Abstract
Pharmacogenetic (PGx) testing may be particularly beneficial in medically underserved populations by reducing the number of appointments required to optimize drug therapy and increasing the effectiveness of less expensive off‐patent drugs. The objective of this study was to identify patient populations with poor health care access and assess prescribing trends for drugs with published PGx testing guidelines. We used electronic health record data from 67,753 University of Florida Health patients, geographic access scores calculated via the 2‐step floating catchment area method, and a composite measure of socioeconomic status. Comparing the poorest (Q4) and greatest (Q1) access score quartiles, poor geographic access was significantly associated with fewer prescriber encounters (incidence rate ratio [IRR] 0.88, 95% confidence interval [CI] 0.86–0.91), fewer total unique drugs (IRR 0.92, 95% CI 0.9–0.95), and fewer PGx guideline drugs (IRR 0.94, 95% CI 0.9–0.99). After correcting for number of unique drugs, patients in low‐access areas were prescribed a greater proportion of PGx guideline drugs (IRR 1.08, 95% CI 1.04–1.13). We detected significant interactions between Black race and access score. Compared to Q1, Black patients with Q4 access scores were disproportionately affected and had fewer encounters (IRR 0.76, 95% CI 0.7–0.82) and a higher proportion of PGx drugs (IRR 1.26, 95% CI 1.13–1.41), creating further disparity. Overall, these results suggest that improved geographic access to PGx testing may allow prescribers to make more efficient use of limited opportunities to optimize therapy for drugs with PGx testing guidelines.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Drugs with pharmacogenetic (PGx) testing guidelines are often off patent and cheaper than newer alternatives. Medically underserved patients may benefit from PGx testing, but little is known about how PGx drugs are currently used in these populations.
WHAT QUESTION DID THIS STUDY ADDRESS?
Are patients with barriers to health care access prescribed more drugs with published PGx guidelines?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Patients with poor geographic access, as determined using the two‐step floating catchment area method, use a higher proportion of PGx drugs. Additionally, this study may serve as a model for the use of geospatial analysis in PGx implementation.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Improved geographic access to PGx testing may allow prescribers to make more efficient use of limited opportunities to optimize therapy for drugs with PGx testing guidelines.
INTRODUCTION
Medically underserved patients experience geographic and socioeconomic barriers to health care access and are among the last to benefit from innovative health technologies. 1 Because of this, innovative technologies have the potential to increase disparities in health care quality and outcomes, especially if there is greater pre‐existing need in the populations unable to access them. 2 This phenomenon, known as the inverse equity hypothesis, will continue to occur unless specific barriers to access are overcome and/or specific populations are targeted when a new technology is implemented. 3 , 4
As more prescribers adopt pharmacogenetic (PGx) testing, there is a chance to reorient research and clinical objectives to be better aligned with public health priorities. 5 Thus far, use of PGx testing is largely limited to academic medical centers, excluding populations that do not live near one. In addition, many health insurers are reluctant to pay for the tests, excluding patients who are unwilling or unable to incur out‐of‐pocket costs. 6 , 7 , 8 However, PGx testing may be particularly beneficial in medically underserved populations by reducing the number of appointments required to optimize drug therapy and increasing the effectiveness of less expensive off‐patent drugs—the type most often with PGx guidelines available. 9
To implement PGx testing in underserved populations, it is necessary to identify them. The Health Resources and Services Administration designates healthcare shortage areas within the United States. Medically underserved areas (MUAs) and medically underserved populations (MUPs) designate geographic areas or specific populations within an area, respectively, as lacking access to primary care services. Health professional shortage areas (HPSAs) designate areas with health workforce shortages. Designation criteria account for both spatial (i.e., provider to population ratio in a defined geographic area) and nonspatial variables (i.e., poverty levels) that affect health care access. MUA/MUP and HPSA designations are given to areas ranging in size from groups of counties to groups of urban census tracts and are used to determine eligibility for federal programs that fund health centers and support health workforce expansion. 10 , 11 , 12 A major limitation of MUA/MUP and HPSA designations is that they are applied to bounded geographic areas. Within large areas, such as counties or groups of counties, there can be considerable variation in socioeconomic status, population density, the location of health care providers relative to the population, and transportation infrastructure. Furthermore, only the healthcare providers within the bounded area are counted as accessible to the resident population with providers in neighboring communities counted as inaccessible. This may not reflect actual utilization patterns, especially in smaller geographic areas, such as census tracts. 13
The two‐step floating catchment area (2SFCA) method was developed to address the limitations imposed by bounded areas. This approach uses geographic information system software to calculate drive times between patient population centers and primary care provider locations and identify pairs that are within a threshold drive time (typically 30 min) from one another. From there, one can calculate a measure of potential spatial accessibility by determining the ratio of the providers accessible to the population to the number of patients potentially served by the accessible providers. An advantage to this approach is that data can be aggregated into more granular geographic units, such as census tracts or ZIP code tabulation areas (ZCTAs), and thus can better account for variation in factors affecting health care accessibility. A second advantage is that although geographic areas can be small, it does not limit accessible providers to those within a bordered area. 14
The purpose of this study was to evaluate whether prescribing patterns for drugs with available PGx testing guidelines differed between medically underserved and served patients within the University of Florida Health (UF Health) system. To identify underserved patients and assess the relative contribution of demographic, geographic, and socioeconomic factors, we incorporated a spatial accessibility measure calculated via the 2SFCA method in combination with patient electronic health record (EHR) data and a measure of area‐level socioeconomic status into a generalized linear model to predict prescriber encounter count, unique drug count, PGx guideline drug count, and the proportion of PGx drugs to total unique drugs.
METHODS
Data collection and variable creation
Data were collected from the EHR of UF Health patients who were 18 years of age or older, had a home address in Florida, and at least one outpatient prescription recorded between September 1, 2016, and September 1, 2018. These data consisted of patient demographics (age, sex, race, abd ethnicity), patient home ZIP codes, drug information (drug name and associated RxNorm Concept Unique Identifier [RXCUI] codes, route of administration, dose, frequency, and flags for ambulatory prescribed and historical drugs), prescriber service locations, and International Classification of Disease (ICD)‐10 codes. De‐identified patient and encounter IDs were generated by the UF Health Integrated Data Repository to facilitate linkage between patient demographics, prescription records, diagnosis codes, and encounter histories. This work was approved by the University of Florida IRB‐01 as an exempt retrospective chart review study.
Weighted Charlson Comorbidity Index scores were calculated from patient ICD‐10 codes using the comorbidity package for R software. 15 When a more severe form of a comorbidity was present, milder forms were assigned a score of 0 to avoid counting the same comorbidity multiple times. Raw scores were converted to index scores of 0, 1–2, 3–4, and greater than or equal to 5 as described by Charlson. 16
In order to standardize drug names, the RxNorm RESTful Web API was used to map RXCUI codes to nonproprietary names (IN or MIN term types). 17 Patients were included in analysis only if at least one of the drugs on their medication lists mapped to an RxNorm term. Drugs were included if flagged as ambulatory prescriptions and excluded if flagged as historical medications in the EHR. Vaccines were also excluded. For each patient, the number of encounters represents the unique encounter ID count and includes encounters where no drugs were prescribed. The number of unique drugs is the unique standardized drug name count. Drugs were coded as Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline drugs, based on CPIC’s genes‐drugs table as of March 2020 (list provided in Table S1). 18 CPIC reviews evidence on drugs affected by genetic variation and publishes clinical guidelines for the use of PGx test results. 19 , 20 Because our goal was to assess current feasibility of implementation in underserved patients, this study focused on drugs for which there are published guidelines available.
Origin‐destination matrix
An origin‐destination driving time matrix was generated using the Network Analyst extension in ArcGIS Pro and road network data from ArcGIS StreetMap Premium 2019. Origin locations were derived from patient ZIP codes converted to ZCTAs using the Uniform Data System Mapper ZIP code to ZCTA crosswalk. 21 ZCTA geographic centroids were derived from United States Census Bureau 2010 TIGER/Line shapefiles using the Calculate Geometry tool in ArcGIS Pro. Population‐weighted centroids were calculated using 2010 Census block group TIGER/Line shapefiles and American Community Survey population estimates that were rasterized to create a gridded population dataset. 22 Using the ArcGIS Mean Center tool, the geographic population center was then calculated for each ZCTA. 23
Providers with practice locations in Florida were identified using the National Plan and Provider Enumeration System (NPPES) database. The provider list was filtered to include only physicians with Healthcare Provider Taxonomy Codes related to family medicine, internal medicine, or general medicine, as well as physician assistants and advanced practice nursing providers. 24 Practice addresses were geocoded using ESRI’s Business Analyst Address Coder software. Because inactive providers may be listed in the NPPES registry, we filtered our list of National Provider Identifiers to include only the providers listed as active in the Florida Agency for Health Care Administration’s Provider Master List. 25 Registration is required for all providers who practice in Florida and bill Medicaid and must be revalidated every 5 years to remain active. 26
We completed a secondary analysis to confirm that state‐wide results were consistent with those of the UF Health catchment area. We used similar methods as described above, but restricted origin and destination locations to those located within counties in UF Health’s approximate catchment area: Alachua, Baker, Bradford, Citrus, Clay, Columbia, Dixie, Duval, Flagler, Gadsden, Gilchrist, Hamilton, Jefferson, Lafayette, Leon, Levy, Madison, Marion, Nassau, Putnam, St. Johns, Sumter, Suwannee, Taylor, Union, Volusia, and Wakulla.
Access score calculation
Geographic access scores were calculated using the 2SFCA method. 14 We used 30‐ and 60‐min cutoffs for drive times from primary care provider locations to define provider catchment and ZCTA geographic and population‐weighted centroids to define patient travel areas. We selected a 30‐min driving time cutoff because it is commonly used to define primary care service areas by and because there is precedence for it in spatial health care access studies. 14 , 27 , 28 , 29 The 60‐min cutoff was included to confirm consistency with 30‐min results and to also account for potentially longer driving times for rural patients. 30
ZCTA‐level population estimates obtained from Census Bureau’s American Community Survey 5‐year Estimates (2013–2017). The basic 2SFCA method uses a binary discrete decay function, where providers within a catchment are considered equally accessible. 31 First, for each service provider (j), the populations (k) of the ZCTAs that have centroids within the 30‐min drive time threshold (do ) are aggregated to define a provider‐to‐population ratio (Rj ), where Sj represents the total number of service providers at a location, and Pk is the total population within catchment j.
Next, an access score (Aj ) is calculated as the sum of all provider to patient ratios (Rj ) for providers located within a 30‐min drive time (do ) from the population location (i), in this case, a ZCTA centroid, are aggregated.
ZCTA‐level socioeconomic measure
We used The Robert Graham Center’s Social Deprivation Index (SDI) to estimate ZCTA‐level socioeconomic status. It is a composite score of 7 variables from the 2011–2015 American Community Survey: percent population less than 100% of the federal poverty level, percent high school dropouts, percent unemployed, percent nonemployed, percent living in crowded housing, percent single‐parent households, percent with no car, percent Black, and percent under age 5 or age 65 and over. 32 , 33
Statistical methods
Data were fitted to negative binomial models using the MASS package for R software. 34 In all models, independent variables included were age, sex, race, ethnicity, Charlson Comorbidity Index, ZCTA SDI score, and ZCTA access score. Age was stratified into groups used in the US Census Bureau table plus an additional category for patients with ages above 89 years in accordance with the Safe Harbor method. 35 The youngest age group was used as the reference level. The reference level for Charlson Comorbidity Index was no comorbidity with additional groups representing mild, moderate, and severe comorbidity. SDI score and access score were both divided into quartiles. For SDI score, quartile 1 (Q1) included the lowest scores, representing the least social deprivation, and for access score, the reference level of Q1 included the highest scores, representing the greatest geographic access. We chose this approach so that the reference level of Q1 represented the most favorable conditions of low social deprivation and high geographic access and Q4 would represent the least favorable conditions of high social deprivation and low geographic access. Quartile breakpoints are provided in Table S2.
PGx guideline drug count was modeled with and without adjusting for total unique prescription count. In the adjusted model, the log number of total unique drugs was used as an offset term. For all models, incidence rate ratios (IRRs) were calculated by exponentiating model coefficients. Exponentiating the offset term results in PGx guideline drug count to be modeled as a proportion of PGx drugs to total unique drugs.
All models include interaction terms for Hispanic ethnicity with access score and SDI as well as Black race with access score and SDI. These variables were tested to assess interactions among demographic, geographic, and socioeconomic variables associated with poor health care access.
Models presented in the main text use access scores calculated with geographic centroids and a 30‐min drive time cutoff as covariates. The primary analysis included ZCTA and provider location data from the entire state of Florida. Additional analyses were calculated using population‐weighted centroids, a 60‐min drive time cutoff, or by limiting the analysis to the UF Health catchment area.
RESULTS
Study cohort
The study cohort included total of 67,753 patients (Table 1). Most commonly, patients were White (73%), not Hispanic or Latino (93%), female (58%), and had a Charlson Comorbidity Index score of 0 (53%). Patients living in areas with poor geographic access tended to be older, were more likely to be White, less likely to be Black, and had more comorbidities. In areas with the highest levels of social deprivation, an opposite trend was observed; patients were younger, less likely to be White, and more likely to be Black. Charlson Comorbidity Index score was similar to that of the full cohort.
TABLE 1.
Patient demographics summarized for full cohort and stratified by healthcare access score and SDI
| Full cohort | Access score | SDI Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 67,753) |
Q1 (best) (N = 14,232) |
Q2 (N = 21,597) |
Q3 (N = 15,465) |
Q4 (worst) (N = 16,459) |
Q1 (best) (N = 18,286) |
Q2 (N = 15,211) |
Q3 (N = 19,537) |
Q4 (worst) (N = 14,719) |
|
| Age (n, %) | |||||||||
| 18–24 | 8920 (13) | 3052 (21) | 3464 (16) | 1258 (8) | 1146 (7) | 1970 (11) | 1116 (7) | 2943 (15) | 2891 (20) |
| 25–44 | 20,734 (31) | 5142 (36) | 7467 (35) | 3944 (26) | 4181 (25) | 5078 (28) | 3898 (26) | 6771 (35) | 4987 (34) |
| 45–64 | 19,602 (29) | 3544 (25) | 5623 (26) | 4817 (31) | 5618 (34) | 5155 (28) | 5037 (33) | 5458 (28) | 3952 (27) |
| 65–89 | 17,605 (26) | 2362 (17) | 4827 (22) | 5291 (34) | 5308 (32) | 5864 (32) | 4994 (33) | 4154 (21) | 2776 (19) |
| 90+ | 892 (1) | 132 (1) | 216 (1) | 155 (1) | 206 (1) | 219 (1) | 166 (1) | 211 (1) | 113 (1) |
| Sex (n, %) | |||||||||
| Female | 40,455 (58) | 8678 (61) | 12,904 (60) | 8840 (57) | 9194 (56) | 10,630 (58) | 8652 (57) | 11,600 (59) | 8734 (59) |
| Male | 28,861 (42) | 5554 (39) | 8693 (40) | 6625 (43) | 7265 (44) | 7656 (42) | 6559 (43) | 7937 (41) | 5985 (41) |
| Race (n, %) | |||||||||
| White | 49,602 (73) | 9090 (64) | 14,008 (65) | 12,466 (81) | 14,038 (85) | 14,746 (81) | 12,559 (83) | 13,567 (69) | 8730 (59) |
| Black | 11,480 (17) | 3434 (24) | 4623 (21) | 1910 (12) | 1513 (9) | 1869 (10) | 1710 (11) | 3256 (17) | 4645 (32) |
| Other | 4389 (6) | 1051 (7) | 1735 (8) | 854 (6) | 749 (5) | 1094 (6) | 752 (5) | 1636 (8) | 907 (6) |
| Asian | 1755 (3) | 499 (4) | 1035 (5) | 152 (1) | 69 (0) | 469 (3) | 111 (1) | 879 (4) | 296 (2) |
| Multiracial | 383 (< 1) | 127 (1) | 147 (1) | 50 (0) | 59 (0) | 80 (0) | 47 (0) | 146 (1) | 110 (1) |
| American Indian | 119 (< 1) | 25 (0) | 41 (0) | 28 (0) | 25 (0) | 24 (0) | 24 (0) | 45 (0) | 26 (0) |
| Pacific Islander | 25 (< 1) | 6 (0) | 8 (0) | 5 (0) | 6 (0) | 4 (0) | 8 (0) | 8 (0) | 5 (0) |
| Ethnicity (n, %) | |||||||||
| Not Hispanic or Latino | 62,925 (93) | 12,938 (91) | 19,734 (91) | 14,559 (94) | 15,694 (95) | 17,174 (94) | 14,427 (95) | 17,741 (91) | 13,583 (92) |
| Hispanic or Latino | 4828 (7) | 1294 (9) | 1863 (9) | 906 (6) | 765 (5) | 1112 (6) | 784 (5) | 1796 (9) | 1136 (8) |
| Charlson Comorbidity Index (n, %) | |||||||||
| 0 | 35,874 (53) | 8604 (60) | 12,289 (57) | 7519 (49) | 7462 (45) | 9989 (55) | 7023 (46) | 10,834 (55) | 8028 (55) |
| 1–2 | 18373 (27) | 3650 (26) | 5586 (26) | 4387 (28) | 4750 (29) | 5069 (28) | 4427 (29) | 5097 (26) | 3780 (26) |
| 3–4 | 6224 (9) | 913 (6) | 1754 (8) | 1637 (11) | 1920 (12) | 1544 (8) | 1786 (12) | 1686 (9) | 1208 (8) |
| 5+ | 7282 (10) | 1065 (7) | 1968 (9) | 1922 (12) | 2327 (14) | 1684 (9) | 1975 (13) | 1920 (10) | 1703 (12) |
Abbreviation: SDI, social deprivation index.
Geographic access scores
Access scores were generated using geographic and population‐weighted centroids and 30‐ and 60‐min drive times. The distance between geographic and population‐weighted centroids ranged from 0 to 15.4 km with a median of 0.62 ± 1.11 km. The largest discrepancies were observed for high landmass ZCTAs, such as those abutting the Everglades in South Florida. As expected, geocoded prescriber locations appeared to cluster around the major population centers within Florida (Figure S1). When access scores were calculated using a 30‐min cutoff for both geographic and population‐weighted centroids, ZCTAs with higher access scores tended to be clustered around larger cities (Figure 1) with more diffuse clustering for the 60‐min drive time cutoff (Figure S2).
FIGURE 1.
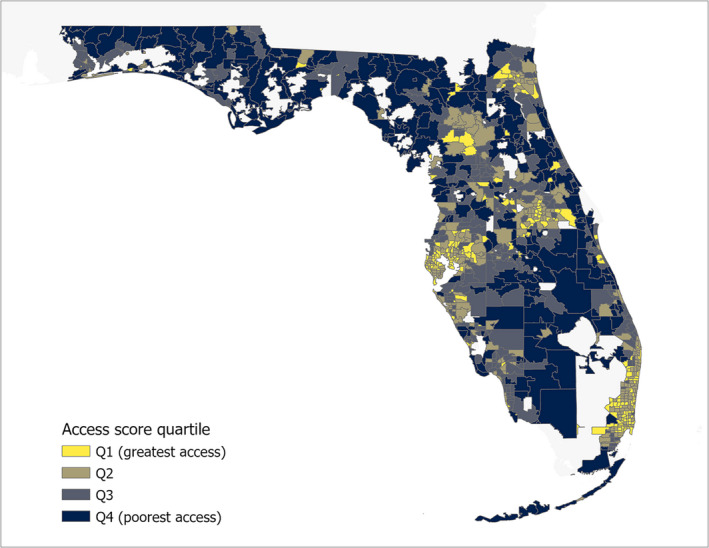
Florida ZCTA access scores. Florida ZCTA boundaries were mapped using United States Census Bureau 2010 TIGER/Line shapefiles and access scores were calculated using ZCTA geographic centroids as origins, geocoded primary care provider locations as destinations, and a 30‐min drive time cutoff. Yellow shading indicates ZCTAs in greatest access quartile and dark blue indicates ZCTAs in the poorest access quartile. ZCTA, ZIP code tabulation area
Demographic, socioeconomic, and clinical associations
Relative to a reference age range of 18–23 years, increased age was significantly associated with a greater number of prescriber encounters and total unique drug counts in all age groups other than 90+ years (Figure 2). After adjusting for total unique drugs, all age groups had fewer adjusted PGx guideline drugs relative to the reference group (Figure 3). Relative to women, men had fewer encounters, fewer unique drugs (Figure 2), and fewer adjusted PGx guideline drugs (Figure 3).
FIGURE 2.
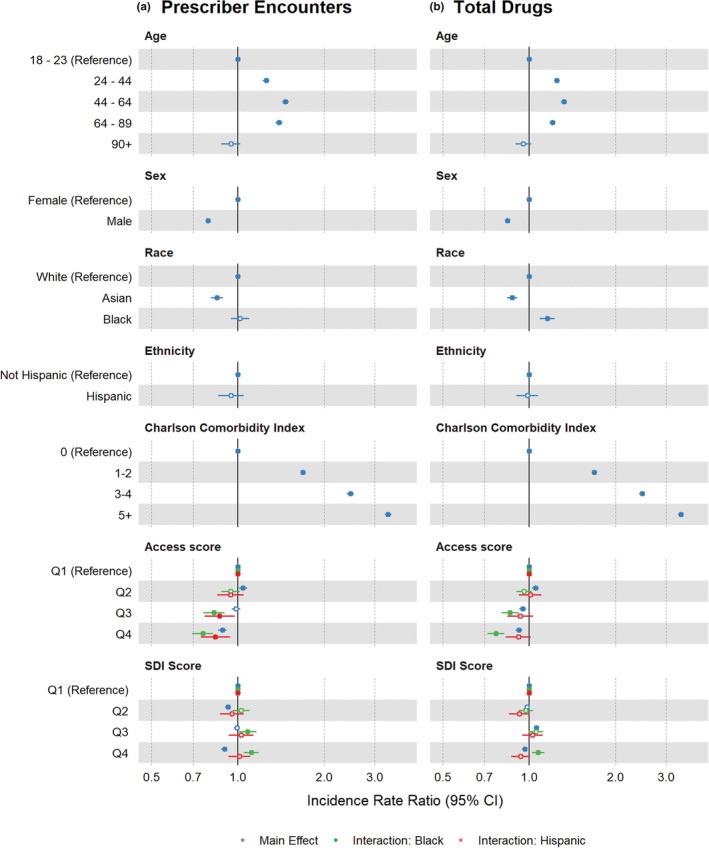
Incidence rate ratios (IRRs) for prescriber encounters and total drugs. IRRs and 95% confidence intervals (CIs) were derived from regression model covariate coefficients and standard errors for the (a) prescriber encounter and (b) total drug models. Main effects are depicted in blue and interaction effects are depicted in green for interaction with Black race or red for interaction with Hispanic or Latino ethnicity. Shaded circles indicate p values <0.05 and open circles indicate p values ≥0.05. SDI, social deprivation index
FIGURE 3.
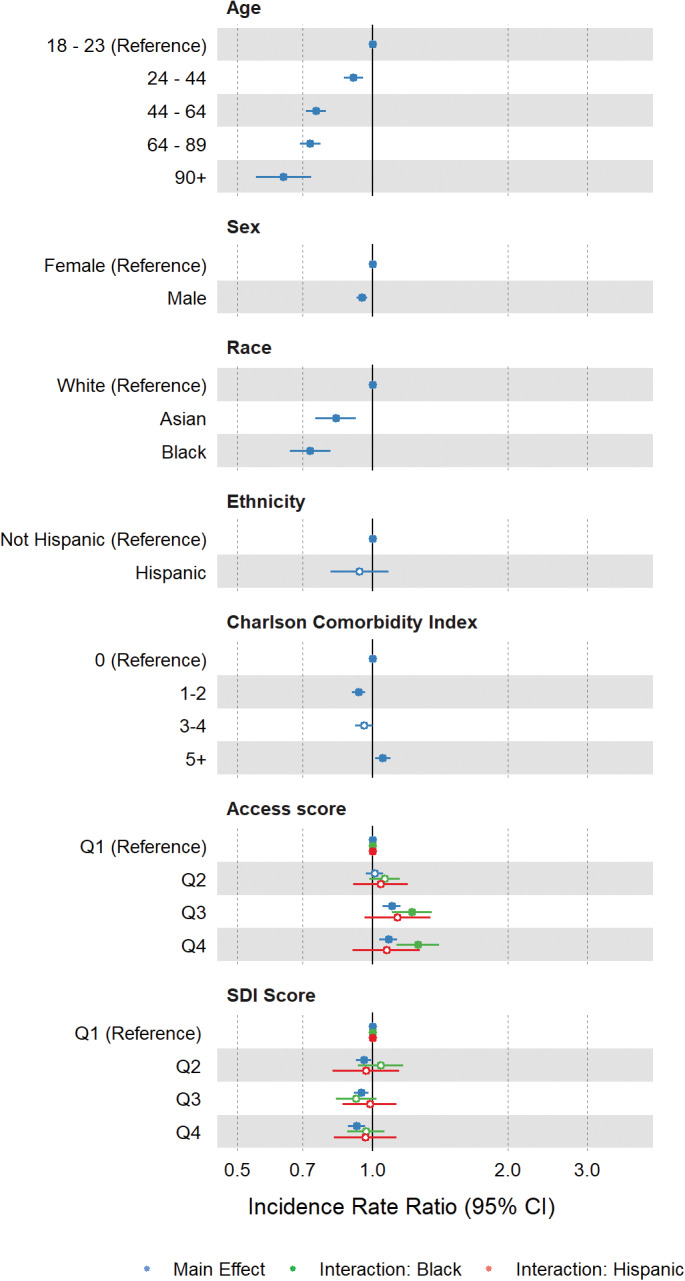
Incidence rate ratios for PGx drug count adjusted for total drug count. Incidence rate ratios and 95% confidence intervals (CIs) were derived from regression model covariate coefficients and standard errors for the adjusted PGx guideline drug count model. Main effects are depicted in blue and interaction effects are depicted in green for interaction with Black race or red for interaction with Hispanic or Latino ethnicity. Shaded circles indicate p values <0.05 and open circles indicate p values ≥0.05. PGx, pharmacogenetic; SDI, social deprivation index
Medical comorbidity, as described by the Charlson Comorbidity Index, was strongly associated with increased prescriber encounters and total drug counts (Figure 2). However, there was comparatively little association between comorbidity and adjusted PGx guideline drugs (Figure 3).
Relative to the first quartile of SDI scores, representing the least deprivation, higher SDI scores were associated with decreased prescriber encounters (Figure 2a) and fewer adjusted PGx guideline drugs (Figure 3).
Lower access scores, representing poorer geographic health care access, were associated with fewer prescriber encounters and fewer overall unique drug prescriptions (Figure 2), and greater adjusted PGx guideline drug counts, indicating higher rates of PGx guideline drug prescribing in low‐access populations (Figure 3; Q3 IRR 1.1, 95% confidence interval [CI] 1.05–1.15; Q4 IRR 1.08, 95% CI 1.04–1.13). Asian race was associated with fewer prescriber encounters, fewer unique drug prescriptions, and fewer adjusted PGx guideline drugs (Figure 2). In preliminary analyses, we did not detect significant interactions between Asian race and SDI score or access score and thus did not include interaction terms in the final models.
Black race, as a main effect, was not significantly associated with prescriber encounter counts, but was significantly associated with a greater number of unique drugs and fewer adjusted PGx guideline drugs. The interaction between Black race and SDI was significant in the prescriber encounter and total drug models where greater social deprivation was associated with increased prescriber encounters and a greater number of total drugs (Figure 2). The interaction between Black race and access score was significant in all models, where poorer access score was associated with fewer prescriber encounters, fewer total drugs (Figure 2), and more adjusted PGx guideline drugs. Of note, Black patients with the lowest access scores were prescribed PGx guideline drugs at a 26% higher rate than reference group patients with the best access scores (Q4 IRR 1.26, 95% CI 1.13–1.41; Figure 3, Table S3).
Hispanic ethnicity, as a main effect, was not significantly associated with prescriber encounters, total drugs, or adjusted PGx guideline drugs. However, the interaction between Hispanic ethnicity and access score was significant in the prescriber encounters model, where poorer access score was associated with fewer prescriber encounters (Figure 2a).
Results were similar for analyses using population‐weighted centroids, a 60‐min drive time, and regional analysis that included only patients and providers within UF Health’s approximate catchment area (Figures S3 and S4).
DISCUSSION
This work demonstrates the feasibility of using a combination of EHR data, geospatial analysis methods, and socioeconomic status indicators to assess the relationship between barriers to health care access and prescribing patterns, with a specific focus on drugs with published PGx testing guidelines. Within a cohort of adult UF Health patients with Florida home addresses, we found that residence in a ZCTA with access scores indicating poorer geographic health care access was significantly associated with fewer prescriber encounters and fewer drug prescriptions. Adjusting for the decreased overall prescription numbers demonstrated that underserved patients are prescribed PGx guideline drugs at a significantly higher rate.
Race, but not ethnicity, appeared to compound the disparities we observed. The interaction between Black race and low access score was associated with greater decreases in prescriber encounters and total drugs, but much higher proportion of PGx guideline drug prescriptions.
These results suggest that poor geographic access may contribute to racial health care disparities and that there is some spatial feature that may influence Black patients being prescribed a higher proportion of PGx guideline drugs relative to other races. A contributing factor may be structural inequality within healthcare systems. For example, a study that evaluated disparities in access to trauma care found that in Chicago and Los Angeles, Black majority census tracts were often in historically Black neighborhoods and were more likely to be located in low‐access areas that corresponded to regions disproportionately affected by trauma center closures. 36 , 37 In addition, a recent systematic review showed that racial minorities have higher rates of adverse drug events and medication dosing errors. 38 Because these geographically underserved patients visit healthcare providers less frequently and are prescribed fewer medications overall, their higher rates of PGx guideline drug prescriptions suggest that improved access to PGx testing may allow prescribers to make more efficient use of limited opportunities to optimize therapy for these patients. An example where PGx would be particularly impactful is treatment for major depressive disorder, where a standard trial‐and‐error approach to prescribing can involve several follow‐up appointments, which may not accommodate patients with health care access barriers. These patients also tend to have the greatest need; major depressive disorder is more prevalent in patients with low socioeconomic status and factors such as low income, low education level, and unemployment are associated with poor treatment outcomes. 39 , 40 A recent meta‐analysis of studies comparing PGx‐guided and unguided treatment for major depressive disorder determined that guided treatment was associated with improved response and remission rates. 41 To facilitate the use of PGx tests, published guidelines are available for selective serotonin reuptake inhibitors, which are commonly used first‐line treatments for major depressive disorder, and tricyclic antidepressants, which may be used for patients with recurrent depression. 42 , 43
Relative to ZCTAs with SDI scores reflecting the lowest levels of social deprivation, residence in a ZCTA with an SDI score representing high levels of social deprivation was associated with fewer prescriber encounters. We observed significant interactions for Black race and SDI score for prescriber encounters and total drugs. Of note, these are the only interactions for which the direction of the interaction effect changed relative to the main effect; Black race and residence in the highest SDI score quartile was associated with more prescriber encounters and total drugs. However, SDI score differed in that less favorable scores were associated with fewer adjusted PGx drugs; furthermore, there was no significant interaction between SDI score and either Black race or Hispanic ethnicity. As reflected in Table 1, it appears that access score and the SDI may be describing different populations, which could contribute to differences in effect size and direction. Patients living in areas with the worst access scores were older, more predominantly White, and had more comorbidities, whereas those in areas with the worst SDI scores were younger and more predominantly Black. It may also be possible that the SDI did not add much additional information beyond the demographic data available in the EHR, consistent with previous work on the use of neighborhood socioeconomic status measures in risk prediction for health care utilization and hospitalizations. 44
Increasing comorbidity, described using the Charlson Comorbidity Index, was associated with greater numbers of encounters and unique prescriptions, as would be expected for populations requiring care for multiple medical conditions. When PGx guideline drug count was adjusted for total drugs, the effect of the Charlson Comorbidity Index score was essentially abolished, suggesting that it is closely correlated with total drug count. The relationship between comorbidity and drug count has been noted previously; for example, the comorbidity‐polypharmacy score is calculated by finding the total count of all known comorbid conditions and associated drugs and is used to predict morbidity and mortality in older trauma patients. 45
An advantage to using ZIP codes is that they can be included in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant limited data set and are thus easier to obtain than more granular geographic data. 46 However, it is important to understand their limitations. ZIP codes are defined by the United States Postal Service and boundaries may be altered over time to accommodate changes in mail delivery routes. This can lead to spatiotemporal mismatches where data collected at a variety of timepoints can refer to the same ZIP code in name but differ in the geographic area covered. 47 , 48 ZCTAs were developed by the US Census Bureau to allow for more precise linkage with socioeconomic datasets using census geography, but because ZIP codes do not necessarily share boundaries with ZCTAs, mismatches are possible. 47 Another challenge is that for origin‐destination matrix generation, ZCTAs must be represented by a single point. This is generally a geographic centroid or population‐weighted centroid. The former is straightforward and objective to calculate but does not take population density into account, whereas the latter is more difficult to calculate and sensitive to variation in population data and processing methodology but is a better representation of where people live within the ZCTA. We did not observe any major differences between our results generated using geographic versus population‐weighted centroids. Results presented in the main text thus used geographic centroid to improve reproducibility.
Because the NPPES database does not include data on whether a provider is actively practicing, there is a risk of overestimating provider supply if additional steps are not taken to exclude those who are inactive. We used Florida’s Provider Master List to limit providers to those who have registered to bill Medicaid within the past 5 years. If analyzing data from a state without a comparable database, a similar approach is to include providers who have claims in the most recent Medicare Provider Utilization and Payment Data dataset. 49 , 50 Although this approach excludes many inactive providers, it will also exclude those who are active but do not bill Medicaid or Medicare. If only Medicare claims are used to filter for active providers, there is a risk of excluding those who see younger patients.
For access score calculation using the 2SFCA method, we chose to use a 30‐min driving time threshold for primary analyses. We selected this value to be consistent with Federal primary medical care service area definitions; for instance, a 30‐min driving time is used by the Health Resources and Services Administration for Health Professional Shortage Area designation and the Department of Veteran Affairs (VA)to define VA facility catchment areas. 28 , 29 This driving time threshold is also consistent with previous health care access studies using the 2SFCA method. 14 , 27 , 51
Limitations of the 30‐min value are that it may not account for providers located further away but within what may be an acceptable driving time for some patients and that it may exclude those in rural areas. 30 Longer driving times can address these issues but limit the ability to detect small but meaningful high‐ or low‐access areas. In our analysis, results were roughly similar when a 60‐min cutoff was used for access score calculation. As shown in Figure 1, we observed a cluster of ZCTAs with favorable access scores in the North center of the state. This cluster overlays Alachua county and is consistent with the county’s status of having the highest concentration of primary care physicians in Florida. 52 Despite this, it is possible that our active provider counts for this region are overinflated due to the presence of UF Health Shands Hospital, a large teaching hospital. A limitation of using the Provider Master List to identify active providers is that the 5‐year window may capture medical residents and fellows that have since moved on to other locations.
An additional limitation is that patient data are only from visits with UF Health providers. It is possible that patients had additional encounters with outside providers and received prescriptions not documented in their UF Health record, and even when a prescription is documented in the EHR, it is possible that it was taken sparsely or never filled at all. In general, the degree to which this is an issue depends on the context and goals of a study. In the present case, our goal was to describe how drugs are prescribed within a single health system. However, this approach is likely too limited if the goal is to assess actual drug usage or make broad conclusions about prescribing in underserved patients.
To address some of these limitations, a natural next step is to apply this method to larger datasets that include data from multiple healthcare systems. This will allow for assessment of whether the approach is generalizable to broader datasets and provide more complete patient data for analysis focused on individual PGx drugs and/or disease states.
As demonstrated in this study, geospatial and neighborhood socioeconomic status data can be used in conjunction with patient EHR data to identify populations most likely to be prescribed drugs with published PGx testing guidelines. To the best of our knowledge, this is the first time these methods have been used in this context. A benefit of this approach is that home ZIP codes are the only EHR‐derived data necessary to determine patients’ geographic access scores. The methodology is generalizable to other geographic areas and can be modified depending on research objectives using publicly available ZCTA boundary shapefiles, ZCTA‐level Census data, and prescriber address data. Ideally, this work will inform future implementation efforts for PGx testing within the UF Health system and serve as a model for other health systems seeking to do similar analyses.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
R.D. and J.D.D. wrote the manuscript. R.D., J.D.B., and J.D.D. designed the research. R.D. performed the research. R.D. analyzed the data.
Supporting information
Supplementary Material
Funding information
R.D. was funded by National Institutes of Health (NIH) grant T32 HG008958; and J.D.D. was funded by K23 GM112014. Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Weiss D, Rydland HT, Øversveen E, Jensen MR, Solhaug S, Krokstad S. Innovative technologies and social inequalities in health: a scoping review of the literature. PLoS One. 2018;13(4):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries: 1989–2000. Med Care. 2005;43(4):320‐329. [DOI] [PubMed] [Google Scholar]
- 3. Victora CG, Joseph G, Silva ICM, et al. The inverse equity hypothesis: analyses of institutional deliveries in 286 national surveys. Am J Public Health. 2018;108(4):464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Victora CG, Fenn B, Bryce J, Kirkwood BR. Co‐coverage of preventive interventions and implications for child‐survival strategies: evidence from national surveys. Lancet. 2005;366(9495):1460‐1466. [DOI] [PubMed] [Google Scholar]
- 5. Fullerton SM, Knerr S, Burke W. Finding a place for genomics in health disparities research. Public Health Genomics. 2012;15(3–4):156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee YM, Manzoor BS, Cavallari LH, Nutescu EA. Facilitators and barriers to the adoption of pharmacogenetic testing in an inner‐city population. Pharmacotherapy. 2018;38(2):205‐216. [DOI] [PubMed] [Google Scholar]
- 7. Park SK, Thigpen J, Lee IJ. Coverage of pharmacogenetic tests by private health insurance companies. J Am Pharm Assoc. 2020;60(2):352‐356. [DOI] [PubMed] [Google Scholar]
- 8. Bielinski SJ, St Sauver JL, Olson JE, et al. Are patients willing to incur out‐of‐pocket costs for pharmacogenomic testing? Pharmacogenomics J. 2017;17(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duconge J, Ruano G. ‘Generic to genetic’ transition in cardiovascular and neuropsychiatric drugs: opportunity for personalized medicine. Pharmacogenomics. 2012;13(10):1097‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Human Resources & Services Administration . Shortage designation application and review process. 2019. https://bhw.hrsa.gov/shortage‐designation/application‐review‐process
- 11. Shortage Designation Scoring Criteria Human Resources & Services Administration . 2019. https://bhw.hrsa.gov/shortage‐designation/hpsa‐criteria.
- 12. Health Resources & Services Administration . 2019. Medically Underserved Areas and Populations (MUA/Ps). https://bhw.hrsa.gov/shortage‐designation/muap.
- 13. Daly MR, Mellor JM, Millones M. Defining primary care shortage areas: do GIS‐based measures yield different results? J Rural Health. 2019;35(1):22‐34. [DOI] [PubMed] [Google Scholar]
- 14. Luo W, Wang F. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the Chicago region. Environ Plann B Plann Des. 2003;30(6):865‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw. 2018;3(23):648. [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 17. Unified Medical Language System (UMLS) Appendix 5 ‐ RxNorm Term Types (TTY). National Library of Medicine. 2019. https://www.nlm.nih.gov/research/umls/rxnorm/docs/appendix5.html.
- 18. Genes‐Drugs . Clinical Pharmacogenetics Implementation Consortium. 2020. https://cpicpgx.org/genes‐drugs/.
- 19. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ZIP Code to ZCTA Crosswalk. UDS Mapper. https://www.udsmapper.org/zcta‐crosswalk.cfm. Accessed January 27, 2020.
- 22. TIGER/Line with selected demographic and economic data. Unites States Census Bureau. https://www.census.gov/geographies/mapping‐files/time‐series/geo/tiger‐data.2010.html.
- 23. Dilts T. Mapping with population‐weighted centroids. ArcUser. 2020;32020:52‐53. [Google Scholar]
- 24. NPPES NPI Registry . https://npiregistry.cms.hhs.gov/.
- 25. Florida Medicaid Web Portal: Provider Information Reports. Agency for Health Care Administration. 2020. http://portal.flmmis.com/FLPublic/Provider_ManagedCare/Provider_ManagedCare_Registration/tabId/77/Default.aspx?linkid=pml.
- 26. Florida Medicaid Web Portal: Providers IDs and Information for Medicaid Health Plans. Agency for Health Care Administration. http://portal.flmmis.com/FLPublic/Provider_ManagedCare/Provider_ManagedCare_Registration/tabId/77/Default.aspx.
- 27. Luo W, Qi Y. An enhanced two‐step floating catchment area (E2SFCA) method for measuring spatial accessibility to primary care physicians. Health Place. 2009;15(4):1100‐1107. [DOI] [PubMed] [Google Scholar]
- 28. CFR Appendix A to Part 5 ‐ Criteria for Designation of Areas Having Shortages of Primary Medical Care Professional(s). https://www.law.cornell.edu/dfr/text/42/appendix‐A_to_part_5.
- 29. CFR § 17.4040 ‐ Designated access standards. https://www.law.cornell.edu/cfr/text/38//17.4040#:~:text=§%2017.4040%20Designated%20access%20standards.%20%28a%29%20The%20following,mental%20health%20care%2C%20and%20non‐institutional%20extended%20care%20services.
- 30. Donohoe J, Marshall V, Tan X, Camacho FT, Anderson RT, Balkrishnan R. Spatial access to primary care providers in Appalachia: evaluating current methodology. J Prim Care Community Health. 2016;7(3):149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang F. Measurement, optimization, and impact of health care accessibility: a methodological review. Ann Assoc Am Geogr. 2012;102(5):1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Social Deprivation Index (SDI) . https://www.graham‐center.org/rgc/maps‐data‐tools/sdi/social‐deprivation‐index.html.
- 33. Phillips RL, Liaw W, Crampton P, et al. How other countries use deprivation indices‐and why the United States desperately needs one. Health Aff (Millwood). 2016;35(11):1991‐1998. [DOI] [PubMed] [Google Scholar]
- 34. Venables W, Ripley D. Modern Applied Statistics with S, Vol. 4. New York: Springer; 2002. [Google Scholar]
- 35. Malin B, Benitez K, Masys D. Never too old for anonymity: a statistical standard for demographic data sharing via the HIPAA Privacy Rule. J Am Med Inform Assoc. 2011;18(1):3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tung EL, Hampton DA, Kolak M, Rogers SO, Yang JP, Peek ME. Race/ethnicity and geographic access to urban trauma care. JAMA Netw Open. 2019;2(3):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsia RY, Shen YC. Rising closures of hospital trauma centers disproportionately burden vulnerable populations. Health Aff (Millwood). 2011;30(10):1912‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chauhan A, Walton M, Manias E, et al. The safety of health care for ethnic minority patients: a systematic review. Int J Equity Health. 2020;19(1):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freeman A, Tyrovolas S, Koyanagi A, et al. The role of socio‐economic status in depression: results from the COURAGE (aging survey in Europe). BMC Public Health. 2016;16(1):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jakubovski E, Bloch MH. Prognostic subgroups for citalopram response in the STAR*D trial. J Clin Psychiatry. 2014;75(7):738‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenblat JD, Lee Y, McIntyre RS. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: a meta‐analysis. J Affect Disord. 2018;241:484‐491. [DOI] [PubMed] [Google Scholar]
- 42. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhavsar NA, Gao A, Phelan M, Pagidipati NJ, Goldstein BA. Value of neighborhood socioeconomic status in predicting risk of outcomes in studies that use electronic health record data. JAMA Netw Open. 2018;1(5):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mubang RN, Stoltzfus JC, Cohen MS, et al. Comorbidity‐polypharmacy score as predictor of outcomes in older trauma patients: a retrospective validation study. World J Surg. 2015;39(8):2068‐2075. [DOI] [PubMed] [Google Scholar]
- 46. CFR § 164.514 ‐ Other requirements relating to uses and disclosures of protected health information. https://www.law.cornell.edu/cfr/text/45/164.514
- 47. Krieger N, Waterman P, Chen JT, Soobader M‐J, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US Census‐Defined Geographic Areas—The Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area‐based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471‐482. [DOI] [PubMed] [Google Scholar]
- 49. Medicare provider utilization and payment data: physician and other supplier. Centers for Medicare and Medicaid Services. 2019. https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Medicare‐Provider‐Charge‐Data/Physician‐and‐Other‐Supplier.
- 50. Bindman AB. Using the National Provider Identifier for health care workforce evaluation. Medicare Medicaid Res Rev. 2013;3(3):E1‐E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen X, Jia P. A comparative analysis of accessibility measures by the two‐step floating catchment area (2SFCA) method. Int J Geographical Information Sci. 2019;33(9):1739‐1758. [Google Scholar]
- 52. County Health Rankings: ratio of population to primary care physicians. 2020. https://www.countyhealthrankings.org/app/florida/2020/measure/factors/4/data?sort=sc‐3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


