Abstract
Aims/Introduction
This 6‐month interventional study aimed to investigate the effectiveness of different educational programs among Saudi women with prediabetes referred by primary care.
Materials and Methods
A total of 253 (100 group education program [GEP], 84 WhatsApp education program [WEP] and 69 control group [CG]) eligible participants were invited to take part in the study, out of whom 120 received intervention (40 GEP, 43 WEP and 37 CG). GEP participants received focused, individualized lifestyle modification advice with bimonthly support sessions, WEP participants received the same intervention, but delivered through social media (WhatsApp). The CG received standard care. Anthropometrics, biochemical profiles and macronutrient intake were measured at baseline, and 3 and 6 months. The primary end‐points were glycated hemoglobin and weight, with lipids and dietary changes as secondary outcomes.
Results
Glycated hemoglobin significantly improved in all groups post‐intervention (GEP baseline 6.0 ± 0.2 vs 6 months 5.5 ± 0.54; P < 0.001, WEP 6.0 ± 0.26 vs 5.3 ± 0.51; P < 0.001, CG 6.0 ± 0.37 vs 5.7 ± 0.49; P < 0.001), but with no difference in between‐group comparisons (P = 0.33). Within‐group comparisons showed a reduction in weight, but only in the GEP group (90.6 kg ± 27.3 vs 84.8 kg ± 24.3; P < 0.01), and this was significant in between‐group comparison (P = 0.003). Significant between‐group comparisons with respect to energy (g) intake (P = 0.005) were also observed, as well as triglycerides (P < 0.001) and low‐density lipoprotein cholesterol (P = 0.001), all in favor of the GEP group.
Conclusions
Diabetes prevention programs, whether delivered through a focused educational group, social media or standard care, are equally efficacious in improving glycated hemoglobin levels among Saudi women with prediabetes, but a focused educational group was more effective in terms of successful weight loss.
Keywords: Arab women, Diabetes prevention, Social media
Education programs whether delivered through a focused educational group, social media or standard care, are equally efficacious in improving HbA1c levels among Saudi women with prediabetes. This may have been due to reduced energy intakes and favourable increases in physical activity. Digital platforms may offer a more accessible and less resource intensive option that is equally effective.
INTRODUCTION
In the present digital age, the opportunity to use internet and smart phone applications to support medical management is limitless. The potential advantages of using digital health interventions include convenience, anonymity and sustained support, which can sometimes be lacking from attending group education sessions 1 . Although these platforms might fail to offer the emotional, psychological and behavioral support that patients need for holistic care, the use of digital technology, such as mobile phone apps and tailored mobile text messaging in particular, have been proven beneficial in the glycemic management of people with type 2 diabetes 2 . In a recent meta‐analysis including 14 studies (n = 1360), the use of mobile apps translated to better self‐management and improved glycemic control through reduced glycated hemoglobin (HbA1c) levels ranging from 0.15–1.87%, as compared with non‐app users 2 . Given that weight management, a healthy diet and appropriate levels of physical activity are the cornerstones in the management and prevention of type 2 diabetes, the use of mobile apps can indeed be effective tools, as it provides ease of monitoring real‐time progress and accurate prospective data collection.
lthough social media is revolutionizing healthcare delivery globally, it is still in its infancy in Saudi Arabia (SA). Among the limited studies undertaken in this area of practice, Al‐Qahtani et al. 3 observed that many Saudi patients use social media to learn more about their illnesses, and that common users were women and young Saudis. There is an emergence of a wide variety of Arabic apps designed for weight loss 4 , 5 . These early apps, however, have been criticized for the lack of sensitivity to the culture and omission of crucial evidence‐informed practices locally, as they were developed as direct translations of existing English‐language weight loss apps 4 . Nevertheless, the increasing popularity of health‐based apps and mobile health in SA has the potential to transform healthcare delivery in the country. Currently, the healthcare system in SA follows the primary healthcare model, which is the basis of universal coverage that includes free diabetes screening for all its citizens 6 . However, despite the rapid digitization of healthcare, policies for its use are not in place due to lack of evidence from studies carried out within the SA community 7 .
Women in SA might particularly benefit from health‐based apps, as they face particular challenges associated with social and cultural factors, which might reduce their physical activity levels and increase their risk for obesity and subsequently type 2 diabetes. Until recently, women in SA were less likely to go outside and had limited access to gyms 8 due to cultural norms of segregation of sexes and women being confined to domestic duties and childcare. The majority of women before King Salman’s reign (2005) were unemployed and required either permission from their guardian (either father, husband or male relative) if they were to leave their home alone or be accompanied by a male relative to go to places. This is despite an increasing population of highly educated women. Sports education is not provided in schools for girls, and girls are not allowed to participate in sports activities in public schools 9 . In terms of healthcare provision, women do not receive a routine medical examination, and almost one in two women with type 2 diabetes are undiagnosed 10 .
The present study aimed to investigate for the first time the differences in the effectiveness of different educational programs versus supervised education through social media among Saudi women with prediabetes.
MATERIALS AND METHODS
This was a 6‐month, multicenter, three‐arm cluster randomized, multi‐intervention study carried out from July 2018 until March of 2019 in Riyadh, SA. The study was approved by the ethics committee of the College of Science, King Saud University; Riyadh, SA (reference# 4/67/231893), KSU‐SE‐18‐07. All procedures implemented were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from each participant before inclusion, before screening and randomization.
Study population
Three centers that were randomly selected out of the total nine governmental primary care centers located in Riyadh were identified for participant recruitment using a cluster randomization scheme. Using this strategy prevents ‘contamination of allocation’, defined as participants in the control group having awareness of the interventions given in the test group and adopting it themselves 11 . In the case of the present study, contamination of allocation could occur if the groups were randomized in the same center, as those in the intensive lifestyle group could be invited by other participants to switch to the WhatsApp group instead. The three centers were King Salman Hospital (allocated for the group intensive lifestyle education program [GEP]), King Salman Social Center (allocated for the WhatsApp Program [WEP]) and one primary healthcare center (allocated for the control group [CG]). It is worthy to note that all primary care centers in SA follow the same national health guidelines issued by the Ministry of Health, and as such, no differences in practices or care are anticipated, regardless of the location. All Saudi women aged 18–60 years diagnosed to have prediabetes (fasting glucose ≥5.6 to ≤6.9 mmol/L) at screening were considered eligible for the study. Men; women already diagnosed with type 2 diabetes; women receiving antihyperglycemic treatment, antihypertensive or lipid‐lowering drugs; pregnant or lactating women; women with known renal, hepatic, pulmonary or cardiac complications; and women who were non‐active users of android or IOS‐based smart phones were excluded.
A total of 1,140 women participants were initially invited to enroll by the practice assigned via brochures and SMS (GEP n = 380, WEP n = 380 and CG n = 380). Out of 1,140 women, just 579 participants (GEP n = 180; WEP n = 237 and CG n = 162) consented for screening, of whom 253 were diagnosed to have prediabetes (GEP n = 100, WEP n = 84 and CG n = 69 CG) and were eligible for intervention. Eligible participants were then contacted to return to their respective centers for collection of baseline information, out of whom just 120 responded and received intervention (GEP n = 40, WEP n = 43 and CG n = 37 CG). A breakdown of participants from selection to analysis has been provided in the flow chart (Figure 1).
Figure 1.
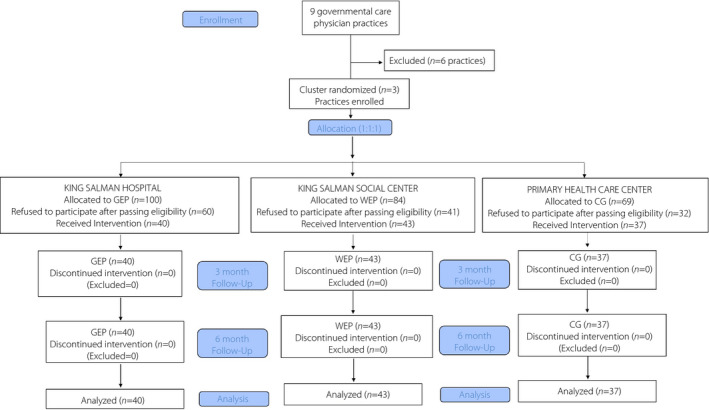
Flow chart of participants. CG, control group; GEP, group education program; WEP, WhatsApp education program.
Interventions
GEP
GEP sessions were carried out at the auditorium of the King Salman Hospital twice a month. Participants received structured group educational instructions and follow‐up support for lifestyle modification from the study dietitian at least six times (at baseline and every 2 weeks during the first 3‐month intervention period). Participants attended a total of six sessions covering the following topics: (i) prediabetes, risk of type 2 diabetes and setting achievable goals; (ii) nutrition for health and weight management; (iii) carbohydrate awareness; (iv) reading and understanding food labels; (v) exercise and the benefits of physical activity (moderate exercise for at least 30 min per day e.g. a walk, 5 times a week was encouraged); and (vi) healthcare essentials that include monitoring and a checklist. All presentations were carried out in Arabic language, and delivered by certified personnel (dietitian and diabetes educator).
WEP
Participants in the WEP group were provided with the same information as the GEP group, but the content was delivered through social media. In addition, the WEP participants were asked to download "Al‐Nahdi Mobile App" an app‐based educational program about lifestyle modifications emphasizing the importance of weight loss, healthy diet and physical activity. WhatsApp messages were sent to the group by the diabetes educator, and the participants were provided with a member card to join the gym in the King Salman Social Center. The messages were written in Arabic and were reviewed by an endocrinologist. The contents of these messages were about diet therapy, exercise, general diabetes and prediabetes care knowledge. Participants were provided with a free consultation, and special offers for the laboratory tests, medicine and Accucheck softclix lancing device for blood sugar monitoring purposes at any time during the study.
CG
Participants in the CG were given lifestyle advice as standard, non‐personalized counseling by the assigned primary healthcare center physician, and interviewed only at baseline and after every 3 months at the primary healthcare center where this allocation was assigned. This advice included distribution of translated pamphlets and booklets with information related to lifestyle changes from proven effective programs 12 , 13 , 14 . A summary of interventions is provided in Table 1.
Table 1.
Interventions given to groups.
| Lifestyle Intervention | CG | GEP | WEP |
|---|---|---|---|
|
Baseline
|
Given as a booklet to the group | Explained by registered dietitian individually | Delivered through WhatsApp group |
| Bimonthly lifestyle education sessions for 3 months | None | ✓ | ✓ |
| Dietary counseling | None | Every 2 weeks for 3 months, then continue to follow up until the 6‐month period | Every 2 weeks for 3 months, (via WhatsApp), then continue to follow up until the 6‐month period |
| Dietary intake record | Baseline, and after 3 and 6 months | ||
| Physical activity record questionnaire | Baseline, and after 3 and 6 months | ||
| Mode of follow up | Group | Group | Group |
| On demand support system | None | ✓ | ✓ |
| Blood extraction | Baseline and after 6 months | ||
| Anthropometrics | Baseline, and after 3 and 6 months | ||
CG, control group; GEP, group educational program; WEP, WhatsApp educational program.
Data collection
Blood collection
Fasting blood samples were collected at the participants’ assigned practice at baseline and 6 months, and were immediately delivered at King Salman Hospital for the analysis of HbA1c. The remaining blood samples were delivered on the same day at the Chair for Biomarkers of Chronic Diseases in King Saud University, Riyadh, SA, for storage at −80°C until further analysis. Routine biochemical tests including fasting blood glucose and lipid profile were analyzed using a regularly calibrated biochemical analyzer (Konelab, Espoo, Finland).
Anthropometrics
Anthropometric measurements were carried out at baseline and after 6 months in all three arms of the study. Height (to the nearest cm) and weight using calibrated scales (to the nearest 100 g) were measured in light clothing without shoes. Waist circumference (cm) was measured at the umbilical level without clothes after exhaling in a relaxed standing position. Blood pressure (mmHg) was measured at each visit after a sufficient rest using the conventional mercurial sphygmomanometer and the average was recorded. Body mass index (BMI) was calculated by dividing weight in kg by the square of height in meters (kg/m2). All measurements were carried out by trained and licensed nurses and dietitians.
Dietary
To measure dietary intake, 3‐day food diaries and a food frequency questionnaire 15 were used to collect dietary information at baseline and after every 3 months until the end of the intervention study. Efforts were made to ensure that participants kept an accurate diary, by advising the participants to write amounts in terms of measurable household items and reminding them to be much more specific and write as much detail as possible. In addition, a self‐administered general questionnaire was submitted during recruitment, and this included demographic information, past and present medical history, as well as medications taken, if any. In term of physical activity advice, a moderate exercise for at least 30 min per day (30‐min walk, 5 times a week) were emphasized.
Data confidentiality, entry and cleaning
Participants' data were sent only to the principal investigator, who allocated a subject code and a number, so participants were completely anonymized before any analysis. All original data were stored under password protection.
Statistical analysis
Descriptive and inferential statistics were carried out using SPSS (version 21; IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation for continuous variables. Categorical variables are presented as frequencies and percentages (%). The χ2‐tests was used to check differences between intervention groups and clinical demographics, obstetric and gynecological characteristics, and physical activity and sleep characteristics. Comparison of groups over time was carried out using per‐protocol analysis, where missing data was dealt with using the last observation carried forward approach. Anthropometric changes in participants over time were tested using repeated measures ancova after adjusting for participants’ age. Furthermore, biochemical and dietary changes in participants before and after the intervention were tested using repeated measures ancova after adjusting for age and baseline BMI. P < 0.05 was considered significant.
We carried out post‐hoc calculations to determine achieved power given n = 37 per arm, α = 0.05 and an effect size of 0.69 (calculated from the actual HbA1c means and standard deviations obtained for the difference between dependent means [matched pairs]), the power acquired for the final sample size was 97.9%. All sample size calculations was carried out using (G*Power software, Dusseldorf, Germany).
RESULTS
Baseline demographic and clinical characteristics
Table 2 shows the baseline demographic and clinical characteristic of participants who completed the intervention according to groups. Participants of the CG were significantly older (50.9 ± 7.1 years) compared with the GEP group (42.9 ± 12.2 years) and WEP group (43.7 ± 8.1 years; P < 0.001). There was a statistically significant difference in the marital status of groups (P = 0.001), with the WEP group having the highest number of married participants (86.2%), the GEP group having the highest number of single participants (32.4%) and the CG having the highest number of widowed participants (18.8%). The presence of consanguineous marriages was high (17%) in all groups, but not significantly different from one another (P = 0.14). With regard to family history, diabetes mellitus was the most common in all groups and highest in the CG (72.5%; P = 0.04). Medical history was unremarkable in all groups, with the exception of anemia, which was highest also in the CG (P = 0.03).
Table 2.
Demographic and clinical characteristics of participants (completers)
| Parameter | CG | GEP | WEP | P‐value |
|---|---|---|---|---|
| n | 37 | 40 | 43 | |
| Age (years) | 50.9 ± 7.1 | 42.9 ± 12.2* | 43.7 ± 8.1* | <0.001 |
| Marital status | ||||
| Divorced | 2 (6.3) | 2 (5.4) | 3 (9.1) | 0.001 |
| Married | 22 (68.8) | 19 (51.3) | 29 (87.9)** | |
| Single | 2 (6.3) | 12 (32.4)* | 1 (3.0)** | |
| Widow | 6 (18.8) | 1 (2.7) | 0A | |
| Married to relative? | ||||
| No | 27 (67.5) | 32 (86.4) | 36 (83.7) | 0.14 |
| Yes | 13 (32.5) | 5 (13.6) | 7 (16.3) | |
| Consanguinity | ||||
| 1st degree | 1 (11.1) | 5 (83.3)* | 2 (28.6) | 0.013 |
| 2nd degree | 8 (88.9) | 1 (16.7)* | 5 (71.4) | |
| Family history | ||||
| Diabetes | 29 (72.5) | 18 (45)* | 22 (55) | 0.04 |
| Hypertension | 10 (25) | 14 (35) | 7 (17.5) | 0.20 |
| Hyperlipidemia | 6 (15) | 7 (17.5) | 6 (15) | 1.00 |
| Asthma | 5 (12.5) | 8 (20) | 3 (7.5) | 0.29 |
| Obesity | 4 (10) | 10 (25) | 6 (15) | 0.23 |
| Coronary heart | 3 (7.5) | 5 (12.5) | 1 (2.5) | 0.29 |
| Medical history | ||||
| Diabetes | 0 | 0 | 1 (2.5) | 1.00 |
| Asthma | 0 | 0 | 1 (2.5) | – |
| Coronary heart | 0 | 0 | 1 (2.5) | – |
| Disease | 1.00 | |||
| Cancer | 0 | 1 (2.5) | 0 | |
| Liver disease | 0 | 0 | 0 | 1.00 |
| Kidney disease | 0 | 0 | 1 (2.5) | – |
| Others | 1.00 | |||
| Anemia | 1 (2.5) | 0 | 0 | |
| Osteoporosis | 4 (10) | 0 | 0 | 1.00 |
| Rheumatoid | 1 (2.5) | 0 | 0 | 0.033 |
| Arthritis | 1 (2.5) | 0 | 0 | 1.00 |
| Thyroid disease | 1.00 | |||
| Smoking history | ||||
| Ex‐smoker | 2 (6.5) | 6 (16.2) | 0 | |
| Never smoked | 29 (93.5) | 25 (67.6) | 18 (100)** | 0.007 |
| Shisha | 0 | 1 (2.7) | 0 | |
| Cigarette | 0 | 5 (13.5) | 0 | |
Frequencies presented as n (%). *Significant difference compared with control; **significant difference compared with group education; significant at P < 0.05.
CG, control group; GEP, group educational program; WEP, WhatsApp educational program.
HbA1c and biochemical changes over time
Table 3 shows the changes in HbA1c and other biochemical parameters over time (baseline vs 6 months) in all groups using per‐protocol analysis. Within‐group comparisons in the CG showed a modest improvement in glucose (P = 0.06) and a significant reduction in HbA1c (P < 0.001). The lipid profile in the control group also showed improvements in triglycerides (P = 0.03). The rest of the parameters remain non‐significant. In the GEP group, an overall significant improvement was observed in all glycemic and lipid parameters (glucose [P = 0.001], HbA1c [P < 0.001], triglycerides [P < 0.001], total cholesterol [P = 0.001], high‐density lipoprotein cholesterol [P = 0.002] and low‐density lipoprotein cholesterol [P = 0.006]). Finally, in the WEP group, within‐group comparisons showed no significant changes in glucose and lipid profile, but a significant improvement in HbA1c over time (P < 0.001).
Table 3.
Glycated hemoglobin and other biochemical changes in completing participants before and after 6‐month intervention
| Parameter | CG (n = 37) | GEP (n = 40) | WEP (n = 43) | P** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P* | Before | After | P* | Before | After | P* | ||
| HbA1c | 6.0 ± 0.37 | 5.7 ± 0.49 | <0.001 | 6.0 ± 0.2 | 5.5 ± 0.54 | <0.001 | 6.0 ± 0.26 | 5.3 ± 0.51 | <0.001 | 0.33 |
| Glucose (mmol/L) | 5.4 ± 1.7 | 5.2 ± 0.88 | 0.06 | 6.0 ± 1.2 | 5.3 ± 1.0 | 0.001 | 5.7 ± 1.0 | 5.9 ± 1.2 | 0.62 | 0.71 |
| Triglycerides (mmol/L) | 1.5 ± 0.93 | 1.4 ± 0.7 | 0.03 | 3.7 ± 2.0 | 2.3 ± 1.3 | <0.001*** | 1.2 ± 0.52 | 1.1 ± 0.57 | 0.58***,**** | <0.001 |
| Total cholesterol (mmol/L) | 5.7 ± 2.2 | 5.2 ± 1.1 | 0.06 | 6.2 ± 1.4 | 5.0 ± 0.83 | 0.001 | 5.7 ± 1.1 | 5.6 ± 1.4 | 0.62 | 0.71 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.43 | 1.2 ± 0.49 | 0.24 | 1.2 ± 0.55 | 1.6 ± 0.75 | 0.002 | 1.27 ± 0.31 | 1.3 ± 0.33 | 0.48 | 0.50 |
| LDL cholesterol (mmol/L) | 3.8 ± 2.0 | 3.4 ± 0.9 | 0.08 | 3.3 ± 1.8 | 2.3 ± 1.4 | 0.006 | 3.9 ± 1.0 | 3.8 ± 1.1 | 0.55**** | 0.001 |
Data presented as the mean ± standard deviation. *P‐value for paired t‐test; **P‐value for repeated measures ancova adjusted for age, baseline body mass index and marital status; ***significant difference compared with control; ****significant difference compared with group education; significant at P < 0.05.
CG, control group; GEP, group educational program; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, high‐density lipoprotein; WEP, WhatsApp educational program.
Between‐group comparisons adjusted for age, baseline BMI and marital status showed no clinically significant differences in glucose, HbA1c, and total and high‐density lipoprotein cholesterol. A clinically significant difference was observed in triglycerides over time in favor of the GEP group (P < 0.001). The GEP group also had a clinically significant improvement in low‐density lipoprotein cholesterol as compared with the WEP group (P = 0.001). The rest of the differences are shown in Table 3.
Anthropometric changes over time
The changes over time in weight and other anthropometric characteristics of participants who completed the intervention are shown in Table 4. Within‐group analysis showed no significant changes in the CG. In the GEP group, a significant reduction in weight was observed over time (P < 0.01), as well as BMI, with weight post‐intervention being significantly lower compared with baseline. A significant improvement was also in the waist circumference (P‐value <0.01). Within‐group comparison in the WEP group showed a modest, but significant, decrease in weight (P = 0.04), and a significant reduction in BMI (P = 0.027), waist (P = 0.007) and hip circumference (P = 0.033). The rest of the anthropometric characteristics in the WEP group did not significantly improve over time.
Table 4.
Weight and other anthropometric changes in completing participants over time
| Parameter | CG (n = 37) | GEP (n = 40) | WEP (n = 43) | P** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | P* | 0 | 3 | 6 | P* | 0 | 3 | 6 | P* | ||
| Weight (kg) | 75.9 ± 12.7 | 75.5 ± 12.2 | 75.7 ± 12.4 | 0.23 | 90.6 ± 27.3 | 86.1 ± 25.2 | 84.8 ± 24.3 | <0.01*** | 74.5 ± 13.8 | 74.3 ± 14 | 73.2 ± 13.8 | 0.044*** | 0.003 |
| BMI (kg/m2) | 31.6 ± 5.8 | 31.4 ± 5.6 | 31.5 ± 5.7 | 0.22 | 34.8 ± 9.0 | 33.2 ± 8.6 | 32.7 ± 8.3 | <0.01 | 30 ± 5.1 | 29.9 ± 5.1 | 29.4 ± 5.1 | 0.027**** | 0.02 |
| Waist (cm) | 93.2 ± 11.3 | 93.3 ± 10.6 | 93.4 ± 10.3 | 1.00 | 100 ± 19.1 | 98.6 ± 18.1 | 96.5 ± 17.8 | <0.01 | 89.9 ± 12.3 | 90.7 ± 12.6 | 88.8 ± 12.3 | 0.007**** | 0.017 |
| SBP (mmHg) | 126 ± 10.9 | 126 ± 11.3 | 126 ± 11.5 | 1.00 | 110 ± 31.2 | 106.4 ± 27 | 104.4 ± 25 | <0.01*** | 117.9 ± 9.4 | 119.7 ± 8.4 | 118.3 ± 7.2 | 0.81**** | <0.01 |
| DBP (mmHg) | 82.9 ± 8.5 | 81 ± 8.3 | 82.6 ± 8.6 | 0.39 | 103 ± 23.4 | 101.4 ± 25 | 98.4 ± 25 | <0.01* | 77.1 ± 7.1 | 73.2 ± 13.8 | 77.4 ± 6.6 | 1.00***,**** | <0.01 |
Data presented as the mean ± standard deviation; 0, 3 and 6 are months of intervention. *P‐value for repeated measures ancova adjusted for age, baseline body mass index (BMI) and marital status; **P‐value for one‐way ancova; ***significant difference compared with control; ****significant difference compared with group education; significant at P < 0.05.
CG, control group; DBP, diastolic blood pressure; GEP, group educational program; SBP, systolic blood pressure; WEP, WhatsApp educational program; WHR, waist‐to‐hip ratio.
Between‐group comparisons adjusted for age, baseline BMI and marital status showed a clinically significant improvement was observed in favor of the GEP group in terms of weight (P = 0.003), BMI (P = 0.02) and waist circumference (P = 0.017), as well as systolic and diastolic blood pressure (P‐values < 0.01) over time. Compared with CG, the WEP group had a clinically significant improvement only in diastolic blood pressure.
Changes in macronutrient intake over time
The 3‐day mean macronutrient intake was recorded for each participant at baseline and every 3 months. The cumulative means per group are presented in Table 5. Within‐group comparisons showed that in the CG group, there was a significant reduction in the total energy intake (P < 0.01) and fat energy (%;P < 0.05). There was also a significant increase in protein energy (%;P < 0.05). Significant reductions in total energy intake, carbohydrates (g), protein (g) and fat (g; P‐values < 0.01) were seen in the GEP group over time. In the WEP group, significant reductions were also seen in the energy intake, carbohydrates (g), protein (g) and fat (g and %; P‐values <0.01, respectively). Between‐group comparisons showed a significant reduction in energy intake and fat (g) in favor of the GEP group (P‐values 0.005 and <0.001, respectively), significant increase in carbohydrate (%) with subsequent decrease in fat (%) in favor of the WEP group (P‐values <0.001, respectively) and significant increase in protein (%) in favor of the CG (P < 0.001). No significance was observed in between‐group comparisons with respect to carbohydrate (g) intake over time. Finally, no differences were observed within and between group comparisons with respect to fiber intake.
Table 5.
Macronutrient changes in participants before and after the 6‐month intervention
| Parameter | CG | GEP | WEP | P** | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| Energy (Kcal) | 3,018.5 ± 828.8 | 2,800.3 ± 733.9** | 2,489.0 ± 805.6 | 1,746 ± 478.5**,*** | 2414.2 ± 946.2 | 2,297.4 ± 915.8** | 0.005 |
| Carbohydrate (g) | 322.9 ± 123.9 | 312.2 ± 120.0 | 308.6 ± 102.3 | 280.3 ± 98.9** | 253.8 ± 107.4 | 248.6 ± 107.1** | 0.066 |
| CHO energy (%) | 47.0 ± 7.4 | 48.1 ± 9.6 | 50.6 ± 11.3 | 51.9 ± 7.7 | 41.8 ± 5.0 | 43.0 ± 5.1**,***,**** | <0.001 |
| Protein (g) | 104.9 ± 35.7 | 103.6 ± 33.8 | 173.7 ± 126.9 | 151.2 ± 111.4**,*** | 123.2 ± 69.1 | 115.7 ± 64.7** | 0.036 |
| Protein Energy (%) | 16.1 ± 5.23 | 17.0 ± 5.8*,**** | 25.6 ± 10.7 | 25.0 ± 6.4 | 19.6 ± 5.4 | 19.4 ± 5.3**** | <0.001 |
| Fat (g) | 108.9 ± 40.7 | 98.7 ± 40.6*,**** | 62.2 ± 19.4 | 52.7 ± 14.2** | 99.9 ± 35.0 | 92.7 ± 33.5**,**** | <0.001 |
| Fat energy (%) | 36.9 ± 7.9 | 34.9 ± 8.0 | 24.2 ± 9.1 | 23.2 ± 5.1*** | 38.5 ± 5.3 | 37.6 ± 6.4**,***,**** | <0.001 |
| Fiber (g) | 24.8 ± 13.8 | 24.5 ± 11.0 | 25.6 ± 14.8 | 23.3 ± 13.2 | 24.9 ± 12.1 | 23.3 ± 8.5 | 0.54 |
Data presented as the mean ± standard deviation. P** denotes P‐value for repeated measures ancova adjusted for age, baseline body mass index and marital status; *P‐value < 0.05; **P‐value < 0.01; ***significant difference compared with control; ***significant difference compared with group education; significant at P < 0.05.
CG, control group; CHO, carbohydrates; GEP, group educational program; WEP, WhatsApp educational program.
DISCUSSION
The main finding of the present study was that the three diabetes prevention education programs, whether implemented intensively as a group, through social media or by standard advice, were equally efficacious in significantly lowering HbA1c and weight, at 6 months, among Arab women with prediabetes. The clinical superiority of GEP was most apparent in terms of favorable changes observed in HbA1c and weight; including clinically significant reductions in BMI, waist circumference, blood pressure and select lipid profile, as compared with the other strategies.
The present findings are more favorable than recent data obtained from SA 12 . In the former study, only the intensive‐lifestyle monitoring program (ILMP) group with metformin achieved a significant reduction in HbA1c after 12 months intervention as compared with ILMP without metformin and the general advice group. Furthermore, although the ILMP group had a substantial reduction in weight and BMI, this was still inferior compared with the ILMP group with metformin. In contrast, the present study obtained significant reductions in HbA1c in all groups, including the controls. These differences could be attributed to the intervention itself, as the former study’s follow‐up system was not as rigorous as the present, and the primary outcome were different (metabolic syndrome vs HbA1c). The improvement in HbA1c in all groups over time is worthy to note, despite lacking in significance when compared between groups.
Changes in dietary habits
There was a significant decrease in total energy intake in all groups over time, but more so in the GEP group, even after adjusting for baseline covariates. It is widely known that the majority of Saudis, whether adults, adolescents or even preschool children, consume an unhealthy diet with a preponderance for meat and fast food over vegetables and fruits 16 , 17 , 18 , 19 . The significant changes in the dietary intake of all groups also highlight how increasing knowledge alone can drastically improve participants’ choices of meals for the prevention of diabetes. A recent review by Alanazi highlighted that despite massive campaigns, the general public knowledge about diabetes and its risk factors remain relatively low in SA 20 , which in turn, translates to poor adherence and glycemic control, even in children with type 1 diabetes 21 . Primary care providers also lack the up‐to‐date management schemes for major chronic diseases, such as diabetes, and this greatly affects the quality of diabetes management in the kingdom overall 22 .
Changes in lipid profile
Favorable changes were observed in the lipid profile of GEP group over time, and when compared with other groups, these improved changes were clinically significant, especially in triglycerides and low‐density lipoprotein cholesterol independent of age, BMI and marital status. The improvement in the lipid profile of the GEP group can be attributed to the parallel decrease in total energy intake that was also observed in the group, which was lesser in other groups, as well as the clinically significant weight loss. The modestly higher baseline fiber intake in the GEP group, albeit non‐significant, might also explain the better lipid profile over time, as fiber intake is known to promote better cardiovascular risk profile and reduced diabetes risk 23 . Caloric restriction, even at modest levels, has been shown to improve lipid metabolism and consequently decrease inflammatory markers 24 . This improvement in lipids is especially true among overweight and obese individuals, as the dietary restriction is significantly associated with acute body fat reduction, which in turn changes the overall metabolic profile, including lipids 25 . The improved cardiometabolic profile, even in the absence of weight changes, has also been verified in long‐term clinical trials. The 5‐year study of Estruch et al. 26 observed that a high‐fat Mediterranean diet did not lead to significant weight and waist circumference changes, but nevertheless improved cardiometabolic health secondary to bodyweight maintenance. Improvement in lipids secondary to lifestyle modification has been previously observed among the Saudis with prediabetes, as well as those with type 2 diabetes 27 . Cumulatively, the favorable changes in the dietary and lipid profile in the GEP group suggests that despite having lower HbA1c levels over time in all groups, improvements in other cardiometabolic risk factors are more evident using the intensified lifestyle strategy than online or other platforms.
The present study had limitations. Baseline differences in characteristics were observed in groups, and although adjustments were made for major confounders, such as age, baseline BMI and marital status, further adjusting from other covariates increases the likelihood of type 2 error, given the small sample size. Hence, bias can affect the results. The findings are applicable only for a short duration period. Hence, more studies with a longer duration would be required to confirm whether a focused education is more effective over a longer period, or whether the use of social media through WhatsApp can complement groups or the individual one‐to‐one approach. Furthermore, the study used only women with prediabetes, and as such, the findings might be different for men in terms of compliance and adherence. The same intervention study could be used to investigate its effects among Saudi adolescents at risk for diabetes, as they are more tech‐savvy when compared with other demographics. Physical activity was not monitored closely, and this could also explain the favorable changes observed during the intervention. By default, Saudi women are disproportionately much more sedentary than their male counterparts due to restrictions in outdoor activities and the barriers of physical activities 28 . Furthermore, an Arab Muslim woman is more than twice likely to be physically inactive as compared with a non‐Arab, non‐Muslim woman 29 . It is therefore expected that the major strategy that Saudi women will more likely use to reduce weight is through caloric restriction, rather than increased physical activity. Caloric restriction alone, whether a low fat or low carbohydrate diet, in the absence of physical activity, has been shown to improve cardiovascular risk factors among overweight and obese individuals with prediabetes 30 .
Despite the limitations, the findings of the present study are robust, and this is the first known trial carried out among Arab women with prediabetes on the efficacy of different lifestyle intervention programs. More intervention trials are required to include other populations at risk, such as adult men, children and the elderly.
In summary, prediabetes education programs, whether delivered through a focused educational group, social media or standard care, are equally efficacious in improving HbA1c levels among Saudi women with prediabetes, whereas improvements in diet and lipids lean toward the focused educational group. These cumulative changes might have been due to reduced energy intakes and favorable increases in physical activity. Digital platforms might offer a more accessible and less resource intensive option that is equally effective in HbA1c reduction, but might not be as efficacious as the traditional intensified lifestyle modification in terms of weight loss.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
The authors are grateful to the Deanship of Scientific Research, King Saud University (KSU), Riyadh, SA, for funding through Vice Deanship of Scientific Research Chairs.
J Diabetes Investig. 2021; 12: 1872–1880
REFERENCES
- 1. Pal K, Dack C, Ross J, et al. Digital health interventions for adults with type 2 diabetes: qualitative study of patient perspectives on diabetes self‐management education and support. J Med Internet Res 2018; 20: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahin C, Courtney KL, Naylor PJ, et al. Tailored mobile text messaging interventions targeting type 2 diabetes self‐management: a systematic review and meta‐analysis. Digit Health 2019; 5: 2055207619845279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Qahtani MF, Al‐Saffar AK, Alshammasi AR, et al. Social media in healthcare: advantages and challenges perceived by patients at a teaching hospital in eastern province. Saudi Arabia. Saudi J Health Sci 2018; 7: 116–120. [Google Scholar]
- 4. Alnasser A, Sathiaseelan A, Al‐Khalifa A, et al. Development of ‘Twazon’: An Arabic app for weight loss. JMIR Res Protoc 2016; 5: e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alturki R, Gay V. The development of an Arabic weight‐loss app Akser Waznk: Qualitative results. JMIR Form Res 2019; 3: e11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al Asmri M, Almalki MJ, Fitzgerald G, et al. The public health care system and primary care services in Saudi Arabia: a system in transition. East Mediterr Health J 2020; 26: 468–476. [DOI] [PubMed] [Google Scholar]
- 7. Alanzi T. mHealth for diabetes self‐management in the Kingdom of Saudi Arabia: barriers and solutions. J Multidiscip Healthc 2018; 11: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Eisa ES, Al‐Sobayel HI. Physical activity and health beliefs among Saudi women. J Nutr Metab 2012; 2012: 642187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mobaraki AE, Soderfeldt B. Gender inequity in Saudi Arabia and its role in public health. East Mediterr Health J 2010; 16: 113–118. [PubMed] [Google Scholar]
- 10. Daoud F, El Bcheraoui C, Tuffaha M, et al. The health status of Saudi women: findings from a national survey. J Public Health (Oxf) 2016; 38: 660–672. [DOI] [PubMed] [Google Scholar]
- 11. Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ 2001; 322: 355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Fawaz HA, Wani K, Alnaami AM, et al. Effects of different dietary and lifestyle modification therapies on metabolic syndrome in prediabetic Arab patients: a 12‐month longitudinal study. Nutrients 2018; 10: pii: E383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Hamdan R, Avery A, Salter A, et al. Identification of education models to improve health outcomes in Arab women with pre‐diabetes. Nutrients 2019; 11: 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuomilehto J, Lindstrom J, Ericksson J. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 15. Gossadi IM, Alatar AA, Otayf MM, et al. Development of a Saudi food frequency questionnaire and testing its reliability and validity. Saudi Med J 2017; 38: 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al‐Rethaiaa AS, Fahmy AE, Al‐Shwaiyat NM. Obesity and eating habits among college students in Saudi Arabia: a cross‐sectional study. Nutr J 2010; 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin TT, Al‐Sultan AI, Ali A. Overweight and obesity and their relation to dietary habits and socio‐demographic characteristics among male primary school children in Al‐Hassa, Kingdom of Saudi Arabia. Eur J Nutr 2008; 47: 310–318. [DOI] [PubMed] [Google Scholar]
- 18. Khalaf A, Westergren A, Berggren V, et al. Prevalence and association of female weight status and dietary habits with sociodemographic factors: a cross‐sectional study in Saudi Arabia. Public Health Nutr 2015; 18: 784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alsubaie ASR. Consumption and correlates of sweet foods, carbonated beverages, and energy drinks among primary school children in Saudi Arabia. Saudi Med J 2017; 38: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alanazi FK, Alotaibi JS, Paliadelis P, et al. Knowledge and awareness of diabetes mellitus and risk factors in Saudi Arabia. Saudi Med J 2018; 39: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Zahrani AM, Al SA. Glycemic control in children and youth with type 1 diabetes mellitus in Saudi Arabia. Clin Med Insights Endocrinol Diabetes 2019; 12: 1179551418825159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Almutairi KM. Quality of diabetes management in Saudi Arabia: A review of existing barriers. Arch Iran Med 2015; 18: 816–821. [PubMed] [Google Scholar]
- 23. Kimura Y, Yoshida D, Hirakawa Y, et al. Dietary fiber intake and risk of type 2 diabetes in a general Japanese population: The Hisayama Study. J Diabetes Investig 2020;. 10.1111/jdi.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park CY, Park S, Kim MS, et al. Effects of mild calorie restriction on lipid metabolism and inflammation in liver and adpose tissue. Biochem Biophys Res Commun 2017; 490: 636–642. [DOI] [PubMed] [Google Scholar]
- 25. Holowko J, Michalczyk MM, Zajac A, et al. Six weeks of calorie restriction improves body composition and lipid profile in obese and overweight former athletes. Nutrients 2019; 11: pii: E1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estruch R, Martinez‐Gonzalez MA, Corella D, et al. Effect of a high‐fat Mediterranean diet on bodyweight and waist circumference: a pre‐specified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: e6–e17. [DOI] [PubMed] [Google Scholar]
- 27. A‐Arifi MN, Al‐Omar HA. Impact of a multi‐disciplinary intensive education program on type 2 diabetes mellitus patients’ glycemic control and cardiovascular risk factors. Saudi Med J 2018; 39: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Hazzaa HM. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int J Health Sci (Qassim) 2018; 12: 50–64. [PMC free article] [PubMed] [Google Scholar]
- 29. Kahan D. Adult physical inactivity prevalence in the Muslim world: Analysis of 38 countries. Prev Med Rep 2015; 2: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Velasquez‐Lopez L, Gonzalez‐Figueroa E, Media‐Bravo P, et al. Low calorie and carbohydrate diet: to improve the cardiovascular risk indicators in overweight or obese adults with prediabetes. Endocrine 2013; 43: 593–602. [DOI] [PubMed] [Google Scholar]


