Abstract
Background
Proximal dental lesions, limited to dentine, are traditionally treated by invasive (drill and fill) means. Non‐invasive alternatives (e.g. fluoride varnish, flossing) might avoid substance loss but their effectiveness depends on patients' adherence. Recently, micro‐invasive approaches for treating proximal caries lesions have been tried. These interventions install a barrier either on top (sealing) or within (infiltrating) the lesion. Different methods and materials are currently available for micro‐invasive treatments, such as sealing via resin sealants, (polyurethane) patches/tapes, glass ionomer cements (GIC) or resin infiltration.
Objectives
To evaluate the effects of micro‐invasive treatments for managing proximal caries lesions in primary and permanent dentition in children and adults.
Search methods
We searched the following databases to 31 December 2014: the Cochrane Oral Health Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE via OVID, EMBASE via OVID, LILACs via BIREME Virtual Health Library, Web of Science Conference Proceedings, ZETOC Conference Proceedings, Proquest Dissertations and Theses, ClinicalTrials.gov, OpenGrey and the World Health Organization (WHO) International Clinical Trials Registry Platform. We searched the metaRegister of Controlled Trials to 1 October 2014. There were no language or date restrictions in the searches of the electronic databases.
Selection criteria
We included randomised controlled trials of at least six months' duration that compared micro‐invasive treatments for managing non‐cavitated proximal dental decay in primary teeth, permanent teeth or both, versus non‐invasive measures, invasive means, no intervention or placebo. We also included studies that compared different types of micro‐invasive treatments.
Data collection and analysis
Two review authors independently screened search results, extracted data and assessed the risk of bias. We used standard methodological procedures expected by Cochrane to evaluate risk of bias and synthesise data. We conducted meta‐analyses with the random‐effects model, using the Becker‐Balagtas method to calculate the odds ratio (OR) for lesion progression. We assessed the quality of the evidence using GRADE methods.
Main results
We included eight trials, which randomised 365 participants. The trials all used a split‐mouth design, some with more than one pair of lesions treated within the same participant. Studies took place in university or dental public health clinics in Brazil, Colombia, Denmark, Germany, Thailand, Greenland and Chile. Six studies evaluated the effects of micro‐invasive treatments in the permanent dentition and two studies on the primary dentition, with caries risk ranging from low to high. Investigators measured caries risk in different studies either by caries experience alone or by using the Cariogram programme, which combines eight contributing factors, including caries experience, diet, saliva and other factors related to caries. The follow‐up period in the trials ranged from one to three years. All studies used lesion progression as the primary outcome, evaluating it by different methods of reading radiographs. Four studies received industry support to carry out the research, with one of them being carried out by inventors of the intervention.
We judged seven studies to be at high overall risk of bias, primarily due to lack of blinding of participants and personnel. We evaluated intervention effects for all micro‐invasive therapies and analysed subgroups according to the different treatment methods reported in the included studies.
Our meta‐analysis, which pooled the most sensitive set of data (in terms of measurement method) from studies presenting data in a format suitable for meta‐analysis, showed that micro‐invasive treatment significantly reduced the odds of lesion progression compared with non‐invasive treatment (e.g fluoride varnish) or oral hygiene advice (e.g to floss) (OR 0.24, 95% CI 0.14 to 0.41; 602 lesions; seven studies; I2 = 32%). There was no evidence of subgroup differences (P = 0.36).
The four studies that measured adverse events reported no adverse events after micro‐invasive treatment. Most studies did not report on any further outcomes.
We assessed the quality of evidence for micro‐invasive treatments as moderate. It remains unclear which micro‐invasive treatment is more advantageous, or if certain clinical conditions or patient characteristics are better suited for micro‐invasive treatments than others.
Authors' conclusions
The available evidence shows that micro‐invasive treatment of proximal caries lesions arrests non‐cavitated enamel and initial dentinal lesions (limited to outer third of dentine, based on radiograph) and is significantly more effective than non‐invasive professional treatment (e.g. fluoride varnish) or advice (e.g. to floss). We can be moderately confident that further research is unlikely to substantially change the estimate of effect. Due to the small number of studies, it does remain unclear which micro‐invasive technique offers the greatest benefit, or whether the effects of micro‐invasive treatment confer greater or lesser benefit according to different clinical or patient considerations.
Plain language summary
Micro‐invasive treatments for managing dental decay on adjacent tooth surfaces in children's and adults' teeth
Review question
The aim of this review is to evaluate the effects of micro‐invasive treatments in the management of tooth decay on adjacent (proximal) teeth in children and adults (primary and permanent teeth).
Background
Decay on tooth surfaces that are next to each other (proximal surfaces) is common. Usually it has not progressed into late stages of decay and the tooth surface does not yet have a cavity.
Different methods are used to manage proximal dental decay. A common method is drilling the affected tooth tissue and inserting a plastic or metal filling. However, a lot of sound tissue can be removed in the process and this method is regarded as invasive). Another non‐invasive methods in use include dental practitioners applying fluoride varnish or advising people to floss regularly. These non‐invasive methods do not require removing any tooth tissue.
More recent approaches (micro‐invasive treatments) involve preparing (conditioning) the tooth surface with an acid and then either placing a sealing (cover) on top of the surface or 'infiltrating' the softer demineralised tissue with resins. These newer methods work by installing a barrier either on the tooth surface or within the demineralised tissue to protect it against acids and avoid the further loss of minerals from within the tooth. This, in theory, should stop the decay. This approach can be performed by a dentist or other dental practitioner and involves the loss of a few micrometers of tooth tissue because of the need to condition the tooth surface with acid.
There is still uncertainty as to how effective micro‐invasive treatments are for managing proximal decay. It is also unclear which if any of these techniques are better than others. For example, a stronger acid is needed to infiltrated porous tissue with resin than when the tooth surface is simply sealed or covered. While infiltration might be a more effective method of protecting the tissue than sealing it, the use of a stronger acid also means losing more tissue. The aim of this review was to investigate the best approach for managing such decay in adults and children.
Study characteristics
This review considered evidence that was up to date at 31 December 2014. We found eight relevant trials with 365 participants. These trials involved children and adults whose decay lesions (tooth decay) were randomly assigned to different micro‐invasive and non‐invasive treatments. There were no studies comparing micro‐invasive interventions with invasive treatment (fillings). Four studies received financial support from intervention inventors or manufacturers to carry out the research.
Key results
The current evidence shows that micro‐invasive treatments can significantly reduce the likelihood of dental decay progression compared with the described non‐invasive methods. There are too few studies to decide which micro‐invasive treatment technique is best or the impact of different clinical and patient considerations. No negative side effects were reported; however, only half of the studies measured this outcome and the follow‐up time of some of the studies was relatively short.
Quality of the evidence
Although further research could possibly change our findings, the available evidence gives us moderate confidence that micro‐invasive treatments are much more effective than non‐invasive treatments for stopping tooth decay.
Summary of findings
Summary of findings for the main comparison. Micro‐invasive versus non‐invasive treatments for managing dental decay in primary and permanent teeth.
| Micro‐invasive versus non‐invasive treatments for managing dental decay in primary and permanent teeth | ||||||
|
Patient or population: people with dental decay on proximal surfaces of primary and permanent teeth
Settings: secondary care setting
Intervention: different micro‐invasive methods (e.g. resin infiltration, resin sealant, sealant patch and glass ionomer) Comparison: non‐invasive treatments (e.g. fluoride varnish, advice to floss) Radiographic follow‐up period: 6 months to 3 years | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Odds Ratio (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Sealing | |||||
| Caries progression measured by DSR > pairwise > visual scoring (12 months to 36 months follow‐up) |
Study population | OR 0.24 (0.14 to 0.41) | 602 (7 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c | The quality of evidence for caries progression measured by scoring (12 to 30 months), including 468 participants (5 RCTs), OR 0.27 (95% CI 0.17 to 0.44), was moderatea,b,c. The quality of evidence for caries progression measured by pairwise (18 to 36 months), including 330 participants (4 RCTs), OR 0.31 (95% CI 0.18 to 0.53), was moderatea,b,c. The quality of evidence for caries progression measured by digital substraction radiography (12 months to 18 months), including 270 participants (3 RCTs), OR 0.18 (95% CI 0.06 to 0.50), was moderatea,b,c. |
|
| 547 per 1000 | 284 per 1000 (230 to 361) | |||||
| Moderate | ||||||
| 649 per 1000 | 337 per 1000 (272 to 428) | |||||
| Change in decayed, missing and filled (DMF/dmf) figures at surface, tooth and whole mouth level. | — | — | — | — | — | No studies reported on caries measured as change in decayed, missing and filled (DMF/dmf) figures at surface, tooth or whole mouth level |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aOne or more studies lacked sufficient blinding of participants, personnel or both. Downgraded one level. bLow number of events. Downgraded one level. cOR < 0.5. Upgraded one level.
Background
Description of the condition
Dental caries
Dental caries is a sugar‐dependent disease that damages tooth structures and may result in cavity formation in the enamel (hard outer tissues of the teeth), dentine (calcified tissue of the tooth body) and cementum (surface layer of the tooth root) (Kidd 2005). Dental plaque is a biofilm formed on the tooth's surface and frequently contains caries‐producing bacteria. These micro‐organisms metabolise dietary sugars and produce acids on the tooth surfaces. The resulting decreasing pH leads to an altered mineral saturation within the biofilm and the dissolution of tooth minerals (that is, demineralisation). Prolonged loss of mineral components will eventually lead to cavitation of the carious lesions, i.e. decay.
Dental caries is the most prevalent disease worldwide (Marcenes 2013). Caries and its sequelae are considered the most important burden of oral health and are increasingly more prevalent in sociodemographically disadvantaged groups (Antoft 1999; Hannigan 2000; Petersen 2005; Ekstrand 2007; Martignon 2010; Sheiham 2010; Schwendicke 2015). Apart from the high cost of treatment, the related pain and discomfort can affect quality of life (Leal 2012).
Proximal tooth surfaces are frequently affected by caries lesions (Ekstrand 2000; Mejàre 2002; Ekstrand 2006; Rehman 2009), which dental practitioners traditionally treat using conventional invasive restorative (drill and fill) methods (Nielsen 2001). The majority of lesions in adolescents and adults occur proximally (Forsling 1999). This, together with the limited life‐span of dental restorations, led to the development of alternative approaches for sealing instead of restoratively managing proximal caries lesions.
Description of the intervention
The treatment of proximal caries lesions can be invasive or non‐invasive. Recently, micro‐invasive treatments for caries lesions have been proposed for the management of dental decay.
Conventional invasive treatment
Conventional invasive treatment usually involves the use of rotary burs, alone or in conjunction with metal hand instruments (e.g. excavators), to remove carious (i.e. infected and demineralised) dentine and then place a filling (restoration). The conventional cavity preparation for caries lesions on proximal tooth surfaces usually leads to removal of marginal ridges of the tooth, thereby sacrificing a considerable amount of sound hard tissue and weakening the tooth structure. The pain and discomfort associated with conventional cavity preparation methods and fear of local anaesthetic injection, which is used to control pain, may discourage people from seeking dental treatment (Berggren 1984). Furthermore, conventional invasive treatment requires expensive equipment (e.g. handpiece), electricity supply and highly trained dental health personnel. These factors limit the access to dental care where financial, human and structural resources are scarce.
Non‐invasive treatment
Non‐invasive treatments aim at 'managing' rather than removing caries lesions. They include biofilm control via mechanical removal of plaque (e.g. dental floss or interdental brushing by patient), antibacterial treatments (e.g. application of chlorhexidine varnishes by dentist or other members of dental team) or remineralisation treatments (e.g. topical fluoride application by dentist or other members of dental team). The efficacy of measures for biofilm control remains uncertain, especially for proximal lesions (Poklepovic 2013), whilst research has generally found fluoride application to be effective in managing caries lesions (Marinho 2009). In general, the dependence on patient adherence compromises the effectiveness of non‐invasive means, especially with regard to biofilm removal on proximal surfaces via oral home care (e.g. flossing). Moreover, the need for periodic visits, for example for repeated application of topical fluoride, increases treatment costs and decreases the cost‐effectiveness of non‐invasive measures (Ellwood 2003; Martignon 2006; Schwendicke 2014a).
Micro‐invasive treatment
Micro‐invasive treatments involve conditioning the tooth surface prior to treating the caries lesion; this conditioning via organic acids involves the loss of few micrometers of tooth substance (enamel). There are two types of micro‐invasive treatments: sealing and resin infiltration.
Conventionally, sealing is performed to prevent dental caries in the pits and fissures on the occlusal or pitted tooth surfaces. Research has found such sealing to be highly efficacious (Ahovuo‐Saloranta 2013). For non‐sound (carious) surfaces, dental practitioners can also apply sealants therapeutically. Such sealing is thought to prevent the diffusion of bacterial acids into the lesion and minerals out of the lesion. Creating a diffusion barrier may prevent dental caries (Handleman 1973; Ripa 1993; Schwendicke 2015).
Based on the identical pathogenesis of occlusal and proximal caries lesions, sealing might also be efficacious in managing dental caries on proximal tooth surfaces (Splieth 2010). A number of sealant materials and techniques have been used as micro‐invasive treatments on proximal surfaces, such as resin sealants, polyurethane patches (tapes), and glass ionomer cement (GIC). These are applied onto the proximal lesion after separating adjacent teeth from each other, for example via orthodontic bands placed between the teeth for a few days prior to sealing (Gomez 2005; Martignon 2010; Splieth 2010; Alkilzy 2011; Trairatvorakul 2011). After separation, practitioners can protect adjacent tooth surfaces and prepare the lesion surface using phosphoric acid or dentine conditioner (for GIC) to increase the micro‐mechanical retention of the sealant (Cueto 1967). Then, they can apply and light cure flowable or fluidic sealant (with or without the patch) or GIC. Available sealant patches are fabricated based on polyurethane and form an elastic pre‐cured adhesive monomer layer that is bonded to the tooth. Compared to conventional sealants, such patches are supposed to be easier to handle and to provide an even layer of resin (Alkilzy 2011).
A second technique for proximal micro‐invasive treatment is resin infiltration (caries infiltration). This technique uses light‐curable low‐viscosity resins (infiltrants), which are soaked up into the porous enamel lesion body. Thus, resin infiltration creates the diffusion barrier inside rather than on top of the caries lesion like other sealants (Meyer‐Lueckel 2007). Another difference in resin infiltration is the fact that the pseudo‐intact surface layer present in non‐cavitated proximal lesions needs to be removed prior to infiltration via acid‐etching, using hydrochloric instead of phosphoric acid (Meyer‐Lueckel 2007). Thus, the amount of lost enamel is greater when infiltrating than when sealing a lesion. After etching, the lesion requires desiccation. Resin infiltration does not necessarily require separation of teeth before treatment, since available kits use wedges and applicator foils to separate and isolate adjacent teeth from each other. Hence, practitioners are supposed to administer resin infiltration in a single visit (Ekstrand 2010; Paris 2010a). Moreover, as resin infiltration is not placed on top, but inside the enamel, retention loss is unlikely to occur (as this would mean losing the 'hybridised' tooth tissue). Some of the described materials are resin based, and may therefore cause skin or mucosal reactions, including anaphylactoid, lichenoid or other allergic reactions (FDI 2009).
How the intervention might work
As described, proximal sealing or infiltrating caries lesions prevents diffusion of acids from biofilms into the lesion or mineral out of the lesion, thereby arresting the lesions or reducing their progression. Current applications of these micro‐invasive techniques are restricted to non‐cavitated lesions only.
Why it is important to do this review
Dental caries in proximal surfaces is an important health problem, especially in children, adolescents and young adults. Micro‐invasive treatment via proximal sealing or infiltrating might reduce the loss of dental hard tissue and the risk for follow‐up treatments emanating from invasive conventional treatment methods (Qvist 2008; Schwendicke 2014a). Consequently, they might help to reduce pain or discomfort during treatment, which could in turn lower dental anxiety and avert costs related to late treatment. Moreover, micro‐invasive treatments could be less dependent on patients' adherence than non‐invasive means, with subsequently increased efficacy. Finally, they should minimise the number of repeated treatment visits, thus increasing the cost‐effectiveness of the therapy.
There is still uncertainty within the dental profession with regard to the efficacy of micro‐invasive treatments compared to invasive or non‐invasive treatments for controlling proximal caries lesions. Therefore, it is important to assess the evidence for such strategies. The findings of this review can inform dental practitioners' clinical decision‐making and enhance evidence‐based practice.
Objectives
To evaluate the effects of micro‐invasive treatments for managing proximal caries lesions in primary and permanent dentition in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials with at least six months follow‐up, as this is the shortest recommended length of intervals between radiographic exposures (ADA 2012). Both parallel group and split‐mouth study designs were eligible for inclusion. The unit of randomisation could be a group (e.g. school, class), an individual, a tooth or lesion, or tooth and lesion pairs.
Types of participants
Dentate children and adults, with proximal carious lesions, extended into enamel or dentine (but not the pulp), as detected by radiographs. We only considered teeth with no existing filling at the site of lesion (primary caries lesions).
Types of interventions
Interventions included proximal micro‐invasive treatments using resin or glass ionomer cement (GIC) sealants, polyurethane tapes or resin infiltration, placed or performed by dentists or dental care professionals (DCPs) (e.g. hygienist, therapist, etc.), versus: another micro‐invasive treatment, non‐invasive measures (e.g. dental floss, fluoride varnish, oral hygiene advice), invasive means (conventional restorations), placebo or no intervention.
Types of outcome measures
Primary outcomes
Progression of existing carious lesion into enamel or dentine, as detected by radiographs, over a minimum period of six months.
Change in decayed, missing and filled (DMF for permanent dentition/dmf for primary dentition) figures at surface, tooth and whole mouth level. Studies were to assess this over a minimum period of six months.
Caries progression could be assessed by any of the following methods.
Lesion progression, as scored via digital subtraction radiography, assessing radiographs obtained at baseline and follow‐up.
Lesion progression, as scored via pairwise comparison of radiographs obtained at baseline and follow‐up.
Lesion progression, as scored by independent assessment of radiographs obtained at baseline and follow‐up.
Clinical lesion progression using visual scoring, for example, the International Caries Detection and Assessment System (ICDAS).
We considered digital subtraction radiography (DSR) to be the most sensitive of the caries progression methods.
Secondary outcomes
Material deficiency (e.g. retention loss, or number of re‐treatments)
Participant and operator perception, as measured by standardised/validated questionnaires
Adverse events
Direct costs of treatment, indirect costs incurred from time off school or work to attend the dental visits by the patient/parent/caregiver (opportunity costs), or both
Search methods for identification of studies
We identified all relevant studies regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
For the identification of studies included or considered for this review, detailed search strategies were developed for each database to be searched. These were based on the search strategy developed for MEDLINE, but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We provide details of the MEDLINE search in Appendix 1. The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs.
The following bibliographic databases and trials registers were searched:
The Cochrane Oral Health Group Trials Register (to 31 December 2014) (see Appendix 2);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2014, Issue 11) (see Appendix 3);
MEDLINE via OVID (1946 to 31 December 2014) (see Appendix 1);
EMBASE via OVID (1980 to 31 December 2014) (see Appendix 4);
LILACS via Bireme Virtual Health Library (1982 to 31 December 2014) (see Appendix 5);
Web of Science Conference Proceedings (1990 to 31 December 2014) (see Appendix 6);
Zetoc Conference Proceedings (1993 to 31 December 2014) (see Appendix 7);
Proquest Dissertations and Theses (1861 to 31 December 2014) (see Appendix 8);
OpenGrey (1985 to 31 December 2014) (see Appendix 9).
There were no language or date limitations in the searches of the electronic databases.
Searching other resources
We searched the following trials registries for ongoing trials (see Appendix 10):
metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) (to 1 October 2014, the database was no longer available after this point);
The US National Institutes of Health Trials Registry (http://www.clinicaltrials.gov/) (to 31 December 2014);
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/default.aspx) (to 31 December 2014).
Handsearching
We only included handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL.
Reference lists
We examined the reference lists of relevant trials in an attempt to identify studies not identified in the previous searches.
Correspondence
We contacted organisations, researchers and experts known to be involved in the field by post, email or in person during scientific meetings and conferences, in an effort to trace unpublished or ongoing studies. We also contacted dental equipment manufacturers to identify any ongoing or unpublished studies. We contacted one company known to manufacture a kit for caries infiltration, and we requested and obtained data and references for all published and unpublished studies on sealants.
Data collection and analysis
We imported the downloaded set of records from each database to the bibliographic software package EndNote and merged them into one core database to remove duplicate records and to facilitate retrieval of relevant articles. For all potentially relevant reports identified when searching other non‐electronic sources (e.g. reference lists of relevant trials, reviews, articles and textbooks), we obtained the records and manually entered them into Endnote.
Selection of studies
Two review authors (MD and FS) independently screened the retrieved studies. Review authors were not blinded to the names of the authors, institutions, journal of publication or results of the studies. We first checked all records identified by the searches on the basis of title, then by abstract, keywords or both. We excluded records that were obviously irrelevant (according to study design/duration, participants, or interventions/comparisons) and obtained the full text of all remaining records. If the information relevant to the inclusion criteria was not available in the abstract or if the title was relevant but the abstract was not available, we obtained the full text of the report. The review authors could read reports in English, Persian, Arabic and German. For publications in other languages, we would have had the title and abstract of the retrieved studies translated by a native speaker who was fluent in English. If we thought the study was potentially eligible, we would have had the full text translated by two translators who were native speakers and fluent in English. One of the authors (MD) was to compare two versions and resolve disagreements, if any, by discussion with the translators. We did not identify any non‐English studies eligible to be included in this review.
MD and FS independently assessed the full reports obtained from all the electronic and other methods of searching to establish whether the studies met the inclusion criteria or not, using an inclusion criteria form that we had previously prepared and piloted. We resolved disagreements by discussion. Where resolution was not possible, we consulted a third review author (SD).
We attempted to contact authors of papers that we could not classify in order to ascertain whether they met inclusion criteria. We identified and checked all reports related to the same included study; in case of any discrepancy, we contacted the authors. If we identified more than one publication of a trial, we reviewed all of them and considered that the paper with the earliest publication date was the primary reference.
We recorded details about the studies rejected at the full‐text or subsequent stages in the 'Characteristics of excluded studies' tables, along with the reasons for exclusion.
Data extraction and management
The review authors (MD, FS and TW) independently extracted data from all included studies using a pilot‐tested data extraction form. Two review authors then separately entered the data into the 'Characteristics of included studies' table in Review Manager 5 (RevMan 2011) and checked for differences. We resolved any disagreements through discussion with another review author (SD) until reaching a consensus. We contacted the trial authors for clarification or missing information, where necessary. We treated studies with duplicate publications as a single source of data. If publications reported data from different time points, we extracted all data sets but only used the last for synthesis (see below).
We recorded the following details for each trial in the data extraction form: study design of randomised controlled trial (e.g. parallel, split‐mouth, cluster); country where trial took place; setting (e.g. primary or secondary care); number of centres; recruitment period; funding source; inclusion criteria; exclusion criteria; number of patients randomised/number of patients evaluated; test and control interventions; mode of administration; duration of interventions; primary and secondary outcomes; time point(s) when outcomes were measured; method of sample size calculation; duration of follow‐up; comparability of groups at baseline; any co‐interventions; risk of bias; and any other relevant data.
Assessment of risk of bias in included studies
Two review authors (MD and FS) independently undertook the 'Risk of bias' assessment for included trials. We resolved disagreements by discussion with a third review author (SD) until reaching a consensus. Where needed, we contacted study authors to obtain further data. We carried out the assessment according to the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Sequence generation: Was the method used to generate the allocation sequence appropriate to produce comparable groups? We graded this domain as having a low risk of bias if the authors described a random component in the sequence generation process (e.g. random number table, coin tossing, drawing of lots).
Allocation sequence concealment: Was the method used to conceal the allocation sequence appropriate to prevent the allocation being known in advance of, or during, enrolment? We graded this domain as having a low risk of bias if the authors described adequate concealment (e.g. by means of central randomisation, sequentially numbered, opaque envelopes), and graded high risk of bias if inadequate concealment was documented (e.g. alternation, use of case record numbers, dates of birth or day of the week) or if allocation was not concealed.
Blinding of participants and personnel: Was knowledge of the allocated intervention adequately prevented during the study? If the study ensured adequate blinding of participants, operators and assessors, for example by using placebo or sham treatments, we graded this domain as having a low risk of bias. For radiographic outcomes, examples of adequate assessment blinding could be anonymising the radiographs, masking the stage of treatment (i.e. baseline or follow‐up) or both. For clinical assessment of sealants, we assumed blinding was not possible. We graded this domain as having a high risk of bias if the study did not use any blinding of participants, operators or assessors.
Incomplete outcome data: How complete were the outcome data for the primary outcomes? Did authors report drop‐out rates and reasons for withdrawals? Did they impute missing data appropriately? We graded this domain as having a low risk of bias if the proportion of the missing outcome data was less than 25% and the groups were balanced in numbers and reasons for drop‐outs, or if investigators imputed missing data using appropriate methods. If drop‐out was above 25% and there was no information on reasons for drop‐outs across groups, but attrition was balanced, we graded the risk of bias as unclear. We graded it as high if the proportion of missing outcome data was over 25% and not balanced between groups.
Selective outcome reporting: Did investigators report appropriate outcomes or were key outcomes missing? We graded this domain as having a low risk of bias if authors reported all pre‐specified outcomes. If they did not report prespecified or expected data, we assumed the risk of bias to be high.
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias? These include information on the baseline characteristics of the intervention and control groups and the similarity in using co‐interventions between groups. We graded the trials as having a high risk of bias if there were important differences in demographic characteristics or caries risk level at baseline between the study groups, or if the groups received different co‐interventions during the trial.
We developed a standardised 'Risk of bias' assessment form, which included the criteria for assessing the above domains, and we entered data in the 'Risk of bias' tables in RevMan (RevMan 2011). We summarised the potential risk of bias for each study and grouped them into the following three categories, as described in Section 8.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Low risk of bias: plausible bias not likely to seriously alter the results (if low risk of bias for all items).
Unclear risk of bias: plausible bias that raises some doubt about the results (if unclear risk of bias for one or more key items).
High risk of bias: plausible bias that seriously weakens confidence in the results (if high risk of bias for one or more key items).
We completed a 'Risk of bias' table for each included study (Characteristics of included studies) and presented the results graphically by domain over all studies and by study.
Measures of treatment effect
According to different outcomes, we planned to convert data obtained from visual analogue scales and any categorical outcomes into dichotomous data prior to analysis. For continuous data, we calculated the effect estimate as the mean difference (MD) and reported it along with 95% confidence intervals (CIs). For dichotomous data, we calculated the effect estimate as the odds ratio (OR) and also reported it along with the 95% CI.
Unit of analysis issues
The unit of analysis for trials with cluster or body part (e.g. split‐mouth design) randomisation was each tooth surface. We anticipated that the majority of studies would be split‐mouth studies that included one or more pairs of tooth surfaces per individual, with the interventions randomly allocated to tooth surfaces within each pair. Strict multiple pairing of tooth surfaces within an individual means that the data are not independent and should be analysed as 'paired data' on an individual basis. However, we decided to analyse the pairs independently, as otherwise we would be excluding most of the trials and losing useful information from these studies (we are unaware of any widely used methods to correct and account for dependence of the tooth pairs when, for example, only marginals are reported). This meant that the confidence intervals would be slightly narrower than they should be, and we took this into consideration when we interpreted the results. We undertook a sensitivity analysis excluding studies where the number of pairs far exceeded the number of individuals.
Dealing with missing data
We contacted the study authors to clarify incompletely reported data related to trial characteristics, methodology and outcomes and to ascertain if data was missing at random or not. We planned, but did not perform, imputation of data not missing at random, since we assumed all missing data to be missing at random.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the characteristics of the studies: the similarity between the types of participants, the interventions and the outcomes as specified in the criteria for included studies. We assessed statistical heterogeneity using a Chi2 test and the I2 statistic, where I2 values over 50% indicate moderate to high heterogeneity (Higgins 2003).
Assessment of reporting biases
We assessed publication bias or small‐study effects graphically using a funnel plot (Egger 1997). A symmetrical plot indicates the absence of bias. An asymmetrical plot may indicate the presence of reporting bias. If there was asymmetry, we planned to further investigate the possible causes. Apart from publication bias, other sources of asymmetry in funnel plots include poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity and chance (Higgins 2011).
Data synthesis
We evaluated the efficacy of micro‐invasive treatments in different subgroups of interventions. Moreover, we synthesised studies according to the measure of radiographic progression used (i.e. digital subtraction radiography, pairwise reading, visual scoring). To calculate one effect estimate for all micro‐invasive interventions versus control interventions regardless of the measure used, we combined different measures, preferring more sensitive rather than less sensitive methods for studies where more than one measure was available (digital subtraction radiography over pairwise reading over scoring). We calculated the number needed to treat for an additional beneficial outcome (NNTB) for the overall pooled estimates.
For the split‐mouth studies, we calculated ORs for differences of paired tooth surfaces being carious or not, along with the appropriate standard errors and 95% CIs. To calculate the ORs, we used the Becker‐Balagtas method outlined in Curtin 2002 by means of R software version 2.13.1. We chose the Becker‐Balagtas method because in this review we also included studies that reported data only in marginal form (as parallel group studies, not as 2 x 2 cross‐classification for paired data) and this method facilitated data synthesis. We chose the intracluster correlation coefficient (ICC) in the studies with data only as marginals to be the conservative 0.05. In the studies with data presented as tooth pairs, we calculated the ICC from the data.
We conducted the meta‐analyses with RevMan 2011, using the generic inverse variance method with either the fixed‐effect or the random‐effects model. In meta‐analyses including two or three studies, we used the fixed‐effect model, and in meta‐analyses including four or more studies, we used the random‐effects model. For the sensitivity analyses, we evaluated the effect on the results of split‐mouth studies with high numbers of pairs compared to the number of subjects as well as the 'Risk of bias' grading. We were unable to meta‐analyse the data from split‐mouth studies with different numbers of lesions in each group.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses for the different types of sealing methods/materials (resin sealant, resin infiltration, sealant patch and GIC).
We had planned to perform subgroup analyses if the included trials had different designs (e.g. split‐mouth or parallel). However, all included studies used a split‐mouth design. Moreover, we had planned to analyse the effects of age (children under 16 or adults), setting (e.g. primary or secondary care), site (e.g. mesial or distal), tooth type (e.g. premolar or molar), operator (e.g. dentist or DCP) and moisture control (e.g. cotton rolls or rubber dam).
In a change to the protocol, we had also planned to undertake a subgroup analysis according to dentition (primary or permanent) and caries risk (low to moderate, moderate, mostly high or high).
Sensitivity analysis
As described, we had planned to compare the effect estimates in studies with overall low risk of bias compared to the effect estimate from studies at any level of overall risk of bias to assess the robustness of our review results.
Summary of findings table and quality of the evidence
In 'Summary of findings' tables, we present a summary of key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on all important outcomes for a given comparison. We used GRADEprofiler software version 3.2 (GRADEpro) to provide the overall grading of the quality of evidence for the caries outcomes according to recommendations outlined by the GRADE network (GRADE 2004). We performed the following comparison: proximal sealing versus placebo or non‐invasive measures (Table 1).
Results
Description of studies
See: 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of ongoing studies'.
Results of the search
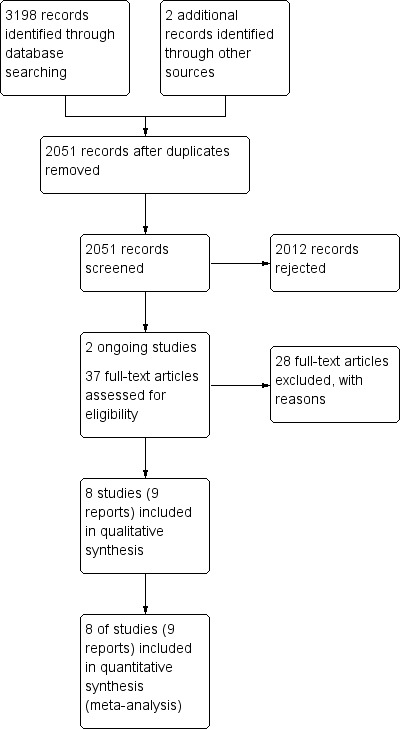
We retrieved 3198 records from searches of the Cochrane Oral Health Group Trials Register (413), CENTRAL (625), MEDLINE (975), EMBASE (587), LILAC (28), Web of Sciences proceedings (543), ZETOC conference proceedings (3), Proquest dissertation and theses services (8), metaRegister of Controlled Trials (2), ClinicalTrials.gov (11), OpenGrey (0) and the WHO International Clinical Trials Registry Platform (3). We identified two further studies through communication with the experts in the field and by checking reference lists from identified trials and review articles (Figure 1).
1.
Study flow diagram
After de‐duplication, we had 2051 records. We screened the title or abstract, or both, and on this basis, rejected 2012 records because they did not meet inclusion criteria. We identified two ongoing studies (see 'Characteristics of ongoing studies'). We obtained and evaluated 37 full‐text manuscripts. We did not identify any non‐English language reports as potentially eligible, and therefore, there were no full‐text reports that needed translation. Of the 37 full‐text articles, we excluded 28 and included 9 (8 studies). The main reasons for exclusion were that the study was a non‐randomised trial, a non‐clinical trial, or it did not include micro‐invasive interventions. We present the reason for the exclusion of each study in 'Characteristics of excluded studies'.
Included studies
We included eight trials in the review (see 'Characteristics of included studies'), all of them using split‐mouth design, some with more than one pair of lesions treated within the same participant. Studies took place in university dental clinics in Brazil, Denmark, Germany, and Thailand, as well as in public health dental clinics in Greenland and Chile. The follow‐up times ranged from one to three years.
The sample sizes in the included studies ranged from 7 to 91 participants, with both males and females evaluated. Participant age ranged from 4 to 39 years, and they had either only enamel or enamel‐dentine lesions extending into the outer third of the dentine. Treated patients had high caries risk in two studies; moderate or high risk in two studies; low, moderate or high risk in two studies; and unknown risk in two studies. Five of the studies assessed the caries risk using the Cariogram programme, which combines several caries‐related factors, including caries experience (number of decayed, missing and filled teeth/surfaces, or DMFT/DMFS); related caries diseases; diet (amount and frequency); plaque amount; fluoride intake; salivary flow rate and buffering capacity (Martignon 2006; Ekstrand 2010; Martignon 2010; Paris 2010a; Martignon 2012). Six studies evaluated the effects of micro‐invasive treatments in the permanent dentition (Gomez 2005; Martignon 2006; Paris 2010a; Alkilzy 2011; Trairatvorakul 2011; Martignon 2012), and two studies focused on the primary dentition (Ekstrand 2010; Martignon 2010). Different control conditions used in the studies included: flossing advice only (three studies); fluoride varnish only (two studies); oral hygiene instruction, flossing and fluoride toothpaste advice (one study); fluoride varnish and fluoride toothpaste advice (one study); fluoride varnish, oral hygiene instruction and diet advice (one study).
Four studies received industry support to carry out the research or had other conflicts of interests. Companies with a financial interest in the study results funded three trials (Ekstrand 2010; Alkilzy 2011; Martignon 2012). The authors of Paris 2010a are inventors of various patents for a resin infiltration technique for dental caries lesions, which is held by Charité‐Universitätsmedizin Berlin. The authors further stated that they receive royalties from the manufacturer of the infiltrant.
All trials used lesion progression as primary outcome and applied radiographic techniques to assess lesion progression. Investigators evaluated caries progression using conventional independent reading of radiographs in five studies (Martignon 2006; Ekstrand 2010; Martignon 2010; Paris 2010a; Trairatvorakul 2011); using pairwise reading of radiographs in three studies (Martignon 2006; Paris 2010a; Martignon 2012) and using digital subtraction radiography in three studies (Martignon 2006; Paris 2010a; Martignon 2012). Ekstrand 2010 additionally assessed lesion progression using ICDAS.
All studies compared micro‐invasive with non‐invasive treatments. Excepting Gomez, the trials also used the non‐invasive intervention in the intervention arm.
Three studies, randomising 212 tooth pairs in 212 participants, compared resin sealant plus flossing advice versus flossing advice alone in secondary care settings (Martignon 2006; Martignon 2010; Martignon 2012). The radiographic follow‐up periods were between one and three years.
One study, randomising 71 lesions in seven participants, compared resin sealant versus fluoride varnish (Gomez 2005). Trials followed up participants for two years with radiograph.
Three studies, randomising 116 tooth pairs in 109 participants evaluated resin infiltration. Ekstrand 2010 compared resin infiltration plus 2.26% sodium fluoride varnish versus 2.26% sodium fluoride varnish alone (48 lesion pairs in 48 children), and Paris 2010a compared resin infiltration plus flossing advice versus sham infiltration plus flossing advice (22 participants and 29 lesions). The study by Martignon 2012 had a third arm for resin infiltration plus flossing advice (39 lesions randomised in 39 participants). The trials followed up participants with radiograph for one to three years. Ekstrand 2010 also followed up participants clinically for one year.
One study, randomising 41 tooth pairs in 26 participants, compared glass ionomer sealant plus 12300 ppm fluoride gel plus 1000 ppm dentifrice versus 12300 ppm fluoride gel plus 1000 ppm dentifrice (Trairatvorakul 2011). Radiographic follow‐up was 6 to 12 months.
One study, randomising 50 tooth pairs in 50 participants, compared adhesive patch versus no treatment (Alkilzy 2011). All participants recieved advice to use dental floss and a fluoridated toothpaste. Clinical follow‐up was 6 to 12 months, and radiographic follow‐up was two to three years.
We included Gomez 2005 in the review as it meets the eligibility criteria, but we could not include it in the meta‐analysis as the data were not presented in the correct format, and there was a very high ratio of tooth pairs to children.
Excluded studies
We excluded 28 studies after full‐text screening, for the following reasons: 18 studies did not use micro‐invasive treatments as intervention, 7 studies were non‐clinical, 2 studies did not use a randomised controlled design, and 1 study did not include proximal lesions. The 'Characteristics of excluded studies' table presents the reasons for the exclusion of each study.
Risk of bias in included studies
Allocation
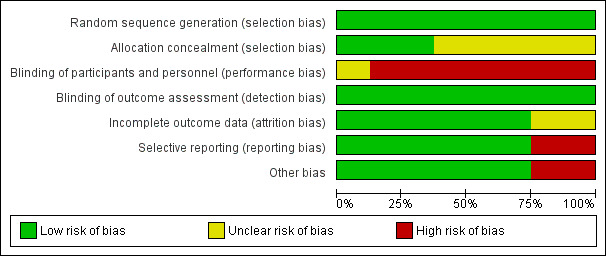
All the included studies reported adequate methods for random sequence generation and we therefore assessed them as being at low risk of bias.
We graded allocation concealment as having a low risk' of bias in three studies (Ekstrand 2010; Paris 2010a; Trairatvorakul 2011). The remaining five studies did not provide sufficient information to judge allocation concealment, so we graded them as having an unclear risk of bias.
Blinding
One study reported blinding participants (sham infiltration), but we assessed the risk of bias for this domain as unclear because operators were not blinded, and it was unclear how this might affect outcomes (Paris 2010a). Ekstrand 2010 assumed that the participating children and their parents were blind to which treatment they were receiving, as the children were young and the parents were at some distance from the dental chair during the treatment. We did not consider this adequate and judged the study to have a high risk of bias in this domain. Martignon 2012 did not blind operators, and it was unclear if participants would have been blinded. We decided to assess this study as at high risk of bias. The remaining five studies did not state they were blinded, and as we considered this possibility unlikely, we also assessed them as being at high risk of bias.
We considered the blinding of clinical assessors to be inadequate for the two studies reporting clinical lesion progression. Investigators reported radiographic assessment of lesion progression to be performed blind, and we therefore rated them as having a low risk of bias. Given that we considered radiographic outcome assessment to be more relevant than clinical assessment, we eventually graded all studies as having a low risk in this domain.
Incomplete outcome data
We judged six studies with low (less than 25%) attrition to have a low risk of attrition bias (Gomez 2005; Martignon 2006; Ekstrand 2010; Paris 2010a; Martignon 2012; Trairatvorakul 2011). Two studies had an unclear risk of bias, since attrition was more than 25%, but it was unclear if this affected overall risk of bias given that the studies used a split‐mouth design (Martignon 2010; Alkilzy 2011).
Selective reporting
Six studies reported all pre‐specified outcomes adequately, so we assessed them as having a low risk of selective reporting bias (Gomez 2005; Martignon 2006; Martignon 2010; Paris 2010a; Alkilzy 2011; Trairatvorakul 2011). Two studies were at high risk of bias for this domain: Ekstrand 2010 did not report the outcomes of the six‐month clinical evaluation, and the radiographic evaluation report was incomplete; Martignon 2012 reported the findings of the digital subtraction radiographic evaluation only after one year, not after the complete follow‐up.
Other potential sources of bias
Gomez 2005 did not provide information on the distribution of different lesion depths among study groups, and there was unbalanced allocation of lesions according to ICDAS scores in Ekstrand 2010. We therefore judged both studies to be at high risk for other sources of bias.
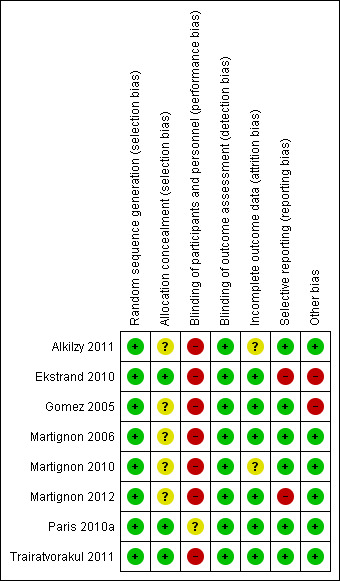
We only judged one study to be at overall unclear risk of bias (Paris 2010a); we considered all other studies to be at overall high risk of bias (Figure 2; Figure 3).
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
See: Table 1
We assessed intervention effects as overall therapies (proximal micro‐invasive treatment) and subgrouped according to different methods (resin sealant, resin infiltration, glass ionomer sealant and sealant patch). We performed separate meta‐analyses for caries progression as assessed by each of three different methods of radiographic readings used in the included studies as well as a meta‐analysis using the most sensitive outcome available (DSR > pairwise > scoring) from each study.
Micro‐invasive treatment versus non‐invasive control/placebo for managing dental decay in primary and permanent teeth
Primary outcomes
Caries progression
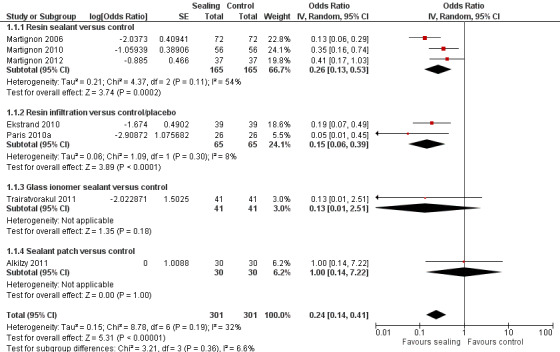
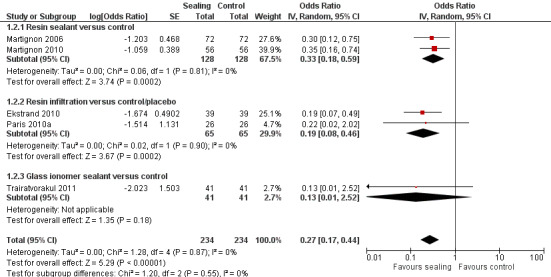
The clinical and radiographic follow‐up times in the included studies ranged from 6 to 12 months, and 6 months to 3 years, respectively. We evaluated lesion progression at the most sensitive level of measurement presented in each of the seven studies reporting data in a format suitable for meta‐analysis (Table 1). Our meta‐analysis showed that micro‐invasive treatment significantly reduced the odds of lesion progression compared with non‐invasive or no treatment (OR 0.24, 95% CI 0.14 to 0.41; 602 lesions; seven studies; I2 = 32%; Analysis 1.1; Figure 4). We also analysed different radiographic measures separately, finding that micro‐invasive treatment was effective where caries progression was measured using radiographic scoring (OR 0.27, 95% CI 0.17 to 0.44; 468 lesions; five studies; I2 = 0%; Analysis 1.2; Figure 5), pairwise reading (OR 0.31, 95% CI 0.18 to 0.52; 330 lesions; four studies; I2 = 3%; Analysis 1.3; Figure 6) and DSR (OR 0.18, 95% CI 0.06 to 0.50; 270 lesions; three studies; I2 = 60%; Analysis 1.4; Figure 7). Ekstrand 2010 reported clinical results of caries progression, finding that resin infiltration plus varnish significantly reduced the odds of lesion progression compared with varnish alone (OR 0.22, 95% CI 0.09 to 0.55; 84 lesions).
1.1. Analysis.
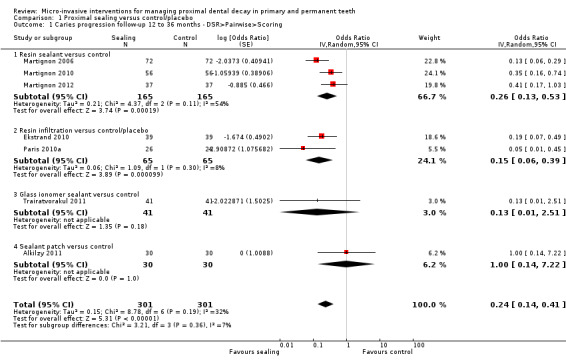
Comparison 1 Proximal sealing versus control/placebo, Outcome 1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring.
4.
Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring
1.2. Analysis.
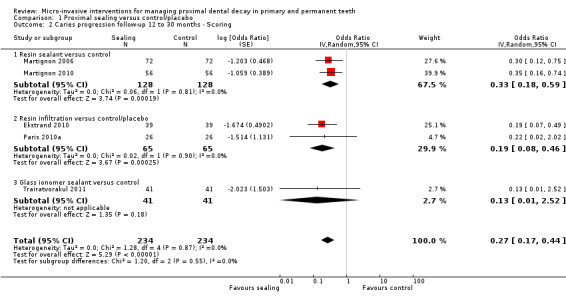
Comparison 1 Proximal sealing versus control/placebo, Outcome 2 Caries progression follow‐up 12 to 30 months ‐ Scoring.
5.
Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Caries progression follow‐up 12 to 30 months ‐ Scoring
1.3. Analysis.
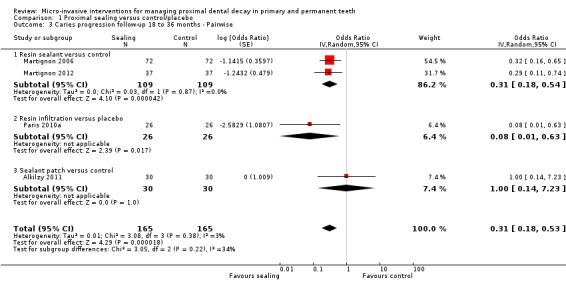
Comparison 1 Proximal sealing versus control/placebo, Outcome 3 Caries progression follow‐up 18 to 36 months ‐ Pairwise.
6.
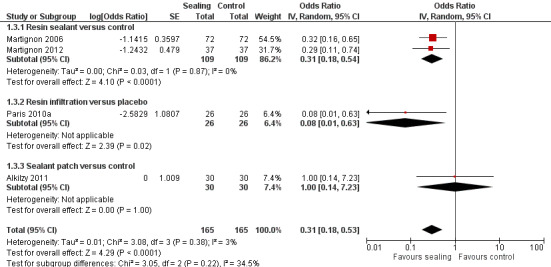
Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Pairwise
1.4. Analysis.
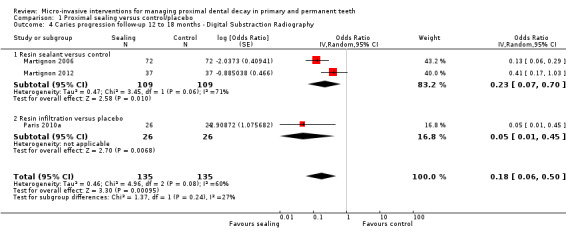
Comparison 1 Proximal sealing versus control/placebo, Outcome 4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography.
7.
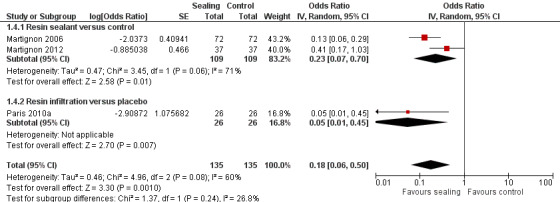
Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography.
We were unable to fully include two studies in the meta‐analysis (Gomez 2005; Martignon 2012). Gomez 2005 compared resin infiltration with fluoride varnish but inappropriately reported the data, and due to the substantial clustering of lesions within participants, we were unable to re‐analyse the data. Martignon 2012 included a third trial arm for resin infiltration. We were unable to include the results of this trial arm in the meta‐analysis due to problems arising from double counting of the control group. However, the results indicated that micro‐invasive treatment significantly reduced the odds of lesion progression compared with non‐invasive measures as assessed by both pairwise reading of radiographs (OR 0.20, 95% CI 0.08 to 0.53; 64 lesions) and digital subtraction radiography (OR 0.20, 95% CI 0.08 to 0.53; 64 lesions).
We calculated the NNTB as 7 (scoring), 4 (pairwise reading) and 3 (DSR), depending on the detection measure. NNTB was 5 when pooling all detection measures.
Change in decayed, missing and filled (DMF/dmf) figures at surface, tooth and whole mouth level
No studies measured this outcome.
Secondary outcomes
No studies reported re‐application of sealant patch or re‐infiltration on follow‐up appointments.
One study comparing proximal sealant patch versus non‐invasive control reported on material deficiency (Alkilzy 2011). Dentists different from operators assessed retention and adaptation of patches according to modified Ryge criteria. Clinically, 10% and 23% of proximal sealants had total and partial loss, respectively, after three years. Also, 53% of the proximal sealants placed had maintained perfect margins, and none was reported to have open margins, after three years.
None of the studies reported on participant or operator perception or costs for micro‐invasive treatment or non‐invasive control were reported.
No adverse events for micro‐invasive treatment or non‐invasive control were reported.
Subgroup analysis
We ran subgroup analyses based on the different interventions for the outcome 'caries progression'. We present results separately according to the method of radiographic measurement.
Micro‐invasive treatments, as assessed by independent scoring of radiographs, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.33, 95% CI 0.18 to 0.59; 256 lesions; two studies) and resin infiltration versus control or placebo (OR 0.19, 95% CI 0.08 to 0.46; 130 lesions; two studies). However, there was no significant difference of the odds of lesion progression between glass ionomer sealant and control (OR 0.13, 95% CI 0.01 to 2.52; 82 lesions; one study). There was no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 1.20, degrees of freedom (df) = 2; P = 0.55; Figure 5).
Micro‐invasive treatments, as assessed by pairwise visual scoring of radiographs, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.31, 95% CI 0.18 to 0.54; 218 lesions; two studies) and resin infiltration versus placebo (OR 0.08, 95% CI 0.01 to 0.63; 52 lesions; one study). However, there was no significant difference of the odds of lesion progression in sealant patch versus control (OR 1.00, 95% CI 0.14 to 7.23; 60 lesions; one study). There was also no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 3.05, df = 2; P = 0.22; Figure 6).
Micro‐invasive treatments, as assessed by digital subtraction radiography, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.23, 95% CI 0.07 to 0.70; 218 lesions; two studies) and resin infiltration versus placebo (OR 0.05, 95% CI 0.01 to 0.45; 52 lesions; one study). There was no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 1.37, df = 1; P = 0.24; Figure 7).
Treated participants varied according to their age, caries risk and the dentition evaluated (primary or permanent teeth treated).
We had planned to perform subgroup analyses as outlined in our Methods section (Subgroup analysis and investigation of heterogeneity); however, as there were a limited number of included studies and a lack of the information in the included trials, we did not perform these subgroup analyses.
Sensitivity analysis
We had planned to compare the effect estimate from studies judged as having an overall low risk of bias to the effect estimate from studies at any level of overall risk of bias to assess the robustness of our review results. However, seven of the eight included studies were at overall high risk of bias, so a sensitivity analysis was not possible.
Discussion
Summary of main results
Overall, this review found that micro‐invasive treatments of proximal caries lesions significantly reduced the risks of lesion progression compared with non‐invasive professional or home care. No studies compared micro‐invasive with invasive treatment. Only four studies indicated measuring adverse events after micro‐invasive treatments. No adverse events were reported after micro‐invasive treatments, which suggests they are not detrimental for gingival or subjective health; however, the limited duration of follow‐up (one to three years) in most studies limits the chance of detecting possible adverse events, especially given the relatively small number of included participants and lesions. Overall, we graded the evidence for micro‐invasive versus non‐invasive interventions as being of moderate quality, which reflects the risks of bias, the potential impact of sponsorship bias, and the limited follow‐up and number of events, while also taking into account the large size of the effect and precision of the estimate.
Our synthesis tended to find resin infiltration slightly more efficacious than the application of resin sealant. Resin sealant patches did not appear to be more efficacious than control interventions, but only one study reported on this therapy (Alkilzy 2011). Glass ionomer sealant seemed to have an efficacy similar to that of infiltration, but only one study reported on that method (Trairatvorakul 2011). Given that only one study directly compared resin sealant versus resin infiltration, it remains unclear as to which technique is more advantageous (Martignon 2012). Network meta‐analysis might be an option to further explore this issue. However, given that control groups differed regarding the non‐invasive treatment used (e.g. professional application of fluoride varnish, flossing advice, standard oral home care), one cannot necessarily assume transitivity, which might limit such analyses.
The study participants who received micro‐invasive treatments differed in terms of age groups, settings and caries risk. Moreover, the treated lesions differed in their depth, which might affect the risk of lesion progression after both test and control treatment. Included studies were not amenable to subgroup or meta‐regression analyses that could explore if such factors confound the efficacy of micro‐invasive treatment, as they were limited in number and reported insufficient data on confounders. Further studies should explore these issues to identify the clinical conditions under which proximal micro‐invasive treatments should be performed or avoided.
Overall completeness and applicability of evidence
As discussed, reporting on confounders was limited in the included studies, so our review could not identify a differential efficacy of micro‐invasive treatments in groups with different caries risk or for lesions of different depths. Four studies reported including only active lesions, and other studies did not indicate the state of the included lesions. Active caries lesions were detected by either gingival inflammations of adjacent papilla or plaque stagnation. Experienced dental examiners performed activity assessment. It therefore remains unclear if less effective activity assessment and the resulting treatment of non‐active lesions would affect the efficacy of micro‐invasive treatments. A lack of power in the performed subgroup comparison, stemming from the low number of studies, might cause the detected indifference of treatment effects in different dentitions, and future studies might be useful to evaluate the influence of participants' age or dentition.
Similarly, the included studies did not allow conclusions regarding which sealant material (resin or GIC sealants) or micro‐invasive technique (sealant or infiltration) is most suitable for treating proximal caries. Given the different retention of sealant materials, combined with potentially different antibacterial or remineralising effects of GIC and resin sealants (Mickenautsch 2013), further well‐designed trials might be needed to elucidate this issue. Similarly, the effects of varying losses of enamel due to the use of different acids when conditioning tooth surfaces for sealant versus infiltration, as well as the potential different retention losses when using these two techniques, merit further investigation.
The discussed duration and sample size of most studies limit their external validity, as both patients and dentists would be interested in long‐term efficacy of lesion arrest. Similarly, the efficacy of micro‐invasive treatments was compared only with non‐invasive treatment, whilst the standard for treating proximal lesions (even those confined to enamel) in many dental practices might be invasive therapy. Therefore, pragmatic practice‐based trials should compare the long‐term effects of performing different interventions for proximal carious lesions. Moreover, such trials should consider different outcomes. For example, instead of using lesion progression as an outcome parameter with limited direct impact on the patient, studies should additionally analyse parameters that might be relevant to patients (e.g. pain, preference, costs), dentists (e.g. applicability, technical feasibility, time required for treatment) and other decision‐makers (e.g. costs, equity effects, adherence‐dependency). This might eventually also shed light on which technique (sealant, patch, infiltration) is most suitable in a real‐life setting. Furthermore, such trials should take place not only in realistic primary care settings, but on patients with different risk profiles, as discussed. To decrease the risk of industry bias, public non‐industrial funding and independent data analysis might be advisable.
If assessing radiographic lesion progression, trials should aim at standardising the outcome measure used (i.e. scoring, pairwise, DSR) to facilitate combining trials for quantitative synthesis. To decide between outcome measures, investigators should explore and compare the relevance of each method's sensitivity, specificity and reliability. A further aspect to consider when choosing an outcome measure in such clinical trials might be its applicability in a non‐trial primary care setting. In this sense, future trials should also aim at reflecting the diagnostic challenges associated with leaving and sealing radiographically detectable lesions (Schwendicke 2014b).
Quality of the evidence
We graded evidence to account for study design, risk of bias, consistency, directness of comparisons, precision, presence of publication bias and magnitude of effect estimate. All studies were randomised controlled trials, but we downgraded their quality due to the high risk of bias associated with insufficient blinding of participants, personnel or both. Moreover, the low number of events, (i.e. the limited quantity of data) led to additional downgrading for imprecision of the effect estimate. It is important to highlight that several trials were prone to bias by industry sponsoring, but we did not downgrade them further for this given that we already rated the overall risk of bias to be high. Rather, we upgraded the evidence, as effect estimates all showed a large effect (OR < 0.5). We graded the overall quality of the evidence as moderate for the comparison of interest: proximal sealing compared with no treatment or placebo. This was consistent over all three different methods of caries progression measurement (visual scoring, pairwise and digital subtraction radiography), as well as for the most sensitive caries progression outcome measurement.
Although we judged the overall risk of bias for the included studies as high, the risk of bias was due to performance bias (seven studies), reporting bias (two studies) and other bias (two studies). Several trials did not report allocation concealment, and presumably none of the trials blinded operators. Indeed, this is very difficult as interventions are clearly distinguishable, with operators possibly being able to identify test and control intervention even if they perform mock‐up sealing or infiltration. Only two trials aimed to blind participants. We found examiner blinding during radiographic assessment to be sufficient in most trials. Blinding of examiners when assessing clinical outcomes might be feasible for infiltration, but it remains unclear if sealants and patches are reliably detectable via clinical (visual‐tactile) means, which would generally impede blinding of clinical outcomes.
Another point of note stemmed from bias caused by industry sponsorship and professional interest. All trials studying resin infiltration received some or all of their funding from the patent‐holding company for this technique, and the inventors of the technique themselves performed one trial. For trials using resin sealant or patches, we assumed (or at least did not exclude) industry bias due to insufficient information in all but two cases. As discussed, there is a need for pragmatic, practice‐based, blinded, independently assessed and reported trials with external, non‐industry funding to overcome this limitation.
Lastly, the small numbers of trials and the short follow‐up period limit the quality of evidence and its external validity. For rare adverse events or relevant late endpoints in cariology trials (e.g. the need to restore or to endodontically intervene, or tooth loss), it is necessary to run longer trials with a larger sample size. Moreover, to evaluate if the existing evidence allows firm conclusions, additional analyses might be applicable (Wetterslev 2008).
Potential biases in the review process
We conducted this review according to guidelines established by Cochrane. However, we cannot exclude certain biases within the review process. Inclusion of only randomised trials certainly narrows the available evidence, especially with regard to outcomes other than efficacy (e.g. applicability, technical feasibility, patients' preferences, costs). However, it increases the overall quality of studies and their internal validity, as lesions have been randomly allocated to different treatment arms. The assessment of risk of industry bias did not follow any guidelines, since none have been published. However, we felt that including this information in the 'Characteristics of included studies' tables was required to reflect the potential influences of industry sponsorship, especially when considering that other means (e.g. standard 'Risk of bias' assessment, funnel plot analyses) do not seem to be able to detect these risks (Lundh 2012).
Agreements and disagreements with other studies or reviews
This review included all available randomised trials regarding micro‐invasive treatment of proximal caries lesions. Our results agree with non‐ or pre‐clinical studies, which found both sealing and infiltration efficacious for arrest of carious lesions in vitro, ex vivo or in situ (Paris 2010b; Meyer‐Lueckel 2012). Moreover, our results confirm that sealing can arrest caries lesions, as reported for occlusal or pitted surfaces (Griffin 2008; Oong 2008).
The lack of data regarding the retention of sealants is worrisome, since other reviews found occlusal sealants—with presumably higher mechanical retention, but also higher masticatory forces and wear—to be prone to loss. However, the same research did not find loss of sealants to necessarily lead to re‐activation of the formerly sealed lesion (Mickenautsch 2013). Given that loss of sealant is only possible for resin sealants or patches, not infiltration, further research should investigate this issue.
Authors' conclusions
Implications for practice.
The available evidence allows us to be moderately confident that micro‐invasive treatment of proximal caries lesions (i.e. caries sealing or infiltration) is effective for arresting non‐cavitated enamel and dentine lesions and has a significant benefit over non‐invasive interventions. The generalisability of the trials was limited by their relatively short duration and the lack of data on other patient‐important outcomes, and current research is insufficient to draw conclusions about the relative benefits of different micro‐invasive techniques and the influence of different patient and clinical characteristics on their effectiveness. Overall, however, dentists can consider micro‐invasive treatments a viable option for treating non‐cavitated proximal lesions, taking into account clinical indication and the feasibility of different techniques.
Implications for research.
Based on the described limitations in the validity and applicability of current evidence, future trials should aim at reducing risk of bias (i.e. increasing internal validity) by aiming to effectively blind participants and outcome assessors. It may not be possible to carry out a placebo‐controlled trial. Pragmatic, practice‐based studies, with independent non‐industrial funding and independent outcome data assessment and reporting, might be suitable to further address the risks of industry and profession bias. Future trials should use a standardised set of outcome measures, defined a priori according to their relevance in clinical decision‐making. Thus, trials should not only measure efficacy (i.e. lesion arrest), but also applicability, time‐ and cost‐related outcomes, and outcomes subjectively reported by participants or dentists. Ideally, studies should have sufficient sample sizes and follow‐up to allow detection of late or adverse events, within primary care settings and in real‐life populations to ensure external validity. Such trials should be suited to explore the impact of tooth‐, participant‐ and dentist‐level confounders on the efficacy of proximal sealing.
Acknowledgements
Review authors would like to thank the Cochrane Oral Health Group and referees Professors Kim Ekstrand, Margherita Fontana, Afshid Anvarkhah and Dr Nicola Innes for their comments, support and assistance in conducting this review. We would also like to thank Professor Helen Worthington for her expertise regarding the data synthesis and Dr Wael Sabbah for his contribution in developing the protocol for this review.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. "Pit and Fissure Sealants"/ 2. (fissure$ adj6 seal$).mp. 3. ((approximal adj6 seal$) or (proximal adj6 seal$)).mp. 4. (dental adj3 sealant$).mp. 5. ((resin$ adj4 sealant$) or (resin$ adj4 infiltrat$)).mp. 6. (compomer$ adj4 sealant$).mp. 7. (composite$ adj4 sealant$).mp. 8. "polyurethane tape$".mp. 9. exp Glass Ionomer Cements/ 10. exp Resins, Synthetic/ 11. ("glass ionomer$" or glassionomer$).mp. 12. 9 or 10 or 11 13. sealant$.mp. 14. 12 and 13 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 14
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. The Cochrane Oral Health Group Trials Registry search strategy
#1 ((fissure and sealant):ti,ab) AND (INREGISTER) #2 (((approximal adj6 seal*) or (proximal and seal*)):ti,ab) AND (INREGISTER) #3 ((dental and sealant*):ti,ab) AND (INREGISTER) #4 (((resin* and sealant*) or (resin* and infiltrat*)):ti,ab) AND (INREGISTER) #5 ((compomer* and sealant*):ti,ab) AND (INREGISTER) #6 ((composite* and sealant*):ti,ab) AND (INREGISTER) #7 ("polyurethane tape*":ti,ab) AND (INREGISTER) #8 ((("glass ionomer*" or glassionomer*) and sealant*):ti,ab) AND (INREGISTER) #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) AND (INREGISTER)
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh ^"Pit and Fissure Sealants"] #2 (fissure* near/6 seal*) #3 ((approximal near/6 seal*) or (proximal near/6 seal*)) #4 (dental near/3 sealant*) #5 ((resin* near/4 sealant*) or (resin* near/4 infiltrat*)) #6 (compomer* near/4 sealant*) #7 (composite* near/4 sealant*) #8 "polyurethane tape*" #9 [mh "Glass Ionomer Cements"] #10 [mh "Synthetic resins"] #11 ("glass ionomer*" or glassionomer*) #12 #9 or #10 or #11 #13 sealant* #14 #12 and #13 #15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #14
Appendix 4. EMBASE (OVID) search strategy
1. "Fissure Sealant"/ 2. (fissure$ adj6 seal$).mp. 3. ((approximal adj6 seal$) or (proximal adj6 seal$)).mp. 4. (dental adj3 sealant$).mp. 5. ((resin$ adj4 sealant$) or (resin$ adj4 infiltrat$)).mp. 6. (compomer$ adj4 sealant$).mp. 7. (composite$ adj4 sealant$).mp. 8. "polyurethane tape$".mp. 9. "Glass Ionomer"/ 10. Resin/ 11. ("glass ionomer$" or glassionomer$).mp. 12. 9 or 10 or 11 13. sealant$.mp. 14. 12 and 13 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 14
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. LILACS via Bireme Virtual Health Library search strategy
("Mh Pit and Fissure sealants" or sealant$ or selladore$ or selante$) [Words] and (approxima$ or proxima$)
Appendix 6. Web of Science Conference Proceedings search strategy
# 9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 # 8 TS=(("glass ionomer*" or glassionomer*) and sealant*) # 7 TS="polyurethane tape*" # 6 TS=(composite* and sealant*) # 5 TS=(compomer* and sealant*) # 4 TS=((resin* and sealant*) or (resin* and infiltrat*)) # 3 TS=(dental and sealant*) # 2 TS=((approximal and seal*) or (proximal and seal*)) # 1 TS=(fissure* and seal*)
Appendix 7. Zetoc Conference Proceedings search strategy
approximal AND seal* proximal AND seal*
Appendix 8. Proquest Dissertations and Theses search strategy
all(dental OR tooth OR teeth) AND all((approximate OR proximal)) AND all(seal*)
Appendix 9. Open Grey search strategy
proximal AND seal proximal AND sealant proximal AND sealing approximal AND seal approximal AND sealant approximal AND sealing
Appendix 10. Trials Registry Search Strategies
The following search strategy was used for the metaRegister of Controlled Trials, the US National Institutes of Health Trials Register (ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform:
dental AND proximal AND seal dental AND proximal AND sealant dental AND proximal AND sealing dental AND approximal AND seal dental AND approximal AND sealant dental AND approximal AND sealing
Data and analyses
Comparison 1. Proximal sealing versus control/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring | 7 | 602 | Odds Ratio (Random, 95% CI) | 0.24 [0.14, 0.41] |
| 1.1 Resin sealant versus control | 3 | 330 | Odds Ratio (Random, 95% CI) | 0.26 [0.13, 0.53] |
| 1.2 Resin infiltration versus control/placebo | 2 | 130 | Odds Ratio (Random, 95% CI) | 0.15 [0.06, 0.39] |
| 1.3 Glass ionomer sealant versus control | 1 | 82 | Odds Ratio (Random, 95% CI) | 0.13 [0.01, 2.51] |
| 1.4 Sealant patch versus control | 1 | 60 | Odds Ratio (Random, 95% CI) | 1.0 [0.14, 7.22] |
| 2 Caries progression follow‐up 12 to 30 months ‐ Scoring | 5 | 468 | Odds Ratio (Random, 95% CI) | 0.27 [0.17, 0.44] |
| 2.1 Resin sealant versus control | 2 | 256 | Odds Ratio (Random, 95% CI) | 0.33 [0.18, 0.59] |
| 2.2 Resin infiltration versus control/placebo | 2 | 130 | Odds Ratio (Random, 95% CI) | 0.19 [0.08, 0.46] |
| 2.3 Glass ionomer sealant versus control | 1 | 82 | Odds Ratio (Random, 95% CI) | 0.13 [0.01, 2.52] |
| 3 Caries progression follow‐up 18 to 36 months ‐ Pairwise | 4 | 330 | Odds Ratio (Random, 95% CI) | 0.31 [0.18, 0.53] |
| 3.1 Resin sealant versus control | 2 | 218 | Odds Ratio (Random, 95% CI) | 0.31 [0.18, 0.54] |
| 3.2 Resin infiltration versus placebo | 1 | 52 | Odds Ratio (Random, 95% CI) | 0.08 [0.01, 0.63] |
| 3.3 Sealant patch versus control | 1 | 60 | Odds Ratio (Random, 95% CI) | 1.0 [0.14, 7.23] |
| 4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography | 3 | 270 | Odds Ratio (Random, 95% CI) | 0.18 [0.06, 0.50] |
| 4.1 Resin sealant versus control | 2 | 218 | Odds Ratio (Random, 95% CI) | 0.23 [0.07, 0.70] |
| 4.2 Resin infiltration versus placebo | 1 | 52 | Odds Ratio (Random, 95% CI) | 0.05 [0.01, 0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alkilzy 2011.
| Methods | Split‐mouth randomised controlled trial Funded partially by manufacturer of sealant patch (Ivoclar, Schaan, Liechtenstein) Clinical follow‐up after 6 and 12 months and radiographic evaluation after 2 and 3 years Drop‐out 30% and 40% after 2 and 3 years, respectively Setting: not specified |
|
| Participants | 50 participants, mean age 21 years (SD 6) with high caries risk (baseline mean DMFT 8.67) Inclusion criteria: two proximal carious lesions, confirmed with radiograph, extending into enamel or outer dentine in teeth with vital pulps Exclusion criteria: general disease, allergies, pregnancy, presence of cavitation on tested or control proximal surfaces |
|
| Interventions | Two treatment arms: Group 1: bonding (Heliobond, Ivoclar) + adhesive polyurethane patch (Ivoclar) + occlusal sealing (and oral health instructions including flossing and use of fluoridated toothpaste) Group 2: oral health instructions including flossing and use of fluoridated toothpaste In group 1, an orthodontic separating rubber ring was placed between teeth in first visit, and after 3‐5 days teeth were cleaned using paste and floss, then sealed (isolation of adjacent tooth by metal matrix band, etching using 37% phosphoric acid for 60 s, application of bonding and patch, light‐curing for 20 s from buccal and oral aspects, respectively, occlusal sealing, recontouring and finishing of proximal seal with finishing discs and strips) |
|
| Outcomes | Radiographic lesion progression as assessed by two blinded dentists according to scoring system (0 = no visible radiolucency; 1 = radiolucency in the enamel; 2 = radiolucency in the outer half of the dentine; 3 = radiolucency in the inner half of the dentine; 4 = restoration) Retention and adaptation of patch according to modified Ryge criteria assessed by dentists different from operators Authors stated that no adverse effect on general or dental health could be recorded. None of the other secondary outcomes were measured. |
|
| Notes | Intraexaminer reproducibility of radiographic assessments of the two examiners was 92% and 82%, and the agreements of each examiner with the gold standard were 92% and 80%. Interexaminer concordance was 90%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by toss of coin" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided; no indication for blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiners were different from the dentist who applied the sealants. Two dentists blindly and randomly assessed the radiographs separately and then compared their readings." Comment: low risk of bias assumed, as radiographic assessment was blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data rate 40% (> 25%) after 3 years; however, split‐mouth design reduces attrition bias |
| Selective reporting (reporting bias) | Low risk | Outcomes reported matched pre‐specified assessed outcomes. |
| Other bias | Low risk | No indication for other bias |
Ekstrand 2010.
| Methods | Split‐mouth randomised controlled trial Partially funded by manufacturer of resin infiltration kit (DMG, Hamburg, Germany) Clinical and radiographic follow‐up for one year Drop‐out 14%, further 6% had no radiographic follow‐up Setting: public dental health service in Greenland |
|
| Participants | 48 children (52% male, 48% female), mean age 7 years (range 5‐8) with high caries risk (mean dmft was 8.1) Inclusion criteria: children who had radiographs taken in January 2008 showing presence of at least 2 proximal lesions in enamel or outer dentine on deciduous molars. Lesions on the mesial surface on primary first molars were not included as the contact area to the canines was found too narrow. Exclusion criteria: presence of caries‐related diseases. |
|
| Interventions | Two treatment arms: Group 1: resin infiltration using Icon pre‐product (DMG) + application of 2.26% sodium fluoride varnish Group 2: 2.26% sodium fluoride varnish For group 1, proximal surfaces were cleaned by floss; rubber dam applied; the adjacent tooth protected by a plastic or metal strip; 15% HCl acid placed on the lesion for 120 s; the surface rinsed, dried and dehydrated twice by treating with 95% ethanol and air‐drying; the infiltrant resin applied to the lesion for 120 s, polymerised according to the manufacturer’s instructions; resin applied again for 30 s and polymerised, and eventually fluoride varnish applied to the lesion. Group 2 received fluoride varnish only. Varnish application was repeated in both groups after 1 year. |
|
| Outcomes | Clinical lesion progression according to ICDAS Radiographic progression according to scoring (1 = radiolucency in the outer half of the enamel; 2 = radiolucency in the inner half of the enamel, 3 = radiolucency in the outer half of the dentine; 4 = radiolucency in the inner half of the dentine; 5 = restoration). Authors stated that no side effects of the infiltration or the Duraphat treatments were observed, either from the participants’ files or from direct questioning of the children and the parents. None of the other secondary outcomes were reported. |
|
| Notes | If more than two lesions were present, lesions were selected using a random number table. ICDAS scoring had reliability of 94%. Clinical calibration was done on 6 children with 48 primary molar teeth. In 4.1% of the recordings the 2 examiners disagreed about the score. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number tables used" |
| Allocation concealment (selection bias) | Low risk | Quote: "prepared lists for allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided; no indication for blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "For all readings the examiner was blinded as to whether the examined radiograph was baseline or final and as to whether the lesion was a test or a control lesion." Comment: low risk of detection bias for radiographic assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[m]issing data rate 19% after 1.5 years" Comment: missing data rate < 25% |
| Selective reporting (reporting bias) | High risk | 6‐month clinical evaluation was not reported, and radiographic evaluation was incomplete as well |
| Other bias | High risk | Unbalanced allocation of lesions according to ICDAS scores |
Gomez 2005.
| Methods | Clustered split‐mouth randomised controlled trial Part of a bigger study involving a total of 50 participants Radiographic follow‐up after 2 years Drop‐out 0% after 2 years Setting: navy dental clinic in Chile |
|
| Participants | 7 participants (72% male, 28% female), mean age 15 years (range 10‐20), with unclear caries risk; 71 lesions randomly allocated to intervention or control. Inclusion criteria: radiographic detection of one or more surface with incipient proximal carious lesions on molars and premolars Unclear who performed initial examination |
|
| Interventions | Two treatment arms: Group 1: resin sealant (Concise, 3M Espe, Neuss, Germany) Group 2: application of fluoride varnish twice yearly In group 1, access was first gained to confirm the presence of enamel lesions and their clinical status by temporary tooth separation. After 1–2 days, the surface was cleaned, dried and a cotton roll or rubber dam applied. The carious tooth area and the 1 mm enamel surrounding the lesion were etched for 20 s with a 35% phosphoric acid gel, washed and dried, whilst the adjacent surface was protected with a nylon adhesive strip. When the proximal surface was completely dried, a light‐cured, low‐viscosity pit and fissure was applied using a brush. After 30 s, the sealant was light cured. During sealing, dental floss was placed in the interdental sulcus space to avoid sealant flow to the cervical zone. After sealing, excess sealant was removed with an explorer, and the margins polished with a fine polishing strip. |
|
| Outcomes | Radiographic progression according to scoring (0 = no visible radiolucency; 1 = radiolucency in the enamel; 2 = radiolucency in the outer half of the dentine; 3 = radiolucency in the inner half of the dentine; and 4 = restoration). Authors did not state if they measured adverse events and none were reported. |
|
| Notes | Intraexaminer reliability showed a kappa of 0.86 when the calculations included carious surfaces (scores 1–4). For surfaces with score 1, kappa was 0.84. Clustering of lesions and unequal distribution; unclear lesion depths |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random numbers were used to decide which surfaces should be treated with sealant or fluoride varnish." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Radiographs were analysed blindly in a random order by one observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data rate 0% after 2 years (< 25%) |
| Selective reporting (reporting bias) | Low risk | No indication for selective reporting |
| Other bias | High risk | Unclear how lesion depths distributed in groups; severe clustering of lesions |
Martignon 2006.
| Methods | Split‐mouth randomised controlled trial Partially funded by Colfuturo and the Universidad El Bosque Radiographic follow‐up after 18 months Drop‐out 12% after 18 months Setting: dental schools in Denmark and Colombia |
|
| Participants | 82 participants (age range 15‐39 years) attending dental faculties in Copenhagen (N = 43) or Bogota (N = 39) with mostly moderate (62% in Denmark and 61% in Colombia) or high (28% in Denmark and 33% in Colombia) caries risk Inclusion criteria: individuals with at least two initial proximal lesions in enamel up to outer third of dentine on the bitewing radiographs and corresponding bleeding after gentle probing in the gingiva next to the lesion |
|
| Interventions | Two treatment arms: Group 1: resin sealant (Gluma One Bond, Heraeus Kulzer, Hanau, Germany, or Concise, 3M Espe) + flossing advice Group 2: flossing advice If necessary, a proximal temporary space was created by means of an elastic orthodontic band placed in the proximal space concerning the selected test lesion for 2 days. At the second appointment, the surface was cleaned, cotton rolls used for partial isolation, a matrix band placed around the lesion tooth or the neighbouring tooth surfaces were covered with a Teflon tape, a wooden edge was placed in the interdental space, the sealing material was applied with the aid of applicator tips and dental floss, the sealants light cured, and the surface polished. |
|
| Outcomes | Radiographic progression as assessed by scoring (1 = enamel, 2 = around enamel‐dentine junction, 3 = outer third dentine, 4 = inner third, 5 = not assessable, 6 = restored) by a blinded independent examiner Radiographic progression as assessed by pairwise radiographic reading: A blinded independent examiner was asked to determine the progression status of the right positioned against the left positioned radiograph, with a further randomisation concerning the position of baseline or follow‐up images. Radiographic progression as assessed via digital subtraction radiography using Compare software (Dental Health Unit, University of Manchester, United Kingdom) by an external trained examiner 10 days after the visual assessment of conventional radiographs None of the secondary outcomes were reported. Authors did not state if they measured adverse events and none were reported. |
|
| Notes | The contact area was wide in most test (75%) and control (88%) lesions. Test and control lesions differed with respect to tooth types (more test lesions on premolars), depth of the lesions (more test lesions were scored 3), location in the jaw (more control lesions were located in the lower jaw) and proximal surface area (more control lesions were on surfaces with a wide contact area to the neighbouring tooth). Intraexaminer reproducibility for the visual independent readings were kappa of 0.84 and 96% agreement; for the paired readings, kappa was 0.44 and the agreement 68%; and for the subtraction readings kappa was 0.87 and agreement 92%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was used." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication for blinding of operator or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessed by an external examiner who was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data rate 12% (< 25%) |
| Selective reporting (reporting bias) | Low risk | No indication for reporting bias |
| Other bias | Low risk | No indication for other bias |
Martignon 2010.
| Methods | Split‐mouth randomised controlled trial No funding information Radiographic follow‐up after 2.5 years Drop‐out 20% and 39% after 1.5 and 2.5 years, respectively Setting: university dental clinic in Colombia |
|
| Participants | 91 preschoolers (51% male, 49% female), mean age 5 years (range 4‐6), with low (23%), moderate (25%) or high (52%) caries risk (mean dmft 1.8) and low socioeconomic status from Bogota, Colombia Inclusion criteria: children aged 4‐6 years with radiographically determined proximal caries on at least 2 distal surfaces on primary first molar teeth with lesion depths involving the enamel up to the outer third of the dentine and gingival bleeding Exclusion criteria: systemic disease, cavitated lesions/restorations involving the mesial surfaces of second primary molar teeth, refusal of radiographs Examiners: dental students |
|
| Interventions | Two treatment arms: Group 1: resin sealant (Single One Bond, 3M Espe) + flossing advice. Group 2: flossing advice In group 1, a proximal temporary elective space was created by means of an elastic orthodontic band placed for 2 days between the first and second primary molar, involving the selected test and control lesions. Surfaces were cleaned, cotton rolls used for partial isolation, the neighbouring tooth surfaces covered with a Teflon tape, and a wooden edge placed in the interdental cervical surface. Adhesive was applied following the manufacturer’s instructions, with the aid of ultrafine applicator tips and dental floss, followed by light‐curing and polishing. |
|
| Outcomes | Radiographic lesion progression according to scoring (0 = no radiolucency, 1 = radiolucency restricted to the outer half of the enamel, 2 = radiolucency involving the inner half of the enamel to the enamel dentine junction, 3 = radiolucency in the outer third of the dentine, and 4 = radiolucency in the inner two thirds of the dentine). Assessment by one blinded independent examiner None of the secondary outcomes were reported. Authors did not state if they measured adverse events, and none were reported. |
|
| Notes | There was no difference in distribution pattern related to lesion depth between test and control lesion. The main author selected two lesions; if more than two were available, a random number table was used for selection. Intraexaminer reproducibility for the radiographic scoring: kappa 0.76. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation sequence generated with a random number table" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided, presumably no blinding performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Lesions were scored on the radiographs independently by an external examiner who was not familiar with the study design." Comment: low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data rate 39% after 2.5 years (> 25%); however, split‐mouth design reduces attrition bias |
| Selective reporting (reporting bias) | Low risk | No indication for reporting bias |
| Other bias | Low risk | No indication for other bias |
Martignon 2012.
| Methods | Multi‐arm split‐mouth randomised controlled trial Partially funded by DMG Radiographic follow‐up after 1, 2, and 3 years Drop‐out 5% after 1 and 3 years Setting: dental school in Colombia |
|
| Participants | 39 participants (72% female, 28% male), mean age 21 years (range 16‐31), with moderate to high caries risk (mean DMFT 4.9) attending the Universidad El Bosque, Colombia Inclusion criteria: presence of three posterior proximal lesions (radiolucency in outer dentine or enamel dentine junction) as detected radiographically Exclusion criteria: leaving the city in the next three years; current orthodontic treatment |
|
| Interventions | Three treatment arms: Group 1: resin infiltration (Icon pre‐product, DMG) + flossing advice Group 2: resin sealant (Prime Bond NT, Dentsply, Konstanz, Germany) + flossing advice Group 3: placebo proximal sealing + flossing advice For group 1, teeth were initially separated using orthodontic elastic bands. At the second visit, rubber dam and plastic wedges were placed, a plastic strip used to isolate the adjacent tooth, the surface etched with 15% hydrochloric acid for 120 s, rinsed and dried, desiccated using 95% ethanol and air‐drying, resin infiltrated for 120 s, light cured, infiltrant re‐applied for 30 sec, and light cured once more. For group 2, pretreatment was similar, with sealing of the lesion performed using an adhesive. For group 3 (placebo), a micro‐brush was passed through the spaces between teeth for 30 sec, with the procedure repeated after 2 min. All individuals received routine instructions on flossing. |
|
| Outcomes | Pairwise subjective comparison by coding the lesions on the most recent film and reading this code against baseline (0 = no radiolucency, 1 = radiolucency restricted to the outer half of the enamel, 2 = radiolucency involving the inner half of the enamel to the enamel‐dentine junction, 3 = radiolucency in the outer third of the dentine, and 4 = radiolucency in the inner two thirds of the dentine) Digital subtraction radiography was performed using Image Tool (UTHSCSA, San Antonio, TX, USA). Authors stated that no adverse events (pain, vitality loss, staining) occurred. None of the other secondary outcomes were reported. |
|
| Notes | If a participant had more than 3 eligible lesions, investigators randomly selected 3. The intra‐rater reliability for the pairwise and the subtraction radiographic methods (kappa values) was 0.74 and 0.78, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Placebo involved moving a microbrush between the teeth; and operator was blind to the radiographic score of lesions." Comment: no blinding of operators; unclear if blinding sufficient to conceal allocation from participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded to the selected treatment groups. Radiographic assessment independently by an external examiner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data rate 5% after 3 years (< 25%) |
| Selective reporting (reporting bias) | High risk | DSR evaluation only after one year |
| Other bias | Low risk | No indication for other bias |
Paris 2010a.
| Methods | Clustered split‐mouth randomised controlled trial Partially funded by DMG Radiographic follow‐up after 18 months with additional follow‐up planned after 36 and 60 months Drop‐out 0% and 9% after 18 and 36 months, respectively Setting: university dental clinic in Germany |
|
| Participants | 22 participants (64% female, 36% male), mean age 25 years (range 18‐35), with low (32%), moderate (36%), and high (32%) caries risk; total of 29 lesion pairs, attending the Berlin University dental clinic Inclusion criteria: presence of 2 or more non‐cavitated proximal caries lesions with radiolucencies involving the inner half of enamel up to the outer third of dentine, aged 18‐35 yrs Exclusion criteria: pregnant, participating in another study, incapable of contracting, institutionalised Radiographs taken using standardised conventional bitewing radiographs with individualised holder. One investigator scored lesions using a light box. |
|
| Interventions | Two treatment arms: Group 1: resin infiltration (Icon pre‐product, DMG) + fluoride varnish application + oral hygiene and dietary advice Group 2: fluoride varnish application + oral hygiene and dietary advice For group 1, rubber dam was applied, teeth were separated by plastic wedges, polyurethane foil placed in the contact area with a plastic holder to protect the adjacent tooth, 15% HCl etching gel applied by syringe in the area below the contact point for 120 s, the gel washed off with air‐water‐spray for 30 s, the lesion desiccated by air‐blowing for 10 s, ethanol applied for 10 s, the lesion desiccated using air‐blowing again for 10 s. Eventually, an infiltrant was applied and after 5 min of penetration time, excess material removed by air‐blowing and flossing. The resin was light cured for 1 min from the buccal, occlusal, and oral aspects. The infiltration step was repeated with a penetration time of 1 min. For group 2, operators performed a sham‐infiltration, with water instead of HCl gel and infiltrant. The operators, however, were aware of this. |
|
| Outcomes | Radiographic progression as assessed by scoring (E1 = up to outer half of enamel, E2 = up to inner half of enamel, D1 = up to outer third of dentine, D2 = up to middle third of dentine, D3 = up to inner third of dentine) of lesions at baseline and follow‐up examination Radiographic progression as assessed by pairwise assessment of lesions for progression, regression, or stability Radiographic progression as assessed by DSR Subjective and clinical adverse events, such as loss of vitality, staining, or gingival alterations were measured and none were reported. None of the other secondary outcomes were reported. |
|
| Notes | Inter‐rater reliability (kappa) was moderate (0.59) for radiographic staging, substantial (0.67) for pairwise comparison, and almost perfect (0.81) for DSR. Intra‐rater reliability ranged from moderate to almost perfect (0.51‐0.89). Potential industry and profession bias (the trialists are appointed as inventors and patent‐holders of the technique) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "From each pair, 1 lesion was allocated to the test and 1 to the control group, respectively, by computer‐generated randomly permuted blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were blinded to lesion allocation throughout the whole study period as a placebo treatment was performed on the control lesions." Comment: Operators were not necessarily blinded, but it is unclear how this might affect outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examiner was blinded with regard to treatment group allocation of teeth." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data rate 9% (< 25%) after 3 years |
| Selective reporting (reporting bias) | Low risk | No indication for reporting bias |
| Other bias | Low risk | No indication for other bias |
Trairatvorakul 2011.
| Methods | Cluster split‐mouth randomised controlled trial Drop‐out 0% after 12 months Radiographic follow‐up after 6 and 12 months Funding by the Postgraduate Research Fund, Faculty of Dentistry, Chulalongkorn University (Thailand) Setting: university paediatric dentistry clinic in Thailand |
|
| Participants | 26 participants, mean age 13 years (range 7‐19). Inclusion criteria: at least one contralateral pair of permanent posterior teeth with proximal caries lesions shown by radiograph to extend into the outer half or inner half of enamel, and plaque stagnation, as detected by explorer. Exclusion criterion: no contact between teeth |
|
| Interventions | Two treatment arms: Group 1: glass ionomer sealant + fluoridation gel + toothpaste advice Group 2: fluoridation gel + toothpaste advice The test teeth were separated using an orthodontic ring. After two days, the lesion was air dried and the roughness assessed by a second, independent examiner. The surface was cleaned using floss and water, dentine conditioner (GC, Tokyo, Japan) was applied for 20 s and rinsed away, and the surface was dried. Glass ionomer sealant (Fuji VII, GC) was then applied on a metal matrix, which was moved up and down between the teeth. The sealant was light cured for 40 s according to manufacturer's instructions and a varnish (GC, Tokyo) applied using a sponge. Then, 1.23% acidulated phosphate fluoride gel was applied using a tray. This was repeated after 6 months. The control teeth only received the fluoride gel application after the initial treatment and after 6 months. |
|
| Outcomes | Radiographic progression as assessed by radiographic scoring (1 = outer half of the enamel, 2 = inner half of the enamel) Radiographic progression as assessed by measurement of lesion depths on a continuous scale None of the secondary outcomes were reported. Authors did not state if they measured adverse events, and none were reported. |
|
| Notes | Intraclass correlation coefficient of the examinations was 0.97 for the 2 ratings at a 95% confidence interval. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "drawing lots" |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was concealed." Comment: no further information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded examination. Glass ionomer sealant was radiographically not detectable." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data rate 0% after 12 months |
| Selective reporting (reporting bias) | Low risk | No indication for reporting bias |
| Other bias | Low risk | No indication for other bias |
dmft/DMFT: decayed, missing or filled teeth (primary/permanent);DSR: digital subtraction radiography;HCl: hydrochloric acid; ICDAS: International Caries Detection and Assessment System; s: second.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abesi 2012 | No intervention using sealant was included |
| Abuchaim 2010 | Not a randomised controlled trial |
| Agustsdottir 2010 | No intervention using sealant was included |
| Bille 1989 | No intervention using sealant was included |
| Bravo 1997 | No proximal sealing was applied |
| Downer 1995 | Not a clinical study |
| Edward 1997 | No intervention using sealant was included |
| Friedman 1976 | Not a randomised controlled trial |
| Ganss 1999 | No proximal sealing was applied |
| Griffin 2008 | Not a clinical study |
| Gustafsson 2000 | No intervention using sealant was included |
| Hintze 1997 | No intervention using sealant was included |
| Hopcraft 2005 | No intervention using sealant was included |
| Kielbassa 2009 | Not a clinical study |
| Kilpatrick 1996 | No proximal lesions were included |
| Li 2002 | No intervention using sealant was included |
| Lith 2002 | No intervention using sealant was included |
| Llena‐Puy 2005 | No intervention using sealant was included |
| Mejare 1990 | No intervention using sealant was included |
| Müller‐Bolla 2006 | Not a clinical study |
| Newmann 2009 | No intervention using sealant was included |
| Paris 2007 | Not a clinical study |
| Ricketts 2006 | Not a clinical study |
| Ridell 2008 | No intervention using sealant was included |
| Splieth 2010 | Not a clinical study |
| Stenlund 2003 | No intervention using sealant was included |
| Vanderas 2003 | No intervention using sealant was included |
| Vidnes‐Kopperud 2011 | No intervention using sealant was included |
Characteristics of ongoing studies [ordered by study ID]
Correia 2012.
| Trial name or title | Resin infiltration on the sealing of proximal early caries lesions: a randomised trial |
| Methods | Split‐mouth randomised controlled trial No funding statement Drop‐out 0% after 12 months |
| Participants | 9 participants (56% female, 44% male), aged 5‐40 years, with ≥ 2 lesions up to the enamel‐dentinal junction. Total of 26 lesions. Exclusion criteria: pregnancy, history of tumours of salivary glands, lesions without adjacent tooth, ongoing orthodontic treatment |
| Interventions | Two treatment arms: Group 1: resin infiltration (Icon, DMG) + fluoride varnish application + oral hygiene and dietary advice Group 2: fluoride varnish application + oral hygiene and dietary advice For group 1, resin infiltration was performed according to manufacturer's instructions. In addition, fluoride varnish, dietary advice and oral hygiene instruction were provided. Group 2 received non‐invasive treatments only. |
| Outcomes | Lesion progression according to pairwise reading and digital subtraction radiography |
| Starting date | NA |
| Contact information | Marisa Maltz, Odontologia Preventiva e Social. Porto Alegre, Universidade Federal do Rio Grande do Sul, marisa.maltz@gmail.com |
| Notes | At 12 months, 2/13 infiltrated and 1/13 non‐invasively treated lesions progressed according to pairwise reading. DSR found 3/13 and 0/13 lesions to progress, respectively. |
Peters 2013.
| Trial name or title | Radiographic progression of infiltrated caries lesions in vivo |
| Methods | Split‐mouth randomised controlled trial Funded by DMG, Hamburg Drop‐out 23% after 12 months |
| Participants | 12 participants (aged 18 to 24 years) with DMFT of 3 or more; having at least two early caries lesions in proximal posterior tooth surfaces; lesions needed to be visible on radiograph Exclusion criteria: current participation in another clinical study; medically compromised subjects; hyposalivation; pregnancy; allergic to methylmethacrylates or latex; symptomatic teeth |
| Interventions | Group 1: resin infiltration (Icon, DMG) plus oral hygiene, diet counselling, fluoride varnish Group 2: control (sham treatment) plus oral hygiene, diet counselling, fluoride varnish |
| Outcomes | Lesion progression according to pairwise reading and DSR. |
| Starting date | March 2013, estimated completion December 2016 |
| Contact information | Mathilde Peters, University of Michigan (mcpete@umich.edu) |
| Notes | ClinicalTrials.gov Identifier: NCT01584024 |
DMFT: decayed, missing or filled teeth (primary/permanent);DSR: digital subtraction radiography.
Differences between protocol and review
Title of the review ‐ changed from 'Proximal sealing for managing dental decay in primary and permanent teeth' to 'Micro‐invasive interventions for managing proximal dental decay in primary and permanent teeth'.
-
Description of interventions ‐ we renamed three groups of interventions from the protocol in the full review, from:
"Conventional restorative management" to "Conventional invasive treatment";
"Non‐operative management" to "Non‐invasive treatment";
"Proximal sealing ‐ a novel approach" to "Proximal sealing (micro‐invasive) treatments".
Electronic searches ‐ we intended to search Google Scholar (http://scholar.google.com/) in addition to the databases listed in Electronic searches; however, as this might have yielded a large number of results with only limited scientific relevance, we did not do so.
Measures of treatment effect ‐ for dichotomous outcomes we used the odds ratio for the measure of effect. This was necessary because of the inappropriate reporting of the included split‐mouth studies, and the method employed to re‐analyse the data for meta‐analysis.
Additional subgroup analysis ‐ post hoc we added dentition (deciduous or permanent) and caries risk (low to moderate, moderate, mostly high or high).
Contributions of authors
Mojtaba Dorri (MD): drafting of the protocol, designing a search strategy, screening search results, selecting studies, writing to authors of papers for additional information, assessing quality, extracting data, carrying out the analysis, interpreting the analysis, drafting the final review, updating the review. Falk Schwendicke (FS) ‐ selecting studies, writing to authors of papers for additional information, assessing quality, extracting data, carrying out the analysis, interpreting the analysis, drafting the final review, updating the review. Stephen Dunne (SD) ‐ drafting the protocol, drafting the final review, updating the review. Tanya Walsh (TW) ‐ extracting data, carrying out the analysis, interpreting the analysis, drafting the final review, updating the review.
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Group Global Alliance, Other.
Through our Global Alliance (http://ohg.cochrane.org/partnerships‐alliances), the Cochrane Oral Health Group has received support from: British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK.
Declarations of interest
Mojtaba Dorri: none known Stephen M Dunne: none known Falk Schwendicke: FS is lecturing dentists for DMG, Hamburg, and has been counselling DMG on future projects not related to techniques evaluated by the present review. Tanya Walsh: none known
New
References
References to studies included in this review
Alkilzy 2011 {published data only}
- Alkilzy M, Berndt C, Splieth CH. Sealing proximal surfaces with polyurethane tape: three‐year evaluation. Clinical Oral Investigations 2011;15(6):879‐84. [DOI] [PubMed] [Google Scholar]
Ekstrand 2010 {published data only}
- Ekstrand KR, Bakhshandeh A, Martignon S. Treatment of proximal superficial caries lesions on primary molar teeth with resin infiltration and fluoride varnish versus fluoride varnish only: efficacy after 1 year. Caries Research 2010;44(1):41‐6. [DOI] [PubMed] [Google Scholar]
Gomez 2005 {published data only}
- Gomez SS, Basili CP, Emilson CG. A 2‐year clinical evaluation of sealed noncavitated approximal posterior carious lesions in adolescents. Clinical Oral Investigations 2005;9(4):239‐43. [DOI] [PubMed] [Google Scholar]
Martignon 2006 {published data only}
- Martignon S, Ekstrand KR, Ellwood R. Efficacy of sealing proximal early active lesions: an 18‐month clinical study evaluated by conventional and subtraction radiography. Caries Research 2006;40(5):382‐8. [DOI] [PubMed] [Google Scholar]
Martignon 2010 {published data only}
- Martignon S, Tellez M, Santamaría RM, Gomez J, Ekstrand KR. Sealing distal proximal caries lesions in first primary molars: efficacy after 2.5 years. Caries Research 2010;44(6):562‐70. [DOI] [PubMed] [Google Scholar]
Martignon 2012 {published data only}
- Martignon S, Ekstrand KR, Gomex J, Lara JS, Cortes A. Infiltrating/sealing proximal caries lesions: a 3‐year randomized clinical trial. Journal of Dental Research 2012;91(3):288‐92. [DOI] [PubMed] [Google Scholar]
Paris 2010a {published data only}
- Meyer‐Lueckel H, Bitter K, Paris S. Randomized controlled clinical trial on proximal caries infiltration: three‐year follow‐up. Caries Research 2012;46(6):544‐8. [DOI] [PubMed] [Google Scholar]
- Paris S, Hopfenmuller W, Meyer‐Lueckel H. Resin infiltration of caries lesions: an efficacy randomized trial. Journal of Dental Research 2010;89(8):823‐6. [DOI] [PubMed] [Google Scholar]
Trairatvorakul 2011 {published data only}
- Trairatvorakul C, Itsaraviriyakul S, Wiboonchan W. Effect of glass‐ionomer cement on the progression of proximal caries. Journal of Dental Research 2011;90(1):99‐103. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abesi 2012 {published data only}
- Abesi F, Mirshekar A, Moudi E, Seyedmajidi M, Haghanifar S, Haghighat N, et al. Diagnostic accuracy of digital and conventional radiography in the detection of non‐cavitated approximal dental caries. Iranian Journal of Radiology 2012;9(1):17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abuchaim 2010 {published data only}
- Abuchaim C, Rotta M, Grande RH, Loguercio AD, Reis A. Effectiveness of sealing active proximal caries lesions with an adhesive system: 1‐year clinical evaluation. Brazilian Oral Research 2010;24(3):361‐7. [DOI] [PubMed] [Google Scholar]
Agustsdottir 2010 {published data only}
- Agustsdottir H, Gudmundsdottir H, Eggertsson H, Jonsson SH, Gudlaugsson JO, Saemundsson SR, et al. Caries prevalence of permanent teeth: a national survey of children in Iceland using ICDAS. Community Dentistry and Oral Epidemiology 2010;38(4):299‐309. [DOI] [PubMed] [Google Scholar]
Bille 1989 {published data only}
- Bille J, Carstens K. Approximal caries progression in 13‐ to 15‐year‐old Danish children. Acta Odontologica Scandinavica 1989;47(6):347‐54. [DOI] [PubMed] [Google Scholar]
Bravo 1997 {published data only}
- Bravo M, Baca P, Llodra JC, Osorio E. A 24‐month study comparing sealant and fluoride varnish in caries reduction on different permanent first molar surfaces. Journal of Public Health Dentistry 1997;57(3):184‐6. [DOI] [PubMed] [Google Scholar]
Downer 1995 {published data only}
- Downer MC. The 1993 national survey of children's dental health. British Dental Journal 1995;178(11):407‐12. [DOI] [PubMed] [Google Scholar]
Edward 1997 {published data only}
- Edward S. Dental caries on adjacent approximal tooth surfaces in relation to order of eruption. Acta Odontologica Scandinavica 1997;55(1):27‐30. [DOI] [PubMed] [Google Scholar]
Friedman 1976 {published data only}
- Friedman M, Merwe EH, Bischoff JI, Fatti LP, Retief DH. Effect of a sealant, used in conjunction with topical fluoride application, on fluoride concentrations in human tooth enamel. Archives of Oral Biology 1976;21(4):237‐41. [DOI] [PubMed] [Google Scholar]
Ganss 1999 {published data only}
- Ganss C, Klimek J, Gleim A. One year clinical evaluation of the retention and quality of two fluoride releasing sealants. Clinical Oral Investigations 1999;3(4):188‐93. [DOI] [PubMed] [Google Scholar]
Griffin 2008 {published data only}
- Griffin SO, Oong E, Kohn W, Vidakovic B, Gooch BF, CDC Dental Sealant Systematic Review Work Group, Bader J, et al. The effectiveness of sealants in managing caries lesions. Journal of Dental Research 2008;87(2):169‐74. [DOI] [PubMed] [Google Scholar]
Gustafsson 2000 {published data only}
- Gustafsson A, Svenson B, Edblad E, Jansson L. Progression rate of approximal carious lesions in Swedish teenagers and the correlation between caries experience and radiographic behavior. An analysis of the survival rate of approximal caries lesions. Acta Odontologica Scandinavica 2000;58(5):195‐200. [DOI] [PubMed] [Google Scholar]
Hintze 1997 {published data only}
- Hintze H. Caries behaviour in Danish teenagers: a longitudinal radiographic study. International Journal of Paediatric Dentistry 1997;7(4):227‐34. [DOI] [PubMed] [Google Scholar]
Hopcraft 2005 {published data only}
- Hopcraft MS, Morgan MV. Comparison of radiographic and clinical diagnosis of approximal and occlusal dental caries in a young adult population. Community Dentistry and Oral Epidemiology 2005;33(3):212‐8. [DOI] [PubMed] [Google Scholar]
Kielbassa 2009 {published data only}
- Kielbassa AM, Muller J, Gernhardt CR. Closing the gap between oral hygiene and minimally invasive dentistry: a review on the resin infiltration technique of incipient (proximal) enamel lesions. Quintessence International 2009;40(8):663‐81. [PubMed] [Google Scholar]
Kilpatrick 1996 {published data only}
- Kilpatrick NM, Murray JJ, McCabe JF. A clinical comparison of a light cured glass ionomer sealant restoration with a composite sealant restoration. Journal of Dentistry 1996;24(6):399‐405. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li G, Yoshiura K, Welander U, Shi X‐Q, McDavid WD. Detection of approximal caries in digital radiographs before and after correction for attenuation and visual response. An in vitro study. Dentomaxillofacial Radiology 2002;31(2):113‐6. [DOI] [PubMed] [Google Scholar]
Lith 2002 {published data only}
- Lith A, Lindstrand C, Grondahl HG. Caries development in a young population managed by a restrictive attitude to radiography and operative intervention: I. A study at the patient level. Dentomaxillofacial Radiology 2002;31(4):224‐31. [DOI] [PubMed] [Google Scholar]
Llena‐Puy 2005 {published data only}
- Llena‐Puy C, Forner L. A clinical and radiographic comparison of caries diagnosed in approximal surfaces of posterior teeth in a low‐risk population of 14‐year‐old children. Oral Health and Preventive Dentistry 2005;3(1):47‐52. [PubMed] [Google Scholar]
Mejare 1990 {published data only}
- Mejare I, Mjör IA. Glass ionomer and resin‐based fissure sealants: a clinical study. Scandinavian Journal of Dental Research 1990;98(4):345‐50. [DOI] [PubMed] [Google Scholar]
Müller‐Bolla 2006 {published data only}
- Muller‐Bolla M, Lupi‐Pégurier L, Tardieu C, Velly AM, Antomarchi C. Retention of resin‐based pit and fissure sealants: a systematic review. Community Dentistry and Oral Epidemiology 2006;34(5):321‐36. [DOI] [PubMed] [Google Scholar]
Newmann 2009 {published data only}
- Newman B, Seow WK, Kazoullis S, Ford D, Holcombe T. Clinical detection of caries in the primary dentition with and without bitewing radiography. Australian Dental Journal 2009;54(1):23‐30. [DOI] [PubMed] [Google Scholar]
Paris 2007 {published data only}
- Paris S, Meyer‐Lueckel H, Kielbassa AM. Resin infiltration of natural caries lesions. Journal of Dental Research 2007;86(7):662‐6. [DOI] [PubMed] [Google Scholar]
Ricketts 2006 {published data only}
- Ricketts DN, Kidd EA, Innes N, Clarkson J. Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003808.pub2] [DOI] [PubMed] [Google Scholar]
Ridell 2008 {published data only}
- Ridell K, Olsson H, Mejare I. Unrestored dentin caries and deep dentin restorations in Swedish adolescents. Caries Research 2008;42(3):164‐70. [DOI] [PubMed] [Google Scholar]
Splieth 2010 {published data only}
- Splieth CH, Ekstrand KR, Alkilzy M, Clarkson J, Meyer‐Lueckel H, Martignon S, et al. Sealants in dentistry: outcomes of the ORCA Saturday Afternoon Symposium 2007. Caries Research 2010;44(1):3‐13. [DOI] [PubMed] [Google Scholar]
Stenlund 2003 {published data only}
- Stenlund H, Mejàre I, Källestål C. Caries incidence rates in Swedish adolescents and young adults with particular reference to adjacent approximal tooth surfaces: a methodological study. Community Dentistry and Oral Epidemiology 2003;31(5):361‐7. [DOI] [PubMed] [Google Scholar]
Vanderas 2003 {published data only}
- Vanderas AP, Manetas C, Koulatzidou M, Papagiannoulis L. Progression of proximal caries in the mixed dentition: a 4‐year prospective study. Pediatric Dentistry 2003;25(3):229‐34. [PubMed] [Google Scholar]
Vidnes‐Kopperud 2011 {published data only}
- Vidnes‐Kopperud S, Tveit AB, Espelid I. Changes in the treatment concept for approximal caries from 1983 to 2009 in Norway. Caries Research 2011;45(2):113‐20. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Correia 2012 {unpublished data only}
- Correa, RT. Resin infiltration on the sealing of proximal early caries lesions: A randomised trial [Selamento de lesões de cárie proximal com infiltrante resinoso: estudo clínico randomizado (Mestrado em programa de pós graduação em odontologia)]. Departamento de Odontologia Preventiva e Social. Porto Alegre: Universidade Federal do Rio Grande do Sul 2012.
Peters 2013 {unpublished data only}
- Peters MC, Tuzzio F, Nedley M, Davis W, Bayne SC. Resin infiltration effects in a caries‐active environment. Journal of Dental Research 2013; 92(A):377. [Google Scholar]
Additional references
ADA 2012
- American Dental Association. Dental Radiographic Examinations: Recommendations for Patient Selection and Limiting Radiation Exposure. US Department of Health and Human Services, 2012. [Google Scholar]
Ahovuo‐Saloranta 2013
- Ahovuo‐Saloranta A, Forss H, Walsh T, Hiiri A, Nordblad A, Mäkelä M, et al. Sealants for preventing dental decay in the permanent teeth. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD001830.pub4] [DOI] [PubMed] [Google Scholar]
Antoft 1999
- Antoft P, Rambusch E, Antoft B, Christensen HW. Caries experience, dental health behaviour and social status: three comparative surveys among Danish military recruits in 1972, 1982 and 1993. Community Dental Health 1999;16(2):80‐4. [PubMed] [Google Scholar]
Berggren 1984
- Berggren U, Meynert G. Dental fear and avoidance: causes, symptoms, and consequences. Journal of the American Dental Association 1984;109(2):247‐51. [DOI] [PubMed] [Google Scholar]
Cueto 1967
- Cueto EI, Buonocore MG. Sealing of pits and fissures with an adhesive resin: its use in caries prevention. Journal of the American Dental Association 1967;75(1):121‐8. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekstrand 2000
- Ekstrand KR, Kuzmina IN, Kuzmina E, Christiansen ME. Two and a half‐year outcome of caries‐preventive programs offered to groups of children in the Solntsevsky district of Moscow. Caries Research 2000;34(1):8‐19. [DOI] [PubMed] [Google Scholar]
Ekstrand 2006
- Ekstrand KR. Knowledge about caries: Is it possible for the Danish Public Dental Health Service for Children to achieve even better results? [Faglig viden om caries: kan den kommunale tandpleje gøre det endnu bedre?]. Tandlaegebladet 2006;110:788‐99. [Google Scholar]
Ekstrand 2007
- Ekstrand KR, Martignon S, Christiansen ME. Frequency and distribution patterns of sealants among 15‐year‐olds in Denmark in 2003. Community Dental Health 2007;24(1):26‐30. [PubMed] [Google Scholar]
Ellwood 2003
- Ellwood R, Fejerskov O. Clinical use of fluoride. In: Fejerskov O, Kidd E editor(s). Dental Caries: The Disease and Its Clinical Management. Copenhagen: Blackwell Munksgaard, 2003:189‐222. [Google Scholar]
EndNote [Computer program]
- Thomas Reuters. http://endnote.com/. New York: Thomas Reuters.
FDI 2009
- FDI (World Dental Federation) Science Committee. FDI Policy Statement: Adverse reactions to resin‐based direct filling materials. World Dental Federation, 2009. Available from www.fdiworldental.org.
Forsling 1999
- Forsling JO, Halling A, Lundin SA, Paulander J, Svenson B, Unell L, et al. Proximal caries prevalence in 19‐year‐olds living in Sweden. A radiographic study in four counties. Swedish Dental Journal 1999;23(2‐3):59‐70. [PubMed] [Google Scholar]
GRADE 2004
- The GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. www.gradepro.org. Version 3.2. McMaster University, 2014.
Handleman 1973
- Handleman SL, Buonocore MG, Schoute PC. Progress report on the effect of a fissure sealant on bacteria in dental caries. Journal of American Dental Association 1973;87(6):1189‐91. [DOI] [PubMed] [Google Scholar]
Hannigan 2000
- Hannigan A, O'Mullane DM, Barry D, Schäfer F, Roberts AJ. A caries susceptibility classification of tooth surfaces by survival time. Caries Research 2000;34(2):103‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
Kidd 2005
- Kidd EAM. Essentials of Dental Caries: The Disease and Its Management. 3rd Edition. London: Wright, 2005. [Google Scholar]
Leal 2012
- Leal SC, Bronkhorst EM, Fan M, Frencken JE. Untreated cavitated dentine lesions: impact on children’s quality of life. Caries Research 2012;46(2):102‐6. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Marcenes 2013
- Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990‐2010: a systematic analysis. Journal of Dental Research 2013;92(7):592‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2009
- Marinho VC. Cochrane reviews of randomized trials of fluoride therapies for preventing dental caries. European Archives of Paediatric Dentistry 2009;10(3):183‐91. [DOI] [PubMed] [Google Scholar]
Mejàre 2002
- Mejàre I. Management of the advanced carious lesion in primary teeth. In: Hugoson A, Falk M, Hohansson S editor(s). Consensus Conference on Caries in the Primary Dentition and Its Clinical Management. Stockholm: Förlagshuset Gothia, 2002:57‐68. [Google Scholar]
Meyer‐Lueckel 2007
- Meyer‐Lueckel H, Paris S, Kielbassa AM. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries Research 2007;41(3):221‐30. [DOI] [PubMed] [Google Scholar]
Meyer‐Lueckel 2012
- Meyer‐Lueckel H, Bitter K, Paris S. Randomized controlled clinical trial on proximal caries infiltration: three‐year follow‐up. Caries Research 2012;46(6):544‐8. [DOI] [PubMed] [Google Scholar]
Mickenautsch 2013
- Mickenautsch, S, Yengopal V. Validity of sealant retention as surrogate for caries prevention – a systematic review. PloS One 2013;8(10):e77103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2001
- Nielsen LA. Caries progression in the deciduous teeth from 3 to 7 years of age [Cariesprogression i det primære tandsæt fra 3‐ til 7‐års‐alderen]. Tandlaegebladet 2001;105:704‐11. [Google Scholar]
Oong 2008
- Oong EM, Griffin SO, Kohn WG, Gooch BF, Caufield PW. The effect of dental sealants on bacteria levels in caries lesions: a review of the evidence. Journal of the American Dental Association 2008;139(3):271‐8. [DOI] [PubMed] [Google Scholar]
Paris 2010b
- Paris S, Meyer‐Lueckel H. Inhibition of caries progression by resin infiltration in situ. Caries Research 2010;44(1):47‐54. [DOI] [PubMed] [Google Scholar]
Petersen 2005
- Petersen PE, Bourgeois D, Ogawa H, Estupinan‐Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization 2005;83(9):661‐9. [PMC free article] [PubMed] [Google Scholar]
Poklepovic 2013
- Poklepovic T, Worthington HV, Johnson TM, Sambunjak D, Imai P, Clarkson JE, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD009857.pub2] [DOI] [PubMed] [Google Scholar]
Qvist 2008
- Qvist V. Longevity of restorations: the 'death spiral'. In: Fejerskov O, Kidd E editor(s). Dental Caries: The Disease and Its Clinical Management. 2nd Edition. Oxford: Blackwell Munksgaard, 2008:444‐55. [Google Scholar]
Rehman 2009
- Rehman K, Khan H, Shah SA. Frequency of class II type carious lesions in first permanent molars and their association with pulp. Pakistan Oral & Dental Journal 2009;29(1):119‐22. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ripa 1993
- Ripa LW. Sealants revised: an update of the effectiveness of pit‐and‐fissure sealants. Caries Research 1993;27(Suppl 1):77‐82. [DOI] [PubMed] [Google Scholar]
Schwendicke 2014a
- Schwendicke F, Meyer‐Lueckel H, Stolpe M, Dörfer CE, Paris S. Costs and effectiveness of treatment alternatives for proximal caries lesions. PloS One 2014;9(1):e86992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwendicke 2014b
- Schwendicke F, Meyer‐Lueckel H, Schulz M, Dörfer CE, Paris S. Radiopaque tagging masks caries lesions following incomplete excavation in vitro. Journal Dental Research 2014;93(6):565‐70. [DOI] [PubMed] [Google Scholar]
Schwendicke 2015
- Schwendicke F, Dörfer C, Schlattmann P, Foster Page L, Thomson M, Paris S. Socioeconomic inequality and caries: A systematic review and meta‐analysis. Journal of Dental Research 2015;94(1):10‐8. [DOI] [PubMed] [Google Scholar]
Sheiham 2010
- Sheiham A, Sabbah W. Using universal patterns of caries for planning and evaluating dental care. Caries Research 2010;44(2):141‐50. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dorri 2013
- Dorri M, Dunne SM, Sabbah W, Kiani B, Schwendicke F. Proximal sealing for managing dental decay in primary and permanent teeth. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010431] [DOI] [PMC free article] [PubMed] [Google Scholar]


