Abstract
Postoperative delirium (POD) and postoperative (neuro‐)cognitive disorder (POCD) are frequent and serious complications after operations. We aim to investigate the association between pre‐operative polypharmacy and potentially inappropriate medications and the development of POD/POCD in elderly patients. This investigation is part of the European BioCog project (www.biocog.eu), a prospective multicenter observational study with elderly surgical patients. Patients with a Mini‐Mental State Examination score less than or equal to 23 points were excluded. POD was assessed up to 7 days after surgery using the Nursing Delirium Screening Scale, Confusion Assessment Method (for the intensive care unit [ICU]), and a patient chart review. POCD was assessed 3 months after surgery with a neuropsychological test battery. Pre‐operative long‐term medication was evaluated in terms of polypharmacy (≥5 agents) and potentially inappropriate medication (defined by the PRISCUS and European list of potentially inappropriate medications [EU(7)‐PIM] lists), and associations with POD and POCD were analyzed using logistic regression analysis. Eight hundred thirty‐seven participants were included for analysis of POD and 562 participants for POCD. Of these, 165 patients (19.7%) fulfilled the criteria of POD and 60 (10.7%) for POCD. After adjusting for confounders, pre‐operative polypharmacy and intake of potentially inappropriate medications could not be shown to be associated with the development of POD nor POCD. We found no associations between pre‐operative polypharmacy and potentially inappropriate medications and development of POD and POCD. Future studies should focus on the evaluation of drug interactions to determine whether patients benefit from a pre‐operative adjustment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Polypharmacy and drugs listed on PRISCUS or European list of potentially inappropriate medications (EU(7)‐PIM) lists are associated with higher hospitalization rates, increased morbidity, and poorer outcome regarding quality of life. Studies examining such medication in addition to polypharmacy and the occurrence of postoperative delirium/postoperative (neuro‐)cognitive disorder (POD/POCD) are lacking.
WHAT QUESTION DID THIS STUDY ADDRESS?
Is there an association between preoperative polypharmacy and potentially inappropriate medications (defined by PRISCUS and EU(7)‐PIM lists) and the development of POD and POCD in elderly patients?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Pre‐operative polypharmacy and intake of potentially inappropriate medications were not associated with the development of POD or POCD. It may be reasonable to focus on the evaluation of drug interactions rather than sole avoidance of listed medications.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Based on these results, simply considering the number of drugs or screening for potentially inadequate medication is insufficient as a preventive strategy regarding POD and POCD. Future studies should consider drug interactions and use randomized controlled approaches to determine the interaction of predisposing and precipitating factors.
INTRODUCTION
Postoperative delirium (POD) is an acute and sudden change in the mental state, characterized by fluctuating levels of attention, consciousness, and cognition. 1 The occurrence of POD is associated with increased complication and mortality rates, 2 , 3 and may be related to the development of mild and major postoperative (neuro‐)cognitive disorder (POCD). 4 , 5 , 6
Incidence depends on predisposing and precipitating risk factors. 7 , 8 Age‐related changes in brain metabolism, such as neurodegeneration, neurotransmitter imbalances, microcirculation disorders, altered penetration of the blood‐brain‐barrier, and modulations in intracellular signal transduction, increase the vulnerability of the brain to stress factors. 9 Therefore, older people are particularly susceptible to POD.
At the same time—due to the accumulation of comorbidities—an increasing amount of medication is prescribed to elderly patients. Polypharmacy and potentially inadequate medication may have an additional influence on cognition. Although there is no uniform definition for polypharmacy, it is generally described in literature as the long‐term intake of greater than or equal to 5 prescription‐free and/or prescription drugs. 10 , 11 To address the increased risk of adverse drug reactions, especially for older people, the German PRISCUS list was published in 2010, 12 which served as basis for the 2015 European list of potentially inappropriate medications (EU(7)‐PIM). 13 Drugs from the PRISCUS and EU(7)‐PIM lists should be avoided whenever possible in elder patients. It has been shown that patients with polypharmacy and drugs listed on the PRISCUS or EU(7)‐PIM lists have a higher hospitalization rate due to adverse drug events, have poorer outcome in terms of quality of life, and an increased morbidity after hospitalization. 14
Awareness and modulation of predisposing and precipitating risk factors 15 in pre‐operative, intra‐operative, and postoperative settings are crucial for the prevention of POD and POCD. Prevention strategies can reduce the in‐hospital incidence of delirium by 30%–40%. 16 Although former studies in the field of peri‐operative medicine focused on the relationship of POD and polypharmacy, there is still a lack of studies using a structured assessment of long‐term medication in terms of potentially inadequate medication, according to the PRISCUS and EU(7)‐PIM lists, in addition to polypharmacy, on the development of POD and POCD. If the application of the lists or the screening for polypharmacy can reliably identify patients at risk for the development of POD and POCD, severe implications can be prevented or reduced through the early initiation of primary prevention strategies.
In this study, we aim to investigate the association between pre‐operative polypharmacy and potentially inappropriate medications (defined by the PRISCUS and EU(7)‐PIM lists) and the development of POD and POCD in elderly patients.
METHODS
Study design and population
This investigation was performed as part of the BioCog project (www.biocog.eu), a prospective multicenter observational study conducted at the Charité – Universitätsmedizin Berlin, Department of Anesthesiology and Operative Intensive Care Medicine, Berlin, Germany, and the University Medical Center Utrecht, Department of Intensive Care Medicine, Utrecht, The Netherlands. The BioCog study aims to establish biomarker panels for risk and clinical outcome prediction of POD and POCD. 17 The study was approved by the local Ethics Committees (ref.: EA2/092/14 and 14–469), and conducted in accordance with the Declaration of Helsinki (ClinicalTrials.gov: NCT02265263). Written informed consent was obtained from each patient, and all local data privacy regulations were followed.
We included patients aged greater than or equal to 65 years of European descent, who underwent elective surgery with an expected surgical duration greater than or equal to 60 min, provided they were able to provide informed consent, and could undergo magnetic resonance imaging. We excluded patients with a Mini‐Mental State Examination score less than or equal to 23 points, homeless patients, and others who could not be reached by telephone or postal services for follow‐up examinations. In addition, we excluded patients enrolled in other prospective interventional studies, those who were accommodated in an institution due to an official or judicial order, and patients with conditions limiting the conduction of neurocognitive testing, such as neuropsychiatric disease, anacusis/hypoacusis, and language barriers.
Baseline measurements
The following baseline measurements were collected to describe the study population: age, sex, physical status according to the American Society of Anesthesiologists Physical Status (ASA PS), Charlson Comorbidity Index (CCI), 18 pre‐existing neurocognitive disorder (NCD; for details see Supplementary Material), frailty status (for details see Supplementary Material), duration of anesthesia, site of surgery (intrathoracic/‐abdominal/‐pelvic, peripheral, and intracranial operations), and education according to the International Standard Classification of Education (ISCED). 19 In addition, Geriatric Depression Scale 20 was recorded 3 months after surgery.
Postoperative delirium
POD was defined according to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) criteria. 1 Patients were considered delirious in case of:
greater than or equal to 2 cumulative points on the Nursing Delirium Screening Scale (Nu‐DESC) and/or
a positive Confusion Assessment Method (CAM) score and/or
a positive CAM for the ICU (CAM‐ICU) and/or
patient chart review that showed descriptions of delirium (e.g., confused, agitated, drowsy, disorientated, delirious, and received antipsychotic therapy).
Delirium screening was started in the recovery room and repeated twice per day at 08:00 and 19:00 (±1 h) up to 7 days after surgery. Delirium assessment was conducted independently of the routine hospital procedures by a research team, which was trained and supervised by psychiatrists and delirium experts.
Postoperative cognitive dysfunction
To identify POCD, a neuropsychological test battery consisting of noncomputerized and computerized tests was performed before surgery, at discharge from the hospital or on the seventh postoperative day, and 3 months after surgery. The composition and retest reliabilities of the test battery are described in an additional publication. 21 Trained doctoral students and study nurses performed the testing based on a standard operating procedure, which was approved by two neuropsychologists.
POCD was defined using an R algorithm described in another publication 22 that applied the Reliable Change Index (RCI) with the International Analysis of Postoperative Cognitive Dysfunction (ISPOCD) criteria proposed by Rasmussen. 23 Missing values that were attributable to technical difficulties or external disturbances were imputed at random using the missForest package in R software environment (R Core Team 2017; R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/). Missing information due to disturbed attention or confusion of the patients (e.g., inability to understand standardized test instructions) for some but not all tests were imputed with the value of the worst performance.
Long‐term medication
The pre‐operative long‐term medication was assessed during the pre‐operative anesthesia consultation by means of anamnesis, patient’s medical record, or medication prescriptions. Long‐term medication included both prescription and prescription‐free drugs, which were taken regularly at the time of the consultation. Long‐term medication was evaluated with regard to polypharmacy and potentially inappropriate medication. Polypharmacy was defined as greater than or equal to 5 agents 10 , 11 and potentially inappropriate medication was defined according to the PRISCUS 12 and EU(7)‐PIM 13 lists. An initial analysis merely regarded the presence of polypharmacy and the intake of (any) potentially inappropriate medication. This was followed by an analysis to consider the number of regularly taken medication and the number of potentially inadequate agents.
For a descriptive analysis, the pre‐operative long‐term medication was additionally divided into groups according to the Anatomic Therapeutic Chemical (ATC) classification system. 24
Statistical analysis
Baseline characteristics were expressed as median and lower and upper quartile, or frequencies with percentages. Differences between the groups were tested using Mann‐Whitney U test or χ2 test.
The associations between pre‐operative polypharmacy and potentially inappropriate medications (defined by the PRISCUS and EU(7)‐PIM lists) and the development of POD and POCD were investigated using multivariable logistic regression analysis, adjusting for possible confounding variables. Confounders were chosen a priori and included age, CCI, frailty status, pre‐existing NCD, duration of anesthesia and site of surgery for the regression analysis of POD, and age, ASA PS, frailty status, depression 3 months after surgery (defined as >4 points in GDS), and education (according to ISCED, with regard to levels 1–4, which corresponds to a lower educational level) for the regression analysis of POCD. A separate regression analysis was performed for each investigated variable (polypharmacy, potentially inappropriate medication, number of agents, and the number of potentially inadequate agents according to the PRISCUS and EU(7)‐PIM lists). No adjustments were made for multiple testing.
All analyses were performed with IBM SPSS Statistics, version 23 (Copyright 1989, 2015 by SPSS Inc., Chicago, IL) and in R software environment.
RESULTS
From October 2014 to April 2017, 1033 participants were enrolled at 2 study centers (Berlin, Germany and Utrecht, The Netherlands). Accounting for early dropouts, missing data, and loss to follow‐up, 837 participants could be included for analysis of POD and 562 participants for analysis of POCD (see Figure 1). Of these, 165 patients (19.7%) fulfilled the criteria of POD and 60 (10.7%) for POCD (see Tables 1 and 2). Patients with POD were significantly older, had higher ASA PS and CCI scores, and longer duration of anesthesia. In addition, there were group differences regarding to frailty status and site of surgery. Patients with POCD were older, had higher ASA PS scores, and were found to fulfill frailty criteria more often than patients without POCD. Patients with POCD did not differ in sex, CCI, duration of anesthesia, and site of surgery compared with patients without POCD.
FIGURE 1.
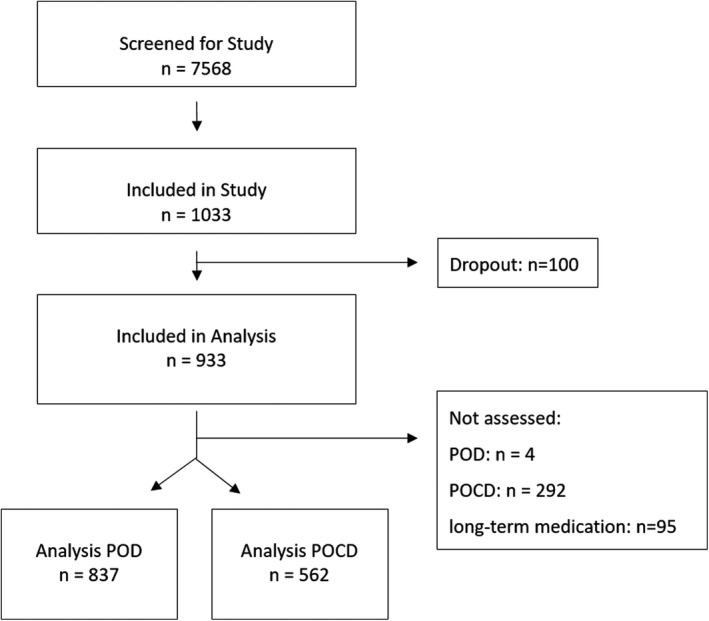
Flow Chart. POCD, postoperative delirium; POD, postoperative (neuro‐)cognitive disorder
TABLE 1.
Patient characteristics for POD analysis (n = 837)
| Characteristic |
POD (n = 165) (19.7%) |
No POD (n = 672) (80.3%) |
p value |
|---|---|---|---|
| n = 837 | |||
| Age [years] | 74 [70; 77] | 71 [68; 75] | <0.001 a |
| Sex | |||
| Female | 79 (47.9%) | 283 (42.1%) | 0.180 b |
| ASA PS | |||
| 1–2 | 77 (46.7%) | 463 (68.9%) | <0.001 b |
| 3–4 | 88 (53.3%) | 209 (31.1%) | |
| CCI | 1.86 ± 1.5 | 1.31 ± 1.5 | <0.001 a |
| Pre‐existing NCD | |||
| No NCD | 102 (69.4%) | 462 (76.7%) | 0.176 b |
| Mild NCD | 25 (17.0%) | 80 (13.3%) | |
| Major NCD | 20 (13.6%) | 60 (10.0%) | |
| Frailty status | |||
| Robust | 37 (22.7%) | 256 (38.6%) | <0.001 b |
| Pre‐frail | 87 (53.4%) | 337 (50.8%) | |
| Frail | 39 (23.9%) | 71 (10.7%) | |
| Duration of anesthesia [min] | 306 [211–473] | 168 [105–255] | <0.001 a |
| Site of surgery | |||
| Intracranial | 2 (1.2%) | 8 (1.2%) | <0.001 b |
| Intrathoracic/‐abdominal /‐pelvic | 105 (63.6%) | 248 (36.9%) | |
| Peripheral | 58 (35.2%) | 416 (61.9%) | |
| Polypharmacy | 88 (53.3%) | 285 (42.4%) | 0.011 b |
| Medication (PRISCUS list) | 22 (11.6%) | 78 (13.3%) | 0.540 b |
| Medication (EU(7)‐PIM list) | 98 (59.4%) | 319 (47.5%) | 0.006 b |
| Number of agents | 5.41 ± 3.7 | 4.44 ± 3.8 | 0.002 a |
| Number of agents according to PRISCUS list | 0.16 ± 0.4 | 0.13 ± 0.4 | 0.503 a |
| Number of agents according to EU(7)‐PIM list | 0.96 ± 1.0 | 0.78 ± 1.0 | 0.008 a |
Data are expressed as median [25th quartile; 75th quartile], or as mean ± SD except for categorical data, which are expressed as frequencies (percentages). Any p ≤ 0.05 was considered as statistically significant.
Abbreviations: ASA PS, physical status according to the American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; EU(7)‐PIM, European list of potentially inappropriate medications; NCD, neurocognitive disorder; POD, postoperative delirium.
The p values refer to the Mann–Whitney U test between patients with or without POD.
The p values refer to the χ2 test between patients with or without POD.
TABLE 2.
Patient characteristics for POCD analysis (n = 562)
| Characteristic |
POCD (n = 60) (10.7%) |
No POCD (n = 502) (89.3%) |
p value |
|---|---|---|---|
| n = 562 | |||
| Age [years] | 74 [70; 79] | 72 [68; 75] | 0.001 a |
| Sex | |||
| Female | 28 (46.7%) | 197 (39.2%) | 0.267 b |
| ASA PS | |||
| 1–2 | 32 (53.3%) | 361 (71.9%) | 0.003 b |
| 3–4 | 28 (46.7%) | 141 (28.1%) | |
| CCI | 1.52 ± 1.6 | 1.19 ± 1.4 | 0.075 a |
| Frailty status | |||
| Robust | 20 (33.3%) | 204 (41.0%) | 0.008 b |
| Pre‐frail | 27 (45.0%) | 249 (50.1%) | |
| Frail | 13 (21.7%) | 44 (8.9%) | |
| Duration of anesthesia [min] | 179 [100; 294] | 188 [117; 277] | 0.476 a |
| Site of surgery | |||
| Intracranial | 2 (3.3%) | 3 (0.6%) | 0.101 b |
| Intrathoracic/‐abdominal /‐pelvic | 23 (38.3%) | 204 (40.6%) | |
| Peripheral | 35 (58.3%) | 295 (58.8%) | |
| Polypharmacy | 32 (53.3%) | 206 (41.0%) | 0.068 b |
| Medication (PRISCUS list) | 9 (15.0%) | 66 (13.1%) | 0.690 b |
| Medication (EU(7)‐PIM list) | 30 (50.0%) | 235 (46.8%) | 0.640 b |
| Number of agents | 5.50 ± 4.0 | 4.32 ± 3.3 | 0.032 a |
| Number of agents according to the PRISCUS list | 0.15 ± 0.4 | 0.14 ± 0.4 | 0.719 a |
| Number of agents according to the EU(7)‐PIM list | 0.95 ± 1.2 | 0.75 ± 1.0 | 0.285 a |
Data are expressed as median [25th quartile; 75th quartile], or as mean ± SD except for categorical data, which are expressed as frequencies (percentages). Any p ≤ 0.05 was considered as statistically significant.
Abbreviations: ASA PS, physical status according to the American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; EU(7)‐PIM, European list of potentially inappropriate medications; POCD, postoperative cognitive dysfunction.
The p values refer to the Mann–Whitney U test between patients with or without POCD.
The p values refer to the χ2 test between patients with or without POCD.
Operations were most frequently performed on the musculoskeletal system (27.8%) and digestive tract (18.5%; see Table S1 for additional information). Surgical procedures were categorized into three types prior to analysis, whereas 43.4% of all operations were intrathoracic, ‐abdominal, or pelvic, 55.5% were peripheral, and 1.1% were intracranial.
Information on long‐term medication was available for 838 patients. Only 87 patients did not take any medication, and the remaining 751 patients with long‐term medication took a median of 4 agents (3851 agents in total; see Table S2). 24 Usually, no adjustment of medication was made at admission. Pantoprazole was the most frequently taken medication (5%), followed by metoprolol (4.2%), simvastatin (4.0%), hydrochlorothiazide (3.2%), levothyroxine (3.2%), and acetylsalicylic acid (2.9%). As shown in Figures 2 and 3, the distribution of the frequencies of the agents did not differ with respect to POD and POCD.
FIGURE 2.
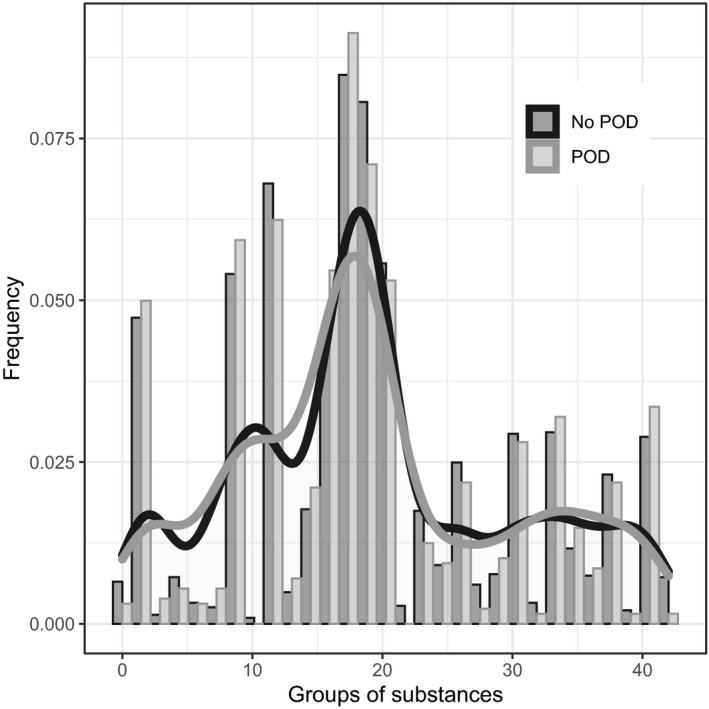
Illustration of frequencies of the groups of substances of the pre‐operative long‐term medication in regard to POD. Groups of substances according to Anatomical Therapeutic Chemical classification system. 24 For an overview of the groups, see Table S2. Presentation as bar plots: grey = POD and black = no POD. The density is additionally shown as a graph. POD, postoperative delirium
FIGURE 3.
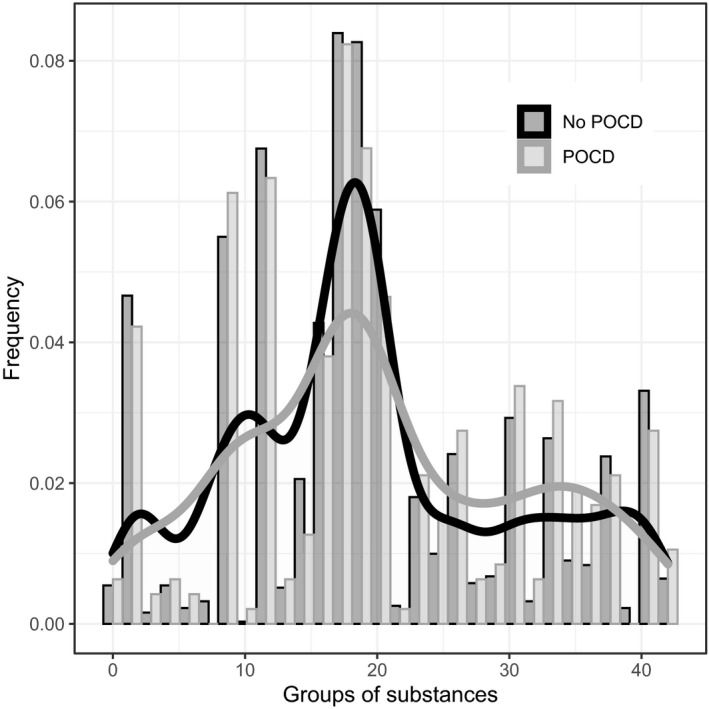
Illustration of frequencies of the groups of substances of the preoperative long‐term medication in regard to POCD. Groups of substances according to Anatomical Therapeutic Chemical classification system. 24 For an overview of the groups, see Table S2. Presentation as bar plots: grey = POCD and black = no POCD. The density is additionally shown as a graph. POCD, postoperative (neuro‐)cognitive disorder
Overall, 45% of all patients (n = 838) fulfilled the criteria for polypharmacy, 11.9% took potentially inappropriate medication according to the PRISCUS list, and 49.8% according to EU(7)‐PIM list. The most common inappropriate drug taken according to the PRISCUS list were amitriptyline (18.2%), doxazosin (12.7%), and temazepam (8.2%). According to the EU(7)‐PIM list, pantoprazole (28.3%), omeprazole (7.6%), and spironolactone (3.8%) were the most commonly consumed drugs.
When analyzing group differences, patients with POD were more often affected by polypharmacy and more often took potentially inappropriate medication according to the EU(7)‐PIM list (see Table 1). Furthermore, patients with POD took more agents in general, as well as more agents according to the EU(7)‐PIM list. There were no group differences with regard to the PRISCUS list. In the multivariable logistic regression analysis, none of the investigated variables (polypharmacy, potentially inappropriate medication according to the PRISCUS and EU(7)‐PIM lists, number of agents, and number of agents according to the PRISCUS and EU(7)‐PIM lists) associated with POD reached a level of significance (see Table 3). However, in each regression model, age, duration of anesthesia, positive frailty criteria, and intrathoracic/‐abdominal/‐pelvic operations (vs. peripheral operations) were significantly associated with POD.
TABLE 3.
Multivariable logistic regression analysis regard to POD
| OR (95% CI) | p value | |
|---|---|---|
| Polypharmacy | 1.076 [0.707; 1.639] | 0.732 |
| Medication (PRISCUS list) | 0.924 [0.511; 1.670] | 0.794 |
| Medication (EU(7)‐PIM list) | 1.217 [0.791; 1.873] | 0.371 |
| Number of agents | 1.025 [0.964; 1.089] | 0.431 |
| Number of agents according to the PRISCUS list | 1.017 [0.619; 1.672] | 0.947 |
| Number of agents according to the EU(7)‐PIM list | 0.949 [0.792; 1.137] | 0.569 |
Entered variables into analysis: age (years), CCI, frailty status, pre‐existing neurocognitive disorder, duration of anesthesia, and site of surgery. Data are expressed as OR and 95% CI. Any p ≤ 0.05 was considered as statistically significant. A separate regression analysis was performed for each investigated variable (polypharmacy, medication [PRISCUS list], medication [EU(7)‐PIM list], number of agents, number of agents according to PRISCUS or EU(7)‐PIM list).
Abbreviations: CI, confidence interval; CCI, Charlson Comorbidity Index; EU(7)‐PIM, European list of potentially inappropriate medications; OR, odds ratio; POD, postoperative delirium.
When analyzing group differences, patients with POCD took more agents in general. No other group differences between POCD and non‐POCD could be identified regarding polypharmacy or potentially inappropriate medication (see Table 2). Similarly, in the multivariable logistic regression analysis, none of the investigated variables displayed an association with POCD (see Table 4). However, in each regression model, age and ASA PS were significantly and consistently associated with POCD.
TABLE 4.
Multivariable logistic regression analysis regard to POCD
| OR (95% CI) | p value | |
|---|---|---|
| Polypharmacy | 0.971 [0.497; 1.896] | 0.931 |
| Medication (PRISCUS list) | 1.139 [0.497; 2.608] | 0.759 |
| Medication (EU(7)‐PIM list) | 0.564 [0.288; 1.105] | 0.095 |
| Number of agents | 1.018 [0.928; 1.117] | 0.702 |
| Number of agents according to PRISCUS list | 0.969 [0.466; 2.015] | 0.933 |
| Number of agents according to EU(7)‐PIM list | 0.932 [0.706; 1.229] | 0.616 |
Entered variables into analysis: age (years), ASA PS, frailty status, depression 3 months after surgery (defined as >4 points in GDS) and education (according to ISCED, with regard to level 1–4 which corresponds to a lower educational level). Data are expressed as OR and 95% CI. Any p ≤ 0.05 was considered as statistically significant.
A separate regression analysis was performed for each investigated variable (polypharmacy, medication [PRISCUS list], medication [EU(7)‐PIM list], number of agents, number of agents according to PRISCUS or EU(7)‐PIM list.
Abbreviations: ASA PS, physical status according to the American Society of Anesthesiologists; CI, confidence interval; EU(7)‐PIM, European list of potentially inappropriate medications; GDS, Geriatric Depression Scale; ISCED, International Standard Classification of Education; OR, odds ratio; POCD, postoperative (neuro‐)cognitive dysfunction.
DISCUSSION
This study aimed to investigate the association between pre‐operative polypharmacy and the intake of potentially inappropriate medications (defined by the PRISCUS and EU(7)‐PIM lists) and the development of POD and POCD in elderly patients. This investigation included patients undergoing elective surgery across a wide range of surgical disciplines.
After adjustment for confounding factors, pre‐operative polypharmacy and the intake of potentially inappropriate medications could not be shown to be associated with the development of POD or POCD. Incidences within our study sample were comparable with other cohorts, with nearly 20% of patients developing POD and nearly 10% developing POCD. 25 , 26 , 27 , 28 Overall, 45% of all patients were affected by polypharmacy. This is in line with other studies investigating elderly patients in peri‐operative context. 10 , 11 In our cohort, 12% took potentially inappropriate medication according to the PRISCUS list. The German PRISCUS list was created with a selective literature search, an analysis of international PIM lists, and a Delphi survey in two rounds (a comprehensive, structured expert survey), and includes 83 drugs. 12 When the PRISCUS list was introduced in 2010, retrospective studies in elderly patients reported prevalences of 22%–25%, 29 , 30 , 31 whereas more recent studies show a prevalence of 10%–14%. 32 , 33 This indicates that after the introduction of the PRISCUS list, prescriptions practices have changed to avoid inadequate drugs. According to the EU(7)‐PIM list, however, 50% of the patients in our cohort took potentially inappropriate medication. The EU(7)‐PIM list was based on the PRISCUS list and other international PIM lists. Experts from seven countries have extended the list, suggesting further medications, which was followed by a Delphi survey in two rounds. The list contains 282 drugs. 13 The number of drugs included in each list help explain the observed difference between the prevalences according to the PRISCUS and EU(7)‐PIM lists, and that in the simple group comparison patients with POD took drugs from the EU(7)‐PIM list more frequently. Furthermore, prevalence of inadequate medication according to the EU(7)‐PIM list varies considerably in literature, with values fluctuating between 37% and 81%. 33 , 34 , 35 , 36 , 37 Similarly to the above described trend, a declining prevalence could be observed in studies conducted after the introduction of the EU(7)‐PIM list in 2015. Large national differences in the prescription of agents listed on the (internationally available) EU(7)‐PIM list have been reported. For example, the prescription prevalence of drugs listed on the EU(7)‐PIM list was 41% in a Swedish cohort, 36 60% in a Brazilian cohort, 34 and 80% in a Belgian cohort. 37 Differences might be explained in part by the age of the respective cohorts (≥80 years in the Belgian study). Furthermore, differences in prescriptions are related to regional differences in guidelines and the availability of drugs. In contrast to the German PRISCUS list, the internationally known EU(7)‐PIM list must consider location as a factor when comparing studies. Pantoprazole, omeprazole, and spironolactone were the most commonly consumed drugs according to the EU(7)‐PIM list in our cohort, compared with apixaban and zopiclone in a Swedish cohort, 38 benzodiazepines, proton pump inhibitors, and tramadol in a Croatian cohort, 39 and proton pump inhibitors, benzodiazepines, and glibenclamide in a Brazilian cohort. 34 In addition, the application of the national PRISCUS list shows differences within Germany. Although in our cohort amitriptyline, doxazosin, and temazepam were the most commonly consumed drugs, according to the PRISCUS list, zopiclone and lorazepam, 40 or zopiclone, zolpidem, and amitriptyline 41 were reported as most common agents in two other German cohorts. In our view, the differences can be explained not only by regional conditions but also by the age and setting of the cohorts. Our study is, to the best of our knowledge, the only one which analyze a peri‐operative cohort. On the other hand, the most frequently used substance classes in general, as well as the frequency of use, are comparable to other European cohorts. 42 , 43 Interestingly, older patients in American cohorts, for example, tend to take significantly more medication than their European counterparts. 44 , 45
So far, there is no evidence that the drugs most frequently taken in our cohort (pantoprazole, metoprolol, simvastatin, hydrochlorothiazide, levothyroxine, acetylsalicylic acid, omeprazole, spironolactone, or doxazosin) could be associated with the development of POD or POCD. According to current hypotheses, only amitriptyline and temazepam would promote delirium through their anticholinergic effects. 46 So, currently, there are no investigations on temazepam. For amitriptyline, no association could be confirmed in another study. 47
In our analysis, special attention should be given to the fact that the incidences of POD and POCD depend on predisposing and precipitating risk factors that are often inter‐related. According to the evidence‐based and consensus‐based guideline on postoperative delirium of the European Society of Anaesthesiology, 7 advanced age and comorbidities are predisposing factors, whereas duration and site of surgery are precipitating risk factors for POD. Advanced age and comorbidities are also risk factors for POCD, in addition to alcohol abuse, low education level, or perioperative complications. 25 , 48 This is also reflected in our results. In addition, we could not find any group differences between patients with POCD and without POCD regarding duration of anesthesia or site of surgery. In this context, it should be noted that there are also indications in the literature that POD and POCD co‐occur and share risk factors, but their relationship remains unclear. 4 In addition, a biphasic pattern was described in the relationship between POD and POCD. 6 Here, we observed that the cognitive performance of patients with POD recovers 3 months after surgery, but shows severe deterioration in the long term. This could help explain why an association of EU(7)‐PIM with POD was observed, but not with POCD in the descriptive, unadjusted analyses.
Furthermore, pre‐existing NCD, reduced functional status, and malnutrition are also considered predisposing risk factors for elderly surgical patients. This could explain why group differences could be observed in descriptive, unadjusted analysis, but not in the logistic regression analysis. Comorbidities are more frequently observed in elderly patients, and medication is often the primary method of treatment. Polypharmacy or medication according to the EU(7)‐PIM list may therefore be an expression of these risk factors and help explain the group differences.
Another plausible approach is that patients with pre‐existing NCD or frailty are particularly vulnerable to the side effects of drugs and their related toxicity, thus increasing the risk of developing POD or POCD. In the context of polypharmacy, this toxicity‐vulnerability interaction might be amplified by drug‐drug interactions, 49 the likelihood of potentially inappropriate medication, and the increase of medication discrepancies (mismatch between the medication actually taken by the patient and the medication recorded in the patient’s medical record or medication prescriptions). 50
Furthermore, although current recommendations call for checking the presence of polypharmacy and to critically review prescription indications (also with regard to potentially inappropriate medication), no general recommendations exists to reduce polypharmacy or the intake of potentially inappropriate medication once identified. 51 , 52 , 53 The “Primary Medical Care” (Primärmedizinische Versorgung [PMV]) Research Group recommends the PRISCUS list “as an aid for critically evaluating medications and not as a list of forbidden drugs.” 54 This recommendation is not yet based on appropriate evidence, but underlines the importance of an individualized treatment of each patient.
Our analysis could not confirm that screening for polypharmacy or the application of the PRISCUS or EU(7)‐PIM list can reliably identify patients at risk for development of POD or POCD prior to surgery. Future analyses should therefore adopt other approaches, considering time‐dependent effects, dose levels of inappropriate drugs, as well as the impact of bridging of inappropriate or beneficial drugs before surgery, in addition to drug interactions. Furthermore, the association of drugs that are not on these lists, but which are known to cause alterations of the physiological processes, should also be investigated in regard to the development of POD and POCD. These approaches could be applied to verify whether patients benefit from a pre‐operative adjustment of long‐term medication aimed at reducing possible harmful and toxic drug reactions in patients with high vulnerability.
Strength and limitations
A key strength of this study is the prospective multicenter design. In addition, POD and POCD were characterized by a comprehensive, standardized, complex, and validated assessment. For POCD, the neuropsychological test battery covered various cognitive domains, and was carried out following a well‐described standard with limited rater effects. A comprehensible algorithm in R is available to improve comparison of our results with other studies. The study database also contains extensive information on possible confounders. In addition, we were able to investigate the associations over a wide range of surgical disciplines, which reflects the conditions that apply to the pre‐operative risk evaluation settings in clinical practice.
Nevertheless, some important limitations must be considered. In our cohort, the incidence of POCD is relatively low with ~ 10%, which limits the statistical power. The wide range of surgical disciplines increased the study population’s variance in characteristics.
CONCLUSIONS
In conclusion, when considering confounders, there were no associations between pre‐operative polypharmacy and the intake of potentially inappropriate medications and the development of POD and POCD. However, further studies are needed to determine whether and how patients benefit from a pre‐operative adjustment of long‐term medication.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
M.H., A.N., F.B., R.M., J.K., G.W., A.J. and C.S. wrote the manuscript. M.H., A.N., G.W., A.J., and C.S. designed the research. M.H., A.N., F.B., and R.M. performed the research. M.H. and J.K. analyzed the data.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
The authors thank Konstanze Scheurer (Charité‐Universitätsmedizin Berlin) for the administrative support with study coordination and management. Judy Veldhuijzen (UMC Utrecht), Antje Kraft (Charité Universitätsmedizin Berlin), Simone Kühn (University of Hamburg) and Insa Feinkohl (Max‐Delbrück Center Berlin) provided additional neuropsychological expertise. Kathrin Scholtz supported the study as the clinical monitor. Data management was provided by Olaf Bender and Alexander Krannich at Koordininierungszentrum für Klinische Studien (KKS Berlin) supported by Pharmaimage Biomarkers Solutions GmbH. Henning Krampe supported the study by recruiting and supervising students for neuropsychological testing. We thank our team of investigators, medical doctoral students and study nurses: Alissa Wolf, Fatima Yürek, Anika Müller, Daniel Hadzidiakos, Ilse Kant, Simone van Montfort, Gunnar Lachmann, Anika Alon, Sina Rosenblender, Tuba Aslan, Markus Laubach, Felix Müller, Emmanuel Keller, Eleftheria Papadaki, Saya Speidel, Bennet Borak, Steffi Herferth, Johannes Lange, Mario Lamping, Helene Michler, Juliane Dörfler, Anton Jacobshagen, Petra Kozma, Marinus Fislage, Wolf Rüdiger Brockhaus, Luisa Rothe, Pola Neuling, Ken‐Dieter Michel, Zdravka Bosancic, Firas Nosirat, Maryam Kurpanik, Sophia Kuenz, Lukas Roediger, Irene Mergele, Leopold Rupp, Marie Graunke, and Victoria Windmann. The authors further wish to thank the team of the student and interns of the Department of Anesthesiology at the Charité‐Universitätsmedizin Berlin. Maria Heinrich is participant in the BIH‐Charité Digital Clinician Scientist Program funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health.
Funding information
This work was conducted with support of the European Union (Seventh Framework Programme) as part of the BioCog project (Biomarker Development for Postoperative Cognitive Impairment in the Elderly), no. 602461.
REFERENCES
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2. Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three‐year prospective study. Dement Geriatr Cogn Disord. 2008;26:26‐31. [DOI] [PubMed] [Google Scholar]
- 3. Moskowitz EE, Overbey DM, Jones TS, et al. Post‐operative delirium is associated with increased 5‐year mortality. Am J Surg. 2017;214:1036‐1038. [DOI] [PubMed] [Google Scholar]
- 4. Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131:477‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long‐term cognitive decline: a meta‐analysis. JAMA Neurol. 2020;77(11):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inouye SK, Marcantonio ER, Kosar CM, et al. The short‐term and long‐term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence‐based and consensus‐based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192‐214. [DOI] [PubMed] [Google Scholar]
- 8. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10:393‐400. [DOI] [PubMed] [Google Scholar]
- 9. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190‐1222. [DOI] [PubMed] [Google Scholar]
- 10. Barnett SR. Polypharmacy and perioperative medications in the elderly. Anesthesiol Clin. 2009;27(3):377‐389. [DOI] [PubMed] [Google Scholar]
- 11. Zafirova Z, Vazquez‐Narvaez KG, Borunda D. Preoperative management of medications. Anesthesiol Clin. 2018;36:663‐675. [DOI] [PubMed] [Google Scholar]
- 12. Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renom‐Guiteras A, Meyer G, Thurmann PA. The EU(7)‐PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71:861‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henschel F, Redaelli M, Siegel M, Stock S. Correlation of incident potentially inappropriate medication prescriptions and hospitalization: an analysis based on the PRISCUS list. Drugs Real World Outcomes. 2015;2:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baron R, Binder A, Biniek R, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS‐Guideline 2015) ‐ short version. Ger Med Sci. 2015;13:Doc19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: a systematic literature review. Age Ageing. 2006;35:350‐364. [DOI] [PubMed] [Google Scholar]
- 17. Winterer G, Androsova G, Bender O, et al. Personalized risk prediction of postoperative cognitive impairment ‐ rationale for the EU‐funded BioCog project. Eur Psychiatry. 2018;50:34‐39. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 19. United Nations Educational, S.a.C.O . International standard classification of education. 1997; 02/05/2020.
- 20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37‐49. [DOI] [PubMed] [Google Scholar]
- 21. Feinkohl I, Borchers F, Burkhardt S, et al. Stability of neuropsychological test performance in older adults serving as normative controls for a study on postoperative cognitive dysfunction. BMC Res Notes. 2020;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spies CD, Knaak C, Mertens M, et al. Physostigmine for prevention of postoperative delirium and long‐term cognitive dysfunction in liver surgery: a double‐blinded randomised controlled trial [published online ahead of print Jan 28, 2021]. Eur J Anaesthesiol. 10.1097/EJA.000000000001456 [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45:275‐289. [DOI] [PubMed] [Google Scholar]
- 24. The World Health Organization Collaborating Center for Drug Statistics Methodology . The Anatomical Therapeutic Chemical (ATC) classification system. November 26, 2019.
- 25. Moller JT, Cluitmans P, Rasmussen LS, et al. Long‐term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post‐Operative Cognitive Dysfunction. Lancet. 1998;351:857‐861. [DOI] [PubMed] [Google Scholar]
- 26. Monk TG, Weldon B, Garvan C, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18‐30. [DOI] [PubMed] [Google Scholar]
- 27. Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin. 2015;33:505‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Zhao X, Dong T, et al. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta‐analysis. Aging Clin Exp Res. 2017;29:115‐126. [DOI] [PubMed] [Google Scholar]
- 29. Amann U, Schmedt N, Garbe E. Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int. 2012;109:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schubert I, Kupper‐Nybelen J, Ihle P, Thurmann P. Prescribing potentially inappropriate medication (PIM) in Germany's elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf. 2013;22:719‐727. [DOI] [PubMed] [Google Scholar]
- 31. Endres HG, Kaufmann‐Kolle P, Steeb V, Bauer E, Böttner C, Thürmann P. Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: an observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One. 2016;11:e0146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graf J, Lucke T, Herrera R, et al. Compatibility of medication with PRISCUS criteria and identification of drug interactions in a large cohort of patients with COPD. Pulm Pharmacol Ther. 2018;49:123‐129. [DOI] [PubMed] [Google Scholar]
- 33. Muhlack DC, Hoppe LK, Saum K‐U, et al. Investigation of a possible association of potentially inappropriate medication for older adults and frailty in a prospective cohort study from Germany. Age Ageing. 2019;49:20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Almeida TA, Reis EA, Pinto IVL, et al. Factors associated with the use of potentially inappropriate medications by older adults in primary health care: an analysis comparing AGS Beers, EU(7)‐PIM List, and Brazilian Consensus PIM criteria. Res Soc Adm Pharm. 2019;15:370‐377. [DOI] [PubMed] [Google Scholar]
- 35. Bobrova V, Heinämäki J, Honkanen O, et al. Older adults using multi‐dose dispensing exposed to risks of potentially inappropriate medications. Res Soc Adm Pharm. 2019;15:1102‐1106. [DOI] [PubMed] [Google Scholar]
- 36. Sonnerstam E, Sjolander M, Gustafsson M. An evaluation of the prevalence of potentially inappropriate medications in older people with cognitive impairment living in Northern Sweden using the EU(7)‐PIM list. Eur J Clin Pharmacol. 2017;73:735‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wauters M, Elseviers M, Azermai M, Vander Stichele R. Availability and actual use in the Belgian market of potentially inappropriate medications (PIMs) from the EU(7)‐PIM list. Eur J Clin Pharmacol. 2016;72:243‐245. [DOI] [PubMed] [Google Scholar]
- 38. Wamil N, Mattsson S, Gustafsson M. Assessment of potentially inappropriate medications using the EU (7)‐PIM list and the Swedish quality indicators. Int J Clin Pharm. 2019;41:903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mucalo I, Hadžiabdić MO, Brajković A, et al. Potentially inappropriate medicines in elderly hospitalised patients according to the EU(7)‐PIM list, STOPP version 2 criteria and comprehensive protocol. Eur J Clin Pharmacol. 2017;73:991‐999. [DOI] [PubMed] [Google Scholar]
- 40. Wickop B, Härterich S, Sommer C, et al. Potentially inappropriate medication use in multimorbid elderly inpatients: differences between the FORTA, PRISCUS and STOPP ratings. Drugs Real World Outcomes. 2016;3:317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pohl‐Dernick K, Meier F, Maas R, Schöffski O, Emmert M. Potentially inappropriate medication in the elderly in Germany: an economic appraisal of the PRISCUS list. BMC Health Serv Res. 2016;16:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mielke N, Huscher D, Douros A, et al. Self‐reported medication in community‐dwelling older adults in Germany: results from the Berlin initiative study. BMC Geriatr. 2020;20:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morin L, Vetrano DL, Rizzuto D, et al. Choosing wisely? Measuring the burden of medications in older adults near the end of life: nationwide, longitudinal cohort study. Am J Med. 2017;130:927‐936.e929. [DOI] [PubMed] [Google Scholar]
- 44. Qato DM, Caleb Alexander G, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over‐the‐counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867‐2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over‐the‐counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The anticholinergic drug scale as a measure of drug‐related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481‐1486. [DOI] [PubMed] [Google Scholar]
- 47. Rothberg MB, Herzig SJ, Pekow PS, et al. Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61:923‐930. [DOI] [PubMed] [Google Scholar]
- 48. Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111:119‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnell K, Klarin I. The relationship between number of drugs and potential drug‐drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911‐918. [DOI] [PubMed] [Google Scholar]
- 50. Franco JVA, Terrasa SA, Kopitowski KS. Medication discrepancies and potentially inadequate prescriptions in elderly adults with polypharmacy in ambulatory care. J Fam Med Prim Care. 2017;6:78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mangin D, Bahat G, Golomb BA, et al. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): position statement and 10 recommendations for action. Drugs Aging. 2018;35:575‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stewart D, Mair A, Wilson M, et al. Guidance to manage inappropriate polypharmacy in older people: systematic review and future developments. Expert Opin Drug Safety. 2017;16:203‐213. [DOI] [PubMed] [Google Scholar]
- 53. Muth C, Blom JW, Smith SM, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. J Intern Med. 2019;285:272‐288. [DOI] [PubMed] [Google Scholar]
- 54. Bergert FW, Braun M, Ehrenthal K, et al. Recommendations for treating adult and geriatric patients on multimedication. Int J Clin Pharmacol Ther. 2014;52(Suppl 1):1‐64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


