Page 7912. In our previous Communication, we inadvertently drew the substrates derived from the molecule pyriproxyfen with incorrect connectivity of the pyriproxyfen molecule (meta instead of para was drawn), omitted a methylene group from compound 13, and drew an epimer of compound 20 in Scheme 3. These drawing errors have been corrected in the corrected Scheme 3 shown here.
Scheme 3. Substrate Scope for the Alkylation of Aryl Thianthrenium Salt.
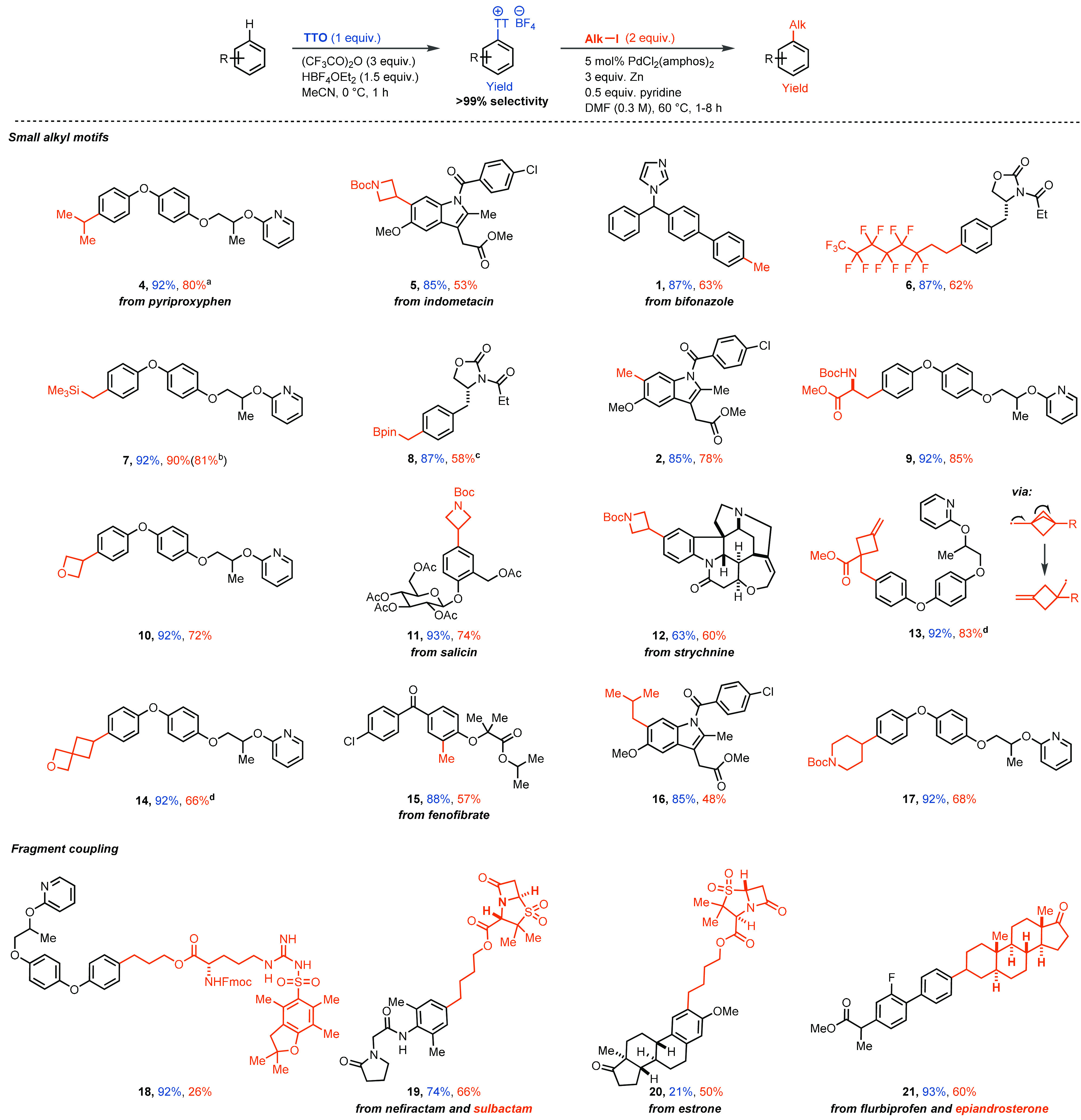
General conditions unless otherwise noted: aryl thianthrenium salt (0.3 mmol), alkyl iodide (0.6 mmol), PdCl2(amphos)2 (15.0 μmol), pyridine (0.15 mmol), DMF (0.3 M). [a]>20:1 ratio of i-PrAr:n-PrAr product. [b]3.0 mmol scale. [c]Pyridine was omitted. [d]Reactions carried with aryl thianthrenium salt (0.2 mmol) and MgCl2 (3 equiv) as additive. Yields in blue correspond to yield of C–H thianthrenation. Yields in orange correspond to yield of alkylation of aryl thianthrenium salts. Yields of thianthrenation were obtained from refs 5b, 6a, 6b, 6c, 6d, and 6e.
As a further clarification, we have commented in the revised Supporting Information about the undefined stereocenter of compound 21.
The findings and conclusions of the original communication remain unchanged. We apologize for the errors and for any inconvenience that this may have caused the readers of JACS.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c06057.
Experimental procedures and spectral data (revised) (PDF)
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


