Abstract
Regenerative engineering has pioneered several novel biomaterials to treat critical-sized bone injuries. However, despite significant improvement in synthetic materials research, some limitations still exist. The constraints correlated with the current grafting methods signify a treatment paradigm shift to osteoinductive regenerative engineering approaches. Because of their intrinsic potential, inductive biomaterials may represent alternative approaches to treating critical bone injuries. Osteoinductive scaffolds stimulate stem cell differentiation into the osteoblastic lineage, enhancing bone regeneration. Inductive biomaterials comprise polymers, calcium phosphate ceramics, metals, and graphene family materials. This review will assess the cellular behavior toward properties of inductive materials.
Keywords: biomaterials, osteoinductive, bone regeneration, stem cell, graphene, osteoblast differentiation
Introduction
Clinical translation of laboratory-engineered inductive biomaterials relies on advances in bioengineering and material science (Khojasteh et al. 2013). Despite innovative progress, synthetic materials have not provided the crucial properties of an ideal material for bone and teeth regeneration (Beachy et al. 2020). Inductive materials have been shown to create a rich bioinductive environment and stimulate osseous differentiation of stem cells and teeth remineralization (Ten Cate 2001; Hosseini, Enderami, et al. 2019). In vivo, the bioinductivity of materials is confirmed upon implantation in nonosseous sites following ectopic bone formation along with remineralization of demineralized teeth (Amini et al. 2012). This review assesses the interaction between inductive biomaterials and cells.
Osteoinduction and Teeth Remineralization
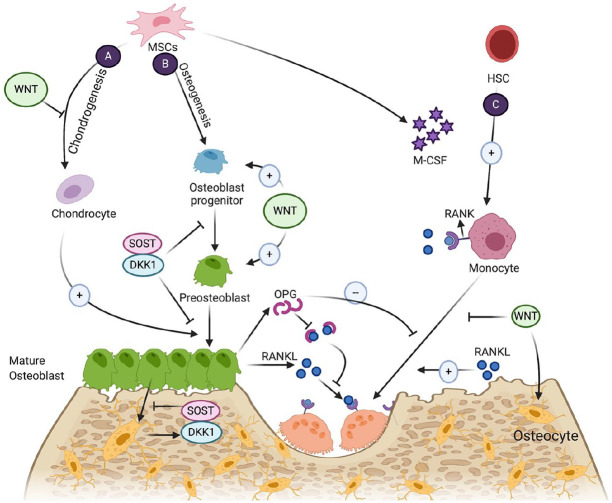
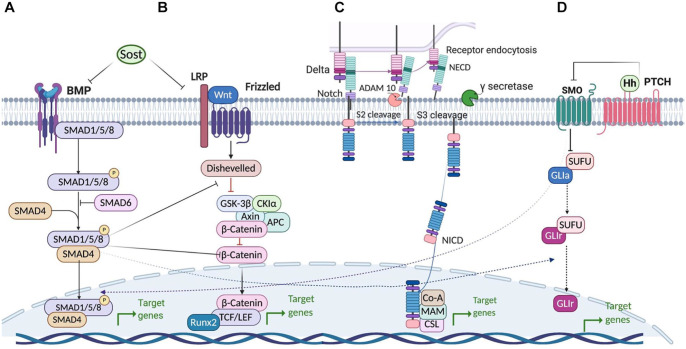
Bone and teeth are the most mineralized tissues in human body. Bone regeneration requires various cell lineages, including osteoblastic and osteoclastic lineages. The cross-talk between these cells regulates bone homeostasis (Botchwey et al. 2003; Harada and Rodan 2003). Different molecular and cellular signaling pathways induce differentiation of mesenchymal stem cells (MSCs) into preosteoblasts, osteoblasts, and osteocytes during osteogenesis (Fig. 1). Osteogenic differentiation of MSCs is regulated through many different molecules, including growth factors, morphogens, cytokines, hormones, and transcription factors. These molecules activate various signaling pathways (i.e., Wnt signaling [Hosseini, Soleimanifar, et al. 2019], Hedgehog signaling [Zhang et al. 2020], bone morphogenic protein [BMP] signaling [Wang et al. 2019] and Notch signaling [Zanotti and Canalis 2016]) to modulate osteogenesis and bone repair (Huang et al. 2014; Chen et al. 2016). Pathways involved in osteogenesis are described in detail in Figure 2.
Figure 1.
Cells and cytokines involved in bone homeostasis. (A) Endochondral ossification via osteoblast differentiation. (B) Intramembranous ossification via osteoblast differentiation. (C) Osteoclastogenesis of monocytes from hematopoietic stem cells (HSCs) to mature osteoclasts. MSC, mesenchymal stem cell.
Figure 2.
Bone homeostasis regulatory pathways. (A) Bone morphogenic protein signaling pathway. (B) Wnt signaling pathway. (C) Notch signaling pathway. (D) Hedgehog signaling pathway.
Osteoclastogenesis represents mononuclear hematopoietic stem cells (HSCs) differentiation to bone marrow–derived macrophages and further into osteoclasts. This process is regulated by macrophage colony-stimulating factor, receptor activator of NF-kappaB ligand (RANKL), receptor activator of NF-kappaB (RANK), and osteoprotegerin (OPG). The cross-talk between osteoclasts and osteoblasts is regulated by the immune and vascular systems. For instance, in response to inflammation, macrophages secrete interleukin (IL)–6 and tumor necrosis factor–alpha (TNF-α), which further stimulates the expression of RANKL by T cells. The soluble RANKL induces osteoclast precursor cells to express RANK and differentiate into osteoclasts. In contrast, endothelial cells secrete IL-13 and IL-4, which further induce stromal cells to expose OPG and suppress osteoclastogenesis (Jacome-Galarza et al. 2019).
The intrinsic osteoinductivity of bone allows for repair and regeneration via osteogenic proteins present in the extracellular matrix (osteonectin, bone sialoprotein, and BMP2). The mechanism of bone induction is not well known, yet the sequence of events may follow the natural regeneration process (Kasir et al. 2017).
Similar to the calcified composition of bone, dentin is composed of collagen-reinforced apatite fibrils comprising the same crystallographic orientation of minerals. The inductive factors secreted by epithelial cells derived from the neural crest cause the differentiation of dental papilla cells to odontoblasts. The odontoblasts secrete collagenous matrix, mostly composed of type I collagen and noncollagenous proteins, such as dentin phosphoproteins, dentin sialoprotein, and dentin matrix protein 1. Mineralization initiates and proceeds by dentin phosphoprotein binding to collagen bundles and originates apatite crystal formation (Goldberg and Smith 2004).
Although demineralization and remineralization are balanced during the entire life of the teeth, in pathological conditions such as the production of acid-fermented carbohydrates by bacteria, demineralization outweighs remineralization. The degradation of collagen fibrils resulting from endogenous cysteine cathepsin and metalloproteinase leads to the progression of carious lesions and weakened mechanical properties of dentin (Tjäderhane et al. 2013). Using bioinductive materials such as the polymer-induced liquid-precursor technique, the remineralized lesion reinstates the intrafibrillar mineral composition of healthy dentin, restoring the elastic modulus of the mineralized dentin (Burwell et al. 2012).
Cellular and Molecular Characteristics of Osteoinductive Materials
Calcium Ions
Calcium (Ca2+) ions stimulate bone mineralization by inducing osteoblastic differentiation markers such as Runt-related transcription factor–2, bone sialoprotein, osteocalcin, osteopontin, alkaline phosphatase (ALP), BMPs, and collagen-I (COL-I). The extracellular gradients of Ca2+ ions act as chemotactic signals and induce bone marrow MSCs and preosteoblast migration in a dose-dependent manner; the higher the concentration, the greater the stem cell maturation and bone regeneration (González-Vázquez et al. 2014).
Ca2+ ions enter the cell via a calcium-sensing receptor (CaSR) and calcium channels. The increased concentration of Ca2+ inside the cell activates the Ca2+/calmodulin-dependent protein kinase type II subunit alpha pathway and induces MSC osteoblastic differentiation through the extracellular signal–regulated kinase1/2 (ERK1/2) and cAMP response element-binding protein pathways. The activation of ERK1/2 results in elevated bone synthesis. Moreover, calcium ions activate the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway in osteoblasts, increasing the life span of the osteoblasts (Yan et al. 2017). The osteoinductive mechanism of Ca2+ ions investigated by microarray analysis revealed that activation of mitogen-activated protein kinase (MAPK)/ERK (MEK1/2) is required for Ca2+ efficacy. Subsequently, after Ca2+ enters into the cytoplasm, the ethanolamine kinase, protein kinase C, and ERK1/2 pathways are activated. Ca2+ then enters the nucleus initiating activator protein–1 and induces Fos gene expression, resulting in BMP-2 upregulation and elevated bone regeneration (Barradas et al. 2012).
The transcellular transport of Ca2+, as the only crucial ion of dentinogenesis, is controlled by calcium channels, Na+/Ca2+ channels, intracellular Ca2+-binding proteins, CaSRs, and ATPase channels, along with extracellular Ca2+ ions support mineralization front of predentin to mature mineralized dentin (Linde and Lundgren 2003). After exposure to increased levels of Ca2+ ions, the number of CaSRs increases on human dental pulp stem cells, causing odontoblastic differentiation. Furthermore, Ca2+ elevate ERK1/2 phosphorylation and dentin mineralization (Mizumachi et al. 2017).
Phosphate Ions
Bone phosphate ions (PO43−) are combined with calcium ions as calcium phosphate (Ca-P) and trigger mineralization-associated genes in osteoblasts via protein kinase C–dependent and ERK1/2 pathways, increasing the expression of BMPs. Moreover, phosphate ions repress osteoclastogenesis through the inhibition of RANK ligand and its receptor, decreasing the bone resorption rate. ALP actively hydrolyzes inorganic pyrophosphate ions to inorganic phosphate ions, increasing the mineralization of collagen type I. Phosphate ions enter the cell by solute carrier family 20 member1, a phosphate transporter protein that acts as an autocrine signaling molecule and joins in adenosine triphosphate synthesis, elevating osteoblastogenesis (Shih et al. 2014). In summary, Ca-P ions stimulate MSCs to express BMP-2 along with other signaling molecules, resulting in osteogenesis and new bone formation.
The extracellular phosphate ions are a key regulator of dentin mineralization through increased BMP-2 expression at the level of both promoter activity and mRNA/protein expression in human dental pulp stem cells. The elevated BMP-2 expression is dependent on the cAMP/protein kinase A pathway. Moreover, a high phosphate ion level independent of cAMP pathway phosphorylates the ERK1/2 pathway, resulting in differentiated odontoblasts and excessive mineralization (Tada et al. 2011).
Inductive Biomaterial Properties Influencing the Cellular Response
The osteoinductivity of materials is supported by ectopic bone formation after implantation in nonosseous sites (e.g., intramuscularly or subcutaneously) without the addition of any osteogenic growth factor (Ameer 2020). In vivo, osteoinductive materials promote de novo ectopic bone formation by inducing stem cell differentiation to the osteoblast lineage (Appendix Fig. S1).
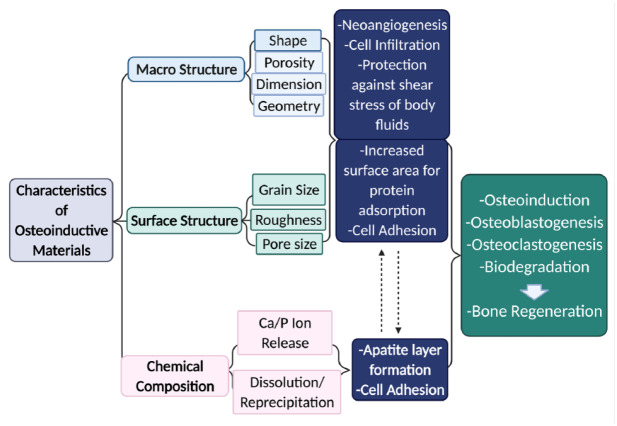
The chemical, structural, and physical properties of osteoinductive material can influence cellular responses (Nelson et al. 2018). Properties such as stiffness, geometry, pore size, grain size, roughness, degradation rate, concentration of released ions, and surface composition modify cellular behavior. Figure 3 summarizes the effect of the characteristics of different materials on osteoinductivity. Porosity (i.e., pore shape, pore size, porosity, and interconnectivity) influences cell behavior during osteogenesis, as it resembles the structure of cancellous bone, and it promotes neovascularization (Butler et al. 2009). The optimal interconnected pore size differs in the range of 100 µm to 200 µm, whereas the desired diameter of the pores varies from 200 µm to 500 µm, leading to the ideal porosity range of 40% to 80%. An appropriate portion of porosity facilitates the mass transport of oxygen and nutrients, cell adhesion, early neovascularization, and bone ingrowth into the scaffold (Deng et al. 2008). Table 1 shows current osteoinductive biomaterials and their advantages and disadvantages in bone regenerative engineering.
Figure 3.
Characteristics of osteoinductive materials influencing the cellular response.
Table 1.
Advantages and Disadvantages of Osteoinductive Grafting Material for Bone Regeneration.
| Osteoinductive Material | Example | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|---|
| Polymers | Synthetic | Poly-HEMA | Osteoinductive | Weak mechanical strength | Kumbar et al. 2006 |
| Bioactive | Degradation products | ||||
| Biodegradable | Reduce pH | ||||
| Biocompatible | |||||
| Ceramics | Calcium phosphate | HA | Biocompatible | Brittle | Bezerra et al. 2017 |
| β-TCP | Biodegradable | Unpredictable degradation rate | Davison et al. 2014 | ||
| BCP | Bioactive | Weak fracture strength | Raucci et al. 2017 | ||
| Osteoinductive | Filgueiras et al. 1993 | ||||
| Osteoconductive | |||||
| Bioglass | Silicate | ||||
| Borate/borosilicate | |||||
| Metal | Titanium and its alloy | Advanced mechanical properties | Corrosion | Fujibayashi et al. 2004 | |
| Biocompatible | Toxicity | ||||
| Osteoinductive | Osteointegration | ||||
| GFMs | Calcium phosphate graphene | Osteoinductive | No data on long-term safety | Lee et al. 2007 | |
| Graphene oxide | Osteoconductive | Unknown biological effect | |||
| Reduced graphene oxide | Biocompatible | ||||
| Biodegradable | |||||
| High specific surface area | |||||
| Thermally conductive | |||||
| Flexible | |||||
| High intrinsic strength | |||||
| Hydrophilic | |||||
BCP, biphasic calcium phosphate; β-TCP, beta tricalcium phosphate, HA, hydroxyapatite; HEMA, hydroxyethyl methacrylate.
Polymers
Natural Polymers
Polymers are macromolecules consisting of repeated subunits that are covalently connected. Natural polymer scaffolds such as fibrin, collagen, chitosan, hyaluronic acid, and starch have outstanding biodegradability and biocompatibility, resembling the original extracellular matrix components. Although bioactive natural polymers interact with the host’s tissue and offer osteoconductivity, their osteoinductivity has not yet been reported (Jiang et al. 2015).
Natural polymers, such as collagen, which are precursors for dentin regeneration, provide nucleation sites for mineral deposition around them. Remineralization of dentin lesion via mineral replacement with COL-I establishes the normal properties of dentin. COL-I acts as a template for intrafibrillar mineralization. Using the polymer-induced liquid-precursor technique procedure, encapsulated liquid nano-poly anionic polymers facilitate dentin remineralization in a time-dependent manner. Applying the acidic polypeptides, the anionic polymer segregates Ca2+ ions and further sequesters phosphate ions, forming Ca-P prenucleation clusters and promote remineralization. Ultimately, the mineral replacement gradually recovers the demineralized lesions’ mechanical properties (Burwell et al. 2012; Niu et al. 2014).
Synthetic Polymers
The unique properties of synthetic polymers, such as control of their molecular weight, biodegradability, and physical, chemical, and mechanical properties, enable manufacturing of the desired polymeric scaffold (Kumbar et al. 2006).
Poly(hydroxyethyl methacrylate) (pHEMA) is a bioinert polymer. Cross-linked pHEMA is fabricated by polymerizing 2-hydroxyethyl methacrylate monomers using a crosslinker such as ethylene glycol dimethacrylate (Macková et al. 2017). Winter and Simpson (1969) showed the potential osteoinductivity of pHEMA in a pig model. The pHEMA sponge subcutaneously implanted was capable of ectopic bone formation. Filmon et al. (2002) used carboxymethylation to modify the neutral electrical surface of pHEMA. They showed that the addition of functionalized negative groups on the backbone of pHEMA represented a Ca2+ binding site. The mineral-nucleating potential of negatively charged pHEMA continuously generates an amorphous Ca-P layer that further induces bone mineralization (Filmon et al. 2002).
Ceramics
Calcium-Based Ceramics
The bioinductivity and biocompatibility of calcium-based ceramics relies on dissolving ratios of structural calcium and phosphate ions in body fluids. The underlying cellular behavior toward different properties of ceramic surfaces is not well established. The long-term cellular fate is dependent on primary cell adhesion events, ceramic degradation, and the release rate of Ca-P ions. The scaffolds’ surface–cellular interaction initiates a cascade of biological responses, including adsorption of proteins, ligand-receptor coupling, and signal transduction (Kasir et al. 2017). Moreover, ceramic surfaces participate in ion solid-solution equilibrium and form an amorphous zone at the tissue–implant interface. Ion exchange in the amorphous zone leads to the deposition of hydroxyapatite (HA) crystals on the implant surface and osseointegration. Osseointegration stabilizes the ceramic through the bone and transfers the mechanical load from the surrounding bone to the implant (Laurencin et al. 1999).
The topology of ceramic surfaces, such as roughness (determined by pore and grain size), porosity, crystallinity, dissolvability, phase composition, phase solubility, and surface energy, affects the rate of extracellular matrix protein adsorption, cell adhesion, proliferation, and new bone formation by osteoblasts (Sethuraman et al. 2007).
Ca-P degrades either by passive chemical dissolution or osteoclastic resorption. Surface topography and scaffold compositions modify cell-resorption degradation. The aqueous solubility (Ksp) of ceramics has an inverse relationship with the Ca-P ratio, crystal size, exterior area, purity, and stability. A higher solubility of Ca-P ceramic leads to increased osteoinductivity due to increased ion concentration, which favorably influences pH and increases protein adsorption, cell adhesion, and osteogenesis. For example, high-soluble biphasic calcium phosphate (BCP) ceramic is more osteoinductive than HA with lower solubility (Yuan et al. 2006). Moreover, environmental conditions such as temperature, acid level, and fluid convection directly modify the BCP’s dissolution (Gustavsson et al. 2012). Appendix Table S1 summarizes the Ca/P ratio and the solubility of various Ca-P ceramics.
Surface roughness plays a critical role in the fate of regenerated bone. The quality and quantity of ceramic cell adhesion depend on its surface roughness, positively influencing protein absorption (Dos Santos et al. 2008). Ceramic with greater surface roughness revealed higher ectopic bone formation. Moreover, increased roughness enhances osteoclast survival and tartrate-resistant acid phosphate (TRAP) activation, resulting in faster degradation (Zhang et al. 2015).
The bioactivity of Ca-P ceramic is influenced by porosity. Increased ceramic porosity elevates fluid body contact with the scaffold surface. Protein adsorption and dissolution rates are enhanced when pore sizes are between 20 µm and 500 µm, whereas ingrowth of the neovasculature and new bone mineralization occurs at 50 µm (Saiz et al. 2007). Ca-P exceeding 100 µm pore size tends to be brittle, has reduced impact resistance, and has weak tensile strength (Ambard and Mueninghoff 2006). Ca-P ceramics representing various crystalline phases are discussed further.
Hydroxyapatite
Hexagonal HA (Ca10[PO4]6[OH]2) is the primary inorganic compound of the Ca-P family and is a naturally derived class of calcium apatite in human bones and teeth. Modified HA composes 50% of volume and 70% of human bone weight with a Ca/P ratio of 1.67, known as a bone mineral. HA is the most stable Ca-P under various physiological states of the body, such as pH, temperature, and body fluid combinations (Deng et al. 2012).
The surface characteristics of hexagonal HA, such as roughness and pore size, influence cell behavior and osteogenesis. Surface topography provides a mineralizing nucleation site that directly affects cell response and collagen matrix formation and mineralization rate. Various sintering temperatures lead to different crystal sizes that influence pore size and surface roughness. Osteoblasts on macro-pore surfaces (50 µm to 700 µm) have stronger cell adhesion and proliferation, whereas micro-pore–sized HA (0.5 µm to 3 µm) upregulate early osteoblastic differentiation (Wang et al. 2013).
Particle size influences the cell pathways. The size of HA particles has been shown to elicit cellular internalization and subcellular distribution responses in human umbilical vein endothelial cells (HUVECs) in vitro. In a study by Qin et al., nano-HA (n-HA) particles were digested via clathrin- and caveolin-mediated endocytosis, whereas macropinocytosis remained the central pathway for micro-HA uptake. The n-HA resulted in downregulation of PI3K, suppressed the synthesis of nitric oxide, and reduced the angiogenic ability of HUVECs (Shi et al. 2017).
The surface wettability of HA plays a vital role in the cell response. The modified wettability of ceramic modulates protein adsorption, osteoblast differentiation, and proliferation. Moreover, HA polarization improves surface wettability and accelerates cell adhesion (Nakamura et al. 2016). Osteoblasts cultured on n-HA respond differently as compared with HUVECs. Li et al. (2018) showed that osteoblasts internalized and agglomerated HA nanocrystals into smaller particles that were further vacuolized by a multilayered membrane. Meanwhile, the internalization process altered the morphology of osteoblasts and showed a decrease in osteoblast gene expression (Li et al. 2018).
Topographical properties of n-HA induce various intracellular signaling pathways with cell-surface connections and enable proper replacement of newly regenerated bone once ceramic is degraded. The n-HA revealed the antiapoptotic impact on preosteoblasts by decreasing the Bcl2-associated X/B-cell lymphoma-2 (Bax/Bcl2) ratio. Furthermore, increased focal adhesion kinase and steroid receptor coactivator signaling transducers promote preosteoblasts. Bezerra and colleagues (2017) treated preosteoblasts with n-HA and showed elevated MAPK/ERK expression. The signal transduction pathway encouraged ALP activity and differentiation rate (Bezerra et al. 2017). Moreover, n-HA stimulated osteogenesis through the BMP/Smad signaling pathway. Bone marrow mesenchymal stem cells on n-HA express a high level of Smad genes (Smad1, Smad4, and Smad5; Wang et al. 2019).
Tricalcium phosphate
Tricalcium phosphate (TCP) (Ca3[PO4]2) is obtained either from the thermal conversion of Ca2+ deficient HA or as a result of heterogeneous solid-state reactions of acidic Ca-Ps with bases. Compared with HA, TCP is more porous, has a faster degradation rate, and has a lower compression yield strength (Bohner et al. 2020). TCP sintered by temperatures greater than 1,125 °C results in the formation of α-TCP with monoclinic space groups. Temperatures ranging from 900 °C to 1,100 °C result in β-TCP patterns with rhombohedral space groups (Horch et al. 2006). As compared with α-TCP, β-TCP is more biodegradable and promotes osteoblast and osteoclast adhesion, differentiation, and proliferation because of higher protein resorption. β-TCP’s high thermodynamic solubility and resorption rate provide excellent biodegradability and enable the proper replacement of newly regenerated bone once the ceramic is degraded (Eliaz and Metoki 2017). Structural topography can also be tailored to modify the osteoclast response on β-TCP. For instance, Davison et al. (2014) found that micro-structured β-TCP attenuated osteoclast survival and TRAP activation, whereas osteoclast cultured on sub-microstructured β-TCP resorbed the ceramic. Moreover, nanoporous β-TCP (0.1 nm to 0.5 nm) elevated osteoclast-mediated degradation (Davison et al. 2014).
β-TCP has unique qualities to induce differentiation of bone marrow stromal cells toward the osteogenic lineage. Fielding et al. (2019) found that silicon (Si) and zinc (Zn) doped with porous β-TCP ceramic increased late-stage osteoblast marker gene expression. In addition, immune cell interaction with biomaterials influenced osteogenic differentiation of osseous cells. β-TCP decreased macrophage-secreted proinflammatory cytokines, IL-6 and TNF-α, and increased osteogenic gene expression of Runt-related transcription factor–2 and bone sialoprotein (Sadowska et al. 2019). Conversely, researchers found that within 10 to 30 d, dental pulp stem cell adhesion was suppressed because of acidic pH resulting from α-TCP hydrolysis as well as initiation and growth of HA crystals (Safronova et al. 2020).
Biphasic calcium phosphate
BCP is a 2-phase bioceramic consisting of low-soluble osteoconductive HA and high-soluble osteoinductive TCPs sintered at greater than 700 °C. Different ratios of β-TCP/HA can be mixed by sintering the precipitation of calcium-deficient apatite of varying Ca/P ratios. The β-TCP/HA ratio can affect the chemical properties of BCP in vivo. For instance, the ceramic degradation rate relies on the β-TCP/HA ratio; increasing β-TCP/HA elevates the solubility of BCP (Eliaz and Metoki 2017). Notably, ceramic solubility influences the osteoclast resorption pattern by the distribution and shape of resorbed lacunae. Compared with pure β-TCP, osteoclasts cultured on BCP 25/75 (25% HA/75% β-TCP) enter the resorption phase quicker and lacunae appear larger and more connected (Yamada et al. 1997).
Calcium and phosphate ions discharged from the degradation of ceramic can induce osteogenic differentiation. Calcium ion elevation affects osteoblasts via increased calcium binding protein expression, allowing differentiation of MSCs to osteoblasts. Furthermore, increased inorganic phosphate ions act as a signaling molecule and excite the expression of genes such as osteopontin (Khoshniat et al. 2011). Modifying the β-TCP/HA ratio can control the biological properties of BCP through the resorption rate and regulated release of calcium and phosphate ions.
BCP sintered at 1,160 °C contains incomplete sintered HA particles that quickly release after implantation. These microparticles induce local inflammation, negatively affecting cells and downregulating osteogenesis. Interestingly, osteoblasts and fibroblasts also internalize microparticles (Fellah et al. 2010). In addition to chemical characteristics, structural topography can modify the osteoclast response on β-TCP. Yokozeki et al. (1998) found that microstructured β-TCP attenuated osteoclast survival and TRAP activation. Osteoclasts cultured on sub-microstructured β-TCP resorbed the ceramic. Moreover, nanoporous β-TCP (0.1 nm to 0.5 nm) elevated osteoclast-mediated degradation (Yokozeki et al. 1998).
Bioactive Glasses
Bioglass is a bioactive ceramic composed of silicon, calcium, sodium, and phosphate with outstanding biocompatibility and osteoinductivity. Bioglass biodegradation releases ions that change to active carbonated HA and promote osteogenesis. The formation of chemical bonds between bioglass-produced HA layers and the surrounding native bone enables bone-material integration (Kaur et al. 2014). Briefly, the exchange between bioglass sodium ions and hydrogen ions leads to the formation of the SiO2 layer on the surface of the bioglass. The Ca2+ and PO43− ions then move toward the surface of SiO2 and initiate an amorphous Ca-P layer. Finally, the amorphous Ca-P crystallizes with extra Ca-P ions by incorporating carbonate ion, forming polycrystalline hydroxycarbonate apatite. The hydroxycarbonate apatite directly binds to collagen in native bone. The mechanical bond strength between bioglass and bone remains equal, sometimes more potent than the native bone (Filgueiras et al. 1993).
Metals
The superior mechanical properties of metals make them suitable candidates for load-bearing orthopedic treatments, but a mismatch of force yield between native bone and metal may eventually drive to shielded stress, resorbed bone, and loose implants. Porous metals resemble the mechanical and morphological properties of the trabecular bone and facilitate early implant fixation via tissue ingrowth. Highly porous tantalum, known as trabecular metal, is used in hip arthroplasty because of advanced clinical results. Although tantalum is not osteoinductive, surface modification coating with tantalum oxide (Ta2O5) improves biocompatibility, anticorrosion, and osteoinductivity (Wawrzynski et al. 2017).
Titanium is the most commonly used metal scaffold for biomedical purposes because of osteoinductivity, biocompatibility, lightness, outstanding resistance to corrosion, and adequate strength. The surface area of titanium is covered with a thin layer of titanium oxide (TiO2), which promotes cell adhesion and osseointegration. The 3-dimensional surface of the microporous structure, the interconnectivity of the macroporous block, and the concentration of phosphate and calcium ions play a significant role in titanium’s osteoinductivity and lamellar bone formation (Fujibayashi et al. 2004).
Graphene Family of Materials
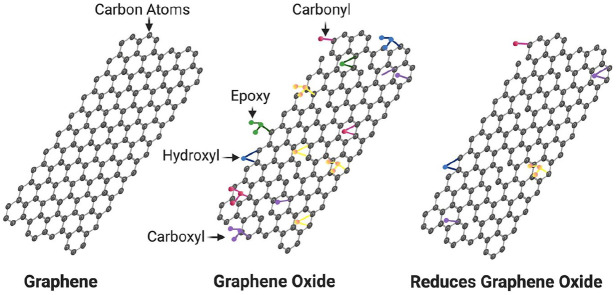
Graphene is a carbon allotrope consisting of an individual atomic thick 2-dimensional layer of tightly bonded carbon atoms in a honeycomb arrangement representing different molecular structural forms (Fig. 4). Graphene oxide (GO) is the most well-known form of graphene family of materials (GFMs) used for drug and gene delivery, biosensing, bioimaging, photodynamic therapy, and regenerative scaffolds. Enhanced biomineralization of GO relates to an oxygen functional group that adsorbs Ca2+, providing an apatite nucleation and growth site. The protein binding of GFMs facilitates excellent cellular adhesion and growth (Sydlik et al. 2015). Interestingly, a study by Wu et al. found high cellular attachment to GFMs in the absence of serum proteins via van der Waals or hydrogen bonding interactions. One proposed protein adsorption mechanism is the chemical bond between noncovalent π-π of GFMs and proteins in osteogenic media, facilitating osteoblast differentiation (Lee et al. 2011). Recently, Arnold et al. (2019) functionalized GO with PO43− through an Arbuzov reaction producing osteoinductive phosphate graphene. The phosphate graphene in vivo induced ectopic bone formation when implanted subcutaneously in mice (Arnold et al. 2019). The osteoinduction’s mechanism of graphene might pass through the activation of the PI3K/Akt/GSK-3β/β-catenin signaling pathway (Wu et al. 2018). In addition, GFM, as a biomaterial, is capable of precipitating apatite crystals on its surface and integrating with the surrounding bone. Ca2+ ions adsorb to the oxygen functional groups of GO through electrostatic interaction and promote nucleation sites for HA crystallization (Raucci et al. 2017).
Figure 4.
Molecular structures of different graphene family of materials (GFMs).
GFMs’ strong mechanical properties nominate them as superior scaffolds for strengthening the mechanical properties of brittle materials, such as bioglass (Wright et al. 2019). GO undergoes enzymatic and hydrolytic degradation through immune cell activity. Nevertheless, the long-term biodistribution and biosafety of GFMs remain a concern.
Conclusion and Future Perspectives
Despite regenerative therapeutic advancements, crucial tissue reconstruction challenges are still encountered when reconstructing critical-size bone defects (Clegg et al. 2017). With the discovery of osteoinductive materials, comprehensive studies have revealed the innate potential of materials to induce stem cells’ osteogenic differentiation (Abdel-Fattah et al. 2007). However, more research is required to expand our knowledge of these scaffolds’ optimum biocompatible-inductive properties. Our current knowledge of cell behavior toward inductive materials has granted opportunities for improving engineered osteoinductive biomaterials to provide optimal conditions for bone regeneration. Uncertainties about the underlying cellular and molecular mechanisms of inductive materials still exist and magnify the importance of further research to explore the unknowns in this field.
Author Contributions
F.S. Hosseini, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; L.S. Nair, C.T. Laurencin, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211010436 for Inductive Materials for Regenerative Engineering by F.S. Hosseini, L.S. Nair and C.T. Laurencin in Journal of Dental Research
Acknowledgments
www.Biorender.com was used to create the figures. The authors would also like to acknowledge the work of Joanne M. Walker, who contributed to reviewing and editing the manuscript.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge support from the National Institutes of Health (NIH), NIH PIONEER (DP1AR068147), for funding work by (C.T. Laurencin). Support from the Raymond and Beverly Sackler Foundation is gratefully acknowledged.
References
- Abdel-Fattah WI, Jiang T, El-Bassyouni GET, Laurencin CT. 2007. Synthesis, characterization of chitosans and fabrication of sintered chitosan microsphere matrices for bone tissue engineering. Acta Biomater. 3(4):503–514. [DOI] [PubMed] [Google Scholar]
- Ambard AJ, Mueninghoff L. 2006. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont. 15(5):321–328. [DOI] [PubMed] [Google Scholar]
- Ameer GA. 2020. Understanding and harnessing variability in regenerative engineering. Regen Eng Transl Med. 6(4):429–432. [Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. 2012. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 40(5):363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AM, Holt BD, Daneshmandi L, Laurencin CT, Sydlik SA. 2019. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc Natl Acad Sci U S A. 116(11):4855–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, Van de Peppel J, Van Leeuwen JP, Van Blitterswijk CA, De Boer J. 2012. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 33(11):3205–3215. [DOI] [PubMed] [Google Scholar]
- Beachy SH, Nair L, Laurencin C, Tsokas KA, Lundberg MS. 2020. Sources of variability in clinical translation of regenerative engineering products: insights from the national academies forum on regenerative medicine. Regen Eng Transl Med. 6(1):1–6. [Google Scholar]
- Bezerra F, Ferreira MR, Fontes GN, da Costa Fernandes CJ, Andia DC, Cruz NC, da Silva RA, Zambuzzi WF. 2017. Nano hydroxyapatite-blasted titanium surface affects pre-osteoblast morphology by modulating critical intracellular pathways. Biotechnol Bioeng. 114(8):1888–1898. [DOI] [PubMed] [Google Scholar]
- Bohner M, Santoni BLG, Döbelin N. 2020. Β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 113:23–41. [DOI] [PubMed] [Google Scholar]
- Botchwey EA, Pollack SR, El-Amin S, Levine EM, Tuan RS, Laurencin CT. 2003. Human osteoblast-like cells in three-dimensional culture with fluid flow. Biorheology. 40(1–3):299–306. [PubMed] [Google Scholar]
- Burwell AK, Thula-Mata T, Gower LB, Habeliz S, Kurylo M, Ho SP, Chien Y-C, Cheng J, Cheng NF, Gansky SA. 2012. Functional remineralization of dentin lesions using polymer-induced liquid-precursor process. PLoS One. 7(6):e38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DL, Goldstein SA, Guldberg RE, Guo XE, Kamm R, Laurencin CT, McIntire LV, Mow VC, Nerem RM, Sah RL. 2009. The impact of biomechanics in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 15(4):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X. 2016. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 23(7):1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JR, Wechsler ME, Peppas NA. 2017. Vision for functionally decorated and molecularly imprinted polymers in regenerative engineering. Regen Eng Transl Med. 3(3):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison NL, ten Harkel B, Schoenmaker T, Luo X, Yuan H, Everts V, Barrère-de Groot F, de Bruijn JD. 2014. Osteoclast resorption of beta-tricalcium phosphate controlled by surface architecture. Biomaterials. 35(26):7441–7451. [DOI] [PubMed] [Google Scholar]
- Deng M, Cushnie EK, Lv Q, Laurencin CT. 2012. Poly (lactide-co-glycolide)-hydroxyapatite composites: the development of osteoinductive scaffolds for bone regenerative engineering. MRS Online Proc Libr Arch. 1417:737. [Google Scholar]
- Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Krogman NR, Singh A, Allcock HR, Laurencin CT. 2008. Miscibility and in vitro osteocompatibility of biodegradable blends of poly [(ethyl alanato)(p-phenyl phenoxy) phosphazene] and poly (lactic acid-glycolic acid). Biomaterials. 29(3):337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos E, Farina M, Soares G, Anselme K. 2008. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J Mater Sci Mater Med. 19(6):2307–2316. [DOI] [PubMed] [Google Scholar]
- Eliaz N, Metoki N. 2017. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 10(4):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellah BH, Delorme B, Sohier J, Magne D, Hardouin P, Layrolle P. 2010. Macrophage and osteoblast responses to biphasic calcium phosphate microparticles. J Biomed Mater Res A. 93(4):1588–1595. [DOI] [PubMed] [Google Scholar]
- Fielding GA, Sarkar N, Vahabzadeh S, Bose S. 2019. Regulation of osteogenic markers at late stage of osteoblast differentiation in silicon and zinc doped porous TCP. J Funct Biomater. 10(4):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueiras MR, La Torre G, Hench LL. 1993. Solution effects on the surface reactions of a bioactive glass. J Biomed Mater Res. 27(4):445–453. [DOI] [PubMed] [Google Scholar]
- Filmon R, Grizon F, Basle M, Chappard D. 2002. Effects of negatively charged groups (carboxymethyl) on the calcification of poly (2-hydroxyethyl methacrylate). Biomaterials. 23(14):3053–3059. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S, Neo M, Kim H-M, Kokubo T, Nakamura T. 2004. Osteoinduction of porous bioactive titanium metal. Biomaterials. 25(3):443–450. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ. 2004. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 15(1):13–27. [DOI] [PubMed] [Google Scholar]
- González-Vázquez A, Planell JA, Engel E. 2014. Extracellular calcium and casr drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 10(6):2824–2833. [DOI] [PubMed] [Google Scholar]
- Gustavsson J, Ginebra M, Planell J, Engel E. 2012. Osteoblast-like cellular response to dynamic changes in the ionic extracellular environment produced by calcium-deficient hydroxyapatite. J Mater Sci Mater Med. 23(10):2509–2520. [DOI] [PubMed] [Google Scholar]
- Harada SI, Rodan GA. 2003. Control of osteoblast function and regulation of bone mass. Nature. 423(6937):349–355. [DOI] [PubMed] [Google Scholar]
- Horch H-H, Sader R, Pautke C, Neff A, Deppe H, Kolk A. 2006. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int J Oral Maxillofac Surg. 35(8):708–713. [DOI] [PubMed] [Google Scholar]
- Hosseini FS, Enderami SE, Hadian A, Abazari MF, Ardeshirylajimi A, Saburi E, Soleimanifar F, Nazemisalman B. 2019. Efficient osteogenic differentiation of the dental pulp stem cells on beta-glycerophosphate loaded polycaprolactone/polyethylene oxide blend nanofibers. J Cell Physiol. 234(8):13951–13958. [DOI] [PubMed] [Google Scholar]
- Hosseini FS, Soleimanifar F, Khojasteh A, Ardeshirylajimi A. 2019. Promoting osteogenic differentiation of human-induced pluripotent stem cells by releasing wnt/β-catenin signaling activator from the nanofibers. J Cell Biochem. 120(4):6339–6346. [DOI] [PubMed] [Google Scholar]
- Huang R, Yuan Y, Tu J, Zou G, Li Q. 2014. Opposing TNF-α/IL-1 β-and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 5(4):e1187–e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M. 2019. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 568(7753):541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Carbone EJ, Lo KW-H, Laurencin CT. 2015. Electrospinning of polymer nanofibers for tissue regeneration. Progr Polym Sci. 46:1–24. [Google Scholar]
- Kasir R, Vernekar VN, Laurencin CT. 2017. Inductive biomaterials for bone regeneration. J Mater Res. 32(6):1047. [Google Scholar]
- Kaur G, Pandey OP, Singh K, Homa D, Scott B, Pickrell G. 2014. A review of bioactive glasses: their structure, properties, fabrication and apatite formation. J Biomed Mater Res A. 102(1):254–274. [DOI] [PubMed] [Google Scholar]
- Khojasteh A, Behnia H, Hosseini FS, Dehghan MM, Abbasnia P, Abbas FM. 2013. The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report.J Biomed Mater Res B Appl Biomater. 101(5):848–854. [DOI] [PubMed] [Google Scholar]
- Khoshniat S, Bourgine A, Julien M, Weiss P, Guicheux J, Beck L. 2011. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci. 68(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbar SG, Nair LS, Bhattacharyya S, Laurencin CT. 2006. Polymeric nanofibers as novel carriers for the delivery of therapeutic molecules. J Nanosci Nanotechnol. 6(9–10):2591–2607. [DOI] [PubMed] [Google Scholar]
- Laurencin CT, Attawia M, Borden MD. 1999. Advancements in tissue engineered bone substitutes. Curr Opin Orthop. 10(6):445–451. [Google Scholar]
- Lee WC, Lim CHY, Shi H, Tang LA, Wang Y, Lim CT, Loh KP. 2011. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 5(9):7334–7341. [DOI] [PubMed] [Google Scholar]
- Li X, Zou Q, Li W, Chen H. 2018. Intracellular interaction of hydroxyapatite-based nanocrystals with uniform shape and traceable fluorescence. Inorg Chem. 57(21):13739–13748. [DOI] [PubMed] [Google Scholar]
- Linde A, Lundgren T. 2003. From serum to the mineral phase: the role of the odontoblast in calcium transport and mineral formation. Int J Dev Biol. 39(1):213–222. [PubMed] [Google Scholar]
- Macková H, Plichta Z, Hlidkova H, Sedláček O, Konefal R, Sadakbayeva Z, Dušková-Smrčková M, Horák D, Kubinová S. 2017. Reductively degradable poly (2-hydroxyethyl methacrylate) hydrogels with oriented porosity for tissue engineering applications. ACS Appl Mater Interfaces. 9(12):10544–10553. [DOI] [PubMed] [Google Scholar]
- Mizumachi H, Yoshida S, Tomokiyo A, Hasegawa D, Hamano S, Yuda A, Sugii H, Serita S, Mitarai H, Koori K. 2017. Calcium-sensing receptor-ERK signaling promotes odontoblastic differentiation of human dental pulp cells. Bone. 101:191–201. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Hori N, Ando H, Namba S, Toyama T, Nishimiya N, Yamashita K. 2016. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater Sci Eng C Mater Biol Appl. 62:283–292. [DOI] [PubMed] [Google Scholar]
- Nelson C, Khan Y, Laurencin CT. 2018. Nanofiber/microsphere hybrid matrices in vivo for bone regenerative engineering: a preliminary report. Regen Eng Transl Med. 4(3):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L-N, Zhang W, Pashley DH, Breschi L, Mao J, Chen J-H, Tay FR. 2014. Biomimetic remineralization of dentin. Dent Mater. 30(1):77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci M, Giugliano D, Longo A, Zeppetelli S, Carotenuto G, Ambrosio L. 2017. Comparative facile methods for preparing graphene oxide–hydroxyapatite for bone tissue engineering. J Tissue Eng Regen Med. 11(8):2204–2216. [DOI] [PubMed] [Google Scholar]
- Sadowska JM, Wei F, Guo J, Guillem-Marti J, Lin Z, Ginebra M-P, Xiao Y. 2019. The effect of biomimetic calcium deficient hydroxyapatite and sintered β-tricalcium phosphate on osteoimmune reaction and osteogenesis. Acta Biomater. 96:605–618. [DOI] [PubMed] [Google Scholar]
- Safronova T, Selezneva I, Tikhonova S, Kiselev A, Davydova G, Shatalova T, Larionov D, Rau J. 2020. Biocompatibility of biphasic α, β-tricalcium phosphate ceramics in vitro. Bioact Mater. 5(2):423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz E, Gremillard L, Menendez G, Miranda P, Gryn K, Tomsia AP. 2007. Preparation of porous hydroxyapatite scaffolds. Mater Sci Eng C. 27(3):546–550. [Google Scholar]
- Sethuraman S, Nair LS, El-Amin S, Nguyen MTN, Greish YE, Bender JD, Brown PW, Allcock HR, Laurencin CT. 2007. Novel low temperature setting nanocrystalline calcium phosphate cements for bone repair: osteoblast cellular response and gene expression studies. J Biomed Mater Res A. 82(4):884–891. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhou K, Huang F, Wang C. 2017. Interaction of hydroxyapatite nanoparticles with endothelial cells: internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int J Nanomed. 12:5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y-RV, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC. 2014. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci U S A. 111(3):990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydlik SA, Jhunjhunwala S, Webber MJ, Anderson DG, Langer R. 2015. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano. 9(4):3866–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Nemoto E, Foster BL, Somerman MJ, Shimauchi H. 2011. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone. 48(6):1409–1416. [DOI] [PubMed] [Google Scholar]
- Ten Cate J. 2001. Remineralization of caries lesions extending into dentin.J Dent Res. 80(5):1407–1411. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR. 2013. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 29(1):116–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang M, Chen F, Wei Y, Chen X, Zhou Y, Yang X, Zhu X, Tu C, Zhang X. 2019. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/Smad signaling pathway. Int J Nanomed. 14:7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xiao Z-W, Fan H-S, Zhang X-D. 2013. Fabrication of micro-grooved patterns on hydroxyapatite ceramics and observation of earlier response of osteoblasts to the patterns. Wuji Cailiao Xuebao (J Inorg Mater). 28(1):51–57. [Google Scholar]
- Wawrzynski J, Gil JA, Goodman AD, Waryasz GR. 2017. Hypersensitivity to orthopedic implants: a review of the literature. Rheumatol Ther. 4(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter GD, Simpson B. 1969. Heterotopic bone formed in a synthetic sponge in the skin of young pigs. Nature. 223(5201):88–90. [DOI] [PubMed] [Google Scholar]
- Wright Z, Arnold A, Holt B, Eckhart K, Sydlik S. 2019. Functional graphenic materials, graphene oxide, and graphene as scaffolds for bone regeneration. Regen Eng Transl Med. 5(2):190–209. [Google Scholar]
- Wu X, Zheng S, Ye Y, Wu Y, Lin K, Su J. 2018. Enhanced osteogenic differentiation and bone regeneration of poly(lactic-co-glycolic acid) by graphene via activation of PI3K/Akt/GSK-3Β/β-catenin signal circuit. Biomater Sci. 6(5):1147–1158. [DOI] [PubMed] [Google Scholar]
- Yamada S, Heymann D, Bouler J-M, Daculsi G. 1997. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials. 18(15):1037–1041. [DOI] [PubMed] [Google Scholar]
- Yan X, Wu H, Wu Z, Hua F, Liang D, Sun H, Yang Y, Huang D, Bian J-S. 2017. The new synthetic H2S-releasing SDSS protects MC3T3-E1 osteoblasts against H2O2-induced apoptosis by suppressing oxidative stress, inhibiting MAPKs, and activating the PI3K/Akt pathway. Front Pharmacol. 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki H, Hayashi T, Nakagawa T, Kurosawa H, Shibuya K, Ioku K. 1998. Influence of surface microstructure on the reaction of the active ceramics in vivo. J Mater Sci Mater Med. 9(7):381–384. [DOI] [PubMed] [Google Scholar]
- Yuan H, Van Blitterswijk C, De Groot K, de Bruijn JD. 2006. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods.J Biomed Mater Res A. 78(1):139–147. [DOI] [PubMed] [Google Scholar]
- Zanotti S, Canalis E. 2016. Notch signaling and the skeleton. Endocr Rev. 37(3):223–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Barbieri D, ten Hoopen H, de Bruijn JD, van Blitterswijk CA, Yuan H. 2015. Microporous calcium phosphate ceramics driving osteogenesis through surface architecture. J Biomed Mater Res A. 103(3):1188–1199. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fan J, Lee CS, Kim S, Chen C, Aghaloo T, Lee M. 2020. Apatite-binding nanoparticulate agonist of hedgehog signaling for bone repair. Adv Funct Mater. 30(12):1909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211010436 for Inductive Materials for Regenerative Engineering by F.S. Hosseini, L.S. Nair and C.T. Laurencin in Journal of Dental Research