Abstract
The cold pressor test (CPT) is widely implemented and offers a simple, experimental acute pain model utilizing cold pain. Previous trials have frequently paired the CPT with opioids in order to investigate the mechanisms underlying pharmacological analgesia, due to their known analgesic efficacy. However, opioid side effects may lead to unblinding and raise concerns about the safety of the experimental setting. Despite the established clinical efficacy of dipyrone (metamizole), its efficacy, tolerability, and safety in cold pressor pain has not been systematically addressed to date. This pooled analysis included data of 260 healthy volunteers from three randomized, placebo‐controlled, double‐blind substudies using the CPT following a pre‐test‐post‐test‐design. These substudies allow for comparing a single dose of 800 mg dipyrone with two different doses of the opioid tilidine/naloxone (50/4 mg and 100/8 mg, respectively). Outcomes included pain intensity ratings, pain tolerance, medication‐attributed side effects, as well as changes of blood pressure and heart rate. We demonstrate that both opioid doses and dipyrone had a comparable, significant analgesic effect on cold pressor pain. However, dipyrone was associated with significantly less self‐reported adverse effects and these were not significantly different from those under placebo. These results indicate that the combination of dipyrone and the CPT provides a safe, tolerable, and effective experimental model for the study of pharmacological analgesia. In combination with a CPT, dipyrone may be useful as a positive control, or baseline medication for the study of analgesic modulation.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The cold pressor test (CPT) has frequently been paired with opioids to investigate analgesia, but opioids are often associated with side effects. Although the pyrazalone derivate dipyrone (metamizole) is used for clinical analgesia, its efficacy, tolerability, and safety in experimental cold pain has not been systematically addressed to date.
WHAT QUESTION DID THIS STUDY ADDRESS?
Which efficacy, tolerability, and safety provide 800 mg dipyrone compared to 50/4 mg and 100/8 mg tilidine/naloxone in a cold pain experiment?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Both opioid doses and dipyrone had a comparable analgesic effect on cold pain. Importantly, dipyrone was associated with less adverse effects.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Combining dipyrone with the CPT offers an effective and safe model of pharmacological analgesia and might be of particular interest to explore contextual modulations of analgesia.
INTRODUCTION
The cold pressor test (CPT), which involves immersing one’s limb in cold water until the individual pain tolerance is reached, represents an established and widely used experimental model for human pain or stress induction. Frequently used outcomes include time of onset of pain (i.e., pain threshold), pain intensity ratings (continuous or retrospective), and time of hand withdrawal (pain tolerance). 1 , 2 , 3 , 4
Since then, the CPT has been used to test the analgesic efficacy of a variety of pharmacological agents in healthy volunteers. Although nonsteroidal anti‐inflammatory drugs (NSAID), 1 , 5 anticonvulsants (i.e., gabapentin 6 and lamotrigine 7 ), and antidepressants (i.e., imipramine 8 ) had little to no analgesic effects, opioids were repeatedly shown to effectively reduce cold pressor pain. 1 , 6 , 7 , 9 , 10 Opioids have therefore frequently been used as active controls in drug development trials using the CPT to test potentially novel analgesic compounds. 6 , 11 , 12 Another potential use of experimental paradigms of analgesia, such as the CPT combined with opioids, represents the investigation of the modulatory effects of treatment context (e.g., open or hidden application of a drug, and patient‐physician interaction), which have been shown to modulate treatment outcomes reviewed in refs. 13, 14, 15.
However, most studies investigating opioid analgesia in a CPT model report significant side effects in healthy volunteers 6 , 9 , 10 raising safety concerns for its use in experimental pain research in humans. Further, opioid side effects have been shown to jeopardize successful blinding, particularly when comparing against non‐opioid control groups. 16 The side effects of opioids may not be detrimental in all research contexts, for instance, when used as a positive control for comparators with comparable side‐effect profile. However, for other research questions, undesired effects can be disadvantageous (e.g., when investing compounds with unknown or no side effects or in mechanistic approaches aiming to properly dissect the contribution of psychological and contextual factors; e.g., expectation, prior experience) to analgesia. These shortcomings question the usefulness of combining CPT with opioid in studies of analgesic modulation in both healthy participants and patients, and underscore the need for a novel tolerable and safe pharmacological model of experimental analgesia.
An analgesic drug, which has not been systematically tested in the context of experimental CPT models is dipyrone (metamizole). As a pyrazolone derivate, it is frequently used to treat postoperative pain, colic pain, cancer pain, and migraine. 17 , 18 Although it is a popular nonopioid first‐line analgesic in many parts of the word, some countries, including the United States, United Kingdom, Sweden, and India, advise against its use because of the potential risk of agranulocytosis. 19 However, a recent meta‐analysis of 79 trials on short‐term use of dipyrone in almost 4000 patients found fewer adverse events for dipyrone than for opioids and no case of agranulocytosis. 20
Here, we present a pooled analysis of three randomized controlled substudies in which we used a two‐step approach. First, we compare the analgesic effect of two different doses of an opioid agonist/antagonist‐combination, tilidine/naloxone (50/4 mg and 100/8 mg, respectively) and dipyrone (800 mg) to placebo treatment on CPT (pain intensity and pain tolerance), side effects, blood pressure, and heart rate. Second, we performed a pooled analysis of the active treatment groups (dipyrone, 50/4 mg tilidine/naloxone, and 100/8 mg tilidine/naloxone) to directly compare the effect of the three analgesic treatments on these outcomes.
METHODS
Participants
This pooled analysis included 264 healthy volunteers from 3 separate substudies (substudy 1: N = 93, substudy 2: N = 90; and substudy 3: N = 81, see Study design). All substudies investigated healthy volunteers aged greater than or equal to 18 years (i.e., inclusion criteria). Exclusion criteria comprised: acute and/or chronic illness or infection, use of any analgesic medication in the last 7 days (e.g., NSAID), alcohol and drug abuse, alcohol use 24 h prior to participation, history of allergic reaction to one of the substances used, participation in a clinical trial during the past 6 months prior to inclusion, general anesthesia in the past 6 months, and insufficient knowledge of the German language in writing and speaking. Pregnant or breast‐feeding women were excluded from participation. Each substudy was performed in accordance to the Declaration of Helsinki and approved by the local ethics committee (17–7918‐BO, 17‐7918_1‐BO, 17‐7918_2‐BO; medical faculty of the University of Duisburg‐Essen). Voluntary written informed consent was obtained from all participants. Participants received a monetary compensation of 75 €. Because this study is a pooled post hoc analysis of three separate studies, it was not registered in a clinical trial register.
Study design
Each substudy followed a randomized, double‐blind, parallel group, pre‐test‐post‐test design (for details, see Figure 1). Randomization was carried out on the basis of an a priori created randomization list (RStudio version 1.1.463, RStudio, Inc.) allowing fully random group allocation. Substudies 1 and 2 were part of a larger project investigating context modulations of pain perception (reported separately): sub‐study 1 aimed to recruit 150 healthy volunteers following a 2:2:1‐balanced randomization into three groups: beyond a control group (N = 30), which received purified water, two experimental groups (each N = 60) each received 100/8 mg tilidine/naloxone (i.e., high‐dose opioid). Notably, the opioid administration of one group was associated with a context modulation (in the form of an additional sublingual placebo treatment). This aforementioned group was not included into this analysis. In substudy 2, again, 150 healthy subjects were recruited and randomly assigned to 3 experimental groups. All group structures were identical to substudy 1, except for the fact that a reduced opioid dose (i.e., 50/4 mg tilidine/naloxone; i.e., low‐dose opioid) replaced the high‐dose opioid. Similarly, the context modulation group was not included into this analysis. Substudy 3, a proof‐of‐principle study, aimed to recruit 80 healthy volunteers following a 1:1‐balanced randomization in 2 groups: the first group (N = 40) received 800 mg dipyrone (dipyrone group) and the second purified aqua (N = 40, control group). All substudies followed identical testing protocol (see section Testing schedule) and were performed by blinded examiners (J.K. in substudy 1, F.B. in substudy 2, and J.W. in substudy 3). A priori power calculations were performed per study to test hypotheses outside the scope of this pooled analysis. Thus, sample sizes used for this post hoc analysis depended on the data available (Figure 1).
FIGURE 1.
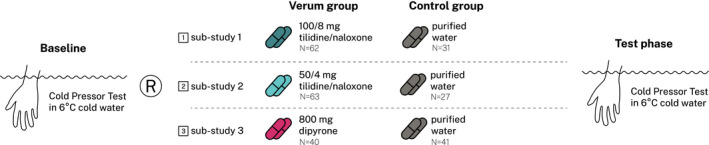
Combined study design of all substudies—after a baseline assessment using the cold pressor test (CPT) with a 6°C cold water bath (see section cold pressor test), the randomization (R) followed. All study medications (tilidine/naloxone, dipyrone, purified aqua) were encapsulated in white tasteless capsules to prevent unblinding through color, taste, or smell. After medication intake, a test phase was performed identically to the baseline assessment
Study medication
In substudies 1 and 2, a tilidine/naloxone combination (Tilidin comp. STADA 50 mg/4 mg per 0.72 ml, Stada Arzneimittel AG) was used based on previous studies with cold pressor pain reviewed in ref. 16. To prevent accidental unblinding, the bitter taste of the tilidine/naloxone combination required concealment. The tilidine/naloxone solution was therefore encapsulated in two taste‐neutral capsules (bovine gelatin‐capsules size “00,” Nagamil). In sub‐study 1, each capsule contained 0.7 ml tilidine/naloxone solution (equaling 50/4 mg tilidine/naloxone), whereas in substudy 2, one capsule was filled with 0.7 ml tilidine/naloxone solution and a second with 0.7 ml purified water.
In substudy 3, a dipyrone (metamizole) solution (Novaminsulfon‐ratiopharm 500 mg/ml, Ratiopharm) was used. In order to prevent any unblinding caused by smell, taste and color, the study medication was encapsulated comparably to substudies 1 and 2. Here, each capsule contained 0.8 ml dipyrone solution (in total 1.6 ml equaling 800 mg dipyrone).
Participants allocated to the control groups of all substudies received purified water. Similar to the treatment groups, purified water was encapsulated in the same capsules to ensure blinding. Each capsule contained 0.8 ml purified water (2 capsules in total).
Testing schedule
All substudies followed the same testing schedule taking place at the University Hospital Essen, Germany. Eligibility was screened via telephone prior to the study day. At the beginning of each study session, participants were, again, screened for eligibility and voluntary, written, informed consent was obtained. Participants were then introduced to the CPT and visual analog scale (VAS) rating procedures according to a standardized protocol. A first CPT was performed as a pretreatment baseline measure. Then, depending on the substudy, either 100/8 mg tilidine/naloxone (substudy 1), or 50/4 mg tilidine/naloxone (substudy 2), or 800 mg dipyrone solution (substudy 3) or purified water (control group, all substudies) was administered orally. Subsequently, all participants filled questionnaires for a resting period of 35 min. The aim of this waiting period was to reach the peak analgesic effect and to allow the participants’ hand to recover from pre‐testing CPT. After the waiting period, the post‐treatment CPT was performed. Last, participants were asked for treatment side effects using a systematic inventory 21 and discharged. Participants of (opioid) substudies 1 and 2 had to remain at the laboratory for an additional 4 h of medical surveillance to ensure participant safety.
Cold pressor test
A refrigerated laboratory water bath was used (WCR‐P22, Witeg, Germany, volume 22 L, temperature precision: ±0.2°C) and water was constantly circulated at a rate of 15 L/min to avoid the formation of a warm‐water layer around the skin. The pre‐ and post‐treatment CPT was performed at 6.0 ± 0.2°C. A laptop equipped with an external screen, Matlab 2015b (MathWorks), and the Psychtoolbox (version 3) 22 was used to record participant’s ratings and to log experimental timings. Participants received CPT instructions following a standardized, partly computerized protocol. Participants were instructed to immerse their nondominant, open hand into the water‐bath up to wrist level and to provide continuous pain ratings during CPT, using a mechanical slider (11 cm, custom construction, sampling rate: 10 Hz), linked to an un‐ticked 101‐point VAS shown on screen (end points: 0 = “no pain,” 100 = “unbearable pain”). Participants were advised to retract their hand from the water‐bath and to provide the maximum VAS pain rating of 100 points if the pain became unbearable. Participants were informed about a time‐limit for the test without knowledge of the maximum duration. Upon hand immersion, the experimenter logged the start of CPT and enabled continuous VAS ratings using a computerized trigger. Voluntary extraction of the hand from the water bath was logged by the experimenter through a second trigger and ended all CPT recordings. Alternatively, CPT was ended upon reaching the 180 s maximum time‐limit; in this case, recordings automatically stopped, and participants were asked to withdraw their hands.
Outcome assessment
Pain intensity: Percent area under the pain curve
Pain intensity was assessed as percent area under the pain curve (%AUPC) according to Koltzenburg et al. 9 The %AUPC corresponds to the averaging pain rating given over the full duration of the CPT, divided by the highest possible AUPC value (= 100 VAS units * 180 s), whereas the maximum VAS rating (100 units) is carried forward to 180 s for participants who terminated testing early. 9 Thus, a higher %AUPC denotes higher individual pain sensitivity, with 0% AUPC denoting complete pain insensitivity for the full testing duration and 100% AUPC denoting the immediate termination of testing due to pain intolerance, or, equivalently, a constant VAS rating of 100 for 180 s. The AUPC has been shown to be a reliable marker for pain intensity in both healthy participants and patients, 23 , 24 , 25 and to be sensitive for detecting opioid analgesia. 9
Pain tolerance
The pain tolerance is defined as the time to hand withdrawal from the water bath in seconds 2 during CPT. For safety reasons the maximum immersion time was limited to 180 s to avoid any injury from the cold. 4
Safety: Blood pressure and heart rate
Blood pressure and heart rate were measured using a multiparametric monitoring system (Infinity Delta, Drägerwerk AG & Co. KGaA) equipped with a noninvasive blood pressure cuff and an oximeter using transmission spectrophotometry (OxiSure SpO2, Drägerwerk AG & Co. KGaA). Prior to baseline, the blood pressure cuff was placed on the dominant upper arm. The heart rate was derived from the oximeter as implemented parameter of the monitor. Both blood pressure and heart rate were recorded after arrival at the laboratory, prior to the baseline and the test phase (35 min after the medication intake).
Tolerability: Side effects
Side effects were assessed by the General Assessment of Side Effects (GASE), 21 a 36‐item (symptom descriptions) standardized questionnaire. These symptoms could be rated as “not present,” “mild,” “moderate,” or “severe.” After rating the presence and severity of a symptom, participants decided whether the symptom was related to the current study medication, leading to a symptom and medication‐attributed symptom count. Participants were asked to fill the GASE at the end of the testing session.
Statistics
Analyses were performed with Matlab 2017b, the Statistics and Machine Learning toolbox, and RStudio (RStudio version 1.1.463, RStudio, Inc.). Individual pain sensitivity was measured as %AUPC (see above). Pain tolerance, heart rate, blood pressure, and side effects were assessed as additional outcomes. The post‐treatment timepoints for all outcomes were tested for differences between the levels (high‐dose opioid vs. control, low‐dose opioid vs. control, dipyrone vs. control, dipyrone vs. high‐dose opioid, dipyrone vs. low‐dose opioid; between‐group) of the factor group using a general linear model (GLM). Pretreatment CPT baseline values were controlled for as a covariate. The room temperature has been identified as systematically different between substudies. Therefore, all outcome models were additionally corrected for room temperature. When applicable, nonsignificant results have been tested for noninferiority via the one‐sided test procedure using mean deltas (Δtest–baseline), SD, and Cohen’s d as test parameters. 26 Therefore, equivalence bounds have been calculated to achieve 90% power. Statistical testing was performed at alpha less than 0.05. Unstandardized (b) estimates ± SEs are provided. Descriptive results are provided as means ± SD.
RESULTS
Dose‐dependent analgesic effects, safety, and tolerability of tilidine/naloxone versus control
In a first step, we aimed at estimating the dose‐dependent effects of tilidine/naloxone on the %AUPC, pain tolerance, blood pressure, heart rate, and side effects. After inclusion of 177 participants (low‐dose opioid = 60, high‐dose opioid = 59, pooled control group = 58; for a participant flow chart see Figure S1). For baseline characteristics and timings of the study groups, see Table 1, Tables S1–S2.
TABLE 1.
Baseline characteristics of the substudies’ treatment and control groups
| Characteristic | Substudies 1 and 2 | Substudy 3 | |||
|---|---|---|---|---|---|
| Low‐dose opioid group | High‐dose opioid group | Pooled control group | Dipyrone group | Control group | |
| N | 60 | 59 | 58 | 40 | 41 |
| Female (%) | 34 (56.7) | 25 (42.4) | 27 (46.6) | 21 (52.5) | 19 (46.3) |
| Age (in years) | 24.3 ± 3.8 | 23.9 ± 2.8 | 24.5 ± 3.3 | 23.5 ± 3.4 | 23.1 ± 3.6 |
| Body mass index (in kg/m2) | 23.3 ± 3.5 | 23.8 ± 2.9 | 24.0 ± 2.8 | 23.2 ± 3.1 | 23.0 ± 3.2 |
| Right‐handed (%) | 59 (98.3) | 50 (86.2) | 54 (93.1) | 34 (85.0) | 34 (82.9) |
| %AUPC (in %) | 71.1 ± 22.4 | 57.7 ± 22.7 | 62.6 ± 24.6 | 59.7 ± 26.0 | 62.1 ± 24.7 |
| Pain tolerance (in seconds) | 100.5 ± 63.7 | 142.7 ± 58.8 | 122.1 ± 65.5 | 125.9 ± 56.5 | 114.5 ± 66.2 |
| Room temperature (in °C) | 23.9 ± 1.4 | 21.6 ± 1.1 | 22.7 ± 1.7 | 22.9 ± 0.7 | 22.9 ± 0.6 |
| Skin temperature (in °C) | 35.2 ± 1.2 | 34.9 ± 1.1 | 35.1 ± 1.2 | 33.7 ± 1.2 | 34.0 ± 0.9 |
| Depression screening (CES‐D score) | 6.6 ± 5.1 | 6.5 ± 4.8 | 8.4 ± 5.1 | 5.2 ± 3.3 | 6.2 ± 3.9 |
| State anxiety (STAI‐S score) | 36.4 ± 6.1 | 35.7 ± 6.4 | 35.7 ± 6.6 | 34.3 ± 5.0 | 37.4 ± 5.6 |
| Trait anxiety (STAI‐T score) | 36.8 ± 9.3 | 34.5 ± 7.1 | 35.7 ± 7.9 | 33.7 ± 6.3 | 35.7 ± 6.3 |
| Sleep quality screening (PSQI score) | 4.8 ± 2.3 | 5.3 ± 2.5 | 5.7 ± 2.9 | 5.4 ± 2.6 | 5.7 ± 2.0 |
Provided are means ± SD. Please note, that the maximum pain tolerance was limited to 180 s.
Abbreviations: %AUPC, percent area under the pain curve; CES‐D, Center for Epidemiologic Studies Depression Scale; STAI‐S and STAI‐T, State Trait Anxiety Inventory, S(tate) and T(rait) version; PSQI, Pittsburgh Sleep Quality Index.
Compared to control, both the low‐ and high‐dose opioid significantly reduced the %AUPC and increased the pain tolerance over the curse of time (for detailed results see Table 2 and Figure 2). In direct comparison, participants allocated to the high‐dose opioid group showed a significantly stronger reduction of %AUPC (−8.8 ± 2.5%AUPC, t(172) = −2.3, p = 0.021, d = −0.35) and a higher increase in pain tolerance (19.4 ± 12.9 s, t(80) = 2.2, p = 0.030, d = 0.48) than those in the low‐dose opioid group, indicating a dose‐dependent effect of tilidine/naloxone on both parameters. Both opioid groups showed significantly higher medication‐attributed symptoms after opioid intake without significant difference between the low‐ and high‐dose groups. Notably, in the high‐dose opioid group, two severe adverse events occurred: one participant needed intravenous antiemetic treatment due to severe nausea and another participant needed intravenous fluid to treat orthostatic dysregulation. In both groups, reported medication‐attributed symptoms included dizziness, nausea, and vomiting. There were no changes in blood pressure and heart rate from baseline to test phase (see Table S3).
TABLE 2.
Main results
| Estimates (b) | df | t | P | d | |
|---|---|---|---|---|---|
| %AUPC (in %) | |||||
| High‐dose opioid versus pooled control group | −10.3 ± 2.6 | 172 | −4.0 | <0.001 | −0.67 |
| Low‐dose opioid versus pooled control group | −6.1 ± 2.6 | 172 | −2.3 | 0.021 | −0.32 |
| Dipyrone (metamizole) versus control group | −7.8 ± 2.8 | 77 | −2.8 | 0.006 | −0.63 |
| Dipyrone (metamizole) versus high‐dose opioid group | 4.6 ± 3.2 | 154 | 1.4 | 0.157 | 0.22 |
| Dipyrone (metamizole) versus low‐dose opioid group | −2.2 ± 3.2 | 154 | −0.7 | 0.482 | −0.01 |
| Pain tolerance a (in seconds) | |||||
| High‐dose opioid versus pooled control group | 55.7 ± 15.6 | 80 | 3.6 | <0.001 | 0.87 |
| Low‐dose opioid versus pooled control group | 27.7 ± 11.9 | 80 | 2.3 | 0.023 | 0.52 |
| Dipyrone (metamizole) versus control group | 41.8 ± 12.1 | 41 | 3.4 | 0.001 | 1.1 |
| Dipyrone (metamizole) versus high‐dose opioid group | −22.1 ± 17.9 | 75 | −1.2 | 0.221 | −0.20 |
| Dipyrone (metamizole) versus low‐dose opioid group | 17.7 ± 14.7 | 75 | 1.2 | 0.233 | 0.23 |
| Side effects (GASE symptom count) | |||||
| High‐dose opioid versus pooled control group | 3.5 ± 0.6 | 173 | 5.4 | <0.001 | 0.84 |
| Low‐dose opioid versus pooled control group | 2.5 ± 0.6 | 173 | 4.0 | <0.001 | 0.87 |
| Dipyrone (metamizole) versus control group | 0.2 ± 0.1 | 78 | 1.2 | 0.229 | 0.25 |
| Dipyrone (metamizole) versus high‐dose opioid group | −4.1 ± 0.8 | 155 | −5.5 | <0.001 | −1.13 |
| Dipyrone (metamizole) versus low‐dose opioid group | −2.7 ± 0.7 | 155 | −3.7 | <0.001 | −1.02 |
Presented are mean, unstandardized GLM estimates (b) ± standard error of group differences. Baseline measurements of %AUPC and pain tolerance were included as covariates into the respective models (no baseline measurements of side effects were obtained). Room temperature was included as a covariate into all models. Analysis of blood pressure and heartrate revealed no statistically significant differences between groups (data not shown). %AUPC, percent area under the pain curve; GASE, General Assessment of Side Effects questionnaire; GLM, general linear model. For complete result tables (including covariates) see Supplementary Material (for substudy 1: Tables S4–S6, for substudy 2: Tables S13–S15, for substudy 3: Tables S16–S18).
To avoid ceiling effects, the analysis of pain tolerance included only participants who did not reach the 180 s limit at baseline.
FIGURE 2.
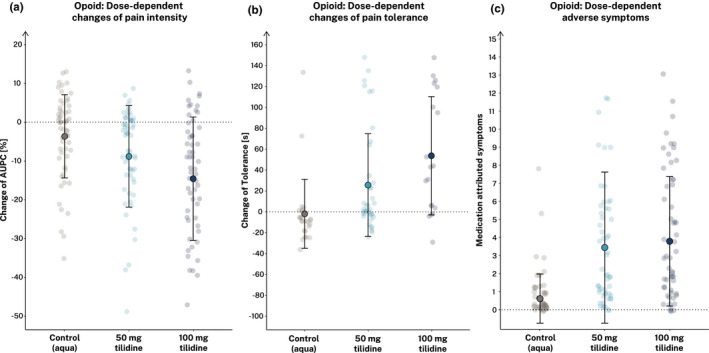
Dose‐dependent analgesic effects, safety, and tolerability of tilidine versus control. Illustrated are changes of the percent area under the pain curve (%AUPC) (a) and the pain tolerance (b) as measures of pain intensity. Self‐reported side effects are illustrated as medication‐attributed symptom count (GASE) (c). Filled dots show means ± SD for each group separately. Light dots show individual participant data
Analgesic effects, safety, and tolerability of dipyrone versus control
For substudy 3, we estimated the analgesic effects of a single 800 mg dipyrone dose on the change of the previously stated outcomes. After inclusion of 81 participants (dipyrone group = 40, control group = 41; see Table 1). Analysis of the group receiving a single dose 800 mg dipyrone compared to the control group revealed significant lower %AUPC ratings and higher pain tolerance, indicating a sufficient analgesic effect of dipyrone on experimentally induced cold pressor pain (see Figure 3 and Table 2). We observed no statistically significant differences of the reported side effects. Furthermore, blood pressure as well as heart rate changes did not significantly differ between the verum and control group (see Table S3).
FIGURE 3.
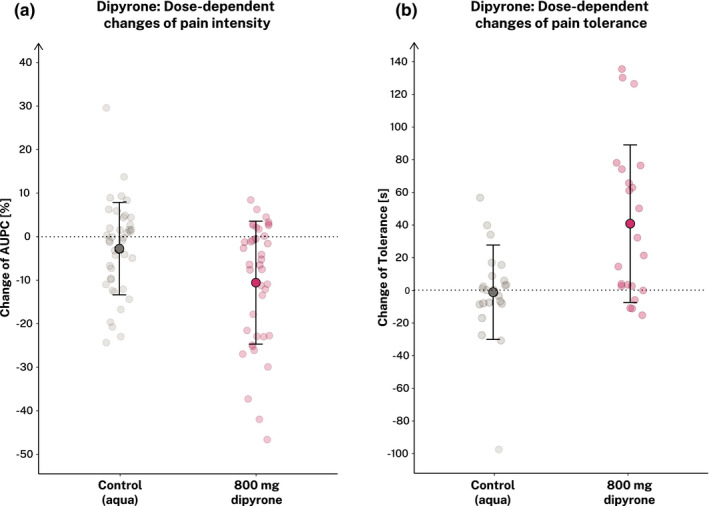
Analgesic effects, safety, and tolerability of dipyrone versus control. Illustrated are changes of the percent area under the pain curve (%AUPC) (a) and the pain tolerance (b) as parameters of pain intensity. Self‐reported side effects did not significantly differ between groups (data not shown). Filled dots show means ± SD for each group separately. Light dots show individual participant data
Pooled analysis: comparison of dipyrone with low‐ and high‐dose tilidine/naloxone
The third, pooled analysis aimed at directly comparing the three pharmacologic agents across substudies. We included 159 participants (low‐dose opioid; [i.e., 50/4 mg tilidine/naloxone] = 60, high‐dose opioid [i.e., 100/8 mg tilidine/naloxone] = 59, and dipyrone group = 40) from all substudies (for details see Figure S1). When comparing participants receiving dipyrone with those receiving low‐dose and high‐dose tilidine/naloxone, we observed no statistically significant differences in %AUPC ratings and pain tolerance. Additionally performed equivalence tests revealed noninferiority of dipyrone compared to low‐dose tilidine regarding %AUPC (equivalence, t(39) = 2.56, p = 0.007) and pain tolerance (equivalence, t(21) = −1.90, p = .041). Compared to high‐dose tilidine, we found significant equivalence regarding pain tolerance (t(21) = 2.11, p = 0.023). Regarding the %AUPC as outcome, the noninferiority test of dipyrone compared to high‐dose tilidine revealed inconclusive results because neither the equivalence nor the null hypothesis test reached significance. Notably, analyses of the reported side effects revealed significantly lower medication‐attributed symptom counts in the dipyrone group compared to both the low‐dose and high‐dose tilidine/naloxone groups (for details see Figure 4, Table 2).
FIGURE 4.
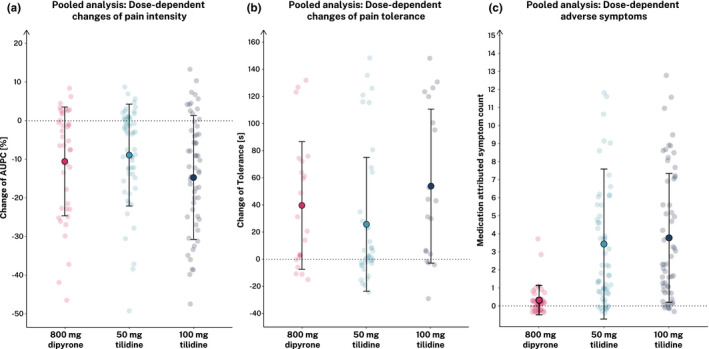
Direct comparison of analgesic effects, safety, and tolerability of dipyrone, low‐ and high‐dose tilidine substudies. Illustrated are changes of the percent area under the pain curve (%AUPC) (a) and the pain tolerance (b) as measures of pain intensity. Self‐reported side effects are illustrated as medication‐attributed symptom count (c). Filled dots show means ± SD for each group separately. Light dots show individual participant data
DISCUSSION
This analysis of three randomized, controlled substudies revealed the following findings. First, both a single 50/4 mg and 100/8 mg tilidine/naloxone combination, and 800 mg dipyrone were effective in reducing experimentally induced cold pain in a CPT. Second, we found significant dosage‐independent opioid‐associated side effects. Third, the analgesic effect of a single 800 mg dipyrone dose did not differ from 50/4 mg tilidine/naloxone (statistical noninferiority). Fourth, participants in the dipyrone substudy showed significantly fewer side effects compared to participants in the opioid substudies and no statistically significant difference between the dipyrone and placebo control group. These results highlight the usability of dipyrone in a safe, tolerable, and effective experimental model of pharmacological analgesia compared to models using opioids.
Tilidine/naloxone is effective in cold pressor pain but likely to induce side effects
In the analyses of substudies 1 and 2, we demonstrate that both a low‐dose and a high‐dose (50/4 mg; 100/8 mg, respectively) tilidine/naloxone combination significantly reduce perceived pain intensity (as indexed by the %AUPC) and significantly increase CPT pain tolerance. These findings are in line with previous studies demonstrating analgesic efficacy of opioids in CPT‐induced pain, albeit in smaller samples. 1 , 10 , 27 , 28 , 29 Thus, our study in a large sample size confirms the analgesic efficacy of opioids in CPT‐induced pain.
However, any assessment of the suitability of an analgesic model for experimental purposes also needs to consider side effects of the drug, which not only affect participant safety but can also lead to premature unblinding. Opioids, such as tilidine/naloxone, have been shown to induce side effects, such as nausea, vomiting, or dizziness, at a significant rate. 6 , 9 , 10 In line with previous reports, our data show side effects of large effect sizes in both opioid groups compared to the control group. About 80% of all participants who received an opioid in our study, reported at least one side effect. Although we did not observe significant changes in blood pressure or heart rate, we found two cases of adverse events, which demanded on‐site medical treatment. In sum, these results demonstrate that tilidine/naloxone is effective in cold pressor pain, but frequent occurrence of side effects has to be considered.
Dipyrone leads to significant analgesia in cold pressor pain with high tolerability
Dipyrone, also known as metamizole, has previously been shown to be efficient in pressure‐evoked pain 30 in experimental settings. To our knowledge, we present the first systematic investigation of dipyrone in cold pressor pain. Our data show a significant reduction of %AUPC and increase of pain tolerance after a single dose of 800 mg dipyrone compared to the control group with a medium analgesic effect size. This finding is remarkable, as most of the previous studies reported incoherent efficacy of non‐opioids, particularly NSAIDs, on cold pain. 1 , 5 , 31 In addition, there were no significant differences between the dipyrone and control group with respect to side effects (20% vs. 12.2%, respectively, reported at least one side effect), blood pressure, and heart rate. These data suggest that dipyrone might be an efficient, safe, and tolerable alternative for pharmacomodulation of the CPT. Comparing dipyrone with low‐ and high‐dose tilidine/naloxone.
In addition to the single comparisons with control, we also directly compared pain intensity, pain tolerance, side effects, as well as changes of blood pressure and heart rate among the three substudies. Treatment allocation to opioid versus was not randomized, therefore any direct comparison has to be interpreted with caution, as systematic differences between the substudies may confound comparisons. The present work is a secondary analysis across three separate studies that aimed to investigate different objectives. The three (sub)studies were fully comparable in many aspects (i.e., performed with identical sample definition), in the same laboratory, with identical protocols, comparable time‐schedules, and identical experimental setup. Still, all comparisons between high‐dose opioids and low‐dose opioids, as well as opioids and metamizole are also comparisons between substudies and therefore vulnerable to systematic bias. Notably, time‐effects (e.g., seasonal effects) and investigator‐specific contextual effects (sub‐\studies were executed by three different investigators J.W., J.K., and F.B.) may have biased the cross‐medication comparisons described here.
Our pooled analysis showed that participants in the dipyrone group did not significantly differ from either those in the low‐ or high‐dose tilidine/naloxone groups regarding changes in %AUPC and pain tolerance. The three groups showed moderate (high‐dose opioid) or small (dipyrone and low‐dose opioid) analgesic effect sizes. Moreover, participants in the dipyrone group showed a significant lower side effect count compared with the opioid groups (percentage of participants reporting any side effects per group; dipyrone: 20%, low‐dose tilidine: 78.3%, high‐dose tilidine: 79,7%). None of the groups showed significant changes in blood pressure and heart rate, indicating sufficient safety of both dipyrone and tilidine/naloxone in our sample. Together, these findings indicate that dipyrone was comparable to the combination of tilidine/naloxone with respect to analgesic efficacy but was significantly better tolerated.
Of note, in order to maintain a high level of comparability, in all substudies, the CPT phase measurement was performed 35 min after the compound’s intake. However, the times to maximum plasma concentrations (T max) differ between the active metabolites of soluble tilidine (nortilidine, T max = 0.7 ± 0.33 SD h 32 ) and soluble dipyrone (4‐methyl‐amino‐antipyrine [MAA)] T max = 1.2 ± 0.5 SD h 33 ). Consequently, the maximum plasma concentration of MAA might not have been reached in our studies, resulting in potential underestimation of the analgesic effect of dipyrone in CPT.
Although dipyrone is frequently used as a first‐line analgesic drug, 17 , 18 its availability is restricted in some countries, due to a reported risk of agranulocytosis. Despite the evidence that the risk of severe adverse events (i.e., agranulocytosis) might have been overestimated in the past, especially with reference to the temporary restricted application in acute pain, 20 this lack of availability of dipyrone in some countries likely affect its use in experimental settings.
CONCLUSION
In this pooled analysis, we investigated the efficacy and tolerability of two pharmacological substances for cold pressor pain. Our data show that all substances (50/4 mg and 100/8 mg tilidine/naloxone, as well as 800 mg dipyrone) led to significant reduction in pain intensity and increase of pain tolerance compared to a placebo control without significant differences between the substances. Safety parameters, such as blood pressure and heart rate changes, did not significantly differ among groups, but side effect reports indicated that dipyrone was better tolerated than both doses of opioids.
In sum, our results suggest that dipyrone in combination with CPT might offer an effective and safe pharmacologic experimental paradigm of analgesia for experimental pain research. The analgesic noninferiority of dipyrone in comparison to low‐dose (50/4 mg) tilidine/naloxone indicates that dipyrone may be used as a positive control condition in combination with cold pressor pain. Finally, the paradigm may be of interest to psychopharmacological research exploring context modulation of analgesia (e.g., social learning, expectation modulation, and placebo interventions), where a minimal level of side effects is desirable.
CONFLICT OF INTEREST
J.K.‐B. received personal fees from Novartis Pharma GmbH outside the submitted work. K.S. received personal fees from Novartis Pharma GmbH and Bayer Pharma AG outside the submitted work. M.Z. is full‐time employee of Takeda Pharma; the present publication has been prepared independently and outside of the employment; the employer is not involved in any of the subjects dealt within this publication and did not provide any form of support. U.B. reports personal fees from Biogen GmbH, personal fees from Lilly Deutschland GmbH, personal fees from Novartis Pharma GmbH, personal fees from Grünenthal GmbH, personal fees from Bionorica SE, personal fees from Chugai Pharma Germany GmbH, personal fees from Eisai GmbH, personal fees from Mylan Germany GmbH, personal fees from Roche Deutschland Holding GmbH, all aforementioned outside the submitted work. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
J.K.‐B., J.W., K.S., M.Z., and U.B. wrote the manuscript. J.K.‐B. and U.B. designed the research. J.K.‐B., J.W., J.K., and F.B. performed the research. J.K.‐B., K.S., M.Z., and U.B. analyzed the data. M.Z. contributed analytic tools.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Lisa Carolin Burgard, Sebastian Wurthmann, and Daniel Müller for excellent medical assistance.
Funding information
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG)—Projektnummer 422744262—TRR 289. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project‐ID 422744262—TRR 289. Author J.K.‐B. was supported as a Clinician Scientist within the University Medicine Essen Academy (UMEA) program, funded by the German Research Foundation (DFG; grant FU356/12‐1) and the Faculty of Medicine, University of Duisburg‐Essen. Authors J.W. and F.B. were supported within the graduate program “Essener Ausbildungsprogramm Labor und Wissenschaft für den ärztlichen Nachwuchs” at the Faculty of Medicine, University of Duisburg‐Essen, funded by the Else Kröner‐Fresenius‐Stiftung.
REFERENCES
- 1. Jones SF, McQuay HJ, Moore RA, Hand CW. Morphine and ibuprofen compared using the cold pressor test. Pain. 1988;34:117‐122. [DOI] [PubMed] [Google Scholar]
- 2. Modir JG, Wallace MS. Human experimental pain models 2: the cold pressor model. Methods Mol Biol. 2010;617:165‐168. [DOI] [PubMed] [Google Scholar]
- 3. Koenig J, Jarczok MN, Ellis RJ, Bach C, Thayer JF, Hillecke TK. Two‐week test‐retest stability of the cold pressor task procedure at two different temperatures as a measure of pain threshold and tolerance. Pain Pract. 2014;14:126‐135. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell LA, MacDonald RAR, Brodie EE. Temperature and the cold pressor test. J Pain. 2004;5:233‐237. [DOI] [PubMed] [Google Scholar]
- 5. Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84:47‐51. [DOI] [PubMed] [Google Scholar]
- 6. Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000;91:185‐191. [DOI] [PubMed] [Google Scholar]
- 7. Webb J, Kamali F. Analgesic effects of lamotrigine and phenytoin on cold‐induced pain: A crossover placebo‐controlled study in healthy volunteers. Pain. 1998;76:357‐363. [DOI] [PubMed] [Google Scholar]
- 8. Enggaard TP, Poulsen L, Arendt‐Nielsen L, et al. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92:277‐282. [DOI] [PubMed] [Google Scholar]
- 9. Koltzenburg M, Pokorny R, Gasser UE, Richarz U. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain. 2006;126:165‐174. [DOI] [PubMed] [Google Scholar]
- 10. Black ML, Hill JL, Zacny JP. Behavioral and physiological effects of remifentanil and alfentanil in healthy volunteers. Anesthesiology. 1999;90:718‐726. [DOI] [PubMed] [Google Scholar]
- 11. Naef M, Curatolo M, Petersen‐Felix S, et al. The analgesic effect of oral delta‐9‐tetrahydrocannabinol (THC), morphine, and a THC‐morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79‐88. [DOI] [PubMed] [Google Scholar]
- 12. Ander F, Magnuson A, Leon AD, Ahlstrand R. Does the b‐receptor antagonist esmolol have analgesic effects? A randomised placebo‐controlled cross‐over study on healthy volunteers undergoing the cold pressor test. Eur J Anaesthesiol. 2018;35:165‐172. [DOI] [PubMed] [Google Scholar]
- 13. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191‐204. [DOI] [PubMed] [Google Scholar]
- 14. Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3:679‐684. [DOI] [PubMed] [Google Scholar]
- 15. Benedetti F. Mechanisms of placebo and placebo‐related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33‐60. [DOI] [PubMed] [Google Scholar]
- 16. Staahl C, Olesen AE, Andresen T, Arendt‐Nielsen L, Drewes AM. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers ‐ an updated review. Br J Clin Pharmacol. 2009;68:149‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards J, Meseguer F, Faura C, Moore RA, McQuay HJ. Single dose dipyrone for acute renal colic pain. Cochrane Database Syst Rev. 2002;2002(4):CD003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derry S, Faura C, Edwards J, McQuay HJ, Moore RA. Single dose dipyrone for acute postoperative pain. Cochrane Database Syst Rev. 2013;2013(11):CD003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med, 2007;146:657‐665. [DOI] [PubMed] [Google Scholar]
- 20. Kötter T, da Costa BR, Fässler M, et al. Metamizole‐associated adverse events: a systematic review and meta‐analysis. PLoS One. 2015;10:e0122918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E Assessing general side effects in clinical trials: Reference data from the general population. Pharmacoepidemiol Drug Saf. 2011;20:405‐415. [DOI] [PubMed] [Google Scholar]
- 22. Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433‐436. [PubMed] [Google Scholar]
- 23. Lydick E, Epstein RS, Himmelberger D, White CJ. Area under the curve: A metric for patient subjective responses in episodic diseases. Qual Life Res. 1995;4:41‐45. [DOI] [PubMed] [Google Scholar]
- 24. Toms L, Derry S, Moore RA, McQuay HJ. Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults. Cochrane Database Syst Rev. 2009;2009(1):CD001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNicol ED, Ferguson MC, Schumann R. Single dose intravenous diclofenac for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;2018:CD012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lakens D, Scheel AM, Isager PM. Equivalence testing for psychological research: a tutorial. Adv Methods Pract Psychol Sci. 2018;1:259‐269. [Google Scholar]
- 27. Luginbühl M, Schnider TW, Petersen‐Felix S, Arendt‐Nielsen L, Zbinden AM. Comparison of five experimental pain tests to measure analgesic effects of alfentanil. Anesthesiology. 2001;95:22–29. [DOI] [PubMed] [Google Scholar]
- 28. Wolff BB, Kantor TG, Jarvik ME, Laska E. Response of experimental pain to analgesic drugs III. Codeine, aspirin, secobarbital, and placebo. Clin Pharmacol Ther. 1969;10:217‐228. [DOI] [PubMed] [Google Scholar]
- 29. Abbott FV, Etienne P, Franklin KB, Morgan MJ, Sewitch MJ, Young SN. Acute tryptophan depletion blocks morphine analgesia in the cold‐pressor test in humans. Psychopharmacology. 1992;108:60‐66. [DOI] [PubMed] [Google Scholar]
- 30. Forster C, Magerl W, Beck A, et al. Differential effects of dipyrone, ibuprofen, and paracetamol on experimentally induced pain in man. Agents Actions. 1992;35:112‐121. [DOI] [PubMed] [Google Scholar]
- 31. Staahl C, Olesen AE, Andresen T, Arendt‐Nielsen L, Drewes AM. Assessing efficacy of non‐opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68:322‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hajda JP, Jähnchen E, Øie S, Trenk D. Sequential first‐pass metabolism of nortilidine: the active metabolite of the synthetic opioid drug tilidine. J Clin Pharmacol. 2002;42:1257‐1261. [DOI] [PubMed] [Google Scholar]
- 33. Levy M, Zylber‐Katz E, Rosenkranz B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet. 1995;28:216‐234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


