Abstract
Aims/Introduction
We estimated the hazards of cardiovascular diseases (CVDs) and early all‐cause mortality in Korean adults according to the presence of recently diagnosed type 2 diabetes (type 2 diabetes for <5 years) and insulin use.
Materials and Methods
We used the Korean National Health Insurance Service–National Sample Cohort database (2002–2015) for this longitudinal population‐based study. Among adults aged ≥40 years without baseline CVD, individuals without diabetes or with recently diagnosed type 2 diabetes were selected (N = 363,919). The hazard ratios (HRs) for myocardial infarction (MI), stroke, and all‐cause mortality during follow‐up were analyzed according to three groups categorized by the presence of type 2 diabetes and insulin use.
Results
Within a mean 7.8 years, there were 5,275 MIs, 7,220 strokes, and 15,834 deaths. The hazards for outcomes were higher in the insulin‐treated type 2 diabetes group than in the non‐diabetes group [HR (95% CI): 2.344 (1.870–2.938) for MI, 2.420 (1.993–2.937) for stroke, and 3.037 (2.706–3.407) for death], higher in the non‐insulin‐treated type 2 diabetes group than in the non‐diabetes group [HR (95% CI): 1.284 (1.159–1.423) for MI, 1.435 (1.320–1.561) for stroke, and 1.135 (1.067–1.206) for death], and higher in the insulin‐treated type 2 diabetes group than in the non‐insulin‐treated type 2 diabetes group [HR (95% CI): 1.914 (1.502–2.441) for MI, 1.676 (1.363–2.060) for stroke, and 2.535 (2.232–2.880) for death].
Conclusions
Recently diagnosed type 2 diabetes patients showed increased risks of incident CVDs and premature mortality, and insulin‐treated group demonstrated an additional increase in the risks of these outcomes in adults with recently diagnosed type 2 diabetes, suggesting the need for intensified cardio‐protective interventions for adults with insulin‐treated type 2 diabetes.
Keywords: Insulin, Mortality, Type 2 diabetes mellitus
In this nationwide, longitudinal, population‐based cohort study including 363,919 individuals either with no diabetes or with recently diagnosed type 2 diabetes, we compared the hazard for myocardial infarction, stroke, and early mortality according to the presence of type 2 diabetes and insulin use in the real‐world utilizing the Korean National Health Insurance Service–National Sample Cohort (NHIS‐NSC) database. Recently diagnosed type 2 diabetes patients showed increased risks of incident myocardial infarction, stroke, and all‐cause mortality during follow‐up, and insulin‐treated group demonstrated an additional increase in the risks of these outcomes in adults with recently diagnosed type 2 diabetes.
![]()
Introduction
Diabetes imposes a burden on society in the form of decreases in labor force participation and productivity and premature mortality due to its potentially fatal complications, including cardiovascular disease 1 , 2 . Previous studies consistently reported an increased risk of cardiovascular and premature all‐cause death associated with diabetes 3 , 4 , 5 . In our previous report 4 , people with type 2 diabetes had an approximately 42% higher hazard of myocardial infarction (MI) and a 51% higher hazard of all‐cause death compared to the population without diabetes within the mean follow‐up of 4.6 years.
In the real‐world, requirement for insulin treatment in people with type 2 diabetes might be associated with increased risk of cardiovascular events and/or early mortality although previous randomized controlled trials (RCTs) that compared insulin to other regimens demonstrated no significant increase in these outcomes in insulin groups 6 , 7 . This is because, in the real‐world, not the randomized controlled setting, individuals with type 2 diabetes requiring insulin treatment based on the decisions of clinicians may have different risk profiles of early all‐cause mortality and cardiovascular disease compared to the individuals with non‐insulin‐treated type 2 diabetes. Nevertheless, to the best of our knowledge, previous real‐world studies that considered the requirement for insulin treatment as a factor for predicting cardiovascular outcomes and early all‐cause mortality that included only people with type 2 diabetes are still scarce, despite potential heterogeneity in hypoglycemia risk 8 , glycemic variability, residual beta‐cell function, and insulin resistance, according to the requirement for insulin treatment in people with type 2 diabetes. Although few studies have explored this aforementioned association among people with type 2 diabetes in non‐randomized settings, their results were not applicable to the overall population with type 2 diabetes in general due to their restricted study populations 9 , 10 , non‐discrimination between diabetes type (type 1 vs type 2) 11 , 12 , lack of information on diabetes duration 10 , marked variance in diabetes duration between groups classified by insulin treatment 9 , and limited treatment regimens 11 . Therefore, to explore whether risk profiles of cardiovascular diseases and early all‐cause mortality vary between individuals with type 2 diabetes required vs not required to be treated with insulin in the real‐world, we compared the risk for cardiovascular diseases (MI and stroke) and all‐cause mortality during follow‐up according to the presence of type 2 diabetes and insulin use through a real‐world nationwide cohort study.
Materials and Methods
Data sources
Data from the Korean National Health Insurance Service–National Sample Cohort (NHIS‐NSC) database from January 2002 to December 2015 were used for this study. All residents in Korea are covered by the Korean National Health Insurance Service (NHIS), the single‐insurer system in Korea 12 . Information associated with healthcare utilization and prescriptions is recorded and stored by the NHIS 12 . This information includes anonymous identification numbers, demographics, monthly household income, primary and secondary diagnoses classified according to the International Classification of Diseases‐10th Revision (ICD‐10), prescriptions, procedures, and dates of hospital visits and hospitalizations for all Korean residents. The NHIS‐NSC was constructed by the Korean NHIS as a representative population‐based sample cohort providing researchers and policy makers with representative datasets with a substantial volume that does not require privacy regulation 12 . From the target population of 48,222,537 individuals that comprised the entire cohort enrolled in the Korean NHIS in 2006, a representative sample cohort of approximately one million was randomly selected using systematic stratified random sampling with proportional allocation within each stratum. These strata were constructed according to variables including age group, sex, residential area (metropolitan, urban, or rural), and income level. Records related to the healthcare utilization of this sample cohort beginning in January 2002 were compiled into NHIS‐NSC datasets. This cohort was followed until December 2015, with the exception of participants whose eligibility was disqualified due to death or emigration.
The Institutional Review Board (IRB) of Korea University approved this study (IRB file number 2018GR0412). An informed consent exemption was granted by the IRB because the NHIS provided the researchers with anonymous, de‐identified data.
Study cohort, outcomes, and follow‐up
In this longitudinal population‐based study, individuals aged ≥40 years, either without diabetes or with recently diagnosed type 2 diabetes (type 2 diabetes for <5 years), were selected from the NHIS‐NSC in 2008 (Figure 1). The index year of 2008 was considered as the baseline. Individuals with any malignancy and those who received organ transplantation throughout all study periods were excluded. In addition, patients with a history of MI or stroke at or before the index year (2008) were also excluded. To unify the type and duration of diabetes, people with T1D (defined according to previous reports 4 , 13 , 14 ) and those with type 2 diabetes duration of ≥5 years were excluded from analysis.
Figure 1.
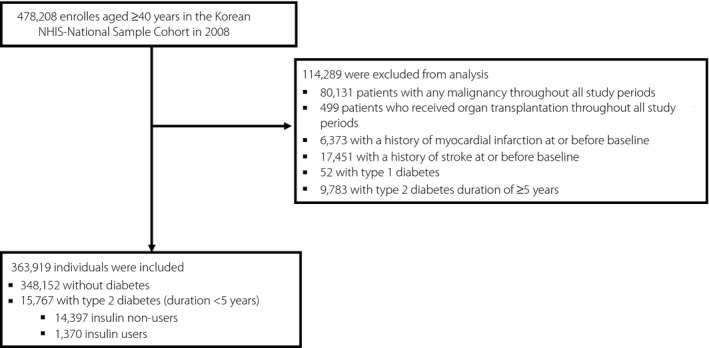
Flow diagram of the study population.
The outcome variables were incident MI, stroke, and all‐cause death during follow‐up. According to previous studies 4 , 15 , 16 , MI was defined as the recording of ICD‐10 codes I21 or I22 during hospitalization or at least two claims under those codes, while stroke was defined as the recording of ICD‐10 codes I63 or I64 during hospitalization with claims for brain magnetic resonance imaging or brain computed tomography. The study population was followed from baseline until the date of death, development of endpoint disease, or December 31, 2015, whichever came first.
Measurements and definitions
The presence of diabetes was assessed at baseline for all participants. According to previous studies 4 , 14 , 17 , 18 , type 2 diabetes was defined as the recording of at least one claim per year for the prescription of antidiabetic drugs under ICD‐10 codes E11–14 or a fasting plasma glucose concentration ≥126 mg/dL, excluding any persons with claims under ICD‐10 code E10. Individuals who did not satisfy the definition of type 2 diabetes were classified into the non‐diabetes group as those with T1D were already excluded from analyses. Insulin use (insulin treatment) was defined for all participants as at least one prescription of insulin per year and a total of at least three prescriptions of insulin in an outpatient setting. Participants were divided into the following three groups according to the presence of recently diagnosed type 2 diabetes and insulin use: no diabetes, non‐insulin‐treated type 2 diabetes, and insulin‐treated type 2 diabetes.
Presence of hypertension 15 , dyslipidemia 15 , atrial fibrillation (AF) 4 , 19 , 20 , hospitalization for heart failure (hHF) 4 , 21 , 22 , dementia 23 , 24 , and end‐stage renal disease (ESRD) 14 were defined according to previous studies. Charlson Comorbidity Index (CCI) was calculated according to the established method 25 , 26 based on the diagnostic codes provided in previous reports 27 , 28 . Low‐income level was assigned to the lowest 20% of the entire population based on monthly household income.
Statistical analyses
The statistical analyses were conducted using SAS software (Version 9.3, SAS Institute, Cary, NC, USA). Two‐tailed P‐values of <0.05 were considered significant. Baseline characteristics are presented according to the three groups categorized by the presence of recently diagnosed type 2 diabetes and insulin use (non‐diabetes group, non‐insulin‐treated type 2 diabetes group, and insulin‐treated type 2 diabetes group). Continuous variables are expressed as means ± standard deviations, and categorical values are presented as frequencies and percentages. The incidence rates of MI, stroke, and all‐cause mortality during follow‐up were estimated using the number of incident cases divided by the follow‐up duration in person‐years. The cumulative incidence of these outcomes according to the three groups categorized by the presence of recently diagnosed type 2 diabetes and insulin use was estimated using Kaplan‐Meier curves; the differences among groups were evaluated using the log‐rank test. Cox regression analyses were performed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of MI, stroke, and all‐cause mortality during follow‐up according to the three pre‐specified groups based on the diabetes status and insulin use. The proportional hazard assumption of the Cox models was ensured by the Schoenfeld residuals. Three regression models were constructed for these analyses: the crude (unadjusted) model, the age and sex‐adjusted model, and the multivariable‐adjusted model, adjusted for age, sex, monthly income, and CCI.
Results
Baseline characteristics of the study population
The study population consisted of a total of 363,919 subjects (Figure 1). Among these, 348,152 had no diabetes, and 15,767 had recently diagnosed type 2 diabetes (diabetes duration <5 years). Of these 15,767 people with recently diagnosed type 2 diabetes, 14,397 were insulin non‐users, and the remaining 1,370 were insulin users. The baseline characteristics of the study population are presented according to the three groups created by the presence of recently diagnosed type 2 diabetes and insulin treatment (Table 1). As the groups advanced from non‐diabetes to non‐insulin‐treated type 2 diabetes to insulin‐treated type 2 diabetes, a trend of increase in the mean age, proportion of individuals aged ≥65 years and with low‐income level, and prevalence of hypertension, dyslipidemia, AF, hHF, dementia, and ESRD was observed.
Table 1.
Baseline characteristics according to the presence of diabetes and insulin use
| No diabetes (n = 348,152) | Type 2 diabetes, insulin non‐user (diabetes duration <5years) (n = 14,397) | Type 2 diabetes, insulin user (diabetes duration <5years) (n = 1,370) | P‐value | P for trend | |
|---|---|---|---|---|---|
| Age (years) | 53.6 ± 11.1 | 59.5 ± 11.3 | 60.3 ± 11.9 | <0.0001 | <0.0001 |
| Age ≥ 65 years | 60,227 (17.30) | 4839 (33.61) | 529 (38.61) | <0.0001 | <0.0001 |
| Men | 164,349 (47.21) | 8011 (55.64) | 724 (52.85) | <0.0001 | <0.0001 |
| Low‐income level (lowest 20%) | 64,059 (18.40) | 3323 (23.08) | 447 (32.63) | <0.0001 | <0.0001 |
| Charlson Comorbidity Index | 0.6 ± 1.0 | 2.1 ± 1.5 | 3.1 ± 1.6 | <0.0001 | <0.0001 |
| Comorbidities | |||||
| Hypertension | 39,971 (11.48) | 5,840 (40.56) | 706 (51.53) | <0.0001 | <0.0001 |
| Dyslipidemia | 16,844 (4.84) | 3,773 (26.21) | 459 (33.50) | <0.0001 | <0.0001 |
| Atrial fibrillation | 1,292 (0.37) | 135 (0.94) | 30 (2.19) | <0.0001 | <0.0001 |
| Previous hospitalization for heart failure | 277 (0.08) | 46 (0.32) | 34 (2.48) | <0.0001 | <0.0001 |
| Dementia | 94 (0.03) | 9 (0.06) | 2 (0.15) | 0.0018 | 0.0006 |
| End‐stage renal disease | 243 (0.07) | 14 (0.10) | 32 (2.34) | <0.0001 | <0.0001 |
Values are presented as number (%) or mean ± standard deviation.
Incidence of cardiovascular disease and all‐cause mortality during follow‐up according to the presence of recently diagnosed type 2 diabetes and insulin use
During a mean follow‐up of 7.79 ± 1.05 years (2,833,111.00 person‐years), 5,275 MI cases developed, whereas 7,220 cases of stroke occurred during a mean follow‐up of 7.77 ± 1.09 years (2,827,787.58 person‐years). During a mean follow‐up of 7.83 ± 0.93 years (2,850,265.46 person‐years), 15,834 deaths were observed in the entire cohort. The cumulative incidence of MI, stroke, and all‐cause death during follow‐up is presented according to the three groups of diabetes status and insulin use using Kaplan‐Meier curves (Figure 2). The incidence rates of MI, stroke, and all‐cause death during follow‐up were higher in people with type 2 diabetes (insulin users and non‐users) compared to individuals without diabetes (Figure 2, Table 2). People with insulin‐treated type 2 diabetes had higher incidence rates of MI, stroke, and all‐cause mortality during follow‐up than those with non‐insulin‐treated type 2 diabetes (Figure 2, Table 3).
Figure 2.
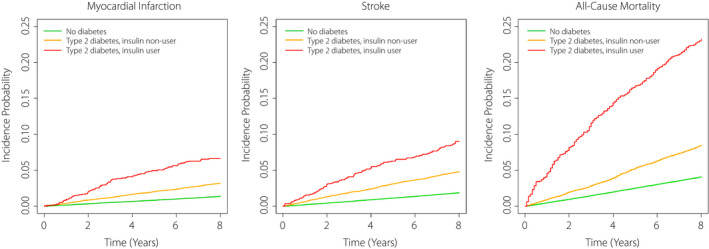
Cumulative incidence of myocardial infarction, stroke, and all‐cause death during follow‐up according to the presence of diabetes and insulin use. Only those with diabetes duration of <5 years were included among people with type 2 diabetes.
Table 2.
Hazard ratios and 95% confidence intervals for the incidence of myocardial infarction, stroke, and all‐cause death during follow‐up, according to the presence of diabetes and insulin use among individuals either without diabetes or with recently diagnosed type 2 diabetes (diabetes for <5 years)
| Group | Events (n) | Follow‐up duration (person‐years) | Incidence rate (per 1000 person‐years) | Unadjusted | Age and sex‐adjusted | Multivariable adjusted* |
|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||
| No diabetes (n = 348,152) | 4,749 | 2,714,833.85 | 1.74928 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 445 | 109,030.08 | 4.08144 | 2.334 (2.117, 2.572) | 1.589 (1.441, 1.751) | 1.284 (1.159, 1.423) |
| Type 2 diabetes, insulin user † (n = 1,370) | 81 | 9,247.07 | 8.75953 | 5.017 (4.029, 6.248) | 3.360 (2.698, 4.186) | 2.344 (1.870, 2.938) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
| Stroke | ||||||
| No diabetes (n = 348,152) | 6,437 | 2,710,183.97 | 2.37510 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 673 | 108,423.56 | 6.20710 | 2.615 (2.416, 2.832) | 1.685 (1.556, 1.825) | 1.435 (1.320, 1.561) |
| Type 2 diabetes, insulin user † (n = 1,370) | 110 | 9,180.05 | 11.98250 | 5.068 (4.198, 6.118) | 3.185 (2.638, 3.846) | 2.420 (1.993, 2.937) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
| All‐cause death | ||||||
| No diabetes (n = 348,152) | 14,290 | 2,730,259.00 | 5.23390 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 1,226 | 110,508.11 | 11.09420 | 2.121 (2.000, 2.248) | 1.257 (1.185, 1.332) | 1.135 (1.067, 1.206) |
| Type 2 diabetes, insulin user † (n = 1,370) | 318 | 9,498.35 | 33.47950 | 6.409 (5.735, 7.162) | 3.620 (3.239, 4.046) | 3.037 (2.706, 3.407) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
Adjusted for age, sex, monthly income, and Charlson Comorbidity Index.
Only those with diabetes duration of <5 years were included among people with type 2 diabetes.
Bold indicates statistically significant values among the hazard ratios.
Table 3.
Hazard ratios and 95% confidence intervals for the incidence of myocardial infarction, stroke, and all‐cause death during follow‐up according to insulin use among people with type 2 diabetes (diabetes duration <5 years)
| Group | Events (n) | Follow‐up duration (person‐years) | Incidence rate (per 1000 person‐years) | Unadjusted | Age and sex‐adjusted | Multivariable adjusted* |
|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 445 | 109,030.08 | 4.08144 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin user † (n = 1,370) | 81 | 9,247.07 | 8.75953 | 2.141 (1.690, 2.713) | 2.123 (1.675, 2.690) | 1.914 (1.502, 2.441) |
| P‐value | <0.0001 | <0.0001 | <0.0001 | |||
| Stroke | ||||||
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 673 | 108,423.56 | 6.20710 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin user † (n = 1,370) | 110 | 9,180.05 | 11.98250 | 1.926 (1.575, 2.357) | 1.89 (1.545, 2.312) | 1.676 (1.363, 2.060) |
| P‐value | <0.0001 | <0.0001 | <0.0001 | |||
| All‐cause death | ||||||
| Type 2 diabetes, insulin non‐user † (n = 14,397) | 1,226 | 110,508.11 | 11.09420 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Type 2 diabetes, insulin user † (n = 1,370) | 318 | 9,498.35 | 33.47950 | 3.020 (2.670, 3.417) | 2.906 (2.569, 3.288) | 2.535 (2.232, 2.880) |
| P‐value | <0.0001 | <0.0001 | <0.0001 | |||
Adjusted for age, sex, monthly income, and Charlson Comorbidity Index.
Only those with diabetes duration of <5 years were included among people with type 2 diabetes.
Bold indicates statistically significant values among the hazard ratios.
The HRs (95% CIs) for incident MI, stroke, and all‐cause death during follow‐up were calculated with respect to the three groups according to diabetes status and insulin use (Table 2). In all models including the multivariable‐adjusted model, the hazards of incident MI, stroke, and all‐cause death during follow‐up were higher among people with type 2 diabetes (insulin users and non‐users) than among people without diabetes. In the multivariable‐adjusted model, which was adjusted for age, sex, monthly income, and CCI, the HRs (95% CIs) in the non‐insulin‐treated type 2 diabetes compared with the non‐diabetes group were 1.284 (1.159–1.423) for MI, 1.435 (1.320–1.561) for stroke, and 1.135 (1.067–1.206) for all‐cause death during follow‐up. The HRs (95% CIs) in the insulin‐treated type 2 diabetes group compared with the non‐diabetes group were 2.344 (1.870–2.938) for MI, 2.420 (1.993–2.937) for stroke, and 3.037 (2.706–3.407) for all‐cause death during follow‐up.
When the people with non‐insulin‐treated type 2 diabetes were set as the reference (Table 3), the HRs (95% CI) of incident MI, stroke, and all‐cause death during follow‐up among those with insulin‐treated type 2 diabetes showed significantly higher values of 1.914 (1.502–2.441) for MI, 1.676 (1.363–2.060) for stroke, and 2.535 (2.232–2.880) for all‐cause death during follow‐up in the multivariable‐adjusted model.
As an additional analysis, only individuals either without diabetes or with longer‐standing type 2 diabetes (type 2 diabetes for ≥5 years) were selected, and HRs (95% CIs) for the incidence of MI, stroke, and all‐cause mortality during follow‐up were calculated according to the three pre‐specified groups based on the diabetes status and insulin use (Table S1). This additional analysis showed results consistent with those from the main analysis.
Discussion
In this nationwide longitudinal study including 363,919 individuals either with no diabetes or with recently diagnosed type 2 diabetes (diabetes duration <5 years), individuals with insulin‐treated type 2 diabetes had a higher hazard of MI, stroke, and all‐cause mortality during follow‐up compared with those with non‐insulin‐treated type 2 diabetes or no diabetes. These findings were consistent after adjusting for age, sex, monthly income, and CCI.
Increased risks of cardiovascular and early all‐cause mortality in people with diabetes have been consistently documented in previous studies 3 , 4 , 5 . People with type 2 diabetes from the Swedish National Diabetes Register showed a 15% increased risk of all‐cause death and a 14% increased risk of cardiovascular mortality compared to the age, sex, and county‐matched controls during a mean follow‐up of <5 years (mean 4.6 years in the diabetes group and 4.8 years in the control group) 5 . In the current study, people with non‐insulin‐treated type 2 diabetes were at a 13.5% increased hazard of all‐cause death during follow‐up compared to the non‐diabetes population, which is similar to an overall 15% increased risk in people with type 2 diabetes from the Swedish National Diabetes Register compared to controls without diabetes 5 . However, individuals with insulin‐treated type 2 diabetes showed a much higher 3.037‐fold (203.7%) increased hazard of all‐cause death during follow‐up compared to the non‐diabetes controls in the current study.
Insulin‐treated patients compared to those without insulin treatment had a significantly higher hazard of MI, stroke, and all‐cause mortality during follow‐up among people with type 2 diabetes in this real‐world study. This finding suggests that people with insulin‐treated type 2 diabetes should be considered as a population with higher risk for cardiovascular disease and early all‐cause mortality, and related monitoring and cardio‐protective interventions should be focused on this population to reduce the social burden associated with these fatal complications. Few previous studies have evaluated insulin use as a predictor of cardiovascular outcomes and all‐cause mortality during follow‐up among people with type 2 diabetes. However, such studies had the following limitations which restricted the application of their results to the general type 2 diabetes population. Although Smooke et al. 9 and Anselmino et al. 10 reported increased premature all‐cause mortality associated with insulin‐treated diabetes, they limited the study population to patients with advanced systolic heart failure referred to a single center 9 , or to patients with established coronary artery disease 10 . Furthermore, the type of diabetes (type 1 vs type 2) was not discriminatively described 9 , 10 , and the duration of diabetes was not presented 10 or was markedly varied between the insulin‐treated and non‐insulin‐treated groups 9 . In a retrospective cohort study, Currie et al. 11 compared the risk of adverse outcomes, including the first major adverse cardiac event and all‐cause mortality during follow‐up in people with type 2 diabetes treated with five different regimens: metformin alone, sulfonylurea alone, insulin alone, a metformin plus sulfonylurea combination regimen, and an insulin plus metformin combination regimen. In their analyses 11 , insulin monotherapy was associated with higher hazards for major adverse cardiac events and all‐cause mortality during follow‐up compared to the metformin monotherapy, and the hazard of all‐cause mortality during follow‐up was higher for insulin monotherapy vs all other regimens. However, prescriptions for people with type 2 diabetes in their study were restricted to only five regimens consisting of metformin, sulfonylurea, insulin monotherapy, and sulfonylurea or insulin combined with metformin, and the implication of overall requirement for insulin treatment in type 2 diabetes as a predictor of adverse outcomes was not fully explored. In an analysis including 8,192 overweight individuals with type 2 diabetes at high risk of cardiovascular disease from the Sibutramine Cardiovascular Outcomes (SCOUT) trial 29 , insulin monotherapy was associated with a higher risk of cardiovascular events than metformin monotherapy or diet‐only treatment. However, analysis of risks associated with overall use of insulin compared with no insulin use in the same study 29 showed neutral effects on cardiovascular events.
Considering that previous RCTs demonstrated no significant increase in cardiovascular outcomes and early mortality associated with insulin treatment itself 6 , 7 , the higher hazards of cardiovascular disease and all‐cause mortality during follow‐up among people with insulin‐treated type 2 diabetes compared to people with non‐insulin‐treated type 2 diabetes in the current study may originate from the following factors rather than the effect of insulin itself. First, differences in glycemic control states possibly due to variations in insulin resistance and/or residual beta‐cell function may have affected the results, although this could not be determined in this study due to a lack of information. Since diabetes is a progressive disease with a wide spectrum, physicians in the real‐world will prescribe treatment regimens reflecting this progression and disease control states in each patient, especially for type 2 diabetes 11 . For example, people with type 2 diabetes who are effectively controlled with lifestyle modification only or one or two oral medications will remain on these treatments, whereas those who develop severe hyperglycemia or complications and those who fail to maintain near euglycemia with previous medications are more likely to be treated more aggressively by initiating insulin therapy. For this reason, insulin users, especially among people with type 2 diabetes, may have unfavorable characteristics in the sense of insulin resistance and/or residual beta‐cell function. Second, higher glycemic variability among people with insulin‐treated type 2 diabetes compared to those with non‐insulin‐treated type 2 diabetes may have contributed to our findings. Recently, glycemic variability in diabetes has emerged as an important factor in predicting adverse outcomes including cardiovascular events and early all‐cause mortality 30 , 31 . Glycemic variability has been associated with plaque vulnerability and subclinical coronary atherosclerosis 30 , 32 , 33 . Glycemic variability in type 2 diabetes is closely correlated with the loss of beta‐cell function 34 , 35 , which might be associated with the requirement for insulin treatment. Therefore, individuals with insulin‐treated type 2 diabetes might have had greater glycemic variability, although our dataset does not include this information. Third, more frequent hypoglycemic episodes among people with insulin‐treated type 2 diabetes than among those with non‐insulin‐treated type 2 diabetes may have affected our findings. According to an analysis of 828 day‐patient glycemic profiles from people with both types of diabetes (T1D, insulin‐treated type 2 diabetes, and non‐insulin‐treated type 2 diabetes) 36 , hypoglycemic episodes were second most frequent in people with insulin‐treated type 2 diabetes and least frequent in those with non‐insulin‐treated type 2 diabetes. Severe hypoglycemia has been closely related to the risk of cardiovascular events and early all‐cause mortality in populations with both types of diabetes 8 , 37 through the effect of low blood glucose itself or activated sympathoadrenal response, promotion of inflammatory and pro‐thrombotic status, and alterations in hemodynamics, electrophysiology, and myocardial perfusion 37 . Lastly, confounding by underlying conditions which affect the requirement for insulin treatment may have influenced the results despite the adjustment for CCI. For instance, the prevalence of ESRD was higher in individuals with insulin‐treated type 2 diabetes than in those with non‐insulin‐treated type 2 diabetes.
Several limitations of our study should be acknowledged. First, due to the study design, clarification of causal relationships and underlying mechanisms is inevitably limited. Second, because only Koreans were included in this study, extrapolation of our findings to different ethnic populations should be conducted with caution. Third, the type 2 diabetes patients included in this study were restricted to individuals that were recently diagnosed (type 2 diabetes for <5 years). However, additional analyses including only individuals either without diabetes or with longer‐standing type 2 diabetes (type 2 diabetes for ≥5 years) demonstrated consistent findings (Table S1). Fourth, considering the nature of an observational study, which is subject to potential confounding, unmeasured confounders might have affected the findings, despite our maximal efforts to adjust for measured potential confounders. For example, measures of glycemic control status including glycated hemoglobin were not collected. In addition, higher prevalence of underlying comorbidities including renal insufficiency in individuals with insulin‐treated type 2 diabetes, which makes the use of oral diabetic medications difficult, may have affected the findings. However, the main objective of our study was not to evaluate the effect of insulin itself in a well‐controlled setting. Rather, we aimed to explore the varied risk profiles for cardiovascular diseases and all‐cause mortality during follow‐up in the real‐world according to the presence of type 2 diabetes and insulin treatment determined by clinicians in real practice. Despite these limitations, our study has major strengths. We used a validated nationwide cohort database, provided by the Korean government, representing the Korean population, and reflecting real‐world practice in Korea. No participants were excluded for having any missing values on at least one variable.
In summary, in this nationwide, population‐based real‐world cohort study that included 363,919 Korean adults, individuals with recently diagnosed type 2 diabetes exhibited higher hazards for incident MI, stroke, and all‐cause mortality during follow‐up than those without diabetes, and among individuals with recently diagnosed type 2 diabetes, insulin‐treated patients demonstrated an additional increase in the hazards of these adverse outcomes. This excess hazard of adverse outcomes in insulin users might have originated from unfavorable features of this population in the sense of comorbidities, insulin resistance and/or residual beta‐cell function, and subsequent glycemic variability and increased hypoglycemic events. The increased hazards of cardiovascular disease and all‐cause mortality during follow‐up in people with diabetes, particularly insulin‐treated diabetes, as demonstrated in this study, represent a substantial burden on society through the loss of labor force participation and productivity. Therefore, our findings advance the argument that close monitoring for cardiovascular risks and cardio‐protective interventions should be intensified for Korean adults with diabetes, especially those with insulin‐treated diabetes.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Table S1 | Hazard ratios and 95% confidence intervals for the incidence of myocardial infarction, stroke, and all‐cause death according to the presence of diabetes and insulin use among individuals either without diabetes or with longer‐standing type 2 diabetes (diabetes for ≥5 years).
Acknowledgments
This work was supported by a grant (G.K., 2018F‐Hyangseol) from the Korean Diabetes Association. This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI19C0543). The funding sources had no role in the design, collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication. This work was performed using a database from the National Health Insurance Service (No. NHIS‐2019‐2‐073), and the results do not necessarily represent the opinion of the National Health Insurance Service.
J Diabetes Investig. 2021; 12: 1855–1863
Contributor Information
Gyuri Kim, Email: star4021@gmail.com.
Jae Hyeon Kim, Email: jaehyeon@skku.edu.
References
- 1. Dall TM, Yang W, Gillespie K, et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2019; 42: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018; 41: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Svane J, Lynge TH, Pedersen‐Bjergaard U, et al. Cause‐specific mortality in children and young adults with diabetes mellitus: a Danish nationwide cohort study. Eur J Prev Cardiol 2019. 10.1177/2047487319836550 [DOI] [PubMed] [Google Scholar]
- 4. Lee Y‐B, Han K, Kim B, et al. Risk of early mortality and cardiovascular disease in type 1 diabetes: a comparison with type 2 diabetes, a nationwide study. Cardiovasc Diabetol 2019; 18: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med 2015; 373: 1720–1732. [DOI] [PubMed] [Google Scholar]
- 6. Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 7. Group UPDSU . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 8. Khunti K, Davies M, Majeed A, et al. Hypoglycemia and risk of cardiovascular disease and all‐cause mortality in insulin‐treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 2015; 38: 316–322. [DOI] [PubMed] [Google Scholar]
- 9. Smooke S, Horwich TB, Fonarow GC. Insulin‐treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149: 168–174. [DOI] [PubMed] [Google Scholar]
- 10. Anselmino M, Ohrvik J, Malmberg K, et al. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29: 177–184. [DOI] [PubMed] [Google Scholar]
- 11. Currie CJ, Poole CD, Evans M, et al. Mortality and other important diabetes‐related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab 2013; 98: 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J, Lee JS, Park SH, et al. Cohort profile: The National Health Insurance Service‐National Sample Cohort (NHIS‐NSC), South Korea. Int J Epidemiol 2017; 46: e15. [DOI] [PubMed] [Google Scholar]
- 13. Lee YB, Han K, Kim B, et al. High proportion of adult cases and prevalence of metabolic syndrome in type 1 diabetes mellitus population in Korea: a nationwide study. Diabetes Metab J 2019; 43: 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee YB, Han K, Kim B, et al. Risk of end‐stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: a comparison with type 2 diabetes and the role of metabolic syndrome. Diabetes Metab Res Rev 2019; 35: e3197. [DOI] [PubMed] [Google Scholar]
- 15. Kim MK, Han K, Kim HS, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population‐based study. Eur Heart J 2017; 38: 3560–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim MK, Han K, Koh ES, et al. Blood pressure and development of cardiovascular disease in Koreans with type 2 diabetes mellitus. Hypertension 2019; 73: 319–326. [PubMed] [Google Scholar]
- 17. Noh J, Han KD, Ko SH, et al. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co‐morbidities during an 8‐year‐follow‐up of nationwide Korean population. Sci Rep 2017; 7: 46656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee YB, Kim DH, Kim SM, et al. Risk of type 2 diabetes according to the cumulative exposure to metabolic syndrome or obesity: a nationwide population‐based study. J Diabetes Investig 2020; 11: 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim TH, Yang PS, Kim D, et al. CHA2DS2‐VASc score for identifying truly low‐risk atrial fibrillation for stroke: a Korean Nationwide Cohort Study. Stroke 2017; 48: 2984–2990. [DOI] [PubMed] [Google Scholar]
- 20. Kim TH, Yang PS, Uhm JS, et al. CHA2DS2‐VASc Score (Congestive Heart Failure, Hypertension, Age >/=75 [Doubled], Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack [Doubled], Vascular Disease, Age 65–74, Female) for Stroke in Asian Patients With Atrial Fibrillation: A Korean Nationwide Sample Cohort Study. Stroke 2017; 48: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Hur H, Lee JW, et al. Long‐term risk of congestive heart failure in younger breast cancer survivors: a nationwide study by the SMARTSHIP group. Cancer 2020; 126: 181–188. [DOI] [PubMed] [Google Scholar]
- 22. Yun JS, Park YM, Han K, et al. Severe hypoglycemia and the risk of cardiovascular disease and mortality in type 2 diabetes: a nationwide population‐based cohort study. Cardiovasc Diabetol 2019; 18: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HM, Lee YH, Han K, et al. Impact of diabetes mellitus and chronic liver disease on the incidence of dementia and all‐cause mortality among patients with dementia. Medicine 2017; 96: e8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weng W, Tian Y, Kong SX, et al. Impact of atherosclerotic cardiovascular disease on healthcare resource utilization and costs in patients with type 2 diabetes mellitus in a real‐world setting. Clin Diabetes Endocrinol 2020; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 26. Fernando DT, Berecki‐Gisolf J, Newstead S, et al. The Australian Injury Comorbidity Indices (AICIs) to predict in‐hospital complications: a population‐based data linkage study. PLoS One 2020; 15: e0238182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sundararajan V, Henderson T, Perry C, et al. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol 2004; 57: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 28. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 29. Ghotbi AA, Kober L, Finer N, et al. Association of hypoglycemic treatment regimens with cardiovascular outcomes in overweight and obese subjects with type 2 diabetes: a substudy of the SCOUT trial. Diabetes Care 2013; 36: 3746–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019; 7: 221–230. [DOI] [PubMed] [Google Scholar]
- 31. Gorst C, Kwok CS, Aslam S, et al. Long‐term glycemic variability and risk of adverse outcomes: a systematic review and meta‐analysis. Diabetes Care 2015; 38: 2354–2369. [DOI] [PubMed] [Google Scholar]
- 32. Gohbara M, Hibi K, Mitsuhashi T, et al. Glycemic variability on continuous glucose monitoring system correlates with non‐culprit vessel coronary plaque vulnerability in patients with first‐episode acute coronary syndrome ‐ optical coherence tomography study. Circ J 2016; 80: 202–210. [DOI] [PubMed] [Google Scholar]
- 33. Yang HK, Kang B, Lee SH, et al. Association between hemoglobin A1c variability and subclinical coronary atherosclerosis in subjects with type 2 diabetes. J Diabetes Complications 2015; 29: 776–782. [DOI] [PubMed] [Google Scholar]
- 34. Marling CR, Schwartz FL. Glycemic variability in type 1 diabetes—does it matter? US Endocrinology 2014; 10: 20. [Google Scholar]
- 35. Kramer CK, Choi H, Zinman B, et al. Glycemic variability in patients with early type 2 diabetes: the impact of improvement in β‐cell function. Diabetes Care 2014; 37: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 36. Monnier L, Colette C, Dejager S, et al. Near normal HbA1c with stable glucose homeostasis: the ultimate target/aim of diabetes therapy. Rev Endocr Metab Disord 2016; 17: 91–101. [DOI] [PubMed] [Google Scholar]
- 37. Hanefeld M, Frier BM, Pistrosch F. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care 2016; 39(Suppl 2): S205–S209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Hazard ratios and 95% confidence intervals for the incidence of myocardial infarction, stroke, and all‐cause death according to the presence of diabetes and insulin use among individuals either without diabetes or with longer‐standing type 2 diabetes (diabetes for ≥5 years).


