Abstract
Background
People with asthma may experience exacerbations or "attacks" during which their symptoms worsen and additional treatment is required. Written action plans may advocate doubling the dose of inhaled steroids in the early stages of an asthma exacerbation to reduce the severity of the attack and to prevent the need for oral steroids or hospital admission.
Objectives
To compare the clinical effectiveness and safety of increased versus stable doses of inhaled corticosteroids (ICS) as part of a patient‐initiated action plan for home management of exacerbations in children and adults with persistent asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register, which is derived from searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) to March 2016. We handsearched respiratory journals and meeting abstracts.
Selection criteria
We included randomised controlled trials (RCTs) that compared increased versus stable doses of ICS for home management of asthma exacerbations. We included studies of children or adults with persistent asthma who were receiving daily maintenance ICS.
Data collection and analysis
Two review authors independently selected trials, assessed quality and extracted data. We contacted authors of RCTs for additional information.
Main results
This review update added three new studies including 419 participants to the review. In total, we identified eight RCTs, most of which were at low risk of bias, involving 1669 participants with mild to moderate asthma. We included three paediatric (n = 422) and five adult (n = 1247) studies; six were parallel‐group trials and two had a cross‐over design. All but one study followed participants for six months to one year. Allowed maintenance doses of ICS varied in adult and paediatric studies, as did use of concomitant medications and doses of ICS initiated during exacerbations. Investigators gave participants a study inhaler containing additional ICS or placebo to be started as part of an action plan for treatment of exacerbations.
The odds of treatment failure, defined as the need for oral corticosteroids, were not significantly reduced among those randomised to increased ICS compared with those taking their usual stable maintenance dose (odds ratio (OR) 0.89, 95% confidence interval (CI) 0.68 to 1.18; participants = 1520; studies = 7). When we analysed only people who actually took their study inhaler for an exacerbation, we found much variation between study results but the evidence did not show a significant benefit of increasing ICS dose (OR 0.84, 95% CI 0.54 to 1.30; participants = 766; studies = 7). The odds of having an unscheduled physician visit (OR 0.96, 95% CI 0.66 to 1.41; participants = 931; studies = 3) or acute visit (Peto OR 0.98, 95% CI 0.24 to 3.98; participants = 450; studies = 3) were not significantly reduced by an increased versus stable dose of ICS, and evidence was insufficient to permit assessment of impact on the duration of exacerbation; our ability to draw conclusions from these outcomes was limited by the number of studies reporting these events and by the number of events included in the analyses. The odds of serious events (OR 1.69, 95% CI 0.77 to 3.71; participants = 394; studies = 2) and non‐serious events, such as oral irritation, headaches and changes in appetite (OR 2.15, 95% CI 0.68 to 6.73; participants = 142; studies = 2), were neither increased nor decreased significantly by increased versus stable doses of ICS during an exacerbation. Too few studies are available to allow firm conclusions on the basis of subgroup analyses conducted to investigate the impact of age, time to treatment initiation, doses used, smoking history and the fold increase of ICS on the magnitude of effect; yet, effect size appears similar in children and adults.
Authors' conclusions
Current evidence does not support increasing the dose of ICS as part of a self initiated action plan to treat exacerbations in adults and children with mild to moderate asthma. Increased ICS dose is not associated with a statistically significant reduction in the odds of requiring rescue oral corticosteroids for the exacerbation, or of having adverse events, compared with a stable ICS dose. Wide confidence intervals for several outcomes mean we cannot rule out possible benefits of this approach.
Plain language summary
Increasing the dose of inhaled steroids or continuing the usual dose to treat asthma attacks in adults and children
Background Previous asthma treatment guidelines recommended doubling the dose of inhaled corticosteroids (ICS) at the first sign of an asthma attack as part of an action plan. We looked for all studies that have assessed whether such an increase is better than and is as safe as carrying on with the usual ICS dose.
Study characteristics This review update added three new studies including 419 participants to the review. We performed the most recent searches in March 2016. In total, we found eight studies involving 1669 people with mild or moderate asthma. Three were conducted in children, and five in adults. These studies provided participants with an inhaler that contained extra doses of ICS (to increase their usual ICS dose) or a placebo that could be used if their symptoms worsened. Participants were then followed for six months to one year to see whether people taking more inhaled corticosteroids during attacks did better than those who took a placebo.
Key results People taking an increased dose of ICS during an attack did not do better than those who took a placebo, regardless of whether we looked at all study participants or only those who actually took the inhalers during an attack. Results showed a lot of variation in studies that focused only on people who took the inhalers, with some studies showing benefit of increasing ICS dose and others showing no benefit. It is unlikely that increasing ICS dose reduces the need for a course of oral steroids to treat the attack, prevents the need for an emergency visit with doctors or at the hospital or reduces the time it takes to recover. We cannot be sure of these last results because few studies reported them. Use of either strategy was not associated with significantly more or less serious and non‐serious side effects, but again we cannot say for sure because we did not find enough studies.
Quality of the evidence We have rated results of this review as having moderate or low quality, depending on the outcome. This means that some of the findings were very uncertain, mainly because the studies included very few people who could say definitively whether increasing the dose was better or worse than, or no different from, keeping the dose stable.
Summary of findings
for the main comparison.
| Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children | ||||||
| Patient or population: adults and children with chronic asthma Setting: outpatient Intervention: increased ICS dose during exacerbations Comparison: stable ICS dose during exacerbations | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with stable ICS | Risk with increased ICS | |||||
|
Treatment failure ‐ need for systemic corticosteroids (ITT) 45 weeks |
179 per 1000 | 163 per 1000 (129 to 205) | OR 0.89 (0.68 to 1.18) | 1520 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa, i, j | Favours increasing ICS but not statistically significant Non‐significant subgroup differences for age, ICS dose (baseline or increased) and ICS fold increase |
|
Treatment failure ‐ need for systemic corticosteroids (of those starting inhaler) 45 weeks |
337 per 1000 | 299 per 1000 (215 to 398) | OR 0.84, (0.54 to 1.30) | 766 (7 RCTs) | ⊕⊕⊝⊝ LOWb,c, i, j | No clear benefit of one strategy over the other. Too imprecise to infer no difference Analysed using random‐effects models because of heterogeneity |
|
Unscheduled physician visits 44 weeks |
147 per 1000 | 142 per 1000 (102 to 195) | OR 0.96 (0.66 to 1.41) | 931 (3 RCTs) | ⊕⊕⊝⊝ LOWd,e, i, j | For both of these outcomes, no clear benefit of one strategy over the other was noted, but the estimate was too imprecise to confirm no differences between them |
|
Unscheduled acute care, ED visit or hospital admission 47 weeks |
18 per 1000 | 18 per 1000 (4 to 67) | OR 0.98 (0.24 to 3.98) | 450 (3 RCTs) | ⊕⊕⊝⊝ LOWf, i, j | |
|
Duration of exacerbation ‐ time to symptom recovery and lung function recovery 52 weeks |
Mean time to symptom recovery was 6.1 days Time to lung function recovery was 7 days |
Time to symptom recovery was 0.7 days longer in the intervention group (1.06 lower to 2.46 higher) Time to lung function recovery was 0.2 days shorter (1.88 lower to 1.48 higher) |
‐ | 207 (1 RCT) | ⊕⊕⊕⊝ MODERATEb,d, i, j | |
|
Serious adverse events 48 weeks |
56 per 1000 | 91 per 1000 (44 to 181) | OR 1.69 (0.77 to 3.71) | 394 (2 RCTs) | ⊕⊕⊕⊝ MODERATEg,h, i, j | Favours stable dose but confidence intervals do not rule out greater safety with increased dose |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; ED: emergency department; ICS: inhaled corticosteroids; ITT: intention‐to‐treat population; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aThe effect was in favour of increasing ICS, but the confidence interval included no effect and the possibility of appreciable benefit of keeping the dose stable (‐1 imprecision)
bUpper and lower confidence intervals include important benefit of both treatments (‐1 imprecision)
cI2 = 55%, P value = 0.04; clear variation was noted between direction and magnitude of study results by visual inspection of the forest plot (‐1 inconsistency)
dSeveral studies did not appear in the analysis, but contact with study authors meant this was unlikely because of selective reporting (no downgrade for publication bias)
eThree studies observed 136 events leading to very wide confidence intervals, which made the result very difficult to interpret (‐2 imprecision)
fOnly eight events in the analysis, leading to a large amount of imprecision in the estimate. Two studies did not observe any events so did not contribute to the effect estimate (‐2 imprecision)
gConfidence intervals included a significant increase in adverse events on increased dose ICS and did not exclude the possibility of no difference against stable ICS. Very few events were included in either of the adverse event analyses (‐1 imprecision)
hOnly two studies explicitly reported serious adverse events separately from the other exacerbation and resource use outcomes (no downgrade for publication bias)
iWe noted some uncertainties regarding allocation concealment and missing data imputation, but only in some studies, and this was not deemed significant enough to have had a serious impact on the results (no downgrade for risk of bias across outcomes)
jAll studies were well matched to the question posed by the review. We resolved uncertainties in the definitions of outcomes through contact with study authors, so we were confident the data were relevant to each outcome of interest (no downgrade for indirectness across outcomes)
Background
Description of the condition
Asthma is a common chronic breathing condition that is estimated to affect as many as 334 million people (Global Asthma Report 2014). Asthma exacerbations involve short‐term worsening of symptoms, which vary from mild to life‐threatening, and are associated with significant morbidity, mortality and healthcare expenditure (Sears 2000). Up to a quarter of patients presenting to the emergency room with asthma exacerbations ultimately require hospitalisation (Pollack 2002), resulting in a three‐fold increase in costs compared with costs of management in a primary care setting (Lane 2006). Asthma exacerbations are very frightening for patients and can have a negative impact on health‐related quality of life (Lloyd 2007). Achieving early control of asthma exacerbations is thus paramount in avoiding hospitalisation and its associated costs, as well as in improving health‐related quality of life.
Description of the intervention
The cornerstone of asthma exacerbations is airway inflammation, often triggered by respiratory virus infection, allergen exposure and/or respiratory irritants (Johnston 2006). This airway inflammation sets up a vicious cycle of bronchial hyper‐responsiveness and mucus hypersecretion, leading to decreased expiratory flow. Although short‐acting beta agonists (SABA) often lead to rapid reversal of airflow obstruction, they do not help the underlying inflammatory changes, so administration of systemic corticosteroids is recommended in patients who have moderate to severe exacerbations and in those who fail to respond promptly to SABA treatment (GINA 2015; NHLBI 2007).
Systemic corticosteroids have potent anti‐inflammatory properties and are the most effective drugs for suppressing the underlying inflammatory response in asthma exacerbations. In comparison with placebo, they result in a faster rate of symptomatic improvement (Fanta 1983), a significant reduction in the number of relapses and decreased beta‐2 agonist use (Rowe 2001) following an acute care hospital visit for acute asthma. However, the well‐recognised adverse effects of repeated short courses of systemic corticosteroids, including hyperglycaemia, psychiatric disturbance, adrenal suppression and occurrence of severe varicella in children, provide the rationale for an alternative management strategy such as use of inhaled corticosteroids (McEvoy 2000). Furthermore, the strategy of utilising short courses of oral prednisone for asthma exacerbations, whether parent‐initiated (Oommen 2003) or administered in the acute care setting (Panickar 2009), has not proved effective in pre‐school‐aged children.
How the intervention might work
Inhaled corticosteroids (ICS) have an established role in the management of chronic asthma. They are considered the most potent and effective long‐term controller medications for asthma (GINA 2015; NHLBI 2007). Clinical benefits of ICS in the management of acute asthma are less well established because systemic corticosteroids are often relied upon as first‐line therapy. Inhaled corticosteroids offer a theoretical advantage in the acute setting in that they are delivered directly to the airways, thus maximising lung deposition and resulting in higher local potency and potentially faster onset of effect (Rodrigo 2006). A previous study demonstrated lower bronchial eosinophilic inflammation within the first 24 hours in participants randomised to high‐dose inhaled fluticasone compared with oral prednisone (Belda 2007). In a Cochrane review comparing use of high‐dose ICS versus systemic corticosteroids for asthma exacerbations following discharge from the emergency department (ED), review authors found no significant differences in relapse rates, beta‐2 agonist use or adverse events (Edmonds 2003). On the basis of these studies, high‐dose ICS might offer a promising alternative to oral corticosteroids.
Why it is important to do this review
With increasing recognition that early treatment of asthma exacerbations is the best strategy for management, written action plans to guide patient self management of exacerbations are recommended (GINA 2015; NHLBI 2007). Most patients with persistent asthma are regular users of ICS; therefore many action plans based on consensus opinion initially advocated doubling the dose of ICS as one of the first steps in treating or preventing progression of exacerbations of asthma (Boulet 1999; BTS 1997). In the light of lack of evidence to support this recommendation, recent guidelines have been more cautious (BTS/SIGN 2014; GINA 2015; NHLBI 2007). We believe that publication of several clinical trials offers an important opportunity to clarify further the role of this strategy in home management of asthma exacerbations. We prepared this update of the Cochrane review originally published in 2010 (Quon 2010) to bring the evidence on this topic up‐to‐date.
Objectives
To compare the clinical effectiveness and safety of increased versus stable doses of inhaled corticosteroids as part of a patient‐initiated action plan for home management of exacerbations in children and adults with persistent asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) reported as full text, those published as abstract only and unpublished data. We included only double‐blinded placebo‐controlled trials to avoid treatment bias with respect to activation of the asthma action plan and determination of subjective treatment outcomes such as treatment failure necessitating rescue systemic corticosteroids.
Types of participants
We included adults and children with asthma exacerbation as defined by guideline criteria such as those outlined in GINA 2015, or by a set of criteria pre‐defined in the included studies. The diagnosis of asthma was confirmed by a physician before the time of enrolment. Participants had to have taken a stable dose of ICS for a minimum of two weeks before enrolment. We excluded studies involving participants treated with continuous daily oral corticosteroids.
Types of interventions
We included studies that compared continuing a stable daily maintenance dose versus increasing the daily dose of ICS as part of an asthma exacerbation action plan. Active or placebo step‐up therapy was to be increased at home at or shortly after the onset of symptoms signalling the beginning of an exacerbation. Other co‐interventions such as long‐acting beta agonists, leukotriene modifiers and other asthma medications were permitted, provided that the dose remained unchanged throughout the study. The only exception to this was the allowance of increased short‐acting beta agonist use during exacerbations. Specifically, inhaled short‐acting beta agonists and short courses of systemic corticosteroids were allowed as rescue medications.
Types of outcome measures
Primary outcomes
Treatment failure ‐ need for rescue systemic corticosteroids* in all randomised participants (i.e. intention‐to‐treat (ITT) analysis).
Secondary outcomes
Treatment failure ‐ need for rescue systemic corticosteroids* in participants using the study inhaler.
Unscheduled physician visits.
Unscheduled acute care or emergency department visits or need for hospital admission.
Serious** and non‐serious adverse events.
Duration of exacerbation as defined by:
recovery of lung function;
recovery of symptoms; or
beta‐2 agonist use back to baseline.
*oral, intramuscular (IM) or intravenous (IV).
**Serious adverse events were defined as fatality, need for hospitalisation, prolongation of hospitalisation, disability and study withdrawal due to the adverse event. We noted in the analysis whether definitions used within these studies differed.
Search methods for identification of studies
Electronic searches
We have detailed search methods used in the previous version of this review in Appendix 1. The previously published version included searches up to October 2009. The search period for this update extended from October 2009 to March 2016.
For this update, we identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (please see Appendix 2 for further details). We searched all records in the CAGR using the search strategy presented in Appendix 3.
We also conducted a search of ClinicalTrials.gov (http://www.clinicaltrials.gov) and the World Health Organization (WHO) trials portal (http://www.who.int/ictrp/en/) for ongoing and unpublished trials. We searched all databases from their inception to the present, with no restriction on language of publication. We conducted the latest search in March 2016.
Searching other resources
We updated additional searches of trial registries and grey literature databases to identify articles that might not have appeared in the main electronic database searches. We searched pharmaceutical company clinical trial registries (AstraZeneca and GlaxoSmithKline) and grey literature databases (Open System for Information on Grey Literature in Europe (OpenSIGLE) and the New York Academy of Medicine). Historical searches for previous versions of this review included http://www.controlled‐trials.com and http://www.clinicalstudyresults.org, which we covered in the new WHO trials portal and ClinicalTrials.gov searches. We also checked reference lists of retrieved articles and reviews and asked field experts if they knew of any relevant ongoing or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (MQ and KK for 2015 update, previously BSQ and NS) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications, and two review authors (MQ and KK for 2015 update, previously BSQ and NS) independently screened the full‐text studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, by consulting a third person (BSQ). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram for Cochrane systematic review updates (Stovold 2014) and Characteristics of excluded studies tables.
Data extraction and management
We used a data collection form for study characteristics and outcome data, which had been piloted on at least one study in the review. Two review authors (MQ and KK for 2015 update, previously BSQ and NS) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest for trial authors.
Two review authors (MQ and KK for 2015 update, previously BSQ and NS) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third person (BSQ). One review author (KK) transferred data into the Review Manager (RevMan 2014) file. We double‐checked that data were entered correctly by comparing data presented in the systematic review with those provided in the study reports.
Assessment of risk of bias in included studies
Two review authors (MQ and KK for 2015 update, previously BSQ and NS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (BSQ). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for an unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review. We brought some sections of the methods up‐to‐date for the most recent version of the review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
We pooled the results of parallel and cross‐over studies when we were satisfied that data could be appropriately analysed to account for intercorrelation in cross‐over studies. We analysed data using participants with one or more events as the unit of analysis. For dichotomous outcomes, when we did not know whether the number of events applied to the entire population or only to those taking the study inhaler, we used the total number randomised per group as the denominator. We performed sensitivity analyses by using the number of participants using their study inhaler at least once as the denominator to test this assumption.
If no events were reported in control or treatment groups, we used the Peto odds ratio to avoid use of the continuity correction.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and when missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We examined homogeneity of effect sizes between pooled studies with the I2 statistic (Higgins 2003). In the absence of heterogeneity (I2 < 25%), we used the fixed‐effect model (Greenland 1985); otherwise we applied summary estimates and reported the DerSimonian and Laird random‐effects model (DerSimonian 1986). Unless otherwise specified, we reported the fixed‐effect model, as it is better equipped than the random‐effects method to detect small effect sizes (Fields 2001).
Assessment of reporting biases
We were not able to pool more than 10 trials; therefore we did not create a funnel plot to explore possible small study and publication biases.
Data synthesis
For dichotomous outcomes, we pooled parallel studies using Mantel‐Haenszel (M‐H) ORs unless few events were reported, thus requiring Peto odds ratios. We obtained ORs from cross‐over studies by comparing the number of participants who needed oral corticosteroids with increased dose (but not with placebo) versus those who needed oral corticosteroids while taking placebo (but not while taking increased ICS dose). We presented ORs with 95% confidence intervals (CIs). For continuous outcomes, such as length of exacerbation, we calculated pooled statistics as MDs and reported them with 95% CIs.
Summary of findings table
We created a 'Summary of findings' table using the following outcomes: rescue systemic corticosteroids (ITT analysis), treatment failure as judged by the need for rescue systemic corticosteroids in participants requiring the study inhaler (modified ITT analysis), unscheduled physician visits, unscheduled acute care or ED visits or hospital admissions, duration of exacerbations and serious adverse events. We used the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) per GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analyses of the primary outcome to identify potential effect modifiers, irrespective of the presence or absence of heterogeneity.
Age group (children < 15 years old vs adults ≥ 15 years old).
Smoking status (smokers vs ex‐smokers or never‐smokers).
Time elapsed before initiation of treatment (< 48 hours vs ≥ 48 hours).
Maintenance ICS dose (ex‐valve) before increase (low vs moderate vs high*).
Achieved daily dose of ICS (ex‐valve) during exacerbation (low vs moderate vs high*).
Fold increase in baseline ICS dose during exacerbation (double dose vs quadruple dose).
In the previous version, subgroup analyses were repeated post hoc for the secondary outcome of treatment failures only within those who started the study inhaler. In this version, we conducted subgroup analyses only on the primary outcome alone.
*ICS dose was classified according to Global Initiative for Asthma Guidelines (GINA 2015) as follows.
High dose ‐ adults: > 1000 mcg/d of chlorofluorocarbon‐propelled beclomethasone dipropionate (CFC‐BDP) dose or equivalent. Children: > 400 mcg/d equivalent CFC‐BDP dose.
Moderate dose ‐ adults: > 500 mcg to 1000 mcg/d CFC‐BDP equivalent. Children: > 200 mcg to 400 mcg/d CFC‐BDP equivalent.
Low dose ‐ adults: 200 mcg to 500 mcg/d CFC‐BDP equivalent. Children: 100 mcg to 200 mcg/d CFC‐BDP equivalent.
Fluticasone propionate was converted to CFC beclomethasone dipropionate (CFC‐BDP) equivalents by multiplying the ex‐valve dose by two because its reported potency in asthmatic patients is two‐fold relative to CFC‐BDP (Barnes 1993). Budesonide was converted to CFC‐BDP equivalents by multiplying the ex‐valve dose by 1.25, as reported in the Canadian Asthma Guidelines (Lemiere 2003).
Sensitivity analysis
We planned the following sensitivity analyses for the primary outcome.
Study design (removing cross‐over studies).
Methodological quality (removing studies at high risk of selection bias).
Source of study funding (removing studies funded by pharmaceutical companies).
Results
Description of studies
Results of the search
The main electronic database update search for October 2009 to March 2016 returned 436 records. We searched 699 additional records found in other resources (365 from AstraZeneca, 164 from GlaxoSmithKline, 143 from clinicaltrials.gov, 24 from the WHO trials portal, one from the New York Academy of Medicine, one from OpenSIGLE and one from study reference lists). We screened all 1135 records and excluded 1119 by looking at the titles and abstracts alone. We reviewed the full texts for 16 records and excluded 13 that did not meet the inclusion criteria. We included three new studies that met the criteria for this review. For the previous version of this review, which covered up to October 2009, review authors screened the titles and abstracts of 882 records, assessed full texts for 39 that were potentially relevant and included five trials that met the inclusion criteria. Together with the three new studies, a total of eight studies met the inclusion criteria for this review. Results of the update search are shown in Figure 1, along with the number of studies brought forward from the previous version (Stovold 2014).
1.
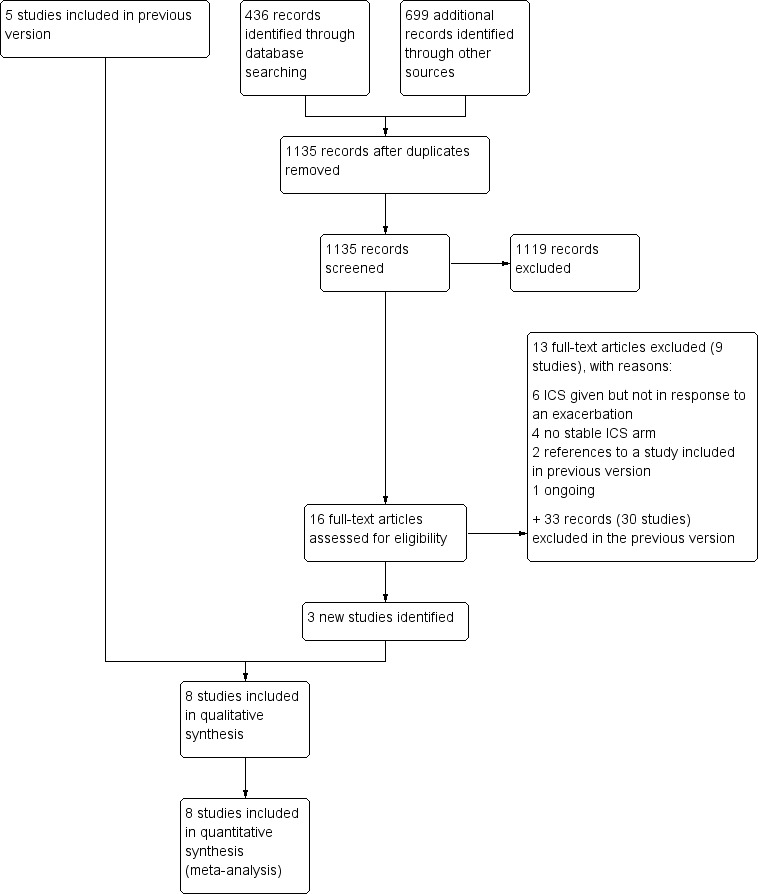
Study flow diagram.
Included studies
This review update added three new studies including 419 participants to the review. In total, eight studies met the eligibility criteria: five adult (Fitzgerald 2004; Foresi 2000; Harrison 2004; Oborne 2009; Rice‐McDonald 2005) and three paediatric studies (Garrett 1998; Martinez 2011; Wainwright 2009). All were published as full‐text papers with the exception of Wainwright 2009, for which study details and results were provided by the lead investigator. The eight studies randomised a total of 1669 participants to the comparison of interest for this review. Of all randomised participants, 58.5% had an exacerbation that led to use of the study inhaler. Four of the eight studies were multi‐centre and four were single‐centre studies. Three were conducted in Australasia, three in Europe and two in North America. The mean number of people randomised to treatment groups relevant to this review was 208 (range 22 to 403).
All included trials compared the efficacy of an increased dose of ICS at the onset of an exacerbation versus placebo as part of an asthma action plan. All other medications, mainly rescue short‐acting beta agonist inhalers, were kept equal between treatment and placebo groups and are noted in individual study characteristics tables.
Details of the countries and centres in which trials were conducted, sample sizes and the percentage with exacerbations in each trial, study treatments, durations and funding are shown in Table 2.
1. Summary of study characteristics.
| Study ID | N randomised* | N (%) who took study inhaler | Country (N centres) | Design | Age range | Maintenance ICS | Exacerbation inhaler | Funding | Asthma severity |
| Fitzgerald 2004 | 290 | 98 (34) | Canada (4) | 6‐month parallel, DB, PC | 13+ | Budesonide 100, 200 or 400 mcg BID (mean 635 mcg/d BDP) |
Budesonide 100, 200 or 400 mcg to double dose for 14 days Control: placebo |
AstraZeneca | FEV1 2.8 L, PEFR 423 L/min, ICS < 1200 mcg/d |
| Foresi 2000 | 142 | 36 (25) | Italy (14) | 6‐month parallel, DB, PC | 18‐65 | Budesonide 100 mcg BID | Budesonide 200 mcg QID to double usual dose Control: placebo |
Astra Farmaceutici | FEV1 74%, PEFR 75%, 41% on LABA, ICS < 1000 mcg/d |
| Garrett 1998 | 28 | 18 (64) | New Zealand (1) | 6‐month cross‐over, DB, PC | 6‐14 | Beclomethasone < 800 mcg/d | Matching beclomethasone to double dose Control: placebo |
New Zealand Asthma Society | FEV1 99%, PEFR 100%, ICS < 800 mcg/d |
| Harrison 2004 | 390 | 207 (53) | UK (1) | 1‐year parallel, DB, PC | 16+ | Usual ICS dose (mean 710 mcg/d BDP) |
Matching ICS inhaler to double dose for 14 days Control: placebo |
NHS Executive | FEV1 2.4 L/80%, PEF 384 L/min, 35% on LABA, ICS 100–2000 mcg/d |
| Martinez 2011 | 143 | 143 (100) | USA (5) | 44‐week parallel, DB, PC | 6‐18 | Beclomethasone 40 mcg BID | Beclomethasone 40 mcg BID to double dose Control: placebo |
NHLBI | 5% on LABA, recent admission or OCS, max 160 mcg bec/d |
| Oborne 2009 | 403 | 94 (23) | UK (1) | 1‐year parallel, DB, PC | 16+ | Usual ICS dose (mean 520 mcg/d BDP) |
Matching ICS inhaler to double dose for 14 days Control: placebo |
Asthma UK | FEV1 2.2 L/82%, PEF 380 L/min, ICS 200‐1000 mcg/d, recent OCS |
| Rice‐McDonald 2005 | 22 | 18 (82) | Australia (1) | Cross‐over until exacerbation in each phase | 18+ | Usual fluticasone dose (range not specified) | Matching ICS inhaler to double dose for 14 days Control: placebo |
Asthma Foundation of Queensland | Excluded mild asthma |
| Wainwright 2009 | 251 | 187 (75) | Australia (8) | 1‐year parallel, PC | 3‐14 | Fluticasone 125 mcg/d, or usual higher dose | Matching fluticasone to double dose for 14 days Control: placebo |
Asthma Foundation of Queensland | Recent ED, OCS or admission; fluticasone at least 125 mcg/d |
DB = double‐blind, NHLBI = National Heart, Lung and Blood Institute, PC = placebo‐controlled, UK = United Kingdom, USA = United States of America. Asthma severity statistics are mean values for the total population in each study. Percentages are means of lung function measured as a percentage of participants' predicted values
* The number randomised to the groups relevant to this review
We describe hereafter the characteristics of studies that contributed data to one or more outcomes in the review. For a full study description of each eligible study, see Characteristics of included studies.
Characteristics of studies
Run‐in
All eight studies included a run‐in period from two weeks to three months, mainly to ensure asthma stability. Three adult studies recruited participants who required low to moderate maintenance doses at baseline, ranging from a mean of 520 mcg/d to 710 mcg/d of CFC‐BDP equivalent (Fitzgerald 2004; Harrison 2004; Oborne 2009). The two other adult studies (Foresi 2000; Garrett 1998) and the three paediatric studies did not report the mean maintenance ICS dose at baseline. In all studies except Foresi 2000, participants continued their usual maintenance dose during the run‐in period. Foresi 2000 required a temporary increase in ICS dose to 1600 mcg/d for four weeks during run‐in, with reduction back to 200 mcg/d after randomisation.
Study design
Six of the eight studies had parallel‐group designs comparing people who were given a placebo inhaler or an active inhaler to increase their ICS dose during exacerbations. Garrett 1998 was a cross‐over design whereby children were randomised to one of two possible treatment sequences for serial exacerbations: placebo then corticosteroid, or corticosteroid then placebo. Rice‐McDonald 2005 also used a cross‐over design with three treatment phases, one of which was not relevant to this review (oral steroid rescue). For this study, we used results from the paper showing the number of people who needed oral steroids in one, neither or both of the two relevant phases, and analysed them to account for correlation.
Study duration
Duration of follow‐up for exacerbations post randomisation was six months for three studies (Fitzgerald 2004; Foresi 2000; Garrett 1998), 44 weeks for one study (Martinez 2011) and 12 months for three studies (Harrison 2004; Oborne 2009; Wainwright 2009). The duration in Rice‐McDonald 2005 was unclear, although investigators stated that the endpoint for each treatment was assessed seven days after the three‐week treatment pack if no treatment failure, or at time of treatment failure in the event of failure.
Characteristics of participants
Age
Four of the five adult studies recruited people from age 16 or 18 years of age onwards, and Fitzgerald 2004 also included adolescents from the age of 13 years. Mean participant age in the adult studies ranged from 32 to 56 (median 46.5) years. The age range in the paediatric studies ranged from six to 14 years (Garrett 1998), from six to 18 years (Martinez 2011) and from three to 14 years (Wainwright 2009). Mean participant ages were 8.2 and 11.2 years in Garrett 1998 and Martinez 2011, respectively, and we calculated a rough mean age from that categorised in Wainwright 2009 as 7.6 years.
Gender
All studies included both male and female participants. All adult studies included more women than men (median percentage male 33%, range 28% to 47%), and all paediatric studies recruited more boys than girls (median percentage male 60%, range 57% to 67%).
Smoking status
Four of the eight trials reported the smoking status of study participants. Never‐smokers made up most of the study samples (61% to 86%), with ex‐smokers making up between 14% and 36%, and active smokers 10% or less of the samples. Rice‐McDonald 2005 and the three paediatric studies did not report smoking status.
Severity
Baseline asthma severity was explicitly stated in just two studies and was reported as mild to moderate in Garrett 1998 and moderate in Foresi 2000. The remainder of studies reported baseline asthma severity as lung function measurements during the stable run‐in period, or informally by minimum medication requirements, which are summarised in the final column of Table 2. The average severity of airway obstruction was mild (forced expiratory volume in one second (FEV1) > 80%) in Harrison 2004 and Oborne 2009. Fitzgerald 2004 reported a mean baseline FEV1 of 2.8 L and a peak expiratory flow rate (PEFR) of 423 L/min, also falling within the mild severity category. Two paediatric studies ‐ Rice‐McDonald 2005 and Wainwright 2009 ‐ required children to have had a recent admission or course of oral steroids for an asthma exacerbation. Rice‐McDonald 2005 excluded people with mild asthma.
Treatment format
Study treatment details
In all eight studies, participants were required to be taking a stable dose of ICS at randomisation, with the dose of ICS increased at the onset of an asthma exacerbation, compared with placebo. In all studies, this was achieved with a study inhaler to be taken alongside the maintenance inhaler that contained additional ICS or placebo, administered at home by participants themselves, or with the aid of a parent or carer for younger children. The dose was increased five‐fold in Foresi 2000 and four‐fold in Oborne 2009 and was doubled in the remaining six studies (Fitzgerald 2004; Garrett 1998; Harrison 2004; Martinez 2011; Rice‐McDonald 2005; Wainwright 2009). The mean ICS dose achieved during exacerbations ranged from 1000 mcg/d to 2075 mcg/d in CFC‐BDP equivalents in the adult studies (Fitzgerald 2004; Foresi 2000; Harrison 2004; Oborne 2009) and from 160 to 500 mcg/d in the paediatric studies (Martinez 2011; Wainwright 2009). Mean dose achieved was not reported in the paediatric study of Garrett 1998, although the maximum dose achieved was 1600 mcg/d. Studies used metered dose or dry powder inhalers, but within studies the treatment or placebo inhaler provided for use during exacerbation was identical to the maintenance corticosteroid inhaler. Moreover, the additional use of a spacer was reported in Garrett 1998 and Wainwright 2009. Inhaled corticosteroid dose was increased for a pre‐defined period of 14 days in Fitzgerald 2000, Harrison 2004 and Rice‐McDonald 2005. In Garrett 1998, it was increased for just three days, in Foresi 2000 for seven days and in Oborne 2009 for just seven days if PEFR had returned to baseline by then, but was continued for 14 days if PEFR had not returned to baseline by day seven. In Martinez 2011, Oborne 2009 and Wainwright 2009, the course of increased ICS dose varied depending on how long it took for symptoms to return to baseline.
Action plan activation
Criteria for an asthma exacerbation that prompted initiation of the study inhaler were pre‐defined in all studies on the basis of a combination of PEFR worsening, increase in asthma symptoms and/or an increase in rescue bronchodilator use relative to run‐in values. In all eight studies, participant measurements or observations obtained alone or with confirmation from a study physician were required for activation of the asthma action plan at the onset of an asthma exacerbation. Participants were required to measure PEFR, to record asthma symptoms and/or to monitor rescue bronchodilator use continuously, or if they believed that their asthma control was deteriorating. One study used a PEFR cut‐off of < 85% of baseline in the criteria of an exacerbation (Harrison 2004), four studies used a cut‐off of PEFR < 80% (Fitzgerald 2004; Garrett 1998; Martinez 2011; Rice‐McDonald 2005) and one study used a cut‐off of < 70% (Foresi 2000). Oborne 2009 used a variable PEFR cut‐off of < 85% of baseline on two consecutive days, or < 70% of baseline on a single day, and Wainwright 2009 did not define a cut‐off. All studies incorporated an increase in asthma symptoms into the criteria of an exacerbation. Three studies incorporated an increase in rescue bronchodilator use among the criteria of an exacerbation (Fitzgerald 2004; Garrett 1998; Rice‐McDonald 2005). All studies provided clear criteria for asthma action plan activation. The minimum time elapsed between onset of asthma deterioration and initiation of increased ICS dose (as recommended by the action plan) varied from immediate use of the study inhaler as a rescue treatment (Martinez 2011; Wainwright 2009) to 24 hours after symptoms worsened (Garrett 1998; Harrison 2004; Rice‐McDonald 2005) to 48 hours (Fitzgerald 2004; Foresi 2000). For Oborne 2009, elapsed time varied from 24 hours to 48 hours, depending on how much PEFR had dropped from baseline.
Concomitant treatment
In all included studies, baseline co‐interventions for asthma were continued, provided that the dose remained unchanged throughout the study period. Four studies permitted the use of long‐acting beta agonists (LABA), and two studies explicitly stated that patients requiring LABA before study entry were excluded (Fitzgerald 2004; Garrett 1998). Martinez 2011 and Wainwright 2009 did not report whether any of the recruited participants were currently taking LABA. Martinez 2011 did report that a small number had taken LABA in the previous year (6% and 4%), and reported usage rates in Harrison 2004 and Oborne 2009 of about 40% in control and treatment groups. One study (Fitzgerald 2004) reported on inclusion of participants requiring oral theophylline at baseline, although usage rates were low at less than 4%, and another study reported on inclusion of participants requiring regular ipratropium at baseline (Fitzgerald 2004). Rice‐McDonald 2005 allowed concomitant use of LABA, theophylline or leukotriene receptor antagonists but did not report the number of participants taking them at baseline. Baseline nasal ICS use was reported in Fitzgerald 2004, with usage rates of 25% and 26% for control and treatment groups, respectively.
Treatment follow‐up
After the action plan was initiated, study investigators provided variable follow‐up periods. In Garrett 1998, participants were visited within the first three days at home and then were seen within one week in the clinic. Fitzgerald 2004 reported post‐treatment surveillance for a period of three months to monitor asthma control and to ensure no late differences between treatment and placebo groups. Wainwright 2009 conducted three‐monthly routine check‐ups, contacted participants two weeks after each exacerbation and took final measurements after 12 months. In Rice‐McDonald 2005, a cross‐over study, participants were contacted fortnightly by a research nurse and were reviewed by a study investigator every eight weeks. Martinez 2011 reviewed participants every four to eight weeks over the 44‐week study period, regardless of exacerbations. Medical follow‐up after the exacerbation was not described in Foresi 2000, Harrison 2004 and Oborne 2009.
Action plan compliance
Four studies monitored compliance with symptom recording and/or study treatment (Fitzgerald 2004; Foresi 2000; Garrett 1998; Rice‐McDonald 2005). Investigators evaluated complianceby reviewing self reported symptom diaries, self reported medication diaries and PEFR recordings and by counting tablets from returned treatment packs. Self reported study treatment compliance was high in three studies, ranging from a mean of 86% in Garrett 1998 to 93% in Fitzgerald 2004, and was not reported in Rice‐McDonald 2005.
Upfront oral corticosteroid use
Participants were required to start oral corticosteroids upfront at the onset of an asthma exacerbation if PEFR was measured at less than 60% in four studies (Fitzgerald 2004; Garrett 1998; Harrison 2004; Oborne 2009) and 50% in one study (Foresi 2000).
Outcome reporting
The primary outcome for this review ‐ the need for rescue systemic corticosteroids ‐ was reported in all studies except Foresi 2000. Generally participants were withdrawn from use of the study inhaler and were started on rescue oral corticosteroids if they failed to respond adequately to an increase in ICS dose, or if their PEFR dropped to below a pre‐defined safety cut‐off (usually 60%). Treatment failure was defined by deterioration or lack of improvement in pulmonary function and/or symptoms. Rescue oral corticosteroids were participant‐initiated if PEFR fell below a pre‐defined threshold of 60% at any point during the treatment period, or after discussion with a study physician based on symptom frequency and PEFR measurements. Harrison 2004 and Oborne 2009 required rescue oral corticosteroid use if participants' asthma control deteriorated to the point that they would usually start oral corticosteroids.
Pre‐defined secondary outcomes were reported less consistently across studies, with no more than three studies included in any of the secondary analyses.
Excluded studies
Reasons for exclusion of 39 studies, including those excluded in the previous version and those excluded in the current update, are documented in the Characteristics of excluded studies section. Of 13 records related to nine studies excluded in this update, the most common reasons for exclusion after viewing of full texts were that ICS were not being given to treat an exacerbation of asthma (N = 6) and no arm was receiving stable ICS (N = 4). Two records were related to a study that was already included in the previous version of this review (Oborne 2009), and one described an ongoing study (NCT02066129). Of the 34 records related to 30 excluded from the previous version of this review, reasons for exclusion included that trials were not placebo‐controlled (N = 15), did not recruit people with asthma (N = 1), did not test ICS to treat an exacerbation (N = 4) and did not recruit people taking maintenance ICS (N = 13). One remaining study that was listed as excluded in the previous version of this review was moved from excluded to included in this update (Rice‐McDonald 2005). The reason for exclusion was that available data did not allow for analysis and study authors could not be contacted for clarification; we were able to rectify this in the current version.
Risk of bias in included studies
We have presented in Figure 2 an overview of the risk of bias in individual studies.. In general, all trials were of high methodological quality and had low risk of bias.
2.
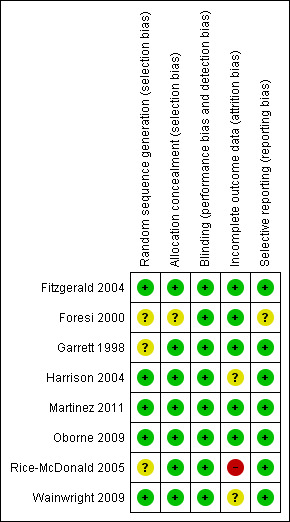
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Five of the eight studies gave sufficient detail regarding random sequence generation to be considered at low risk of bias, stating that computer‐generated codes or random number tables were used (Fitzgerald 2004; Harrison 2004; Martinez 2011; Oborne 2009; Wainwright 2009). We did not have sufficient information from the remaining three studies, which were rated as having unclear risk.
For concealment of the allocation, we considered all except Foresi 2000 to be at low risk of bias because details given suggested that randomisation was done through a central system or by an independent pharmacist not otherwise involved in the study.
Blinding
Seven studies explicitly stated their double‐blind design and masking procedures and therefore were at low risk of bias. Wainwright 2009 was not described as double‐blind, but investigators described matching placebo inhalers, which implies that blinding procedures were used; hence we also considered this study to be at low risk of bias.
Incomplete outcome data
We considered five studies to be at low risk of bias as the result of incomplete data. Withdrawal rates in Fitzgerald 2004, Martinez 2011 and Oborne 2009 ranged between 11% and 22% across groups, but rates were fairly balanced within studies and appropriate imputation was used to adhere to the ITT principle. Dropout was not given per group in Foresi 2000, but overall dropout was low (10.6%) and the ITT analysis included 98% of those randomised. Garrett 1998 was rated as having low risk because, although several participants were not included in their analyses, this occurred because of their cross‐over design and as a result of their plan to include only participants who had exacerbations in both study phases.
Harrison 2004 was rated as having unclear risk because, although around 10% dropped out of each group, which is a relatively low and balanced dropout rate, investigators did not make clear whether they had used the protocol or ITT analyses. We rated Rice‐McDonald 2005 as having high risk of bias because 13 of the 35 people randomised (37%) dropped out and were not included in the analysis.
Selective reporting
We were satisfied that no selective outcome reporting had occurred in seven included studies, either because stated outcomes were well defined and reported in the published papers after study authors provided additional data upon request, or because we were able to confirm with study authors that the outcomes we were interested in had not been measured. We rated none of the studies as having high risk of bias and only one as having unclear risk for these reasons.
Other potential sources of bias
The cross‐over study by Garrett 1998 did not state the time lapse between treatments and did not comment on any possible carry‐over effect. If the effective intervention is followed closely by placebo, the therapeutic effect could be carried over into the placebo period, thereby minimising any possible differences between placebo and treatment.
Effects of interventions
See: Table 1
Primary outcome
Treatment failure ‐ need for systemic corticosteroids (ITT analysis)
People randomised to an increased ICS dose during an exacerbation were not significantly less likely to require rescue oral corticosteroids compared with those assigned to placebo (OR 0.89, 95% CI 0.68 to 1.18; participants = 1520; seven studies; I2 = 0%; Analysis 1.1). Just under 60% of randomised participants actually required use of the study inhaler (mean 58.5%, range 23% to 100%). The estimate slightly favoured increased ICS dose, but confidence intervals did not rule out the possibility that keeping the dose stable was better, so we downgraded the evidence once for imprecision and rated the study as having moderate quality.
1.1. Analysis.
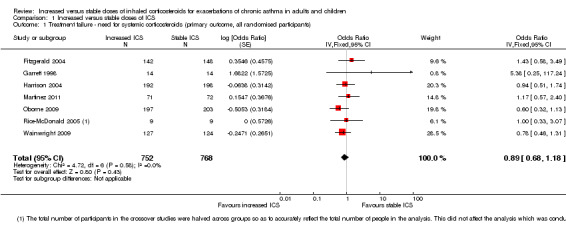
Comparison 1 Increased versus stable doses of ICS, Outcome 1 Treatment failure ‐ need for systemic corticosteroids (primary outcome, all randomised participants).
Subgroup analysis
When the primary outcome was used with all randomised participants as the analysis denominator, five out of six subgroup analyses had sufficient data for analysis. Findings of tests for subgroup differences in age (Analysis 2.1), time to treatment initiation (Analysis 2.2), maintenance ICS dose (Analysis 2.3) and exacerbation ICS dose (Analysis 2.4) were all non‐significant. Garrett 1998 could not be included in maintenance or achieved ICS dose subgroups because of the large dose range, which included no details about average doses on which to base a categorisation. The estimate favoured an ICS increase more if the dose was quadrupled rather than doubled, but only one study quadrupled the dose, and the difference between dose subgroups was not statistically significant (I2 = 47, P value = 0.17). We could not examine the impact of smoking status on the odds of requiring oral corticosteroids during an exacerbation because all studies recruited non‐smokers or ex‐smokers.
2.1. Analysis.
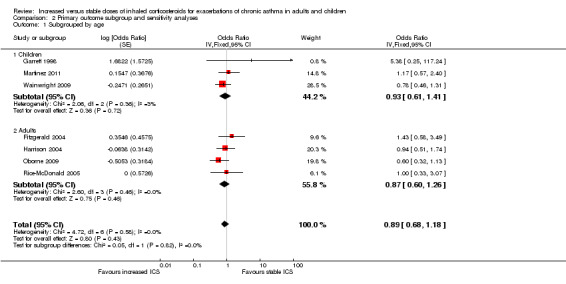
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 1 Subgrouped by age.
2.2. Analysis.
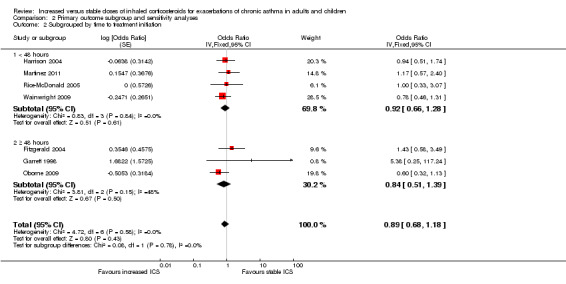
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 2 Subgrouped by time to treatment initiation.
2.3. Analysis.
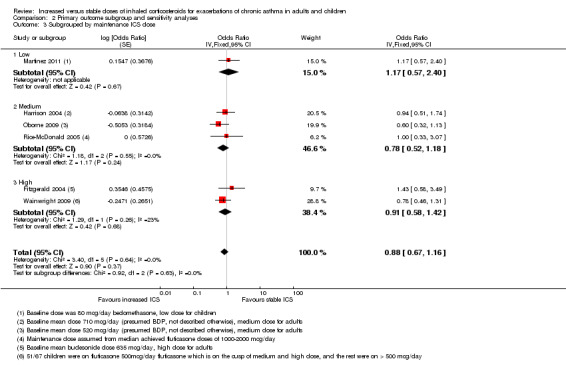
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 3 Subgrouped by maintenance ICS dose.
2.4. Analysis.
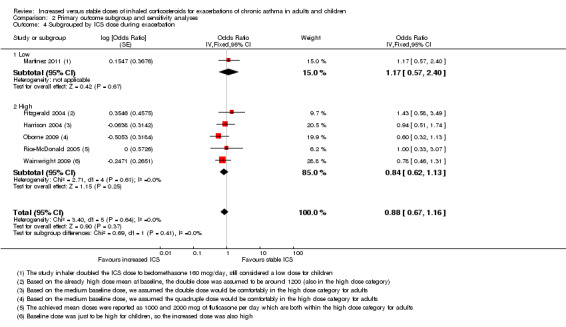
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 4 Subgrouped by ICS dose during exacerbation.
Sensitivity analysis
Study design
Removing the two cross‐over studies (Garrett 1998 and Rice‐McDonald 2005) from the primary analysis had very little effect on direction, size or precision of the estimate size for the primary outcome (OR 0.87, 95% CI 0.66 to 1.16; Analysis 2.6).
2.6. Analysis.
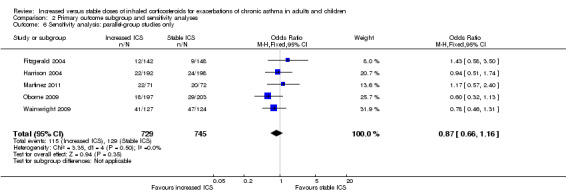
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 6 Sensitivity analysis: parallel‐group studies only.
Methodological quality
The two cross‐over studies were the only studies with uncertainties regarding risk of selection bias, so the result was the same as for the study design sensitivity analysis above.
Source of study funding
Three studies were funded by pharmaceutical companies involved in the sales of ICS (Fitzgerald 2004; Foresi 2000; Garrett 1998). Fitzgerald 2004 and Garrett 1998 contributed data to the primary outcome. Their exclusion slightly increased the size of the effect in favour of increasing ICS dose and the precision of the estimate, but it did not alter the conclusions drawn (OR 0.84, 95% CI 0.62 to 1.12).
Publication bias
Studies were too few for review authors to determine whether publication bias was present or to identify a systematic difference between smaller and larger studies via funnel plot analysis.
Secondary outcomes
Asthma exacerbations requiring rescue systemic corticosteroids (modified ITT analysis)
We included the same seven studies in this outcome (Fitzgerald 2004; Garrett 1998; Harrison 2004; Martinez 2011; Oborne 2009; Rice‐McDonald 2005; Wainwright 2009), when looking at the effect of doubling ICS in participants who took their study inhaler rather than all those randomised. In two studies, all randomised participants took their study inhaler, so the data were the same as those entered for the primary outcome. The analysis included 766 people who had exacerbations meeting the study criteria rather than all 1520 randomised to the studies. Significant inconsistency between study results also contributed to imprecision in the estimate, meaning that the evidence was considered of low quality. The pooled estimate did not suggest that participants randomised to increase their ICS dose were less likely to require rescue systemic corticosteroids compared with those assigned to placebo (OR 0.84, 95% CI 0.54 to 1.30; participants = 766; seven studies); I2 = 42%; random‐effects method; Analysis 1.2).
1.2. Analysis.
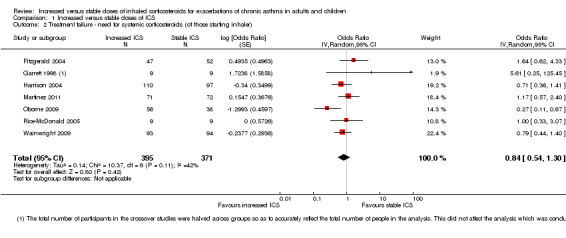
Comparison 1 Increased versus stable doses of ICS, Outcome 2 Treatment failure ‐ need for systemic corticosteroids (of those starting inhaler).
We did not perform subgroup and sensitivity analyses on this outcome as was done in the previous version of the review, as these analyses were not originally planned in the protocol.
Unscheduled physician visits
Three parallel‐group studies measured this outcome and showed no significant differences in the odds between groups (OR 0.96, 95% CI 0.66 to 1.41; participants = 931; three studies; I2 = 0%; Analysis 1.3). Harrison 2004 and Wainwright 2009 reported unscheduled visits only for people who took their study inhaler, but we used the total number randomised as the denominator. We performed a post hoc sensitivity analysis using only those taking the study inhaler as the denominator for these two studies, and our conclusions did not change (OR 0.89, 95% CI 0.59 to 1.35). The width of the confidence intervals made it very difficult to determine where the true effect may lie, so we downgraded the evidence twice for imprecision and rated the studies as low quality.
1.3. Analysis.
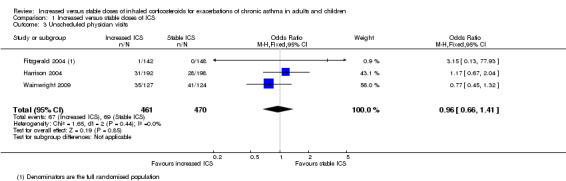
Comparison 1 Increased versus stable doses of ICS, Outcome 3 Unscheduled physician visits.
Unscheduled acute care or emergency department visits or need for hospital admission
Three studies collected data on unscheduled acute care or emergency department visits, but only one paediatric study observed any events (Wainwright 2009). We could not draw a meaningful conclusion because the study estimate was based on only four visits in either group (Peto OR 0.98, 95% CI 0.24 to 3.98; participants = 450; three studies), and we downgraded the evidence to low for this imprecision. It made very little difference when only the number taking the study inhaler was used as the denominator (Peto OR 1.01, 95% CI 0.25 to 4.15; participants = 386; three studies).
Duration of exacerbation
Three studies reported data on the duration of exacerbation following initiation of study inhaler, as defined by the time required for PEFR to return to baseline values (Garrett 1998; Harrison 2004; Oborne 2009). However, group mean and standard deviation values were available only for Harrison 2004 and show no benefit for recovery time with increased ICS (Analysis 1.5).
1.5. Analysis.
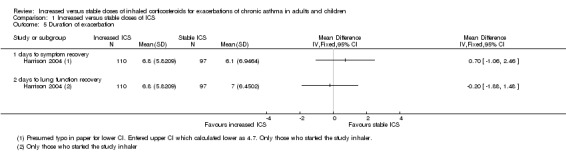
Comparison 1 Increased versus stable doses of ICS, Outcome 5 Duration of exacerbation.
Two studies provided data on the duration of exacerbation following initiation of study inhaler, as defined by time required for symptoms to return to baseline values (Fitzgerald 2004; Harrison 2004). Again, only mean and standard deviation values were reported (Harrison 2004), and results show no benefit of increased ICS when this definition was used (also in Analysis 1.5).
Imprecision in both estimates made it difficult for review authors to be certain of the true effect, so we downgraded both once for imprecision and rated the studies as moderate quality.
No studies reported data for the duration of exacerbations defined as reduction in beta2 agonist use back to baseline requirements.
Serious and non‐serious adverse events
Participants assigned to an increased ICS dose following onset of an asthma exacerbation did not have significantly more serious adverse events (OR 1.69, 95% CI 0.77 to 3.71; participants = 394; two studies). Serious adverse events in Martinez 2011 included bronchitis in the increased dose group and viral meningitis in the stable daily dose group. We classified study‐defined serious adverse events in Wainwright 2009 as follows, some of which might not generally be considered serious adverse events: upper respiratory tract infection/otitis media/croup (six in double‐dose group), ear/nose/throat surgery (one in usual dose group, three in double‐dose group), fracture (one in usual dose group), other orthopaedic events (one in each group), chest infection/pneumonia (four in each group), other (three in usual dose group, two in double‐dose group) and death (one in double‐dose group). The only serious adverse events, which were reported in Rice‐McDonald 2005, were noted in the oral steroid rescue group, which was not included in this review.
Taking increased ICS did not significantly increase the odds of having any non‐serious adverse event (OR 2.15, 95% CI 0.68 to 6.73; participants = 142; two studies) compared with keeping the dose stable. We have summarised specific non‐serious adverse events narratively because they were reported inconsistently across studies. Three studies reporting lists of specific side effects generally showed low occurrence (one or two people) in either group (Foresi 2000; Oborne 2009; Rice‐McDonald 2005). The odds of occurrence of specific adverse effects including oral irritation, headaches, psychiatric disturbance, gastrointestinal discomfort, dysphonia and change in appetite were not significantly higher in the increased ICS versus stable dose groups. Results of Rice‐McDonald 2005 must be interpreted with caution because of the study's cross‐over design and the oral steroid treatment phase, which was not included in this review.
Adverse events were not reported in detail in Fitzgerald 2004, but the participant flow diagram showed that one person in the double‐dose group and three in the stable dose group dropped out because of unspecified adverse events, none of whom had exacerbations requiring the need for the study inhaler. Garrett 1998 and Harrison 2004 provided minimal information regarding adverse events, although Garrett 1998 stated that no child was hospitalised during the study (for asthma or for other reasons).
In addition to the data on serious adverse events, the Martinez 2011 paediatric study reported linear growth but not specifically for the two groups compared in this review.
Discussion
Summary of main results
This review update added to the review three new studies including 419 participants. In total we identified eight randomised controlled trials (RCTs), most of which were at low risk of bias involving 1669 participants with mild to moderate asthma. We identified three paediatric (n = 422) and five adult (n = 1247) studies; six were parallel‐group trials, and two had a cross‐over design; all but one study followed participants for six months to one year. Allowed maintenance doses of inhaled corticosteroids (ICS) varied in adult and paediatric studies, as did use of concomitant medications and achieved ICS doses initiated during exacerbations. Investigators gave participants a study inhaler containing additional ICS or placebo to be started as part of an action plan to treat exacerbations.
Available evidence suggests that an increased ICS dose was not associated with a statistically significant difference in the odds of needing rescue oral corticosteroids or in other effectiveness outcomes compared with a continued stable dose of ICS. Subgroup analyses of the primary outcome based on age (children vs adults), time elapsing before treatment initiation (< 48 hours vs ≥ 48 hours), baseline ICS dose and magnitude of the dose increase (doubling vs quadrupling) showed no significant differences between subgroups. The modified intention‐to‐treat analysis, with the denominator restricted to participants who used the study inhaler for at least one exacerbation to mimic an efficacy study, still failed to demonstrate any overall benefit. The post hoc subgroup analysis performed on the modified intention‐to‐treat analysis of the primary outcome suggested but did not confirm greater benefit with quadrupling over doubling the ICS dose, as no head‐to‐head comparison of different ICS dose fold increase and no dose increase were associated with a statistically significant reduction in odds. As the subgroup analysis was based on the magnitude of dose increase (doubling or quadrupling) rather than on absolute dose increase or absolute dose achieved, it remains unclear whether magnitude of dose increase or absolute dose increase/achieved would have greater impact, if any, on apparent benefit. Whilst the apparent benefit could be explained by the absence of a significant reduction in odds, under‐powering of the primary analysis due to inclusion of participants who never used the study drug, known limitations of subgroup analyses (particularly post hoc) and multiple statistical testing require care in interpretation of this finding (Wang 2007). The comparative benefit of ICS dose‐doubling versus quadrupling would be best examined by a head‐to‐head comparison in a large RCT.
Confidence intervals around the primary outcome estimate for the seven studies were wide; therefore one cannot exclude a possible reduction or increase by about 30% in the odds of requiring rescue oral corticosteroids associated with increased ICS dosing. For secondary outcomes, the ICS dose increase did not significantly reduce the frequency of unscheduled physician visits or unscheduled acute care visits or hospital admissions, although the effect estimates were imprecise. Studies were insufficient for aggregation of data on other secondary outcomes, including duration of asthma exacerbation as defined by return of lung function, symptoms or rescue bronchodilator use back to baseline.
Participants allocated to an increased ICS dose during exacerbations did not experience a statistically significant increase in the odds of serious and overall or specific non‐serious side effects, namely, headaches, dysphonia, pharyngitis, glossitis, oral candidiasis, change in appetite, upper respiratory tract infection, psychiatric disturbance (depression, anxiety) and gastrointestinal symptoms (nausea and abdominal discomfort). Moreover, with the exception of linear growth (Martinez 2011), prospective data on specific adverse events were not collected, likely leading to under‐reporting of adverse events. No studies reported data on the number of people experiencing hyperglycaemia, adrenal dysfunction or pneumonia.
Overall completeness and applicability of evidence
To our knowledge, this is the update of the only systematic review and meta‐analysis in the literature examining the safety and effectiveness of increasing versus maintaining the same ICS dose at the onset of an asthma exacerbation as part of a patient‐initiated action plan. Since this review was first published, the Global Initiative for Asthma (GINA) Guidelines have been updated and no longer recommend temporary doubling of the ICS dose (GINA 2015). Our results contrast with those of a pre‐school trial demonstrating that risk of the need for rescue systemic corticosteroids was reduced by half when high‐dose fluticasone versus placebo was used as a pre‐emptive strategy in children with viral‐induced asthma, although none of these young children were using daily ICS (Ducharme 2009).
Lack of overall benefit from an increased ICS dose strategy demonstrated in this systematic review might be explained in several ways. First, regular use of ICS in asthma has proved very effective in preventing exacerbations and specifically reducing the need for rescue oral corticosteroids (Adams 1999): Daily ICS may indeed be the most effective preventive strategy with minimal additional benefit of pre‐emptive increased ICS dose during exacerbations. For example, just one‐half of participants randomised in the included studies required step‐up therapy with the study inhaler. This low exacerbation rate in turn may have led to possible under‐powering of data to detect a significant difference in odds between groups, if present. Second, the small number of studies contributing data to this outcome led to wide confidence intervals for most outcomes, attesting to the lack of power to conclude firmly on the absence of beneficial effect. Third, despite low heterogeneity between studies on the main outcome, participant, treatment or design characteristics could have influenced the magnitude of effect, which could not be adequately explored because of the small number of studies. Finally, although self reported compliance with the action plan protocol and study inhaler was high (86% in Garrett 1998, 100% in Fitzgerald 2004), actual compliance was not measured and may have been lower. Indeed, in a previous study looking at asthma action plan compliance in a family practice setting, less than 40% properly implemented their action plan (Turner 1998). In other words, included studies were primarily effectiveness trials; consequently absence of effect may be due to non‐efficacy or to poor or delayed implementation of the intervention, which was documented only by participant reports rather than as an objective measure of adherence.
With regards to applicability of the findings, most data were derived from non‐smoking adults with mild to moderate asthma who were taking low to moderate stable doses of ICS at baseline, in addition to other asthma therapy. Study results may not apply to children and adolescents, as just two included studies involved children (Garrett 1998; Wainwright 2009) and one accepted an unspecified number of adolescents.
Quality of the evidence
According to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) method, our confidence in the evidence across outcomes was moderate or low, meaning that true effects may be substantially different from pooled estimates. The most common limitation across outcomes was lack of precision, which was a result of the small number of identified studies and observed events. None of the outcomes were thought to be compromised by risk of bias within studies; although some uncertainties regarding allocation concealment and missing data imputation were evident in some studies, review authors did not deem this significant enough to have had a serious impact on the results. Similarly, although we noted that several trials did not contribute to secondary outcomes, contact with study authors confirmed that this was unlikely to be due to publication bias. We did not downgrade any of the outcomes because of indirectness of study populations, interventions or outcomes for the review question. At least two review authors made study inclusion decisions to ensure that studies were relevant to the review, and resolved with study authors any uncertainties in the definitions of outcomes.
Our confidence in the primary outcome was reduced from high to moderate because the confidence interval around the null effect did not exclude the possibility of appreciable benefit of either option. This imprecision affected our confidence both when the need for oral steroids was assessed as a proportion of the total intention‐to‐treat population, and when assessment was limited to those starting their study inhaler. However, statistical heterogeneity in the latter analysis was much higher, so our confidence in this analysis was low.
Within secondary outcomes, our confidence in the effect of increasing ICS dose on unscheduled physician visits and unscheduled acute visits was low, and was reduced substantially by the numbers of studies and events included in the analyses. We also judged the effect of increasing ICS dose on duration of exacerbation and serious and non‐serious adverse events as low because of imprecision of the estimates.
Potential biases in the review process
The main strength of this review is its low opportunity for bias. Although one cannot firmly rule out publication bias, our systematic search of published trials and unpublished reports was undertaken with a high likelihood of identifying all relevant studies, thus minimising this type of bias. Indeed, we found no abstracts whose results were not published afterwards. The rigorous eligibility criteria requiring double‐blinding resulted in the inclusion of generally high‐quality trials, further strengthening the validity of our findings.
This systematic review had a few limitations that could have introduced bias. Inherent to the cross‐over design of included studies, individual participants experienced multiple exacerbations/treatments within the same study. Potential non‐independence of events due to inadequate wash‐out may have resulted in undue influence on study results in one direction or another. To obviate to the issue of non‐independence, all analyses were performed per participant, not per event. Second, the limited response of study authors or sponsors to requests for providing data contributed to lack of precision for our primary outcome.
Agreements and disagreements with other studies or reviews
We have identified no other non‐Cochrane reviews addressing the efficacy of increasing the dose of inhaled corticosteroids at the onset of an asthma exacerbation as part of a patient‐initiated action plan. This is an update of a Cochrane review first published in 2010 (Quon 2010). We included in this review three additional studies (Martinez 2011; Rice‐McDonald 2005; Wainwright 2009) with an additional 419 participants, but overall study findings and conclusions are consistent with those of our prior review.
Authors' conclusions
Implications for practice.
Evidence does not support increasing the dose of ICS as part of a self initiated action plan to treat exacerbations in adults or children with mild to moderate asthma. Increased ICS dose is not associated with a statistically significant reduction in the odds of requiring rescue oral corticosteroids for the exacerbation, or of having adverse events, compared with maintenance of a stable ICS dose. Wide confidence intervals for several outcomes mean that we cannot rule out possible benefits of this approach.
Implications for research.
Additional RCTs comparing increased versus stable ICS doses at the onset of an exacerbation in specific subgroups (children, adolescents, smokers) are needed, along with RCTs comparing various ICS doses in head‐to‐head comparisons by a parallel design. Randomised controlled trials should report detailed subgroup analyses on variables that may affect response to therapy, such as triggers for exacerbation, maintenance ICS doses and achieved ICS doses following step‐up therapy.
Future studies should investigate a step‐up in ICS dose in excess of doubling or above a certain ICS threshold dose because the high‐quality studies identified in this review failed to show clinical benefit with dose doubling. A strategy similar to that of Oborne 2009, with quadrupling of baseline ICS dose during step‐up therapy to an achieved ICS dose in the range of 2000 mcg/d or higher in chlorofluorocarbon‐propelled beclomethasone dipropionate (CFC‐BDP) equivalents, might prove more effective, as previously suggested, among steroid‐naive pre‐school‐aged children given 1500 mcg/d (Ducharme 2009).
Future studies should provide documentation on important outcomes such as the need for rescue oral corticosteroids, unscheduled medical resource use and duration of exacerbation as defined by return of symptoms, lung function and rescue medication use. These studies should also prospectively document the numbers of participants experiencing serious and non‐serious adverse events, objectively document compliance with the treatment regimen using dose counters and clarify the cost‐effectiveness of such a strategy.
Feedback
feedback, 27 October 2010
Summary
The abstract and document appear to mix up use of mg and mcg throughout the document. I assume the units should be mcg throughout but mg is used widely, particularly in the abstract. This could potentially lead to significant error and risk to patient safety. Could you confirm whether these are errors?
Reply
We are very grateful to the author for highlighting the typo in the review, along with others who pointed this out. We have corrected the typo and apologise for any confusion caused.
Contributors
Vanessa Chapman
What's new
| Date | Event | Description |
|---|---|---|
| 24 March 2016 | New search has been performed | Three new studies (Martinez 2011; Rice‐McDonald 2005; Wainwright 2009) including 419 additional participants were included in this review update |
| 24 March 2016 | New citation required but conclusions have not changed | Although additional data have been included in this review, the original conclusions remain unchanged |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 8 November 2010 | New citation required but conclusions have not changed | Feedback has triggered a new citation version |
| 8 November 2010 | Feedback has been incorporated | We received feedback and corrected several typos by which mcg was confused with mg |
Acknowledgements
We are very grateful to the study authors Vernon Chinchilli, Fernando Martinez, Claire Wainwright, Tim Harrison and Janet Oborne, who kindly responded to requests for additional information for the previous version and this review update.
We thank the Cochrane Airways Review Group, namely, Liz Stovold, for assistance with the literature search and ongoing support.
We would like to acknowledge Neal Shahidi for his contributions to study assessment and characterisation and data extraction for the previous version of the review.
Christopher Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Review Group.
Appendices
Appendix 1. Search strategy for the previous version of this review
All records in the Specialised Register coded as ‘asthma’ were searched using the following terms:
(exacerbat* OR acute* or status* or severe* OR worsen* OR emergenc* OR attack* or crisis) and (dose* or dosing or dosage) and (doubl* or increas*) OR "dose response" or "drug dose") and (glucocorticoid* OR corticosteroid* OR "inhaled steroid*" OR fluticasone OR Flovent OR beclomethasone OR Becloforte OR budesonide OR Pulmicort OR flunisolide OR Aerobid OR triamcinolone OR Beclovent OR Azmacort OR Vanceril OR Becotide OR Flixotide OR Aerobec OR Mometasone OR Qvar or ciclesonide or Alvesco)
Appendix 2. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All
#6 ICS:TI,AB
#7 (beclomethasone* or beclometasone* OR triamcinolone* OR fluticasone* OR budesonide* OR betamethasone* OR flunisolide* OR ciclesonide* OR mometasone*)
#8 (inhal*) NEAR5 (steroid* or corticosteroid* or glucocorticoid*)
#9 #5 or #6 or #7 or #8
#10 MeSH DESCRIPTOR Dose‐Response Relationship, Drug
#11 (dose* or dosing or dosage) AND (doubl* or increas*)
#12 step‐up* OR (step* NEXT up*)
#13 dose* NEXT reponse*
#14 drug* NEXT dose*
#15 #10 or #11 or #12 or #13 or #14
#16 MeSH DESCRIPTOR Disease Progression
#17 exacerbat* OR acute* or status* or severe* OR worsen* OR emergenc* OR attack* or crisis
#18 #16 or #17
#19 #4 AND #9 AND #15 AND #18
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Increased versus stable doses of ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure ‐ need for systemic corticosteroids (primary outcome, all randomised participants) | 7 | 1520 | Odds Ratio (Fixed, 95% CI) | 0.89 [0.68, 1.18] |
| 2 Treatment failure ‐ need for systemic corticosteroids (of those starting inhaler) | 7 | 766 | Odds Ratio (Random, 95% CI) | 0.84 [0.54, 1.30] |
| 3 Unscheduled physician visits | 3 | 931 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 4 Unscheduled acute care, ED visit or hospital admission | 3 | 450 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.24, 3.98] |
| 5 Duration of exacerbation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 days to symptom recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 days to lung function recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Serious and non‐serious adverse events | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Serious adverse events | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.77, 3.71] |
| 6.2 Non‐serious adverse events | 2 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.68, 6.73] |
1.4. Analysis.
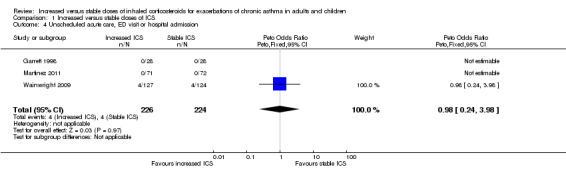
Comparison 1 Increased versus stable doses of ICS, Outcome 4 Unscheduled acute care, ED visit or hospital admission.
1.6. Analysis.
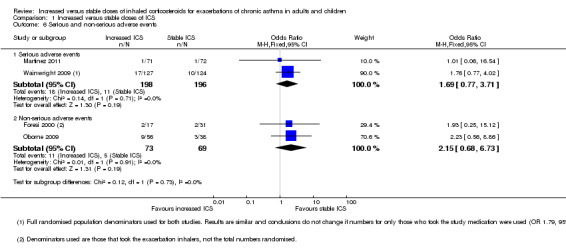
Comparison 1 Increased versus stable doses of ICS, Outcome 6 Serious and non‐serious adverse events.
Comparison 2. Primary outcome subgroup and sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subgrouped by age | 7 | Odds Ratio (Fixed, 95% CI) | 0.89 [0.68, 1.18] | |
| 1.1 Children | 3 | Odds Ratio (Fixed, 95% CI) | 0.93 [0.61, 1.41] | |
| 1.2 Adults | 4 | Odds Ratio (Fixed, 95% CI) | 0.87 [0.60, 1.26] | |
| 2 Subgrouped by time to treatment initiation | 7 | Odds Ratio (Fixed, 95% CI) | 0.89 [0.68, 1.18] | |
| 2.1 < 48 hours | 4 | Odds Ratio (Fixed, 95% CI) | 0.92 [0.66, 1.28] | |
| 2.2 ≥ 48 hours | 3 | Odds Ratio (Fixed, 95% CI) | 0.84 [0.51, 1.39] | |
| 3 Subgrouped by maintenance ICS dose | 6 | Odds Ratio (Fixed, 95% CI) | 0.88 [0.67, 1.16] | |
| 3.1 Low | 1 | Odds Ratio (Fixed, 95% CI) | 1.17 [0.57, 2.40] | |
| 3.2 Medium | 3 | Odds Ratio (Fixed, 95% CI) | 0.78 [0.52, 1.18] | |
| 3.3 High | 2 | Odds Ratio (Fixed, 95% CI) | 0.91 [0.58, 1.42] | |
| 4 Subgrouped by ICS dose during exacerbation | 6 | Odds Ratio (Fixed, 95% CI) | 0.88 [0.67, 1.16] | |
| 4.1 Low | 1 | Odds Ratio (Fixed, 95% CI) | 1.17 [0.57, 2.40] | |
| 4.2 High | 5 | Odds Ratio (Fixed, 95% CI) | 0.84 [0.62, 1.13] | |
| 5 Subgrouped by ICS fold increase | 7 | Odds Ratio (Fixed, 95% CI) | 0.89 [0.68, 1.18] | |
| 5.1 Double dose | 6 | Odds Ratio (Fixed, 95% CI) | 0.98 [0.72, 1.34] | |
| 5.2 Quadruple dose | 1 | Odds Ratio (Fixed, 95% CI) | 0.60 [0.32, 1.13] | |
| 6 Sensitivity analysis: parallel‐group studies only | 5 | 1474 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.16] |
| 7 Sensitivity analysis: independently funded studies only | 5 | Odds Ratio (Fixed, 95% CI) | 0.84 [0.62, 1.12] |
2.5. Analysis.
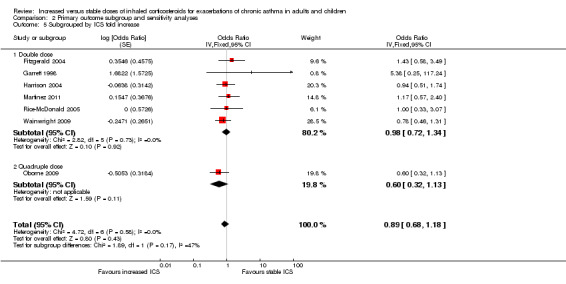
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 5 Subgrouped by ICS fold increase.
2.7. Analysis.
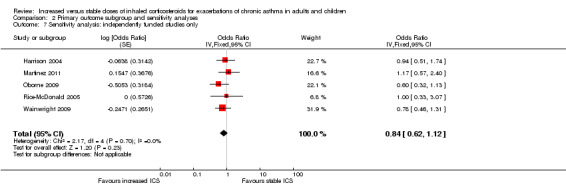
Comparison 2 Primary outcome subgroup and sensitivity analyses, Outcome 7 Sensitivity analysis: independently funded studies only.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fitzgerald 2004.
| Methods | This 6‐month, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study compared a continued maintenance dose of inhaled corticosteroid vs a dose doubled at the time of an asthma exacerbation Conducted at 4 teaching units in Canada |
|
| Participants |
Population 290 participants were randomised; 98 participants experienced an exacerbation and contributed to the analysis Participants were 13 years or older. Mean age was 32 years. 28% were male. 14% were ex‐smokers of fewer than 10 pack‐years, and 86% were non‐smokers Baseline asthma severity Mean dose of budesonide: 635 mcg Mean FEV1: 2.8 L Mean PEFR: 423 L/min At least 1 previous asthma exacerbation with mean duration from recent exacerbation to visit 1 of 131 days Stable dose of ICS (< 1200 mcg/d of beclomethasone or equivalent twice daily) for 1 month before visit 1 Inclusion criteria Age ≥ 13; documentation of the diagnosis of asthma within the previous year based on FEV1 reversibility post bronchodilator, methacholine provoking a fall in FEV1 and/or diurnal PEF variability Exclusion criteria Severe or near fatal asthma; current smokers and ex‐smokers > 10 pack‐years; baseline use of LABA; pregnant or lactating women; women of child‐bearing potential not on effective birth control; exacerbation due to chronic sinusitis; hospitalisation in previous 3 months; respiratory tract infection ≤ 1 month before visit 1 |
|
| Interventions |
Run‐in period Three‐ to six‐week period whereby participants using other forms of inhalers were switched to budesonide turbuhaler at an equivalent dose and placed on a twice‐daily dose regimen Study period Control arm: maintenance inhaler of budesonide (100, 200 or 400 mcg BID) + placebo inhaler BID for exacerbations Study arm: maintenance inhaler of budesonide + inhaler with budesonide to double dose of ICS (200, 400 or 800 mcg BID) for exacerbations Other medications allowed Terbutaline sulphate inhaler as rescue medication; theophylline; anticholinergics; nasal corticosteroids |
|
| Outcomes |
Primary outcome The proportion of participants with treatment failure as judged by the need for treatment with oral methylprednisolone or an unscheduled visit to a physician or medical emergency department due to asthma or unstable asthma after 14 days of treatment Secondary outcomes None |
|
| Notes | Funding source: AstraZeneca Canada Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised to treatment groups at visit 2 according to a blocked computer generated randomisation list for each centre" |
| Allocation concealment (selection bias) | Low risk | Central randomisation ‐ assumed that this meant randomisation was separate from those dealing with participant details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind trial" ‐ "The maintenance dose (MD) group received a maintenance inhaler of budesonide dispensing 100, 200, or 400 mg/dose (depending on their maintenance therapy) plus an additional inhaler containing placebo for twice daily use. The double dose (DD) group received the same maintenance inhaler as the first group, but the additional inhaler dispensed 100, 200, or 400 mg/dose of budesonide as well" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Statistical analysis used the "all patients treated" (APT) approach. Since patients were "treated" only if they had an exacerbation, all patients who had at least one asthma exacerbation after randomisation and were treated with at least one dose of additional study drug are included" ‐ Of the 148 randomised to the control group, 115 completed the study (22% dropout), and 117/142 in the intervention group completed the study (17.6% dropout). This was considered relatively low and balanced |
| Selective reporting (reporting bias) | Low risk | The primary outcome and outcomes of interest to this review were well reported. Some secondary outcomes not relevant to our review were presented only graphically. Peak expiratory flow rate data not reported for unforeseen technical reasons. These data were not required as a pre‐defined primary or secondary outcome |
Foresi 2000.
| Methods | This multi‐centre, randomised, double‐blind, parallel‐group study was designed to compare effects of 6‐month treatment with low vs standard dose budesonide in controlling symptoms and lung function in a group of asthmatic patients with moderate asthma previously treated with inhaled beclomethasone Conducted at 14 outpatient clinics in Italy Moreover, a comparison was made between a continued low maintenance dose of budesonide vs a short‐term increase in daily dose at the time of an asthma exacerbation |
|
| Participants |
Population 213 participants were randomised to 3 treatment groups, and 47 participants experienced an exacerbation. Groups 2 and 3 accounted for 36 exacerbations and contributed to the analysis Participants were 18 to 65 years of age. Mean age was 39 years. 47% were male. 70% were non‐smokers, 22% ex‐smokers and 8% smokers Baseline asthma severity Moderate asthma Duration of asthma: 28% < 5 years, 22% 5 to 10 years, 50% > 10 years Mean FEV1: 74% predicted Mean PEFR: 75% predicted 41% taking salmeterol, 17% theophylline Inclusion criteria Age 18 to 65 years; baseline FEV1 ≥ 50% and ≤ 90% of predicted values; daily PEF variability ≥ 20% on at least 4 different days during a 2‐week period; daily requirement of inhaled β2 agonist; presence of wheeze, cough, chest tightness, shortness of breath that interfered with normal daily activity during a 2‐week pre‐study observation period Exclusion criteria Treatment with a high dose of beclomethasone (> 1000 mcg/d); history of seasonal asthma |
|
| Interventions |
Run‐in period Four‐week pre‐study treatment period whereby participants were asked to inhale budesonide 800 mcg twice daily Study period Control arm (Group 3): maintenance inhaler of budesonide 100 mcg BID + placebo inhaler QID for exacerbations Study arm (Group 2): maintenance inhaler of budesonide 100 mcg BID + budesonide 200 mcg QID for exacerbations Other medications allowed Inhaled β2 agonist; LABA; theophylline; anticholinergics |
|
| Outcomes |
Primary outcome: not specified Secondary outcomes
|
|
| Notes | Funding source: Astra Farmaceutici | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Seven patients were withdrawn during the run‐in phase and four patients just after randomization. Thus, the intention‐to‐treat analysis was based on 209 patients." "A group of 22 patients discontinued their treatment: 10 patients were lost at follow‐up, 4 patients for adverse events and 8 patients for other reasons. Therefore, out of 213 randomised patients, a group of 191 patients completed the study." "Protocol violations were detected in 38 patients. Thus the per‐protocol analysis was performed on 175 patients: 56 patients were included in group 1, 55 patients in group 2, and 64 in group 3." Dropout was not given per group but the overall dropout was low (10.6%) and the ITT group included 98% of those randomised. |
| Selective reporting (reporting bias) | Unclear risk | The study did not report many of the outcomes of interest for this review but there was no evidence to suggest selective reporting based on the list of outcomes given in the paper, although no trial registration could be found to check what was stated in advance. |
Garrett 1998.
| Methods | This single‐centre, randomised, double‐blind, placebo‐controlled, cross‐over trial investigated the efficacy of an increased dose of inhaled corticosteroid used within the context of an asthma self management plan for treating exacerbations of asthma | |
| Participants |
Population Participants were recruited from a paediatric outpatient department, a department of respiratory medicine and a local general practice 28 participants were randomised and 18 pairs of exacerbations were available for analysis The analysis sample revealed participants 6 to 14 years old with a mean age of 8.2 years. 67% were male Baseline asthma severity Mild to moderate severity Mean FEV1: 99% predicted Mean PEFR: 100% predicted Inclusion criteria Age 6 to 14 years; currently taking inhaled corticosteroid prophylaxis (not exceeding 800 mcg/d) Exclusion criteria Taking oral corticosteroids, sodium cromoglycate or LABA; any previous ICU admission, recent inpatient care for asthma or any change in dose of inhaled corticosteroids in the past 2 months; any concurrent illness |
|
| Interventions |
Run‐in period Two‐week run‐in period during which participants were required to use beclomethasone via MDI and spacer and a salbutamol MDI. Participants previously taking budesonide were switched to beclomethasone, but the child's daily dose was not changed Study period Sequence 1: maintenance beclomethasone inhaler (< 800 mcg/d) + placebo inhaler for exacerbation 1, followed by maintenance beclomethasone inhaler + inhaler with beclomethasone to double dose of ICS for exacerbation 2 Sequence 2: maintenance beclomethasone inhaler + inhaler with beclomethasone to double dose of ICS for exacerbation 1. Maintenance beclomethasone inhaler (< 800 mcg/d) + placebo inhaler for exacerbation 2 Other medications allowed Salbutamol MDI |
|
| Outcomes |
Primary outcome: not specified Secondary outcomes
|
|
| Notes | Funding source: New Zealand Asthma Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After stratification for age and sex, the children were randomised by the hospital pharmacist to one of two possible treatment sequences for serial exacerbations, placebo then steroid, or steroid then placebo." |
| Allocation concealment (selection bias) | Low risk | ''By the hospital pharmacist" implies allocation was not done by those conducting the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, placebo‐controlled". "The investigators were blinded to this allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of any children dropping out of the trial. "Each child acted as their own control in a crossover design, and only children who had exacerbations in both treatment phases were included in the main analyses." |
| Selective reporting (reporting bias) | Low risk | The study reported the outcomes stated in the methods but there was no trial registration to check that they were consistent. There was no clear evidence of selective reporting. |
Harrison 2004.
| Methods | This single‐centre, randomised, placebo‐controlled, parallel‐group study investigated whether doubling the dose of inhaled corticosteroid when asthma control starts to deteriorate reduces the number of patients needing prednisolone, and sought to establish effects on the severity and duration of the subsequent exacerbation | |
| Participants |
Population Participants were recruited from local general practices and the asthma research register 390 participants were randomised; 207 experienced an exacerbation and contributed to the analysis Participants were 16 years or older. Mean age was 49 years. 33% were male. 3% were smokers, 36% ex‐smokers and 61% never‐smokers Baseline asthma severity Mean ICS dose: 710 mcg Mean FEV1: 2.4 L or 80% predicted Mean PEFR: 384 L/min Mean symptom score (range 0 to 7): 0.5 35% on LABA 55% required oral corticosteroids, 42% doubled inhaled corticosteroids and 2% did both in the previous 12 months to treat or prevent asthma exacerbation Inclusion criteria Age ≥ 16 years; clinical diagnosis of asthma; taking an inhaled corticosteroid (100 to 2000 mcg/d) on a regular basis; previous course of oral corticosteroids or doubled dose of inhaled corticosteroid in the previous 12 months for treatment or prevention of an asthma exacerbation Exclusion criteria History of smoking > 10 pack‐years; unstable asthma during a 2‐week run‐in period |
|
| Interventions |
Run‐in period Two‐week period whereby participants continued their usual dose of inhaled corticosteroid and recorded morning peak flow and daytime symptom scores to ensure asthma stability Study period Control arm: maintenance inhaled corticosteroid (100 to 2000 mcg/d) + identical placebo inhaler for exacerbations Study arm: maintenance inhaled corticosteroid (100 to 2000 mcg/d) + identical inhaler with corticosteroid to double dose of ICS for exacerbations Participants were to use study inhaler for 14 days in addition to usual treatment when peak flow or symptoms deteriorated Other medications allowed Not specified |
|
| Outcomes |
Primary outcome Proportion of individuals who needed prednisolone in each group Secondary outcomes
|
|
| Notes | Funding source: NHS Executive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent pharmacist randomly allocated individuals to active or placebo inhalers using computer‐generated random number tables" |
| Allocation concealment (selection bias) | Low risk | "independent pharmacist" implies allocation was not done by those conducting the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose"" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8.9% and 10.1% withdrew from intervention and placebo groups, respectively, which is relatively low and balanced. However, statistical analyses state, "Our analysis was by intention‐to‐treat and per protocol (i.e. patients who used their study inhaler as instructed before starting prednisolone)", leaving it unclear which was the primary and how missing data were imputed |
| Selective reporting (reporting bias) | Low risk | The study reported outcomes stated in the methods; several were required by our review. However, again no trial registration was available to confirm that outcome reporting was consistent with what was stated in the protocol. We found no clear evidence of selective reporting |
Martinez 2011.
| Methods | This 44‐week, randomised, double‐blind, placebo‐controlled, 4‐treatment trial used a 2‐by‐2 factorial design (2 arms were not relevant to the review and were not included) The study was conducted at 5 clinical centres in the USA |
|
| Participants |
Population Participants were recruited from 5 clinical centres 288 were randomised to 1 of 4 groups, of which 143 contributed to this analysis (71 combined group, 72 daily group) Aged between 5 and 18 years. Mean age was 11.2. 56.6% were male Baseline asthma severity Mean dose of budesonide: NR (≤ 160 μg daily equivalent) Mean FEV1 (pre‐BD): 101.5 (11.7) active, 100.1 (10.8) control Mean PEFR: 321.0 (113.1) active, 301.8 (125.9) control Approx 5% were taking long‐acting beta‐agonists. In the previous year, 82% had taken ICS, 10% had taken a leukotriene inhibitor, 1% had taken salmeterol and none had taken theophylline or sodium cromoglycate. Participants were required to have had 1 or 2 exacerbations in the previous year Inclusion criteria Children and adolescents 6 to 18 years of age, history of mild persistent asthma during the previous 2 years, qualified for interruption or discontinuation of controller treatment because their illness was well controlled (as defined in US National Asthma Education and Prevention Program asthma care guidelines), naive to controller treatment with a history of 1 to 2 exacerbations in the previous year, those treated for the previous 8 weeks with monotherapy other than inhaled corticosteroids, and those whose illness was controlled for the previous 8 weeks on low‐dose corticosteroids as monotherapy (≤ 160 mcg daily with a beclomethasone equivalent) Exclusion criteria Pre‐bronchodilator FEV1 < 60% predicted at the first visit; admitted to hospital for asthma in the previous year; any asthma exacerbation in the previous 3 months or more than 2 in the previous year; history of life‐threatening asthma exacerbations that required intubation or mechanical ventilation, or that resulted in a hypoxic seizure |
|
| Interventions |
Run‐in period Four‐week run‐in period, during which participants received twice‐daily treatment with 1 puff of beclomethasone dipropionate and rescue treatment with a placebo inhaler added to rescue albuterol every time they needed albuterol Study period Control arm: maintenance inhaler of beclomethasone 40 mcg BID + placebo BID inhaler and albuterol as rescue for exacerbations Study arm: maintenance inhaler of beclomethasone 40 mcg BID + 40 mg beclomethasone BID and albuterol as rescue for exacerbations (combined group) Other medications allowed Low‐dose ICS or other monotherapy in previous 8 weeks. ICS > 160 mcg beclomethasone equivalent not allowed (daily beclomethasone group) Definition of exacerbation: use of more than 12 puffs of albuterol in 24 hours (excluding preventive use before exercise), peak expiratory flow < 70% of consecutive days, peak expiratory flow < 50% of reference value despite relief treatment, emergency room visit due to worsening of asthma symptoms |
|
| Outcomes |
Primary outcome Time to first exacerbation that required treatment with prednisone Secondary outcomes Spirometry FEV1, fractional exhaled nitric oxide (FENO), symptom diaries and control and quality of life questionnaires, linear growth |
|
| Notes |
Funding source: grants from the National Heart, Lung and Blood Institute; TEVA Pharmaceutical Industries Ltd provided beclomethasone dipropionate‐HFA and placebo Study identifiers: TREXA, NCT00394329 The study used a factorial design, which had implications for the independence of treatments and subsequent analysis of results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation sequence, stratified by clinical centre and age group, was used to randomly assign participants to one of four treatment groups" ‐ "The Data Coordinating Center (DCC; Penn State Hershey College, PA, USA) generated the random allocation sequence" |
| Allocation concealment (selection bias) | Low risk | "The DCC had no interaction with participants, but was responsible for management of data and statistical analyses" ‐ "A pharmaceutical vendor was selected to package, code, and ship the drug packets to each clinical centre. When a clinical centre deemed that a participant was eligible for randomisation, the clinical centre coordinator logged onto the secure CARE Network website, entered the relevant information to confirm participant eligibility, and received the appropriate drug packet code to be assigned to the participant" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind, placebo‐controlled trial" ‐ "Drug groups were labelled as A, B, C, and D to mask statisticians to treatment group during the first complete run‐through of data analyses" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals from the study were relatively low and even between the 2 groups included in this review: 11.3% in the intervention group (double ICS) and 12.5% in the control group (stable ICS) |
| Selective reporting (reporting bias) | Low risk | The study was prospectively registered (NCT00394329) and results were well reported in accordance with the protocol |
Oborne 2009.
| Methods | This randomised, double‐blind, placebo‐controlled, parallel‐group study investigated whether a 4‐fold increase in the dose of inhaled corticosteroids, started when asthma control deteriorates, can prevent the need for oral corticosteroids | |
| Participants |
Population 403 participants were randomised and 94 participants experienced an exacerbation, for a total of 121 exacerbations contributed to the analysis Participants 16 years of age or older. Mean age was 56 years. 32% of participants were male. 10% were smokers, 21% ex‐smokers and 69% never‐smokers Baseline asthma severity Mean ICS dose: 520 mcg Mean FEV1: 2.2 L or 82% predicted Mean PEFR: 380 L/min Inclusion criteria: age > 16 years, stable asthma, treated with ICS (200 to 1000 mcg budesonide or equivalent), taken a course of oral corticosteroid or doubled dose of ICS in the previous 12 months but not in the preceding 4 weeks Exclusion criteria: > 20 pack‐year smoking history, other clinically significant medical conditions, pregnant or lactating |
|
| Interventions |
Run‐in period: 2‐week period whereby participants continued their usual dose of inhaled corticosteroid and recorded morning peak flow and daytime symptom scores to ensure asthma stability Study period Control arm: maintenance inhaled corticosteroid (200 to 1000 mcg/d) + identical placebo inhaler for exacerbations Study arm: maintenance inhaled corticosteroid (200 to 1000 mcg/d) + identical inhaler with corticosteroid to quadruple dose of ICS for exacerbations Participants were to use study inhaler for 14 days in addition to usual treatment when peak flow or symptoms deteriorated Other medications allowed Not specified |
|
| Outcomes |
Primary outcome Number of partcipants who had exacerbations of asthma treated with oral corticosteroids (ITT analysis) Secondary outcomes Number of participants who started the study inhaler and went on to require treatment with oral corticosteroids (per‐protocol or modified ITT analysis) |
|
| Notes | Funding source: Asthma UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An allocation sequence of random permuted blocks of 10 was generated using a random number table by an independent pharmacist and implemented by one of the study investigators once participants were enrolled into the trial" |
| Allocation concealment (selection bias) | Low risk | Independent pharmacist randomly allocated individuals; "The authors thank…Sarah Pacey for providing the randomization schedule and concealed allocation of masked inhalers" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind, placebo‐controlled trial" ‐ "Drug groups were labelled as A, B, C, and D to mask statisticians to treatment group during the first complete run‐through of data analyses" ‐ "Active and placebo inhalers were…identical apart from the presence or absence of inhaled corticosteroid, to achieve allocation concealment and blinding of investigators and participants" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Thirty‐eight (19.3%) and 39 participants (18.9%) in the active and placebo groups withdrew from the study but contributed data for the intention‐to‐treat analysis up to the point at which they left the study. All participants received their allocated intervention, although 3 were lost to follow‐up with no outcome data (all in the placebo group), leaving 197 and 203 participants in the groups receiving active and placebo inhalers, respectively, for the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes reported match those stated in the prospectively registered protocol and are relevant to the review |
Rice‐McDonald 2005.
| Methods | This single‐centre, randomised, double‐blind, placebo‐controlled, cross‐over study was conducted in Australia. The duration of each phase was unclear | |
| Participants |
Population 22 participants were randomised; 18 experienced an exacerbation in both phases and contributed to the analysis Participant mean age 46.5. 40.9% were male Baseline asthma severity Mean dose of budesonide: not reported Mean FEV1: 73% predicted Mean PEFR: not reported Inclusion criteria: consenting adults ≥ 18 years of age; physician diagnosed asthma; reversible airways obstruction evidenced by (i) ≥ 15% reversibility in FEV1 or (ii) ≥ 20% variability in PEF over the 2‐ to 4‐week run‐in period (% variability defined as highest PEF–lowest PEF/highest PEF 3100); assessment by investigator that ongoing treatment with ICS was appropriate; participant did not meet any exclusion criteria Exclusion criteria: mild asthma when exacerbations with PEF < 80% of best were thought to be unlikely during the course of the study; demonstration by potential volunteers of erroneous or falsified PEF entries during a 2– to 4‐week reliability check; reliability was determined by comparison of self recorded PEF with actual PEF as recorded on personal Vitalograph 2110 Electronic PEF/FEV1 Diaries (Vitalograph, Buckingham, UK); participants were unaware that the Diaries recorded all PEF values; asthma requiring continuous oral steroids or immunosuppressive‐type therapies; concomitant use of long‐acting beta agonists, theophylline or leukotriene receptor antagonists did not exclude participants from participation |
|
| Interventions |
Run‐in period: Two‐ to 4‐week run‐in period to ensure inclusion criteria, demonstrate competence in taking ICS via spacer and ensure that asthma was stable Study period Control phase: maintenance ICS inhaler (usual type/dose) + same number of placebo inhalations for 14 days during exacerbations Study phase: maintenance ICS inhaler (usual type/dose) + same number of ICS inhalations for 14 days during exacerbations Participants also received placebo oral steroids for 7 days during these phases and their usual SABA inhaler Other medications allowed: concomitant use of long‐acting beta agonists, theophylline or leukotriene receptor antagonists was not exclusionary |
|
| Outcomes | Treatment failure rates; PEF at endpoint; adverse events. The endpoint was assessed at 7 days if no treatment failure, or at time of treatment failure in the event of failure Outcomes were not defined as primary and secondary |
|
| Notes | Funding source: Asthma Foundation of Queensland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "order and allocation of treatment by concealed randomisation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, double dummy" design ‐ placebos were used to blind each of the study medications. "Participants were then given the first of three treatment packs in a concealed randomised order" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 35 randomised participants, 13 subsequently withdrew before any asthma exacerbations because of: (i) personal choice (6); (ii) inadequate compliance (5); (iii) development of disease unrelated to asthma (1); and (iv) relocation precluding continuation in the study (1). Baseline characteristics of the 22 participants contributing data and described subsequently are found in Table 1 |
| Selective reporting (reporting bias) | Low risk | The study reported outcomes stated in the methods; several were those required by our review. However, again no trial registration was available to confirm that outcome reporting was consistent with what was stated in the protocol. We found no clear evidence of selective reporting |
Wainwright 2009.
| Methods | This 12‐month, randomised, double‐blind, placebo‐controlled, parallel study compared continued maintenance dose of inhaled corticosteroid vs doubled dose at the time of childhood asthma exacerbations. It was conducted at 8 centres in south east Queensland | |
| Participants |
Population 251 children were randomised; 187 participants experienced an exacerbation and contributed to the analysis 38% of children were 3 to 5 years of age, 43% between 6 and 11 and 19% between 12 and 14 years. 60% were male Baseline asthma severity Mean dose of ICS: minimum 125 mcg fluticasone/d Mean FEV1: not reported Mean PEFR: not reported (other severity metrics, e.g. baseline ICS requirement or exacerbation frequency) Inclusion criteria: informed consent obtained from parent/carer and assent from child when possible. age between 3 and 14 years, doctor diagnosis of asthma and taking regular ICS (minimum 125 mcg fluticasone/d), at least 1 exacerbation in previous 12 months requiring admission to hospital, presentation to emergency department + use of oral steroids Exclusion criteria: children with co‐morbidities that may affect growth; children with other respiratory illness; unable to obtain informed consent; unable to speak English |
|
| Interventions |
Run‐in period: 3‐month run‐in period including 2 weeks of peak flow measurement Study period Control arm: maintenance fluticasone inhaler at child's usual dose + placebo inhaler to keep dose stable during exacerbations Study arm: maintenance fluticasone inhaler at child's usual dose + study puffer to double dose during exacerbations. Continued until back to baseline Other medications allowed: not reported |
|
| Outcomes |
Primary outcome: use of oral steroid rescue and admission to hospital Secondary outcomes: growth over 12 months; time off work for parents, school for children; time for peak flow to return to baseline |
|
| Notes |
Funding source: Asthma Foundation Queensland; RCH Foundation Brisbane; fluticasone propionate, placebo and peak flow meters provided by GlaxoSmithKline Study identifier: ACTRN12605000631606 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre" ‐ "Sequential study number allocated from a list according to blocking details" |
| Allocation concealment (selection bias) | Low risk | "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne" ‐ "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but not explicitly described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 in the intervention group (8.7%) and 10 in the control group (8.1%) withdrew from the study ‐ low and balanced. Only those having an exacerbation were included in the main analyses. No information about whether an ITT analysis was undertaken and, if so, how missing data were imputed |
| Selective reporting (reporting bias) | Low risk | The study has not been fully published yet, but it was prospectively registered and study authors were able to provide us with data for the outcomes of interest |
BID = twice a day. d = day. FEF = forced expiratory flow. FENO = fractional exhaled nitric oxide. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. HFA = ICS = inhaled corticosteroids. ICU = intensive care unit. ITT = intention‐to‐treat. LABA = long‐acting beta agonist. LTRA = leukotriene receptor antagonist. MDI = metered dose inhaler. PEF = peak expiratory flow. PEFR = peak expiratory flow rate. QID = four times a day.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bateman 2008 | Comparison of 2 doses of ciclesonide; not placebo‐controlled; uncontrolled asthma at baseline |
| Boushey 2005 | Budesonide vs LTRA for mild persistent asthma |
| Brand 2011 | ICS stopped during run‐in; therefore no baseline ICS |
| Bullard 1996 | Systemic corticosteroids vs placebo for COPD, not asthma exacerbations |
| Clearie 2010 | Stopped ICS for 2 weeks before trial. Not focused on exacerbations |
| Condemi 1999 | Low‐ vs high‐dose ICS; not placebo‐controlled; uncontrolled asthma at baseline |
| Connett 1993 | No use of ICS at baseline |
| Currie 2003 | Salmeterol‐fluticasone vs fluticasone for uncontrolled asthma (not exacerbations) |
| De Benedictis 2005 | Nebulised fluticasone vs budesonide; not placebo‐controlled; no use of ICS at baseline |
| Devidayal 1999 | Nebulised budesonide vs oral prednisone; not placebo‐controlled; no use of ICS at baseline |
| Fitzgerald 2000 | Use of systemic corticosteroids first; not placebo‐controlled |
| Greening 1994 | BDP + salmeterol vs high‐dose BDP; not placebo‐controlled; uncontrolled asthma at baseline |
| GSK 2005 | Not placebo‐controlled; uncontrolled asthma at baseline |
| Hedlin 1999 | Inhaled budesonide vs oral betamethasone; not placebo‐controlled |
| Heinig 1999 | Budesonide vs fluticasone; not placebo‐controlled; uncontrolled asthma at baseline |
| Karpel 2007 | Severe persistent asthma (not exacerbations); participants on oral corticosteroids at baseline |
| La Rosa 1997 | Salbutamol‐flunisolide vs salbutamol; not placebo‐controlled |
| Lee‐Wong 2002 | Inhaled flunisolide vs systemic corticosteroids following IV corticosteroids; not placebo‐controlled |
| Lemanske 2010 | Three step‐up options and no stable arm. ICS increased but not in response to exacerbation |
| Leuppi 2002 | Unstable dose of ICS (dose reduction) before exacerbation |
| Levy 1996 | Fluticasone vs oral prednisolone; not placebo‐controlled; not all participants on ICS at baseline |
| Manjra 2000 | Nebulised fluticasone vs oral prednisolone; not placebo‐controlled; not all participants on ICS at baseline |
| Matz 2001 | Salmeterol‐fluticasone vs high‐dose fluticasone for stable asthma (not exacerbations) |
| Milani 2004 | No use of ICS at baseline |
| Nana 1998 | Inhaled budesonide vs oral prednisolone; not placebo‐controlled |
| Nuhoglu 2001 | No use of ICS at baseline |
| O'Connor 2010 | ICS given but not in response to exacerbation |
| Pedersen 2009 | ICS given but not in response to exacerbation |
| Razi 2008 | Two dosing regimens of nebulised budesonide; not placebo‐controlled |
| Rodrigo 1998 | No use of ICS at baseline |
| Rodrigo 2005 | Inhaled fluticasone vs IV hydrocortisone; no use of ICS at baseline |
| Schuh 2000 | Inhaled fluticasone vs oral prednisolone; not all participants on ICS at baseline |
| Schuh 2006 | Inhaled fluticasone vs oral prednisolone; not all participants on ICS at baseline |
| Sekerel 2005 | Not all participants on ICS at baseline |
| Singhi 1999 | Not all participants on ICS at baseline |
| Svedmyr 1995 | ICS started at onset of URTI but not a confirmed asthma exacerbation; no ICS use at baseline |
| Volovitz 1998 | Inhaled budesonide vs oral prednisone; not placebo‐controlled; no ICS use at baseline |
| Wilson 1990 | Not all participants on ICS at baseline |
| Yousef 2012 | No stable ICS arm |
BDP = beclomethasone dipropionate. COPD = chronic obstructive pulmonary disease. ICS = inhaled corticosteroids. IV = intravenous. LTRA = leukotriene receptor agonists. URTI = upper respiratory tract infection.
Characteristics of ongoing studies [ordered by study ID]
NCT02066129.
| Trial name or title | Step‐up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | This is a double‐blind, parallel‐group trial, including a total of 250 participants, 5 to 11 years of age, with a diagnosis of asthma and a history of at least 1 asthma exacerbation treated with oral corticosteroids in the prior year |
| Interventions | All participants will be treated for 48 weeks with open‐label fluticasone 44 mcg 2 puffs twice daily. During the 48‐week treatment period, participants will receive randomised blinded therapy for 7 days each time they enter the "yellow zone" (at the onset of symptoms previously associated with upper respiratory illnesses and subsequent asthma exacerbations). Yellow zone therapy will be fluticasone 44 or 220 mcg 2 puffs twice daily |
| Outcomes | The primary outcome is listed as the rate of severe asthma exacerbations treated with oral corticosteroids during the 48‐week treatment period |
| Starting date | July 2014 |
| Contact information | David Mauger, PhD (dtm5@psu.edu) |
| Notes |
Differences between protocol and review
The data synthesis section of our protocol initially read as follows "we will report fixed‐effect rate ratios, such as the rate of the need for rescue systemic corticosteroids per person‐years of follow up in treatment and control groups". We have made two changes to this statement. We used the fixed‐effect model if we found no significant heterogeneity, otherwise we used the random‐effects model. As we could not obtain individual participant level data, we could not calculate event rates; therefore we defaulted to comparing frequency of events (numbers of participants requiring rescue systemic corticosteroids in treatment and control groups).
We did not discuss in the protocol unit of analysis issues for parallel and cross‐over studies. We did not pool parallel and cross‐over studies in previous versions, but we were able to pool them in this update. For dichotomous outcomes, we pooled studies using Mantel‐Haenszel (M‐H) odds ratios unless investigators reported a low number of events requiring Peto odds ratios. In the previous version, we obtained the marginal odds ratio from the cross‐over trial by comparing the number of participants who needed increased doses of oral corticosteroids (but not receiving placebo) with the number needing oral corticosteroids while receiving placebo (but not given double‐dose ICS). In this version, we included cross‐over data by obtaining these two‐by‐two data and applying a formula to account for inter‐correlation of matched pairs (Elbourne 2002).
The previous version of this review included a funnel plot, which we did not include this time, as the protocol stated that this would be done only if more than 10 trials were included.
For the primary outcome, we changed the denominator from the number of participants requiring study inhaler to the number of participants randomised, consistent the intention‐to‐treat analysis. As a secondary outcome, we re‐analysed treatment failure on the basis of the number of participants requiring the study inhaler.
We added magnitude of ICS dose increase (two‐fold vs four‐fold) as a subgroup analysis. Post hoc subgroup analyses were previously performed on the modified intention‐to‐treat analysis of the primary outcome, but this was not repeated for the current update.
For this update, we defined the sensitivity analyses to clarify which studies would be removed from the primary analysis. The meaning of the study design and the source of study funding did not change, but we chose to remove studies at high risk of selection bias from the methodological quality sensitivity analysis. We considered this to be an important factor related to bias in these studies, as inadequate allocation procedures could have resulted in unbalanced groups, which would have had an important effect on the numbers having exacerbations for each treatment.
We encountered uncertainty about the number of participants included in analyses, especially for dichotomous outcomes. For this reason, we took the following approach, which we added to the methods: "For dichotomous outcomes, where we did not know whether the number of events were for the entire population or only those taking the study inhaler, we used the total number randomised per group as the denominator. We performed sensitivity analyses using the number taking their study inhaler as the denominator to test this assumption."
We extended the definition of serious adverse events in the list of outcomes to include prolongation of hospitalisation or disability as the standard definition. We also noted in the analysis whether definitions used within studies differed.
In Types of participants, we extended the definition of exacerbations to include a set of criteria pre‐defined in the included studies, because guidelines were not always cited, but it was clear that a list of criteria had to be met before the study medication could be initiated.
Contributions of authors
KK: lead for the 2015 update. Sift and study selection, data extraction, analysis, risk of bias and GRADE assessment, write‐up.
MQ: review work for the 2015 update. Sift and study selection, data extraction, analysis, risk of bias assessment, write‐up.
BQ: protocol development, study assessment, data extraction and write‐up of the previous version, critical appraisal of this update.
FD: protocol development; interpretation of data, write‐up and editorial sign‐off of the previous version, critical review of data interpretation and write‐up of this update.
Sources of support
Internal sources
-
St George's, University of London, UK.
Kayleigh Kew was supported by her employer, St George's, University of London
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Kayleigh Kew: none.
Michael Quinn: none.
Bradley Quon: none.
Francine Ducharme: grant support for investigator‐initiated studies from Merck and Co., unrestricted donations from Merck, GlaxoSmithKline, and Takeda, to support an electronic database of children consulting for asthma; member of the advisory boards of Boehringer Ingelheim.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Fitzgerald 2004 {published data only}
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J, et al. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 2004;59(7):550‐6. [PUBMED: 15223858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foresi 2000 {published data only}
- Foresi A, Morelli MC, Catena E. Low‐dose budesonide with the addition of an increased dose during exacerbations is effective in long‐term asthma control. Chest 2000;117(2):440‐6. [PUBMED: 10669688] [DOI] [PubMed] [Google Scholar]
Garrett 1998 {published data only}
- Garrett J, Williams S, Wong C, Holdaway D. Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid. Archives of Disease in Childhood 1998;79(1):12‐7. [PUBMED: 9771245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2004 {published data only}
- Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004;363(9405):271‐5. [PUBMED: 14751699] [DOI] [PubMed] [Google Scholar]
Martinez 2011 {published data only}
- Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double‐blind, placebo‐controlled trial. Lancet 2011;377:650‐7. [PUBMED: 21324520] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oborne 2009 {published data only}
- Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double blind, placebo controlled, parallel group, clinical trial. American Journal of Respiratory and Critical Care Medicine 2009;180(7):598‐602. [PUBMED: 19590019] [DOI] [PubMed] [Google Scholar]
Rice‐McDonald 2005 {published data only}
- Rice‐McDonald G, Bowler S, Staines G, Mitchell C. Doubling daily inhaled corticosteroid dose is ineffective in mild to moderately severe attacks of asthma in adults. Internal Medicine Journal 2005;35(12):693‐8. [PUBMED: 16313543] [DOI] [PubMed] [Google Scholar]
Wainwright 2009 {unpublished data only}
- Wainwright C. A multicentre randomised controlled trial of treatment of acute exacerbations of asthma in children with a doubling of the usual inhaled corticosteroid (ICS) dose to identify efficacy of intervention. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630 (accessed 18 June 2015).
References to studies excluded from this review
Bateman 2008 {published data only}
- Bateman ED, Cheung D, Lapa e Silva J, Göhring UM, Schäfer M, Engelstätter R. Randomized comparison of ciclesonide 160 and 640 mug/day in severe asthma. Pulmonary Pharmacology and Therapeutics 2008;21(3):489‐98. [PUBMED: 18178494] [DOI] [PubMed] [Google Scholar]
Boushey 2005 {published data only}
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as‐needed corticosteroids for mild persistent asthma. New England Journal of Medicine 2005;352(15):1519‐28. [PUBMED: 15829533] [DOI] [PubMed] [Google Scholar]
Brand 2011 {published data only}
- Brand PL, Luz Garcia‐Garcia M, Morison A, Vermeulen JH, Weber HC, Brand PLP. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105(11):1588‐95. [DOI] [PubMed] [Google Scholar]
Bullard 1996 {published data only}
- Bullard MJ, Liaw SJ, Tsai YH, Min H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. American Journal of Emergency Medicine 1996;14(2):139‐43. [PUBMED: 8924134] [DOI] [PubMed] [Google Scholar]
Clearie 2010 {published data only}
- Clearie KL. Airway challenges in different clinical phenotypes and their relationship to markers of disease and treatment [PhD thesis]. Dundee, UK: University of Dundee, 2010. [Google Scholar]
Condemi 1999 {published data only}
- Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Annals of Allergy, Asthma and Immunology 1999;82(4):383‐9. [PUBMED: 10227337] [DOI] [PubMed] [Google Scholar]
Connett 1993 {published data only}
- Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Archives of Disease in Childhood 1993;68(1):85‐7. [PUBMED: 8435016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Currie 2003 {published data only}
- Currie GP, Bates CE, Lee DK, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. European Journal of Clinical Pharmacology 2003;59(1):11‐5. [PUBMED: 12743669] [DOI] [PubMed] [Google Scholar]
De Benedictis 2005 {published data only}
- Benedictis FM, Giudice MM, Vetrella M, Tressanti F, Tronci A, Testi R, et al. Nebulized fluticasone propionate vs. budesonide as adjunctive treatment in children with asthma exacerbation. Journal of Asthma 2005;42(5):331‐6. [PUBMED: 16116682] [DOI] [PubMed] [Google Scholar]
Devidayal 1999 {published data only}
- Devidayal, Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatrica 1999;88(8):835‐40. [PUBMED: 10503681] [DOI] [PubMed] [Google Scholar]
Fitzgerald 2000 {published data only}
- FitzGerald JM, Shragge D, Haddon J, Jennings B, Math JLM, Bai T, et al. A randomized, controlled trial of high dose, inhaled budesonide versus oral prednisone in patients discharged from the emergency department following an acute asthma exacerbation. Canadian Respiratory Journal 2000;7(1):61‐7. [PUBMED: 10700672 ] [DOI] [PubMed] [Google Scholar]
Greening 1994 {published data only}
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher‐dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344(8917):219‐24. [PUBMED: 7913155] [DOI] [PubMed] [Google Scholar]
GSK 2005 {published data only}
- GlaxoSmithKline. A multicentre, double‐blind, randomised, four‐week, parallel‐group comparison of inhaled fluticasone propionate and inhaled beclomethasone dipropionate in patients with severe asthma controlled by high‐dose inhaled corticosteroids (FLIP04). http://www.gsk‐clinicalstudyregister.com/study/FMS30049#rs (accessed 17 December 2015).
Hedlin 1999 {published data only}
- Hedlin G, Svedmyr J, Ryden AC. Systemic effects of a short course of betamethasone compared with high‐dose inhaled budesonide in early childhood asthma. Acta Paediatrica 1999;88(1):48‐51. [PUBMED: 10090547 ] [DOI] [PubMed] [Google Scholar]
Heinig 1999 {published data only}
- Heinig JH, Boulet LP, Croonenborghs L, Mollers MJ. The effect of high‐dose fluticasone propionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respiratory Medicine 1999;93(9):613‐20. [PUBMED: 10542974] [DOI] [PubMed] [Google Scholar]
Karpel 2007 {published data only}
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628‐37. [PUBMED: 16875813] [DOI] [PubMed] [Google Scholar]
La Rosa 1997 {published data only}
- Rosa M, Ranno C, Mandair G, Barbato A, Biraghi M. Double‐blind study of inhaled salbutamol versus salbutamol plus high‐dose flunisolide in exacerbation of bronchial asthma: a pilot study. Pediatric Asthma, Allergy and Immunology 1997;11(1):23‐30. [EMBASE: 1997208440] [Google Scholar]
Lee‐Wong 2002 {published data only}
- Lee‐Wong M, Dayrit FM, Kohli AR, Acquah S, Mayo PH. Comparison of high‐dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest 2002;122(4):1208‐13. [PUBMED: 12377843] [DOI] [PubMed] [Google Scholar]
Lemanske 2010 {published data only}
- Lemanske R, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step‐up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. New England Journal of Medicine 2010;362(11):975‐85. [DOI: 10.1056/NEJMoa1001278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leuppi 2002 {published data only}
- Leuppi JD, Downie SR, Salome CM, Jenkins CR, Woolcock AJ. A single high dose of inhaled corticosteroids: a possible treatment of asthma exacerbations. Swiss Medical Weekly 2002;132(1‐2):7‐11. [PUBMED: 11901445] [DOI] [PubMed] [Google Scholar]
Levy 1996 {published data only}
- Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax 1996;51(11):1087‐92. [PUBMED: 8958890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manjra 2000 {published data only}
- Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respiratory Medicine 2000;94(12):1206‐14. [PUBMED: 11192957] [DOI] [PubMed] [Google Scholar]
Matz 2001 {published data only}
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low‐dose fluticasone versus higher‐dose fluticasone: an analysis of asthma exacerbations. Journal of Allergy and Clinical Immunology 2001;107(5):783‐9. [PUBMED: 11344343] [DOI] [PubMed] [Google Scholar]
Milani 2004 {published data only}
- Milani GK, Rosario Filho NA, Riedi CA, Figueiredo BC. Nebulized budesonide to treat acute asthma in children. Jornal de Pediatria 2004;80(2):106‐12. [EMBASE: 2006186583] [PubMed] [Google Scholar]
Nana 1998 {published data only}
- Nana A, Youngchaiyud P, Charoenratanakul S, Boe J, Lofdahl C, Selroos O, et al. High‐dose inhaled budesonide may substitute for oral therapy after an acute asthma attack. Journal of Asthma 1998;35(8):647‐55. [PUBMED: 9860085] [DOI] [PubMed] [Google Scholar]
Nuhoglu 2001 {published data only}
- Nuhoglu Y, Bahceciler NN, Barlan IB, Mujdat Basaran M. The effectiveness of high‐dose inhaled budesonide therapy in the treatment of acute asthma exacerbations in children. Annals of Allergy, Asthma, and Immunology 2001;86(3):318‐22. [PUBMED: 11289332] [DOI] [PubMed] [Google Scholar]
O'Connor 2010 {published data only}
- O'Connor BJ, Kilfeather S, Cheung D, Kafé H, Blagden MD, Schlösser N, et al. Efficacy and safety of ciclesonide in patients with severe asthma: a 12‐week, double‐blind, randomized, parallel‐group study with long‐term (1‐year) follow‐up. Expert Opinion on Pharmacotherapy 2010;11(17):2791‐803. [DOI: 10.1517/14656566.2010.526603] [DOI] [PubMed] [Google Scholar]
Pedersen 2009 {published data only}
- Pedersen S, Engelstätter R, Weber HJ, Hirsch S, Barkai L, Emeryk A, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulmonary Pharmacology and Therapeutics 2009;22(3):214‐20. [DOI] [PubMed] [Google Scholar]
Razi 2008 {published data only}
- Razi CH, Turktas I, Bakirtas A. Comparison of single 2000‐microg dose treatment vs. sequential repeated‐dose 500‐microg treatments with nebulized budesonide in acute asthma exacerbations. Annals of Allergy, Asthma, and Immunology 2008;100(4):370‐6. [PUBMED: 18450124] [DOI] [PubMed] [Google Scholar]
Rodrigo 1998 {published data only}
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):698‐703. [PUBMED: 9517578] [DOI] [PubMed] [Google Scholar]
Rodrigo 2005 {published data only}
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(11):1231‐6. [PUBMED: 15764724] [DOI] [PubMed] [Google Scholar]
Schuh 2000 {published data only}
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. New England Journal of Medicine 2000;343(10):689‐94. [PUBMED: 10974132] [DOI] [PubMed] [Google Scholar]
Schuh 2006 {published data only}
- Schuh S, Dick PT, Stephens D, Hartley M, Khaikin S, Rodrigues L, et al. High‐dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics 2006;118(2):644‐50. [PUBMED: 16882819] [DOI] [PubMed] [Google Scholar]
Sekerel 2005 {published data only}
- Sekerel BE, Sackesen C, Tuncer A, Adalioglu G. The effect of nebulized budesonide treatment in children with mild to moderate exacerbations of asthma. Acta Paediatrica 2005;94(10):1372‐7. [PUBMED: 16299866] [DOI] [PubMed] [Google Scholar]
Singhi 1999 {published data only}
- Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. Journal of Paediatrics and Child Health 1999;35(5):483‐7. [PUBMED: 10571764] [DOI] [PubMed] [Google Scholar]
Svedmyr 1995 {published data only}
- Svedmyr J, Nyberg E, Asbrink Nilsson E, Hedlin G. Intermittent treatment with inhaled steroids for deterioration of asthma due to upper respiratory tract infections. Acta Paediatrica 1995;84(8):884‐8. [PUBMED: 7488811] [DOI] [PubMed] [Google Scholar]
Volovitz 1998 {published data only}
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. Journal of Allergy and Clinical Immunology 1998;102(4 Pt 1):605‐9. [PUBMED: 9802368 ] [DOI] [PubMed] [Google Scholar]
Wilson 1990 {published data only}
- Wilson NM, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Archives of Disease in Childhood 1990;65(4):407‐10. [PUBMED: 2189367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousef 2012 {published data only}
- Yousef E, Hossain J, Mannan S, Skorpinski E, McGeady S. Early intervention with high‐dose inhaled corticosteroids for control of acute asthma exacerbations at home and improved outcomes: a randomized controlled trial. Allergy and Asthma Proceedings 2012;33(6):508‐13. [DOI] [PubMed] [Google Scholar]
- Yousef E, Hossain J, Mannnan S. Ineffectiveness of high‐dose inhaled corticosteroids for control of pre‐exacerbation asthma symptoms. Journal of Allergy and Clinical Immunology (Abstract) 2011;127(2 Suppl 1):AB85. [Google Scholar]
References to ongoing studies
NCT02066129 {published data only}
- Step‐up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations. https://clinicaltrials.gov/ct2/show/NCT02066129 (accessed 10 June 2015).
Additional references
Adams 1999
- Adams N, Bestall J, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD003274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1993
- Barnes NC, Marone G, Maria GU, Visser S, Utama I, Payne SL. A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma. European Respiratory Journal 1993;6:877‐85. [PUBMED: 8339809] [PubMed] [Google Scholar]
Belda 2007
- Belda J, Margarit G, Martínez C, Bellido‐Casado J, Casan P, Torrejón M, et al. Anti‐inflammatory effects of high‐dose inhaled fluticasone versus oral prednisone in asthma exacerbations. European Respiratory Journal 2007;30(6):1143‐9. [PUBMED: 17690122] [DOI] [PubMed] [Google Scholar]
Boulet 1999
- Boulet LP, Becker A, Bérubé D, Beveridge R, Ernst P. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. CMAJ 1999;161(Suppl 11):S1‐61. [PUBMED: 10906907] [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society, The National Asthma Campaign, The Royal College of Physicians of London in association with The General Practitioner in Asthma Group, The British Association of Accident and Emergency Medicine, The British Paediatric Respiratory Society, and the Royal College of Paediatrics and Child Health. British guidelines on asthma management. Thorax 1997;52(Suppl 1):S1‐21. [PubMed] [Google Scholar]
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. http://sign.ac.uk/pdf/SIGN141.pdf (accessed 17 Feb 2015).
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Ducharme 2009
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high‐dose fluticasone for virus‐induced wheezing in young children. New England Journal of Medicine 2009;360(4):339‐53. [DOI] [PubMed] [Google Scholar]
Edmonds 2003
- Edmonds ML, Camargo CA, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002308] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fanta 1983
- Fanta CH, Rossing TH, McFadden ER Jr. Glucocorticoids in acute asthma. A critical controlled trial. American Journal of Medicine 1983;74(5):845‐51. [PUBMED: 6340496] [DOI] [PubMed] [Google Scholar]
Fields 2001
- Fields AP. Meta‐analysis of correlation coefficients: a Monte Carlo comparison of fixed‐ and random‐effects methods. Psychological Methods 2001;6(2):161‐80. [DOI] [PubMed] [Google Scholar]
GINA 2015
- Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/local/uploads/files/GINA_Report_2015.pdf (accessed 17 Feb 2015).
Global Asthma Report 2014
- Global Asthma Network. Global Asthma Report 2015. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf (accessed 10 March 2016).
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnston 2006
- Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax 2006;61(8):722‐8. [PUBMED: 16877691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lane 2006
- Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respiratory Medicine 2006;100(3):434‐50. [PUBMED: 16099149] [DOI] [PubMed] [Google Scholar]
Lemiere 2003
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult Asthma Consensus Guidelines Update 2003. Canadian Respiratory Journal May/June 2004;11(Suppl A):9A‐33A. [DOI] [PubMed] [Google Scholar]
Lloyd 2007
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health‐related quality of life in moderate to severe asthma patients in the UK. Primary Care Respiratory Journal 2007;16(1):22‐7. [PUBMED: 17297523] [DOI] [PMC free article] [PubMed] [Google Scholar]
McEvoy 2000
- McEvoy CE, Niewoehner DE. Corticosteroids in chronic obstructive pulmonary disease. Clinical benefits and risks. Clinical Chest Medicine 2000;21(4):739‐52. [PUBMED: 11194783] [DOI] [PubMed] [Google Scholar]
NHIS 2005
- National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention 2005. National Health Interview Survey (NHIS 2005). Hyattsville, MD. http://www.cdc.gov/nchs/about/major/nhis/reports_2005.htm (accessed 14 March 2008).
NHLBI 2007
- National Heart, Lung, and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). Bethesda, MD: National Institutes of Health, 2007. Publication no. 08‐4051.
Oommen 2003
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent‐initiated oral prednisolone for viral wheeze in children aged 1‐5 years; randomised controlled trial. Lancet 2003;362(9394):1433‐8. [PUBMED: 14602435 ] [DOI] [PubMed] [Google Scholar]
Panickar 2009
- Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. New England Journal of Medicine 2009;360:329‐38. [DOI] [PubMed] [Google Scholar]
Pollack 2002
- Pollack CV Jr, Pollack ES, Baren JM, Smith SR, Woodruff PG, Clark S, et al. Multicenter Airway Research Collaboration Investigators. A prospective multicenter study of patient factors associated with hospital admission from the emergency department among children with acute asthma. Archives of Pediatric & Adolescent Medicine 2002;156(9):934‐40. [PUBMED: 12197803] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigo 2006
- Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: an evidence‐based evaluation. Chest 2006;130(5):1301‐11. [PUBMED: 17099004] [DOI] [PubMed] [Google Scholar]
Rowe 2001
- Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002178] [DOI] [PubMed] [Google Scholar]
Sears 2000
- Sears M. Natural history and epidemiology. In: Fitzgerald JM, Ernst P, Boulet L‐P, O'Byrne PM editor(s). Evidence based asthma management. Hamilton: BC Decker Inc., 2000:1‐12. [Google Scholar]
Stovold 2014
- Stovold E, Beecher D, Foxlee R, Noel‐Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Systematic Reviews 2014;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 1998
- Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow and symptom self‐management plans for patients with asthma attending a primary care clinic. American Journal of Respiratory and Critical Care Medicine 1998;157:540‐6. [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang R, Lagakos SW, Hare JH, Hunter DJ, Drazen JM. Special report. Statistics in medicine ‐ reporting of subgroup analyses in clinical trials. New England Journal of Medicine 2007;357(21):2189‐94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Quon 2010
- Quon BS, FitzGerald JM, Lemière C, Shahidi N, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007524.pub3] [DOI] [PubMed] [Google Scholar]


