Abstract
Calcium silicate cements (CSCs) are the choice materials for vital pulp therapy because of their bioactive properties, promotion of pulp repair, and dentin bridge formation. Despite the significant progress made in understanding CSCs’ mechanisms of action, the key events that characterize the early interplay between CSC-dentin-pulp are still poorly understood. To address this gap, a microfluidic device, the “tooth-on-a-chip,” which was developed to emulate the biomaterial-dentin-pulp interface, was used to test 1) the effect of CSCs (ProRoot, Biodentine, and TheraCal) on the viability and proliferation of human dental pulp stem cells, 2) variations of pH, and 3) release within the pulp chamber of transforming growth factor–β (TGFβ) as a surrogate of the bioactive dentin matrix molecules. ProRoot significantly increased the extraction of TGFβ (P < 0.05) within 24 to 72 h and, along with Biodentine, induced higher cell proliferation (P > 0.05), while TheraCal decreased cell viability and provoked atypical changes in cell morphology. No correlation between TGFβ levels and pH was observed. Further, we established a biofilm of Streptococcus mutans on-chip to model the biomaterial-biofilm-dentin interface and conducted a live and dead assay to test the antimicrobial capability of ProRoot in real time. In conclusion, the device allows for direct characterization of the interaction of bioactive dental materials with the dentin-pulp complex on a model of restored tooth while enabling assessment of antibiofilm properties at the interface in real time that was previously unattainable.
Keywords: stem cells, microfluidics, calcium silicate cements, growth factors, organs-on-a-chip, bioassays
Introduction
The tooth is a complex organ characterized by mineralized tissues surrounding the dental pulp, a highly vascularized and innervated connective tissue responsible for the tooth’s vital responses. Most of these responses are elaborated at the interface that forms between dentin and the pulp tissue, which has a unique configuration in the body, with odontoblasts forming a pseudostratified layer along the dentin walls (Fig. 1A). Dental caries is a biofilm-induced disease that destroys the mineralized dental tissues (Kassebaum et al. 2015). When fueled by dietary sugar, cariogenic bacteria such as Streptococcus mutans in the plaque biofilms produce acids, which decay the tooth structure, leading to cavitation and further dentin destruction, eventually infecting pulpal tissues (Fig. 1B; Bowen et al. 2018; Lamont et al. 2018) The treatment for decayed teeth involves the removal of the infected necrotic tissue, protection of the dentin-pulp complex with a pulp-capping material, and restoration of the tooth anatomy (Carvalho et al. 2016). Currently, some of the most effective options to protect the dentin-pulp complex involve the use of calcium silicate cements (CSCs; Fig. 1C). The ability of these types of cement to set in hydrated environments, along with their low solubility, high pH, good biocompatibility, bioactivity, and antimicrobial properties, positions these materials as the standard of care for various clinical indications, such as pulp capping (Çalışkan and Güneri 2017), root resorptions (Hansen et al. 2011), root perforations (Mente et al. 2014), and apexification (Bonte et al. 2015).
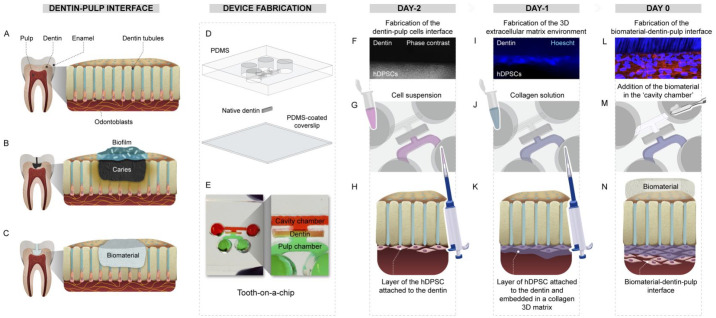
Figure 1.
Fabrication process of the “tooth-on-a-chip” and the biomaterial-dentin-pulp interface. (A) Dentin-pulp interface of a sound tooth. (B) Decayed tooth showing a biofilm-dentin-pulp interface. (C) After restoration, a biomaterial-dentin-pulp interface. (D) A polydimethylsiloxane (PDMS) mold, dentin, and a PDMS spin-coated coverslip are plasma bonded to assemble a device that has 2 chambers separated by a dentin fragment (E). To form a cell layer attached to the dentin (F), human dental pulp stem cells (hDPSCs) are seeded in the pulp side (G) and left to adhere to the dentin (H). After 24 h, to emulate the 3-dimensional environment for the cells, a solution of collagen was added onto the hDPSCs layer (I–K). On the following day, the pulp environment was well-formed (L), then a calcium silicate cement was placed on the opposite side of the dentin (M, N), emulating a pulp-capping treatment.
Biomaterials containing mineral trioxide aggregate (MTA) belong to the CSC family, which have ProRoot as one of its most popular examples. They have gained extensive popularity because of their capacity to stimulate dentin repair and the formation of a mineral barrier in the pulp. However, many materials in this category have a long setting time and are difficult to handle (Dawood et al. 2017). To overcome the clinical disadvantages of MTA, another CSC product, Biodentine, was developed to have improved handling properties, reduced setting time (Dawood et al. 2017), and a high rate of Ca2+ release (Camilleri et al. 2014). TheraCal LC, another CSC, was later developed to be an easy-to-use light-curable cement that can be used as a liner in deep cavities or pulp exposures.
CSCs are mixed with water, and the setting reaction under such hydrated conditions produces a calcium silicate hydrate and calcium hydroxide (Camilleri 2011) releasing calcium ions (Gandolfi et al. 2015), which are responsible for alkalization of the pH, formation of apatite, and biocompatibility. Yet, as the setting reaction happens, it is challenging to evaluate the simultaneous effect of CSCs on dentin and dental pulp stem cells in real time using conventional cell culture systems or standard ISO dentin models, such as Boyden chambers, because both the cement and dentin are opaque, making it difficult or impossible to image the system. Moreover, the ability of CSCs to extract host growth factors from intact dentin (Cooper et al. 2010) and direct these and other bioactive molecules across the dentin barrier and into the pulp chamber, mostly as a function of time, remains difficult to quantify. Therefore, although the interactions between CSCs and pulp cells, CSCs and dentin, or CSCs and biofilm have been evaluated extensively (Laurent et al. 2012; Pedano et al. 2019), the critical interactions of CSCs with the dentin-pulp complex or dentin biofilm, including its simultaneous effect on dentin matrix molecules, microorganisms, and pulp cells, remain poorly understood.
Here, we used a recently developed tooth-on-a-chip model (França et al. 2020) to perform real-time analyses of the response of pulp cells to dental materials at the biomaterial-dentin-pulp interface. We tested the hypothesis that CSCs stimulate the release of dentin matrix molecules from intact dentin and stimulate pulp cells’ biological activity in a simulated pulp chamber. To test this hypothesis, we compared the effects of 3 different CSCs on the viability and proliferation of human dental pulp stem cells (hDPSCs) and investigated whether these events correlate with pH variations and the release of transforming growth factor–β (TGFβ) on-chip. Furthermore, we tested the antimicrobial activity of ProRoot against biofilms on-chip as a proof of concept to illustrate the capability of this device to serve as a platform to investigate the biological effects of CSC on a biomaterial-biofilm-dentin interface.
Materials and Methods
Fabrication of the Tooth-on-a-Chip
We first emulated the biomaterial-dentin-pulp interface on-chip (Fig. 1). To that end, a master mold was created from a 1-mm-thick sheet of polymethyl methacrylate (PMMA) using a laser cutter as previously published (França et al. 2020). The PMMA molds were used to make an impression on a transparent layer of polydimethylsiloxane (PDMS; Fig. 1D, E). The PDMS mold has 2 parallel channels, 2 perfusable chambers, and a central groove to fit the dentin. According to the institutional ethics committee guidelines, human dentin from third molars extracted for orthodontic reasons was used. Sound teeth were sectioned into fragments of 500 µm in thickness perpendicular to the dentin tubules. Subsequently, PDMS-positive mold and PDMS–spin-coated coverslips were plasma cleaned, and a dentin fragment was inserted into the groove of each PDMS mold. The fully assembled microdevice replicates the interface of dentin with the dental pulp on one side and the dental material with dentin on the other, thus forming 2 accessible chambers representing the “pulp side” and the “cavity side,” respectively (Fig. 1E). Details are in the Appendix.
Cell Culture and Device Seeding
hDPSCs (P3–6) were cultured in an odontogenic medium for 7 d. Dentin was treated with 17% ethylenediaminetetraacetic acid (EDTA) to remove the smear layer, then rinsed thoroughly with deionized water (DIW). A suspension with 105 hDPSCs was seeded onto the “pulp side” of dentin to form the pulp cell layer (Fig. 1F–H). On the following day, to emulate the dental pulp’s 3-dimensional (3D) extracellular matrix (ECM) environment, a solution of 1.5 mg/mL collagen I was inserted into the “pulp side” (Fig. 1I–K). Collagen gelated for 15 min, the reservoirs were filled with odontogenic medium, and the devices were incubated for 24 h (Fig. 1(I-K)). Afterward, to fabricate a biomaterial-dentin-pulp interface, we treated the opposite side of the dentin of cell-laden devices with ProRoot, Biodentine, or Theracal LC on day 0 (Fig. 1(L-N)). Devices with no CSC were used as controls. See the Appendix for details.
pH, Cell, and Molecular Analyses
Cell viability was determined using a live and dead assay on days 1 and 7. For morphology, cells were stained with actin red and DAPI on days 1 and 7 and imaged using a confocal microscope. We used TGFβ as a target surrogate protein marker to evaluate the potential of each cement to influence the cumulative release of bioactive molecules from the dentin matrix. Briefly, we assembled the biomaterial-dentin interface on the device (without cells), incubated the system at 37 °C, and collected the supernatant on the “pulp side” of the device at 6, 24, 48, 72 h and 7 d. Immediately after collection, we tested the pH and froze the aliquots at −80 °C. Samples were then tested for TGFβ using an enzyme-linked immunosorbent assay. See Appendix for details.
Bacteria and Biofilm Culture
S. mutans UA159 (ATCC 700610), a biofilm-forming and cariogenic human pathogen, was used to develop the biofilm model at the biomaterial-dentin-pulp interface. S. mutans was grown in ultrafiltered (10-kDa molecular-weight cutoff membrane) buffered tryptone-yeast extract broth (UFTYE; pH 7.0) supplemented with 1% (w/v) glucose at 37 °C and 5% CO2 to the mid-exponential phase before use. Each device had the dentin surface on the cavity side treated with filter-sterilized human saliva to form the salivary-acquired pellicle. The cavity chamber was inoculated with 105 colony-forming units/mL.S. mutans in UFTYE containing 1% (w/v) sucrose and the biofilm were grown for 19 h at 37 °C and 5% CO2 (Liu et al. 2018). The 3D architecture of the biofilm, bacterial viability, and the impact of biomaterial treatment were examined via confocal microscopy with live/dead fluorescence imaging and quantitative computational analyses (Ren et al. 2019). See the Appendix for details.
Statistics
All groups were done in quadruplicate. Results were analyzed using 2-way analysis of variance (ANOVA) followed by Tukey post hoc tests (α = 0.05) on GraphPad Prism 8.
Results
Cell Morphology at the Biomaterial-Dentin-Pulp Interface
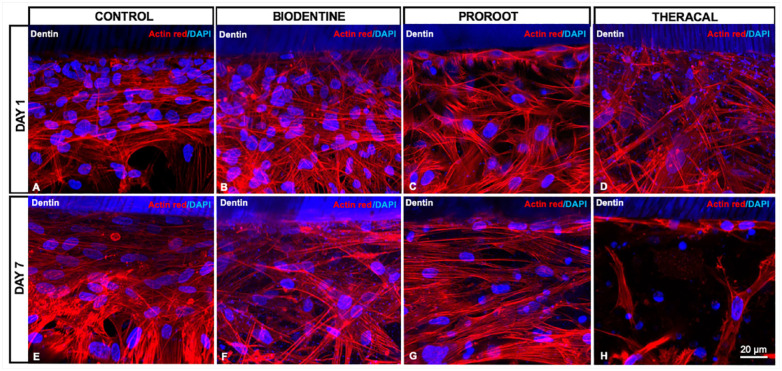
On day 1, a layer of cells juxtaposed to the dentin was observed in all groups, while the cells in Biodentine and ProRoot groups were similar to the control (Fig. 2A–C), the Theracal group had fewer cells and pyknotic nuclei (Fig. 2D).
Figure 2.
Human dental pulp stem cell (hDPSC) layer formation on-chip. On day 1, hDPSCs in all groups presented a similar morphology consisting of polygonal-shaped cells, densely packed at the dentin surface (A–D). On day 7, cells in the Biodentine (F) and ProRoot (G) groups were similar to the control in cell density and shape (A, E), whereas cells in the Theracal (H) group were fewer and mostly presenting atypical morphology.
On day 7, few cells remained at the Theracal-dentin-pulp interface (Fig. 2H), in contrast with the other groups that showed cells densely packed at the interface with the cytoskeleton parallel to the dentin surface (Fig. 2E–G). Moreover, cells from the Theracal group were randomly distributed. They had heterogeneous morphology, either with a large polygonal cytoplasm and extensive filopodia or elongated shape, with scarce cells juxtaposed with the dentin (Fig. 2H).
Cell Viability
To confirm the effect of CSC on the viability of hDPSCs at the dentin-pulp interface, we fabricated separate chips and stained cells with a live and dead stain on days 1 and 7. As early as day 1, it was observed that the dentin-pulp interface treated with ProRoot had more cells than the other groups did (Appendix Fig. 1A–D, I); however, the percentage of live cells among the groups was statistically similar.
Variation of pH and Release of Growth Factor from Dentinal Tissue into the Pulp Chamber
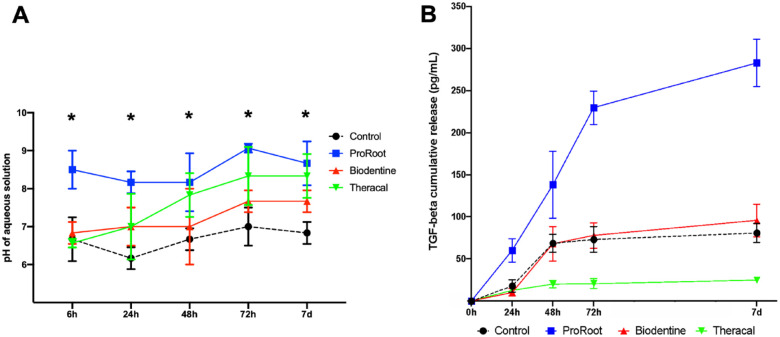
We investigated the pH variation and the release of TGFβ in the solution in contact with dentin inside the device’s pulp chamber. ProRoot promoted a pH of about 8 to 9 throughout the 7 d (Fig. 3A). At 6 h, Biodentine had a pH of about 6.8, which increased to 8 at 72 h and remained stable after 7 d. Theracal started at 6 h with a pH close to 6.5, rising to about 7.8 at 48 h and 8.3 from 72 h on. For the first 24 h, ProRoot had a higher pH, with the other groups being similar to Theracal. The pHs of ProRoot, Biodentine, and Theracal were statistically similar at 48, 72, and 7 d, and all were higher than in the control group (P < 0.05).
Figure 3.
pH measurements and transforming growth factor–β (TGFβ) release according to the calcium silicate cement (CSC). (A) At 6 h, the pulp chamber for the control group, Biodentine, and Theracal had a similar pH at about 6.5, whereas that of ProRoot had a pH between 8.0 and 9.0. The pH for Theracal rose daily and appeared to level off near 8.0 by the third day. The pH for ProRoot remained high and steady at about 8.0 to 9.0 throughout the experiment. On day 7, the CSC groups presented similar pH levels, all higher than in the control group. (B) For the 7 d of the experiment, dentin from chips treated with ProRoot steadily released more TGFβ than that exposed to the other materials (P < 0.05). At 24, 48, and 72 h, Biodentine and the control had a similar release of TGFβ. Theracal had the lowest release of TGFβ throughout the experiment (P < 0.05; 2-way analysis of variance with Tukey post hoc test, α = 0.05).
At 24 h, ProRoot showed the highest release of TGFβ, being about 3 to 4 times greater than the other groups (Fig. 3B). The release from ProRoot increased almost linearly with time until 72 h and showed a stable profile after that. The control and Biodentine groups had a similar increase in the TGFβ levels up to 48 h with a stable profile after that for the control and continued slight increases for Biodentine up to 7 d. Theracal, in contrast, showed low levels of TGFβ at all time points. Overall, ProRoot induced the highest levels of TGFβ release during all time points.
Effect of CSC on Biofilm
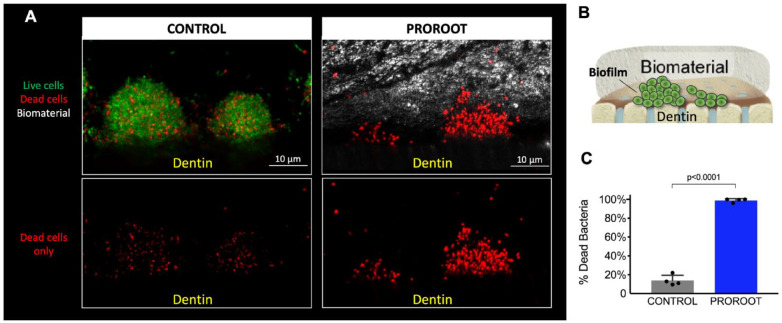
We developed a biofilm of S. mutans onto the “cavity side” of the dentin for 19 h (Fig. 4A) to simulate microbial infection at the biomaterial-dentin interface. S. mutans formed structured clusters of densely packed bacterial cells (typically found in intact biofilms) on the dentine surface (Fig. 4A). Next, the “cavity side” was restored with ProRoot by applying the cement at the interface, immediately followed by live and dead assay to determine the impact on bacterial viability across the biofilm structure (Fig. 4C). The application of ProRoot disrupted the integrity of the biofilm structure, likely by compressing the physical space (Fig. 4A; the cement is shown as gray color). Furthermore, we found that the bacterial cells within biofilms treated by ProRoot were mostly killed, both at the biofilm’s outer and inner layers (dead cells in red, >90%), likely due to the high-pH microenvironment created by ProRoot and the exposure to calcium ions released from the material (Salehi et al. 2015). In contrast, the bacteria in control biofilms are composed predominantly of live cells (in green).
Figure 4.
Modeling of the biomaterial-biofilm-dentin interface. (A) A biofilm of S. mutans was grown on-chip, emulating microbial infection at the interface, then treated with ProRoot, while an untreated group served as a control; green, red, and gray indicate live cells, dead cells, and the cement, respectively. (B) The diagram illustrated the biofilm grown on the dentin surface and capped with the biomaterial (ProoRoot). (C) Antimicrobial activity of ProRoot as determined by live and dead quantification assay (Student’s t test, α = 0.05).
Discussion
The tooth-on-a-chip can emulate the biomaterial-biofilm-dentin-pulp cell interface making it possible to mimic several clinical treatments of both sound and infected dentin while analyzing the structural organization of the microbial community, cell morphology, and biomaterial using high-resolution and time-resolved microscopy. Furthermore, it is possible to collect the supernatant to investigate the presence and type of cellular and microorganism secretome and dentin matrix molecules and correlate with morphological data of the biointerface. All this can be done spatiotemporally, by varying the biomaterial, microorganism of interest, time points, and dentin treatment, allowing for a systematic evaluation of the dynamic interactions that are occurring in situ. Ultimately, it will be possible to achieve an unprecedent understanding of how dentin, pulp cells, biofilms, and biomaterial affect one another, simultaneously using this platform, addressing key issues to the development of improved biomaterials or microbial dysbiosis.
There is a complex interaction between biomaterials placed in the dental cavity as a coronal barrier with stem cells and dentin in regenerative procedures. One of the essential aspects of this interaction is the ability of CSCs to promote the proliferation of hDPSCs. On day 7, all groups, except Theracal, had more than twice the cell number than on the first day (Appendix Fig. 1J), with no significant differences between Biodentine, ProRoot, and the control group (Appendix Fig. 1). Biodentine and ProRoot are known to induce hDPSC proliferation and differentiation (Athanasiadou et al. 2018). MTA elicits a continuous release of calcium ions in the cell medium, which is a potent proliferative stimulus to dental pulp cells and stem cells (Takita and Parirokh 2006). Moreover, cells respond to MTA and MTA extracts by secreting cytokines and growth factors related to cell proliferation, such as interleukin (IL)–1, IL-2, IL-6, and TGFβ (Torabinejad and Parirokh 2010). Conversely, previous studies have shown that Theracal promoted little proliferation of pulp fibroblast and low cell viability or migration of stem cells from deciduous teeth (Collado-González et al. 2017). Theracal was also associated with more inflammation and low bioactivity (Giraud et al. 2018), probably because it contains approximately 45% monomers; thus, free remaining monomers due to incomplete photopolymerization may injure pulp cells, hampering their viability (Gurcan and Seymen 2019). A technical consideration of the Theracal group is that the light-curing step, which is only applied to this material, may lead to some heat buildup during the 20 s of photopolymerization, potentially injuring the cells on-chip at a higher rate than cells in a native tooth. To exclude that hypothesis, we performed additional experiments, finding that light exposure does not affect cell viability (Appendix Fig. 2).
It is noteworthy that the model system used here further builds on our recent report (França et al. 2020). The current device includes a photopolymerized collagen hydrogel encapsulating dental pulp cells in a controllable 3D microenvironment as a natural scaffold, mimicking the dental pulp ECM and its proximity with the dentinal tissue (Prescott et al. 2008). Collagen is a significant component of the pulp ECM that works as a scaffolding with controllable porosity and physical properties to enable cell growth and proliferation (Fahimipour et al. 2018). During the optimization experiments, we observed that extending the fabrication process to 2 d, with a 24 h interval between cell seeding and collagen addition, was necessary to achieve the morphology of a consistent odontoblast-like layer interfaced with the dentin. Cells needed 24 h to adhere to dentin fully, and if collagen was added any sooner, the loading process tended to disrupt the cell layer. Experimental days were counted from the moment the dentin was treated with the biomaterial.
Potent angiogenic and vasculogenic growth factors, such as TGFβ (Smith et al. 2016) vascular endothelial growth factor (VEGF; Zhang et al. 2011) and fibroblast growth factor (Ferracane et al. 2013), have been identified in the dentin matrix. The TGF family has pleiotropic functions such as cell adhesion, cytoskeletal organization, migration, proliferation, differentiation, and chemotaxis (Subbiah et al. 2020). TGFβ can induce odontogenic cell migration and differentiation (Niwa et al. 2018). TGFβ is being studied as a surrogate of the release of a variety of bioactive molecules, and it is only one of several molecules involved, although it would be completely impractical to study all of these molecules concurrently; hence, the use of a surrogate marker (Smith et al. 2012; Mullaguri et al. 2016). CSCs have shown the potential to stimulate the release of TGFβ by dental pulp stem cells (Laurent et al. 2012; Mullaguri et al. 2016). Also, CSC produce elevated pH (Asgary et al. 2014) and calcium levels that can upregulate TGFβ genes in dental pulp cells (Mizuno and Banzai 2008). However, several questions remain unanswered regarding the interplay between CSC and dentin matrix.
It is known that growth factors can be extracted from powdered dentin (Ferracane et al. 2013; Salehi et al. 2016). Latent TGFβ within powdered dentin can be activated and solubilized by CSCs, because of their capacity to make the medium more alkaline (Tomson et al. 2017) or via collagen degradation (Huang et al. 2018). Our results showed that ProRoot elicited a steady release of TGFβ for 7 d, while control and Biodentine induced a release profile that peaked at 72 h, with detectable levels of TGFβ continuing for 7 d. Another study that also used dentin fragments treated with MTA or Biodentine to investigate the extraction of TGFβ for 14 d found that Biodentine promoted the highest extraction of TGFβ, whereas MTA and control groups were not significantly different (Wattanapakkavong and Srisuwan 2019). The apparent differences between these results and ours can be explained by the fact that our study evaluated a much earlier time point, and the temperature at which the devices were incubated was different from our study.
The fact that we treated the dentin with EDTA before placing the cements may have induced the release of the TGFβ in the control group, explaining the similar values with Biodentine. When the dentin was not treated with EDTA, ProRoot still induced the highest release of TGFβ, followed by Biodentine, with the control and Theracal releasing the lowest levels of TGFβ (Appendix Fig. 3). It is noteworthy that both conditions of our study (with and without EDTA) and other studies have detected the presence of TGFβ in untreated control groups, suggesting that dentin naturally releases low, but steady, levels of TGFβ in the dental pulp microenvironment (Wattanapakkavong and Srisuwan 2019). This is an essential aspect for future investigations on dentin healing behavior in the absence of dental materials. Overall, we could argue that the TGFβ early release within 24 h is an essential outcome to the pulp healing, which can be confirmed by the cell proliferation.
For the 3 CSCs tested, it was expected that the pH would rise in the pulp chamber because of the ions release and the patency of dentin tubules (Gandolfi et al. 2015). In the native pulp, there would be buffering effects within the pulp chamber that were not present in water, but since we wanted to test whether the release of TGFβ was related to pH or not, we incubated the chips in DIW. The rise in the pH levels is directly associated with calcium ions release. ProRoot was the fastest to raise the pH, suggesting the fastest ion release, probably because it has the slowest setting and is thus mostly soluble at 6 h. Interestingly, Theracal promoted a rise in the pH, which became similar to ProRoot at 48 h, even though Theracal is partially polymerized and likely less soluble than the others. Our findings relative to the pH levels corroborate others that found a constant calcium release for ProRoot, Theracal, and Biodentine, which was significantly higher than untreated dentin (Gandolfi et al. 2015).
Because ProRoot was the most potent material that promoted high pH in the microenvironment that could kill the bacteria, we decided to test the antibiofilm activity of this cement as a proof of concept to demonstrate the capacity of the tooth-on-a-chip to investigate the antibiofilm effects of CSCs at the biomaterial-biofilm-dentin interface. Our results suggest that the application of ProRoot as capping material could disrupt the structural integrity of the biofilms at the biomaterial-dentine interface and simultaneously kill the bacteria cells within. This proof-of-concept experiment paves the way for investigations to address several questions regarding the interplay of biomaterials, biofilm, dentin, and dental pulp cells in the interfaces were these interactions occur.
Conclusion
In summary, the tooth on-a-chip enables direct visualization and analyses of biomaterials interactions with dentin, pulp cells, as well as live biofilms. ProRoot was capable of inducing the highest release of TGFβ for 7 d, with significant antibiofilm activity, which will play a key role in the early proliferation and differentiation of pulp cells during tertiary dentin formation.
Author Contributions
N.S. Rodrigues, C.M. França, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Tahayeri, Z. Ren, contributed to data acquisition and analysis, drafted and critically revised the manuscript; V.P.A. Saboia, A.J. Smith, J.L. Ferracane, contributed to data analysis and interpretation, critically revised the manuscript; H. Koo, L.E. Bertassoni, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211016429 for Biomaterial and Biofilm Interactions with the Pulp-Dentin Complex-on-a-Chip by N.S. Rodrigues, C.M. França, A. Tahayeri, Z. Ren, V.P.A. Saboia, A.J. Smith, J.L. Ferracane, H. Koo and L.E. Bertassoni in Journal of Dental Research
Acknowledgments
We acknowledge expert technical assistance from Dr. Crystal Chaw in the Advanced Light Microscopy Core at the Jungers Center at Oregon Health & Science University.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by funding from the National Institute of Dental and Craniofacial Research (R01DE026170 and 3R01 DE026170-03S1 to L.E.B.), the Oregon Clinical & Translational Research Institute (OCTRI)—Biomedical Innovation Program (BIP), the Innovation in Oral Care Awards sponsored by GlaxoSmithKline (GSK), International Association for Dental Research (IADR), the OHSU Fellowship for Diversity and Inclusion in Research (OHSU-OFDIR to C.M.F.). H.K.’s work is supported by funding from the National Institute of Dental and Craniofacial Research (R01DE025220, R01DE018023, and R01DE025848). This work was conducted during a scholarship supported by the International Cooperation Program CAPES/PDSE. Financed by CAPES—Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil.
ORCID iDs: Z. Ren  https://orcid.org/0000-0002-3553-1958
https://orcid.org/0000-0002-3553-1958
L.E. Bertassoni  https://orcid.org/0000-0003-2732-8164
https://orcid.org/0000-0003-2732-8164
References
- Asgary S, Nazarian H, Khojasteh A, Shokouhinejad N. 2014. Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J Endod. 40(3):387–392. [DOI] [PubMed] [Google Scholar]
- Athanasiadou E, Paschalidou M, Theocharidou A, Kontoudakis N, Arapostathis K, Bakopoulou A. 2018. Biological interactions of a calcium silicate based cement (Biodentine™) with stem cells from human exfoliated deciduous teeth. Dent Mater. 34(12):1797–1813. [DOI] [PubMed] [Google Scholar]
- Bonte E, Beslot A, Boukpessi T, Lasfargues JJ. 2015. MTA versus Ca(OH)2 in apexification of non-vital immature permanent teeth: a randomized clinical trial comparison. Clin Oral Investig. 19(6):1381–1388. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26(3):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çalışkan MK, Güneri P. 2017. Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2- to 6-year follow-up. Clin Oral Investig. 21(1):357–367. [DOI] [PubMed] [Google Scholar]
- Camilleri J. 2011. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater. 27(8):836–844. [DOI] [PubMed] [Google Scholar]
- Camilleri J, Laurent P, About I. 2014. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 40(11):1846–1854. [DOI] [PubMed] [Google Scholar]
- Carvalho JC, Dige I, Machiulskiene V, Qvist V, Bakhshandeh A, Fatturi-Parolo C, Maltz M. 2016. Occlusal caries: biological approach for its diagnosis and management. Caries Res. 50(6):527–542. [DOI] [PubMed] [Google Scholar]
- Collado-González M, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Álvarez-Muro T, Lozano A, Forner L, Llena C, Moraleda JM, Rodríguez-Lozano FJ. 2017. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int Endod J. 50(suppl 2):e19–e30. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. 2010. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. 38(9):687–697. [DOI] [PubMed] [Google Scholar]
- Dawood AE, Parashos P, Wong RHK, Reynolds EC, Manton DJ. 2017. Calcium silicate-based cements: composition, properties, and clinical applications.J Investig Clin Dent. 8(2). doi: 10.1111/jicd.12195 [DOI] [PubMed] [Google Scholar]
- Fahimipour F, Dashtimoghadam E, Rasoulianboroujeni M, Yazdimamaghani M, Khoshroo K, Tahriri M, Yadegari A, Gonzalez JA, Vashaee D, Lobner DC, et al. 2018. Collagenous matrix supported by a 3D-printed scaffold for osteogenic differentiation of dental pulp cells. Dent Mater. 34(2):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracane JL, Cooper PR, Smith AJ. 2013. Dentin matrix component solubilization by solutions of pH relevant to self-etching dental adhesives. J Adhes Dent. 15(5):407–412. [DOI] [PubMed] [Google Scholar]
- França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL, Bertassoni LE. 2020. The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 20(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C. 2015. Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations.J Appl Biomater Funct Mater. 13(1):43–60. [DOI] [PubMed] [Google Scholar]
- Giraud T, Jeanneau C, Bergmann M, Laurent P, About I. 2018. Tricalcium silicate capping materials modulate pulp healing and inflammatory activity in vitro. J Endod. 44(11):1686–1691. [DOI] [PubMed] [Google Scholar]
- Gurcan AT, Seymen F. 2019. Clinical and radiographic evaluation of indirect pulp capping with three different materials: a 2-year follow-up study. Eur J Paediatr Dent. 20(2):105–110. [DOI] [PubMed] [Google Scholar]
- Hansen SW, Marshall JG, Sedgley CM. 2011. Comparison of intracanal Endosequence Root Repair Material and ProRoot MTA to induce pH changes in simulated root resorption defects over 4 weeks in matched pairs of human teeth. J Endod. 37(4):502–506. [DOI] [PubMed] [Google Scholar]
- Huang XQ, Camba J, Gu LS, Bergeron BE, Ricucci D, Pashley DH, Tay FR, Niu LN. 2018. Mechanism of bioactive molecular extraction from mineralized dentin by calcium hydroxide and tricalcium silicate cement. Dent Mater. 34(2):317–330. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. 2015. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 94(5):650–658. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Koo H, Hajishengallis G. 2018. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Camps J, About I. 2012. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 45(5):439–448. [DOI] [PubMed] [Google Scholar]
- Liu Y, Naha PC, Hwang G, Kim D, Huang Y, Simon-Soro A, Jung HI, Ren Z, Li Y, Gubara S, et al. 2018. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun. 9(1):2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mente J, Leo M, Panagidis D, Saure D, Pfefferle T. 2014. Treatment outcome of mineral trioxide aggregate: repair of root perforations-long-term results. J Endod. 40(6):790–796. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Banzai Y. 2008. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J. 41(11):933–938. [DOI] [PubMed] [Google Scholar]
- Mullaguri H, Suresh N, Surendran S, Velmurugan N, Chitra S. 2016. Role of pH changes on transforming growth factor-β1 release and on the fibrin architecture of platelet-rich fibrin when layered with biodentine, glass ionomer cement, and intermediate restorative material. J Endod. 42(5):766–770. [DOI] [PubMed] [Google Scholar]
- Niwa T, Yamakoshi Y, Yamazaki H, Karakida T, Chiba R, Hu JC, Nagano T, Yamamoto R, Simmer JP, Margolis HC, et al. 2018. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci Rep. 8(1):4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedano MS, Li X, Jeanneau C, Ghosh M, Yoshihara K, Van Landuyt K, About I, Van Meerbeek B. 2019. Survival of human dental pulp cells after 4-week culture in human tooth model. J Dent. 86:33–40. [DOI] [PubMed] [Google Scholar]
- Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A. 2008. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 34(4):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Kim D, Paula AJ, Hwang G, Liu Y, Li J, Daniell H, Koo H. 2019. Dual-targeting approach degrades biofilm matrix and enhances bacterial killing. J Dent Res. 98(3):322–330. [DOI] [PubMed] [Google Scholar]
- Salehi S, Cooper P, Smith A, Ferracane J. 2016. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent Mater. 32(3):334–342. [DOI] [PubMed] [Google Scholar]
- Salehi S, Davis HB, Ferracane JL, Mitchell JC. 2015. Sol-gel-derived bioactive glasses demonstrate antimicrobial effects on common oral bacteria. Am J Dent. 28(2):111–115. [PubMed] [Google Scholar]
- Smith AJ, Duncan HF, Diogenes A, Simon S, Cooper PR. 2016. Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J Endod. 42(1):47–56. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. 2012. Dentine as a bioactive extracellular matrix. Arch Oral Biol. 57(2):109–121. [DOI] [PubMed] [Google Scholar]
- Subbiah R, Hipfinger C, Tahayeri A, Athirasala A, Horsophonphong S, Thrivikraman G, França CM, Cunha DA, Mansoorifar A, Zahariev A, et al. 2020. 3D printing of microgel-loaded modular microcages as instructive scaffolds for tissue engineering. Adv Mater. 32(36):e2001736. [DOI] [PubMed] [Google Scholar]
- Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K. 2006. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 39(5):415–422. [DOI] [PubMed] [Google Scholar]
- Tomson PL, Lumley PJ, Smith AJ, Cooper PR. 2017. Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int Endod J. 50(3):281–292. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Parirokh M. 2010. Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod. 36(2):190–202. [DOI] [PubMed] [Google Scholar]
- Wattanapakkavong K, Srisuwan T. 2019. Release of transforming growth factor beta 1 from human tooth dentin after application of either proroot MTA or biodentine as a coronal barrier. J Endod. 45(6):701–705. [DOI] [PubMed] [Google Scholar]
- Zhang R, Cooper PR, Smith G, Nör JE, Smith AJ. 2011. Angiogenic activity of dentin matrix components. J Endod. 37(1):26–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211016429 for Biomaterial and Biofilm Interactions with the Pulp-Dentin Complex-on-a-Chip by N.S. Rodrigues, C.M. França, A. Tahayeri, Z. Ren, V.P.A. Saboia, A.J. Smith, J.L. Ferracane, H. Koo and L.E. Bertassoni in Journal of Dental Research