Abstract
Aims
Prediabetes is a multifactorial condition. Current guidelines for diabetes screening recommend either the use of glycated hemoglobin (HbA1c), or blood glucose level (BGL). This research aimed to identify if p66shc a component of the Human SHC‐Transforming Protein 1 (Shc1), a mitochondrial associated oxidative stress biomarker, is significantly altered in patients with elevated BGL. Furthermore, we evaluated if inflammatory and oxidative stress markers, such as p66shc, are a useful addition to the regularly used biomarkers to increase sensitivity for identification of prediabetes.
Methods
All participants attended the Diabetic Health Screening at Charles Sturt University (CSU), Australia. The cross‐sectional clinical study collected demographic and clinical variables from 346 participants and classified into control or prediabetes based on fasting BGL. Blood and urine samples were analyzed for oxidative stress and inflammation markers. Logistic regression was used to compare multidimensional diagnostic models for prediabetes, including p66shc/Shc1, to the current HbA1c‐only model in terms of sensitivity, specificity and predictive accuracy. Significance was set at P ≤ 0.05.
Results
A significant decrease of p66shc/Shc1 was determined in prediabetes compared to controls (P ≤ 0.05). HbA1c testing resulted in an accuracy of 62%, while adding p66shc and triglycerides increased predictive accuracy to 88.05%. When HbA1c was omitted and Shc1 was combined with 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) and monocyte chemo‐attractant protein‐1 (MCP‐1), a predictive accuracy of 89.5% was achieved.
Conclusion
Our findings showed a major improvement of sensitivity to identify prediabetes by including oxidative stress and inflammatory biomarkers underlining beneficial diagnostic information, which most likely improves prevention and early treatment options in prediabetes.
Keywords: Inflammation, Oxidative stress, Prediabetes
SHC‐1 is associated with oxidative stress, which in conjunction with inflammatory markers is involved in the pathogenesis of prediabetes.
Introduction
Prediabetes and type 2 diabetes mellitus are multifactorial conditions affecting multiple organ systems 1 , 2 , 3 . Current guidelines for diabetes screening recommend either impaired fasting glucose, impaired glucose tolerance or glycated hemoglobin testing 4 . Applying these guidelines, prediabetes can be classified by impaired fasting glucose level (IFG) of 100–125 mg/dL (5.6–7 mmol/L), impaired glucose tolerance of 140–199 mg/dL (7.8–11.0 mmol/L) and/or glycated hemoglobin (HbA1c) level between 5.7% and 6.4% 5 . Current clinical screening usually includes only blood glucose level (BGL) or HbA1c 4 , 6 . This has been shown to underestimate the presence of prediabetes or type 2 diabetes mellitus and only provides limited information about the complex pathophysiology associated with disease progression 7 . Knowledge of the presence of oxidative stress and inflammatory processes, forming a complex pattern of disease progression, is therefore an important step towards early, effective treatment 8 , 9 , 10 .
In prediabetes, an elevated BGL already leads to increased reactive oxygen species (ROS) production. The adaptor protein p66shc has recently been shown to play a role in regulating intracellular reactive oxygen species and oxidative stress 11 . p66shc is an isoform of the Human SHC‐Transforming Protein 1 (Shc1). Shc1 is an intracellular scaffold protein, which is involved in downstream pathways of cell surface signaling receptors, such as the Insulin signaling pathway. The interaction of p66shc with the electron transport chain in mitochondria leads to the production of reactive oxygen species (ROS), especially during high glucose loads 2 . This mechanism can lead to oxidative stress induced cell death in prediabetes 12 . Balancing this increased p66shc activity and oxidative stress is the oxidative stress‐induced increase in SIRT1 activity which down regulates p66shc 13 . The value of p66shc as a potential prognostic marker combined with inflammatory and oxidative stress markers in prediabetes has not been reported. Figure 1 illustrates the metabolic interactions of p66shc 14 .
Figure 1.
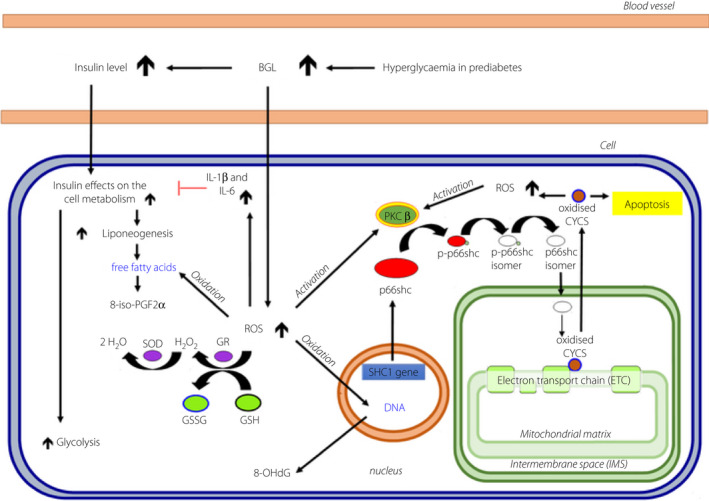
Transfer pathway of p66shc into the mitochondrion. Cell, muscle‐, fat‐ or endothelial cells; 8‐iso‐PGF2α, 8‐isoprostaglandin F2α; 8‐OHdG, 8‐hydroxy‐2′‐deoxyguanosine; CYCS, cytochrome c, somatic; GSH, reduced glutathione; GSR, glutathione‐disulfide reductase; GSSG, oxidized glutathione; IL‐1β, interleukin‐1β; IL‐6, interleukin‐6; PKCβ, protein kinase c beta; p‐p66shc, phosphorylated p66shc; ROS, reactive oxygen species; Shc1 gene, Human SHC‐Transforming Protein 1. gene; SOD, superoxide dismutase. Blue, oxidized; Red, inhibition.
As a redox protein, the Shc1 isoform p66shc is implicated in mitochondrial reactive oxygen species (ROS) production and translation of oxidative signals into apoptosis, which was previously shown to occur during prolonged hyperglycemia such as in diabetes 15 , 16 . In addition, Shc1 isoform p52shc is altered in diabetic cardiovascular disease and targets the insulin IGF‐1 signaling pathways associated with insulin resistance 17 . Moreover, Shc1 isoform p46shc, is associated with reduced adiposity and insulin sensitivity in ShcP mice 18 .
Diabetes has now been recognized for some years as an inflammatory disease 19 , 20 and the Insulin Resistance and Atherosclerosis Study (IRAS) studies reported that the levels of some inflammatory markers were independently associated with insulin sensitivity and linearly related to a number of the metabolic syndrome components 21 , 22 . Similarly, oxidative stress has been reported as a strong biomarker for diabetes prognosis 23 . However, inflammation and oxidative stress are not independent factors in the pathogenesis of diabetes. The accumulation of mitochondrial ROS in prediabetes may increase interleukin‐1 β (IL‐1β) and interleukin‐6 (IL‐6) levels, as well as other inflammatory markers, such as Insulin Growth factor‐1 (IGF‐1), monocyte chemo‐attractant protein‐1 (MCP‐1) and C‐reactive protein (CRP), which can mediate insulin resistance in type 2 diabetes mellitus and promote disease progression 24 .
The current research investigated whether inflammatory and oxidative stress markers, in particular p66shc, show significant differences in patients with prediabetes compared to a control group. Furthermore, we examined if an additional use of inflammatory and oxidative stress markers improves the sensitivity of HbA1c to identify prediabetes.
Methods
Participant recruitment
This cross‐sectional study was performed at the Charles Sturt University (CSU) Diabetes Screening Clinic, Australia between July and September 2017. Demographic and clinical variables were collected from 346 participants following a local media announcement, and were classified by measuring blood glucose into control and prediabetes groups. The control group was made up of participants with a blood glucose level below 5.6 mmol/L. Inclusion and exclusion criteria were kept to a minimum to reflect routine patient presentation to general practice in a typical Australian rural population. Patients had to be over 40 years of age and were requested to have fasted overnight. The design of the study conformed to the general ethical standards, as outlined by the Helsinki Convention on ethical conduct in human research. All methods were carried out in accordance with the relevant guidelines and regulations and as approved by the Charles Sturt University Human Ethics Committee (Ethics Approval # 2006‐042). Attendance at the clinic was voluntary, and all participants declared informed written consent prior to being enrolled.
Anthropogenic measurement
Height and weight were recorded to calculate body mass index (BMI). Blood pressure (BP) readings were obtained after five minutes rest by using a standard mercury sphygmomanometer and a cuff of appropriate size. Hypertension was defined as a SBP of 140 mmHg or higher or DBP 90 mmHg or higher in line with Australian guidelines. Waist circumference was measured with a flexible centimeter tape at the midpoint between the lowest rib and the iliac crest.
Biochemical analyses
Venous blood was collected and stored in either ethylenediaminetetraacetic acid (EDTA) or citric acid tubes as required for the biochemical assays. Urine samples were taken, stored at −20°C and analyzed within 2–3 days using appropriate enzyme‐linked immunosorbent assay (ELISA) kits as outlined previously 25 . ELISA kits were stored at 2–8°C until required. The assays applied a quantitative sandwich enzyme technique, and samples were measured in duplicate. All markers were averaged over two wells with samples and standards. Color change was measured by photometry. Final biomarker concentrations were analyzed by Curve Expert 1.3, (Flarebio Biotech LLC, College Park, MD, USA). The CSU Diabetes Screening Clinic analyzed several pro‐and anti‐inflammatory markers, such as IL‐1β, IL‐6, MCP‐1 and CRP in blood plasma samples. In addition, venous blood samples were taken to determine oxidative stress markers, including glutathione (GSH) and glutathione disulphide (GSSG), as well as the cell proliferation related biomarker Insulin like growth factor 1 (IGF‐1).
Midflow urinary samples were analyzed at the CSU Diabetes Screening Clinic to determine the oxidative stress markers 8‐OHdG and p66shc. Measurements of p66shc were performed with a Human SHC‐Transforming Protein 1 ELISA kit (CUSABIO; Flarebio Biotech LLC) that recognizes primarily Shc1 and its isoform p66shc as reported by the developers. Photometry detection rate was between 25 and 1600 pg/mL. Sensitivity of the kit was at a concentration of 6.25 pg/mL. Avidin conjugated horseradish peroxidase was used with biotin in a color reaction, and photometry was performed to establish the concentration of the compound, as specified by the supplier, at 450 nm wavelength 26 .
For analysis of Blood glucose level (BGL), HbA1c, total cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), and triglycerides venous blood samples were sent to the local Dorevitch Pathology Laboratory, Albury, NSW.
Statistical analyses
Statistical analyses were performed with IBM SPSS (V22) and R version 3.4.2 (R Core Team, 2017) 27 . Sample size was determined using G*Power (https://stats.idre.ucla.edu/other/gpower/) with effect size set at 0.6, power at 0.8 and P < 0.05, and data distribution was determined by applying the Shapiro–Wilk test. Statistical methods included A Chi‐square test was used to analyse medication use in the two groups. General linear modelling (GLM) compared the means of the two groups. For further advanced investigation and modelling, logistic regression modelling was applied. A good predictive power for the model was assumed for an overall correct prediction of ≥70 precent. Significant results were set at P < 0.05. Model results are presented in terms of accuracy, sensitivity and specificity.
Results
General clinical data
Participants with a BGL below 5.6 mmol/L were classified as controls (n = 242), while those with BGL between 5.6 and <7 mmol/L were categorized as prediabetic (n = 105). Besides BGL of the physiological variables measured, only supine SBP was significantly different between the two groups (Table 1).
Table 1.
General clinical data
| Control (mean ± SD) | Prediabetes (mean ± SD) | |
|---|---|---|
| Age (yrs.) | 62.4 ± 1.1 | 64.7 ± 1.5 |
| Sex (m/f) | (96/146) | (42/62) |
| BGL (mmol/L) | 4.9 ± 0.4 | 6.1 ± 0.3*** |
| p66shc (pg/mL) | 52.3 ± 2.9 | 42.3 ± 2.3** |
| HbA1c (%) | 5.6 ± 0.02 | 5.7 ± 0.04 |
| WC (cm) | 90.4 ± 1.1 | 93.8 ± 1.7 |
| BMI (kg/m2) | 25.8 ± 0.4 | 26.4 ± 0.6 |
| Lying SBP (mmHg) | 122.3 ± 1.4 | 128.3 ± 2.4* |
| Lying DBP (mmHg) | 74.6 ± 0.7 | 77.2 ± 1.2 |
Values are means ± standard deviation. Significance: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs control group.
BGL, blood glucose level; BMI, body mass index; f, female; HbA1c, glycosylated haemoglobin; m, male; Supine DBP, supine diastolic blood pressure; Supine SBP, supine systolic blood pressure; WC, waist circumference; Yrs., year.
Comorbidities, medication use and common pathology markers
In order to analyze common comorbidities of type 2 diabetes mellitus, we determined the occurrence of hypertension (HT) and cardio‐vascular diseases (CVD), such as coronary heart disease, cerebrovascular disease, peripheral arterial disease, in our investigated population. In addition, we determined the use of long‐term medication and the levels of common pathology markers (Table 1).
For antidepressants (AD), antihypertensives (AHT), thyroid hormones (TH) and proton‐pump inhibitors (PPIs) the control group presented with higher regular use, whilst the use of statins (ST) and nonsteroidal anti‐inflammatory drugs (NSAIDs) was higher in the prediabetes (Table 2).
Table 2.
Medication
| Control n (%) | Prediabetes n (%) | |
|---|---|---|
| AA | 1 (0.4) | 2 (1.9) |
| AC | 6 (2.5) | 2 (1.9) |
| AD | 36 (14.9) | 10 (9.5) |
| AHT | 113 (46.7) | 45 (42.9) |
| BDZ | 9 (3.7) | 6 (5.7) |
| CS | 7 (2.9) | 4 (3.8) |
| GTN | 3 (1.2) | 3 (2.9) |
| HRT | 2 (0.8) | 1 (0.9) |
| NSAIDs | 94 (38.8) | 46 (43.8) |
| ST | 58 (24) | 31 (29.5) |
| TH | 28 (11.6) | 11 (10.5) |
| PD | 1 (0.4) | 1 (0.9) |
| PPIs | 54 (22.3) | 20 (19) |
| XOI | 7 (2.9) | 4 (3.8) |
| O | 1 (0.41) | 0 (0.00) |
| NM | 122 (50.41) | 54 (51.43) |
AA, Antiarrhythmic agents; AC, anticonvulsants; AD, antidepressants; ATH, antihypertensives; BDZ, benzodiazepines; CS, corticosteroids; GTN, glyceryl trinitrate; HRT, hormone replacement therapy; NM, no regularly medication use; NSAIDs, nonsteroidal anti‐inflammatory drugs; O, opioids; PD, Parkinson disease medication; PPIs, proton‐pump inhibitors; ST, statins; TH, thyroid hormones; XOI, xanthine oxidase inhibitor.
Of the common pathology markers, such as total cholesterol (TC), triglycerides (TG), high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL), only TG was significantly higher in the prediabetes group (Figure 2).
Figure 2.
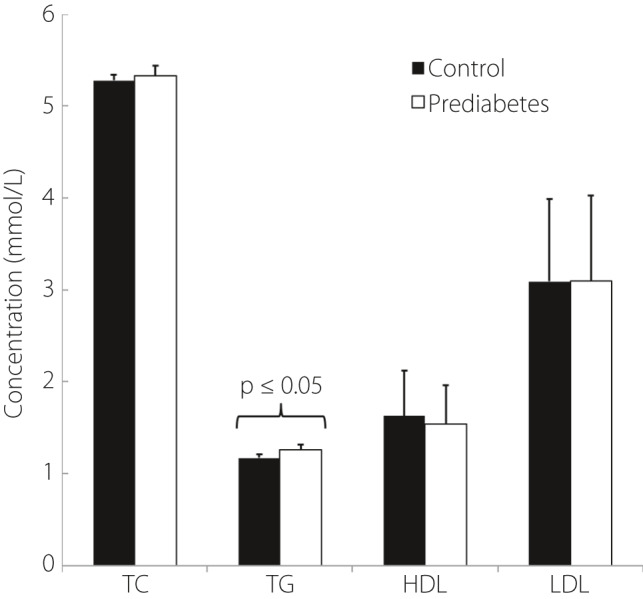
Physiological variables. BGL, blood glucose level; HbA1c, glycosylated hemoglobin; HDL, high‐density Lipoprotein; LDL, low‐density lipoprotein; TC, total cholesterol; TG, triglycerides.
Inflammatory and oxidative stress markers
Inflammatory markers were not significantly different between the two groups (Figure 3).
Figure 3.
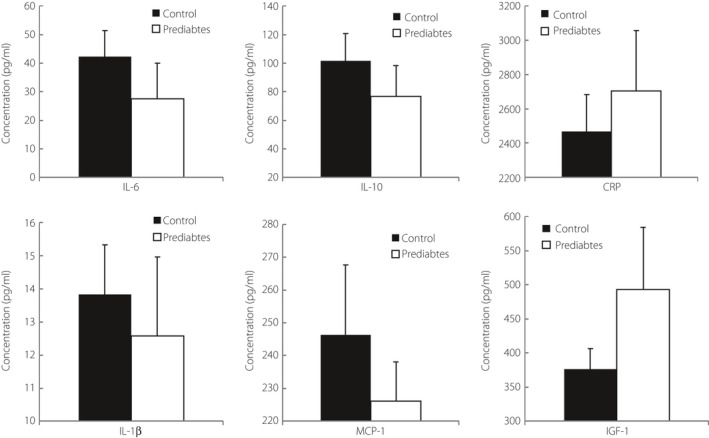
Inflammatory markers. CRP, C‐reactive protein; IL‐10, interleukin‐10; IL‐1β, interleukin‐1β; IL‐6, plasma interleukin‐6; MCP‐1, Monocyte Chemotactic Protein‐1.
Glutathione was significantly decreased in the prediabetes as compared to the control group, suggesting mild oxidative stress (1643.19 ± 67.25 µM & 1838.07 ± 55.25 µM). Similarly, the GSH/GSSG ratio was significantly decreased (7.47 ± 0.41 & 5.86 ± 0.38; Figure 4). Shc1/p66shc was also significantly decreased in the prediabetes group (52.30 ± 2.87 pg/mL vs 42.25 ± 2.33 pg/mL; Figure 4, Table 1).
Figure 4.
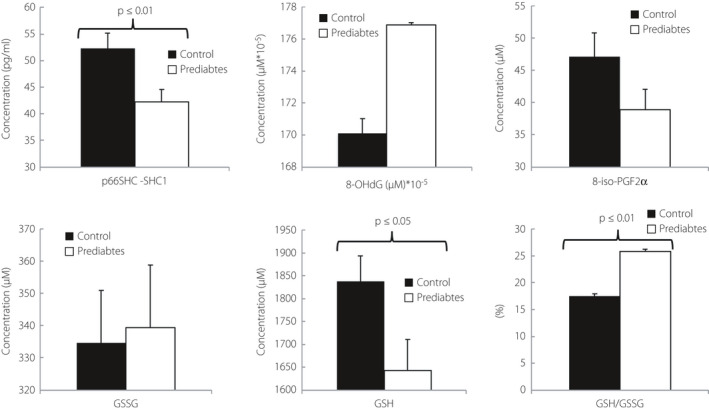
Oxidative stress markers. 8‐iso‐PGF2α, 8‐isoprostaglandin F2α; 8‐OHdG, Hydroxy‐2′‐deoxyguanosine; GSH, glutathione; GSSH, Glutathione disulphide; p66shc‐Shc1, Human SHC‐Transforming Protein 1.
Logistic regression models of p66Shc and other biomarkers
The logistic regression model with only HbA1c as a factor was significant (P < 0.05), but showed a sensitivity of only 50% and a specificity of 74.30%, with an overall accuracy of 62.1%. Accuracy was significantly improved when Shc1/p66shc was included leading to a sensitivity of 74.8%, a specificity of 75% and an overall accuracy of 74.9%. The best HbA1c‐inclusive model for the predication of prediabetes was a combination HbA1c, Shc1/p66shc and triglycerides, with an accuracy of 88.05%. All models that included HbA1c had a significance of P < 0.05 (Table 3).
Table 3.
Models of p66shc and other biomarkers
| Sensitivity (%) | Specificity (%) | Accuracy (%) | |
|---|---|---|---|
| Model (HbA1c included) | |||
| HbA1c* | 50 | 74.3 | 61.2 |
| HbA1c + p66shc** | 74.8 | 75 | 74.9 |
| HbA1c + p66shc + 8‐iso‐PGF2α** | 73.6 | 80 | 76.8 |
| HbA1c + p66shc + TC** | 75.4 | 100 | 87.7 |
| HbA1c + p66shc + TG** | 76.1 | 100 | 88.05 |
| Model (HbA1c excluded) | |||
| p66shc + BMI** | 100 | 73.2 | 86.5 |
| p66shc + CRP + TC** | 73 | 100 | 86.5 |
| p66shc + 8‐OHdG + MCP‐1** | 100 | 78.9 | 89.5 |
Significance of models: *P ≤ 0.05; **P ≤ 0.01.
8‐iso‐PGF2α, 8‐isoprostaglandin F2α; 8‐OHdG, urinary 8‐hydroxy‐2′‐deoxyguanosine; BMI, body mass index; CRP, C‐reactive protein; HbA1c, glycated hemoglobin; IGF‐1, insulin like growth factor‐1; IL‐1β, Interleukin‐1β; IL‐6, Interleukin‐6; MCP‐1, monocyte chemo‐attractant protein‐1; TC, total cholesterol; TG, triglycerides.
Exclusion of HbA1c improved the performance of the model. The best model combined Shc1/p66shc with 8‐OHdG and MCP‐1 with an accuracy of 89.5%, sensitivity of 100% and specificity of 78.9% (P < 0.01). Combining Shc1/p66shc with other common markers including BMI had an overall accuracy of 86.5%, with a sensitivity of 100% and a specificity of 73.2% (P < 0.05). The combination of Shc1/p66shc, CRP and total cholesterol had a sensitivity of 73%, a specificity of 100%, and an overall correct predicate of 86.5% (P < 0.01; Table 3).
Discussion
Recent studies have demonstrated that current diabetes diagnostic protocols, based only on impaired fasting glucose or HbA1c, may not be sensitive enough to identify prediabetes or type 2 diabetes mellitus because of individual variances in plasma glucose levels and the associated biochemical changes 28 .
Shc1 and its isoforms have a close relation to oxidative stress and to the pathology of diabetes. p66shc has been implicated in type 2 diabetes mellitus, potentially playing a major role in the development of retinopathy, nephropathy and cardiovascular complications 2 , 29 , 30 , 31 .
Combining Shc1/p66shc and BMI as predictive biomarkers had a sensitivity of 100% to identify prediabetes patients (P < 0.05). In contrast, HbA1c showed a sensitivity of only 50% to identify prediabetes (P < 0.05). Moreover, our best predictive models included Shc1/p66shc, oxidative stress, beta‐oxidation and inflammatory biomarkers, suggesting a strong link between p66shc and the role of inflammation and oxidative stress in prediabetes.
Pagnin et al. 31 previously assessed the p66shc mRNA in diabetes mellitus type 2. They reported that the level of p66shc mRNA was significantly higher in the DMT2 compared to the control group. Similarly, increased p66shc levels with increased glucose concentration in the renal tubules has also been reported 16 . Interestingly, our results in a prediabetes group displayed a significant decreased Shc1/p66shc level compared to control. Several physiological mechanisms may lead to this finding. Shc1 is a scaffold protein of the insulin receptor pathway, which may be permanently down regulated during the prediabetes stage 17 , 32 . Permanent hyperinsulinemia accrues in the metabolic stage of prediabetes, due the high glucose levels stimulating the insulin production. The down‐regulation of the Shc1 by hyperinsulinemia, and phosphorylation of p66shc might cause the significant decrease of plasma p66shc found in our prediabetes group, which is possibly mediated by SIRT1 (Sirtuin1 lysine deacetylase) 33 , 34 , 35 , 36 .
The stage of disease progression and insulin responsiveness is an important pathophysiological factor that determines levels of inflammation. As such, patients with a decreased acute insulin response were reported to have lower levels of inflammatory proteins compared with those with high insulin secretion 22 , which was also found in the current research where several cytokines included in our model for prediabetes, such as IL‐6, IL‐10, IL‐1β and MCP‐1, had lower concentrations compared to the control group, but displayed no significant P‐values 37 . This lower level of inflammatory proteins might also indicate insufficient stimulation of p66shc by inflammatory processes in our prediabetes group and explain the significant decreased of p66shc in the prediabetes group. Increased BGL may also lead to an increase in SIRT1, which suppresses p66shc prior to the full diabetic state 13 .
Our results indicate that accuracy in identifying prediabetes significantly improved, when Shc1/p66shc was included in the oxidative stress‐inflammatory model with or without HbA1c, emphasizing the importance of the complex multifactorial signaling pathway associated with Shc1/p66shc and confirming previous results of ours and others 38 , 39 , 40 . The current work underlines that inflammatory factors such as MCP‐1, IL‐1β, IL‐6 and CRP, even in a group with mild increases in BMI but with BGL levels in the prediabetes range, may contribute to disease progression 41 , 42 . MCP‐1 was included in our best models suggesting a possible role for inflammation in prediabetes 43 .
Previous studies have pointed out the high sensitivity of 8‐OHdG for oxidative stress, and demonstrated the positive impact on type 2 diabetes mellitus diagnostics in combination with HbA1c 28 . The relevance of 8‐OHdG to oxidative stress and prediabetes pathophysiology is emphasized by the inclusion of 8‐OHdG in our model, in combination with p66shc and MCP‐1. Future considerations to include are refining the role of oxidative stress by comparing 8‐OHdG (DNA oxidation) to 8‐oxo‐7,8‐dihydroguanosine (8‐oxoGuo, RNA oxidation) as RNA oxidation, rather than DNA oxidation may be associated with type 2 diabetes mellitus using LC‐MS methodology rather than ELISA 44 . However, the current study was geared to utilizing low level of equipment available especially in small, rural communities and whether significant differences in results between RNA and DNA 8‐hydroxy guanine are found in clinical studies that lead to a change in treatment is debatable 45 . Similarly, the use of spot urine may have led to elevated results and a future study can use 24‐h urine collection and control with creatinine. 24‐h urine collection however is not a routine screening tool and has not been used in the current study. In addition, previous work has shown that unless chronic kidney disease is present the difference in results of both tests is not significant 46 .
Our study was conducted in a community screening clinic, and therefore, some limitations need to be acknowledged. Recruitment limitations consisted of restriction to the geographical area where the clinic was located. Therefore, the current study focused on a clinical population, where participants attended a free community screening program from within 100 km from the CSU clinic. Other limitations consisted of not being able to include a number of pathophysiological variables, such as an oral glucose tolerance test (OGTT). Selecting a medication free group may also provide clearer results in future studies.
Conclusion
In conclusion, a more comprehensive assessment of prediabetes, based on its multifactorial condition and the availability of recently identified biomarkers significantly increases identification of prediabetes. Including p66shc/Shc1, 8‐OHdG, CRP or MCP‐1 in our diagnostic models showed a major improvement of sensitivity to identify prediabetes. Our findings underline beneficial, diagnostic information of oxidative stress and inflammatory biomarkers, which most likely improves prevention and early treatment options in prediabetes. It is recommended to include p66shc/Shc1, oxidative stress and inflammatory markers in prediabetes risk models to benefit patients by enabling earlier lifestyle and pharmacological intervention and more effective personalized, targeted treatment.
Disclosure
The authors have declared that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
The authors would like to acknowledge Simon McDonald from the Spatial Analysis Unit, CSU, Australia, for statistical advice. Bev de Jong and Eugene Butkowski provided technical assistance.
J Diabetes Investig. 2021; 12: 1881–1889
References
- 1. Xu X, Zhu X, Ma M, et al. p66Shc: a novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci Rep 2016; 6: 29302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Francia P, Cosentino F, Schiavoni M, et al. p66(Shc) protein, oxidative stress, and cardiovascular complications of diabetes: the missing link. J Mol Med 2009; 87: 885–891. [DOI] [PubMed] [Google Scholar]
- 3. Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract 2008; 14: 933–946. [DOI] [PubMed] [Google Scholar]
- 4. Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011; 34: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ADA . Classification and diagnosis of diabetes. Diabetes Care 2015; 38(Suppl 1): S8–S16. [DOI] [PubMed] [Google Scholar]
- 6. Lim W‐Y, Ma S, Heng D, et al. Screening for diabetes with HbA1c: test performance of HbA1c compared to fasting plasma glucose among Chinese, Malay and Indian community residents in Singapore. Sci Rep 2018; 8: 12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1c. Diabetes Care 2011; 34(Suppl 2): S184–S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab J Res Rev 2010; 26: 3–6. [DOI] [PubMed] [Google Scholar]
- 9. Voigt A, Jelinek HF. Humanin: a mitochondrial signaling peptide as a biomarker for impaired fasting glucose‐related oxidative stress. Physiol Rep 2016; 4: e12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng H, Tong R, Tong W, et al. Metabolic biomarkers for prognostic prediction of pre‐diabetes: results from a longitudinal cohort study. Sci Rep 2017; 7: 6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magenta A, Greco S, Capogrossi MC, et al. Nitric oxide, oxidative stress, and p66(Shc) Interplay in diabetic endothelial dysfunction. BioMed Res Int 2014; 2014: 193095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minami Y, Sonoda N, Hayashida E, et al. p66Shc signaling mediates diabetes‐related cognitive decline. Sci Rep 2018; 8: 3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J 2013; 37: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelicci G, Lanfrancone L, Grignani F, et al. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 1992; 70: 93–104. [DOI] [PubMed] [Google Scholar]
- 15. Fadini GP, Albiero M, Menegazzo L, et al. The redox enzyme p66Shc contributes to diabetes and ischemia‐induced delay in cutaneous wound healing. Diabetes 2010; 59: 2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun L, Xiao LI, Nie J, et al. p66Shc mediates high‐glucose and angiotensin II‐induced oxidative stress renal tubular injury via mitochondrial‐dependent apoptotic pathway. Am J Physiol Renal Physiol 2010; 299: F1014–F1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neumann‐Haefelin E, Qi W, Finkbeiner E, et al. SHC‐1/p52Shc targets the insulin/IGF‐1 and JNK signaling pathways to modulate life span and stress response in C. elegans . Genes Develop 2008; 22: 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomilov AA, Ramsey JJ, Hagopian K, et al. The Shc locus regulates insulin signaling and adiposity in mammals. Aging Cell 2011; 10: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest 2003; 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11: 98–107. [DOI] [PubMed] [Google Scholar]
- 21. Festa A, D’Agostino R, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: The insulin resistance atherosclerosis study (IRAS). Circulation 2000; 102: 42–47. [DOI] [PubMed] [Google Scholar]
- 22. Festa A, Hanley AJG, Tracy RP, et al. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation 2003; 108: 1822–1830. [DOI] [PubMed] [Google Scholar]
- 23. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004; 24: 816–823. [DOI] [PubMed] [Google Scholar]
- 24. Barry JC, Shakibakho S, Durrer C, et al. Hyporesponsiveness to the anti‐inflammatory action of interleukin‐10 in type 2 diabetes. Sci Rep 2016; 6: 21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maschirow L, Khalaf K, Al‐Aubaidy HA, et al. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes – biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem 2015; 48: 581–585. [DOI] [PubMed] [Google Scholar]
- 26. Bratthauer GL. The avidin‐biotin complex (ABC) method and other avidin‐biotin binding methods. Meth Mol Biol 2010; 588: 257–270. [DOI] [PubMed] [Google Scholar]
- 27. R_Team. R . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 28. Stranieri A, Yatsko A, Jelinek HF, et al. Data‐analytically derived flexible HbA1c thresholds for type 2 diabetes mellitus diagnostic. J Artif Intell Res 2016; 5: 111–134. [Google Scholar]
- 29. Li X, Zhang X, Zheng L, et al. Hypericin‐mediated sonodynamic therapy induces autophagy and decreases lipids in THP‐1 macrophage by promoting ROS‐dependent nuclear translocation of TFEB. Cell Death Dis 2016; 7: e2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim Y‐R, Kim C‐S, Naqvi A, et al. Epigenetic upregulation of p66shc mediates low‐density lipoprotein cholesterol‐induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol 2012; 303: H189–H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pagnin E, Fadini G, de Toni R, et al. Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab 2005; 90: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 32. Kubota T, Kubota N, Kadowaki T. Imbalanced insulin actions in obesity and type 2 diabetes: key mouse models of insulin signaling pathway. Cell Metab 2017; 25: 797–810. [DOI] [PubMed] [Google Scholar]
- 33. Morita M, Matsuzaki H, Yamamoto T, et al. Epidermal growth factor receptor phosphorylates protein kinase C δ at Tyr332 to form a trimeric complex with p66Shc in the H2O2‐stimulated cells. J Biochem 2007; 143: 31–38. [DOI] [PubMed] [Google Scholar]
- 34. Kumar S, Kim Y‐R, Vikram A, et al. Sirtuin1‐regulated lysine acetylation of p66Shc governs diabetes‐induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci 2017; 114: 1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wright KD, Staruschenko A, Sorokin A. Role of adaptor protein p66Shc in renal pathologies. Am J Physiol Renal Physiol 2018; 314: F143–F153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kong X, Guan J, Li J, et al. P66(Shc)‐SIRT1 regulation of oxidative stress protects against cardio‐cerebral vascular disease. Mol Neurobiol 2017; 54: 5277–5285. [DOI] [PubMed] [Google Scholar]
- 37. Zhao G, Dharmadhikari G, Maedler K, et al. Possible role of interleukin‐1β in type 2 diabetes onset and implications for anti‐inflammatory therapy strategies. PLoS Comp Biol 2014; 10: e1003798‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Butkowski EG, Al‐Aubaidy HA, Jelinek HF. Interaction of homocysteine, glutathione and 8‐hydroxy‐2′‐deoxyguanosine in metabolic syndrome progression. Clin Biochem 2017; 50: 116–120. [DOI] [PubMed] [Google Scholar]
- 39. Paneni F, Volpe M, Luscher TF, et al. SIRT1, p66Shc, and Set7/9 in vascular hyperglycemic memory: bringing all the strands together. Diabetes 2013; 62: 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Marchi E, Baldassari F, Bononi A, et al. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxid Med Cell Longev 2013; 2013: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panee J. Monocyte chemoattractant protein 1 (MCP‐1) in obesity and diabetes. Cytokine 2012; 60: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Radhakrishnan P, Srikanth P, Seshadri KG, et al. Serum monocyte chemoattractant protein‐1 is a biomarker in patients with diabetes and periodontitis. Indian J Endocrinol Metab 2014; 18: 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rathmann W, Kowall B, Heier M, et al. Prediction models for incident type 2 diabetes mellitusin the older population: KORA S4/F4 cohort study. Diabet Med 2010; 27: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 44. Broedbaek K, Siersma V, Henriksen T, et al. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population‐based cohort study. Diabetes Care 2013; 36: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sørensen A‐S, Kjær LK, Petersen KM, et al. The effect of smoking on the urinary excretion of 8‐oxodG and 8‐oxoGuo in patients with type 2 diabetes. Scandinavian J Clin Lab Invest 2017; 77: 253–258. [DOI] [PubMed] [Google Scholar]
- 46. Ying T, Clayton P, Naresh C, et al. Predictive value of spot versus 24‐hour measures of proteinuria for death, end‐stage kidney disease or chronic kidney disease progression. BMC Nephrol 2018; 19: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]


