Abstract
Background
Acquired factor X deficiency is an uncommon condition, and affected individuals have severe and spontaneous bleeding. The associated conditions include malignancy, infection, burn, and inflammatory bowel disease. Many previous studies reported association between lymphoproliferative disease and factor X disappearance. Amyloid deposition causing factor X absorption was the most common mechanism. Here, we report a case of stage IV lymphoplasmacytic lymphoma (LPL) with factor X deficiency who was successfully treated with bendamustine plus rituximab (BR) regimen.
Case Presentation
A 52-year-old Thai woman presented with heavy menorrhea, hoarseness, and widespread ecchymosis at her extremities. On physical examination, the patient had bilateral periorbital purpura and vocal cord hematoma. Coagulation testing showed prolonged prothrombin time (PT) and prolonged activated thromboplastin time (aPTT); however, after mixing with 1:1 normal pooled plasma, PT and aPTT were both corrected to normal levels. Factor assays demonstrated markedly decreased factor X levels, but no presence of factor X inhibitor. Bone marrow examination revealed numerous abnormal lymphoplasmacytoid lymphocytes with kappa light chain expression. Serum free light chain assay also showed kappa light chain restriction [kappa 716.16 mg/L, lambda 16.96 mg/L, ratio 42.23 (0.26–1.65)]. The patient was diagnosed as lymphoplasmacytic lymphoma with factor X deficiency. She received chemotherapy with 6 cycles of bendamustine plus rituximab (BR) regimen. The patient responded favorably to treatment, she remains in lymphoma remission at one year after diagnosis, and her factor X level was more than 20%.
Conclusion
We performed a literature review to identify previous case reports about lymphoma-associated factor X deficiency or inhibitor to determine a possible explanation in our patient. It is important to emphasize that when patients present with acquired factor deficiency, including factor X, lymphoproliferative disease is commonly one of the underlying conditions. Furthermore, the recovery of coagulation factor deficiency is possible if successful remission of lymphoma can be achieved.
Keywords: factor X, lymphoproliferative disorder, lymphoplasmacytic lymphoma, amyloidosis
Introduction
Factor X, which is a vitamin k-dependent coagulation factor, is synthesized by hepatocytes.1 It is activated by tissue factor and FVIIa from the extrinsic pathway.2 Factor IXa and factor VIIIa from the intrinsic pathway work concomitantly to change factor X to active form.2 The resulting effect is the formation of thrombin and fibrin clots.2 Bleeding manifestation in deficient patients is well correlated with the amount of residual factor X activity.3 Patients with a factor X level less than 10–20 IU/dL usually experience severe and spontaneous bleeding.4–6 The estimated prevalence of congenital factor X deficiency (Stuart-Prower disease) is approximately 1 in 1 million; however, data specific to factor X acquired deficiency/inhibitor remains scarce.3,6 Being different from other coagulation factors, the presence of factor X auto-inhibitor is less common than factor X deficiency.7 Previous studies reported factor X inhibitor to be associated with respiratory tract infection, leprosy, gastrointestinal lymphoma, burn, and inflammatory bowel disease.8–11 In contrast, the most common cause of acquired factor X deficiency is light chain amyloidosis, which is a plasma cell dyscrasias that is characterized by deposition of amyloid fibrils from abnormal monoclonal immunoglobulins.12 The proposed mechanism of factor X deficiency in systemic amyloidosis is rapid clearance of factor X by amyloid fibril absorption and probably splenic sequestration.13,14 Here, we report a case of an adult Thai female patient who was recently diagnosed as lymphoplasmacytic lymphoma with factor X deficiency due to suspected amyloid deposition.
Case Presentation
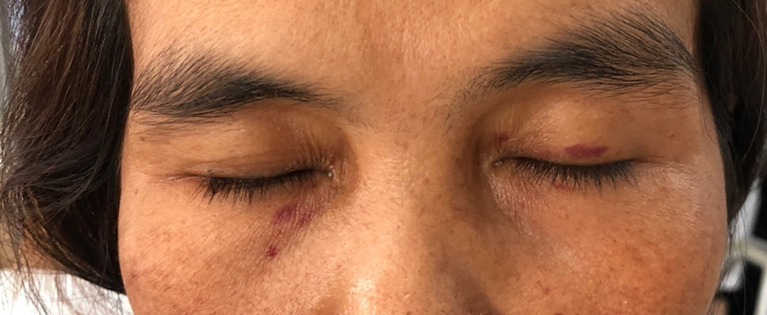
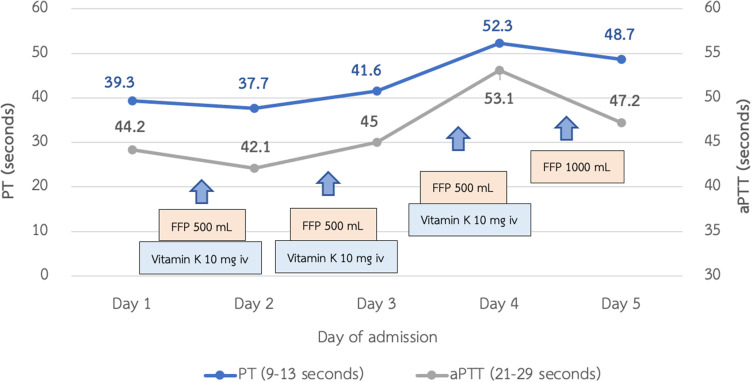
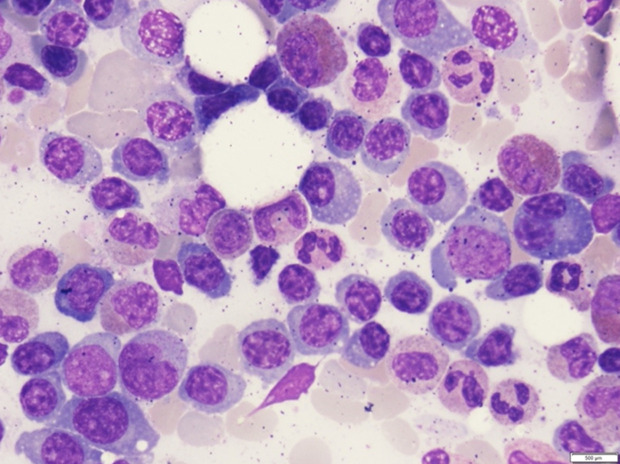
A 52-year-old Thai woman presented with heavy menorrhea for 4 months. Two months later, she felt fatigue, anorexia, and noticed spontaneous hematoma along her extremities. Two weeks ago, she had a low-grade fever and odynophagia, so she visited a primary hospital and physical examination showed a cachectic woman with an 8 cm hematoma at her left forearm. Throat exam showed ecchymosis at the epiglottis and vallecula. Non-blanchable erythematous patches were noticed at both upper eyelids (Figure 1). Other organ systems were within normal limits. During admission at that primary hospital, her laboratory tests showed prolonged prothrombin time (PT) and prolonged activated thromboplastin time (aPTT) with no recovery after plasma and vitamin K replacement (Figure 2). However, her hematoma was improved without new bleeding symptoms. She was then transferred to our hospital, which is a national tertiary referral center, for further investigation. Her initial complete blood count showed hemoglobin of 8.5 g/dl, mean corpuscular volume of 80 femtolitres, a white blood cell count of 6.9 × 109/L (neutrophil 65.4%, lymphocyte 62%, monocyte 5%), and increased platelet count of 800 × 109/L. Blood chemistry showed LDH level of 235 U/L (normal 135–214 U/L), normal liver function tests, and normal creatinine level. Coagulation study showed prolonged PT and prolonged aPTT (65.7 seconds (10.5–12.5) and 85.1 seconds (22.0–31.0), respectively). After mixing with 1:1 normal pooled plasma, PT and aPTT was 13.8 seconds and 28.8 seconds, respectively. The fibrinogen level was 410 mg/dl (200–400). The levels from coagulation factor II, V, and X assay were 117.7% (50–150%), 125.5% (50–150%), and 2.1% (50–150%), respectively. The Bethesda assay of factor X inhibitor was negative. The result of the mixing study suggested that this patient had no functional auto-antibodies. However, our patient’s PT and aPTT levels did not improve after plasma or vitamin K replacement. Bone marrow Wright’s stain and pathological examination showed 50–60% infiltrations of kappa+/CD20+ small lympho-plasmacytoid lymphoid cells (Figure 3). Congo red stain was negative. Serum protein electrophoresis and immunofixation were normal. Free kappa light chain was markedly elevated [Kappa 716.16 mg/L, Lambda 16.96 mg/L, ratio 42.23 (0.26–1.65)]. A diagnosis of stage IV lymphoplasmacytic lymphoma was made. Based on the presence of typical skin changes and light chain monoclonal gammopathy, factor X deficiency from systemic amyloidosis was suspected. To demonstrate amyloid deposition, we carefully performed an abdominal fat pad biopsy immediately after fresh frozen plasma replacement. No bleeding consequence developed after the procedure, and the result was negative. More extensive biopsy was not performed due to a concern about bleeding complication. Echocardiography showed no specific pattern of cardiac amyloidosis. Computed tomography scan of neck, chest, and abdomen revealed multiple enhancing mediastinal lymph nodes, the largest of which was 1.5 cm in diameter.
Figure 1.
Periorbital purpura; skin changes in this patient.
Figure 2.
Treatment and coagulogram at previous hospital.
Figure 3.
Bone marrow aspirate smear.
Systemic chemotherapy of bendamustine plus rituximab (BR) for 6 cycles was the chosen regimen to minimize toxicities. A good correlation between factor X normalization and disease remission was demonstrated. Improvement in abnormal laboratory results after treatment are shown in Table 1. Since our patient’s residual factor X increased to more than 10 IU/dL, she had no spontaneous bleeding. At 12 months after diagnosis, she maintained a good response after BR regimen with a factor X level of approximately 22%.
Table 1.
Follow-Up Laboratory Investigations After Treatment
| Lab | Normal | At Diagnosis | After 1st BR | After 2nd BR | After 3rd BR | After 4th BR | After 5th BR | After 6th BR |
|---|---|---|---|---|---|---|---|---|
| PT | 10.5–12.5 s | 65.7 | 67.6 | 47.7 | N/A | 25.4 | 22.5 | 18.7 |
| APTT | 22.0–31.0 s | 85.1 | 64.6 | 66.2 | N/A | 44.1 | 38.8 | 40.7 |
| Fibrinogen | 200–400 mg/dl | 410.1 | 279.0 | N/A | N/A | N/A | N/A | N/A |
| Factor X assay | 50–150% | 2.1 | 1.1 | 1.5 | N/A | 5.4 | 9.8 | 13.9 |
| Serum free light chain (mg/L) | κ: 3.30–19.40; λ: 5.71–26.30 κ:λ ratio: 0.26–1.65 |
κ 716.16; λ 16.96 (Ratio 42.23) | κ 131.22; λ 15.95 (Ratio 8.23) | N/A | κ 20.47; λ 7.51 (Ratio 2.73) | N/A | N/A | κ 12.07; λ 5.53 (Ratio 2.18) |
| Bone marrow study | Small B-cell lymphoid neoplasm with plasmacytic differentiation, favor lympho-plasmacytic lymphoma (LPL) 50–60% of total nucleated marrow cells | N/A | N/A | A small number of scattered PAX5+ small and medium-sized B cells 2–3% of total nucleated marrow cells | N/A | N/A | No abnormal lymphoid cells seen | |
| CT neck, chest, abdomen | Multiple mediastinal nodes at left upper paratracheal, both lower paratracheal, and subcarinal regions, the largest one is measured about 1.5 cm in diameter | No significant intrathoracic and intraabdominal lymph node enlargement | No significant intrathoracic and intraabdominal lymph node enlargement |
Abbreviations: aPTT, activated partial thromboplastin time; BR, bendamustine-rituximab; N/A, not available; PT, prothrombin time.
Discussion
We have described a case that well represents lymphoproliferative disorders associated with coagulation factor deficiency. Many previously published studies reported acquired coagulation inhibitors accompanying lymphoma, mostly acquired hemophilia A or factor VIII inhibitors.15–20 Similarly, acquired factor X deficiencies or inhibitors showed association with lymphoproliferative diseases via three mechanisms.7,21 The most prevalent mechanism was amyloid fibril absorption.22 The second most commonly reported mechanism was non-functional antibody that enhanced factor X clearance from circulation.23 The third and least common mechanism involves a pathologic functional antibody that was difficult to detect by citrate-based assays because it is a calcium-ion dependent anti-FX IgG antibody.8,24,25 All of these cases required complicated treatments not only to manage severe bleeding, but also to control the primary disease (Table 2).
Table 2.
Previous Case Reports of Lymphoma with Factor X Deficiency/Inhibitor
| Name of Author and Year of Publication | Age and Sex of Patients | Type of Lymphoma | Bleeding Manifestation | Factor X Level at Diagnosis (%) | Treatment | Outcome After Treatment (Follow-Up Time) | Etiology of Decreased Factor X Level |
|---|---|---|---|---|---|---|---|
| Meenhuis A et al (2015)23 | 81-year-old man | Nodal marginal zone lymphoma | Soft tissue and intra-muscular hematoma | 3–4% | Chlorambucil and rituximab | Normalization of factor X level (1 year after diagnosis) | Non-inhibitory antibody |
| Tashiro H et al (2018)22 | 37-year-old woman | Lympho-plasmacytic lymphoma | Not mentioned | 2% | -Steroids - Cyclophosphamide - Rituximab - Fludarabine - Cladribine - Bortezomib - Thalidomide -Allogeneic SCT with matched sibling donor (RIC conditioning regimen) |
Not improved | Sequestration by amyloid fibrils |
Abbreviations: SCT, stem cell transplantation; RIC, reduced intensity conditioning regimen; CR, complete remission.
In our case, the in vitro mixing study demonstrated correctable aPTT to normal value, and PT to near-normal value by normal pool plasma. It showed factor deficiency pattern. However, both prolonged values were uncorrectable after in vivo plasma and vitamin k replacement even though bleeding was clinically improved. The cause of factor X disappearance could be from either non-functional antibody or amyloid substances deposition. We hypothesized that the cause of factor X deficiency in this patient was from amyloid deposition. The evidence to support our hypothesis is that periorbital purpura, which was the pathognomonic skin sign, indicated weakening vascular integrity by amyloid deposition even though it occurred in only 15% of patients.26 Moreover, the persistent low level of factor X after immunosuppressive treatment implied that it was not the natural course of autoimmune disease.23 However, the level was high enough to prevent spontaneous bleeding. Due to concerns about patient safety, we decided not to perform any further biopsy to confirm our presumption of amyloidosis. A previous study by TashiroH22 also could not demonstrate amyloid deposition in bone marrow or skin from antemortem biopsy; however, autopsy showed amyloid depositions in multiple organs, including liver, spleen, kidney, bone marrow, lymph nodes, adrenal glands, lungs, and heart. Those authors identified factor X accompanied by amyloid fibrils. Similarly, their patient’s mixing study was correctable, but the coagulogram was not improved after replacement with substantial amounts of plasma and anti-inhibitor coagulant complex. The mechanism may be rapid clearance of factor X from circulation with subsequent rapid distribution of amyloid throughout the body.13
Our case demonstrates successful treatment of lymphoplasmacytic lymphoma and bleeding complication by BR regimen. However, a strategy to prolong our patient’s survival poses an additional and more complex challenge. Autologous and allogeneic stem cell transplantation should be carefully considered due to the high rate of non-relapse mortality in lymphoplasmacytic lymphoma.27 The benefits and drawbacks of this treatment alternative will be discussed with our patient so that a plan can be in place when and if our patient relapses.
Conclusion
Acquired factor deficiency is an acquired bleeding disorder that frequently presents with occult primary cause, especially hematologic malignancies. We presented an interesting case of factor X deficiency associated with lymphoproliferative disorder. The patient was treated with 6 cycles of BR regimen, she remains in lymphoma remission at one year after diagnosis, and her factor X level has increased to more than 20%.
Acknowledgments
The authors are grateful to Ms. Suthirak Sitaposa, Ms.Tussnem Binhama, and Mrs.Yupa Nakkinkun for their laboratory technical support.
Abbreviations
aPTT, activated thromboplastin time; PT, prothrombin time.
Data Sharing Statement
The datasets used in the current study are available upon reasonable request from the corresponding author.
Ethics Approval and Informed Consent
In Thailand, a case report does not require ethics approval. The patient gave written permission to publish her personal data.
Consent for Publication
Written informed consent was obtained from the patient for publication of the report and accompanying images. A copy of that consent form is available for review via a request from the Editor-in-Chief.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Bajaj SP, Mann KG. Simultaneous purification of bovine prothrombin and factor X. Activation of prothrombin by trypsin-activated factor X. J Biol Chem. 1973;248(22):7729–7741. doi: 10.1016/S0021-9258(19)43250-X [DOI] [PubMed] [Google Scholar]
- 2.Sucker C, Zotz RB. The Cell-Based Coagulation Model. In: Marcucci CE, Schoettker P, editors. Perioperative Hemostasis: Coagulation for Anesthesiologists. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015:3–11. [Google Scholar]
- 3.Palla R, Peyvandi F, Shapiro AD. Rare bleeding disorders: diagnosis and treatment. Blood. 2015;125(13):2052–2061. doi: 10.1182/blood-2014-08-532820 [DOI] [PubMed] [Google Scholar]
- 4.Peyvandi F, Palla R, Menegatti M, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. J Thromb Haemost. 2012;10(4):615–621. doi: 10.1111/j.1538-7836.2012.04653.x [DOI] [PubMed] [Google Scholar]
- 5.Menegatti M, Peyvandi F. Treatment of rare factor deficiencies other than hemophilia. Blood. 2019;133(5):415–424. doi: 10.1182/blood-2018-06-820738 [DOI] [PubMed] [Google Scholar]
- 6.Peyvandi F, Auerswald G, Austin SK. et al. Diagnosis, therapeutic advances, and key recommendations for the management of factor X deficiency. Blood Rev;2021. 100833. doi: 10.1016/j.blre.2021.100833 [DOI] [PubMed] [Google Scholar]
- 7.Menegatti M, Biguzzi E, Peyvandi F. Management of rare acquired bleeding disorders. Hematology Am Soc Hematol Educ Program. 2019;2019(1):80–87. doi: 10.1182/hematology.2019000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan IS, Ogunsile FJ. An Acquired Factor X Inhibitor: the Importance of Understanding Coagulation. Am J Med. 2017;130(7):e307–e8. doi: 10.1016/j.amjmed.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 9.Hsia CC, Keeney M, Bosco AA, Xenocostas A. Treatment of acquired factor X inhibitor by plasma exchange with concomitant intravenous immunoglobulin and corticosteroids. Am J Hematol. 2008;83(4):318–320. doi: 10.1002/ajh.21105 [DOI] [PubMed] [Google Scholar]
- 10.Lankiewicz MW, Bell WR. A unique circulating inhibitor with specificity for coagulation factor X. Am J Med. 1992;93(3):343–346. doi: 10.1016/0002-9343(92)90244-6 [DOI] [PubMed] [Google Scholar]
- 11.Ness PM, Hymas PG, Gesme D, Perkins HA. An unusual factor-X inhibitor in leprosy. Am J Hematol. 1980;8(4):397–402. doi: 10.1002/ajh.2830080408 [DOI] [PubMed] [Google Scholar]
- 12.Patel G, Hari P, Szabo A, et al. Acquired factor X deficiency in light-chain (AL) amyloidosis is rare and associated with advanced disease. Hematol Oncol Stem Cell Ther. 2019;12(1):10–14. doi: 10.1016/j.hemonc.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Furie B, Greene E, Furie BC. Syndrome of acquired factor X deficiency and systemic amyloidosis in vivo studies of the metabolic fate of factor X. N Engl J Med. 1977;297(2):81–85. doi: 10.1056/NEJM197707142970203 [DOI] [PubMed] [Google Scholar]
- 14.Rosenstein ED, Itzkowitz SH, Penziner AS, Cohen JI, Mornaghi RA. Resolution of factor X deficiency in primary amyloidosis following splenectomy. Arch Intern Med. 1983;143(3):597–599. doi: 10.1001/archinte.1983.00350030211041 [DOI] [PubMed] [Google Scholar]
- 15.Nixon CP, Prsic EH, Guertin CA, Stevenson RL, Sweeney JD. Acquired Factor XIII inhibitor associated with mantle cell lymphoma. Transfusion. 2017;57(3):694–699. doi: 10.1111/trf.13947 [DOI] [PubMed] [Google Scholar]
- 16.Gesierich W, Munker R, Geiersberger U, Pohlmann H, Brack N, Hartenstein R. Spontaneous Bleeding in a Patient with Malignant Lymphoma: a Case of Acquired Hemophilia. Onkologie. 2000;23(6):584–588. [DOI] [PubMed] [Google Scholar]
- 17.Aljohani NI, Matthews JH. Acquired factor V inhibitor in a patient with mantle cell lymphoma presenting with hematuria followed by thrombosis: a case report. Int Med Case Rep J. 2014;7:27–30. doi: 10.2147/IMCRJ.S59236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regina S, Colombat P, Fimbel B, Guerois C, Gruel Y. Acquired inhibitor to factor VIII in a patient with Hodgkin’s disease following treatment with interferon-alpha. Haemophilia. 2001;7(5):526–527. doi: 10.1046/j.1365-2516.2001.00555.x [DOI] [PubMed] [Google Scholar]
- 19.Kelsey PR, Leyland MJ. Acquired inhibitor to human factor VIII associated with paraproteinaemia and subsequent development of chronic lymphatic leukaemia. Br Med J. 1982;285(6336):174–175. doi: 10.1136/bmj.285.6336.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee ES, Hibsman BK, Liebman HA. Acquired bleeding disorder in a patient with malignant lymphoma: antibody-mediated prothrombin deficiency. Cancer. 2001;91(4):636–641. doi: [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Duan-Porter W, Metjian AD. Acquired, non-amyloid related factor X deficiency: review of the literature. Haemophilia. 2012;18(5):655–663. doi: 10.1111/j.1365-2516.2012.02773.x [DOI] [PubMed] [Google Scholar]
- 22.Tashiro H, Shirasaki R, Watanabe M, Kawasugi K, Takahashi Y, Direct Factor SN. X sequestration by systemic amyloid light-chain amyloidosis. Clin Case Rep. 2018;6(3):513–515. doi: 10.1002/ccr3.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meenhuis A, van Vliet R, Hudig F, Ypma PF, Schipperus MR, Hollestelle MJ. Successful treatment of a noninhibitory antibody-mediated acquired factor X deficiency in a patient with marginal-zone lymphoma. Clin Case Rep. 2015;3(7):587–593. doi: 10.1002/ccr3.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broze GJ Jr. An acquired, calcium-dependent, factor X inhibitor. Blood Cells Mol Dis. 2014;52(2–3):116–120. doi: 10.1016/j.bcmd.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakou DS, Alexandrakis MG, Passam FH, et al. Acquired inhibitors to coagulation factors in patients with gastrointestinal diseases. Eur J Gastroenterol Hepatol. 2002;14(12):1383–1387. doi: 10.1097/00042737-200212000-00016 [DOI] [PubMed] [Google Scholar]
- 26.Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. doi: 10.1038/s41572-018-0034-3 [DOI] [PubMed] [Google Scholar]
- 27.Parrondo RD, Reljic T, Iqbal M, et al. Efficacy of Autologous and Allogeneic Hematopoietic Cell Transplantation in Waldenström Macroglobulinemia: a Systematic Review and Meta-analysis. Clin Lymphoma Myeloma Leuk. 2020;20(10):e694–e711. doi: 10.1016/j.clml.2020.05.021 [DOI] [PubMed] [Google Scholar]