Abstract
The last 50 years have witnessed the translation of stem cell therapy from the laboratory to the clinic for treating brain disorders, in particular stroke. From the focal stereotaxic transplantation to the minimally invasive intravenous and intraarterial delivery, stem cells display the ability to replenish injured cells and to secrete therapeutic molecules, altogether promoting brain repair. The increased stroke incidence in COVID-19 survivors poses as a new disease indication for cell therapy, owing in part to the cells’ robust anti-inflammatory properties. Optimization of the cell transplant regimen will ensure the safe and effective clinical application of cell therapy in stroke and relevant neurological disorders.
Keywords: Brain repair, cerebral ischemia, neurological disorders, regenerative medicine, stem cells
Stem cell-based regenerative medicine arguably is the most exciting innovative treatment for human diseases over the last 50 years. Since the first successful allogeneic bone marrow transplants to treat leukemia in 1957, research breakthroughs have advanced stem cell transplantation as a standard of care for many hematological diseases. In part, this success has paved the way for the expanded application of cell therapies to other organ systems including the brain. In particular, cell therapy initially targeted Parkinson’s disease (PD) due to its relatively localized etiology characterized by the degeneration of dopaminergic neurons in the substantia nigra. Subsequently, focal or lacunar stroke ischemic stroke became the disease indication for cell therapy. 1 The efforts to develop a cell therapy for PD and stroke reached clinical trials in the late 1980s and 1990s using the stereotaxic implantation of human fetal ventral mesencephalon tissue and teratocarcinoma derived-neurons in the putamen of PD and stroke patients, respectively. 2 , 3 Although fetal and teratocarcinoma cell grafts have produced a modest level of efficacy in patients, logistical, safety, and ethical issues have hindered their large-scale clinical applications. 4 With these limitations in mind, an array of transplantable candidates for the regeneration of the injured brain has been introduced including neural stem cells (NSC), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and mesenchymal stromal cells (MSCs).
Cell replacement served as the original mechanism of tissue repair ascribed to cell therapy based on evidence that transplanted stem cells—depending on the tissue source—have been shown to differentiate into the cell phenotype of the injured tissue. However, the last two decades have witnessed by-stander effects of stem cells, specifically the cells’ secretion of anti-inflammatory cytokines and molecules that promote tissue repair (e.g. neurogenesis, angiogenesis, oligodendrogenesis, vasculogenesis, synaptogenesis). 5 Such robust secretory function of stem cells accompanies the functional recovery of the transplanted injured brain. A key pathological feature of many neurological disorders manifests as aberrant inflammation leading to neurodegeneration. That stem cells secrete factors that sequester the inflammatory response may prove beneficial against the secondary cell death of brain diseases. 6 , 7 The heterogenicity of stem cells has made distinguishing cell replacement from by-stander effects challenging. However, single-cell omic studies may help tease out the distinct brain repair processes that can be ascribed to specific stem cell populations and reveal the discrete signaling pathways mediating the cells’ therapeutic effects. 8 , 9 These advancements would guide the optimization of cell therapy for abrogating inflammation-plagued neurological disorders.
Major translational bottlenecks in producing a clinical-grade cell product overlap between neurological diseases. Patient safety is the biggest concern, especially when using highly proliferative cells like ESCs and iPSCs. Manufacturing logistics like scaling up a cell product for commercial use, storing cells without compromising efficacy, and optimizing delivery methods warrant equal considerations. Despite their common pathological features, recognizing the heterogenic nature of many neurological disorders is critical for selecting ideal target patient populations and adjusting transplant regimens to cater for each specific disease when designing clinical trials. Establishing consortia for stem cell-targeted diseases involving academic, clinical, industry, and regulatory agencies may provide direction for an expedited entry of safe and effective stem cell products to the clinic. 10
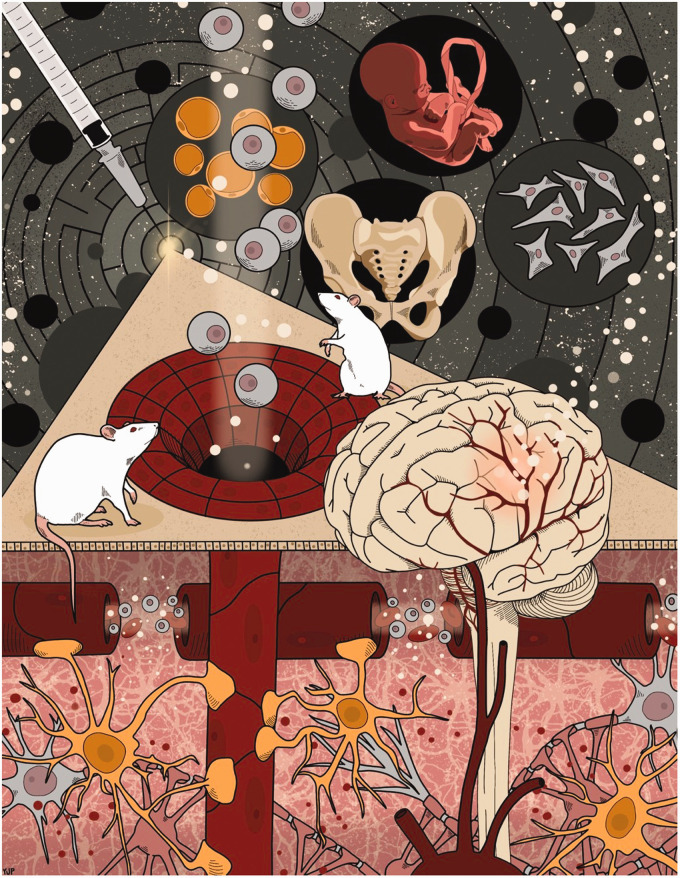
Although many research hurdles still exist in the translation of stem cell therapy from bench to clinic, it is undoubtedly an opportune time for the field of regenerative medicine for brain diseases. In light of the current pandemic, MSC transplantation has emerged as a potent treatment for various inflammatory sequelae of COVID-19 including acute respiratory distress syndrome and stroke. In navigating a new scientific frontier, translational guidelines that create transparent safety and efficacy standards will usher stem cell therapy a step closer from its status as an experimental treatment to becoming a proven therapeutic option (Figure 1).
Figure 1.
Stem cell-based regenerative medicine stands as a potential treatment for a variety of human diseases. In particular, advancements in stem cell therapy for central nervous system disorders have led to the development of multiple transplantable candidate cells for diseases like PD and stroke. Collaboration amongst academic, clinical, industry, and regulatory agencies that provide guidelines and standards of safety and efficacy are of utmost importance and will likely expedite lab-to-clinic applications of cell therapy as researchers and clinicians navigate this new frontier of regenerative medicine.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.V.B. declares patents and patent applications related to stem cell therapy. Additionally, C.V.B. was funded and received royalties and stock options from Astellas, Asterias, Sanbio, Athersys, KMPHC, and International Stem Cell Corporation; and also received consultant compensation for Chiesi Farmaceutici. The other author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
ORCID iD: Cesar V Borlongan https://orcid.org/0000-0002-2966-9782
References
- 1.Borlongan CV, Tajima Y, Trojanowski JQ, et al. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol 1998; 149: 310–321. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 1990; 247: 574–577. [DOI] [PubMed] [Google Scholar]
- 3.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000; 55: 565–569. [DOI] [PubMed] [Google Scholar]
- 4.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet 1999; 353: SI29–SI30. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Sato Y, Kushida Y, et al. Intravenously delivered multilineage-differentiating stress enduring cells dampen excessive glutamate metabolism and microglial activation in experimental perinatal hypoxic ischemic encephalopathy. J Cereb Blood Flow Metab 2021; 41: 1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonsack B, Jiang RH, Borlongan CV. A gut feeling about stroke reveals gut-brain axis’ active role in homeostasis and dysbiosis. J Cereb Blood Flow Metab 2020; 40: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlongan CV, Nguyen H, Lippert T, et al. May the force be with you: transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab 2019; 39: 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauch A, Mandrup S. Transcriptional networks controlling stromal cell differentiation. Nat Rev Mol Cell Biol 2021; 22: 465–482. [DOI] [PubMed] [Google Scholar]
- 9.Andrzejewska A, Nowakowski A, Grygorowicz T, et al. Single-cell, high-throughput analysis of cell docking to vessel wall. J Cereb Blood Flow Metab 2019; 39: 2308–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsbury C, Farooq J, Sadanandan N, et al. Aging, obesity, and male: co-morbidities and treatments for COVID-19. Cond Med 2020; 3: 252–257. [Google Scholar]