Abstract
Difelikefalin, a selective kappa opioid receptor agonist designed to limit central nervous system (CNS) penetration, is under development for the treatment of pruritus. Its hydrophilic, small‐peptidic structure limits CNS entry, minimizing potential CNS‐mediated adverse events (AEs). This study assessed the effect of difelikefalin on key relevant measures of respiratory depression in healthy volunteers. This single‐center, randomized, double‐blind, placebo‐controlled, three‐way crossover study enrolled healthy, nonsmoking volunteers. Subjects were randomized to 1 of 3 treatment sequences of difelikefalin (1.0 or 5.0 mcg/kg i.v.) or placebo on sequential days with an intervening 24 (±2) h washout period. The primary end points included incidence of increased end‐tidal carbon dioxide (ETCO2) greater than or equal to 10 mm Hg versus baseline or a level greater than 50 mm Hg sustained greater than or equal to 30 seconds, and incidence of reduction in saturation of peripheral oxygen (SpO2) to less than 92% sustained greater than or equal to 30 seconds. Secondary end points included incidence of reduced respiratory rate and other safety assessments. Fifteen subjects were randomized and completed the study. No subject on placebo or difelikefalin met the increased ETCO2 or reduced SpO2 primary end point criteria for respiratory depression. All respiratory measures in each group remained near baseline values during 4‐h postdose observations. No subject met the reduced respiratory rate criterion or experienced clinically significant changes in ETCO2, SpO2, or respiratory rate. The most commonly reported treatment‐emergent AEs (TEAEs; ≥20% of subjects) were paresthesia, hypoesthesia, and somnolence in the difelikefalin arms. All TEAEs were mild and resolved without intervention. Difelikefalin 1.0 and 5.0 mcg/kg i.v. did not produce respiratory depression.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Severe respiratory depression is a life‐threatening complication of inappropriate use of mu opioid receptor agonists. Difelikefalin, a peripherally restricted kappa opioid receptor (KOR) agonist, has not demonstrated evidence of compromised respiratory safety.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study evaluated whether difelikefalin, a selective and potent KOR agonist, induces respiratory depression.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study helps to expand on the safety profile for difelikefalin. Difelikefalin did not produce respiratory depression in healthy volunteers at doses that were 2 to 10 times higher than those observed to be therapeutically effective in clinical trials of patients with chronic kidney disease–associated pruritus.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
KOR agonists may be potentially safe and effective therapeutics. Difelikefalin is currently being evaluated for chronic kidney disease‐associated pruritus and other chronic pruritic conditions.
INTRODUCTION
Opioid receptors are involved in the modulation of pruritus and pain signals and consist of mainly 4 types, classified as mu, kappa, delta, and opioid receptor like‐1 (ORL1). 1 , 2 , 3 , 4 , 5 Expression of opioid receptors occurs in the central nervous system (CNS), on sensory ganglionic neurons and their nerve fibers in the peripheral nervous system, and on certain immune cells. 2 , 3 , 4 Most clinically used opioid analgesics act primarily via activation of mu opioid receptors located in the CNS and peripheral nervous system and are associated with a wide array of side effects, such as euphoria, potential for abuse, and respiratory depression. 2 , 4 , 6 , 7
Severe respiratory depression is a life‐threatening complication associated with the inappropriate use of mu opioid receptor agonists, such as morphine, hydrocodone, and fentanyl. 7 , 8 , 9 Mu opioid receptor agonists inhibit respiration via stimulation of mu opioid receptors within the CNS that control respiration, primarily in the pons and brainstem. 7 Kappa opioid receptor (KOR) agonists, however, have different properties compared with mu opioid receptor agonists and could potentially provide alternative analgesic options as well as novel therapies for the treatment of pruritus. 4 , 6 , 10 Centrally acting KOR agonists do not cause euphoria or respiratory depression but may produce dysphoria and hallucinations, which have limited their clinical development. 6 , 11
Difelikefalin is a novel, selective KOR agonist that does not readily enter into the CNS by virtue of its hydrophilic D‐amino acid peptidic structure. 12 , 13 Difelikefalin is thought to activate KORs primarily in peripheral sensory neurons and immune cells. 12 Antipruritic and analgesic effects of difelikefalin have been demonstrated in clinical studies of i.v. difelikefalin, with no dysphoria observed and no meaningful evidence of abuse potential. 14 , 15 , 16 In clinical studies of difelikefalin, no reductions in pulse oximetry were observed. However, pulse oximetry is relatively insensitive to early signs of respiratory depression. 17 Here, we report the results of a phase I clinical study that assessed the effects of difelikefalin compared with placebo using end‐tidal carbon dioxide (ETCO2), a more sensitive measure of respiratory depression than pulse oximetry, 17 in healthy volunteers. This study used doses 2 to 10 times higher than the dose (0.5 mcg/kg) found to be therapeutically effective in the phase III clinical study of difelikefalin for the treatment of chronic kidney disease‐associated pruritus (CKD‐aP). 18 , 19
METHODS
Study ethics
The study protocol was reviewed and approved by the institutional review board prior to initiating any study‐related activities and by Health Canada. The study was conducted in accordance with Good Clinical Practice and all applicable regulations, including the Declaration of Helsinki. All study subjects provided signed, written informed consent before enrolling in the study.
Study subjects
The study enrolled healthy male or female subjects 18 to 55 years of age, inclusive, with a body mass index range of 19.0 to less than or equal to 33.0 kg/m2 and body weight between 60.0 and less than or equal to 135.0 kg. The subjects were nonsmokers for at least the previous 3 months. Subject eligibility was determined during an outpatient visit at the clinical research unit of the study site. Excluded from the study were subjects with a self‐reported history or current diagnosis of substance or alcohol use disorder within the previous 2 years and/or having ever been in a treatment program for substance or alcohol dependence, a history or evidence of sleep‐disordered breathing, a history of pulmonary and/or cardiac disease or dysfunction, or use of any opioids in the 30 days prior to screening.
Study design
This was a single‐center, randomized, double‐blind, placebo‐controlled, 3‐way crossover study comparing 2 doses of difelikefalin, 1.0 mcg/kg and 5.0 mcg/kg, administered i.v. versus placebo on key measures of respiration in healthy volunteers. The study design is illustrated in Figure 1. The doses used in the current study (1.0 mcg/kg and 5.0 mcg/kg) are expected to cover the expected systemic exposure for the maximum target dose of i.v. difelikefalin for the treatment of CKD‐aP in patients undergoing hemodialysis based on findings from pharmacokinetic analyses, in which difelikefalin demonstrated generally comparable maximum plasma concentration in healthy subjects and in patients undergoing hemodialysis. 20 , 21
FIGURE 1.
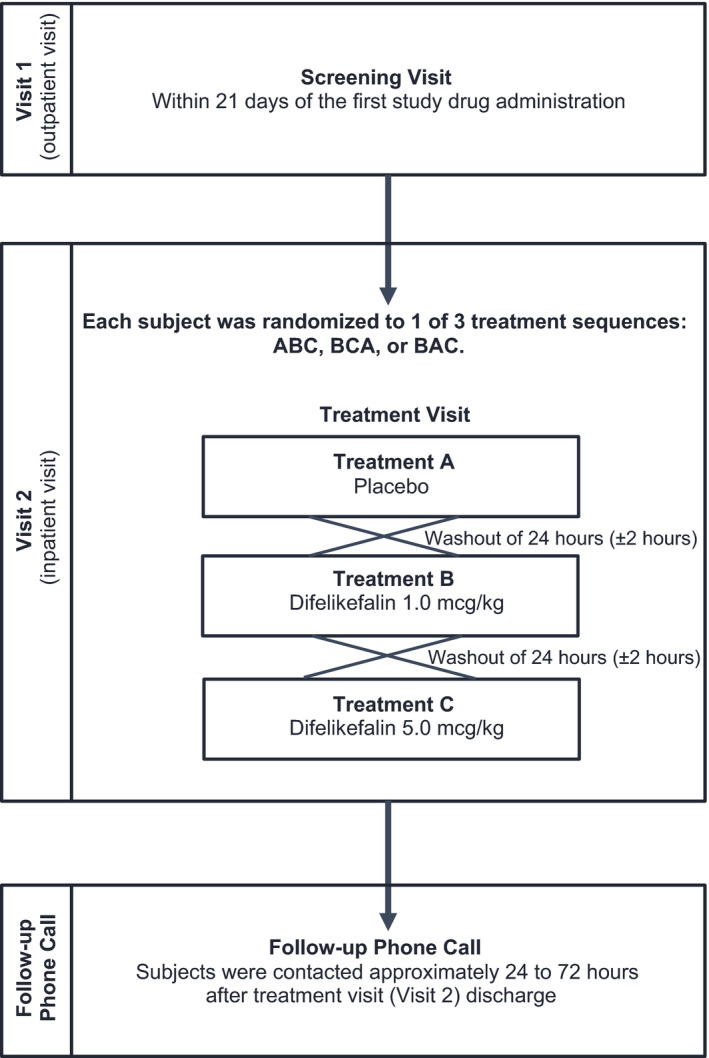
Schematic representation of study design (e.g., ABC sequence). Each subject was randomized to 1 of 3 treatment sequences: ABC, BCA, or BAC, where A = placebo, B = difelikefalin 1.0 mcg/kg, and C = difelikefalin 5.0 mcg/kg. All treatments were single dose and were administered intravenously. Subjects were discharged ~ 24 h after the last study drug administration
Eligible subjects who completed the screening visit returned to the clinical research unit to complete the inpatient treatment visit. During the treatment visit, each subject was randomized to 1 of 3 treatment sequences: ABC, BCA, or BAC, where A = placebo, B = difelikefalin 1.0 mcg/kg, and C = difelikefalin 5.0 mcg/kg. Thus, each subject received a single dose of all study drugs. In each treatment sequence, subjects received the lower dose of difelikefalin (1.0 mcg/kg) before the higher dose (5.0 mcg/kg) to ensure subject safety before exposing them to the higher dose. Treatment was administered at ~ 8:00 a.m. each day and each subject fasted for 2 h prior to and until 4 h after each dose. Respiratory rate (i.e., breaths per minute), ETCO2, and saturation of peripheral oxygen (SpO2; a surrogate measure of oxygenation) were measured using capnography and pulse oximetry. Measurements of ETCO2, which is the concentration of CO2 at the end of exhalation, 22 , 23 were obtained using a nasal cannula while the subjects breathed room air, and supplemental oxygen was available. This setup allowed for undiluted end‐tidal gas sampling with good plateau waveforms approximating arterial CO2 partial pressure and represents a sensitive and reliable measure of respiratory depression. 17 Baseline measurements were recorded ~ 1 h before dose administration, and each parameter was monitored continuously up to 2 h prior to dosing and continued for at least 4 h after each dose. Subjects were discharged from the clinical research unit on study day 4 (i.e., 24 h after the last dose day). Subjects received a follow‐up telephone call from the clinical research unit staff ~ 24 to 72 h postdischarge to check on the subjects’ health status. Subjects were instructed to contact the clinical research unit at any time to report an adverse event (AE). The primary end points were defined as a subject’s increase in ETCO2 greater than or equal to 10 mm Hg from baseline or an absolute ETCO2 value greater than 50 mm Hg (sustained for at least 30 s), and a subject’s reduction in SpO2 to less than 92% (sustained for at least 30 s). Secondary end points included a subject’s absolute respiratory rate of less than 10 breaths/min or a reduction of at least 30% compared with baseline (sustained for at least 30 s); and mean ETCO2, SpO2, and respiratory rate values at baseline and during the post‐treatment assessments based on continuous measurement of these respiratory parameters. Continuous monitoring was conducted from 2 h prior to dosing up to 4 h postdosing or longer, if deemed medically necessary. The 4‐h postdose window for ETCO2 measurement was selected based on the standard used by anesthesiologists, as the recovery and monitoring of patients in the postanesthesia care unit after surgical anesthesia with opioids is usually less than 4 h. Investigators did not expect significant respiratory depression with difelikefalin based on prior data, 24 and prior clinical studies support an observation period of less than 4 h when evaluating respiratory depression. 25 , 26 The primary and secondary end points were defined based on parameters commonly used to evaluate respiratory depression in prior studies. 22 , 27 , 28 Additional safety assessments included AEs, vital signs, clinical laboratory assessments, physical examination findings, and electrocardiograms (ECGs). Emergency medication (e.g., i.v. naloxone, diphenhydramine, epinephrine, methylprednisolone, ranitidine, and rescue medication required for advanced cardiac life support) could be administered or patients could be transported to a hospital if deemed necessary.
Fifteen (15) subjects were to be randomized to 3 different treatment sequences to ensure evaluable data from at least 12 subjects. A total of 12 subjects (4 in each crossover sequence) was considered sufficient to provide descriptive information on the safety and tolerability of the dose levels of i.v. difelikefalin. The randomization codes were generated by the study sponsor and a designated unblinded statistician, and provided in sealed code break envelopes prior to the start of the study, using an altered 3 × 3 Williams’ design, to guarantee the lower dose of difelikefalin (1.0 mcg/kg) always preceded the higher dose of difelikefalin (5.0 mcg/kg). All subjects and study personnel, with the exception of the study pharmacist and the designated unblinded statistician, were blinded to the randomization codes. Efforts were made to achieve at least 25% female subjects for study enrollment.
Data analysis
Demographic data were summarized for all subjects who received at least 1 dose of the study drug by randomization sequence. End points for respiratory safety were summarized by descriptive statistics (mean, SD, number, and/or percentage). Differences in respiratory safety variables between placebo and difelikefalin groups were determined using analysis of variance with repeated measures (post hoc analysis). The absence of relevant respiratory depression events did not warrant any statistical analysis comparing the incidence of such events between treatment groups. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 19.1 or higher). The number and percentage of subjects with treatment‐emergent AEs (TEAEs) were summarized by system organ class, preferred term, and treatment, and for each treatment by maximum severity and relationship to difelikefalin. All data were collected at the clinical research unit.
RESULTS
Subjects
A total of 15 subjects were randomized, 5 to each of the 3 treatment sequences. All subjects completed the three treatment sequences and had data available for analysis of the primary end points. The study began on February 6, 2017, and was completed on February 25, 2017. Subject demographics are presented in Table 1. There were no major differences in demographic characteristics among the three treatment sequences. Subjects were predominantly male (11/15 [73.3%]), with a majority being either White (6/15 [40.0%]) or African American (6/15 [40.0%]). The mean (SD) age was 38.3 (7.8) years and mean (SD) body mass index was 25.6 (2.4) kg/m2.
TABLE 1.
Subject demographics
| Parameter |
Overall N = 15 |
Sequence ABC n = 5 |
Sequence BCA n = 5 |
Sequence BAC n = 5 |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 11 (73.3) | 3 (60.0) | 4 (80.0) | 4 (80.0) |
| Female | 4 (26.7) | 2 (40.0) | 1 (20.0) | 1 (20.0) |
| Race, n (%) | ||||
| White | 6 (40.0) | 1 (20.0) | 3 (60.0) | 2 (40.0) |
| African American | 6 (40.0) | 3 (60.0) | 1 (20.0) | 2 (40.0) |
| Asian | 3 (20.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) |
| Age, mean (SD), years | 38.3 (7.8) | 41.6 (6.6) | 35.4 (5.9) | 38.0 (10.6) |
| Body weight, mean (SD), kg | 77.7 (8.9) | 76.6 (10.7) | 74.9 (5.9) | 81.5 (9.9) |
| BMI, mean (SD), kg/m2 | 25.6 (2.4) | 27.2 (3.6) | 24.8 (0.6) | 25.0 (1.4) |
Abbreviations: A, placebo; B, difelikefalin 1.0 mcg/kg i.v.; BMI, body mass index; C, difelikefalin 5.0 mcg/kg i.v.
Primary and secondary end points
There were no subjects who met the primary or secondary end point criteria for respiratory depression, whether receiving placebo or difelikefalin. Mean ETCO2 was between 36 and 38 mm Hg at baseline, and no statistically significant (p ≥ 0.8102) differences between treatments were observed during continuous monitoring up to 4 h after dosing (Figure 2). Highest mean (SD) ETCO2 in any subject during the study occurred 3 h following administration of difelikefalin 1.0 mcg/kg and was 39 (2.5) mm Hg, well below the 50 mm Hg predefined respiratory depression threshold. Likewise, mean SpO2 ranged from 98% to 99% at baseline and was similar in each group following each treatment (p ≥ 0.8896; Figure 3). The lowest mean (SD) SpO2 observed in any subject during the study occurred 10 h following administration of the difelikefalin 1.0‐mcg/kg dose, and was 97% (1.7%), considerably above the 92% predefined respiratory depression threshold. Across the difelikefalin and placebo groups, there were no subjects who met the reduced respiratory rate criterion during the study; similar mean respiratory rates were observed among the difelikefalin and placebo groups at baseline and after administration of each treatment (p ≥ 0.8952; Figure 4). The lowest mean (SD) respiratory rate observed in any subject during the study occurred 15 min following administration of difelikefalin 1.0 mcg/kg and was 14 (2.7) breaths/min. This was well above the 10 breaths/min predefined assessment limit for respiratory safety. In addition, no medical interventions for respiratory depression were necessary during the study.
FIGURE 2.
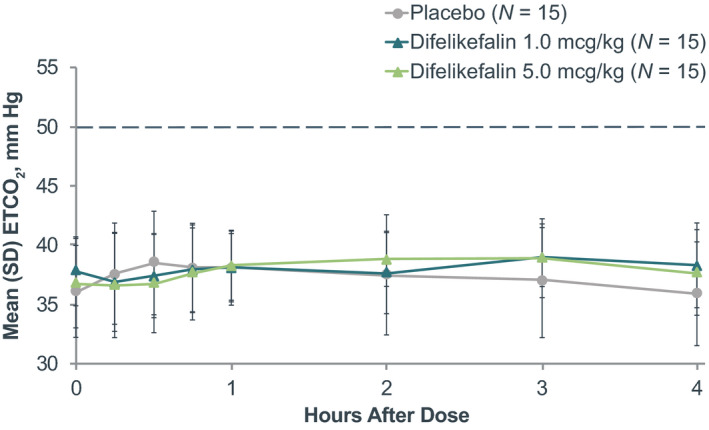
Mean ETCO2 before and following treatment. The dotted line represents the threshold for a respiratory depression event. ETCO2, end‐tidal carbon dioxide
FIGURE 3.
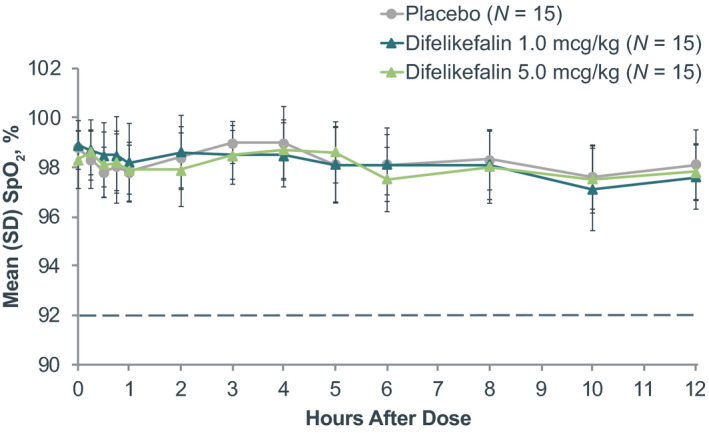
Mean SpO2 before and following treatment. The dotted line represents the threshold for a respiratory depression event. SpO2, saturation of peripheral oxygen
FIGURE 4.
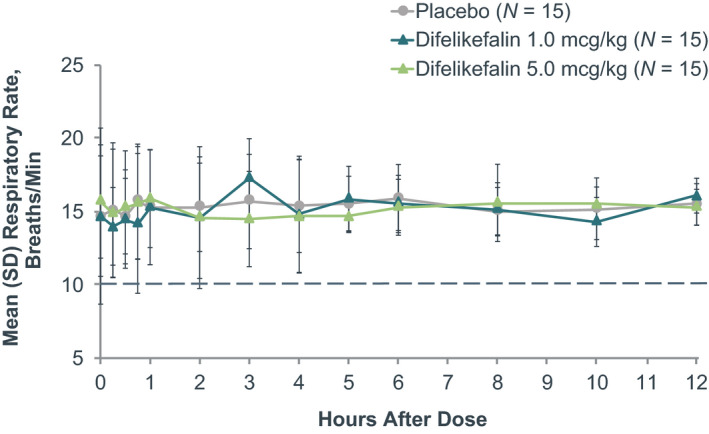
Mean respiratory rate before and following treatment. No subjects met the reduced respiratory rate criterion (<10 breaths/min or reduction ≥30% from baseline [sustained for ≥30 seconds]) and no subjects had clinically significant respiratory rate during the study. Several subjects experienced abnormal (not clinically significant) respiratory rate (reference range: 12–20 breaths/min) over the course of the study, but the incidence was similar both pre‐ and postdose and did not show a dose‐response relationship
Safety end points
Treatment‐emergent AE sreported in 2 or more subjects during any of the 3 treatments in any treatment group are presented in Table 2. Overall, 20.0% of subjects in the placebo group (n/N = 3/15) and 80.0% of subjects in each of the difelikefalin dose groups (n/N = 12/15 for each dose) experienced at least 1 TEAE. The most frequently reported TEAEs with difelikefalin 1.0 mcg/kg and 5.0 mcg/kg (≥20%) were paresthesia (n/N = 5/15 [33.3%] and n/N = 9/15 [60.0%]), hypoesthesia (n/N = 3/15 [20.0%] and n/N = 5/15 [33.3%]), and somnolence (n/N = 3/15 [20.0%] and n/N = 2/15 [13.3%]). Events of paresthesia and hypoesthesia demonstrated a dose‐response relationship and were transient in nature, ranging from less than 1 min to less than 90 min; none were considered serious. 18 All TEAEs were mild and resolved without intervention.
TABLE 2.
TEAEs reported by two or more subjects during any of the three treatments in any treatment group
| TEAE, n (%) | Placebo N = 15 | Difelikefalin 1.0 mcg/kg N = 15 |
Difelikefalin 5.0 mcg/kg N = 15 |
|---|---|---|---|
| Any TEAE | 3 (20.0) | 12 (80.0) | 12 (80.0) |
| Paresthesia | 1 (6.7) | 5 (33.3) | 9 (60.0) |
| Hypoesthesia | 0 | 3 (20.0) | 5 (33.3) |
| Dysgeusia | 0 | 2 (13.3) | 1 (6.7) |
| Headache | 1 (6.7) | 2 (13.3) | 0 |
| Dizziness | 0 | 0 | 2 (13.3) |
| Somnolence | 1 (6.7) | 3 (20.0) | 2 (13.3) |
| Discomfort | 0 | 0 | 2 (13.3) |
| Pruritus | 0 | 2 (13.3) | 0 |
Incidence is reported as number (%) of subjects.
Abbreviation: TEAE, treatment‐emergent adverse event.
There were no serious AEs reported during the study, and no subject was discontinued due to a TEAE. There were no clinically relevant abnormalities in vital signs, laboratory tests, or ECG findings for any subject during the study.
DISCUSSION
In this single‐center, randomized, double‐blind, placebo‐controlled crossover study, there was no indication of respiratory depression in healthy volunteers during treatment with either dose of difelikefalin as compared with placebo. Medical interventions were not indicated or required for any subject at any time throughout this study. The 2 doses of difelikefalin in the current study are 2 to 10 times higher than the doses assessed in the phase III study of difelikefalin in patients with CKD‐aP. 18 , 19 In previous clinical studies, bolus i.v. doses of difelikefalin 0.5 to 40.0 mcg/kg have been administered to healthy volunteers (unpublished data) or patients with chronic kidney disease without any indication of compromised respiratory safety compared with placebo. 18 , 19
SpO2 is less sensitive to changes in acute respiratory events than the ETCO2 parameter. 17 During a study of 60 emergency department patients undergoing procedural sedation and analgesia, patients were monitored for SpO2, respiratory rate, and ETCO2. 17 There were 20 acute respiratory events recorded that were consistent with apnea or hypoventilation. 17 Seventeen (85%) of these patients had abnormal ETCO2 findings, which when observed, occurred before changes in SpO2 or respiratory rate. 17 Thus, ETCO2 monitoring may provide the earliest indication of compromised respiration. 17 , 29 The use of ETCO2 in the present study, a more sensitive index of respiratory depression than pulse oximetry, 17 demonstrated no clinically relevant changes after difelikefalin administration and confirmed the negligible changes seen with SpO2 and respiratory rate, further reinforcing the respiratory safety observed with difelikefalin.
This study was conducted in healthy volunteers and evaluated single doses of difelikefalin. However, difelikefalin has also been evaluated in patients with chronic pruritus and pain. 18 , 30 , 31 Intravenous difelikefalin was evaluated in two 12‐week, phase III, double‐blind, placebo‐controlled studies (KALM‐1, United States only; and KALM‐2, global) in patients with moderate‐to‐severe pruritus who were undergoing hemodialysis. 18 , 32 In KALM‐1, AEs that were reported in at least 1.5% of patients did not include events indicative of respiratory compromise, 18 as rates of acute respiratory failure (0.5% and 1.1%), hypoxia (0.5% and 1.1%), and respiratory failure (1.1% and 0%) were comparable at 12 weeks (end of double‐blind treatment phase) between i.v. difelikefalin and placebo, respectively. The patients included in these phase III studies were older, had multiple comorbidities, and were taking a significant number of concomitant medications compared with the healthy volunteers included in this respiratory depression study. 18 In KALM‐1, no patient discontinued difelikefalin due to acute respiratory failure or respiratory failure. 18 The incidence of respiratory depression‐related AEs with longer‐term repeated exposure remains unknown.
In this study, difelikefalin did not produce respiratory depression and was well‐tolerated by healthy volunteers when administered i.v. at supratherapeutic doses compared with the doses used in studies of CKD‐aP. 18 , 19 Prior clinical studies demonstrated significant antipruritic effects of i.v. difelikefalin versus placebo and lack of dysphoria or evidence of abuse potential in patients with end‐stage renal disease. 18 , 19 Our findings in healthy volunteers further establish the safety profile of difelikefalin by demonstrating that it does not produce respiratory depression.
CONFLICTS OF INTEREST
C.L.M. and S.N.B. are employees of Cara Therapeutics and had input into the content of the report. E.R.V. is a scientific consultant for Cara Therapeutics. M.C.T. is a scientific consultant for Cara Therapeutics. B.S.S. was an employee of Syneos Health, Toronto, Ontario, Canada, at the time of this study and had input on the design, conduct, and data analysis of this study. J.W.S. was an employee of and held stock in Cara Therapeutics at the time of this study, and had input on the design, execution, and analysis of this trial. He is currently employed by Antibe Therapeutics, Toronto, Ontario, Canada.
AUTHOR CONTRIBUTIONS
E.R.V., M.C.T., C.L.M., J.W.S., B.S.S., and S.N.B. wrote the manuscript. E.R.V., M.C.T., J.W.S., B.S.S., and S.N.B. designed the research. S.N.B. and B.S.S. performed the research. C.L.M., S.N.B., B.S.S., J.W.S., E.R.V., and M.C.T. analyzed the data. E.R.V., J.W.S., S.N.B., and C.L.M. contributed new reagents/analytical tools.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Edward Weselcouch, PhD, Gregory A. Kopia, PhD, CMPP, and Diana Talag, ELS, CMPP (PharmaWrite, LLC, Princeton, NJ) and Amy Shaberman, PhD (Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ), for medical writing and editorial support, which was funded by Cara Therapeutics, under the direction of the authors.
Clinical trial number and registry URL: Not applicable (NA).
Funding information
Cara Therapeutics sponsored this study.
DATA AVAILABILITY STATEMENT
Please contact Cara Therapeutics for data inquiries.
REFERENCES
- 1. Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18(2):69‐70. [PubMed] [Google Scholar]
- 2. Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23(2):121‐145. [DOI] [PubMed] [Google Scholar]
- 3. Stein C. Opioid receptors on peripheral sensory neurons. Adv Exp Med Biol. 2003;521:69‐76. [PubMed] [Google Scholar]
- 4. Phan NQ, Lotts T, Antal A, Bernhard JD, Stander S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol. 2012;92(5):555‐560. [DOI] [PubMed] [Google Scholar]
- 5. Al‐Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor‐dependent signaling and behavior. Anesthesiology. 2011;115(6):1363‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology. 2010;210(2):109‐119. [DOI] [PubMed] [Google Scholar]
- 7. Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non‐analgesic effects of opioids: opioid‐induced respiratory depression. Curr Pharm Des. 2012;18(37):5994‐6004. [DOI] [PubMed] [Google Scholar]
- 8. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid‐induced respiratory depression in the acute care setting: a compendium of case reports. Pain Management. 2014;4(4):317‐325. [DOI] [PubMed] [Google Scholar]
- 9. Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug Poisoning Deaths in the United States, 1980‐2008. NCHS data brief, number 81. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 10. Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(Suppl 10):S10‐S15. [DOI] [PubMed] [Google Scholar]
- 11. Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology. 2001;157(2):151‐162. [DOI] [PubMed] [Google Scholar]
- 12. Aldrich JV, McLaughlin JP. Opioid peptides: potential for drug development. Drug Disc Today Technol. 2012;9(1):e23‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albert‐Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa‐opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41(4):371‐382. [DOI] [PubMed] [Google Scholar]
- 14. Webster L, Menzaghi F, Spencer R. CR845, a novel peripherally‐acting kappa opioid receptor agonist, has low abuse potential compared with pentazocine [abstract 421]. J Pain. 2015;16(4 suppl):S81. [Google Scholar]
- 15. Menzaghi F, Spencer R, Abrouk N, Lewis M, Chalmers D. CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy [abstract 422]. J Pain. 2015;16(4 suppl):S81. [Google Scholar]
- 16. Jones JB, Menzaghi F, O’Connor SJ, Spencer RH, Chalmers DT. Analgesic and morphine‐sparing effects of the peripherally‐restricted kappa opioid agonist CR845 after intravenous administration in women undergoing a laparoscopic hysterectomy [poster PT 426]. Presented at: Biennial World Congress on Pain; August 27‐31, 2012; Milan, Italy.
- 17. Burton JH, Harrah JD, Germann CA, Dillon DC. Does end‐tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad Emerg Med. 2006;13(5):500‐504. [DOI] [PubMed] [Google Scholar]
- 18. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222‐232. [DOI] [PubMed] [Google Scholar]
- 19. Fishbane S, Mathur V, Germain MJ, et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spencer RH, Mathur VS, Tumlin JA, Stauffer JW, Menzaghi F. CR845, a novel kappa opioid receptor agonist, reduces moderate‐to‐severe pruritus and improves quality of life in chronic kidney disease patients undergoing hemodialysis [poster SA‐PO1117]. Presented at: Annual Meeting of the American Society of Nephrology; November 5‐8, 2015; San Diego, CA.
- 21. O’Connor S. CR845: Novel peripherally acting kappa opioid receptor agonist for the treatment of pain and pruritus [oral presentation]. Presented at: Boulder Peptide Symposium; September 27, 2016; Boulder, CO.
- 22. Sivilotti ML, Messenger DW, van Vlymen J, Dungey PE, Murray HE. A comparative evaluation of capnometry versus pulse oximetry during procedural sedation and analgesia on room air. CJEM. 2010;12(5):397‐404. [DOI] [PubMed] [Google Scholar]
- 23. Siobal MS. Monitoring exhaled carbon dioxide. Respir Care. 2016;61(10):1397‐1416. [DOI] [PubMed] [Google Scholar]
- 24. O’Connor S, Labissiere G, Spencer R, Chalmers D, Menzaghi F. Safety, pharmacokinetic and pharmacodynamic profile of CR845, a novel peptidergic kappa opioid agonist in development for the treatment of acute pain and itch [poster PT371]. Presented at: Biennial World Congress on Pain; August 28‐September 2, 2010; Montreal, QC, Canada. [Google Scholar]
- 25. Mora CT, Torjman M, White PF. Sedative and ventilatory effects of midazolam infusion: effect of flumazenil reversal. Can J Anaesth. 1995;42(8):677‐684. [DOI] [PubMed] [Google Scholar]
- 26. Goldberg ME, Torjman M, Bartkowski RR, Mora CT, Boerner T, Seltzer JL. Time‐course of respiratory depression after an alfentanil infusion‐based anesthetic. Anesth Analg. 1992;75(6):965‐971. [DOI] [PubMed] [Google Scholar]
- 27. Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93(2):212‐223. [DOI] [PubMed] [Google Scholar]
- 28. Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient‐controlled analgesia. Anesth Analg. 2007;105(2):412‐418. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko Y. Clinical perspectives on capnography during sedation and general anesthesia in dentistry. Anesth Prog. 1995;42(3–4):126‐130. [PMC free article] [PubMed] [Google Scholar]
- 30. Stauffer JW, Spencer RH, Menzaghi F. CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy [abstract]. Postgrad Med. 2015;127:S96‐S97. [Google Scholar]
- 31. Stauffer JW, Spencer RH, Menzaghi F. Analgesic efficacy of the peripheral kappa‐opioid agonist CR845 in laparoscopic hysterectomy [abstract]. Postgrad Med. 2015;127:S97. [Google Scholar]
- 32. Wooldridge TD, McCafferty K, Schoemig M, et al. Efficacy and safety of difelikefalin for moderate‐to‐severe CKD–associated pruritus: a global phase 3 study in hemodialysis patients (KALM‐2) [abstract FR‐OR24]. J Am Soc Nephrol. 2020;31(suppl):22‐23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact Cara Therapeutics for data inquiries.


