Abstract
Plasminogen is involved in the process of angiogenesis; however, the underlying mechanism is unclear. Here, we investigated the potential contribution of plasmin/plasminogen in mediating angiogenesis and thereby contributing to functional recovery post-stroke. Wild-type plasminogen naive (Plg+/+) mice and plasminogen knockout (Plg−/−) mice were subjected to unilateral permanent middle cerebral artery occlusion (MCAo). Blood vessels were labeled with FITC-dextran. Functional outcomes, and cerebral vessel density were compared between Plg+/+ and Plg−/− mice at different time points after stroke. We found that Plg−/− mice exhibited significantly reduced functional recovery, associated with significantly decreased vessel density in the peri-infarct area in the ipsilesional cortex compared with Plg+/+ mice. In vitro, cerebral endothelial cells harvested from Plg−/− mice exhibited significantly reduced angiogenesis assessed using tube formation assay, and migration, as evaluated using Scratch assays, compared to endothelial cells harvested from Plg+/+ mice. In addition, using Western blots, expression of thrombospondin (TSP)-1 and TSP-2 were increased after MCAo in the Plg−/− group compared to Plg+/+ mice, especially in the ipsilesional side of brain. Taken together, our data suggest that plasmin/plasminogen down-regulates the expression level of TSP-1 and TSP-2, and thereby promotes angiogenesis in the peri-ischemic brain tissue, which contributes to functional recovery after ischemic stroke.
Keywords: Ischemic stroke, angiogenesis, functional recovery, plasminogen, thrombospondin-1, thrombospondin-2
Introduction
As a serine protease, tPA catalyzes the conversion of the zymogen plasminogen into the active plasmin to lyse the fibrin component of a blood clot in the intravascular space. 1 Our previous studies have demonstrated that tPA/plasmin system also acts as a neurorestorative agent for stroke.2–4
Angiogenesis plays a key role in the repair of brain tissue after stroke. 5 , 6 The process of angiogenesis is controlled by the balance between various angiogenesis-promoting factors and angiogenesis-inhibiting factors, 7 , 8 which led to the concept of “angiogenesis switch”, that is, the activation of cerebral endothelial cells depends on the regulation of angiogenic factors and/or the reduction of angiogenesis inhibitors. 9 Studies indicate that the tPA/plasmin system is indispensable in mediating angiogenesis in neurological diseases and cancer.10–16 However, the specific mechanism of tPA/plasmin system regulation and its contribution of angiogenesis to neurological recovery after stroke remain unclear.
Thrombospondin (TSP)-1 and TSP-2 have similar structure and function and are important inhibitors of angiogenesis.17–23 Moreover, TSP-1 and TSP-2 have been postulated to impact post-ischemic angiogenesis and functional recovery after stroke. 23 , 24 Since plasmin causes proteolytic cleavage of TSP-1, 25 we hypothesized that the tPA/plasmin system may be involved in angiogenesis by regulating TSPs. As plasminogen (Plg) is the precursor of the active enzyme plasmin, in this study, plasminogen knockout (Plg−/−) mice and their Plg-naive littermates (Plg+/+), were used to investigate the expression of TSP-1 and TSP-2 to elucidate the role of plasminogen in angiogenesis after stroke. Here we report that lack of plasminogen causes upregulation of TSP-1 and TSP-2 after ischemic stroke, thereby inhibiting angiogenesis in the peri-ischemic brain tissue which leads to poor functional recovery.
Materials and methods
All experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital (IACUC No. 1494), and complied with guidelines set forth in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, as well as the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines. 26 All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Animal groups were blinded to investigators who participated in behavioral tests and endpoint data collections.
Animal stroke model
Plg heterozygous (Plg+/–) mice (F1 generation) was purchased from Jackson Laboratory (Bar Harbor, ME). The Plg+/– mice were intercrossed and their F2 offspring genotyped by Southern blot analysis of tail-tip DNA. Male Plg−/− mice and their corresponding Plg+/+ littermates at 8-10 weeks of age were subjected to permanent right intraluminal monofilament middle cerebral artery occlusion (MCAo), which was induced by advancing a 6-0 surgical nylon suture (8.0 to 9.0 mm determined by body weight) with an expanded (heated) tip from the right external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA. 27 We excluded Plg−/− mice exhibiting rectal prolapse before or during the experiments. Within the first week after surgery, 9 mice died out of the 75 subjected to MCAo (3 in Plg+/+ group and 6 in Plg−/− group). The remaining mice were divided into different groups according to their sacrifice date, i.e., 10 mice each in the 7 and 28 day group, 13 mice each in the 14 day group. Additionally, 5 naive Plg+/+ or Plg−/− mice each were used as normal control for FITC labeling; 3 naive Plg+/+ or Plg−/− mice each were used for Western blot and 2 naive Plg+/+ or Plg−/− mice each were used for tube formation (a total of 95 mice).
Neurologic functional tests
We used Foot-fault test 2 and Adhesive removal test 27 to monitor neurological functional deficits and recovery after stroke. An investigator who was blinded to the experimental group performed these tests at 1 day prior to MCAo (baseline) and at day 1, 7, 14, 28 after MCAo. The Foot-fault test measures the accuracy of forepaw placement on a non-equidistant grid as the percentage of fault of the left forepaw to total steps. 2 The Adhesive removal test is employed to detect the sensorimotor deficits after MCAo; a small quarter-circle adhesive-backed paper dot was placed onto the impaired forepaw, and the time to remove the dot was recorded. 27
Measurement of FITC-perfused vessel density in the peri-infarct cortex
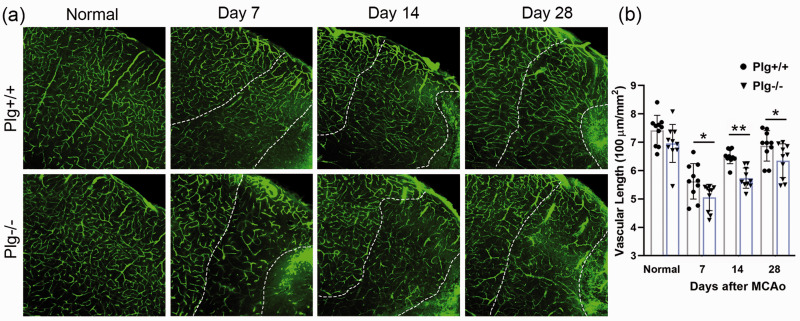
In Plg+/+ and Plg−/− mice without MCAo (n = 5/group) or at day 7, 14 and 28 after MCAo (n = 10/group), fluorescein isothiocyanate–dextran (FITC-dextran; MW 2000 kDa; 25mg/ml, 0.1 ml/mouse; Sigma-Aldrich, St. Louis, MO) was injected into a tail vain, and the mice were sacrificed 10 minutes later. The brain was removed and embedded in 4% paraformaldehyde overnight and processed for vibratome traverse section (100 µm). Since the vessels are unequally distributed in the brain, especially in the striatum due to the structures of axonal bundles and nuclei, we focused on the peri-infarct cortical area (200-300 µm away from the infarct border) wherein vessel density was reduced after stroke. The sections were digitized with an Olympus laser-scanning confocal imaging system (FV1200; Tokyo, Japan). Since there was no detectable change in vessel density in the contralesional cortex (Data not shown), and between non-stroke Plg+/+ and Plg−/− mice (Figure 2), the vascular lengths in the peri-infarct cortical area in the ipsilesional cortex were measured with skeletonized FITC-perfused vessels on 16 single layer confocal images per mouse using ImageJ software, 28 and the density of microvasculature was calculated and compared between animal groups.
Figure 2.
Single layer confocal images showing FITC-perfused cerebral vessels in the cortex of normal and stroke Plg+/+ and Plg−/− mice (a). Cerebrovascular density in the peri-infarct cortical area between the infarct core and normal brain tissue indicated by dotted lines was measured on 16 single layer confocal images per mouse using ImageJ software (b). Compared to Plg+/+ mice, Plg deficient mice exhibited a reduced cerebrovascular density in the peri-infarct cortical area at 7, 14, and 28 days after MCAo (n = 10/group; *p < 0.05, **p < 0.001; one-way ANOVA). Scale bar = 100 µm.
Tube formation assay
The entire brains from both Plg+/+ mice and Plg−/− mice were collected. The cortical tissue was isolated and digested in collagenase/dispase, and the microvessels were separated by centrifugation in a Percoll gradient (Sigma-Aldrich). Cerebral microvascular endothelial cells were seeded in flasks coated with rat-tail collagen (Sigma-Aldrich) and the medium was changed every 2–3 days. Capillary tube formation assay 29 was performed. Briefly, 0.1 ml growth factor reduced Matrigel (BD Bioscience, San Jose, CA) was added per well to a 96 well plate, and microvascular endothelial cells (2 × 104 cells) were incubated for 5 hours (n = 6/group). For quantitative measurements of capillary tube formation, Matrigel wells were digitized under a 4× objective (Olympus BX40) for measurement of total tube length of capillary tube formation using ImageJ. The total length of tube formation was quantitated as the average of randomly selected 3 microscopic fields for each well.
In vitro scratch assay
The primary cerebral microvascular endothelial cells isolated from Plg+/+ and Plg−/− mice were seeded into three 6-well chambers separately until confluent. In vitro Scratch assay 30 was performed by using a sterile 200-µl pipette tip to scratch several straight lines on the cell monolayer. Immediately after scratching, one 6-well chamber was washed with PBS and fixed with 4% paraformaldehyde for 20 minutes and used as a baseline control. Cells in the other 6-well chamber were fixed and incubated for 12h and 24h. The images were captured using a fluorescence phase contrast microscope. The area of the wound healing was measured using ImageJ, and the percentage of wound closure was calculated for each time point.
Western blot assay
The right (ischemic) and left brain tissues were collected separately at 14 days after MCAo (n = 3/group). Proteins were isolated using RIPA lysis buffer (Sigma-Aldrich) containing protease inhibitor cocktail I (Calbiochem; Billerica, MA), separated on 10% Bis-Tris acrylamide gels and transferred onto PVDF membranes. Membranes were incubated with either a primary monoclonal mouse anti-TSP-1 antibody (1:250, BD Transduction Laboratories), a mouse anti-TSP-2 antibody (1:250, BD Transduction Laboratories), or a mouse anti-actin antibody (1:500, BD Transduction Laboratories), followed by a secondary alkaline phosphatase–conjugated anti-rabbit IgG antibody (1:5000, Promega). Blots were imaged using chemiluminescence and the Protein Simple Imager (Pierce, Rockford, IL, USA). The experiment was repeated in triplicate. β-Actin was used as the internal control. The densities of bands were analyzed using ImageJ. 31
Statistical analysis
Data analysis was performed using the statistical software package PRISM (GraphPad, San Diego, CA, USA). Shapiro-Wilk test was used to assess data distribution for normality. All data are sufficiently normally distributed and presented as mean ± SD. One-way analysis of variance (ANOVA) was performed to test functional recovery measured for both behavioral task (foot-fault test and adhesive removal test) between two different genotype mice, vascular density at each time point after MCAo, percentage of wound area, as well as expression of TSP-1 and TSP-2 measured using Western blot. Tube length in tube formation assay was analyzed with the Student's t-test. To test the relationship between behavioral outcome and cerebrovascular density, the correlation coefficients between the performance scores of the stroke impaired forepaw and the cerebrovascular density in the peri-infarct cortical area were calculated by Pearson’s correlation coefficients after MCAo. P < 0.05 was considered statistically significant.
Results
Motor functional recovery after ischemia stroke is reduced in Plg deficient mice
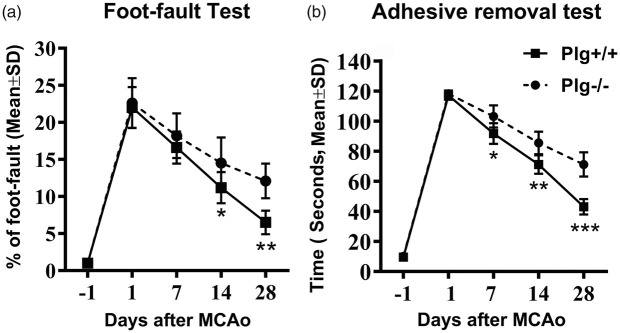
To measure the neurological deficit and recovery, we conducted Foot-fault test and Adhesive removal test prior to stroke and on days 1, 7, 14, 28 after stroke. As shown in Figure 1, the behavioral performance was comparable in both Plg+/+ and Plg−/− mice prior to MCAo and on day 1 after MCAo. Of both genotypes, the functional deficits reached peak at day 1 after MCAo and then gradually recovered with time. However, the recovery in Plg−/− mice post-stroke was significantly worse than in Plg+/+ mice, assessed in Foot-fault test (A, p < 0.05 at day 14 and p < 0.01 at day 28) and Adhesive removal test (B, p < 0.05 at day 7, p < 0.01 at day 14, p < 0.001 at day 28).
Figure 1.
Profile of behavioral deficit and recovery after MCAo. Motor performance of the stroke-impaired left forepaw was assessed with Foot-fault test (a) for motor performance with accuracy of forepaw placement on a non-equidistant grid as the percentage of foot-faults of the stroke-impaired forepaw to total steps; and Adhesive removal test (b) for sensory and motor deficits of the stroke-impaired forepaw with a small quarter-circle adhesive-backed paper dot, as the time to remove the dot. Note that significant behavioral deficits were evident one day post stroke, and were then followed by continuous, gradual, however, incomplete recovery as assessed using both tests. Plg deficiency significantly reduced functional improvements compared with wild type mice (n = 10/group; *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA).
Cerebrovascular density is decreased in Plg deficient mice after stroke
Since the blood supply in the peri-ischemic brain area is important for neurological recovery after stroke, 32 we investigated the density of actively perfused cerebral vessels in the peri-ischemic cortical area in both Plg+/+ and Plg−/− mice. As shown in Figure 2, there was no difference in the cerebrovascular density between Plg+/+ mice and Plg−/− mice without MCAo (P > 0.05, Normal). The cerebrovascular density in both genotypes was significantly reduced at day 7 after MCAo and then gradually increased with time. However, the increase of cerebrovascular density in Plg−/− mice post-stroke was significantly less than that in Plg+/+ mice (P < 0.05 at day 7 and 28 P < 0.001 at day 14).
Behavioral recovery after stroke significantly correlates to cerebrovascular density
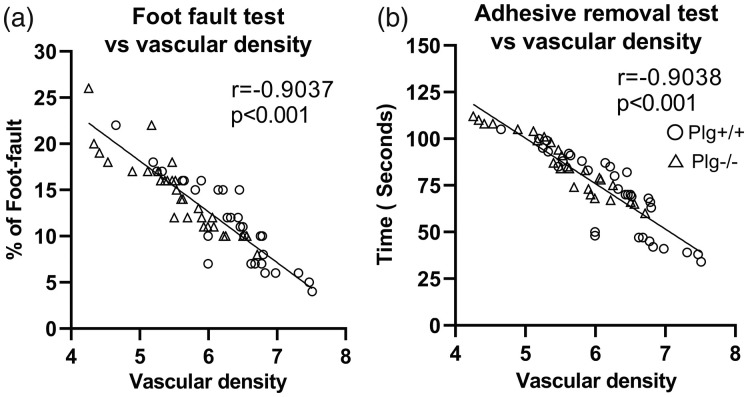
Pearson’s correlation test was used to evaluate the correlations between individual behavioral tests and cerebrovascular density in the peri-infarct cortical area (Figure 3). The results showed that both Foot-Fault test and Adhesive Removal test were strongly negatively correlated with cerebrovascular density in the peri-infarct cortical area of Plg+/+ and Plg−/− mice after MCAo (p < 0.001), indicating that cerebrovascular density in the peri-infarct cortical area may contribute to the motor and sensitive recovery after stroke.
Figure 3.
Correlations between cerebrovascular density and behavioral recovery. Pearson correlation analysis showed significant correlations between cerebrovascular density in the peri-infarct cortical area and performance of the Foot Fault test (a) and the Adhesive Removal test (b) in both Plg+/+ and Plg−/− mice at 28 days after MCAo.
Tube formation is reduced in Plg deficient mice
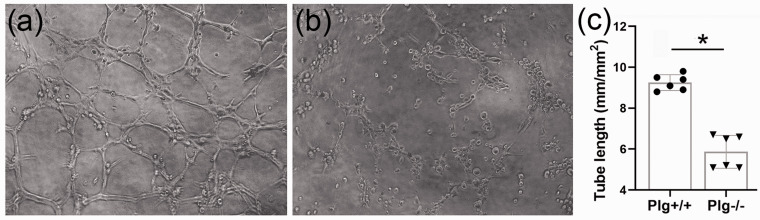
In order to demonstrate that the post stroke decrease of vessel density is due to reduced angiogenesis in Plg−/− mice, we assessed the capillary-like vessel formation ability of cerebral microvascular endothelial cells harvested from both Plg+/+ and Plg−/− mice. As shown in Figure 4, the capillary-like tubes formed by primary capillary endothelial cells of Plg+/+ mice aggregated into distinct reticular structures, with obvious migration track and hollow cavities (a). In contrast, the capillary-like tubes formed by primary capillary endothelial cells of Plg−/− mice were dispersed. Although there are migration trajectories and some hollow cavities, there is no obvious reticular structure formation (b). The total length of capillary unit area in Plg+/+ mice was 9.2 + 1.1 mm/mm2, while that in Plg−/− mice was 5.9 + 1.4 mm/mm2 (c; P < 0.01).
Figure 4.
The capillary-like tubes formed by primary capillary endothelial cells of Plg+/+ mice aggregated into distinct reticular structure, with obvious migration track and hollow official cavity (a), while endothelial cells from Plg−/− mice displayed a dispersed reticular structure formation (b). Ratio of tube length to the area of unit was calculated as an index for tube density. Compared to Plg+/+ endothelial cells, the ability to form tubes was significantly reduced from that from Plg deficient mice (c, n = 6/group; *p < 0.01; Student's t-test).
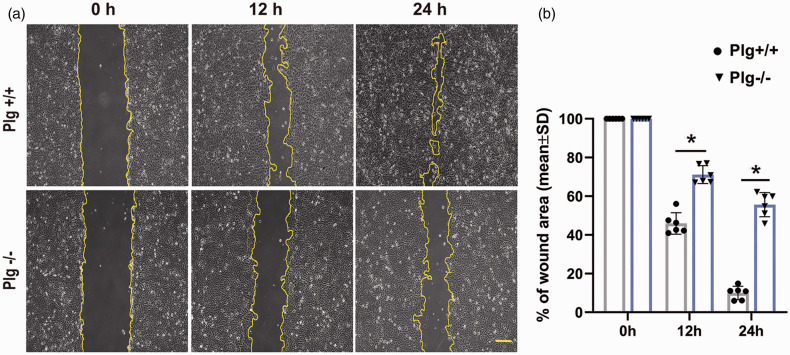
Migration ability is reduced in Plg deficient mice
In order to demonstrate that the decrease of the vessel formation of Plg−/− mice is due to reduced migration of endothelial cells in Plg−/− mice, we assessed the migration of microvascular endothelial cells harvested from both Plg+/+ and Plg−/− mice by conducting a cell Scratch assay. As shown in Figure 5, the average percentage of wound area of microvascular endothelial cells isolated from Plg+/+ mice were 54.06 ± 5.56% at 12 h, 89.98 ± 3.44% at 24 h in culture. In parallel, the average percentage of wound closure of microvascular endothelial cells isolated from Plg−/− mice was 28.83 ± 4.60% at 12h, 44.38 ± 6.22% at 24h, indicating that Plg deficiency significantly reduced the migration of vascular endothelial cells.
Figure 5.
Representative images showing migration of cerebral microvascular endothelial cells isolated from Plg+/+ and Plg−/− mice at 0 h, 12 h and 24 h (a). The gap area was measured with ImageJ. Quantitative data show that Plg deficiency significantly reduced endothelial cell migration than that from Plg+/+ mice (b, *p < 0.001 vs. WT; n = 6). Scale bar = 200 µm.
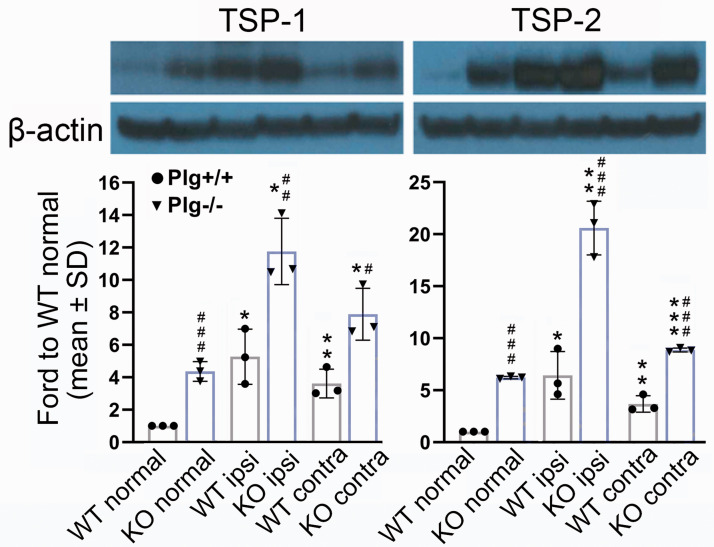
Expression of TSP-1 and TSP-2 are upregulated in Plg deficient mice
To identify molecular regulators of the suppressed angiogenesis in Plg deficient mice, we examined the expression of TSP-1 and TSP-2 in the brain in normal and stroke mice. As shown in Figure 6, in mice without MCAo, the expression of both TSP-1 and TSP-2 were significantly increased in Plg−/− mice compared to that in Plg+/+ mice (p < 0.001). After MCAo, the expression of TSP-1 and TSP-2 significantly increased in both ipsilesional and contralesional brain tissues of Plg+/+ and Plg−/− mice compared to that in their normal mice, respectively (p < 0.05). Moreover, compared to Plg+/+ mice after MCAo, the expression of TSP-1 and TSP-2 in the Plg−/− group was significantly increased, especially in the ipsilesional brain tissue in Plg−/− mice (p < 0.01).
Figure 6.
Western blots showing TSP-1 and TSP-2 expression in the brain of Plg+/+ and Plg−/− mice. Note that on day 14 after MCAo, the expression of TSP-1/2 significantly increased in both contralesional and infarcted brain tissues of both genotype mice compared to that in non-stroke mice (*P < 0.05, **P < 0.01, ***p < 0.001), while Plg−/− mice showed substantially higher expression of TSP-1/2 than Plg+/+ mice in groups of non-stroke and stroke mice, especially in the ipsilesional brain tissue (n = 3/group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs WT; one-way ANOVA).
Discussion
In the present study, to directly examine the role of plasminogen in angiogenesis and neurological recovery after stroke, we investigated the differences of behavioral outcome and cerebrovascular density in young adult Plg+/+ and genetically matched Plg−/− mice subjected to MCAo, as well as the angiogenic-like endothelial cell tube formation potential of microvessels harvested from both Plg+/+ and Plg−/− mice. Our data indicated that mice lacking plasminogen showed reduced neurological recovery and decreased cerebrovascular density in the peri-ischemic cortical area after stroke, as well as reduced in vitro endothelial cell tube formation. Moreover, Plg deficiency upregulated the expression of TSP-1 and TSP-2 after ischemic stroke. This suggests that the lack of plasminogen reduces migration of endothelial cells and thus reduces post-stroke angiogenesis.
We have previously shown that the volume of cerebral infarction induced by the same model of stroke as used in the present study, i.e., permanent occlusion of the MCA, 2 does not differ between Plg+/+ and Plg−/− mice. In the present study, the required vibratome processing of brain tissue for confocal microscopy and Western blots precluded using the present tissue for analysis of infarct volumes. The volumes of cerebral infarction for Plg+/+ mice are 20.4 ± 3.9% (Range 15.8 to 26.2%) and for and Plg−/− mice are 22.6 ± 4.1% (Range 13.4 to 27.3%). 2
In normal non-stroke Plg+/+ and Plg−/− mice, there were no apparent differences in the cerebrovascular density between Plg+/+ mice (741 ± 0.40 µm/mm2) and Plg−/− mice (718 ± 0.17 µm/mm2). We also conducted several behavioral tests on both genotype sets of mice. The results showed that there was no significant difference in functional scores between the two groups prior to MCAo, which demonstrates that the absence of plasminogen did not change the development of postnatal neuromotor ability, motor coordination and spontaneous activity in mice. 33
Angiogenesis occurs within 4 to 7 days in peri-infarct regions after cerebral ischemia. 32 , 34 In our study, FITC labeling was performed to identify those vessels that are actively perfused by the circulation after ischemic stroke in both genotype mice. We also observed the functional recovery of mice improved alongside angiogenesis after ischemic stroke. With the increase of vessel density, the functional performance of the stroke-impaired forelimb gradually improved. Pearson’s correlation test was used to evaluate the correlations between individual behavioral tests and cerebrovascular density in the peri-infarct cortical area. Our results showed that the functional performance of stroke-impaired forelimb was highly positively correlated with cerebrovascular density after MCAo (p < 0.001), supporting the hypothesis that post-ischemic angiogenesis contributes to the functional recovery after stroke. Our results are consistent with numerous studies that showed that post-ischemic angiogenesis contributes to neuronal remodeling to improve functional recovery by affecting microvessels. 34 In addition, angiogenesis also affects neurogenesis by supplying oxygen, nutrients, and soluble factors to support the migration of neural stem/progenitor cells toward the peri-infarct region.34–36 Since the functional recovery and vessel density in Plg−/− mice were reduced compared to Plg+/+ mice, our data suggest that the lack of plasminogen affects vessel density after cerebral ischemia, leading to poor functional prognosis. Interestingly, the foot-fault test data suggest that at the subacute stage of cerebral infarction (7 days after MCAo), there was no significant difference in motor outcome between the two groups, at a time that cerebrovascular density between these groups were significantly different. This may, in-part be attributed to the possibility that in the early stage after stroke, blood flow was not fully restored, because the newly born cerebral vessels are not fully functional as is the mature cerebrovasculature prior to ischemia. 37 , 38
The vessel density in the peri-ischemic area in plasminogen deficient mice was less than that in wild type mice after ischemic stroke. To support the hypothesis that the decreased vessel density is due to reduced angiogenesis in Plg−/− mice, we performed tube formation assay to assess the vessel formation ability of primary capillary endothelial cells of both Plg+/+ and Plg−/− mice. We found that compared to Plg+/+ mice, aggregated reticular structure and the density of capillary-like tubes formed from endothelial cells harvested from the Plg−/− mice were significantly decreased, indicating that the angiogenesis ability of Plg−/− mice was reduced compared with Plg+/+ mice. This finding is consistent with a previous study performed in an in vivo mouse corneal model showing that capillary germination rates are significantly reduced in Plg−/− genotype mice, 39 indicating that plasminogen may play as a key factor in angiogenesis.
In order to demonstrate that the decrease of the vessel formation ability of Plg−/− mice may in-part be attributed to reduced migration of microvascular endothelial cells in Plg−/− mice, we performed an in vitro Scratch assay in cultured primary microvascular endothelial cells harvested from both Plg+/+ and Plg−/− mice. The migration of microvascular endothelial cells of Plg−/− mice was significant reduced than it of Plg+/+ mice in the Scratch assay. This result indicates that plasminogen deficiency decreases endothelial cell migration, and therefore reduces the process of angiogenesis. Plasmin specifically binds to the adhesion molecule αvβ3 through the kringle domains and induces migration of endothelial cells. 40 In addition, as the plasminogen/plasmin system contributes to proteolysis, our results are consistent with a prior study showing migration of endothelial cells is associated with significant upregulation of proteolysis. 13
TSP-1 and TSP-2 are well-known antiangiogenic factors and their upregulation after ischemia in rats has been postulated to regulate angiogenesis. 41 , 42 Therefore, to better understand the mechanism that plasminogen plays in angiogenesis, we used Western blot assay to examine the expression of TSP-1/2 in both genotypes of mice before and 14 days after MCAo. In naïve mice without MCAo, expression of TSP-1 and TSP-2 was increased in Plg−/− mice compared to Plg+/+ mice, although there were no significant differences detected in cerebrovascular density and sensorimotor performance between Plg+/+ mice and Plg−/− mice. However, our observation indicated a further increased expression of TSP-1 and TSP-2 in the ipsilesional side of the brain 14 days after MCAo in Plg−/− mice in association with reduced behavioral outcome and reduced vascular density in the peri-ischemic area. This may, at least in part, be explained by the proteolytic cleavage of plasmin to TSP. 25 Thus, our results provide a potential therapeutic target to promote neurological recovery after stroke, via enhancing plasmin-TSPs regulated angiogenesis in the peri-ischemic area of the brain.
There are a number of caveats and limitations of the present studies which include the following considerations. Since there is no significant influence of gender on angiogenesis response in Plg−/− mice, 39 we only used male mice in the present study. We chose 14 days after MCAo as the time point to measure the expression of TSP-1 and TSP-2, because prior reports demonstrated that TSP-1 reached peak at 1 and 72 hours after focal cerebral ischemia, while TSP-2 peaked at 14 days after focal cerebral ischemia. 31 Angiogenesis occurs within 4 to 7 days in peri-infarct regions after cerebral ischemia. 32 , 34 Therefore, measurements of TSP-1 and TSP-2 at 14 days after MCAo seemed reasonable. Since our observation showed a higher TSP expression in non-stroke Plg−/− mice than that in Plg+/+ mice, but no difference in vessel density as well behavioral outcomes between the phenotypes, consideration of potential differing roles of TSPs during development and recovery after lesion warrant investigation. In addition, it has been well demonstrated that pro-angiogenic factors such as vascular endothelial growth factor and erythropoietin play a pivotal role in the tightly controlled angiogenic process. 43 , 44 Thus, further investigations on dynamic regulation between such angiogenic factors and inhibitory TSP signaling are warranted to better understand the recovery process post-stroke. The present study only examined the effects of Plg deficit on angiogenesis and neurological recovery after stroke. Further studies on the effects of Plg on neurogenesis, oligodendrogenesis and neural plasticity are warranted. In addition, we cannot exclude the possibility that the reduced functional recovery of the Plg deficient mice may also be attributed to its effect on other restorative processes. Due to technical difficulties of specifically measuring the vessel density in the peri-infarct area with irregular shape on 3D images where the vessel density is reduced after stroke, we employed an effective method using ImageJ software, 28 to manually identify the peri-lesion area on single layer confocal images. We then measured the length of skeletonized FITC-labeled vessels in this area, and calculated the vessel density within the area. However, more advanced measurement of the density at the 3D level requires development and implementation in future studies.
In summary, we conclude that although the lack of plasminogen does not alter the postnatal development of neuromotor ability, motor coordination and spontaneous activity, plasminogen deficiency up-regulates the expression of TSP-1 and TSP-2, thus reduces post-stroke angiogenesis, which contributes to reduced functional recovery. Taken together, our data suggest that the plasminogen/plasmin system contributes to neurorestorative angiogenesis via down-regulation of expression level of TSP-1 and TSP-2, which may play an important role in functional recovery after stroke.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Experimental design: ZL, MC and LH.
Experimental performance: JF, HX, LZ and WG.
Data Acquisition and analysis: JF and FW.
Manuscript writing: JF and ZL.
Manuscript revising: MC and ZGZ.
ORCID iDs: Hongqi Xin https://orcid.org/0000-0003-3172-416X
Li He https://orcid.org/0000-0002-2034-1027
Zhongwu Liu https://orcid.org/0000-0002-8571-8112
References
- 1.Lack CH, Ali SY. Tissue activator of plasminogen. Nature 1964; 201: 1030–1031. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Li Y, Qian J, et al. Plasminogen deficiency causes reduced corticospinal axonal plasticity and functional recovery after stroke in mice. PLoS One 2014; 9: e94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Chopp M, Xiong Y, et al. Subacute intranasal administration of tissue plasminogen activator improves stroke recovery by inducing axonal remodeling in mice. Exp Neurol 2018; 304: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng Y, Chopp M, Zhang Y, et al. Subacute intranasal administration of tissue plasminogen activator promotes neuroplasticity and improves functional recovery following traumatic brain injury in rats. PLoS One 2014; 9: e106238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H-J, Zhang H-N, Tang T, et al. Alteration of thrombospondin-1 and -2 in rat brains following experimental intracerebral hemorrhage. Laboratory investigation. J Neurosurg 2010; 113: 820–825. [DOI] [PubMed] [Google Scholar]
- 6.Murphy S, Durand M, Negro F, et al. The relationship between blood flow and motor unit firing rates in response to fatiguing exercise post-stroke. Front Physiol 2019; 10: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257. [DOI] [PubMed] [Google Scholar]
- 8.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–364. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol 1997; 273: H2091–H2104. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000; 57: 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 2001; 21: 1104–1117. [DOI] [PubMed] [Google Scholar]
- 13.Rakic JM, Maillard C, Jost M, et al. Role of plasminogen activator-plasmin system in tumor angiogenesis. Cell Mol Life Sci 2003; 60: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrovets T, Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell Mol Life Sci 2004; 61: 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost 2007; 97: 336–342. [PubMed] [Google Scholar]
- 16.Hildenbrand R, Allgayer H, Marx A, et al. Modulators of the urokinase-type plasminogen activation system for cancer. Expert Opin Investig Drugs 2010; 19: 641–652. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Wu Z, Sorenson CM, et al. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 2003; 228: 630–642. [DOI] [PubMed] [Google Scholar]
- 18.Tang T, Liu X-J, Zhang Z-Q, et al. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res 2007; 1175: 134–142. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, Tang T, Guo C, et al. Expression of Angiopoietin-1 and the receptor Tie-2 mRNA in rat brains following intracerebral hemorrhage. Acta Neurobiol Exp (Wars) 2008; 68: 147–154. [DOI] [PubMed] [Google Scholar]
- 20.Iruela-Arispe ML, Lombardo M, Krutzsch HC, et al. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999; 100: 1423–1431. [DOI] [PubMed] [Google Scholar]
- 21.Qian X, Wang TN, Rothman VL, et al. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp Cell Res 1997; 235: 403–412. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein P, Armstrong LC, Hankenson KD, et al. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol 2000; 19: 557–568. [DOI] [PubMed] [Google Scholar]
- 23.Yang A-L, Zhou H-J, Lin Y, et al. Thrombin promotes the expression of thrombospondin-1 and -2 in a rat model of intracerebral hemorrhage. J Neurol Sci 2012; 323: 141–146. [DOI] [PubMed] [Google Scholar]
- 24.Liauw J, Hoang S, Choi M, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab 2008; 28: 1722–1732. [DOI] [PubMed] [Google Scholar]
- 25.Venkatraman L, Chia S-M, Narmada BC, et al. Plasmin triggers a switch-like decrease in thrombospondin-dependent activation of TGF-β1. Biophys J 2012; 103: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Percie Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab 2005; 25: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Wang H, Jiang H, et al. Quantitative analysis of conjunctival microvasculature imaged using optical coherence tomography angiography. Eye Vis (Lond) 2019; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Chopp M, Zacharek A, et al. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis 2011; 43: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Jiang H, Lu D, et al. Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery 2009; 65: 1087–1096; discussion 1096–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin T-n, Kim G-M, Chen J-J, et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 2003; 34: 177–186. [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa M, Takahashi T, Ishikawa M, et al. Angiogenesis in the ischemic core: a potential treatment target?. J Cereb Blood Flow Metab 2019; 39: 753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoover-Plow J, Wang N, Ploplis V. Growth and behavioral development in plasminogen gene-targeted mice. Growth Dev Aging 1999; 63: 13–32. [PubMed] [Google Scholar]
- 34.Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res 2020; 15: 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan L, Wang B, ZhuGe Q, et al. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 2015; 1623: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 2008; 28: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner D-C, Deten A, Härtig W, et al. Changes in T2 relaxation time after stroke reflect clearing processes. Neuroimage 2012; 61: 780–785. [DOI] [PubMed] [Google Scholar]
- 38.Engedal TS, Hjort N, Hougaard KD, et al. Transit time homogenization in ischemic stroke—a novel biomarker of penumbral microvascular failure?. J Cereb Blood Flow Metab 2018; 38: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh C-W, Hoover-Plow J, Plow EF. The role of plasminogen in angiogenesis in vivo. J Thromb Haemost 2003; 1: 1683–1687. [DOI] [PubMed] [Google Scholar]
- 40.Tarui T, Majumdar M, Miles LA, et al. Plasmin-induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J Biol Chem 2002; 277: 33564–33570. [DOI] [PubMed] [Google Scholar]
- 41.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med 2012; 2: a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolsma SS, Volpert OV, Good DJ, et al. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol 1993; 122: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 2012; 43: 2270–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosky EEP, Ding Y. The role of vascular endothelial growth factor in angiogenesis and brain circulation after stroke. Brain Circ 2018; 4: 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]