Abstract
The association between baseline perfusion measures and clinical outcomes in patients with acute small subcortical infarcts (SSIs) has not been studied in detail. Post-processed acute perfusion CT and follow-up diffusion-weighted imaging of 71 patients with SSIs were accurately co-registered. Relative perfusion values were calculated from the perfusion values of the infarct lesion divided by those of the mirrored contralateral area. The association between perfusion measures with clinical outcomes and the interaction with intravenous thrombolysis were studied. Additionally, the perfusion measures for patients having perfusion CT before and after thrombolysis were compared. Higher contralateral hemispheric cerebral blood flow (CBF) was the only independent predictor of an excellent clinical outcome (modified Rankin Scale of 0-1) at 3 months (OR = 1.3, 95% CI 1.1–1.4, P = 0.001) amongst all the perfusion parameters, and had a significant interaction with thrombolysis (P = 0.04). Patients who had perfusion CT after thrombolysis demonstrated a better perfusion profile (relative CBF ≥1) than those who had perfusion CT before thrombolysis (After:45.5%, Before:21.1%, P = 0.03). This study implies that for patients with SSIs, hemispheric CBF is a predictor of clinical outcome and has an influence on the effect of intravenous thrombolysis.
Keywords: Small subcortical infarct, perfusion, intravenous thrombolysis, clinical outcome, collateral flow
Introduction
Small subcortical infarcts (SSIs), resulting from occlusion of a single perforating artery due to cerebral small vessel disease (CSVD), are often associated with milder symptoms and better clinical outcomes compared with other stroke subtypes.1–3 However, a considerable number of patients with SSIs still suffer from disability. 4 Many studies have been made to explore the prognostic factors of SSIs, such as blood biomarkers, demographic profiles and imaging markers.5–11 Nevertheless, unlike stroke with large vessel occlusion, perfusion measures from acute perfusion imaging have not been correlated with the outcomes of patients with SSIs. Higher contralateral hemispheric cerebral blood flow (CBF) was associated with better clinical outcomes in acute ischemic stroke patients with large vessel occlusions. 12 It was considered that hemispheric CBF could probably represent capacity to recruit collaterals, cardiac function and severity of underlying chronic diseases (e.g., CSVD).12–16 It is becoming more apparent that CSVD may be considered a global rather than a focal disease. 17 Therefore, we hypothesized that hemispheric CBF might also predict outcomes of SSIs, an acute manifestation of CSVD.
The efficacy of intravenous thrombolysis using recombinant tissue plasminogen activator (rtPA) in SSIs has long been controversial. Recent randomized clinical trials demonstrated inconclusive results.18,19 Moreover, no studies have investigated the effect of rtPA on the perfusion status of SSIs.
Since previous studies of perfusion patterns of SSIs only used visual inspection or primary qualitative analysis through co-registration of perfusion and follow-up imaging,20–25 the necessity of a study with accurate co-registration and quantitative calculation was warranted. Through an analysis using accurate co-registration of baseline perfusion imaging and follow-up magnetic resonance imaging (MRI), we tested the hypothesis that higher hemispheric CBF was associated with a better clinical outcome in SSIs, and had an interaction with the effect of intravenous rtPA. Additionally, an explorative analysis was made using patients who had perfusion imaging before intravenous rtPA and patients who had perfusion imaging after intravenous rtPA to investigate whether intravenous rtPA could improve the perfusion status in SSIs.
Material and methods
Patient selection
Consecutive acute ischemic stroke patients presenting within 24 hours of symptom onset who were admitted to Department of Neurology, Huashan Hospital, Fudan University from December 2011 to December 2020 were included if they 1) underwent multimodal computed tomography (CT) scanning (including non-contrast CT, CT Angiography [CTA] and CT perfusion [CTP]) when arriving at the Emergency Room (ER), before or after intravenous rtPA (patients without intravenous thrombolysis were included in the pre-rtPA-group); 2) had a SSI in the basal ganglia, thalamus, corona radiata and internal capsule identified using diffusion-weighted imaging (DWI) performed within 7 days after stroke onset. The exclusion criteria were 1) patients with coexisting severe stenosis (>50% of the vessel caliber) or occlusion of the ipsilateral extracranial arteries and intracranial arteries identified by baseline neck and head CTA; 2) patients with potential sources of cardioembolism (atrial fibrillation, recent myocardial infarction, dilated cardiomyopathy, valvular heart disease and infective endocarditis, etc.). SSIs in the brainstem were not included in this study since the co-registration of post-processed CTP maps and DWI images is more problematic. Demographic data and clinical profiles were collected from each patient. Baseline National Institutes of Health Stroke Scale (NIHSS) was assessed by the stroke neurologist when patients arrived in the ER. Written informed consent was obtained from each participant for data collection, analysis and publishment. The study was approved by the ethics committee of Huashan Hospital under the principles of the Helsinki Declaration of 1975/1983. Patients were thrombolyzed with intravenous rtPA according to the latest Chinese guidelines of management of acute ischemic stroke. A 3-month modified Rankin scale (mRS) 0-1 was considered as an excellent functional outcome.
Imaging acquisition
For patients admitted between December 2011 and January 2015, a 256-slice detector scanner (Brilliance iCT; Philips Medical Systems, Cleveland, Ohio) was used for multimodal CT scan, where the scanning parameters were as follows: Jog mode, 80-kV/150 mAs, 13 cycles for 50 s; 325 slices. A dual-head power injector (Stellant Injection System; Medrad Inc., Indianola, Pennsylvania) was used to inject 40 mL of a nonionic contrast agent (Ultravist, iodine 370 mg I/mL; Bayer Healthcare, Berlin, Germany) at a rate of 5 mL/s, followed by a 20-mL saline flush into the cubital vein. CT scanning was initiated 5 seconds after the start of the injection. For patients admitted between January 2015 and December 2020, a 64-slice detector scanner (Discovery CT750 HD; GE Medical Systems, Waukesha, Wisconsin) was used for multimodal CT scan due to the reconstruction of our ER, where the scanning parameters were as follows: Jog mode, 80 kVp/220 mAs; 26 cycles for 42 s; 312 slices. A dual-head power injector (Stellant Injection System; Medrad Inc., Indianola, Pennsylvania) was used to inject 40 mL of a nonionic contrast medium (Ultravist, iodine 370 mg I/mL; Bayer Healthcare, Berlin, Germany) at 4.5 mL/s, followed by 20 mL saline. CT scan was initiated at 7 s after the contrast agent bolus. CTA with acquisition from aorta arc to vertex was performed immediately after perfusion CT. Brain standard reconstruction was then performed. To avoid radiation exposure to the lens, the gantry angle was parallel to and above the orbital roof. Whether CTP and CTA were acquired before or after intravenous rtPA depended on the judgement of stroke neurologists.
All participants underwent follow-up magnetic resonance imaging (MRI) scan within 7 days after stroke onset on a 3.0 T MRI scanner (Magnetom Verio; Siemens Healthcare, Erlangen, Germany). The MRI imaging protocol included DWI and Fluid-Attenuation Inversion Recovery (FLAIR) imaging.
Imaging analysis
CTP postprocessing
CTP images were post-processed using the commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Australia) with manual selection of the arterial input function in the anterior cerebral artery and venous output function in the straight sinus. Singular value decomposition with delay and dispersion correction was applied to generate CBF, cerebral blood volume (CBV), mean transit time (MTT) and delay time (DT) maps. Areas of vessels, skulls and cerebrospinal fluid regions were masked from the perfusion maps.
MRI analysis
Images of MRI were also analyzed using MIStar (Apollo Medical Imaging Technology, Melbourne, Australia). SSI was identified as a single ovoid DWI hyperintensity lesion with a maximum diameter less than 20 mm. The infarct volume and volume of the global white matter hyperintensity were also calculated using planimetric techniques by semi-automatically drawing regions of interests (ROIs) in DWI and FLAIR images respectively. Additionally, ROIs of the contralateral hemisphere, ROIs that were mirrored contralaterally to the infarct lesion were also drawn using the same technique.
Imaging co-registration
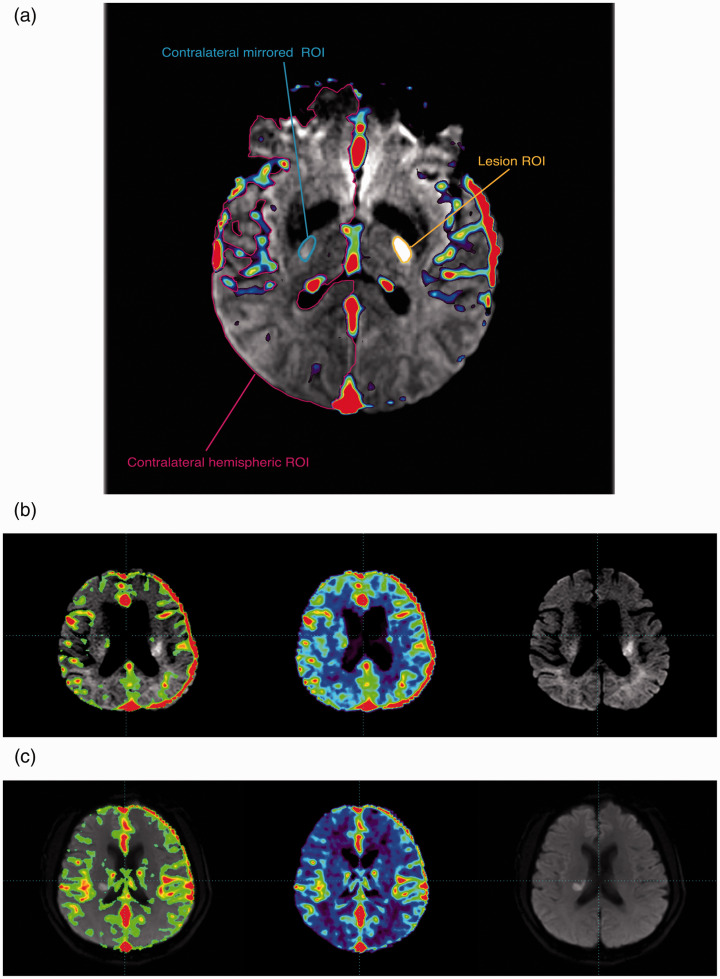
Postprocessed CTP maps, including CBF, CBV, MTT and DT maps were respectively co-registered with images of DWI. Then, previously drawn ROIs were applied on the co-registered images to calculate the voxel-wise mean CBF/CBV/MTT/DT values within those ROIs. (Figure 1(a))
Figure 1.
Co-registration illustration. (a) ROI Illustration. (b) Illustration figure of a patient with a 3-month mRS 0 (Left to Right: Co-registration of CBF map and DWI, CBF map and DWI image; contralateral hemispheric vmCBF = 28.4 ml/min/100 g). (c) Illustration figure of a patient with a 3-month mRS 2 (Left to Right: Co-registration of CBF map and DWI, CBF map and DWI image; contralateral hemispheric vmCBF = 16.4 ml/min/100 g).
ROI: region of interest; mRS: modified Rankin Scale; vmCBF: voxel-wise mean cerebral blood flow; DWI: diffusion-weighted imaging.
The voxel-wise mean (abbreviated as ‘vm’ in the following context) CBF/CBV/MTT/DT values represented the weighted average value of each ROI using the following formula (taking CBF in the infarct area as an example):
n = layers of the lesion; = average value of CBF of each voxel in layer k; Vk = number of voxels within the infarct lesion in layer k.
All the imaging was analyzed by Dr. Lan Hong who was blinded to the clinical data.
Statistical analysis
Statistical Analysis was performed on STATA v15.1 (StataCorp, Ltd, College Station, Texas). Figures were prepared using STATA v15.1 (StataCorp, Ltd, College Station, Texas) and Adobe Illustrator 2020 (Adobe.inc, San Jose, CA). Since this is a retrospective cohort study with an exploratory purpose to find imaging predictors of outcomes in patients with SSIs, this study was not previously planned with sample size or registered a priori. All p-values constitute exploratory data analysis. A two-tailed P < 0.05 was considered as significant. Continuous variables were described as mean and standard deviation (SD) if normally distributed, or median and interquartile range (IQR) if skewed. Normality was tested using Shaprio-Wilk test. Differences of demographic and clinical data were compared using student’s t-test, Welch’s t-test or Wilcoxon rank-sum test for continuous variables, and χ2 test or Fisher’s exact test for categorical variables. The associations among continuous variables were evaluated using linear regression model adjusted for potential confounders with all the continuous variables being log-transformed because of their skewed distribution.
Independent predictors of 3-month mRS 0-1
Multivariate adjusted and backward stepwise logistic regression model using a stepwise removal probability of P < 0.05 were applied to explore the independent predictors of an excellent functional outcome. Variables with P < 0.05 in univariate analyses or with clinical significance were included in the stepwise multivariate logistic regression model. The interaction between the independent predictor(s) and intravenous rtPA on excellent functional outcome was also tested. And interaction plots were drawn to visualize the interaction association. As a post-hoc sensitivity analysis (demanded by reviewer) we explored whether results change if mRS 0-2 was used as dichotomization threshold.
The role of intravenous rtPA in the perfusion status of SSIs
The relative CBF/CBV/MTT/DT (rCBF/rCBV/rMTT/rDT) values of the infarct lesion were calculated through dividing the perfusion parameters in the infarct area by the perfusion parameters in the contralateral corresponding ROI. Since repeated CTP scan within 24 hours of admission was not permitted in patients with SSIs in Huashan Hospital concerning their mild symptoms and radiation dose, patients who had perfusion imaging before rtPA and patients who had patients imaging after rtPA were compared in order to indirectly explore the role of intravenous rtPA on the perfusion status of SSIs. A better perfusion status was considered when the rCBF value was not lower than 1. The percentage of patients who had rCBF ≥ 1 of these two groups was univariately compared using χ2 test and multivariate adjusted with different types of CT scanner using multivariate logistic regression model. Perfusion parameters of the infarct lesion and contralateral area were also compared using paired t-test or paired Wilcoxon signed-rank test in these two groups, respectively.
Results
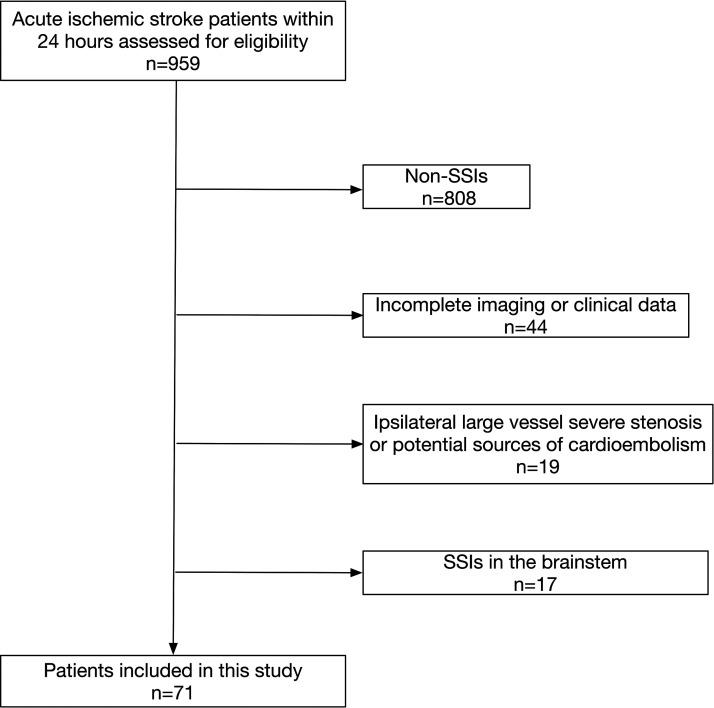
From December 2011 to December 2020, a total number of 959 acute ischemic stroke patients within 24-hour onset time were prospectively registered in the acute ischemic stroke database of Huashan Hospital, Fudan University if they underwent baseline multimodal CT imaging and had follow-up CT/MRI scan. Through careful inspection of clinical and imaging data, 71 patients were included in this study (Figure 2). The mean [SD] age was 62.5 [10.0] years, and 48 (67.6%) were male with median [IQR] baseline NIHSS of 4.0 [3.0, 7.0]. The median [IQR] lesion volume was 1.3 [0.6, 2.4] mL. Forty-six (65.6%) patients received intravenous rtPA, and 13 of these patients had perfusion CT before the initiation of thrombolysis. The correlation between perfusion parameters and onset to perfusion imaging time was listed in Supplementary Table 1. Among all the included patients, 61 had 3-month follow-up, and 41 (67.2%) achieved a 3-month mRS of 0-1. No difference in white matter hyperintensity volume was found between patients with 3-month mRS 0-1 and 3-month mRS 2-6. The baseline demographic, clinical data and perfusion imaging parameters are listed in Table 1.
Figure 2.
Flow-chart of patient selection.
SSI: small subcortical infarction.
Table 1.
Baseline demographic, clinical data and perfusion parameters.a
| n = 71 | |
|---|---|
| Age, mean (SD), yrs | 62.5 (10.0) |
| Male | 48 (67.6%) |
| Baseline NIHSS, median (IQR) | 4.0 (3.0, 7.0) |
| Baseline SBP, mean (SD), mmHg | 154.0 (29.5) |
| Baseline DBP, mean (SD), mmHg | 88.1 (15.3) |
| Onset to imaging time, median (IQR), min | 226.0 (154.0, 311.0) |
| Wake-up stroke | 9 (12.7%) |
| Medical history | |
| History of smoking | 31 (43.7%) |
| History of hypertension | 47 (66.2%) |
| History of dyslipidemia | 18 (25.4%) |
| History of diabetes mellitus | 15 (21.1%) |
| Past history of stroke | 7 (9.9%) |
| Intravenous thrombolysis | 46 (67.8%) |
| Baseline leukocyte count, median (IQR), ×109/L | 6.7 (5.7, 8.3) |
| Lesion volume, median (IQR), mL | 1.3 (0.6, 2.4) |
| White matter hyperintensity volume, median (IQR), mL | 6.1 (3.1, 10.5) |
| 3-month mRS 0-1 (n = 61) | 41 (67.2%) |
| Perfusion parametersb | |
| Infarct lesion | |
| CBV, mean (IQR), ml/1000g | 19.9 (16.4, 23.2) |
| CBF, median (IQR), ml/min/100g | 17.0 (14.5, 23.0) |
| MTT, mean (SD), sec/100 | 669.8 (145.8) |
| DT, median (IQR), sec/100 | 67.6 (31.5, 101.2) |
| ROIs contralaterally mirrored to the infarct lesion | |
| CBV, median (IQR), ml/1000g, | 19.8 (15,6, 23.4) |
| CBF, median (IQR), ml/min/100g | 19.6 (15.7, 23.3) |
| MTT, mean (SD), sec/100 | 604.8 (140.4) |
| DT, median (IQR), sec/100 | 48.7 (14.7,72.0) |
| Relative perfusion values | |
| rCBV, median (IQR) | 1.0 (0.8,1.3) |
| rCBF, median (IQR) | 0.9 (0.8, 1.1) |
| rMTT, median (IQR) | 1.1 (1.0, 1.2) |
| rDT, median (IQR) | 1.7 (0.8, 2.4) |
| Contralateral hemisphere | |
| CBV, median (IQR), ml/1000g | 26.2 (23.6, 28.3) |
| CBF, median (IQR), ml/min/100g | 25.3 (20.6, 29.1) |
| MTT, median (IQR), sec/100 | 578.3 (503.2, 656.5) |
| DT, median (IQR), sec/100 | 47.0 (22.4, 68.6) |
IQR: interquartile range; SD: standard deviation; NIHSS: National Institutes of Health Stroke Scale; SBP: systolic blood pressure; DBP: diastolic blood pressure; mRS modified Rankin Scale; CBV: cerebral blood volume; CBF: cerebral blood flow; MTT: mean transit time; DT: delay time; rCBV: relative cerebral blood volume; rCBF: relative cerebral blood flow; rMTT: relative mean transit time; rDT: relative delay time.
aData are presented as number (percentage) of patients unless otherwise indicated.
bAbsolute values of perfusion parameters are presented as voxel-wise mean values.
The role of hemispheric CBF and its interaction with intravenous thrombolysis on clinical outcome
Sixty-one patients had 3-month follow-up. The differences of demographic, clinical or imaging data between patients with 3-month follow-up and patients without 3-month follow-up are listed in Supplementary Table 2. After univariate analysis, patients with mRS 0-1 showed higher vmCBF of the infarct lesion, the area contralaterally mirrored to the infarct lesion and contralateral hemisphere, and shorter vmMTT of the contralateral hemisphere compared with patients who had 3-month mRS 2-6 (Table 2). Higher contralateral hemispheric vmCBF and lower baseline NIHSS were the only independent predictors of an excellent clinical outcome after multivariate stepwise logistic regression analysis with baseline NIHSS, age and intravenous rtPA entering the model as clinically relevant variables (contralateral hemispheric vmCBF OR = 1.3, 95% CI 1.1–1.4, P = 0.001; baseline NIHSS OR = 0.8, 95%CI 0.7–1.0, P = 0.04; Figure 1(b) and (c)). The adjusted R2 was 0.23 in the logistic regression model with contralateral hemispheric CBF and baseline NIHSS. The results remained same when P = 0.1 was used instead of p = 0.05 in the univariate analysis (data not shown).
Table 2.
Baseline demographic and clinical profiles and perfusion imaging data between patients with 3-month mRS 0–1 and mRS 2–6 (n = 61).a
| mRS 0–1(n = 41) | mRS 2–6(n = 20) | P | |
|---|---|---|---|
| Age, mean (SD), yrs | 61.9 (9.1) | 63.9 (11.6) | 0.48b |
| Male | 28 (68.3%) | 14 (70.0%) | 0.89c |
| Baseline NIHSS, median (IQR) | 4.0 (2.5,5.0) | 5.0 (2.3, 8.0) | 0.27d |
| Baseline SBP, mean (SD), mmHg | 151.5 (29.4) | 156.8 (26.7) | 0.50b |
| Baseline DBP, mean (SD), mmHg | 87.4 (15.6) | 88.7 (12.5) | 0.75b |
| Onset to imaging time, median (IQR), min | 235.0 (165.0, 295.5) | 241.0 (173.8, 367.0) | 0.41d |
| Wake-up stroke | 5 (12.2%) | 3 (15.0%) | 1.00e |
| Medical history | |||
| History of smoking | 18 (43.9%) | 8 (40.0%) | 0.77c |
| History of hypertension | 24 (58.5%) | 13 (65.0%) | 0.63c |
| History of dyslipidemia | 11 (26.8%) | 4 (20.0%) | 0.75e |
| History of diabetes mellitus | 8 (19.5%) | 4 (20.0%) | 0.96e |
| Past history of stroke | 4 (9.8%) | 3 (15.0%) | 0.67e |
| Intravenous thrombolysis | 27 (67.9%) | 11 (55.0%) | 0.41c |
| Baseline leukocyte count, median (IQR), ×109/L | 6.8 (5.9, 8.3) | 6.6 (5.5, 8.3) | 0.47d |
| White matter hyperintensity volume, median (IQR), mL | 6.1 (3.4, 9.4) | 3.7 (2.1, 8.4) | 0.17d |
| Lesion volume, median (IQR), mL | 1.1 (0.6, 1.9) | 1.3 (0.6, 2.4) | 0.66d |
| Perfusion parametersf | |||
| Infarct lesion | |||
| CBV, mean (SD), ml/1000g | 21.0 (6.9) | 19.2 (6.3) | 0.34b |
| CBF, mean (SD), ml/min/100g | 19.8 (6.6) | 15.4 (4.1) | 0.01b |
| MTT, mean (SD), sec/100 | 634.1 (159.4) | 710.0 (109.4) | 0.06b |
| DT, median (IQR), sec/100 | 84.6 (50.2, 118.4) | 59.1 (26.1, 105.2) | 0.18d |
| ROIs contralaterally mirrored to the infarct lesion | |||
| CBV, median (IQR), ml/1000g | 20.0 (16.0, 27.7) | 17.4 (14.2, 22.4) | 0.20d |
| CBF, mean (SD), ml/min/100g | 20.7 (5.6) | 16.7 (3.9) | 0.01b |
| MTT, mean (SD), sec/100 | 572.9 (151.3) | 643.3 (121.6) | 0.08b |
| DT, median (IQR), sec/100 | 50.3 (31.0, 110.0) | 39.9 (5.0, 81.0) | 0.24d |
| Relative perfusion values | |||
| rCBV, median (IQR) | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.2) | 0.94d |
| rCBF, median (IQR) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.0) | 0.81d |
| rMTT, mean (SD) | 1.2 (0.3) | 1.1 (0.1) | 0.65b |
| rDT, median (IQR) | 1.8 (1.0, 2.6) | 1.8 (1.0, 2.7) | 0.68d |
| Contralateral hemisphere | |||
| CBV, median (IQR), ml/1000g | 26.3 (24.0, 29.2) | 24.3 (23.3, 27.2) | 0.10d |
| CBF, median (IQR), ml/min/100g | 28.2 (22.7, 31.8) | 20.6 (17.7, 24.9) | 0.001d |
| MTT, median (IQR), sec/100 | 538.6 (481.9, 624.7) | 611.3 (562.5, 709.7) | 0.02d |
| DT, mean (SD), sec/100 | 54.4 (28.8) | 41.8 (28.2) | 0.11b |
IQR: interquartile range; SD: standard deviation; NIHSS: National Institutes of Health Stroke Scale; SBP: systolic blood pressure; DBP: diastolic blood pressure; mRS: modified Rankin Scale; CBV: cerebral blood volume; CBF: cerebral blood flow; MTT: Mean transit time; DT: Delay time; rCBV: relative cerebral blood volume; rCBF: relative cerebral blood flow; rMTT: relative mean transit time; rDT: relative delay time.
aData are presented as number (percentage) of patients unless otherwise indicated.
bP-value was calculated using student’s t-test.
cP-value was calculated using chi-square test.
dP-value was calculated using Wilcoxon rank-sum test.
eP-value was calculated using Fisher’s exact test.
fAbsolute values of perfusion parameters are presented as voxel-wise mean values.
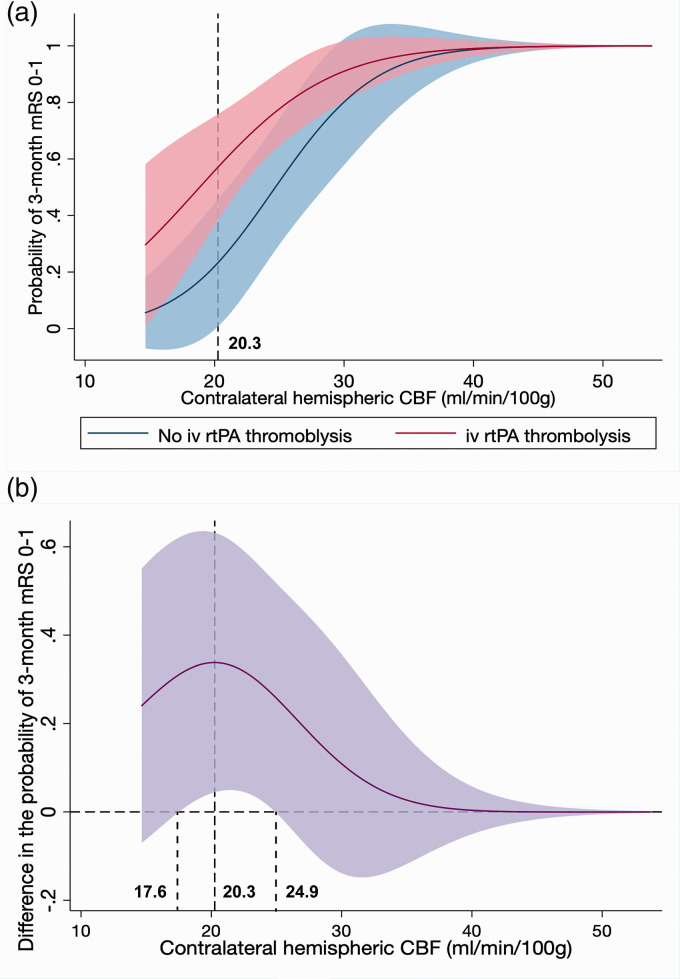
Contralateral hemispheric vmCBF had a statistically significant interaction with intravenous rtPA on outcome (adjusted for baseline NIHSS and age, P for interaction =0.04; Figure 3(a)). The interaction was complex, with the difference in the probability of achieving mRS 0-1 between patients with or without intravenous rtPA alongside the increasing hemispheric CBF was demonstrated in Figure 3(b) with an inverse-U shape. There was a ‘sweet spot’ of contralateral hemispheric CBF (ranging from 17.6 to 24.9 ml/min/100 g) where the outcomes of patients with intravenous rtPA were significantly better than patients without intravenous rtPA. When contralateral hemispheric CBF was lower than 17.6 ml/min/100 g, the outcomes of patients were relatively poor regardless of thrombolysis. Meanwhile, when contralateral hemispheric CBF was higher than 24.9 ml/min/100 g, the outcomes of patients were relatively good regardless of rtPA. This interaction revealed that intravenous rtPA might only be effective when contralateral hemispheric CBF fell in the ‘sweet spot’ range.
Figure 3.
Interaction plot between thrombolysis and contralateral hemispheric vmCBF concerning 3-month mRS 0–1. (a) Probability of achieving 3-month mRS 0-1 in patients with or without rtPA thrombolysis with the increasing of contralateral hemispheric vmCBF. Color margins indicate 95% CI. (b) Difference in the probability between patients with intravenous rtPA thrombolysis and without intravenous rtPA thrombolysis with the increasing of contralateral hemispheric vmCBF. Color margins indicate 95% CI.
iv rtPA: intravenous recombinant tissue plasminogen activator vmCBF: voxel-wise mean cerebral blood flow; mRS: modified Rankin Scale.
Additionally, a relationship between higher contralateral hemispheric vmCBF and smaller final infarct volume was found after adjustment for baseline NIHSS (log-transformed adjusted coefficient = −0.8, 95%CI −1.7–−0.003, p = 0.049).
An analysis using perfusion measures of the ipsilateral hemisphere showed similar results (See Supplementary Appendix 1).
When the outcome is defined as mRS 0-2, only CBF in the infarct lesion and baseline NIHSS were the independent predictors of 3-month mRS 0-2 (CBF in the lesion infarct: OR = 1.3, 95%CI [1.0, 1.6], P = 0.03; Baseline NIHSS: OR = 0.7,95%CI [0.6,0.9], P = 0.01), though only 8 patients had a 3-month mRS of 3–6, making these results be interpreted with caution.
The relationship between intravenous thrombolysis and SSI perfusion
Among the 71 patients included, 38 patients had perfusion CT before intravenous rtPA (including 25 patients who did not receive thrombolysis) and 33 patients had perfusion CT after rtPA. There was no difference concerning demographic or clinical profiles between the imaging pre-rtPA group and imaging post-rtPA group, except that the imaging pre-rtPA group had a much longer onset to imaging time, due to more wake-up stroke patients and more patients with onset time beyond the 4.5-hour intravenous rtPA window (Supplementary Table 3). The mean [SD] time from the initiation of thrombolysis to perfusion imaging in imaging-post-rtPA group was 46.4 [27.2] minutes. The proportion of patients with rCBF ≥ 1 in the infarct (i.e., not lower than the CBF in the contralateral mirrored region) in the imaging post-rtPA group (15/33, 45.5%) was much higher than the proportion in the imaging pre-rtPA group (8/38, 21.1%, p = 0.03; Table 3). This difference remained significant after being adjusted for scanner types (P = 0.03).
Table 3.
Perfusion parameters of infarct lesion and contralaterally mirrored ROIs in patients with perfusion imaging before or after intravenous rtPA (n = 71).a
| Infarct lesion | Contralaterally mirrored ROI | P | |
|---|---|---|---|
| Imaging before intravenous rtPA (n = 38) | |||
| CBV, mean (SD), ml/1000 g | 21.9 (7.4) | 22.0 (8.5) | 0.95b |
| CBF, median (IQR), ml/min/100 g | 17.4 (15.6, 24.0) | 20.7 (17.0,26.3) | 0.01c |
| MTT, mean (SD), sec/100 | 669.3 (146.2) | 60,9.0 (157.0) | 0.003b |
| DT, median (IQR), sec/100 | 73.6 (24.9, 105.3) | 41.0 (10.9, 71.3) | 0.001c |
| Imaging after intravenous rtPA (n = 33) | |||
| CBV, mean (SD), ml/1000 g | 19.1 (4.8) | 18.3 (4.1) | 0.39b |
| CBF, mean (SD), ml/min/100 g | 17.2 (5.2) | 18.2 (4.1) | 0.25b |
| MTT, mean (SD), sec/100 | 670.4 (147.7) | 600.0 (120.8) | 0.001b |
| DT, median (IQR), sec/100 | 67.4 (38.6, 100.2) | 49.8 (16.1, 78.0) | 0.04c |
rtPA: recombinant tissue plasminogen activator; IQR: interquartile range; SD: standard deviation; CBV: cerebral blood volume; CBF: cerebral blood flow; MTT: mean transit time; DT: delay time; ROI: region of interest.
aAbsolute values of perfusion parameters are presented as voxel-wise mean values.
bP-value was calculated using paired t-test.
cP-value was calculated using paired Wilcoxon signed-rank test.
Discussion
This retrospective study of a single-center cohort of acute ischemic stroke patients with SSIs has demonstrated that: 1) Higher hemispheric vmCBF was independently associated with a better clinical outcome and had an interaction with intravenous rtPA, and; 2) patients with perfusion CT after intravenous rtPA demonstrated a better perfusion profile (rCBF ≥ 1) in the infarct compared with patients who had CTP before rtPA. To our knowledge, this is the first ever study quantitatively assessing perfusion imaging to delineate the perfusion characteristics of patients with SSI and examining the association of brain perfusion with intravenous rtPA and clinical outcomes.
The role of contralateral hemispheric CBF was previously assessed by the Imaging Collaterals in Acute Stroke (iCAS) study investigators in 77 acute ischemic stroke patients with a median NIHSS of 13 (implicating large vessel occlusions). The iCAS study demonstrated that a higher contralateral hemispheric CBF was correlated with a better clinical outcome, leading to the hypothesis that higher hemispheric CBF might reflect a greater capacity to recruit collaterals and is an overall proxy of a good ‘brain health’. 12 It is well established that collateral status is key to the infarct progression and clinical outcomes of patients with large vessel occlusions.26,27 However, recent studies using dynamic 4-dimentional angiography derived from perfusion CT acquisitions have visualized collateral flow in SSIs, with compensatory blood supply to the ischemic area from adjacent perforator arteries.20,28 Moreover, CSVD has now been recognized to reflect whole-brain endothelial dysfunction, which is strongly correlated with loss of brain reactivity, and is reflected by a more globally compromised CBF. 29 Therefore, the higher hemispheric CBF seen in our study might also reflect better ‘collateral reserve’ in patients with SSIs, and hence the association with better clinical outcome.
The pathogenesis of SSI has been attributed to microatheroma and lipohyalinosis for decades, 30 which has raised doubts about the biologic efficacy of clot-dissolving therapy with intravenous rtPA. There is a study demonstrating that intravenous rtPA was not associated with more favorable outcome in patients without identified baseline vessel occlusion compared to those not treated with tPA. 31 However, this included patients with small cortical lesion as well as SSIs. Moreover, the potential of rtPA for Ischemic Strokes With Mild Symptoms (PRISMS) trial has concluded that for acute ischemic patients with non-disabling symptoms, rtPA was not superior to aspirin 18 and recent guidelines suggest withholding rtPA in patients with mild non-disabling symptoms. 32 Unfortunately the PRISMS trial did not perform subgroup study according to stroke subtypes, so it is unclear what proportion of patients with SSIs were included. Using a clinical diagnosis of SSI (which is not very accurate), many have concluded that patients with SSIs could be equally benefitted from intravenous thrombolysis compared with other stroke subtypes.1,33–37 Importantly, the sub-group analysis of the Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial has demonstrated that intravenous rtPA might be effective in patients with MRI-identified SSIs. 19 In our cohort, around 45.5% patients with perfusion imaging post-rtPA demonstrated higher infarct CBF than contralaterally, a much higher proportion than that seen in the patients with perfusion imaging pre-rtPA. This is an indirect evidence that thrombolysis has improved perfusion in the SSI territory.
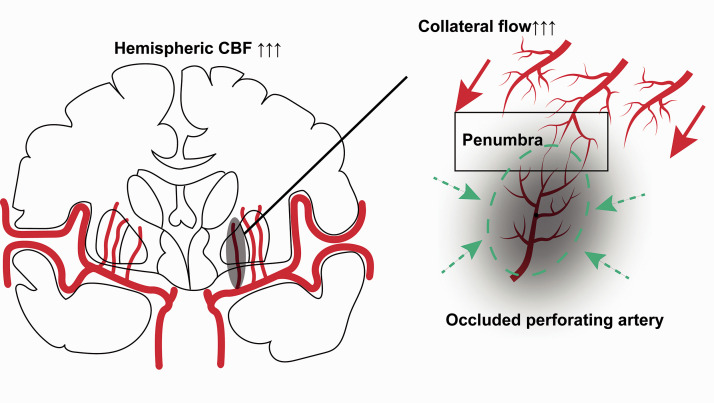
The interaction analysis revealed that there was a ‘sweet spot’ range of hemispheric CBF where the efficacy of intravenous rtPA was significant. The outcomes of patients with hemispheric CBF below that range were relatively poorer, while the outcomes of patients with hemispheric CBF beyond that range were relatively better, regardless of intravenous rtPA. Additionally, higher contralateral hemispheric CBF was also associated with a smaller infarction volume. Therefore, we propose a hypothesis that for patients with SSIs a higher baseline hemispheric CBF represents better ‘collateral reserve’. It is well accepted that penumbral existence depends on sufficient collateral blood supply. When an occlusion occurs in a perforator artery, on one hand, a higher hemispheric CBF may more effectively recruit collateral flow from adjacent perforators to maintain penumbra. It follows that, only when penumbral tissue is present, will any reperfusion achieved by intravenous rtPA result in benefit (by preventing penumbra progressing to infarction). At lower hemispheric CBF values there is less collateral flow and hence less (or any) penumbra. Hence reperfusion (with rtPA or not) will not result in any penumbral salvage (as there is none to salvage). At the other end of the inverse U-curve (patients with a higher baseline hemispheric CBF), intravenous rtPA may not result in any benefit for a different reason, as tissue is not critically hypoperfused due to excellent collateral flow. This might be analogous to previously described ‘benign oligemia’ seen in cortical ischemia. 38 Thus higher CBF ‘protects’ the perforator artery territory from infarction, and the natural history is that there will be smaller infarcts and higher rates of excellent clinical outcome (independent of rtPA, Figure 4). In other words, the beneficial effect of thrombolysis drops after hemispheric CBF surpasses a certain value. Although needing further validation, these results imply that hemispheric CBF probably should be taken into account in order to select the optimal treatment strategies in acute SSIs. Moreover, since the calculation of CBF using perfusion imaging is considered indirect (derived from CBV and MTT), “gold standard” CBF measurement using positron-emission tomography (PET) would be considered optional to further elucidate the pathophysiological mechanism underlying hemispheric CBF and SSIs for research purposes.39,40
Figure 4.
Illustration of how hemispheric CBF works in SSI.
When an occlusion occurs in a perforated artery, on one hand, a higher hemispheric CBF can effectively recruit collateral flow to maintain penumbra (red arrowhead). On the other hand, for patients with a fairly high baseline hemispheric CBF, there would be very limited hypoperfusion area (due to excellent collateral flow), which would naturally lead to a smaller infarct and higher probability of excellent clinical outcome (green dotted oval and green dotted arrowhead).
SSI: small subcortical infarcts; CBF: cerebral blood flow.
There are some inevitable limitations in our study. First of all, the sample size is limited with a single-center cohort, and only 61 patients had 3-month follow-up, limiting the generalizability of our results. Noteworthily, the aim of this study was to explore the potential interaction of hemispheric CBF and effect of rtPA, rather than to derive hemispheric CBF thresholds where rtPA is of benefit. Therefore, the absolute values of contralateral hemispheric CBF in the current study (which did correlate with the effect of intravenous thrombolysis) should be considered as references for future study. Additionally, our inclusion criteria were very strict, resulting in the exclusion of a large number of patients from the primary 959 candidates. Moreover, this sample size is comparable with similar studies.20,28 Second, the perfusion changes before or after rtPA was measured in different patients with only one perfusion imaging scan. It would be ideal to perform perfusion imaging before and after rtPA, but this is not considered routine care due to radiation dose. Thus alternative methods of perfusion imaging might be considered to confirm our result. Third, only 61 patients had a 3-month follow-up, and the DT of patients without 3-month mRS was much lower than patients with 3-month mRS. However, since hemispheric CBF and rCBF were not different between the two groups, the main results were not influenced. Fourth, only patients with visible SSI lesions on the follow-up DWI were included. The exclusion of patients with negative DWI after rtPA might lead to biased results where the effects of rtPA could be underestimated since some patients might have fully reperfused after rtPA with no visible infarction on DWI. Fifth, two types of scanners were used in this cohort, which might result in different perfusion results. Therefore, results were adjusted for scanner types when perfusion parameters were considered as dependent variables in this study.
Conclusion
In patients with acute SSIs, baseline hemispheric CBF was a predictor of excellent 3-month outcome and also had an influence on the effect of intravenous thrombolysis.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by National Key R&D Program of China (2016YFC1300503, 2017YFC1308201), National Natural Science Foundation of China (81971123), Shanghai Municipal Committee of Science and Technology (20Z11900800), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJ Lab. Dr. Longting Lin is funded by Medical Research Future Fund (MRFF) BioMedTech Horizons (BMTH) program.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Parsons reports other from Siemens, other from Canon, other from Apollo Medical Imaging, outside the submitted work.
Other authors have nothing to disclose.
Authors’ contributions: LH, YL, YS, LY, LL, MP, XC, QD participated in data collection, critical review and revision of this manuscript.
XC, MP, QD designed the study.
LH, YL analyzed the data.
LH drafted this manuscript, prepared tables and figures.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Lan Hong https://orcid.org/0000-0002-4002-4627
Longting Lin https://orcid.org/0000-0001-7104-9846
Xin Cheng https://orcid.org/0000-0001-7816-0547
References
- 1.Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA stroke trial. Stroke 1997; 28: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 2.Mustanoja S, Meretoja A, Putaala J, et al.; Helsinki Stroke Thrombolysis Registry Group. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke 2011; 42: 102–106. [DOI] [PubMed] [Google Scholar]
- 3.Petty GW, Brown RD, Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000; 31: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 4.Del Bene A, Palumbo V, Lamassa M, et al. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke 2012; 7: 321–329. [DOI] [PubMed] [Google Scholar]
- 5.Helenius J, Mayasi Y, Henninger N. White matter hyperintensity lesion burden is associated with the infarct volume and 90-day outcome in small subcortical infarcts. Acta Neurol Scand 2017; 135: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu YP, Tan L. The infarct shape predicts progressive motor deficits in patients with acute lacunae-sized infarctions in the perforating arterial territory. Intern Med 2015; 54: 2999–3004. [DOI] [PubMed] [Google Scholar]
- 7.Serena J, Leira R, Castillo J, et al. Neurological deterioration in acute lacunar infarctions: the role of excitatory and inhibitory neurotransmitters. Stroke 2001; 32: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Lee KY, Koh SH, et al. The role of matrix metalloproteinase 9 in early neurological worsening of acute lacunar infarction. Eur Neurol 2006; 55: 11–15. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos M, Castillo J, García MM, et al. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke 2002; 33: 982–987. [DOI] [PubMed] [Google Scholar]
- 10.Audebert HJ, Pellkofer TS, Wimmer ML, et al. Progression in lacunar stroke is related to elevated acute phase parameters. Eur Neurol 2004; 51: 125–131. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Ohara T, Hamanaka M, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci 2010; 288: 170–174. [DOI] [PubMed] [Google Scholar]
- 12.Thamm T, Guo J, Rosenberg J, et al.; on behalf of the iCAS Study Investigators. Contralateral hemispheric cerebral blood flow measured with arterial spin labeling can predict outcome in acute stroke. Stroke 2019; 50: 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016; 36: 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang OY, Saver JL, Buck BH, et al.; for the UCLA Collateral Investigators. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2007; 79: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaharchuk G, Do HM, Marks MP, et al. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011; 42: 2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson AL, Liu D, Gupta DK, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 2017; 89: 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ter Telgte A, van Leijsen EMC, Wiegertjes K, et al. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol 2018; 14: 387–398. [DOI] [PubMed] [Google Scholar]
- 18.Khatri P, Kleindorfer DO, Devlin T, et al.; PRISMS Investigators. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. Jama 2018; 320: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barow E, Boutitie F, Cheng B, et al.; WAKE-UP Investigators. Functional outcome of intravenous thrombolysis in patients with lacunar infarcts in the WAKE-UP trial. JAMA Neurol 2019; 76: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudilosso S, Laredo C, Mancosu M, et al. Cerebral perfusion and compensatory blood supply in patients with recent small subcortical infarcts. J Cereb Blood Flow Metab 2019; 39: 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudilosso S, Urra X, San Roman L, et al. Perfusion deficits and mismatch in patients with acute lacunar infarcts studied with whole-brain CT perfusion. AJNR Am J Neuroradiol 2015; 36: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson JC, Payabvash S, Mortazavi S, et al. CT perfusion in acute lacunar stroke: detection capabilities based on infarct location. AJNR Am J Neuroradiol 2016; 37: 2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao W, Yassi N, Sharma G, et al. Diagnosing acute lacunar infarction using CT perfusion. J Clin Neurosci 2016; 29: 70–72. [DOI] [PubMed] [Google Scholar]
- 24.Forster A, Kerl HU, Wenz H, et al. Diffusion- and perfusion-weighted imaging in acute lacunar infarction: is there a mismatch? PLoS One 2013; 8: e77428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JP, Kim SJ, Lee JJ, et al. Diffusion-perfusion mismatch in single subcortical infarction: a predictor of early neurological deterioration and poor functional outcome. Eur Neurol 2015; 73: 353–359. [DOI] [PubMed] [Google Scholar]
- 26.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab 2020; 40: 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faizy TD, Kabiri R, Christensen S, et al. Perfusion imaging-based tissue-level collaterals predict ischemic lesion net water uptake in patients with acute ischemic stroke and large vessel occlusion. J Cereb Blood Flow Metab 2021; 41: 2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forster A, Murle B, Bohme J, et al. Perfusion-weighted imaging and dynamic 4D angiograms for the estimation of collateral blood flow in lacunar infarction. J Cereb Blood Flow Metab 2016; 36: 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696. [DOI] [PubMed] [Google Scholar]
- 30.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965; 15: 774–784. [DOI] [PubMed] [Google Scholar]
- 31.Tian H, Parsons MW, Levi CR, et al. Intravenous thrombolysis may not improve clinical outcome of acute ischemic stroke patients without a baseline vessel occlusion. Front Neurol 2018; 9: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 33.Matusevicius M, Paciaroni M, Caso V, et al. Outcome after intravenous thrombolysis in patients with acute lacunar stroke: an observational study based on SITS international registry and a meta-analysis. Int J Stroke 2019; 14: 878–886. [DOI] [PubMed] [Google Scholar]
- 34.Shobha N, Fang J, Hill MD. Do lacunar strokes benefit from thrombolysis? Evidence from the registry of the Canadian stroke network. Int J Stroke 2013; 8: 45–49. [DOI] [PubMed] [Google Scholar]
- 35.Hsia AW, Sachdev HS, Tomlinson J, et al. Efficacy of IV tissue plasminogen activator in acute stroke: does stroke subtype really matter? Neurology 2003; 61: 71–75. [DOI] [PubMed] [Google Scholar]
- 36.Fuentes B, Martinez-Sanchez P, Alonso de Lecinana M, et al.; on behalf of the Madrid Stroke Network. Efficacy of intravenous thrombolysis according to stroke subtypes: the Madrid stroke network data. Eur J Neurol 2012; 19: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 37.Lahoti S, Gokhale S, Caplan L, et al. Thrombolysis in ischemic stroke without arterial occlusion at presentation. Stroke 2014; 45: 2722–2727. [DOI] [PubMed] [Google Scholar]
- 38.Bandera E, Botteri M, Minelli C, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke 2006; 37: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 39.Sobesky J. Refining the mismatch concept in acute stroke: lessons learned from PET and MRI. J Cereb Blood Flow Metab 2012; 32: 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner P, Saur D, Zeisig V, et al. Simultaneous PET/MRI in stroke: a case series. J Cereb Blood Flow Metab 2015; 35: 1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X211029884 for Hemispheric cerebral blood flow predicts outcome in acute small subcortical infarcts by Lan Hong, Yifeng Ling, Ya Su, Lumeng Yang, Longting Lin, Mark Parsons, Xin Cheng and Qiang Dong in Journal of Cerebral Blood Flow & Metabolism