Abstract
Abstract
Before the first generic version of a drug is marketed, patent litigation often occurs. The process begins when generic manufacturers notify the US Food and Drug Administration (FDA) of their intent to market a generic copy of a brand‐name drug protected by patents, which they allege to be invalid or not infringed (called a Paragraph IV certification). Assuming the brand‐name manufacturer responds with litigation within 45 days, a 30‐month stay period is triggered, which bars the FDA from authorizing generic entry until the stay period expires or litigation is resolved in favor of the generic manufacturer. To understand whether 30‐month stays delay generic entry, we examined the timing of major legal events leading to generic entry for a cohort of 46 generic drugs, including the timing of Paragraph IV certification filings, stay period expirations, the FDA approvals of generics, and generic product launches. We found Paragraph IV certifications were filed a median of 5.2 years after the brand drug’s FDA approval. There was a median of 3.2 years between the stay period expiration and subsequent generic launch. Because stay periods generally expire well in advance of when generic entry typically occurs, 30‐month stays are unlikely to delay the timing of generic entry. Patent litigation could begin even earlier, however, if litigation was allowed to start immediately following a brand‐name drug’s FDA approval; but by law currently, the soonest this can begin is 4 years after the brand drug’s FDA approval.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Before generic versions of new drugs reach the market, patent litigation often occurs. Once litigation has been initiated, a 30‐month regulatory stay period is triggered that bars the US Food and Drug Administration (FDA) from approving the generic application until litigation resolves or the stay period expires.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the timing of key legal events in the regulatory approval process for generic drugs in relation to the eventual launch of the generic product?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We identified the typical timing of the initiation of patent litigation and expiration of the 30‐month stay period prior to the eventual launch of generic products. Litigation is often initiated as soon as legally possible (i.e., 4 years after the launch of the brand product), and stay periods typically expire well before generic entry occurs.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Stay periods are unlikely to delay generic entry directly because stay expirations often occur well before the time of generic launch. Allowing the submission of generic drug applications immediately following a brand drug’s FDA approval would facilitate earlier patent dispute resolution and prevent unnecessary delays in the anticipated generic product launch date.
INTRODUCTION
The process of introducing generic drugs into the US market is critically important for patients, because generic drugs increase competition, lower drug prices, and create an incentive for brand‐name drugmakers to further innovate. Generic availability marks the end of the brand‐name drug’s period of market exclusivity, the length of which is often determined by the expiration of key patents. High prices charged during the exclusivity period allow brand‐name manufacturers to recover research and development costs and earn a profit.
In 1984, Congress passed the Drug Price Competition and Patent Term Restoration Act (Hatch‐Waxman Act) to facilitate the approval of generic drugs. Under the Hatch‐Waxman Act, generic drug manufacturers could obtain approval via a streamlined process that required much simpler and less expensive trials showing bioequivalence to the brand‐name drug product. The new law restricted submission to the US Food and Drug Administration (FDA) of an application for a generic drug during the first 4 years after a branded drug’s approval for the vast majority of drugs that are covered by patents (Figure 1), and provided for patent extensions of up to 5 years to account for time lost during the clinical trial and FDA approval process.
FIGURE 1.
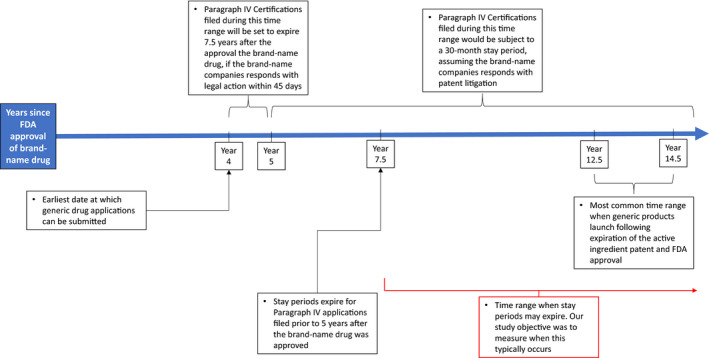
Key events and policies surrounding the processes leading to generic entry. Notes: The process of generic entry begins for more than half of new drugs with the filing of a Paragraph IV certification with the US Food and Drug Administration (FDA), which provides notice of a generic manufacturer’s intent to market a copy of a new brand drug protected that is protected by patents that the generic manufacturer alleges to be invalid or not infringed. By law, the soonest this can begin is 4 years after the brand drug’s FDA approval. If the Paragraph IV certification is filed between 4 and 5 years following the brand drug’s FDA‐approval and the brand manufacturer responds with patent litigation, a stay period is triggered that bars the FDA from authorizing generic entry until 7.5‐years after the brand drug’s FDA‐approval or until litigation is resolved. If the Paragraph IV certification is filed 5 or more years following the brand drug’s FDA‐approval and the brand manufacturer responds with patent litigation, a stay period is triggered that bars the FDA from authorizing generic entry until 30 months have elapsed or until litigation is resolved, whichever occurs sooner. Generic entry typically occurs between 12.5 and 14.5 years after a branded drug's FDA‐approval date. Our study’s objective was to measure when stay periods typically expire and how much time remains prior to generic product launch in order to understand the extent to which stay periods could potentially delay the timing of generic entry. FDA, US Food and Drug Administration
For patent‐protected drugs, the generic manufacturer can assert that the patents are invalid or would not be infringed by the proposed generic version (called a “Paragraph IV certification”). If the brand‐name manufacturer disagrees, it can initiate litigation. To incentivize earlier litigation, the brand‐name manufacturer receives the benefit of a 30‐month regulatory “stay” if it brings suit within 45 days of receivingnotice of the Paragraph IV certification, during which time the FDA cannot approve the generic drug. The law provides that the stay will not terminate until at least 7.5 years after the approval of the brand‐name product, unless litigation resolves sooner or a court orders otherwise. The duration of the 30‐month stay period was considered to be a reasonable amount of time for patent litigation to resolve. 1 If the stay expires before litigation ends, the FDA can approve the generic drug product and its manufacturer can launch “at‐risk,” entering the market while risking substantial damages if a court rules that the relevant patents are valid and infringed. At‐risk launches are therefore likely only when the generic manufacturer is confident in the strength of its legal position in the ongoing litigation and when generic applications are far enough along to be reviewed and approved by the FDA.
The stay period has been a particular point of controversy because it links the drug regulatory system with the patent system. Supporters of the stay emphasize that it creates an incentive for patent litigation to begin (and therefore be resolved) sooner, because the stay is available only if the patent holder brings a legal suit within 45 days of receiving notice of the Paragraph IV challenge, before any actual infringement has occurred. Opponents of the stay argue that it leads to delays in generic entry when patents are later held to be invalid or not infringed. Linkage is also practiced in Australia, Canada, Japan, Mexico, Peru, Singapore, Taiwan, Ukraine, and Vietnam, and is being considered by China, Thailand, and Russia. 2 By contrast, in Brazil, Indonesia, the European Union, and Switzerland, among others, the patent and drug regulatory systems are kept separate so that drug regulatory bodies can approve applications for market entry from generic manufacturers once the products in question have satisfied regulatory requirements, irrespective of patent status. Following approval, disputes over intellectual property are resolved via the judicial system, and at‐risk launches can more freely occur.
Almost 40 years after the Hatch‐Waxman Act, policymakers continue to debate its strengths and weaknesses in facilitating generic entry, 3 , 4 , 5 , 6 , 7 , 8 but these debates often occur without systematic data on the various steps and legal milestones that must first occur. We therefore examined the timing of the major legal or regulatory events following Paragraph IV certification, the prevalence of stay periods, and the frequency with which generic products launch immediately after stay periods expire. One hypothesis was that stay periods would delay the timing of generic entry, which we sought to examine by calculating the time between stay expiration and generic launch.
METHODS
We tracked the timing of four major milestones leading to the availability of generic drugs for US patients, including the date of: (i) the original generic drug application submission to the FDA; (ii) expected stay expiration (absent earlier judicial resolution); (iii) FDA approval of the generic drug application; and (iv) generic drug launch. Finally, we tested whether stay periods were associated with shorter or longer timeframes among these four milestone events.
Cohort selection
Based on the findings of previous studies of generic market dynamics that recorded the timing of Paragraph IV certifications and generic launches, 9 we reasoned that 4.6–4.9 years would be required following FDA approvals of the generic drug application (assuming FDA approvals occured 30 months after the Paragraph IV certifications were filed) before observing generic product launch for most drugs. Therefore, we selected an observation window starting with first FDA generic approvals during the years 2013–2015 to the time of study initiation in 2020.
Data extraction
First generic approvals of new molecular entities were identified using the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (“Orange Book”) 10 and the Drugs@FDA online database. 11 These data sources contain information on therapeutic equivalence between brand‐name and generic products, the approval dates for brand‐name and generic products, and copies of the FDA’s approval letters. To determine which generic drugs were approved following a Paragraph IV certification, we scoured the FDA’s List of Paragraph IV Drug Product Applications and copies of the FDA approval letters. 12 The FDA approval letters from Drugs@FDA were also used to determine whether brand‐name companies responded with patent litigation within 45 days after the Paragraph IV certification filing, which was used to create a binary stay variable. Expected stay expirations were calculated according to the specifications in the Hatch‐Waxman Act: 30 months after Paragraph IV certification submission date, or 7.5 years after the FDA approval date of the brand‐name reference product in question, whichever was later. (The 30‐month period begins to run from receipt of notice by the patent owner that a Paragraph IV certification has been made, which notice must be given within 20 days after the date of the postmark on the notice sent by the FDA to the generic drug manufacturer that its application has been filed, but such notice receipt dates are not readily available.) Finally, to identify generic product launch dates, we performed searches on the generic manufacturers’ websites for press releases. Occasionally, there were multiple first generics simultaneously approved by the FDA. We considered each first generic application as a separate unit of analysis, rather than combining it with other applications approved on the same day, as there may be individual variations for the other major events leading to generic entry. Additional detail on our data collection strategy is available in the Supplementary Information.
Analysis
Descriptive statistics are reported on the number of Paragraph IV certifications that led to stay periods. Dates of the key events (i.e., Paragraph IV certification submission date, expected stay expiration, FDA approval of the generic application, and generic product launch) relating to each generic drug were placed along a common timeline by calculating years between their occurrence and the date when their brand‐name reference product was FDA‐approved. We report our results using medians and interquartile ranges (IQRs) as the data were not normally distributed. To measure the relevance of the 7.5‐year provision and its potential impact on the timing of stay expiration, we also calculated the proportion of Paragraph IV certifications that were affected by the 7.5‐year provision.
We used the Mann–Whitney–Wilcoxon rank sum test to determine whether the observed differences between drugs with and without stay periods were significantly different (95% confidence level, two‐tailed). For drugs with stay periods, we measured the number of years between the expected stay expiration and generic launch. We further noted any cases when generic launch occurred immediately after the expiration of the stay period.
RESULTS
There were 87 first generic approvals of new molecular entities between 2013 and 2015 (Figure 2). Fifty‐one (59%) involved a Paragraph IV certification, and 46 of these launched during our time window, making up our final drug cohort (the remaining 5 were approved but not yet launched by 2020). (These 46 generic products were equivalents to 34 brand‐name drugs; the FDA simultaneously approved multiple generic equivalents to 5 brand‐name products on the same day.) For 17 (37%) of the 46 drugs in our final cohort, the brand‐name manufacturers did not respond with litigation within 45 days of the Paragraph IV certification and a stay period was therefore not triggered. For the remaining 29 drugs (63% of 46), the 30‐month stay was triggered.
FIGURE 2.
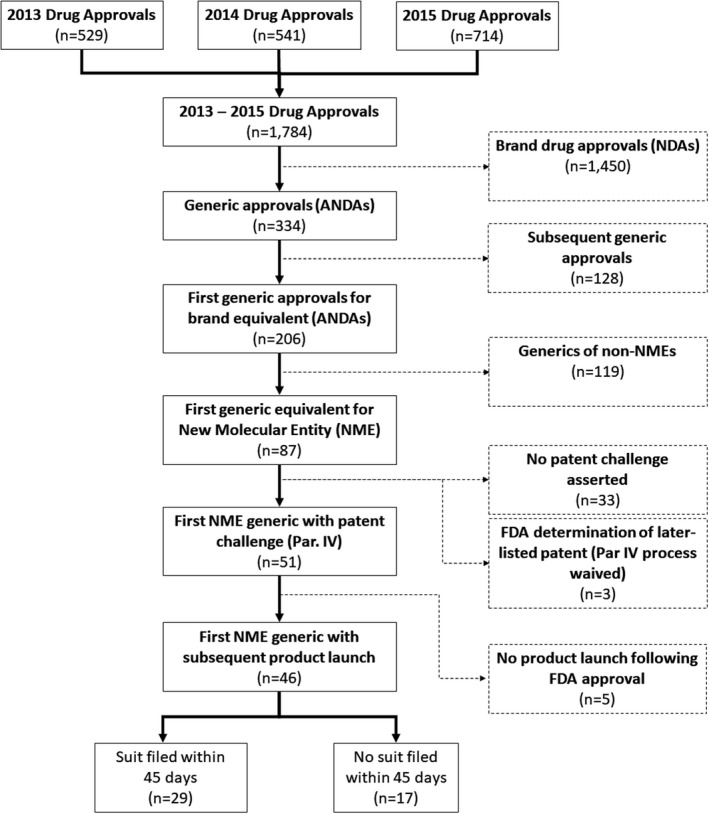
Identification of first generic approvals of New Molecular Entities, 2013–2015. ANDAs, abbreviated new drug application; FDA, US Food and Drug Administration; NDAs, new drug application
Timing of milestone events
Paragraph IV certification initiating the litigation process to facilitate generic entry was filed a median of 5.2 years (IQR: 4.0–8.0 years) after the approval of the brand‐name drug (Table 1, Figure 3). Of the 46 Paragraph IV certifications, a plurality (14/46, 30%) were filed 4 years after the brand‐name reference product was approved. Among the 29 applications with stay periods, the expected expiration of the stay occurred a median of 7.7 years (IQR: 7.5–10.2 years) after the approval of the brand‐name drug. Eleven stay periods were extended by the 7.5‐year minimum requirement. The FDA approvals of the 46 generic applications occurred a median of 11.5 years (IQR: 9.4–14.5 years) after the brand‐name drug approval. Generic product launch occurred a median of 14.1 years (IQR: 11.1–15.2 years) after brand‐name drug approval. There were no significant differences in the timing of these milestone events when comparing cases when the stay period was in force (n = 29) versus when it was absent (n = 17; Table 1).
TABLE 1.
Time of and between major milestone events leading to generic entry following submission of a Paragraph IV application
| Measure | Overall | Stay period | No stay period | Mann–Whitney–Wilcoxon rank sum test, p‐value |
|---|---|---|---|---|
| Number of first generic applications, n | 46 | 29 | 17 | — |
| Timing of milestones leading to generic entry, years (IQR) | ||||
| Paragraph IV filing | 5.2 (4.0–8.0) | 5.2 (4.0–9.2) | 5.1 (4.0–7.5) | 0.640 |
| Stay expiration | — | 7.7 (7.5–10.2) | — | — |
| FDA approval of Paragraph IV application | 11.5 (9.4–14.5) | 11.5 (9.4–13.4) | 12.2 (9.4–15.0) | 0.399 |
| Generic product launch | 14.1 (11.1–15.2) | 13.7 (11.5–14.5) | 14.6 (11.0–15.3) | 0.400 |
| Time intervals between milestone events, years (IQR) | ||||
| Stay expiration to FDA approval | — | 2.1 (0.8–3.1) | — | — |
| Stay expiration to generic launch | — | 3.2 (1.9–6.0) | — | — |
| Paragraph IV filing to generic launch | 7.0 (5.0–8.5) | 5.4 (4.4–8.5) | 7.2 (6.4–8.1) | 0.090 |
Abbreviations: FDA, US Food and Drug Administration; IQR, interquartile range.
Eleven of these “30‐month” stays expired after 7.5 years (i.e., after more than 30 months) because the Paragraph IV application was filed between 4 and 5 years after the approval of the reference brand name product and because brand‐name manufacturer filed suit within 45 days.
FIGURE 3.
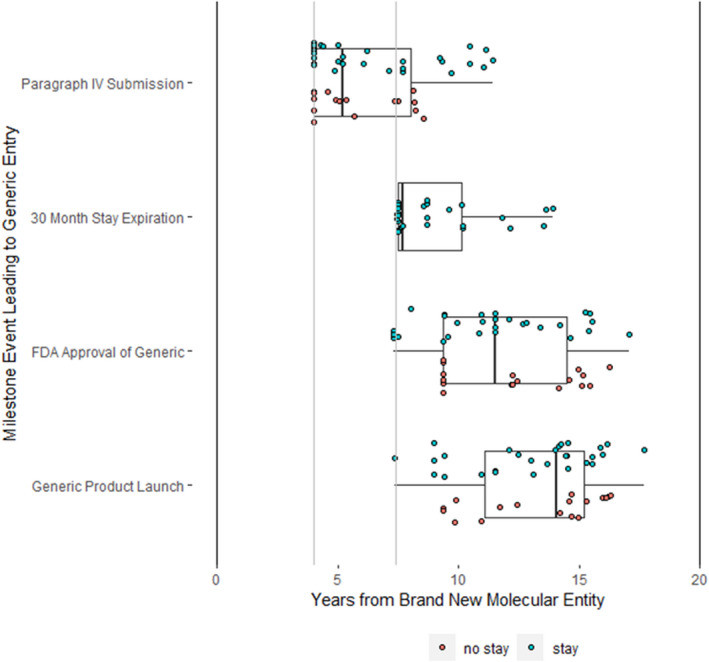
Timing of major milestone events leading to generic entry for drugs experiencing Paragraph IV challenges in years since approval of brand equivalent
Timing of generic launches versus stay period expirations
The 29 stay periods expired a median of 3.2 years (IQR: 1.9–6.0 years) prior to generic launch (Table 1). There was one case (nebivolol, a beta‐blocker used to treat hypertension) in which generic launch occurred when litigation was resolved via a settlement shortly before its stay period was set to expire (7.5 years after the approval of its brand‐name reference product) (Figure 3). Otherwise, no other launches occurred within 1 month of the expected stay expiration, and the next shortest gap in time between expected stay expiration and launch is 10 months.
DISCUSSION
In our review of the milestones leading to generic entry, we found that more than half (59%) of first generic drug approvals were subject to Paragraph IV patent challenges, and that of these challenges, about one‐third were initiated by generic manufacturers as soon as permitted by law (4 years after the reference brand drug approval). These challenges resulted in a median expected stay period expiring 7.7 years after approval of the first generic drug product. This means that most new brand‐name drugs with litigated patent challenges should expect a minimum of 7.5 years of generic‐free market exclusivity, although most will be longer. Nearly all (28/29) stay periods expired several years before the generic launch date, suggesting they did not delay generic entry.
The timing of the milestone events in our cohort is consistent with previous research. For example, a previous study of generic entry found that the time between brand name drug approval and Paragraph IV certification has been decreasing since 1995, with a 3‐year moving average of 6.5 years in 2012 and 5.9 years in 2014, compared to the 5.2‐year period described in our study based on a more recent cohort of drugs. 9 This decreasing period suggests intensifying competition in the market for generic drugs, which may in turn reflect a separate Hatch‐Waxman incentive that provides the first‐filer of a generic drug application with 180‐days of exclusivity with respect to other generic drug manufacturers.
Our study also found that generic launch, on average, occurred long after (median 3.2 years) the expected expiration of the 30‐month stay period. Possible reasons for this finding include that some patents are found valid, forcing generic drug companies to wait until patent expiration; that some patent litigation is settled, with agreed‐upon generic drug entry dates falling between the end of stay periods and patent expiration dates; that patent litigation is ongoing and generic drug manufacturers decline to launch at‐risk; and, perhaps most importantly, that the FDA approval of the generic drug application occurred, on average, several years after stay expirations (11.5 vs. 7.7 years after brand‐name drug approval).
Our results illustrate some of the advantages of early drug patent dispute resolution. Aside from allowing ample time for court proceedings to reach their natural conclusion so that conflicts over market protections are fully resolved well before generic entry is set to occur, early patent dispute resolution will minimize the amount of time that weak patents later deemed to be invalid or not infringed appear in the Orange Book, create barriers to market entry, and enable high prices, limiting drug accessibility and exerting a public health impact. Early patent dispute resolution provides additional certainty surrounding when future generic entry is likely to occur, which is a benefit for multiple stakeholders including generic manufacturers, practitioners, and patients. Furthermore, earlier filing of applications for generic entry allows more time for FDA review and for generic applicants to respond to requests by the FDA for more information. A policy change that could facilitate earlier patent dispute resolution and earlier FDA review of generic applications would be to remove the 4‐year period after brand‐name drug approval during which generic drug applications cannot be submitted.
Our study is subject to certain limitations. First, our analysis was based on the 52% (46 of 87) of first generic drugs for which both a Paragraph IV certification was filed and generic launch occurred, and the focus of our calculations was the 33% (29 of 87) of generic drugs for which a 30‐month stay was triggered. Assessment of these subsets may not be representative of drugs not subject to certification, stay, or launch. This may help to explain why we found a median period of market time prior to generic entry (14.1 years) that was on the high end of the range described in previous studies not limited to drugs subject to Paragraph IV certification (12–14.5 years). 9 , 13 , 14 , 15 , 16 , 17 Second, our study examined only new molecular entities. As generic entry tends to occur sooner for modified versions of existing drugs (e.g., after about 8.25 years 13 , 16 ), litigation could play a different role in the timing of generic entry for such drugs. Third, our study did not consider the possibility that courts can shorten or prolong stay periods if either litigant fails to reasonably cooperate in expediting the proceedings, nor did we attempt to measure how frequently this occurs. 3 Finally, whereas our study found that generic manufacturers often (30% of first generic drugs) began the patent challenge process as soon as legally permitted, the study’s focus upon marketed products did not consider the possibility that stay periods could discourage generic applicants from initiating or continuing with the Paragraph IV process.
CONCLUSION
When 30‐month stay periods are triggered, which occurred for 29 (33%) of 87 first generics in our study, they nearly always (28 of 29) expire well before generic entry occurs. Nevertheless, stays can be important if they delay generic entry for high‐cost or high‐volume brand‐name drugs. Early patent resolution has several advantages, including that stay periods are unlikely to directly impact the timing of generic entry and that questionable patent claims can be tested before patentees accrue additional time on an exclusive market (a right reserved for true innovations). One way to facilitate earlier patent dispute resolution is to remove the stipulation that generic drug applications can be submitted only after 4 years have elapsed from the brand‐name drug's FDA approval date.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
S.K., J.J.D., A.S.K., and R.F.B. wrote the manuscript. S.K., J.J.D., A.S.K., and R.F.B. designed the research. S.K. and R.F.B. performed the research. S.K., J.J.D., A.S.K., and R.F.B. analyzed the data. S.K. and R.F.B. contributed new analytical tools.
Supporting information
Supplementary Material
Funding information
Sunand Kannappan’s work was funded by a studentship grant from the O’Brien Institute for Public Health at the University of Calgary. Dr. Beall’s work is funded by an O’Brien Institute for Public Health catalyst grant for equitable drug innovation and access. Dr. Darrow and Dr. Kesselheim’s work is funded by Arnold Ventures. Drs. Darrow and Kesselheim are also funded by a Novo Nordisk Foundation grant for a scientifically independent Collaborative Research Programme (grant NNF17SA0027784), by West Health, and by the Commonwealth Fund.
REFERENCES
- 1. 21 U.S.C. § 355., authors
- 2. Melling P, Khabarov D, Trusov A, Ermolina D. Global guide to patent linkage. Baker McKenzie. 2019. https://www.bakermckenzie.com/en/insight/publications/guides/global‐guide‐to‐patent‐linkage. Accessed February 1, 2021.
- 3. Lewis J, Ikahihifo‐Bender N. When courts allow changes to Hatch‐Waxman 30‐Month Stay. Law360. 2018. https://www.law360.com/articles/1080769/when‐courts‐allow‐changes‐to‐hatch‐waxman‐30‐month‐stay. Accessed February 1, 2021.
- 4. Schacht WH, Thomas JR, Resources S, Division I. The Hatch‐Waxman Act: proposed legislative changes affecting pharmaceutical patents. Washington, DC: Congressional Research Service; 2003. [Google Scholar]
- 5. Hui YF. FDA's proposed rules on patent listing requirements for new drug and 30‐month stays on ANDA approval (Proposed Oct. 24, 2002). Ann Health Law. 2003;12:325. [PubMed] [Google Scholar]
- 6. Young AK, Andrus MS. Pharmaceutical pricing and Hatch‐Waxman reform: the right prescription. J Generic Med. 2004;1(3):228‐237. [Google Scholar]
- 7. Bhat VN. Patent term extension strategies in the pharmaceutical industry. Pharm Policy Law. 2005;6:109‐122. [Google Scholar]
- 8. Wirz M. Are patents really limited to 20 years: a closer look at pharmaceuticals. Okla JL & Tech. 2003;1:1. [Google Scholar]
- 9. Grabowski HG, Long G, Mortimer R, Boyo A. Updated trends in US brand‐name and generic drug competition. J Med Econ. 2016;19(9):836‐844. [DOI] [PubMed] [Google Scholar]
- 10. United States Food and Drug Administration . Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. United States Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Published 2021. Accessed February 1, 2021. [Google Scholar]
- 11. United States Food and Drug Administration . Drugs@FDA: FDA approved drug products. United States Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/. Published 2021. Accessed February 1, 2021.
- 12. United States Food and Drug Administration . Paragraph IV patent certifications. United States Food and Drug Administration. 2021. https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/abbreviatednewdrugapplicationandagenerics/ucm047676.htm. Accessed February 1, 2021.
- 13. Beall RF, Darrow JJ, Kesselheim AS. A method for approximating future entry of generic drugs. Value Health. 2018;21(12):1382‐1389. [DOI] [PubMed] [Google Scholar]
- 14. Grabowski HG, Kyle M. Generic competition and market exclusivity periods in pharmaceuticals. MDE Manage Decis Econ. 2007;28(4–5):491‐502. [Google Scholar]
- 15. Grabowski HG, Vernon JM. Effective patent life in pharmaceuticals. Int J Technol. 2000;19(1–2):98‐120. [Google Scholar]
- 16. Hemphill CS, Sampat BN. Evergreening, patent challenges, and effective market life in pharmaceuticals. Health Econ. 2012;31(2):327‐339. [DOI] [PubMed] [Google Scholar]
- 17. Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top‐selling prescription drugs in the United States. JAMA Intern Med. 2015;175(4):635‐637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


