Abstract
Evidence from clinical cases indicates an association between the low success rate of in vitro fertilization (IVF) and ovarian injury due to previous methotrexate (MTX) administration. Therefore, it is necessary to develop and propose reasonable clinical drug guidelines to improve the quality of oocytes and the development of embryos before pregnancy. In this study, we established a mouse model with previous MTX exposure to validate the effects of MTX on reproductive function in female mice. We observed that MTX administration could result in a decrease in the success rate of fertilization and an aberrant embryonic development in both natural fertilization and IVF, even after completion of five to six ovulation cycles after MTX withdrawal. Further research revealed senescence and apoptosis of follicular granulosa cells (GCs), accompanied by arrested follicle development and aberrant estradiol and anti‐Mullerian hormone levels. Supportive evidence indicated that MTX administration induced senescence and apoptosis of human GCs in vitro, and the effects were consistent with the high levels of p21, p53, and oxidative stress. We further demonstrated that folic acid (FA) could improve oocyte function and embryonic development in vivo and in vitro by protecting GCs against apoptosis and senescence. Based on these findings, we propose the implementation of extended intervals between MTX exposure and conception or IVF and recommend FA as a special dietary supplement during this interval period; however, prospective inquiry in humans is necessary to further understand the relationship between MTX and FA recovery.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Clinical cases have demonstrated an association between aberrant embryo development or poor in vitro fertilization (IVF) outcome and ovarian injury due to previous methotrexate (MTX) administration, with no reports on the elucidation of the mechanism.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the mechanism involved in oocyte dysfunction and aberrant embryo development which persists after MTX withdrawal? How can we improve oocyte function and embryonic development of patients with previous MTX medication?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Granulosa cell senescence and apoptosis have been proven to persist after MTX withdrawal with the involvement of p53/p21 expression, which leads to oocyte dysfunction and aberrant embryonic development. Folic acid (FA) reduces ovarian injury caused by MTX administration.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
It is recommended to reevaluate the applicability of an interval of three to six menstrual cycles between MTX discontinuation and pregnancy stated in the current clinical guidelines. Extend the interval between MTX exposure and conception or IVF while monitoring hormone levels. FA should be provided as a dietary supplement during the interval.
INTRODUCTION
Methotrexate (MTX), with a chemical structure similar to that of dihydrofolate, affects one‐carbon metabolism by competitively inhibiting dihydrofolate reductase activity, which further disrupts intracellular metabolic pathways, including nucleotide acid synthesis, amino acid metabolism, and methylation modifications. 1 MTX mainly targets rapidly dividing cells and leads to cell cycle‐specific death; owing to these reasons, MTX is widely used in the treatment of tumors, 2 early medical abortion, 3 ectopic pregnancy, 4 and autoimmune and lymphoproliferative diseases. 5 Concurrently, MTX affects female reproductive health based on its effects exerted on all rapidly dividing cells and via direct interference with folate metabolism. 6
It has been documented that MTX is teratogenic if administered during the first trimester of pregnancy. 7 , 8 However, the data from studies using assisted reproductive technology have shown discrepancies on the safety of MTX administration around the time of conception. On one hand, some researchers observed that a poor response to ovarian stimulation occurred within 18 months following MTX treatment for ectopic pregnancy, and the success of oocyte retrieval in an in vitro fertilization (IVF) cycle depended on the number of days after completion of MTX treatment. 9 Hence, it is recommended that there should be a treatment‐free interval of 3 or more months between discontinuation of MTX regimen and conception. 9 , 10 On the other hand, some researchers suggested that MTX exerted no adverse effects on ovarian reserve and outcomes in assisted reproductive technology cycles in a large fertility cohort, 11 , 12 and female fertility was not negatively affected by MTX treatment based on the results of an anti‐Müllerian hormone (AMH) test. Nevertheless, although these statistical data suggest a low possibility of occurrence of detrimental effects on female reproductive mechanisms due to previous MTX medication, the conclusion based on these may not be applicable to every patient as prescription of medication in the clinical settings varies because of individual differences. 13 In this case, to propose a more reasonable introduction of MTX applicable to more patients, we explored the alteration of ovaries and oocytes that occurred even after discontinuation of MTX administration for a long time and the related mechanism.
The direct effects of MTX on mouse oocyte maturation in vitro have been previously described. 14 However, the effects of MTX on follicular granulosa cells (GCs), a type of somatic cell found in the sex cord, should not be overlooked because GCs may be negatively affected by MTX as GCs rapidly divide during ovarian development. It is well known that GCs provide essential nutrients for oocyte development and regulate follicular development through secreting AMH and estrogen. 15 Several studies have reported that apoptosis in GCs can result in disorders in follicle development and may predict poor embryonic development. 16 , 17 , 18 , 19 Therefore, it is possible that MTX may affect oocyte maturation and quality by inflicting injury on follicular GCs in vivo. Considering that the use of folic acid (FA) might reduce the systemic toxicity of MTX, 20 , 21 we further explored whether FA could rescue injured GCs effectively and reverse ovarian dysfunction and embryonic disorder.
Briefly, to validate whether it was necessary to extend the interval between MTX exposure and conception, which comprises three to six ovulation cycles as suggested by the US Food and Drug Administration (FDA), 22 we established a mouse model with MTX exposure, attempted to explore the specific mechanism of MTX‐induced persistent dysfunction of oocytes, and proposed possible oocyte functional recovery measures.
METHODS
Animal experiments
All animal experiments were performed in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals and were approved by the Bioethics Committee of Second Military Medical University. The procedures also adhered to the relevant guidelines and regulations of the Second Military Medical University.
Four‐week‐old Institute of Cancer Research (ICR) female mice (mean weight ± SD: 16 ± 2.1 g) were purchased from the Shanghai Research Center for Model Organisms and were raised in the specific pathogen‐free (SPF) animal facility of the Department of Cell Biology, SMMU, under a controlled environment (14‐h light/10‐h dark cycle, 24°C).
Establishment of mouse models
MTX group (MTX withdrawal group)
ICR female mice were intraperitoneally injected with 10 mg/kg MTX (MedChemExpress, Lot #23838) every other day for 8 days; the administration is based on low‐dose prolonged regimen MTX medication for ectopic pregnancy in the clinic. 23 A dose of 10 mg/kg MTX could induce reproductive damage without causing the death of mice, using the selected series of MTX dosage calculated while estimating the survival rate of mice (Figure S1A). All mice were used in the experiment 20 days after MTX withdrawal. A period of 20 days after MTX withdrawal is equivalent to 5–6 ovulation cycles in mice. 24
FA group (treatment with FA)
ICR mice were intraperitoneally injected with 10 mg/kg MTX every other day for 8 days and then intragastrically administered with 10 mg/kg of FA (SIGMA, Lot #WXBB4821V) every 2 days for 20 days.
After completion of six ovulation cycles following MTX withdrawal or FA treatment, we observed that there was no significant weight loss (Figure S1B) and decrease in mating ability (Figure S1C) in mice of the MTX and FA groups.
Quantification of ovarian follicles
Each of the 5 continuous 3‐µm sections of the mouse ovaries was stained using hematoxylin‐eosin (H&E staining), and the follicles with visible nuclei were counted. The follicles were classified into distinct stages based on previously established standards. 25
Embryo collection from mice
Male and female mice were mated at a ratio of 1:1. The vaginal plug developed after mating was observed and 108 h after this observation, the fallopian tubes and the uterus of female mice were douched with 5 ml of 4% paraformaldehyde (PFA; douched for a total of 5 times, 1 ml PFA was added each time). After collection of the douche and determination of the embryonic development under the microscope (dropwise addition of the sample used for microscopic examination, 50 µl volume of each drop), we determined the number of 2–16‐cell stage embryos, morula, and blastocysts, and calculated the total number of embryos in each mouse.
Oocyte collection and observation
After the mice were injected with pregnant mare serum gonadotropin (PMSG; Solarbio, P9970) for 48 h, they were injected with human chorionic gonadotropin (hCG; PROSPEC, hor‐250‐a). After 14 h, the oocytes were collected and placed in modified phosphate‐buffered saline (PBS) containing hyaluronidase. 26 The diameter of the oocytes was determined using a micrometer under a microscope. Each oocyte was measured three times, and the average value was determined and used to represent oocyte diameter.
In vitro fertilization
Mouse sperms were collected from the epididymis of healthy 10‐week‐old male mice and incubated at 37°C for 10 min. Then, oocytes were added to the IVF medium comprising at least 2 × 106 sperms/ml, and the mixture was incubated for 4–6 h at 37°C and 5% CO2. Thereafter, the oocytes were added to fresh IVF medium. 26 The number of two‐cell embryos was counted, which represents the number of fertilized oocytes. Then, the 2‐cell embryos were transferred to the KSOMAA medium (Amyjet Scientific, IVL04) and cultured for another 96 h with the enumeration of the embryo count at each stage (2 cells, 4 cells, 8/16 cells, morula, and blastocyst) every 24 h.
Enzyme‐linked immunosorbent assays
Mice were anesthetized via intraperitoneal injection of 2% tribromoethanol at a dose of 0.016 ml/g body weight. Then, 1.5–2 ml of blood from the orbital vein was collected and stored at 4°C. After 12 h, the blood was centrifuged at 825 g for 10 min, and the supernatant containing the serum was collected. The levels of follicle‐stimulating hormone (FSH), AMH, and estradiol in the serum were determined using FSH (Xitang Biology, F10450), AMH (EIAab, E0228 m), and estradiol (Xitang Biology, F10440) enzyme‐linked immunosorbent assay (ELISA) kits, respectively.
Detection of apoptosis in ovaries
The terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick‐end labeling (TUNEL) kit (KeyGEN BioTECH, KGA702) was used to investigate cellular apoptosis in the ovaries. After the integral sections with complete tissue outline were selected and stained with H&E staining, TUNEL staining was performed according to the manufacturer’s instructions.
Cell culture
KGN cells (human ovarian granulosa cell line, GCs) were originally obtained from the Cell Bank at the Shenzhen Institute, Chinese Academy of Sciences (Shenzhen, People’s Republic of China) and cultured in DMEM medium (Gibco, C11995500BT) containing 10% fetal bovine serum (Gibco, 10091–148) and 1% 100× penicillin‐streptomycin solution (Gibco, 15140‐122) in an incubator (37°C, 5% CO2, 95% humidity). The medium was replaced with fresh medium every 48 h.
Establishment of MTX‐sustained group, MTX cell (MTX withdrawal), and FA cell (treatment with FA) model
KGN cells were treated with 50 nM MTX for 48 h (MTX‐sustained group) and then incubated in an MTX‐free medium for 72 h (MTX group). A concentration of 1000 nM of FA was selected as the optimal dose to validate (the specific process of FA dose selection is described in detail in the Results section) the rescue effect on the MTX‐treated KGN cells by culturing the cells in a medium with FA for 72 h (FA treatment group). KGN cells cultured in DMEM were used as controls. Thereafter, cell proliferation was assessed using the cell counting kit‐8 (CCK‐8; MedChem Express) assay.
Cell apoptosis detection
KGN cells were collected and stained using reagents of a flow cytometry kit (MULTI SCIENCES, AP105‐60‐kit) to evaluate cellular apoptosis. The proportion of apoptotic cells was calculated using fluorescence‐activated cell sorting (FACS) and data from three independent experiments.
Cell senescence detection
KGN cells were incubated in six‐well plates, rinsed three times, and stained with reagents of a beta‐galactosidase senescence test kit (Beyotime). Five independent microscopic fields of view were selected for each well. The number of total cells and β‐galactosidase (β‐gal)‐positive cells were determined.
Reactive oxygen species detection
The reactive oxygen species (ROS) assay kit (Beyotime, S0033) was used for ROS detection. Adherent KGN cells in a 6‐well plate were incubated with a fluorescent probe, DCFH‐DA, in a serum‐free medium at 37°C. After cellular entry, DCFH‐DA can be hydrolyzed by intracellular esterases to produce DCFH. The ROS in cells can oxidize nonfluorescent DCFH to produce fluorescent DCF. After 25 min of incubation, DCF‐positive cells were observed and photographed under a fluorescence microscope. For FACS, the percentage of DCF‐positive cells was evaluated using flow cytometry (Beckman) at 488‐nm excitation and 525‐nm emission wavelengths. ROS levels were quantified as the mean fluorescence intensity (MFI) for DCF staining.
Real‐Time reverse transcriptase‐polymerase chain reaction
Total RNA was extracted using the TRIzol reagent (Takara), and cDNA was synthesized from 100 ng of total RNA using a cDNA synthesis kit (TaKaRa). Quantitative polymerase chain reaction (PCR) was performed using the One‐Step TB Green PrimeScript reverse transcriptase‐polymerase chain reaction (RT‐PCR Kit; TaKaRa). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as an endogenous RNA reference gene to normalize gene expression levels. Primers for the genes encoding p16, p21, and p53 as well as the reference gene are summarized below. GAPDH (F) 5′‐TCAACGACCACTTTGTCAAGC‐3′; GAPDH(R) 5′‐TACTTTATTGATGGTACATGACAAGG‐3′; p16(F) 5′‐GCCCAACGCACCGAATAGTT‐3′, p16(R) 5′‐ATGGTTACTGCCTCTGGTGC‐3′; p21(F) 5′‐CTGGGGATGTCCGTCAGAAC‐3′; p21(R) 5′‐CATTAGCGCATCACAGTCGC‐3′; p53(F) 5′‐TGGCCATCTACAAGCAGTCACA‐3′; and p53(R) 5′‐GCAAATTTCCTTCCACTCGGAT‐3′.
Western blotting
The cell lysates were loaded onto a 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gel. After electrophoresis, the proteins from the gels were transferred to membranes via application of 250 mA current for 30 min. The membranes were blocked, followed by incubation with anti‐p16 (CST, #92803), anti‐p21 (CST, #8831), and anti‐p53 (CST, #2524). The bound antibodies were detected using HRP‐conjugated anti‐rabbit and peroxidase‐conjugated anti‐mouse antibodies, and analyzed using chemiluminescence (ECL System) via autoradiography. The same blots were used to evaluate GAPDH (CST, #5174) as a protein loading control.
Experimental design and statistical analysis
Each experiment was repeated at least three times. Oocytes/embryos and serum samples collected from six mice were used for each in vivo experiment, and the cell samples were obtained from three parallel culture dishes for each in vitro experiment. The data are expressed as mean, and the error bars indicate SD of the mean for the individual results. Statistical analysis was performed using the Student’s t‐test with the SPSS/GraphPad Prism software.
RESULTS
MTX‐induced aberrant embryo development after natural fertilization can be partially mitigated by FA treatment
We observed that the total number of embryos obtained from the MTX group decreased by 35%, and the number of blastocysts was reduced by 69% compared with the control group (Figure 1b), which indicated that previous MTX administration delayed or blocked pre‐implantation embryo development. We then observed the morphology of the embryo under a microscope and observed that the aberrant embryos were present in the MTX group, and the embryos manifested as flattened morulas, blastocysts with atypical inner cell mass volume, and embryos without zona pellucida (Figure 1a,c). Thereafter, we observed that the proportion of blastocysts recovered after a 20‐day FA treatment (Figure 1c), and there was a negligible proportion of aberrant embryos in the FA group (Figure 1c).
FIGURE 1.
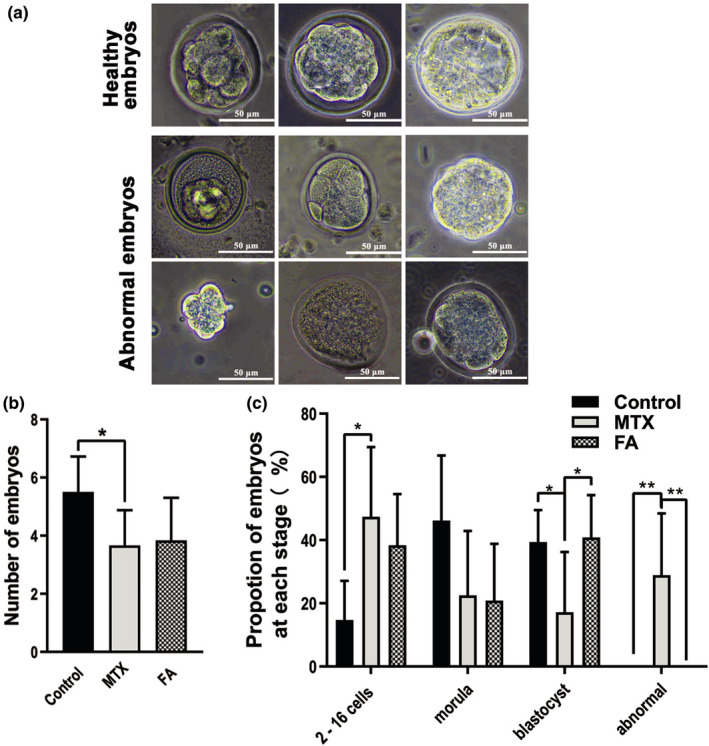
Methotrexate (MTX)‐induced aberrant embryo development after natural fertilization could be partially mitigated by folic acid (FA). (a) Representative images of healthy embryos, which represent the criteria for staging embryos obtained from natural conception. Aberrant embryos observed in the MTX group represented by flattened morulas, blastocysts with atypical inner cell mass, and embryos without zona pellucida. (b) Statistical analysis of the number of embryos after 108 h of mating showing a significant reduction in the total number of embryos of the MTX group, which could not be restored by FA treatment. (c) The percentage of ~ 8 to 16‐cell embryos increased, whereas the blastocyst proportion decreased in the MTX group. FA treatment mainly recovered the proportion of blastocysts. Aberrant embryos were only found in the MTX group. (*p < 0.05, **p < 0.01, Student’s t‐test, n = 6; Scale bars: 50 μm)
Oocytes from MTX group mice exhibit a lower IVF success rate and abnormal embryo development, which is recovered by FA treatment
IVF is an effective method for the evaluation of oocyte quality and the direct observation of the development of the embryo, and the method further eliminates influences of the in vivo fertilization environment. After observing and measuring oocytes obtained through superovulation, we found that although MTX exposure did not significantly change the morphology or diameter of the oocytes (Figure 2A a, b), the proportion of oocytes from the MTX mice that progressed to the 2‐cell stage after overnight incubation decreased by 9.69% compared with the control group (Figure 2B b). As only fertilized oocytes could progress to the two‐cell stage, the reduced percentage showed a decreasing fertilization ability of mouse oocytes. After 2‐cell embryos were incubated for 120 h, compared with the control group, the proportion of the 2‐cell stage embryos and blastocysts from the MTX group increased by 22.2% and decreased by 19.3%, respectively, indicating that fewer 2‐cell embryos from the MTX mice could develop into blastocysts (Figure 2c). Meanwhile, FA treatment increased the proportion of blastocysts by 13% compared with that in the MTX group (Figure 2c). Furthermore, MTX increased the rate of death in embryos showing inner cell mass lysis (Figure 2D a) by 22.3%, whereas FA treatment decreased the rate by 9.5% (Figure 2D b). Additionally, few malformed embryos exhibit deformity, such as shrinkage of inner mass and absence of zona pellucida (Figure 2E a), and this was observed in the malformed embryos in the natural fertilization experiment (Figure 1e). Moreover, malformed embryos were found only in the MTX and FA groups. Interestingly, FA treatment reduced the proportion of MTX‐induced malformed embryos from 7.4% to 2.5% (Figure 2E b).
FIGURE 2.
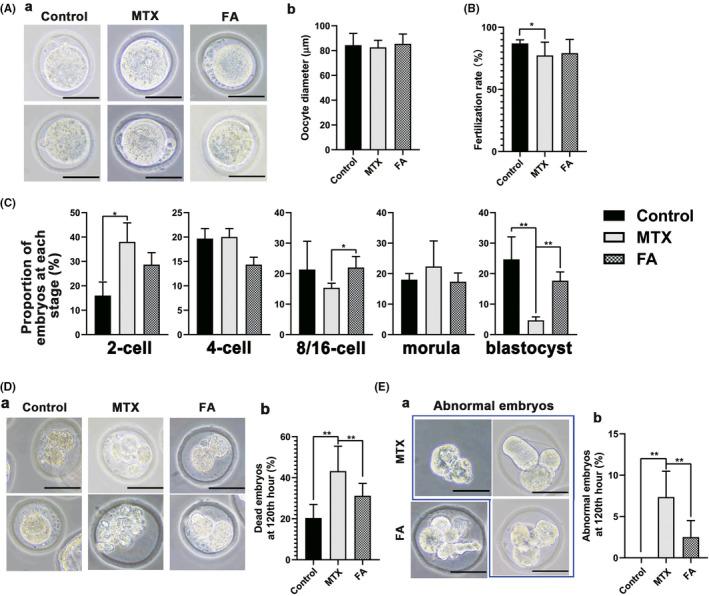
Oocytes from the methotrexate (MTX) group mouse exhibited lower in vitro fertilization (IVF) success rate and abnormal embryo development, which was recovered by folic acid (FA). (A) (a) Representative images of oocytes collected via superovulation after removing cumulus cells. (b) No significant differences were observed in the diameter of oocytes among the three groups. (B) The MTX group exhibited a significantly reduced fertilization rate. FA treatment could not significantly restore the fertilization rate of the oocyte. (C) The percentage of embryos at different stages. The proportion of blastocysts was significantly lower than that of the control group, which was restored after FA treatment. (D) (a) The morphology of dead embryo in each group, the inner cell mass of the dead embryo was fragmented. (b) The statistical chart showed that the proportion of dead embryos in the group was significantly higher than that in the FA treatment group and the control group. (E) (a) Representative images show aberrant embryos from MTX and FA treatment groups including embryos without zona pellucida or with atypical inner cell mass. (b) The statistical chart showed the proportion of aberrant embryos in the MTX group 120 h after IVF, which was much higher than that of the control and FA groups (*p < 0.05, **p < 0.01, Student’s t‐test, n = 6; Scale bars: 50 μm)
The data mentioned above indicated the presence of a greater percentage of dysfunctional oocytes in mice that were previously exposed to MTX, evidenced by decreasing success rate of fertilization, retardation of embryonic development, increasing death rate, and specific malformations in embryos. Meanwhile, FA could partially rescue oocytes by alleviating the retardation and blockage of embryonic development.
MTX induces senescence and apoptosis of human GCS in vitro, which can be released by FA antagonism
KGN cells (human GCs) were then used to validate the detrimental effects exerted by MTX exposure and to assess the recovery mechanisms of FA. To imitate the MTX plasma concentration in patients prescribed with MTX, 27 we conducted an in vitro analysis wherein we supplemented a medium with 50 nM MTX and performed cell culture experiments. Forty‐eight hours after incubation in a medium with 50 nM MTX, KGN cells were transferred to a medium supplemented with 200–4000 nM FA. After 120 h, based on the recovery of cell proliferation (Figure 3a), we selected 1000 nM FA as the optimal dose to validate the effect of FA‐mediated rescue on KGN cells treated with MTX (FA treatment group). As shown in Figure 3b, KGN cell proliferation was inhibited during 48 h of MTX treatment and seemed to be restored after MTX withdrawal, even though it was not restored to the same level as the control group. Simultaneously, senescent KGN cells with positive β‐galactosidase staining were observed at 24 h of MTX treatment (Figure 3C a), along with the increasing proportion of apoptotic KGN cells in a time‐dependent manner (Figure 3D a). The senescent and apoptotic cells were detectable in the MTX group at 72 h after MTX withdrawal (Figure 3D b, E b). Further, the expression of p21 and p53, but not that of p16, increased after MTX treatment for 48 h (Figure 4A a, B), and this observation was consistent with the senescence and apoptosis of GCs after MTX treatment. Additionally, high expression of p21 and p53 was maintained for 72 h after MTX withdrawal (Figure 4A b, B). ROS production is one of the most important mechanisms causing cell senescence and apoptosis; in the present study, ROS produced in KGN cells could be detected 24 h after MTX treatment (MTX sustained group) as shown in Figure 4C. Seventy‐two hours after MTX withdrawal, that was equivalent to 120 h after MTX exposure, ROS‐positive KGN cells could be still detected (Figure 4c). Furthermore, FA supplement reversed the damage of KGN cells attributed to MTX administration in vitro. After 72 h of FA addition to the medium, the proliferation rate of KGN cells increased significantly (Figure 3B b), the percentage of senescent KGN cells decreased from 16% to 6%, and the percentage of apoptotic GCs decreased from 73.3% to 59.3% (Figure 3C b, D b), which was accompanied by downregulation of p53 and p21 expression (Figure 4A b, B). Concurrently, ROS generation by KGN cells was significantly inhibited by FA addition to the culture medium (Figure 4C).
FIGURE 3.
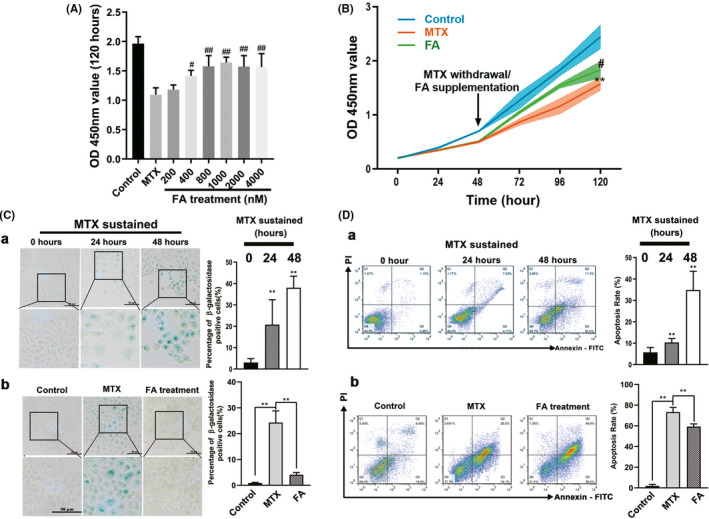
Methotrexate (MTX) caused the senescence and the apoptosis of human granulosa cells (GCs; KGN cells), which can be released by folic acid (FA) antagonism. (A) Forty‐eight hours after continuous treatment with 50 nM MTX, KGN cells were replaced with different concentrations of FA and cultured in FA‐containing medium for 72 h. The activity of KGN cells was determined. It was found that 1000 nM FA had the best recovery ability to KGN cells activity. (B) Detection of KGN cells proliferation in the MTX, FA treatment, and control group using cell counting kit‐8 (CCK‐8) assay. KGN cells proliferation was inhibited at 48 h after MTX administration and the effect was sustained till 72 h after MTX withdrawal. The proliferation of KGN cells was partially restored with the addition of FA. (C) (a) Senescent KGN cells appeared at 48 h after MTX sustained treatment accompanied by morphological changes and mainly defined as β‐ galactosidase positive cells. (b) Senescent KGN cells could still be observed at 72 h after MTX withdrawal. Senescent KGN cells no longer appeared 72 h after FA was added. (D) (a) Apoptosis of KGN cells induced by MTX appeared in large numbers at 48 h after MTX treatment. (b) Seventy‐two hours after MTX withdrawal, KGN cells still appeared in the early apoptosis stage. The apoptosis of KGN cells could be significantly reduced by the addition of FA after MTX withdrawal. (Symbol * indicates a difference between MTX processed group and control group, **p < 0.01; Symbol # indicates a difference between MTX and FA treatment groups, # p < 0.05, ## p < 0.01. Student’s t‐test; Scale bars: 50 μm)
FIGURE 4.
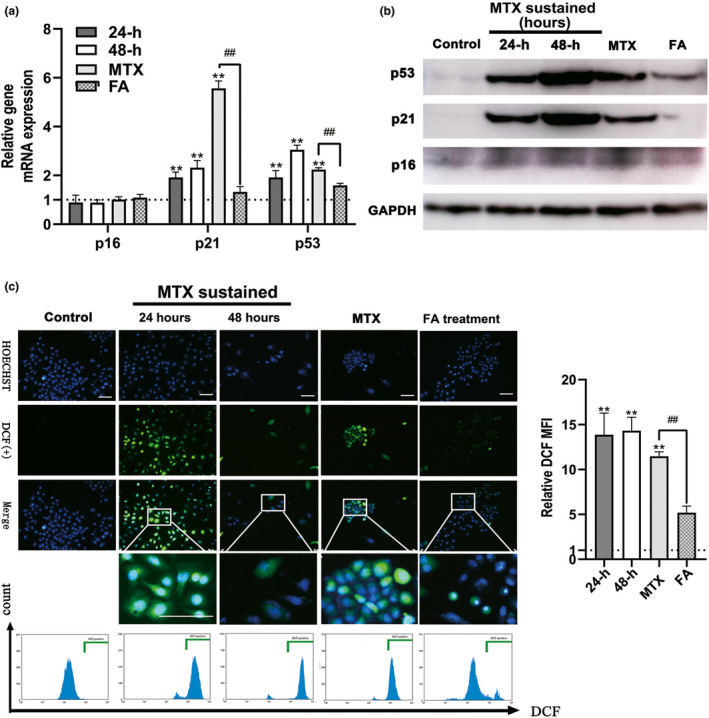
Methotrexate (MTX) caused the OS and increased p53/p21 expression of human granulosa cells (GCs; KGN cells), which can be released by folic acid (FA) antagonism. (A) Detecting the expression of p16, p21, and p53 genes using real time polymerase chain reaction (PCR). MTX upregulated the expression of p21 and p53 but not p16 genes in the KGN cells, p21 and p53 genes maintained the expression at a high level at 72 h after MTX withdrawal. In contrast, the expression of the genes reduced significantly after 72 h of incubation in FA‐medium. (B) Detecting the expression of p16, p21, and p53 genes using Western blot. (C) Detection of OS of KGN cells in MTX, FA treatment, and control group. The reactive oxygen species (ROS)‐positive cells in the green box show the nucleus with blue fluorescence and cytoplasm with green fluorescence. Relative DCF MFI shows quantitative analysis of ROS expression of KGN cells in each group. OS of GCs could be detected as early as 24 h after MTX treatment (MTX sustained group) and still be detected 72 h after MTX withdrawal (MTX group). The addition of FA could eliminate almost all the reactive oxygen species. (Symbol * indicates a difference between MTX processed groups and control group, **p < 0.01; Symbol # indicates a difference between MTX and FA treatment groups, # p < 0.05, ## p < 0.01. Student’s t‐test; Scale bars: 50 μm)
GC apoptosis in mice ovaries due to MTX exposure leads to the development of follicular dysplasia
We investigated whether the GCs in the mouse ovarian tissue would also be damaged after MTX exposure, and found that apoptotic GCs were present not only in pre‐antral follicles but were present also in primary follicles as evidenced by TUNEL assay results (Figure 5A), which indicated the pathogenicity of the injured follicles caused by MTX administration. Considering the histological and functional association between GCs and oocytes in follicles, we investigated the changes in follicles and ovaries in the MTX group mice. First, the ovaries of the MTX group mice exhibited an evident weight reduction compared with the control group (Figure 5C). Second, MTX exposure led to a significant reduction in estradiol and AMH levels (Figure 5B), which are mainly secreted by GCs, which further confirmed the existence of GC damage. 15 Additionally, we determined the number of follicles at different developmental stages. The results showed that even though there was no significant difference in the total number of follicles among the three groups (Figure 5D b), the proportion of atretic follicles increased significantly because of MTX exposure, whereas the proportion of pre‐antral and antral follicles rather than primordial and primary follicles decreased dramatically (Figure 5D c).
FIGURE 5.
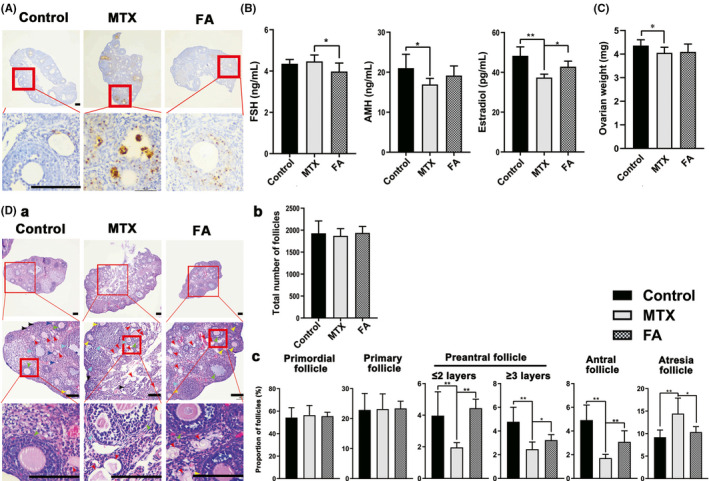
Granulosa cells (GCs) apoptosis in mice ovaries due to methotrexate (MTX) led to follicular dysplasia. (A) Apoptotic GCs in the ovaries, apoptotic GCs appear brown. Apoptotic GCs were already visible in both primary and secondary follicles. Folic acid (FA) treatment could decrease the proportion of apoptotic GCs dramatically. (B) Hormone levels in the serum. MTX administration decreased the estradiol and anti‐Müllerian hormone (AMH) significantly, and FA treatment restored the level of estradiol in mouse serum instead of AMH. (C) The ovary weight of the MTX group decreased compared with the control group. (D) (a) Representative images of hematoxylin‐eosin (H&E)‐stained serially sectioned ovaries. No significant pathological changes were found in the mouse ovaries after MTX administration. Follicles at different stages are indicated with arrows. Atresia follicles (red), primordial follicles (grey), primary follicles (dark blue), pre‐antral follicles (≤2 layers GCs, yellow; greater than 2 layers GCs, light blue), and antral follicles (green). (b) Statistical chart showing the total number of follicles. No difference was observed among the three groups. (c) Statistical chart showing the percentage of follicles at different stages in three groups. Although there was no significant difference in the proportion of primordial follicles and primary follicles among the three groups, the percentage of atresia follicles in the MTX group increased significantly. In contrast, that of the pre‐antral follicles and antral follicles decreased. FA treatment decreased the proportion of the atresia follicles and increased that of pre‐antral and antral follicles. (*p < 0.05, **p < 0.01, Student’s t‐test, n = 6; Scale bars: 50 μm)
Similar to the results obtained for the KGN cell experiments in vitro, FA supplementation remarkably decreased the percentage of apoptotic GCs in vivo (Figure 5A). Meanwhile, FA treatment not only decreased the FSH level and partially restored the estradiol level (Figure 5B), but also recovered the proportion of mature follicles and reduced the proportion of atretic follicles (Figure 5D c).
DISCUSSION
Based on the low‐dose prolonged regimen of MTX medication prescribed for ectopic pregnancy in clinical settings, 23 we developed the MTX withdrawal mouse model without variation in body weight to mimic the conditions reported in female patients with a history of MTX medication. The results of IVF and embryo development of MTX mice revealed that oocyte dysfunction presented as a decreasing rate of successful fertilization, retardation in embryonic development, increasing embryo death rate, and malformed embryos, which continue to be detected even after completion of five to six ovulation cycles after MTX withdrawal. Notably, five to six ovulation cycles in mice may be equivalent to five to six menstrual cycles in humans. 24 Considering these conditions, the implementation of intervals spanning three to six menstrual cycles between MTX discontinuation and pregnancy for patients with a history of MTX medication stated in the clinical guidelines 22 may warrant re‐evaluation.
Ovarian reserve, as a response to gonadotropin stimulation, and clinical pregnancy or live birth rate are usually used to validate the reproductive toxicity of MTX in the clinic. However, the reason for the worse outcome of IVF results caused by MTX is not clear. Tian et al. reported that the changes in oocytes in response to MTX administration in vitro included delayed maturation time, chromosome arrangement, aberrant karyotype, spindle morphology, and localization of microtubule‐organizing centers. 14 However, considering the metabolism of MTX in vivo and the integrity of ovaries, we focused on studying follicular changes and found that MTX administration reduced the proportion of mature follicles and increased the proportion of atretic follicles. Combined with the decrease in AMH and estradiol levels, we proposed that GC function was impaired because of prior MTX exposure, which was supported by the following results. First, MTX treatment induced GC apoptosis and senescence in vitro. Consistent with this phenomenon, increased apoptosis of GCs in mouse follicles was observed, which resulted in the formation of atretic follicles. Additionally, we observed the generation of ROS and increased expression of p53 and p21 genes due to MTX exposure in vitro, which are markedly related to cellular senescence and apoptosis, as revealed by previous studies. 28 , 29 These data suggest that the p53/p21 expression may be involved in the infliction of ovarian granulosa cell damage, thereby contributing to oocyte dysfunction and aberrant embryo development. In addition to follicular developmental disorders, we also observed oocyte dysfunction in the IVF experiments. As follicles are the histologic and functional units for the development of mammalian oocytes, we proposed that MTX exposure may induce the elimination of developing follicles and subsequent development into atretic follicles and further decrease the quality of oocytes.
Following this observation, aberrant embryonic development was noted after natural fertilization in mice. As reported by previous studies, GC senescence and further apoptosis could result in follicular atresia, aberrant embryo development, and poor IVF outcome. 30 , 31 , 32 We proposed that previous MTX administration induced persistent senescence and apoptosis of GCs for a prolonged duration even after MTX withdrawal, which could further lead to abnormal follicular development and dysfunction of oocytes in mouse ovaries.
It has been reported that FA medication could reduce the systemic toxicity of MTX. 20 , 21 Interestingly, we demonstrated that FA treatment after MTX withdrawal could inhibit senescence and apoptosis of GCs, restore ovary function, improve the developmental environment of oocytes, and finally improve embryonic development in mice and IVF success rates.
Results of the present study not only provide an improved interpretation of the mechanisms of reproductive toxicity induced by previous treatment with MTX medication, but also imply that the interval between MTX discontinuation and conception currently suggested by the FDA in the clinic may not be sufficient for the ovary to recover physiologically from the damage without any drug intervention. Based on the results, we suggest that after MTX exposure, patients should be treated with an appropriate dose of FA as soon as possible after MTX withdrawal. Further, it should be noted that the dose of FA reported to be protective in mice is much higher than the currently recommended dietary supplement dose for pregnant women after being converted to the appropriate dosage in humans. However, in our present study, FA was used to effectively eliminate MTX‐mediated reproductive toxicity effects, and it might be necessary to increase the dosage of FA to a level higher than that prescribed as a daily supplementation. Moreover, according to the literature reviewed, there is no evidence that high doses of FA are toxic because of the pharmacokinetic properties of FA in vivo. 20 , 21 , 33 Given that FA appears low risk, it is reasonable to consider high‐dose FA supplementation when trying to use it to decrease the reproductive toxicity of previous MTX administration in humans. In addition, the current data should be considered when discussing the optimal interval between MTX discontinuation and pregnancy seeking with patients. Although the FDA currently recommends 3–6 months, our findings suggest that the interval may be at least 6 months, and monitoring of hormone level may be necessary to be used. However, understanding the impact of a longer interval requires further studies, particularly in humans.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
J.F., Y.W., H.Z., and H.Z. wrote the manuscript. J.F., Y.W., and H.Z. designed the research. J.F., Y.L., and H.Z. performed the research. Y.L. and C.W. analyzed the data. Y.L., Y.W., H.Z., H.Z., and B.Y. contributed new reagents/analytical tools.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Wenlin Li for valuable scientific support, to Dr. Zhangjing Ma for assistance with the Western‐blot experiments, and to Dr. Mingxin Sun for performing qRT‐PCR.
Jingbo Fu, Yang Liu, and Chen Wang have contributed equally to the work.
Funding information
This work was supported by the National Natural Science Foundation of China (grants no. 31471284 and 81472771) as well as the Institute of Translational Medicine of Second Military Medical University Fund (No. 2017JZ48).
Contributor Information
Ye Wang, Email: nature_wangye@139.com.
Haiying Zhu, Email: hyzhu@smmu.edu.cn.
REFERENCES
- 1. Visentin M, Zhao R, Goldman ID. The antifolates. Hematol Oncol Clin North Am. 2012;26(3):629‐648, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mack F, Baumert BG, Schäfer N, et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;43:83‐91. [DOI] [PubMed] [Google Scholar]
- 3. Borgatta L, Burnhill MS, Tyson J, Leonhardt KK, Hausknecht RU, Haskell S. Early medical abortion with methotrexate and misoprostol. Obstet Gynecol. 2001;97:11‐16. [DOI] [PubMed] [Google Scholar]
- 4. Cecchino GN, Araujo Júnior E, Elito Júnior J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290(3):417‐423. [DOI] [PubMed] [Google Scholar]
- 5. Grim J, Chladek J, Martinkova J. Pharmacokinetics and pharmacodynamics of methotrexate in non‐neoplastic diseases. Clin Pharmacokinet. 2003;42:139‐151. [DOI] [PubMed] [Google Scholar]
- 6. Donnelly JG. Folic acid. Crit Rev Clin Lab Sci. 2001;38(3):183‐223. [DOI] [PubMed] [Google Scholar]
- 7. Bachman EA, Barnhart K. Medical management of ectopic pregnancy: a comparison of regimens. Clin Obstet Gynecol. 2012;55(2):440‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gromnica‐ihle EAKK Use of methotrexate in young patients with respect to the reproductive system. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S80. [PubMed] [Google Scholar]
- 9. McLaren JF, Burney RO, Milki AA, Westphal LM, Dahan MH, Lathi RB. Effect of methotrexate exposure on subsequent fertility in women undergoing controlled ovarian stimulation. Fertil Steril. 2009;92(2):515‐519. [DOI] [PubMed] [Google Scholar]
- 10. Weber‐Schoendorfer C, Chambers C, Wacker E, et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014;66(5):1101‐1110. [DOI] [PubMed] [Google Scholar]
- 11. Boots CE, Hill MJ, Feinberg EC, et al. Methotrexate does not affect ovarian reserve or subsequent assisted reproductive technology outcomes. J Assist Reprod Genet. 2016;33(5):647‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill MJ, Cooper JC, Levy G, et al. Ovarian reserve and subsequent assisted reproduction outcomes after methotrexate therapy for ectopic pregnancy or pregnancy of unknown location. Fertil Steril. 2014;101(2):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brouwer J, Laven JSE, Hazes JMW, Schipper I, Dolhain RJEM. Levels of serum anti‐müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res. 2013;65(9):1534‐1538. [DOI] [PubMed] [Google Scholar]
- 14. Tian N, Yu J, Zhang S, Ma WY, Wang T, Wang YM. Effects of methotrexate on the quality of oocyte maturation in vitro. Eur Biophys J. 2018;47(3):249‐260. [DOI] [PubMed] [Google Scholar]
- 15. Clarke HJ. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip Rev Dev Biol. 2018;7(1):e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng EH, Chen SU, Lee TH, et al. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28(4):929‐936. [DOI] [PubMed] [Google Scholar]
- 17. Tatone C, Amicarelli F. The aging ovary‐the poor granulosa cells. Fertil Steril. 2013;99(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 18. Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 19. Almeida CP, Ferreira MCF, Silveira CO, et al. Clinical correlation of apoptosis in human granulosa cells‐A review. Cell Biol Int. 2018;42(10):1276‐1281. [DOI] [PubMed] [Google Scholar]
- 20. Bayram M, Ozogul C, Ercan ZS, Dilekoz E, Soyer C, Bayram O. Examination of the rescue effects of folic acid on derangement of the tubo‐ovarian ultrastructural architecture caused by methotrexate. Adv Ther. 2006;23(5):772‐777. [DOI] [PubMed] [Google Scholar]
- 21. Bagshawe KD, Wilde CE. Infusion therapy for pelvic trophoblastic tumors. J Obstet Gynaecol Br Commonw. 1964;71:565‐570. [DOI] [PubMed] [Google Scholar]
- 22. Methotrexate dosage. 2018. https://www.drugs.com/dosage/methotrexate.html. Accessed January 17, 2018.
- 23. Kim TJ, Seong SJ, Lee KJ, et al. Clinical outcomes of patients treated for cervical pregnancy with or without methotrexate. J Korean Med Sci. 2004;19(6):848‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine‐mediated event. Physiol Rev. 1999;79(2):263‐323. [DOI] [PubMed] [Google Scholar]
- 25. Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reprod. 2004;127(5):569‐580. [DOI] [PubMed] [Google Scholar]
- 26. Taft R. In vitro fertilization in mice. Cold Spring Harb Protoc. 2017;2017(11):pdb.prot094508. [DOI] [PubMed] [Google Scholar]
- 27. Huang Z, Tong HF, Li Y, et al. Effect of the polymorphism of folylpolyglutamate synthetase on treatment of high‐dose methotrexate in pediatric patients with acute lymphocytic leukemia. Med Sci Monit. 2016;22:4967‐4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertram C, Hass R. Cellular responses to reactive oxygen species‐induced DNA damage and aging. Biol Chem. 2008;389(3):211‐220. [DOI] [PubMed] [Google Scholar]
- 29. Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age‐related diseases. Oxid Med Cell Longev. 2016;2016:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93(2):382‐389. [DOI] [PubMed] [Google Scholar]
- 32. Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF‐ET. J Assist Reprod Genet. 2001;18(9):490‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Obeid R, Herrmann W. The emerging role of unmetabolized folic acid in human diseases: myth or reality? Curr Drug Metab. 2012;13(8):1184‐1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


