habitats was the distance to the nearest house. Thus, larval control programmes should aim at targeting aquatic habitats near human dwellings to reduce the abundance of adult An. funestus. This study serves as a guide for the control of aquatic stages of An. funestus using larval source management or larviciding to complement existing vector control strategies. The relative abundance, high sporozoite rate, and HBI also confirm the importance of An. funestus in malaria transmission and the need for continuous vector surveillance before implementing vector control interventions.
Supporting information
(TIF)
Acknowledgments
We extend our sincerest gratitude to all community leaders, farmland owners and heads of households of the Bungoma and Kombewa communities for permitting us to collect mosquitoes and larvae from their houses and farmlands.
Data Availability
All sequences of ITS2 are available at GenBank under accession numbers MZ435355-MZ435414.
Funding Statement
GY This study was supported by grants from the National Institute of Health (R01 A1123074, U19 AI129326, R01 AI050243, and D43 TW001505). There was no additional external funding received for this study. The funders, however did not play any role in designing, data collection and manuscript writing.
References
- 1.WHO, World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. 2020.
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526: 207. doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7. doi: 10.1371/journal.pmed.1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitron U, Spielman A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Rev Infect Dis. 1989;11: 391–406. doi: 10.1093/clinids/11.3.391 [DOI] [PubMed] [Google Scholar]
- 5.Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. The potential impact of integrated malaria transmission control on entomologic inoculation rate in highly endemic areas. Am J Trop Med Hyg. 2000;62: 545–551. doi: 10.4269/ajtmh.2000.62.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zogo B, Koffi AA, Alou LPA, Fournet F, Dahounto A, Dabiré RK, et al. Identification and characterization of Anopheles spp. breeding habitats in the Korhogo area in northern Côte d’Ivoire: a study prior to a Bti-based larviciding intervention. Parasit Vectors. 2019;12: 146. doi: 10.1186/s13071-019-3404-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu W, Utzinger J, Novak RJ. Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg. 2008;78: 2–6. [PubMed] [Google Scholar]
- 8.Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21: 2–21. doi: 10.1111/j.1365-2915.2007.00674.x [DOI] [PubMed] [Google Scholar]
- 9.Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Anophelinae Afr South Sahara Ethiop Zoogeographical Reg. 1968. [Google Scholar]
- 10.Dia I, Guelbeogo MW, Ayala D. Advances and Perspectives in the Study of the Malaria Mosquito Anopheles funestus. Anopheles Mosquitoes-New Insights Malar Vectors. 2013;10: 55389. [Google Scholar]
- 11.Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, et al. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol. 2014;43: 200–206. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Lee M-C, Githeko AK, Atieli HE, Yan G. Insecticide-treated net campaign and malaria transmission in western Kenya: 2003–2015. Front Public Health. 2016;4: 153. doi: 10.3389/fpubh.2016.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, et al. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68: 16–22. [PubMed] [Google Scholar]
- 14.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90: 597–604. doi: 10.4269/ajtmh.13-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol. 2001;38: 282–288. doi: 10.1603/0022-2585-38.2.282 [DOI] [PubMed] [Google Scholar]
- 16.Minakawa N, Sonye G, Dida GO, Futami K, Kaneko S. Recent reduction in the water level of Lake Victoria has created more habitats for Anopheles funestus. Malar J. 2008;7: 1–6. doi: 10.1186/1475-2875-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juma SG, Kelonye F. Projected rainfall and temperature changes over Bungoma county in western Kenya by the year 2050 based precis modeling system. Ethiop J Environ Stud Manag. 2016;9: 625–640. [Google Scholar]
- 18.Okiro EA, Alegana VA, Noor AM, Snow RW. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar J. 2010;9: 285. doi: 10.1186/1475-2875-9-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, Zhou G, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: Implication on malaria vector control measures. PloS One. 2020;15: e0224718. doi: 10.1371/journal.pone.0224718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochomo E, Bayoh MN, Brogdon WG, Gimnig JE, Ouma C, Vulule JM, et al. Pyrethroid resistance in Anopheles gambiae ss and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med Vet Entomol. 2013;27: 156–164. doi: 10.1111/j.1365-2915.2012.01039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisumu County. Kisumu County Weather Forecast 2020. Ministry Of Environment and Forestry Kenya Meteorological Department–Kisumu County; 2020 pp. 1–4.
- 22.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14: 244. doi: 10.1186/s12936-015-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Entomological field techniques for malaria control. Part II. Tutor’s guide. World Health Organization; 1992. [Google Scholar]
- 24.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ Afr Inst Med Res. 1987;55: 1–143. [Google Scholar]
- 25.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19: 1–20. doi: 10.1186/s12936-019-3075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver JB. Mosquito ecology: field sampling methods. springer science & business media; 2007. [Google Scholar]
- 27.WHO. Manual on practical entomology in malaria. Part 2, Methods and techniques. Geneva: World Health Organization; [London: ]: [H.M.S.O.], 1975.; 1975. [Google Scholar]
- 28.Service M. Medical Entomology for Students. fifth. Cambridge University Press, New York, www.cambridge.org; 2012. [Google Scholar]
- 29.Musapa M, Kumwenda T, Mkulama M, Chishimba S, Norris DE, Thuma PE, et al. A simple Chelex protocol for DNA extraction from Anopheles spp. JoVE J Vis Exp. 2013; e3281. doi: 10.3791/3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins FH, Mehaffey PC, Rasmussen MO, Brandling-Bennett AD, Odera JS, Finnerty V. Comparison of DNA-probe and isoenzyme methods for differentiating Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae). J Med Entomol. 1988;25: 116–120. doi: 10.1093/jmedent/25.2.116 [DOI] [PubMed] [Google Scholar]
- 31.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66: 804–811. doi: 10.4269/ajtmh.2002.66.804 [DOI] [PubMed] [Google Scholar]
- 32.Cohuet A, Simard F, Toto J-C, Kengne P, Coetzee M, Fontenille D. SPECIES IDENTIFICATION WITHIN THE ANOPHELES FUNESTUS GROUP OF MALARIA VECTORS IN CAMEROON AND EVIDENCE FOR A NEW SPECIES. Am J Trop Med Hyg. 2003;69: 200–205. doi: 10.4269/ajtmh.2003.69.200 [DOI] [PubMed] [Google Scholar]
- 33.Daibin Z, Hemming-Schroeder E, Wang X, Solomon K, Guofa Z, Harrysone A, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci Rep Nat Publ Group. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol. 2009;121: 346–351. doi: 10.1016/j.exppara.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 35.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47: 975–980. doi: 10.1128/JCM.01858-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP, et al. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol. 1988;25: 9–16. doi: 10.1093/jmedent/25.1.9 [DOI] [PubMed] [Google Scholar]
- 37.Beier JC, Koros JK. Visual assessment of sporozoite and bloodmeal ELISA samples in malaria field studies. J Med Entomol. 1991;28: 805–808. doi: 10.1093/jmedent/28.6.805 [DOI] [PubMed] [Google Scholar]
- 38.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28: 1647–1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol Biol Evol. 2021. doi: 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4: 406–425. doi: 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 41.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. evolution. 1985;39: 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35: 1547. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol. 2002;39: 162–172. doi: 10.1603/0022-2585-39.1.162 [DOI] [PubMed] [Google Scholar]
- 44.Imbahale SS, Paaijmans KP, Mukabana WR, Van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10: 81. doi: 10.1186/1475-2875-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76: 450–460. [PubMed] [Google Scholar]
- 46.Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector, Anopheles funestus in rural south-eastern Tanzania. 2020. doi: 10.1186/s12936-020-03295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musiime AK, Smith DL, Kilama M, Otto G, Kyagamba P, Rek J, et al. Identification and characterization of Anopheles larval aquatic habitats at three sites of varying malaria transmission intensities in Uganda. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Getachew D, Balkew M, Tekie H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J. 2020;19: 1–13. doi: 10.1186/s12936-019-3075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess AD, Hall TF. The intersection line as a factor in anopheline ecology. J Natl Malar Soc. 1943;2: 93–8. [Google Scholar]
- 50.Hall TF. The influence of plants on anopheline breeding. Am J Trop Med Hyg. 1972;21: 787–794. doi: 10.4269/ajtmh.1972.21.787 [DOI] [PubMed] [Google Scholar]
- 51.Orr BK, Resh VH. Influence ofMyriophyllum aquaticum cover onAnopheles mosquito abundance, oviposition, and larval microhabitat. Oecologia. 1992;90: 474–482. doi: 10.1007/BF01875440 [DOI] [PubMed] [Google Scholar]
- 52.Balling SS, Resh VH. Seasonal patterns of pondweed standing crop and Anopheles occidentalis densities in Coyote Hills Marsh. Proceedings and papers of the annual conference of the California Mosquito and Vector Control Association (USA). 1984.
- 53.Manguin S, Roberts DR, Peyton EL, Rejmankova E, Pecor J. Characterization of Anopheles pseudopunctipennis larval habitats. UNIFORMED SERVICES UNIV OF THE HEALTH SCIENCES BETHESDA MD DEPT OF …; 1996. [PubMed]
- 54.Aklilu E, Kindu M, Gebresilassie A, Yared S, Tekie H, Balkew M. Environmental Factors Associated with Larval Habitats of Anopheline Mosquitoes (Diptera: Culicidae) in Metema District, Northwestern Ethiopia. J Arthropod-Borne Dis. 2020;14: 153. doi: 10.18502/jad.v14i2.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58: 433–453. doi: 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 56.Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am J Trop Med Hyg. 1999;61: 1010–1016. doi: 10.4269/ajtmh.1999.61.1010 [DOI] [PubMed] [Google Scholar]
- 57.Munga S, Minakawa N, Zhou G, Barrack O-OJ, Githeko AK, Yan G. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J Med Entomol. 2014;43: 221–224. [DOI] [PubMed] [Google Scholar]
- 58.Kweka EJ, Zhou G, Gilbreath TM, Afrane Y, Nyindo M, Githeko AK, et al. Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasit Vectors. 2011;4: 1–7. doi: 10.1186/1756-3305-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaalan EA-S, Canyon DV. Aquatic insect predators and mosquito control. Trop Biomed. 2009;26: 223–261. [PubMed] [Google Scholar]
- 60.Koenraadt CJM, Takken W. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med Vet Entomol. 2003;17: 61–66. doi: 10.1046/j.1365-2915.2003.00409.x [DOI] [PubMed] [Google Scholar]
- 61.Ndenga BA, Simbauni JA, Mbugi JP, Githeko AK, Fillinger U. Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS One. 2011;6: e19473. doi: 10.1371/journal.pone.0019473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dida GO, Gelder FB, Anyona DN, Abuom PO, Onyuka JO, Matano A-S, et al. Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara River, Kenya and Tanzania. SpringerPlus. 2015;4: 1–14. doi: 10.1186/2193-1801-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahgoub MM, Kweka EJ, Himeidan YE. Characterisation of larval habitats, species composition and factors associated with the seasonal abundance of mosquito fauna in Gezira, Sudan. Infect Dis Poverty. 2017;6: 23. doi: 10.1186/s40249-017-0242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar R, Muhid P, Dahms H-U, Tseng L-C, Hwang J-S. Potential of three aquatic predators to control mosquitoes in the presence of alternative prey: a comparative experimental assessment. Mar Freshw Res. 2008;59: 817–835. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All sequences of ITS2 are available at GenBank under accession numbers MZ435355-MZ435414.