Abstract
The liver is the primary organ responsible for clearing most drugs from the body and thus determines systemic drug concentrations over time. Drug clearance by the liver appears to be directly related to organ size. In children, organ size changes as children age and grow. Liver volume has been correlated with body surface area (BSA) in healthy children and adults and has been estimated by functions of BSA. However, these relationships were derived from “typical” populations and it is unknown whether they extend to estimations of liver volumes for population “outliers,” such as children with overweight or obesity, who today represent one‐third of the pediatric population. Using computerized tomography or magnetic resonance imaging, this study measured liver volumes in 99 children (2–21 years) with normal weight, overweight, or obesity and compared organ measurements with estimates calculated using an established liver volume equation. A previously developed equation relating BSA to liver volume adequately estimates liver volumes in children, regardless of weight status.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Liver anatomy and physiology are key determinants of hepatic drug metabolizing capacity captured by physiologically‐based pharmacokinetic (PBPK) models. Liver volumes in children with normal weight have been estimated as a function of body surface area (BSA), age, and other anthropometric features. These relationships have not been established for children with overweight or obesity, a growing patient population for whom PBPK modeling could provide valuable new pharmacology knowledge.
WHAT QUESTION DID THIS STUDY ADDRESS?
Are liver volumes in children with overweight or obesity accurately estimated by a previously published BSA‐based equation derived from children with normal weight?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study demonstrates that liver volumes in children with overweight or obesity can be accurately estimated using BSA‐based equations previously established in children with normal weight, whereas estimations of liver volume as a function of other parameters, such as age, require corrections for weight status.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Accurate estimates of liver volume are critical scalars for predicting hepatic clearance with PBPK models, which are used to help approve and improve pharmaceutical therapies for children, 30% whom have overweight/obesity. The strong correlation of liver volumes with BSA across weight strata, paired with a weak correlation with body mass index z‐score, indicates that liver volume increases proportionally with overall body size (i.e., BSA) not obesity status per se. Our findings suggesting that separate obesity‐specific liver volume equations are not necessary for PBPK model development, as long as BSA is accounted for.
INTRODUCTION
Prior to the Best Pharmaceuticals for Children Act (BPCA) in 2002, drugs had not been frequently or extensively investigated in children. Therefore, there remains much to learn regarding pediatric drug disposition (pharmacokinetics [PK]) and response (pharmacodynamics [PD]. Additionally, there are some challenges in fully characterizing pediatric PK and PD. The pediatric population represents a dynamic and heterogenous group of patients whose inherent and dynamic characteristics (e.g., age, size, stage of biologic development, and organ maturation) can significantly influence drug PK and PD. Furthermore, it is often more challenging to conduct pediatric pharmacology trials compared with adults and investigations may be especially difficult to implement for subpopulations of children, resulting in small study sample size. 1 In such scenarios, computational approaches can be applied to simulate drug concentrations and responses in children when it is difficult to obtain adequate data or sample sizes to evaluate the PK/PD of a pharmaceutical agent of interest. 1 Physiologically‐based pharmacokinetic (PBPK) modeling is one computational approach that simulates drug concentrations with respect to time and can be applied across the pediatric age range. 2 It has, thus, garnered increased interest and use for pediatric populations. Currently, pediatric PBPK models are predominately representative of children with normal weight, but nearly 30% of children in the United States are overweight or obese. 3 Due to comorbid conditions, children with overweight and obesity are more likely to require treatment with medications than normal‐weight peers 4 and it is therefore necessary to identify if physiologic model inputs (e.g., organ volumes) require adjustments in children with overweight or obesity.
Pediatric PBPK models use the expected anatomy and physiology of children (e.g., organ sizes, blood flows, etc.) in combination with drug‐related properties (e.g., physiochemical characteristics, lipid solubility, enzyme and transporter kinetics, etc.) to mechanistically simulate the absorption, distribution, metabolism, and elimination of the drug in question. 5 These models evolve as pediatric anatomy/physiology data become available to more accurately capture changes in physiology over age, disease status, etc., and thus more accurately simulate PK in a broad spectrum of children, including those with overweight/obesity.
Because the liver is responsible for the elimination of greater than 70% of drugs, 6 anatomic and physiological changes in the liver expected during the course of childhood are essential elements for PBPK model development. For drugs eliminated by hepatic mechanisms (e.g., phase I metabolism), a PBPK model estimates in vivo clearance by applying scaling factors, including microsomal protein per gram of liver (MPPGL) and liver volume, to the intrinsic clearance evaluated in an in vitro system (e.g., hepatic microsomes). 7 MPPGL values are available from in vitro studies 8 and may vary with factors such as age. 8 , 9 Reliable estimates of liver volumes in children are needed in PBPK model development to help estimate hepatic capacity for drug clearance and thus systemic drug concentrations for children. 10 , 11 , 12
Several studies have evaluated liver volume and mass in adults and normal‐weight children, and demonstrated that these important PBPK parameters can be estimated as a function of anthropometric features such as height, 13 body mass index (BMI), 14 age, 14 and sex. 13 , 14 A meta‐analysis of 9 pediatric sources (5036 patients) and 11 models that generated estimates of pediatric liver volumes using variables, including body surface area (BSA), age, sex, and weight determined that liver volume (LV) in children was most accurately estimated as a function of BSA, which depends on height and weight, regardless of sex (LV = 0.722*BSA1.176). 15 The model also performed well predicting liver volume in adults compared to other liver size models in an independent verification. 16 However, despite the prevalence of overweight and obesity in the pediatric population, an algorithm to describe LV has not been established for these children. Thus, the primary aim of this study was to assess the applicability of an existing BSA‐based liver volume equation derived from children with normal weight 17 , 18 to estimate liver volumes measured via magnetic resonance imaging (MRI) and computerized tomography (CT) in children with overweight or obesity; the relationship of liver volumes with other anthropometric descriptors was also considered for comparison and completeness.
METHODS
Two independently collected datasets of liver volumes and anthropometric features were collated for this study. Normal weight, overweight, or obesity status for children was defined using standard BMI criteria for age and sex. 3 , 19 , 20
Dataset 1 collection and liver volume determination
A retrospective chart review of patients aged 2–20 years, who underwent MRI or CT imaging of the abdomen and were not known to have conditions that could impact hepatic size or function (e.g., abdominal tumors and hepatitis) were included in the study. Using software that searched all radiology reports at our institution (PS360 Montage, Nuance), we identified consecutive MRIs and CTs, which included the whole abdomen in the field of view from 2010 to 2017. Height, weight, age, sex, race/ethnicity, and comorbidity information were collected from a detailed review of the electronic medical record. BMI (kg/m2) was calculated and children were categorized to have normal weight, overweight, or obesity based on BMI percentile for age and sex: BMI less than 85th percentile (normal weight), 85–94th percentile (overweight), or greater than or equal to 95th percentile (obese).
Z‐scores 21 were calculated for height, weight, and BMI for each record. When the absolute value of a z‐score was greater than 3, the records were examined in the context of other growth measures for that child and removed if they were incorrectly recorded.
Images were analyzed in the PACS system (InteleViewer, Intelerad Medical Systems Incorporated). Axial images from CT studies and T2 MRI sequences were reviewed and liver measurements were made with the volume of interest tool. Liver contours were traced on each axial slice with coronal and sagittal reformats available for reference. Slice thickness and skip was interpolated by the volume tool. The inferior vena cava (IVC) was excluded from measurement when it could be clearly separated from the adjacent liver parenchyma. When the IVC could not be easily excluded, or in the case of branch veins, they were included in the measurement. The portal vein was included in the region of interest measurements in all cases. Regions of interest were drawn to exclude the hilum (Figure 1).
FIGURE 1.
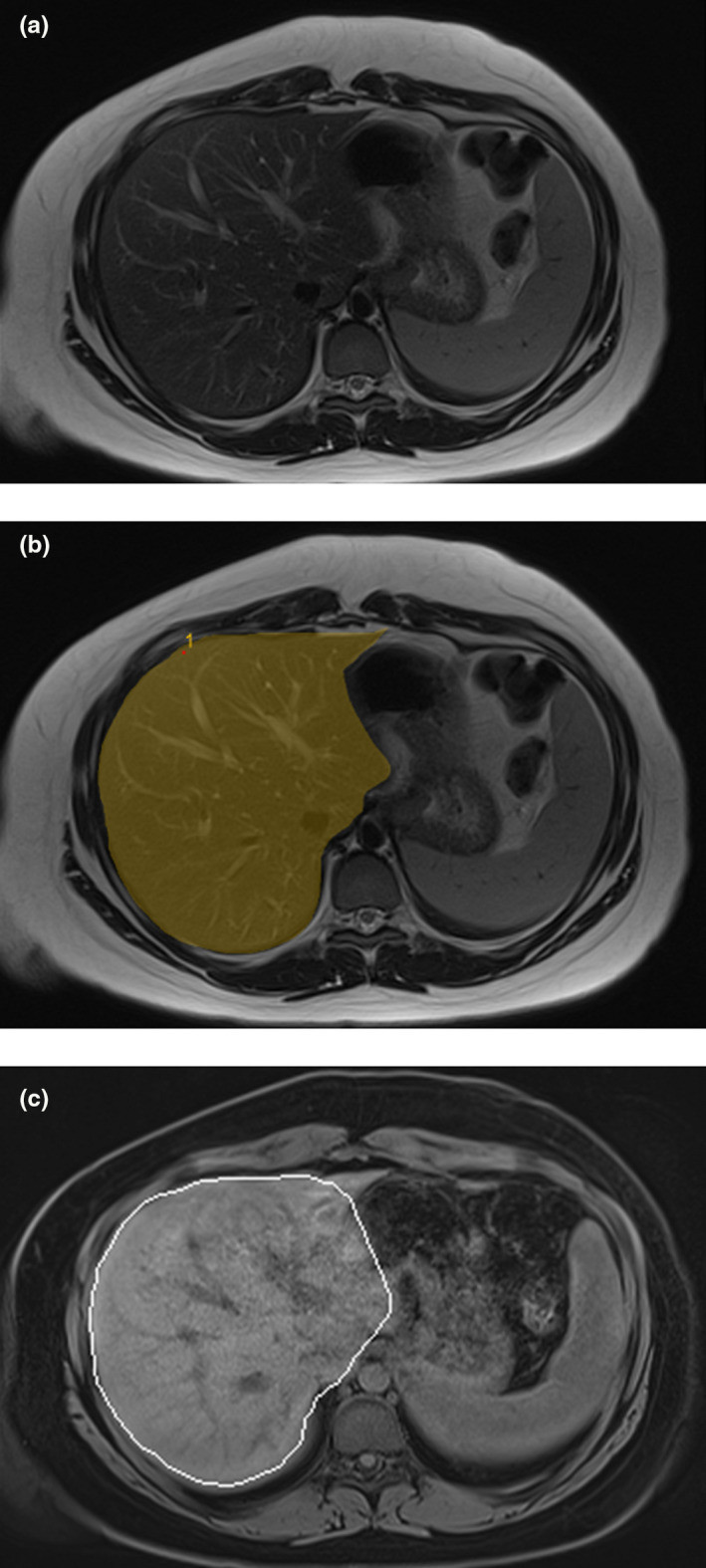
(a) Axial T2 magnetic resonance (MR) images through the level of the mid liver from a from a 15 year old girl with obesity. (b) Axial MR images from the same patient with an overlay showing an example of the manually drawn contours that were used to calculate liver volumes. (c) Axial t1 fat saturated MR images from the same patient at the same level showing the automated contours (white) that were also used to calculate liver volumes. Note the similarity between the contours on images in (b) and (c). In a subset of patients who had both automated and manually contoured liver volumes available (n = 22), the volumes were within 1% of each other.
This study was approved by the institutional review board at Children’s Mercy Hospital as minimal risk with a waiver of permission, consent, and assent.
Dataset 2
All procedures for dataset 2 were identical except that all images were acquired from T2 MRI sequences performed for research purposes in children participating in a prospective pharmacology trial of proton pump inhibitors in pediatric obesity (NCT04248335). Liver volumes measured using tracings of the liver contour on imaging were compared to liver volumes reported as direct output from the MRI sequence and were within 1% of each other (Figure 1).
This study was approved by the institutional review board at Children’s Mercy Hospital. Informed consent and permission/assent were obtained prior to any study‐related procedures.
Analysis
The dataset compositions were compared using a student t‐test for age, and χ2 test for sex, weight class, and distribution of self‐reported race.
Liver volumes measured on imaging were compared to the volumes estimated from the methods published in the meta‐analysis by Johnson et al. 15 : BSA was calculated by the Haycock 22 equation for children less than 15 kg, and the Dubois and Dubois 23 equation for children greater than 15 kg and liver volume was calculated as 0.722*BSA1.176. A twofold difference from estimated values was quantified and the image‐measured liver volumes were compared to the estimated volumes using twofold error analysis. Image‐measured liver volumes greater than 2‐fold or less than 1/2‐fold from the estimated volumes were tallied. The coefficients of variation (CV) of the image‐measured liver volumes compared to the liver volumes estimated by each subject’s BSA were calculated for each weight group. A nonlinear regression, analogous to that already published (LV = A*BSAB), was applied and the coefficients (A and B) were fit using GraphPad Prism. The extra sum‐of‐squares F test was used to determine whether one regression, or regressions specific to each weight class were required. The nonlinear regressions and analyses were applied to subgroups of children based on commonly employed age bins: child (6–12 years), adolescent (12–18 years), and young adult (18–21 years).
The following variables were independently examined for their ability to estimate liver volume in each weight class: age, height, weight, BMI, BMI z‐score, lean body mass (LBM), and fat‐free mass (FFM). We also included BSA calculated following the methods as described by Johnson et al. 15 as a comparator. LBM was calculated using the equation by Peters et al. 24 FFM was calculated using the equation by Al Sallami et al. 25 Pearson correlation coefficients were calculated for each variable as an estimator of liver volume. Linear equations were fit for each variable using the generalized linear model (glm) function in R Studio as an estimator of liver volume for the entire dataset and subdivided into normal weight, overweight, and obese datasets. The CVs were calculated for each equation (composite, normal weight, overweight, and obese).
RESULTS
Datasets
Liver volume data were collected for 48 children (ages 2 to 20 years) in dataset 1. Data from six children were removed due to comorbid conditions that could affect liver size independent of weight status (hepatoblastoma, lymphoma and hyperbilirubinemia, metastatic leukemia, alpha‐1‐antitrypsin deficiency, Ewing’s sarcoma with rapid weight loss, and known non‐alcoholic fatty liver disease), resulting in a dataset of 42 patients. Age was available for every patient and height and weight were available for 40 patients (Figure 2). Twenty of the final images were acquired from MRI and 22 from CT. MRI and demographic data were obtained for all 51 subjects (ages 6 to 21 years) in dataset 2. Descriptive statistics for each dataset are reported in Table 1 and the distribution of the datasets into age bins is shown in Figure 3.
FIGURE 2.
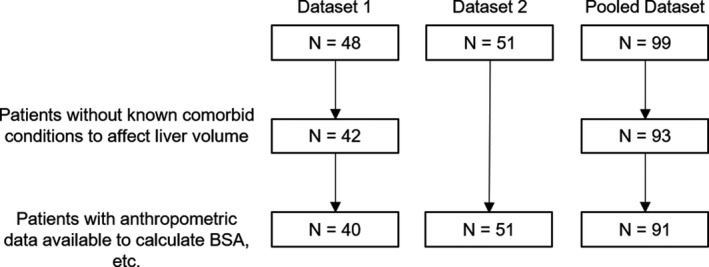
Flow of the data available after liver volumes were collected in the retrospective study (dataset 1) and the prospective study (dataset 2). BSA, body surface area
TABLE 1.
Descriptive statistics of measured liver volumes in pediatric patients
| Set 1 | Set 2 | P | |
|---|---|---|---|
| n | 42 | 51 | |
| % Male | 52 | 49 | Ns |
| Age | 11.7 ± 4.6 | 14.8 ± 3.0 | <0.001 |
| Range | 2.6–19.1 | 8.8–20.3 | |
| Weight class a | Ns | ||
| Normal | 13 | 17 | |
| Overweight | 11 | 11 | |
| Obese | 16 | 23 | |
| Race | <0.05 | ||
| Non‐Hispanic White | 30 | 27 | |
| Black | 5 | 18 | |
| Hispanic | 4 | 0 | |
| Asian | 1 | 1 | |
| Native American | 0 | 0 | |
| Pacific Islander | 0 | 2 | |
| White/Hispanic | 0 | 1 | |
| White/Black | 0 | 2 | |
| Multiple race, unspecified | 2 | 0 |
When height and weight were available, n = 40 in dataset 1.
FIGURE 3.
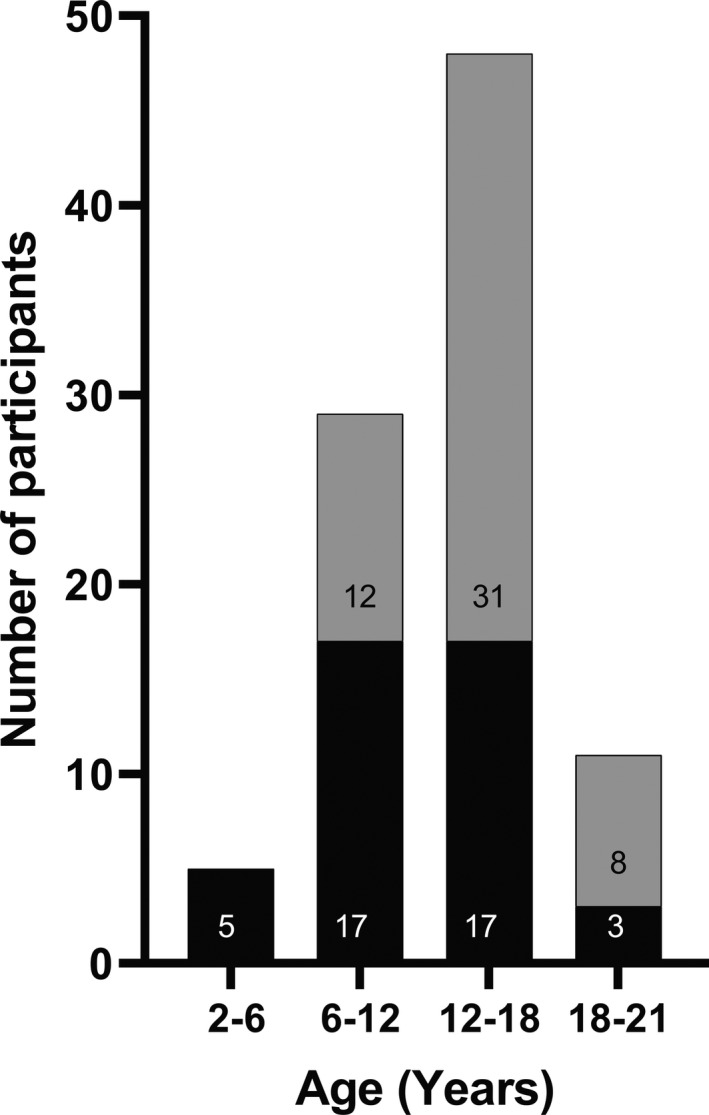
Age distribution of datasets 1 (black bars) and 2 (grey bars)
The datasets were similar with respect to sex and weight class distribution. Given differences in age inclusion criteria for the 2 studies, dataset 2 had a statistically significantly higher mean subject age than dataset 1. A significant difference in the distribution of race was also noted between datasets, with dataset 2 having a higher proportion of Black participants.
Associations of liver volume with anthropometric features
BSA and LBM were most highly correlated to liver volume (Table 2). When linear equations for each anthropometric variable were regressed to image‐measured liver volumes, BSA and LBM had the lowest errors (CVs) and remained among the best predictors of liver volume for each weight status group, with no discernible difference in CV between weight statuses (Table 2). Additionally, the correlation between BMI z‐score—a continuous indicator of weight status—and liver volume was weak (0.32–0.47), indicating that liver volume is not dependent on weight status. With no other anthropometric predictor performing substantially better than BSA for all weight classes, and a robust meta‐analysis previously establishing a significant relationship between BSA and liver volume for predominantly normal weight children, 15 we evaluated these previously established estimation equations of liver volume against image‐measured volumes from our independent pediatric datasets.
TABLE 2.
Anthropometric features as estimators of liver volume in children: correlation coefficients and coefficients of variation fit to simple linear regressions for each variable
| Set 1 | Set 2 | Combined Sets | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CV (%) | CV (%) | CV (%) | |||||||||||||
| R | All | Normal | Overweight | Obese | R | All | Normal | Overweight | Obese | R | All | Normal | Overweight | Obese | |
| Johnson Estimate a | 0.88 | 20 | 20 | 15 | 21 | 0.85 | 17 | 12 | 26 | 16 | 0.84 | 18 | 16 | 21 | 18 |
| Variable | |||||||||||||||
| LBM (kg) | 0.88 | 19 | 20 | 12 | 18 | 0.85 | 15 | 15 | 11 | 10 | 0.84 | 18 | 18 | 17 | 15 |
| BSA (m2) | 0.88 | 19 | 20 | 12 | 19 | 0.85 | 15 | 15 | 11 | 10 | 0.84 | 18 | 18 | 17 | 16 |
| FFM | 0.86 | 20 | 22 | 13 | 20 | 0.85 | 15 | 11 | 13 | 15 | 0.83 | 18 | 17 | 20 | 18 |
| Weight (kg) | 0.87 | 19 | 22 | 12 | 15 | 0.83 | 16 | 15 | 11 | 11 | 0.83 | 19 | 19 | 17 | 14 |
| BMI (kg/m2) | 0.77 | 25 | 26 | 14 | 18 | 0.68 | 21 | 20 | 10 | 20 | 0.71 | 24 | 24 | 15 | 20 |
| Height (cm) | 0.76 | 26 | 19 | 14 | 24 | 0.65 | 22 | 20 | 12 | 10 | 0.69 | 24 | 21 | 19 | 20 |
| Age (days) | 0.69 | 29 | 23 | 14 | 24 | 0.40 | 26 | 23 | 13 | 23 | 0.58 | 28 | 25 | 16 | 23 |
| BMI z‐score | 0.32 | 37 | 43 | 26 | 28 | 0.47 | 25 | 20 | 13 | 23 | 0.38 | 31 | 32 | 22 | 25 |
Abbreviations: BMI, body mass index; BSA, body surface area; CV, coefficient of variation; FFM, fat‐free mass; LBM, lean body mass; LV, liver volume.
The Johnson estimate is a nonlinear regression fit by LV (cm3) = 722*BSA1.176.
Liver volumes of patients with normal, overweight, and obesity compared to those from a reference estimation
All estimations of liver volume using the equation LV = 0.722*BSA1.176. 15 fell within 2‐fold of the image‐measured liver volumes (Figure 4). The CVs for the BSA‐estimated liver volumes compared to the image‐measured liver volumes are similar between weight statuses and are less than 30% (Table 2).
FIGURE 4.
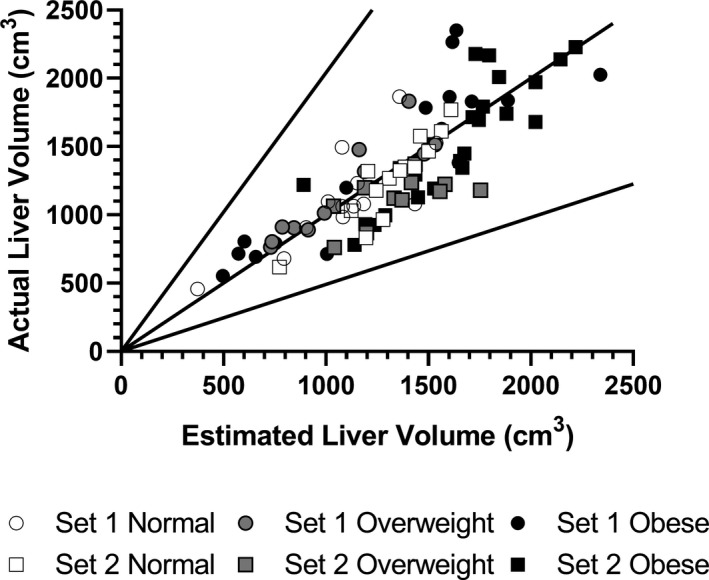
Measured liver volume versus Johnson estimation of liver volume with twofold error
Nonlinear regressions fit to the analogous function LV = A*BSAB were best fit by a single curve for all weight classes in dataset 1 (p = 0.86). For dataset 2, the model performed best with different curves for different weight classes (p = 0.02). However, this was driven by a regression line for overweight children, which was significantly different from the regressions for both normal‐weight (p = 0.0003) and children with obesity (p = 0.04), whereas a single curve can describe the liver volume as a function of BSA for both children with normal weight and children with obesity (p = 0.29; Figure 5). Nonlinear regressions fit to the analogous function LV = A*BSAB were best fit by a single curve for all weight classes when subgroup analysis was applied based on age bins: 6–12 years (p = 0.61), 12–18 years (p = 0.33), and 18–21 years (p = 0.38).
FIGURE 5.
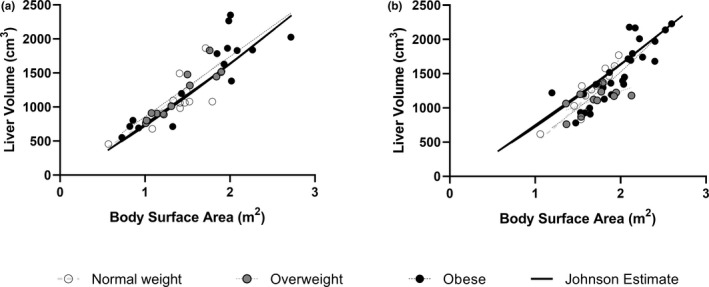
Liver volumes versus body surface area (BSA). Dataset 1 (panel a) and dataset 2 (panel b). BSA was calculated using the method described by Johnson et al. Regression lines are fit to weight class (normal, overweight, or obese) using LV = A*BSAB. The solid line shows the estimates of liver volume as a function of BSA as published by Johnson et al. 15
There were no significant differences in the above regressions for males and females in dataset 1, whereas boys and girls were fit by different regressions in dataset 2. In dataset 2, boys had greater liver volume in relation to BSA when BSA was greater than 1.66, whereas liver volume was greater in girls with a smaller BSA. Both datasets showed significant differences in the relationships between liver volume and BSA for White and Black participants. Although dataset 1 showed that at similar BSAs, the liver volume was larger in children who were Black compared to the liver volume of children who were White, dataset 2 showed that at similar BSAs, the liver volume of children who were White was larger than the liver volume of children who were Black. There were no significant differences in liver volume as a function of BSA in dataset 1 for the liver volumes collected by CT or by MRI (Figure S1).
DISCUSSION
BSA has been proposed as a measure to estimate liver volume. 15 , 26 , 27 However, equations estimating liver volumes in children have thus far not considered weight status, stratified categorically as normal, overweight, and obese according to current BMI percentile‐based criteria, as a covariate. Studies in adults suggest that weight status may alter liver volume. For instance, liver volume significantly decreased with weight loss in adults following bariatric surgery 28 and following a low‐ or very‐low‐calorie diet prior to bariatric surgery. 29 Our data confirm that the published BSA‐dependent liver volume equation reliably estimates liver volume for children independent of weight status, with every child in our dataset having less than twofold error for estimated versus measured volume (Figures 4 and 5) as well as consistently low CVs (<30%) across weight strata (Table 2). The strong correlation of liver volumes with BSA, paired with a weak correlation with BMI z‐score, indicates that liver volume increases proportionally with overall body size (i.e., BSA) not obesity status per se, suggesting that separate obesity‐specific liver volume equations are not necessary for PBPK model development. These findings are strengthened by observed reliability of the liver volume equation independent of the measuring image modality used (CT or MRI).
This study also independently evaluated other anthropometric variables as estimators of liver volume in children with normal weight, overweight, or obesity. Size‐related variables, particularly BSA and LBM, were the most reliable estimators and superior to age. Although several established models estimate liver volume solely by age, 30 , 31 , 32 , 33 our results suggest that when pediatric liver volumes are estimated on the basis of age alone, adjustments must also be made for weight status (Figure S2). Moreover, subgroup analysis in commonly used age groups reaffirmed that liver volume can be adequately estimated by a single regression to BSA, despite weight status. LBM estimated liver volume with similar reliability to BSA. It has been suggested that, because metabolic processes are primarily confined to lean tissues, LBM, or FFM may be useful in understanding the clearance of drugs in children with obesity. Because LBM is often approximated by FFM, FFM might be a preferred scalar. 25 In the current study, there was little difference in the reliability of LBM or FFM in estimating liver volume.
Both race 12 , 15 , 34 , 35 , 36 and sex 29 have been reported to significantly impact liver volume. A scaling factor was applied to the Johnson et al. equation to correct for liver size in a Chinese population, 36 but further analysis based on data from Wang et al. 37 and Li et al. 38 has shown that the scaling factor is only needed for boys (unpublished results of Pan X, Salem F, Johnson TN, et al., Certara UK limited, 2020). In our cohort, we could not confirm that race could affect liver volume in any consistent manner. In addition, boys have been reported to have larger livers 29 ; however, this appears to be a function of differences in body composition (i.e., boys have larger LBM or BSA), 39 and when these covariates are accounted for, sex differences are not apparent. 15 We did not observe differences in liver volumes, when predicted as a function of BSA, for boys versus girls in dataset 1, whereas boys had larger livers at larger BSAs and smaller livers at smaller BSAs than girls in dataset 2. Thus, the primary determinant of liver volume appears to be body size, as best described by BSA.
A potential limitation of this study is that we assessed liver volume, as opposed to mass. However, organ volume measurement is readily available in vivo, which is important for developing clinically applicable PBPK models. Organ volumes have been noted to be good predictors of organ mass and function, and densities allow conversion from volume to mass (1.08 for liver). However, it is possible that these densities may not be consistent across normal, overweight, and obese weight groups as increases in free fat content in obese individuals could alter tissue density. As such, further research into potential variability in organ density as a consequence of obesity is warranted for optimal obesity PBPK model development.
Drug dosages in children are often prescribed according to BSA, weight, or LBM. 40 It is often uncertain if adjustments to dosing algorithms need to be made for children with obesity. This study suggests that liver size correlates well with both BSA and LBM. Because liver size is associated with drug metabolizing capacity in the liver, it appears that either measure may appropriately account for liver functionality and thus hepatic clearance. However, this study was not designed to address the cellular composition of liver volumes. Excess deposits of adipose tissue, which may not be as metabolically active as hepatocytes, may contribute to the volumes of livers of children with overweight or obesity and thus increases in metabolic activity may not be directly proportional. However, weight gain and obesity affect many systems in the body, where they may exert differential effects and extrapolation of our observations beyond the liver warrants caution. Because PBPK models may use liver volumes as critical scalars to estimate drug clearance and concentrations, this study suggests that liver volume estimations as a function of BSA do not need adjustment for weight class, whereas estimations as a function of age may require additional anthropometric input to accurately estimate drug concentrations in children with overweight or obesity.
CONCLUSIONS
The relationship previously developed to estimate liver volume as a function of BSA (LV = 722*BSA1.176) in children applies to children of all weight status, including overweight and obesity.
Reliability of this liver volume Equation 2 is replicated across different imaging modalities and volume‐determining methodology.
DISCLAIMER
As an Associate Editor of Clinical and Translational Science, Valentina Shakhnovich was not involved in the review or decision process for this paper.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
C.H.C., S.S.C., A.R., N.M., T.N.J., J.S.L., and V.S. wrote the manuscript. C.H.C., S.S.C., A.R., N.M., J.S.L., and V.S. designed the research. C.H.C., V.S., C.S.F., A.R., V.W., E.S., D.O., J.R., and N.M. performed the research. C.H.C., T.N.J., J.S.L., and V.S. analyzed the data.
Supporting information
Fig S1
Fig S2
Funding information
This work was supported in part by the George Ferry Young Investigator Development Award from the NASPGHAN foundation (V.S. and P.I.), a career development award from NIDDK (5K23DK115827; V.S. and P.I.), and a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# UL1TR002366) with V.S. as recipient. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
REFERENCES
- 1. Grimstein M, Yang Y, Zhang X, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration's Office of Clinical Pharmacology. J Pharm Sci. 2019;108:21‐25. [DOI] [PubMed] [Google Scholar]
- 2. Templeton IE, Jones NS, Musib L. Pediatric dose selection and utility of PBPK in determining dose. AAPS J. 2018;20:31. [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solmi F, Morris S. Association between childhood obesity and use of regular medications in the UK: longitudinal cohort study of children aged 5–11 years. BMJ Open. 2015;5:e007373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamei M. Recent advances in development and application of Physiologically‐Based Pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2:161‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005;4:825‐833. [DOI] [PubMed] [Google Scholar]
- 7. Rostami‐Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6:140‐148. [DOI] [PubMed] [Google Scholar]
- 8. Barter ZE, Chowdry JE, Harlow JR, et al. Covariation of human microsomal protein per gram of liver with age: absence of influence of operator and sample storage may justify interlaboratory data pooling. Drug Metab Dispos. 2008;36:2405‐2409. [DOI] [PubMed] [Google Scholar]
- 9. Barter ZE, Bayliss MK, Beaune PH, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8:33‐45. [DOI] [PubMed] [Google Scholar]
- 10. Jamei M, Dickinson GL, Rostami‐Hodjegan A. A framework for assessing inter‐individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of bottom‐up vs top‐down recognition of covariates. Drug Metab Pharmacokinet. 2009;24:53‐75. [DOI] [PubMed] [Google Scholar]
- 11. Johnson TN, Rostami‐Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931‐956. [DOI] [PubMed] [Google Scholar]
- 12. Small BG, Wendt B, Jamei M, Johnson TN. Prediction of liver volume ‐ a population‐based approach to meta‐analysis of paediatric, adult and geriatric populations ‐ an update. Biopharm Drug Dispos. 2017;38:290‐300. [DOI] [PubMed] [Google Scholar]
- 13. Malpique R, Bassols J, Lopez‐Bermejo A, et al. Liver volume and hepatic adiposity in childhood: relations to body growth and visceral fat. Int J Obes (Lond). 2018;42(1):65‐71. [DOI] [PubMed] [Google Scholar]
- 14. Joshi M, Dillman JR, Singh K, et al. Quantitative MRI of fatty liver disease in a large pediatric cohort: correlation between liver fat fraction, stiffness, volume, and patient‐specific factors. Abdom Radiol (NY). 2018;l43(5):1168‐1179. [DOI] [PubMed] [Google Scholar]
- 15. Johnson TN, Tucker GT, Tanner MS, Rostami‐Hodjegan A. Changes in liver volume from birth to adulthood: a meta‐analysis. Liver Transpl. 2005;11:1481‐1493. [DOI] [PubMed] [Google Scholar]
- 16. Pomposelli JJ, Tongyoo A, Wald C, Pomfret EA. Variability of standard liver volume estimation versus software‐assisted total liver volume measurement. Liver Transpl. 2012;18:1083‐1092. [DOI] [PubMed] [Google Scholar]
- 17. Jackowski C, Thali MJ, Buck U, et al. Noninvasive estimation of organ weights by postmortem magnetic resonance imaging and multislice computed tomography. Invest Radiol. 2006;41:572‐578. [DOI] [PubMed] [Google Scholar]
- 18. Tang H, Vasselli JR, Wu EX, Boozer CN, Gallagher D. High‐resolution magnetic resonance imaging tracks changes in organ and tissue mass in obese and aging rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R890‐R899. [DOI] [PubMed] [Google Scholar]
- 19. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1‐190. [PubMed] [Google Scholar]
- 20. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bi C, Leeder JS. Large‐Scale Computation of Pediatric Growth Percentiles with Fuzzy Logic Justification of Parameter Selection. Proceedings of the 2012 IEEE Computational Intelligence Symposium on Bioinformatics & Computational Biology (CIBCB). 2012: 43‐46. [Google Scholar]
- 22. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height‐weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62‐66. [DOI] [PubMed] [Google Scholar]
- 23. Dubois D, Dubois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863‐871. [Google Scholar]
- 24. Peters AM, Snelling HL, Glass DM, Bird NJ. Estimation of lean body mass in children. Br J Anaesth. 2011;106:719‐723. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Sallami HS, Goulding A, Grant A, et al. Prediction of Fat‐Free Mass in Children. Clin Pharmacokinet. 2015;54:1169‐1178. [DOI] [PubMed] [Google Scholar]
- 26. Park CW, Yu N, Yun SW, et al. Measurement and estimation of renal size by computed tomography in Korean children. J Korean Med Sci. 2017;32:448‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lizarraga‐Mollinedo E, Martinez‐Calcerrada JM, Padros‐Fornieles C, et al. Renal size and cardiovascular risk in prepubertal children. Sci Rep. 2019;9:5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer‐Gerspach AC, Peterli R, Moor M, et al. Quantification of liver, subcutaneous, and visceral adipose tissues by MRI before and after bariatric surgery. Obes Surg. 2019;29:2795‐2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gils Contreras A, Bonada Sanjaume A, Montero Jaime M, et al. Effects of two preoperatory weight loss diets on hepatic volume, metabolic parameters, and surgical complications in morbid obese bariatric surgery candidates: a randomized clinical trial. Obes Surg. 2018;28:3756‐3768. [DOI] [PubMed] [Google Scholar]
- 30. Haddad S, Restieri C, Krishnan K. Characterization of age‐related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Health A. 2001;64:453‐464. [DOI] [PubMed] [Google Scholar]
- 31. Kanamori M, Takahashi H, Echizen H. Developmental changes in the liver weight‐ and body weight‐normalized clearance of theophylline, phenytoin and cyclosporine in children. Int J Clin Pharmacol Ther. 2002;40:485‐492. [DOI] [PubMed] [Google Scholar]
- 32. Noda T, Todani T, Watanabe Y, Yamamoto S. Liver volume in children measured by computed tomography. Pediatr Radiol. 1997;27:250‐252. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi H, Ishikawa S, Nomoto S, et al. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68:541‐555. [DOI] [PubMed] [Google Scholar]
- 34. Heinemann A, Wischhusen F, Puschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366‐368. [DOI] [PubMed] [Google Scholar]
- 35. Davidson LE, Kelley DE, Heshka S, et al. Skeletal muscle and organ masses differ in overweight adults with type 2 diabetes. J Appl Physiol. 2014;1985(117):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barter ZE, Tucker GT, Rowland‐Yeo K. Differences in cytochrome p450‐mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2013;52:1085‐1100. [DOI] [PubMed] [Google Scholar]
- 37. Wang J, Li B, Chen R. Reference values of main internal organs for Chinese. Chinese J Radiol Med Protection. 1995;15:248‐254. [Google Scholar]
- 38. Li GF, Zheng QS, Yu Y, et al. Impact of ethnicity‐specific hepatic microsomal scaling factor, liver weight, and cytochrome P450 (CYP) 1A2 content on physiologically based prediction of CYP1A2‐mediated pharmacokinetics in young and elderly Chinese adults. Clin Pharmacokinet. 2019;58:927‐941. [DOI] [PubMed] [Google Scholar]
- 39. Schmidt IM, Mølgaard C, Main KM, Michaelsen KF. Effect of gender and lean body mass on kidney size in healthy 10‐year‐old children. Pediatr Nephrol. 2001;16:366‐370. [DOI] [PubMed] [Google Scholar]
- 40. Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292‐307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


