Abstract
Background
Asthma is a common long‐term breathing condition that affects approximately 300 million people worldwide. People with asthma may experience short‐term worsening of their asthma symptoms; these episodes are often known as ‘exacerbations’, ‘flare‐ups’, ‘attacks’ or 'acute asthma'. Oral steroids, which have a potent anti‐inflammatory effect, are recommended for all but the most mild asthma exacerbations; they should be initiated promptly. The most often prescribed oral steroids are prednisolone and dexamethasone, but current guidelines on dosing vary between countries, and often among different guideline producers within the same country. Despite their proven efficacy, use of steroids needs to be balanced against their potential to cause important adverse events. Evidence is somewhat limited regarding optimal dosing of oral steroids for asthma exacerbations to maximise recovery while minimising potential side effects, which is the topic of this review.
Objectives
To assess the efficacy and safety of any dose or duration of oral steroids versus any other dose or duration of oral steroids for adults and children with an asthma exacerbation.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) and reference lists of all primary studies and review articles. This search was up to date as of April 2016.
Selection criteria
We included parallel randomised controlled trials (RCTs), irrespective of blinding or duration, that evaluated one dose or duration of oral steroid versus any other dose or duration, for management of asthma exacerbations. We included studies involving both adults and children with asthma of any severity, in which investigators analysed adults and children separately. We allowed any other co‐intervention in the management of an asthma exacerbation, provided it was not part of the randomised treatment. We included studies reported as full text, those published as abstract only and unpublished data.
Data collection and analysis
Two review authors independently screened the search results for included trials, extracted numerical data and assessed risk of bias; all data were cross‐checked for accuracy. We resolved disagreements by discussion with the third review author or with an external advisor.
We analysed dichotomous data as odds ratios (ORs) or risk differences (RDs) using study participants as the unit of analysis; we analysed continuous data as mean differences (MDs). We used a random‐effects model, and we carried out a fixed‐effect analysis if we detected statistical heterogeneity. We rated all outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system and presented results in 'Summary of findings' tables.
Main results
We included 18 studies that randomised a total of 2438 participants ‐ both adults and children ‐ and performed comparisons of interest. Included studies assessed higher versus lower doses of prednisolone (n = 4); longer versus shorter courses of prednisolone (n = 3) or dexamethasone (n = 1); tapered versus non‐tapered courses of prednisolone (n = 4); and prednisolone versus dexamethasone (n = 6). Follow‐up duration ranged from seven days to six months. The smallest study randomised just 15 participants, and the largest 638 (median 93). The varied interventions and outcomes reported limited the number of meaningful meta‐analyses that we could perform.
For two of our primary outcomes ‐ hospital admission and serious adverse events ‐ events were too infrequent to permit conclusions about the superiority of one treatment over the other, or their equivalence. Researchers in the included studies reported asthma symptoms in different ways and rarely used validated scales, again limiting our conclusions. Secondary outcome meta‐analysis was similarly hampered by heterogeneity among interventions and outcome measures used. Overall, we found no convincing evidence of differences in outcomes between a higher dose or longer course and a lower dose or shorter course of prednisolone or dexamethasone, or between prednisolone and dexamethasone.
Included studies were generally of reasonable methodological quality. Review authors assessed most outcomes in the review as having low or very low quality, meaning we are not confident in the effect estimates. The predominant reason for downgrading was imprecision, but indirectness and risk of bias also reduced our confidence in some estimates.
Authors' conclusions
Evidence is not strong enough to reveal whether shorter or lower‐dose regimens are generally less effective than longer or higher‐dose regimens, or indeed that the latter are associated with more adverse events. Any changes recommended for current practice should be supported by data from larger, well‐designed trials. Varied study design and outcome measures limited the number of meta‐analyses that we could perform. Greater emphasis on palatability and on whether some regimens might be easier to adhere to than others could better inform clinical decisions for individual patients.
Plain language summary
Different doses and durations of oral steroids for asthma attacks
Background: People with asthma sometimes have asthma attacks, wherein their symptoms such as cough, chest tightness and difficulty breathing become worse. Many patients with asthma attacks are treated with steroids, which are usually given as a short course of tablets or liquid medicine. Steroids work by reducing inflammation in the airways in the lungs, but they can have side effects (e.g. reduced growth in children, hyperactivity, nausea).
Review question: We set out to compare different doses or durations of oral steroids given to people having asthma attacks. This is an important issue because different doses and durations of oral steroids are used for asthma attacks in different countries, and we do not know which regimen is most likely to improve symptoms while minimising unpleasant side effects.
Study characteristics: We included 18 studies involving 2438 adults and children. Studies compared two types of steroid ‐ prednisolone and dexamethasone ‐ or two different doses or durations of either drug. The smallest study included just 15 people, and the largest 638. Studies followed people for between seven days and six months to see what happened to them. The evidence presented here is current to April 2016.
Key results: It was difficult to combine the results of studies in a useful way because investigators used a variety of doses and durations of steroids and measured their results in different ways. Also, events such as hospital admissions and serious side effects happened very rarely in these studies, making it difficult to tell whether longer or shorter courses or higher or lower doses are better or safer, or if prednisolone is generally better or worse than dexamethasone. Some studies were old and did not use steroid doses or durations used by medical practitioners today.
Any changes to the way in which asthma attacks are currently managed with oral steroids would need to be supported by larger studies than have been conducted so far.
Quality of the evidence: Evidence presented in this review is generally considered to be of low or very low quality, which means we are not very sure whether the results are accurate, mostly because we have not been able to combine many studies. Some studies did not clearly explain how trial organisers decided which people would receive which dose of steroids, and in some studies, both participants and trial organisers knew which dose they were getting. This may have affected study results.
Summary of findings
Background
Description of the condition
Asthma is a common long‐term breathing condition that affects approximately 300 million people worldwide and causes an estimated 250,000 deaths every year (WHO 2007). Between 1% and 18% of people in different countries are affected by asthma (GINA 2015), which is characterised by chronic airway inflammation and airway hyperresponsiveness, leading to shortness of breath, wheeze, chest tightness and cough. Symptoms are typically worse at night and in the early morning and may vary over time (CDC 2012; GINA 2015). Treatments are largely aimed at reducing airway smooth muscle constriction through the use of inhaled bronchodilators (e.g. short‐ and long‐acting beta2‐agonists) and reducing airway inflammation through the use of corticosteroids, which usually are also inhaled (BTS/SIGN 2014).
People with asthma may experience short‐term worsening of their asthma symptoms; these episodes are known as ‘exacerbations’, ‘flare‐ups’, ‘attacks’ or 'acute asthma'. Exacerbations are characterised by episodes of “progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms” (NAEPP 2007). International consensus on the definition of an attack or exacerbation has not been reached, but a working group in the USA recently suggested the definition as “a worsening of asthma requiring the use of systemic corticosteroids to prevent a serious outcome” (Fuhlbrigge 2012).
In the USA in 2008, more than half of adults and children with asthma had at least one asthma exacerbation (CDC 2011). Asthma exacerbation triggers vary from person to person but commonly include tobacco smoke, respiratory tract infection, house dust mites, air pollution, pets and mould (CDC 2006). Depending on severity, asthma exacerbations usually require a temporary change in the medication regimen for a person with asthma, for example, increased use of short‐acting bronchodilators such as salbutamol and a course of systemic steroids. More severe exacerbations may require treatment in an emergency department or admission to the hospital (BTS/SIGN 2014).
Description of the intervention
Oral steroids are recommended for all but the most mild asthma exacerbations (BTS/SIGN 2014); they should be initiated promptly (Rowe 2001). It is thought that the intravenous or intramuscular route offers no advantage over the oral route unless compliance with treatment or intestinal absorption is a matter of concern (Krishnan 2009; Lahn 2004). It is advised that oral steroids be taken as a single dose after breakfast (BNF).
Current guidelines on dosing vary slightly between countries, and often among different guideline producers within the same country. In the UK, the most recent (BTS/SIGN 2014) guidelines recommend for adults 40 to 50 mg daily oral prednisolone for at least five days, or until recovery. The same guidelines recommend a dose of 20 mg of prednisolone for children two to five years old, and 30 to 40 mg for children older than five years. GINA 2015 recommendations are similar and suggest a dose of 1 mg/kg for adult patients, up to a maximum daily dose of 50 mg, and 1 to 2 mg/kg for children aged six to 11 years, up to a maximum daily dose of 40 mg. GINA 2015 guidance advises that a five‐ to seven‐day course in adults and three to five days in children is usually adequate.
Currently evidence is insufficient to suggest that alternative steroids, such as dexamethasone, offer any advantage over prednisolone (BTS/SIGN 2014). Prednisolone is widely used internationally and is relatively inexpensive; a packet 28 × 5 mg tablets costs just £1.29 in the UK (BNF). It is not necessary to taper the dose when stopping, provided the patient is already using inhaled corticosteroids, is not taking long‐term oral steroids or has required an acute course of over three weeks’ duration (BTS/SIGN 2014; GINA 2015).
How the intervention might work
Glucocorticoids, including prednisolone, are potent inhibitors of inflammation and are used to treat a wide variety of inflammatory and autoimmune conditions, including asthma (Barnes 2003; van der Velden 1998). Glucocorticoids are thought to work by binding to a cellular glucocorticoid receptor, leading to down‐regulation of the expression of various genes involved in maintaining the inflammatory process. This in turn leads to decreased inflammatory cell recruitment and activation, up‐regulation of beta2‐receptors, decreased microvascular permeability and decreased mucus production (Barnes 1992). Research findings suggest more rapid resolution of symptoms and reduced relapse rates among patients treated with oral steroids (Alangari 2014; Krishnan 2009; Rowe 2007).
Why it is important to do this review
Despite their proven efficacy, use of steroids needs to be balanced against their potential to cause important adverse events. The problems associated with longer‐term steroid therapy are well established and include diabetes, osteoporosis, muscle wasting, Cushing’s syndrome and linear growth restriction in children (BNF). Indeed, regular use of even low to moderate daily doses of inhaled corticosteroids is associated with a mean reduction in linear growth velocity of 0.48 cm/y among children (Zhang 2014). However, many important adverse events are associated with shorter‐term use, which is commonly recommended for asthma exacerbations. These side effects include insomnia, nausea, abdominal distension, dyspepsia, malaise, vertigo, headache and (especially in children) behavioural changes (BNF; Kayani 2002).
Current evidence regarding optimal dosing of oral steroids for asthma exacerbations is somewhat limited. Bowler 1992 randomised 76 participants to receive low‐, medium‐ or high‐dose intravenous hydrocortisone in an inpatient setting for 48 hours, followed by low, medium or high doses of oral steroids give over 12 days. Study authors concluded that low‐dose hydrocortisone (50 mg, four times a day for 48 hours), followed by low‐dose prednisolone (20 mg daily, reduced to 5 mg over 12 days), was as effective as higher doses. In a similar study of 20 participants in the year 2000, researchers concluded that a one‐week course of oral steroids after a three‐day course of intravenous steroids was as effective as a two‐week course (Hasegawa 2000). A study of 86 children aged two to 16 years concluded that an oral prednisolone dose of 1 mg/kg was equally effective as 2 mg/kg but was associated with fewer behavioural adverse events (Kayani 2002). Similarly, Hewer 1998 identified no advantage of a 1 or 2 mg/kg dose over a 0.5 mg/kg dose in a study of 98 children admitted to hospital with acute asthma.
An overview or 'umbrella review' of corticosteroid use in acute asthma also addressed this question, suggesting that no evidence shows that doses above 50 to 100 mg daily are beneficial, and that a course duration of five to 10 days is sufficient for most discharged patients (Krishnan 2009). Similar findings were reported in Manser 2001. However, the conclusions presented in both of these reviews are based on studies of hospitalised patients wherein participants in at least one of the trial arms were receiving parenteral steroids.
Objectives
To assess the efficacy and safety of any dose or duration of oral steroids versus any other dose or duration of oral steroids for adults and children with an asthma exacerbation.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs), both blinded and unblinded, that evaluated any dose or duration of oral steroids versus any other dose or duration of oral steroids for management of an asthma exacerbation. We excluded cross‐over trials because of the long‐term effects of treatment with oral steroids and the unpredictable timing of a second exacerbation. We included studies reported as full text, those published as abstract only and unpublished data.
Types of participants
We included studies of both adults and children with asthma, diagnosed by clinician or according to national or international guidelines, who were experiencing an exacerbation. We recorded the severity of the exacerbation and the criteria used to define this. We excluded studies that recruited participants with other respiratory co‐morbidities and those taking long‐term oral steroids.
Types of interventions
We included studies comparing any dose or duration of oral steroids with any other dose or duration of oral steroids. We included studies that allowed any other co‐interventions for management of an asthma exacerbation, such as inhaled or nebulised short‐acting beta2‐agonists, provided they were not part of the randomised treatment.
We included participants who had presented to a primary care‐based healthcare facility or emergency department and those who had been admitted to hospital. We included participants who had received intravenous or intramuscular steroid therapy before commencing oral steroids, provided this was not part of the randomised treatment and this route of administration had ceased before randomisation to different oral dose or duration arms.
Eligable study comparisons included, but were not limited to, the following examples.
Short versus long duration of the same dose, e.g. 40 mg oral prednisolone daily for five days versus 40 mg oral prednisolone daily for 10 days.
High versus low dose of the same duration, e.g. 20 mg oral prednisolone daily for five days versus 40 mg oral prednisolone daily for five days.
Short duration and high dose versus long duration and low dose, e.g. 50 mg oral prednisolone for three days versus 20 mg oral prednisolone daily for 10 days.
Types of outcome measures
Primary outcomes
Admission/re‐admission to hospital.
Asthma symptoms at end of steroid course.
Serious adverse events.
Secondary outcomes
New exacerbation during post‐treatment follow‐up period.
Lung function tests at end of treatment/follow‐up period (trough forced expiratory volume in one second (FEV1) preferred if available).
All adverse events/side effects.
Reporting by investigators of one or more of the outcomes listed here was not an inclusion criterion for the review. Outcomes were chosen as those most important to patients after consultation with a patient representative.
If more than one scale measuring the same construct was reported within a study, or if different scales were used across studies, we analysed them together using standardised mean differences, provided clinical heterogeneity was sufficiently low to make a pooled analysis meaningful (e.g. we avoided combining different un‐validated symptom scales).
When possible, we extracted the types of adverse events experienced; our user group research suggests that psychological/emotional/behavioural side effects can be particularly troublesome during short‐term steroid courses. This has been reported narratively when meta‐analysis was not possible.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for details). We searched all records in the CAGR using the search strategy presented in Appendix 2. We performed the search in April 2016.
We conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/), also in April 2016.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. In a change to our protocol, we did not search manufacturers' websites, as the intervention medication is made generically by a large number of manufacturers worldwide.
We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) in April 2016 and identified no errata or retractions.
Data collection and analysis
Selection of studies
Two review authors (RN and KMK or GM) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications; two review authors (RN and KMK or GM) independently screened full‐text reports and identified studies for inclusion, while identifying and recording reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted the third review author. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least two studies in the review to record study characteristics and outcome data. In a change from the protocol, one review author (RN) extracted study characteristics from included studies and another review author (KMK) independently spot‐checked the extracted information for accuracy. We extracted the following information.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of all trial authors.
Two review authors (RN and KMK or GM) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving the third person (RN, KMK or GM). One review author (RN or KMK) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review with data provided in the study reports. We ensured that KMK was not involved in both transferring data into RevMan and spot‐checking for accuracy.
Assessment of risk of bias in included studies
Two review authors (RN and KMK or GM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussing them or by involving another review author (RN, KMK or GM). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios or (for very rare events) as risk differences, which takes into account the zero cells in an analysis. We analysed continuous data as mean differences or standardised mean differences. We entered data presented as a scale with a consistent direction of effect. We extracted change from baseline scores in preference to endpoint scores, if both were reported.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we planned to include only the relevant arms. However, no included study reported a treatment arm irrelevant to this review. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) are combined in the same meta‐analysis, we will halve the control group to avoid double‐counting.
We dealt with children (i.e. average age of participants younger than 16) and adults separately in the review.
For our analyses, we attempted to group data into 'high‐dose' courses (e.g. > 50 mg daily dose in adults or > 2 mg/kg in children, i.e. higher than current recommendations) versus 'low‐dose' courses (i.e. within current recommendations), and 'longer duration' courses (e.g. > 7 days, again longer than most recommendations) versus 'short duration' courses.
Further grouping, determined by comparisons made within the studies, will be described later in the review.
Unit of analysis issues
The unit of analysis was the patient (i.e. number of participants admitted to hospital at least once rather than number of admissions per participant).
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, we reported this. We were not able to carry out any of our pre‐specified subgroup analyses because combinable data were lacking.
Assessment of reporting biases
We were unable to pool more than 10 trials, and so we could not create a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
Summary of findings table
We created a 'Summary of findings' table using the following outcomes.
Admission/re‐admission to hospital.
Asthma symptoms at end of steroid course.
Serious adverse events.
New exacerbation in post‐treatment follow‐up period.
All adverse events/side effects.
Lung function tests at end of treatment/follow‐up period.
We used the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), while using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses if we found significant heterogeneity. However, we anticipated correctly that we would identify few studies contributing data to each outcome within the possible comparisons outlined under Types of interventions. Therefore, we did not attempt to perform these subgroup analyses and instead presented information on these potential effect modifiers in Table 5.
1. Summary of included study characteristics.
| Study ID | Total n | Country | Age range, years | Duration of follow‐up | Comparison | Total dose comparison (converted to prednisolone equivalent) |
| Aboeed 2014 | 58 | USA | Not reported | 4 weeks | Prednisone 40 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 200 mg vs 213 mg |
| Altamimi 2006 | 134 | Canada | 2 to16 | 3 weeks (maximum) | Predisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 1 day | 200 mg vs 80 mg (based on 20 kg child) |
| Chang 2008 | 201 | Australia | 2 to15 | 4 weeks | Prednisolone 1 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 3 days | 100 mg vs 60 mg (based on 20 kg child) |
| Cronin 2015 | 226 | Ireland | 2 to 16 | 2 weeks | Prednisolone 1 mg/kg daily for 3 days vs 0.3 mg/kg dexamethasone once daily for 1 day | 60 mg vs 40 mg (based on 20 kg child) |
| Cydulka 1998 | 15 | USA | 19 to 50 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg |
| Ghafouri 2010 | 125 | USA | 2 to 17 | 1 week | Dexamethasone 0.6 mg/kg once daily for 2 doses (days 1 and 3) versus dexamethasone 0.6 mg/kg once daily for 1 day | 160 mg vs 80 mg (based on 20 kg child) |
| Greenberg 2008 | 167 | USA | 2 to 18 | 1.5 weeks | Prednisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 200 mg vs 160 mg (based on 20 kg child) |
| Hasegawa 2000 | 20 | Japan | Not reported | 26 weeks | Prednisolone 0.5 mg/kg daily for 14 days vs prednisolone 0.5 mg/kg once daily for 7 days | 490 mg vs 245 mg (based on 70 kg adult) |
| Jones 2002 | 47 | UK | 16 to 60 | 4‐6 weeks | Prednisolone 40 mg once daily for 10 days vs prednisolone 40 mg once daily for 5 days | 400 mg vs 200 mg |
| Karan 2002 | 26 | India | 17 to 70 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg |
| Kayani 2002 | 88 | USA | 2 to 18 | 4 weeks (maximum) | Prednisolone 2 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 5 days | 200 mg vs 100 mg (based on 20 kg child) |
| Kravitz 2011 | 285 | USA | 18 to 45 | 2 weeks | Prednisolone 50 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 250 mg vs 213 mg |
| Langton Hewer 1998 | 98 | UK | 1 to 15 | 2 weeks | Prednisolone 2 mg/kg once daily vs prednisolone 1 mg/kg once daily vs prednisolone 0.5 mg/kg once daily while inpatient and for up to 3 days post discharge | 200 mg vs 100 mg vs 50 mg (based on 20 kg child receiving a 5‐day course) |
| Lederle 1987 | 43 | USA | 30 to 78 | 12 weeks | Prednisolone 45 mg daily reducing to 0 mg daily over 7 weeks vs prednisolone 45 mg daily reducing to 0 mg daily over 7 days | 1575 mg vs 225 mg |
| NCT00257933 | 152 | USA | 2 to 18 | 2 weeks | Prednisolone 4 mg/kg daily for 2 days, then 2 mg/kg daily for duration of admission vs prednisolone 2 mg/kg daily for duration of admission | 400 mg vs 200 mg (based on 20 kg child receiving a 5‐day course) |
| O’Driscoll 1993 | 39 | UK | 16 to 55 | 4‐6 weeks | Prednisolone 40 mg daily for 10 days followed by 7‐day taper vs prednisolone 40 mg daily for 10 days | 540 mg vs 400 mg |
| Qureshi 2001 | 628 | USA | 2 to 18 | 2 weeks | Prednisolone 2 mg/kg initial dose, then 1 mg/kg daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 120 mg vs 160 mg (based on 20 kg child) |
| Viska 2008 | 86 | Indonesia | "Adults" | 6 weeks | Prednisolone 36 mg daily for 2 weeks vs prednisolone 12 mg daily for 2 weeks | 504 mg vs 168 mg |
Severity of asthma exacerbation according to mean baseline characteristics (e.g. mild vs moderate vs severe).
Hospitalised participants versus non‐hospitalised participants.
Treatment with intramuscular or intravenous steroids before randomisation versus no treatment with intramuscular or intravenous steroids before randomisation.
Asthma severity according to reported background characteristics (e.g. Global Initiative for Asthma (GINA) 1 and 2 vs GINA 3 and 4).
We planned to use the following outcomes in subgroup analyses.
Admission/re‐admission to hospital.
Asthma symptoms at end of treatment course.
Serious adverse events.
All adverse events.
We planned to use the formal test for subgroup interactions in Review Manager 5 (RevMan 2014), had subgroup analysis been possible.
We included all adverse events as an outcome in the subgroup analysis, as user group feedback suggests that many of the adverse events experienced would not be classified as 'serious' according to standard definitions in research, but can nonetheless have a substantial impact on daily functioning.
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
Studies at high risk of selection bias.
Unpublished data (i.e. no peer‐reviewed full paper available).
Results
Description of studies
Full details of the conduct and characteristics of each included study can be found in the Characteristics of included studies tables and reasons for exclusion when full texts had to be viewed are given in the Characteristics of excluded studies table.
Results of the search
We identified 1297 references through electronic database searches and an additional 109 records through searches of clinicaltrials.gov and the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/). We excluded most (n = 1335) of these references on the basis of title and abstract. We retrieved 71 full texts for more detailed assessment and at this stage excluded 47 additional references (related to 39 individual studies). Reasons for exclusion included wrong comparator, wrong intervention and not a randomised controlled trial. We also excluded three studies that were ongoing, and one study (reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria. We present trial flow in Figure 1.
1.
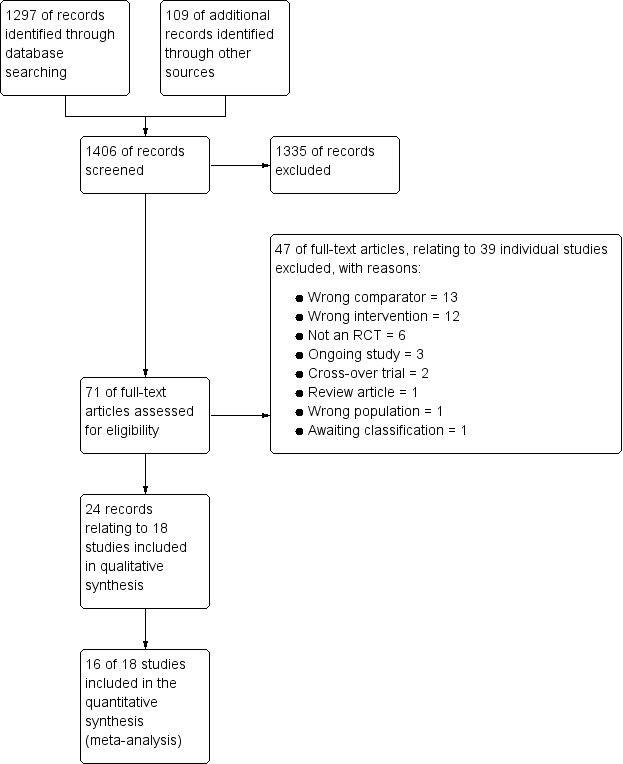
Study flow diagram.
Included studies
Eighteen studies met our inclusion criteria, 16 of which contributed data to at least one meta‐analysis. These studies included a total of 2438 participants who were randomly assigned to comparisons of interest in this review. The largest study included 628 participants, and the smallest just 15. The mean total number of participants was 135, and the median 93. Investigators reported 14 trials as full peer‐reviewed articles, three as abstracts only (Aboeed 2014; Ghafouri 2010; Viska 2008) and one on the clinicaltrials.gov website (NCT00257933), for which we obtained additional unpublished data directly from the trial contact person. We present a summary of the characteristics of included studies in Table 5.
Methods
As per our protocol, all included trials were RCTs with parallel design that compared one dose or duration of oral steroids versus another dose or duration. One study included three relevant arms: high‐, medium‐ and low‐dose oral prednisolone. Trial duration varied, with oral steroid treatment courses ranging from just a single dose to seven weeks of treatment. All studies included a post‐treatment follow‐up period, which ranged in duration from seven days to six months. No studies reported a run‐in period, as recruitment was triggered by an unscheduled presentation with an acute exacerbation of asthma. Outcomes data were extracted at the end of steroid treatment or at the last time point reported, or at both times if available. Trials were conducted in a variety of countries worldwide, but most were carried out in the USA (Aboeed 2014; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Kayani 2002; Kravitz 2011; Lederle 1987; NCT00257933; Qureshi 2001) and the UK (Jones 2002; Langton Hewer 1998; O’Driscoll 1993). The remainder were carried out in Australia (Chang 2008), Canada (Altamimi 2006), Japan (Hasegawa 2000), Indonesia (Viska 2008), India (Karan 2002) and Ireland (Cronin 2015).
Participants
We included studies involving both children and adults. Nine studies (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001) recruited only children (age range one to 18 years depending on the individual study), and seven studies (Cydulka 1998; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993; Viska 2008) recruited only adults (age range 16 to 78 years depending on the individual study). Two studies (Aboeed 2014; Hasegawa 2000) did not report the age range of participants, but the steroid doses administered in Aboeed 2014 would be consistent with adult participants. Most studies did not specify the ethnicity of participants.
All studies included participants with acute exacerbations of asthma. Although reported as having asthma, most of the participants in Lederle 1987 were older men who were current smokers or ex‐smokers, and many may in fact have had chronic obstructive pulmonary disease (COPD) with a degree of reversibility. In most cases, researchers did not report baseline asthma severity and severity of the asthma attack. However, in the majority of studies (Aboeed 2014; Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Karan 2002; Kayani 2002; Kravitz 2011; Qureshi 2001), researchers recruited participants in the emergency department (ED) or at an outpatient clinic, and the inclusion criteria in most of these studies required that they must be well enough to be discharged home. Four studies (Jones 2002; Langton Hewer 1998; Lederle 1987; O’Driscoll 1993) recruited participants and commenced randomised treatment on an inpatient basis but completed treatment at home. In one study (NCT00257933), randomised steroid treatment was continued for 48 hours or until discharge, whichever came sooner, followed by five to 10 days of standard oral steroid treatment at the discretion of the treating physician. One study did not report the specific setting in which treatment was commenced (Viska 2008), and in Hasegawa 2000, treatment was initiated in hospital, but it is not clear whether participants remained as inpatients for the duration of their steroid treatment.
Interventions
Studies included a variety of comparisons: longer versus shorter course of prednisolone (Chang 2008; Hasegawa 2000; Jones 2002); higher versus lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933; Viska 2008); longer course of prednisolone versus shorter course of dexamethasone (Aboeed 2014; Altamimi 2006; Cronin 2015; Greenberg 2008; Kravitz 2011; Qureshi 2001); tapering versus non‐tapering course of prednisolone (Cydulka 1998; Karan 2002; O’Driscoll 1993); long‐tapering versus short‐tapering course of prednisolone (Lederle 1987); and finally long versus short course of dexamethasone (Ghafouri 2010). Dosing also varied across studies; we have extracted this information and presented it in the Characteristics of included studies tables, along with the 'prednisolone‐equivalent' total dose received. All participants in Hasegawa 2000 received three days of intravenous methylprednisolone before commencing randomised oral steroid treatment.
Although we did not set out to compare different types of oral steroids, we included the dexamethasone versus prednisolone comparison because these agents were given over different durations, and this was part of our scope. We meta‐analysed these trials separately because, unlike studies that compared a different dose or duration of the same drug, most of these studies gave almost equivalent total doses of steroid in each intervention arm, so any between‐group differences may be related to drug‐specific factors including adherence or palatability. We recognise that in a clinical setting, drug‐specific factors, such as convenience for the patient, may affect an individual practitioner’s choice of drug or regimen.
Most studies stated that participants were allowed to continue use of specified rescue and preventer medication for asthma throughout the study, and in some trials, frequency of use of rescue medication, such as a short‐acting beta2‐agonist, was an efficacy outcome.
Outcomes
Outcomes reported were not consistent across reviews, and validated scales were not always used. Most studies (n = 13) reported some measure of asthma symptoms, at the end of treatment or follow‐up, or time taken for resolution of symptoms. Most (n = 13) also reported relapse rates, defined usually as an unscheduled visit to the ED or another healthcare provider during the follow‐up period. Three studies specifically reported hospitalisation during the follow‐up period, and seven studies reported new exacerbations or another course of oral steroids prescribed during the follow‐up period. Various measures of lung function were also frequently reported (n = 10), as was compliance with prescribed steroid therapy (n = 6). Adverse events were explicitly stated as an outcome measure in only six studies. Four studies recorded rescue medication use, four reported vital signs and three reported asthma severity scores. Two studies assessed adrenal suppression. One study reported Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), two reported school days or workdays missed and another used the asthma control test.
Excluded studies
We excluded 46 references (related to 38 individual studies) after assessment of full‐text articles. We excluded 13 studies, as they used a comparator not of interest in this review, for example, intravenous or inhaled steroids were compared with oral steroids. We excluded 12 studies because the intervention was not of interest in this review, for example, studies comparing different doses of intravenous steroids in the acute setting, or interventions including additional randomised treatments not of interest in this review. We excluded six studies as they were not randomised controlled trials and another two because they used a cross‐over trial design. One study was in fact a review article, and another study recruited a mixed population of patients with COPD and asthma. We excluded two studies that were ongoing (NCT01241006; NCT02192827), and one study (Tanifuji 2001; reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria.
Risk of bias in included studies
For details of the risk of bias rating for each study and the supporting evidence for each rating, see the Characteristics of included studies table. A summary of risk of bias judgements by study and domain (sequence generation, allocation concealment, blinding, incomplete data and selective reporting) can be found in Figure 2.
2.
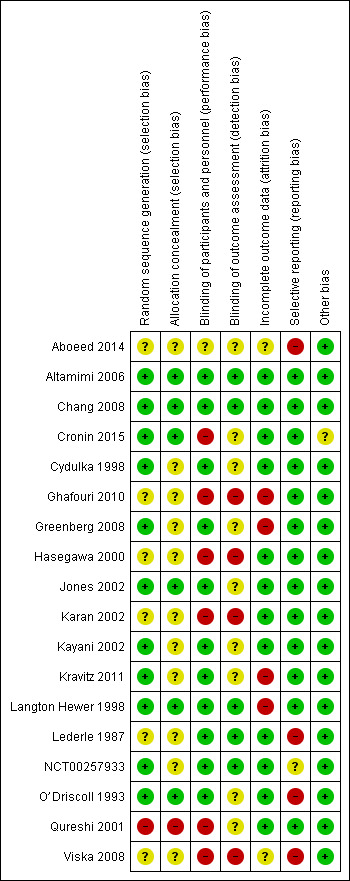
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six studies (Altamimi 2006; Chang 2008; Cronin 2015; Jones 2002; Langton Hewer 1998; O’Driscoll 1993) described the generation of a random sequence and concealment of allocation of participants in sufficient detail for review authors to assess them as having low risk of selection bias. We considered five other studies (Cydulka 1998; Greenberg 2008; Kayani 2002; Kravitz 2011; NCT00257933) to be at low risk of bias for random sequence generation but at unclear risk of bias for allocation concealment, which was not described in sufficient detail to allow a judgement.
Six studies (Aboeed 2014; Ghafouri 2010; Hasegawa 2000; Karan 2002; Lederle 1987; Viska 2008) did not provide sufficient details of random sequence generation or allocation concealment for review authors to make a judgement, and so we considered these studies to be at unclear risk of bias in both domains. We assessed Qureshi 2001 as having high risk of bias for random sequence generation and allocation concealment, as participants were allocated to the two intervention arms on the basis of the day of the month they presented to the ED.
Blinding
We judged most studies (n = 11; Altamimi 2006; Chang 2008; Cydulka 1998; Greenberg 2008; Jones 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Lederle 1987; NCT00257933; O’Driscoll 1993) to be at low risk of performance bias, as participants and trial personnel were adequately blinded. Five studies (Altamimi 2006; Chang 2008; Langton Hewer 1998; Lederle 1987; NCT00257933) clearly described blinding of outcome assessors, and we judged these studies to be at low risk of detection bias. We assessed the remaining six studies as having unclear risk of detection bias, as blinding of outcome assessors was not clearly described.
We considered Aboeed 2014 to be at unclear risk of bias for both performance and detection bias, as the abstract did not contain enough detail to allow a judgement. Four studies (Ghafouri 2010; Hasegawa 2000; Karan 2002; Viska 2008) were open‐label and were considered to be at high risk of performance and detection bias. In Cronin 2015, also an open‐label study, outcome assessors for the primary outcome (paediatric respiratory assessment measure (PRAM)) at day 4 were unaware of group allocation, but other participant‐reported or influenced outcomes (e.g. decision to re‐present to a healthcare practitioner) may have been affected by knowledge of group allocation, so we rated this study as having unclear risk of detection bias and high risk of performance bias. We considered one study (Qureshi 2001) to be at high risk of performance bias, as the trial was unblinded, but the primary outcome ‐ decision to seek medical care for deteriorating symptoms ‐ was assessed independently of study investigators, and so we rated the risk of detection bias as unclear.
Incomplete outcome data
We assessed 12 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Lederle 1987; NCT00257933; O’Driscoll 1993; Qureshi 2001) to be at low risk of attrition bias, as they had low and balanced withdrawal, and all participants who withdrew were clearly accounted for in the trial flow. We assessed Aboeed 2014 and Viska 2008, both conference abstracts, as having unclear risk, as they did not describe the number randomised to, or withdrawn from, each treatment arm.
We assessed Langton Hewer 1998 to be at high risk; attrition in the intervention groups was unbalanced (< 10% in the medium‐ and low‐dose groups and 20% in the higher‐dose group), and although all withdrawals were accounted for in the text of the report, this imbalance may have affected the findings. We assessed Ghafouri 2010, a conference abstract, to be at high risk of attrition bias because of unbalanced attrition in intervention groups, and because the reasons for withdrawal were not stated. We assessed Greenberg 2008 also to be at high risk, as approximately half of the participants randomised to each treatment did not complete the trial, and although baseline details are given for those who completed and those who did not, how this high level of attrition may have affected the findings is unclear. Finally, we assessed Kravitz 2011 as having high risk of attrition bias, as 30% (85 out of 285) of all randomised participants did not complete the trial as the result of admission to hospital after they were randomised or loss to follow‐up, and their outcomes remain unknown.
Selective reporting
We assessed 13 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Qureshi 2001) to be at low risk of reporting bias, although we were able to find prospectively registered protocols only for Chang 2008, Cronin 2015 and Ghafouri 2010.
We assessed Aboeed 2014 and Viska 2008, both conference abstracts, to be at high risk, as they provided minimal details and could not be included in the quantitative synthesis. We assessed NCT00257933 to be at unclear risk, as the trial has not yet been published. Some results are posted on clinicaltrials.gov, and the study authors kindly provided us with an unpublished manuscript, but some listed outcomes are as yet not fully reported (peak flow, clinical asthma score).
We considered Lederle 1987 to be at high risk, as not all outcomes were reported in a way that allowed meta‐analysis, including FEV1 (reported as percentage of baseline value without variance) and diary outcomes (reported narratively in the text with minimal supporting numerical data). Similarly, we assessed O’Driscoll 1993 to be at high risk, as many of the diary outcomes were not reported numerically, and data were displayed graphically with no variance.
Other potential sources of bias
Most studies did not report their funding source, and for those that did, this was not considered to be a likely source of bias. We assessed Cronin 2015 as being at unclear risk of other bias, as investigators allowed participants to enrol more than once in the trial. This may have led to the same participant contributing to outcomes twice; how the trial authors adjusted the analyses to take this into account is not clear, as they simply state that a "descriptive analysis of the patients enrolled multiple times was performed".
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma.
| Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
|
Patient or population: adults with an acute exacerbation of asthma
Setting: inpatient or community
Intervention: higher dose/longer course of prednisolone
Comparison: lower dose/shorter course of prednisolone Duration range: 3 to 26 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission in follow‐up period | Longer vs shorter course prednisolone | OR 1.35 (0.38 to 4.79) | 142 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 74 per 1000 | 97 per 1000 (29 to 275) | |||||
|
Asthma symptoms Asthma severity score |
Longer vs shorter course prednisolone | ‐ | 44 (1 RCT) | ⊕⊕⊝⊝ Lowc,d | Higher score = Worse symptoms | |
| Mean asthma severity score was 2.6 | Mean asthma severity score in the longer course group was 0.7 lower (1.28 lower to 0.12 lower) | |||||
|
Asthma symptoms Complete resolution by day 28 |
Longer vs shorter course prednisolone | OR 0.55 (0.13 to 2.26) | 35 (1 RCT) | ⊕⊕⊝⊝ Lowb,e | ||
| 412 per 1000 | 278 per 1000 (83 to 613) | |||||
|
New exacerbation in follow‐up period Requiring visit to healthcare provider |
Longer vs shorter course prednisolone | OR 0.98 (0.17 to 5.56) | 55 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,f,g | ||
| 111 per 1000 | 109 per 1000 (21 to 410) | |||||
| Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | OR 3.56 (0.34 to 37.36) | 41 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,f,g | No events were reported in the tapered arm and only 2 events in the stable arm, so we were unable to calculate a baseline risk | ||
| No events | Risk difference in the stable (higher total dose) group was 9% (0 to 26%) | |||||
|
New exacerbation in follow‐up period Oral corticosteroids prescribed |
Longer vs shorter course prednisolone | OR 0.62 (0.23 to 1.68) | 122 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Lederle 1987 dominates this analysis, as the event rate was much higher than in the other 2 studies, possibly reflecting co‐morbid COPD in the study population. Result should be interpreted with caution | |
| 241 per 1000 | 165 per 1000 (68 to 348) | |||||
|
Lung function tests FEV1% predicted |
Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | ‐ | 41 (2 RCTs) | ⊕⊝⊝⊝ Very lowf,g,h | Higher percentage = Better lung function | |
| Mean FEV1% predicted was 70.6 | Mean FEV1% predicted in the stable dose (higher total dose) was 1.02 lower (4.62 lower to 2.58 higher) | |||||
| All adverse events | Longer vs shorter course prednisolone | OR 4.15 (0.94 to 18.41) | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowe,i | ||
| 143 per 1000 | 409 per 1000 (135 to 754) | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; OR: Odds ratio; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aLederle 1987 carried a large proportion of the analysis weight for this outcome because event rates were higher in both groups. This may reflect co‐morbid COPD (participants were older and most had an extensive smoking history). Downgraded once for indirectness
bConfidence intervals include no difference and an important benefit of a longer or shorter course. Downgraded once for imprecision
cConfidence intervals excluded possible benefit of a shorter course, but the effect was based on only 1 study of 44 people. Downgraded once for imprecision
dA 1‐7 scale of symptom severity averaged over days 6‐21 was used, making clinical benefit difficult to interpret. Downgraded once for indirectness
eNeither treatment regimen used in the one study in this analysis is consistent with current international guidance. Downgraded once for indirectness
fThe study contributing most of the analysis weight was unblinded and uncertainties surrounded the selection procedure. Downgraded once for risk of bias
gBoth trials contributing to the analysis used a treatment regimen that was inconsistent with current international guidance. Downgraded once for indirectness
hThe effect was derived from 2 very similar studies including 41 people in total. Studies had smaller standard deviations than would be expected given the sample sizes. Downgraded once for imprecision
iThe result is based on 1 small study and has wide confidence intervals, which do not exclude the possibility of no difference or an important increase in adverse events in the longer course arm, Downgraded twice for imprecision
Summary of findings 2. Adults: prednisolone compared with dexamethasone for acute asthma.
| Adults: prednisolone compared with dexamethasone for acute asthma | ||||||
|
Patient or population: adults with an acute exacerbation of asthma
Setting: inpatient or community
Intervention: prednisolone
Comparison: dexamethasone Duration: 2 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Re‐admission during follow‐up period | 29 per 1000 | 10 per 1000 (1 to 93) | OR 0.35 (0.04 to 3.47) | 200 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
|
Asthma symptoms Returned to normal activities within 3 days |
901 per 1000 | 800 per 1000 (634 to 902) | OR 0.44 (0.19 to 1.01) | 191 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | |
|
New exacerbation during follow‐up period Any ED visit after discharge |
48 per 1000 | 63 per 1000 (19 to 184) | OR 1.32 (0.39 to 4.47) | 200 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
|
New exacerbation during follow‐up period Unscheduled visit to primary healthcare provider |
29 per 1000 | 52 per 1000 (13 to 191) | OR 1.85 (0.43 to 7.96) | 200 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; ED: emergency department; OR: Odds ratio; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aOnly 1 study contributed to this outcome with very few events reported in total, resulting in an imprecise estimate with confidence intervals including both important harms and benefits of either regimen. Downgraded twice for imprecision
bOnly contributing study judged to be at high risk of attrition bias because of post‐randomisation exclusions and large numbers lost to follow‐up. Downgraded once for risk of bias
cOnly 1 study contributed to this outcome with imprecise estimate and confidence intervals not completely excluding the possibility of no differences. Downgraded once for imprecision
Summary of findings 3. Children: higher dose/longer course compared with lower dose/shorter course for acute asthma.
| Children: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
|
Patient or population: children with an acute exacerbation of asthma
Setting: inpatient or community
Intervention: higher dose/longer course of oral steroids
Comparison: lower dose/shorter course of oral steroids Duration range: 1 to 4 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission during follow‐up period | Higher‐ vs lower‐dose prednisolone | Not estimable | 98 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Only one 3‐arm study (Langton Hewer 1998) contributed events to this analysis. Two lower‐dose arms pooled for this outcome. OR 1.55 (0.24 to 9.78) favouring lower dose | |
| Not pooled | Not pooled | |||||
| Longer vs shorter course prednisolone | OR 0.33 (0.01 to 8.28) | 201 (1 RCT) | ⊕⊕⊝⊝ Lowc | |||
| 10 per 1000 | 3 per 1000 (0 to 76) | |||||
| Longer vs shorter course dexamethasone | OR 2.22 (0.19 to 25.27) | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | |||
| 19 per 1000 | 42 per 1000 (4 to 331) | |||||
|
Asthma symptoms Symptom free by 7 days |
Longer vs shorter course prednisolone | OR 1.22 (0.67 to 2.19) | 201 (1 RCT) | ⊕⊕⊕⊝ Moderatee | One other study (Langton Hewer 1998) randomising 98 children to high‐ vs medium‐ vs low‐dose prednisolone reported clinical asthma score at discharge. Small differences in scores were reported with uncertain clinical importance and no consistent dose‐response effect | |
| 307 per 1000 | 351 per 1000 (229 to 492) | |||||
| Serious adverse events | Longer vs shorter course prednisolone | Not estimable | 201 (1 study) | No events occurred in either trial arm | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
|
New exacerbation during follow‐up period Oral corticosteroids prescribed |
Higher‐ vs lower‐dose prednisolone | OR 1.38 (0.25 to 7.47) | 231 (2 RCTs) | ⊕⊕⊝⊝ Lowf | ||
| 17 per 1000 | 24 per 1000 (4 to 116) | |||||
| Longer vs shorter course prednisolone | OR 0.61 (0.19 to 1.94) | 201 (1 RCT) | ⊕⊕⊕⊝ Moderatee | |||
| 79 per 1000 | 50 per 1000 (16 to 143) | |||||
| Longer vs shorter course dexamethasone | OR 0.24 (0.05 to 1.19) | 100 (1 RCT) | ⊕⊕⊝⊝ Lowd,g | |||
| 154 per 1000 | 42 per 1000 (9 to 178) | |||||
|
New exacerbation during follow‐up period Unscheduled visit to healthcare provider |
Longer vs shorter course dexamethasone | OR 2.17 (0.67 to 7.01) | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | ||
| 96 per 1000 | 188 per 1000 (67 to 427) | |||||
| Lung function tests FEV1% predicted at discharge | High vs medium vs low dose | ‐ | 34 (1 study) | This outcome includes only 1 small study (Langton Hewer 1998) in which a subset of participants were able to perform PFTs. Reported between‐group differences were small and of uncertain clinical importance with no consistent dose‐response effect. | ||
| ‐ | ‐ | |||||
| All adverse events | Longer vs short course prednisolone | OR 0.67 (0.11 to 4.08) | 201 (1 RCT) | ⊕⊕⊕⊝ Moderatee | ||
| 30 per 1000 | 20 per 1000 (3 to 111) | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; FEV1: forced expiratory volume in 1 second; OR: Odds ratio; PFTs: pulmonary function tests; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aOnly 1 study contributed events to this outcome and was assessed to be at high risk of attrition bias because of unbalanced drop‐out from intervention arms. Downgraded once for risk of bias
bThe study contributing events had 3 different dose arms, 1 of which is outside the current dosing guidelines. Two other studies reported no events, but intervention involved much higher doses of prednisolone. Downgraded once for indirectness
cOnly 1 study contributed to this analysis. Imprecise estimate with confidence intervals including possibility of important harms or benefits. Downgraded twice for imprecision
dOnly contributing study considered at high risk of bias in multiple domains. Downgraded once for risk of bias
eOnly 1 study contributed to this outcome, resulting in imprecise estimate and confidence intervals including the possibility of important harms or benefits. Downgraded once for imprecision
fOnly 2 studies contributed to this outcome with few events, resulting in imprecise estimate and wide confidence intervals including the possibility of important harms or benefits. Downgraded twice for imprecision
gOnly 1 study contributed to this outcome, resulting in imprecise estimate, which does not exclude the possibility of no difference. Downgraded once for imprecision
Summary of findings 4. Children: prednisolone compared with dexamethasone for acute asthma.
| Children: prednisolone compared with dexamethasone for acute asthma | ||||||
|
Patient or population: children with acute exacerbation of asthma
Setting: inpatient or community
Intervention: prednisolone
Comparison: dexamethasone Duration range: 1.5 to 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Admission at initial presentation | 116 per 1000 | 124 per 1000 (89 to 172) | OR 1.08 (0.74 to 1.58) | 1007 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Re‐admission during follow‐up period | 22 per 1000 | 10 per 1000 (3 to 29) | OR 0.44 (0.15 to 1.33) | 985 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
|
Asthma symptoms scores Pulmonary Index Score (PIS); Patient Self Assessment Score (PSAS); Paediatric Respiratory Assessment Measure (PRAM) |
Not pooled | Not pooled | ‐ | 328 (2 RCTs) |
⊕⊝⊝⊝ Very lowc,d,e,f |
Altamimi 2006 reported PIS and PSAS Cronin 2015 reported PRAM (we extracted the result, which excluded re‐enrolments) No between‐group differences were detected |
|
Asthma symptoms Persistent cough, wheeze, chest tightness, night‐time wakening and difficulty maintaining normal activities |
Not pooled | Not pooled | ‐ | 533 (1 RCT) | The number of people experiencing these symptoms at day 10 was not found to be significantly different between the 2 intervention arms | |
| Serious adverse events | Not pooled | Not pooled | Not estimable | 255 (2 studies) | No events were reported in either study | |
|
New exacerbation during follow‐up period Unscheduled visit to healthcare provider |
97 per 1000 | 83 per 1000 (55 to 126) | OR 0.85 (0.54 to 1.34) | 981 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aThe 2 studies contributing most events to this outcome were considered to be at high or unclear risk of selection (Qureshi 2001) and performance and detection bias (Cronin 2015; Qureshi 2001). In addition, Cronin 2015 allowed 19 participants to enrol more than once in the study. Downgraded once for risk of bias
bConfidence intervals include possible harms or benefits of either intervention. Downgraded once for imprecision
cThe pulmonary index score may lack rigorous evaluation, so clinical interpretation of this score is limited. Downgraded once for indirectness
dConfidence intervals for PIS and PSAS include no difference, but we are unsure whether either end of the confidence intervals includes a clinically important effect. Downgraded once for imprecision
eThe PSAS score has been adapted from National Institute of Health guidelines and may lack rigorous evaluation, so clinical interpretation is limited. Downgraded once for indirectness
fWe were unable to combine the results of these different scales. Downgraded once for inconsistency
Structure of the analysis
We chose to analyse trials in adults and trials in children completely separately in this review.
Structure of the meta‐analysis
We created four main comparison headings within the analysis tree. For each comparison, we chose to meta‐analyse results only when the interventions and outcomes measured were sufficiently similar for pooling to make sense.
Adults: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in adults that compared a higher dose or a longer course with a lower dose or a shorter course of the same oral steroid (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), for example, 40 mg of prednisolone once daily for 10 versus five days, or 36 mg versus 12 mg of prednisolone daily for two weeks.
Adults: prednisolone versus dexamethasone
This comparison included all studies in adults that compared prednisolone with dexamethasone (Aboeed 2014; Kravitz 2011), for example, 40 mg prednisolone daily for five days versus 16 mg of dexamethasone daily for two days.
Children: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in children that compared a higher dose or a longer course with a lower dose of a shorter course of the same oral steroid (Chang 2008; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933), for example, 1 mg/kg daily prednisolone for five versus three days, or 2 mg/kg daily versus 1 mg/kg daily prednisolone for five days.
Children: prednisolone versus dexamethasone
This comparison included all studies in children that compared prednisolone with dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001), for example, 1 mg/kg prednisolone twice daily for five days versus dexamethasone 0.6 mg/kg once daily for one day.
Structure of the narrative synthesis
Below, we present the results narratively according to our pre‐specified outcomes. We begin with the primary outcomes: admission/re‐admission to hospital; asthma symptoms; and serious adverse events. Within each outcome, we describe effects of the interventions in adults, followed by effects in children, clearly specifying which of the above comparisons yielded the extracted data. We then describe the secondary outcomes: new exacerbation in the follow‐up period; lung function tests; and all adverse events/side effects, according to the same pattern.
Primary outcomes
Admission/re‐admission to hospital
Overall, our results demonstrated no difference in admission or re‐admission to hospital between participants prescribed a longer course or a higher dose of oral steroids and those prescribed a shorter course or a lower dose, or between those prescribed prednisolone and those prescribed dexamethasone. The requirement for admission at initial presentation was an exclusion criterion for many of the included studies. In those reporting admissions or re‐admissions, events were generally rare, and differences between interventions and populations in the included studies precluded meaningful meta‐analysis, resulting in imprecise estimates and low confidence in the result.
Admission at initial presentation: children
Four studies in children (Altamimi 2006; Cronin 2015; Ghafouri 2010; Qureshi 2001) reported admission at initial presentation. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ studies comparing prednisolone and dexamethasone ‐ did not detect a difference in admission rates between intervention groups (Analysis 4.1; odds ratio (OR) 1.08, 95% confidence interval (CI) 0.74 to 1.58; participants = 1007; I2 = 0%), but the confidence intervals include an important reduction and increase in admissions. In addition, one of the studies contributing to this analysis (Qureshi 2001) was considered to be at high risk of selection bias, and another study (Cronin 2015) was open‐label and therefore was at high risk of performance and detection bias for this outcome. We therefore have low confidence in the finding. Ghafouri 2010, a study comparing a longer course versus a shorter course of the same dose of dexamethasone, also reported no difference in admissions at initial presentation (Analysis 3.1; OR 1.66, 95% CI 0.60 to 4.61; participants = 125) but again with wide confidence intervals. It is important to note that admission at initial presentation would have been measured before the differing durations of treatment would have had an impact, and so this result is of limited value.
4.1. Analysis.
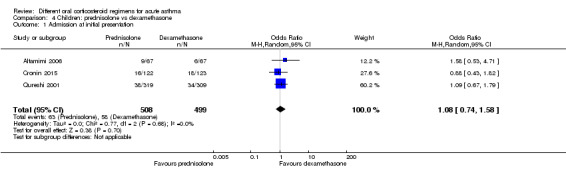
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 1 Admission at initial presentation.
3.1. Analysis.
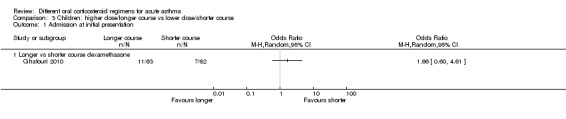
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 1 Admission at initial presentation.
Re‐admission during follow‐up period: adults
Re‐admission to hospital during the follow‐up period was reported by five studies of adult participants (Hasegawa 2000; Jones 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993).
In four studies that compared a longer course versus a shorter course of prednisolone (Hasegawa 2000; Jones 2002; Lederle 1987; O’Driscoll 1993), no difference in re‐admissions was found between intervention groups, but events were rare and confidence intervals include the possibility of harm and the possibility of benefit from a longer or a shorter course (Analysis 1.1; OR 1.35, 95% CI 0.38 to 4.79; participants = 142; studies = 4; I2 = 0%). Of note, the study carrying the greatest weight in this analysis (Lederle 1987) likely recruited participants with co‐morbid COPD, so this outcome was additionally downgraded for indirectness of the study population. Similarly, the study comparing prednisolone versus dexamethasone in adults (Kravitz 2011) reported infrequent re‐admissions to hospital and consequently an imprecise result, and was considered to be at high risk of attrition bias (Analysis 2.1; OR 0.35, 95% CI 0.04 to 3.47; participants = 200).
1.1. Analysis.
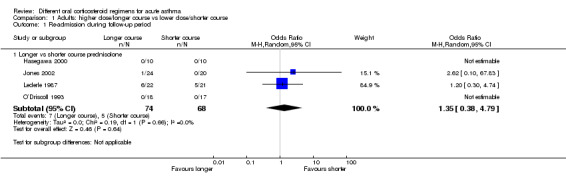
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 1 Re‐admission during follow‐up period.
2.1. Analysis.
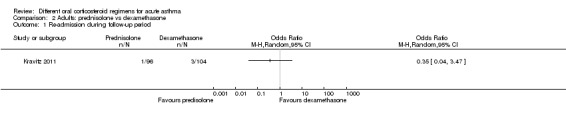
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 1 Re‐admission during follow‐up period.
Re‐admission during follow‐up period: children
Re‐admission to hospital during the follow‐up period was reported by eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001).
Three studies in children compared a higher dose versus a lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933), one compared a longer course versus a shorter course of prednisolone (Chang 2008) and one compared a longer course versus a shorter course of dexamethasone (Ghafouri 2010). Again, events were rare, with only nine participants requiring re‐admission across all five studies (with two studies reporting no events), resulting in wide confidence intervals in each of the three studies reporting events (Analysis 3.2). As the interventions were not sufficiently similar, we did not perform a meta‐analysis and our confidence in these estimates is low or very low.
3.2. Analysis.
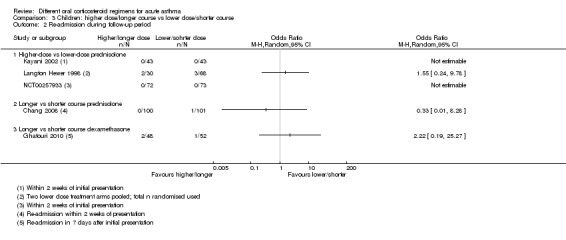
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 2 Re‐admission during follow‐up period.
Altamimi 2006, Cronin 2015 and Qureshi 2001 compared prednisolone versus dexamethasone, and although all three studies reported re‐admissions, they were infrequent, resulting in wide confidence intervals (Analysis 4.2; OR 0.44, 95% CI 0.15 to 1.33; participants = 985; I2 = 0%), and our confidence in the finding was further reduced by the risk of selection bias identified in Qureshi 2001 and by lack of blinding in Cronin 2015.
4.2. Analysis.
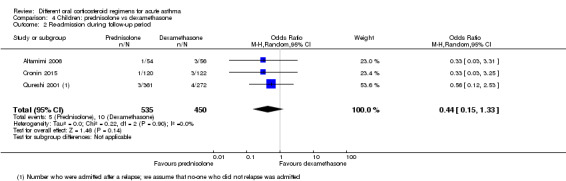
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 2 Re‐admission during follow‐up period.
Asthma symptoms
Asthma symptoms were reported by several included studies, but investigators used a variety of measures and time points, limiting meaningful meta‐analysis. In general, individual studies did not detect an important difference between intervention arms but with a high level of imprecision.
Adults
In adults, asthma severity score was reported by Jones 2002 (mean of individuals' mean overall severity 1 to 7; 1 = no symptoms, 7 = worst symptoms) on days six to 21; Analysis 1.2). The result showed modest benefit with a longer course of prednisolone over a shorter course, but the clinical importance of this is not clear (mean difference (MD) ‐0.70, 95% CI ‐1.28 to ‐0.12; participants = 44), and our confidence in this estimate is low. O’Driscoll 1993, a small study comparing a tapered (longer) course of prednisolone versus a non‐tapered (shorter) course, reported the number of participants with complete resolution of asthma symptoms by day 28 but provided insufficient data to allow conclusions (Analysis 1.3; OR 0.55, 95% CI 0.13 to 2.26; participants = 35), and again we have low confidence in this estimate. Kravitz 2011, a trial that compared prednisolone versus dexamethasone, reported the number of participants who had resumed normal activities within three days. Results suggest a modest benefit of dexamethasone over prednisolone (Analysis 2.2; OR 0.44, 95% CI 0.19 to 1.01; participants = 191), but the confidence intervals do not fully exclude no differences, and the one study contributing to this outcome was assessed to be at high risk of attrition bias.
1.2. Analysis.
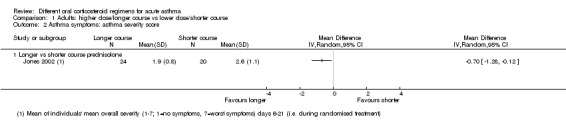
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 2 Asthma symptoms: asthma severity score.
1.3. Analysis.
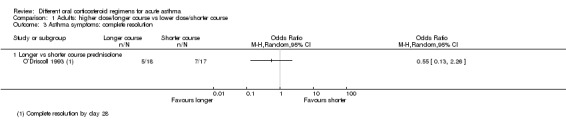
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: complete resolution.
2.2. Analysis.
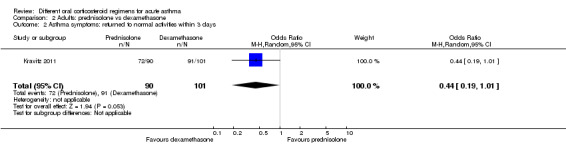
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 2 Asthma symptoms: returned to normal activities within 3 days.
Children
In children, clinical asthma score at discharge was reported by Langton Hewer 1998, a study that compared high‐, medium‐ and low‐dose prednisolone. These findings are inconsistent, have uncertain clinical importance and show no clear benefit of a higher or a lower dose (Analysis 3.3). Chang 2008, a trial of a five‐ versus three‐day course of prednisolone, reported the number of children symptom free at seven days and did not detect a difference between intervention groups (Analysis 3.4; OR 1.22, 95% CI 0.67 to 2.19; participants = 201). We downgraded this outcome once for imprecision, but we are otherwise moderately confident in this estimate. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ reported asthma symptoms using different scales. Altamimi 2006 reported both the pulmonary index score (PIS) at day five and the mean number of days for the patient self assessment sheet (PSAS) score to return to normal. Researchers detected no between‐group differences (Analysis 4.3; MD ‐0.10, 95% CI ‐0.45 to 0.25; participants = 110; Analysis 4.4; MD 0.01, 95% CI ‐0.67 to 0.69; participants = 110), but we have low confidence in both estimates as the result of imprecision and lack of clarity about the rigorous validation of the scoring systems used. Cronin 2015 reported the paediatric respiratory assessment measure (PRAM) score at day four as the primary outcome for which the study was powered and detected no between‐group differences (Analysis 4.5; MD 0.00, 95% CI ‐0.36 to 0.36). Qureshi 2001, again a trial of prednisolone versus dexamethasone, reported separately persistent cough, wheeze, chest tightness, night wakening and difficulty maintaining normal activities (Analysis 4.6). This study detected no between‐group differences, but we assessed this trial as having high risk of selection and performance bias.
3.3. Analysis.
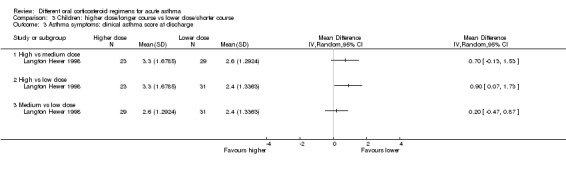
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: clinical asthma score at discharge.
3.4. Analysis.
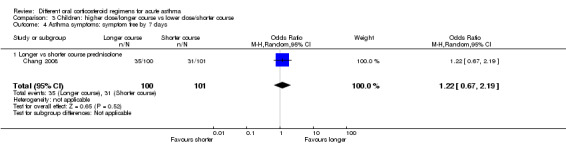
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 4 Asthma symptoms: symptom free by 7 days.
4.3. Analysis.
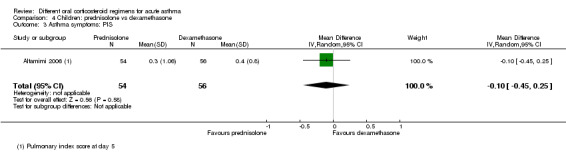
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 3 Asthma symptoms: PIS.
4.4. Analysis.
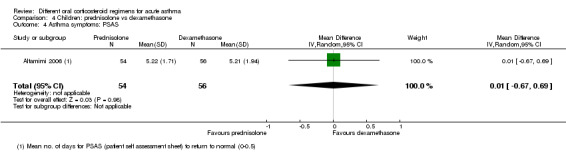
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 4 Asthma symptoms: PSAS.
4.5. Analysis.
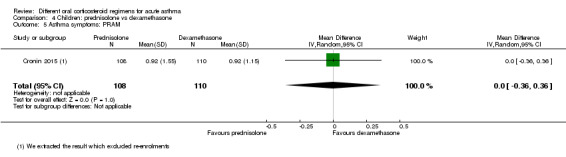
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 5 Asthma symptoms: PRAM.
4.6. Analysis.
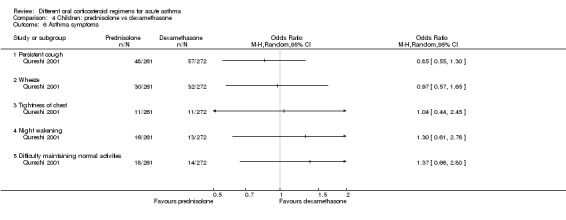
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 6 Asthma symptoms.
Serious adverse events
Included studies infrequently reported serious adverse events, and none of the studies in adults specifically reported this outcome. Five studies in children (Altamimi 2006; Chang 2008; Langton Hewer 1998; NCT00257933; Qureshi 2001), including a total of 695 participants, reported that there were no serious adverse events.
Secondary outcomes
New exacerbations during the follow‐up period
New exacerbations during the follow‐up period were reported by seven studies in adults (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993) and eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; NCT00257933; Qureshi 2001). New exacerbations were classified in two main ways: those requiring an unscheduled visit to a healthcare provider, and those requiring the prescription of additional oral corticosteroids. Overall, no included study reported a clear, unbiased benefit of one regimen over another, and varied interventions and definitions of an exacerbation prevented a unifying meta‐analysis.
Exacerbation requiring a visit to a healthcare provider: adults
Four small studies in adults (Cydulka 1998; Hasegawa 2000; Karan 2002; O’Driscoll 1993; total n = 96; Analysis 1.4) that compared longer versus shorter courses of prednisolone or stable versus tapered prednisolone reported exacerbations requiring a visit to a healthcare professional during the follow‐up period. Only eight events were reported across the four studies, resulting in insufficient data to ascertain possible differences between interventions for this outcome. Our confidence in these estimates was further reduced by concerns about selection, performance and detection bias in two of the contributing studies (Hasegawa 2000; Karan 2002) and by indirectness of the treatment regimens used, which deviated widely from current standard practice.
1.4. Analysis.
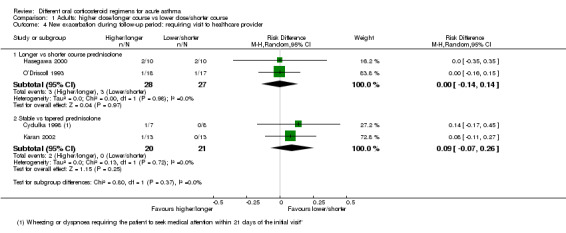
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 4 New exacerbation during follow‐up period: requiring visit to healthcare provider.
Kravitz 2011, a study involving adults that compared prednisolone versus dexamethasone, separately reported exacerbations requiring an emergency department visit and those requiring a visit to a primary healthcare provider. Investigators detected no differences between the two interventions for this outcome, but confidence intervals did not exclude the possibility of risk or harm for either intervention (Analysis 2.3; Analysis 2.4); we assessed this study to be at high risk of attrition bias, further limiting our confidence in this estimate.
2.3. Analysis.
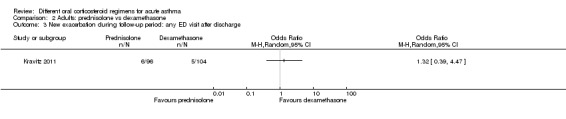
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 3 New exacerbation during follow‐up period: any ED visit after discharge.
2.4. Analysis.
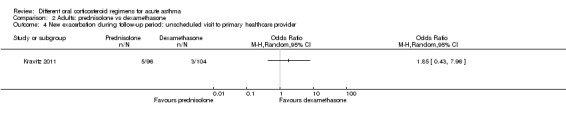
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider.
Exacerbation requiring a visit to a healthcare provider: children
Five studies in children ‐ one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010; Analysis 3.7) and four comparing prednisolone versus dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001; Analysis 4.8) reported exacerbations requiring an unscheduled visit to a healthcare provider during the follow‐up period. The results reported by Ghafouri 2010 favoured a shorter over a longer course of dexamethasone for this outcome but with wide confidence intervals, which do not exclude the possibility that the longer course may be more beneficial (OR 2.17, 95% CI 0.67 to 7.01; participants = 100). In addition to our serious concerns about imprecision, we considered this study to be at high risk of bias in several domains.
3.7. Analysis.
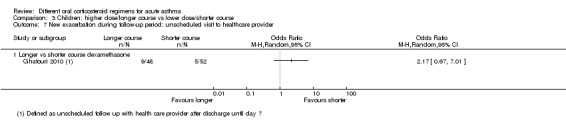
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.
4.8. Analysis.
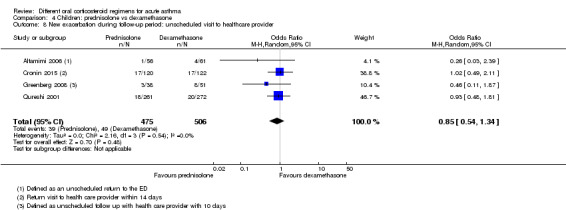
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.
The four studies investigating prednisolone versus dexamethasone favoured prednisolone, but again the confidence intervals did not exclude potential risk or benefit of either steroid for this outcome (Analysis 4.8; OR 0.85, 95% CI 0.54 to 1.34; participants = 981; I2 = 0%). Of note, Qureshi 2001 carried out an intention‐to‐treat analysis for this outcome, assuming that all children excluded because of vomiting or lost to follow‐up had a relapse; this analysis favoured dexamethasone, but confidence intervals did not exclude the possibility of no differences (OR 0.61, 95% CI 0.35 to 1.05), and we assessed this study as having high risk of selection and performance bias. We also rated Cronin 2015 as having high risk of performance and detection bias for this outcome, and we are uncertain about the effect that repeated enrolment of the same participants may have had on this outcome.
Exacerbation requiring additional oral corticosteroids: adults
Three studies in adults (Jones 2002; Lederle 1987; O’Driscoll 1993) that compared longer courses versus shorter courses of prednisolone reported exacerbations requiring an additional course of oral steroids during the follow‐up period. Results favoured a longer course of steroids, but the confidence intervals did not exclude the possibility of no differences or benefit derived from a shorter course (Analysis 1.5; OR 0.62, 95% CI 0.23 to 1.68; participants = 122; I2 = 0%). In addition, as already described, our confidence in the applicability of this finding to a population with asthma is reduced by the likelihood that many of the participants in Lederle 1987 had co‐morbid COPD, and that the higher event rate in this study dominated the analysis.
1.5. Analysis.
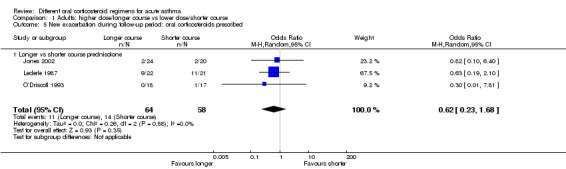
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 5 New exacerbation during follow‐up period: oral corticosteroids prescribed.
Viska 2008, a conference abstract, also reported 'relapse'. We did not include this study in the quantitative synthesis, as the total 'n' for each intervention group (higher‐ vs lower‐dose prednisolone) was not given. However, the abstract reported no differences between the two treatment arms for this outcome.
Exacerbation requiring additional oral corticosteroids: children
Finally, five studies in children ‐ two comparing higher versus lower doses of prednisolone (Kayani 2002; NCT00257933), one comparing a longer versus a shorter course of prednisolone (Chang 2008), one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010) and one comparing prednisolone and dexamethasone (Cronin 2015) ‐ reported exacerbations requiring an additional course of oral steroids. As for previous outcomes, events in Chang 2008, Ghafouri 2010, Kayani 2002 and NCT00257933 were rare, and none of these analyses demonstrated a conclusive benefit of one regimen over the other (Analysis 3.6). Our confidence in these estimates is moderate (Chang 2008) or low (Ghafouri 2010; Kayani 2002; NCT00257933) because of concerns about imprecision and risk of bias. Cronin 2015 detected benefit in favour of prednisolone (Analysis 4.9; OR 0.29, 95% CI 0.10 to 0.81; participants = 242). However, as the study authors discuss, this finding may be related to unblinded clinicians who tended to favour prednisolone over dexamethasone and were more inclined to prescribe additional steroids for those in the dexamethasone intervention group, reducing our confidence in this result.
3.6. Analysis.
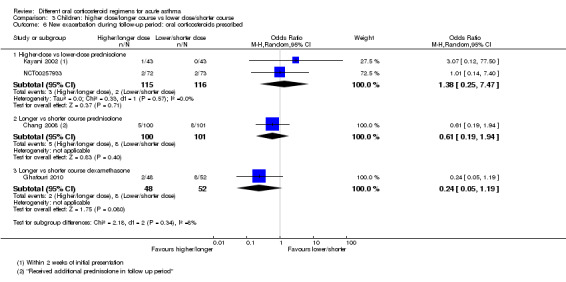
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 6 New exacerbation during follow‐up period: oral corticosteroids prescribed.
4.9. Analysis.
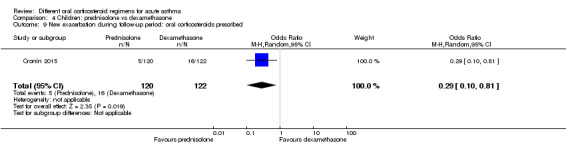
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 9 New exacerbation during follow‐up period: oral corticosteroids prescribed.
Lung function tests
Some included studies reported lung function test results, predominantly peak expiratory flow rates (PEFRs) and forced expiratory volume in one second (FEV1), but overall these studies did not identify a conclusive benefit of one steroid regimen over another.
PEFR: adults
Two studies of adult participants that compared longer courses versus shorter courses of prednisolone (Jones 2002; O’Driscoll 1993) reported trough PEFR. Although a combined analysis of results of these two studies did not suggest differences between treatment regimens, the confidence intervals did not rule out a perceivable difference between trial arms (Analysis 1.6; MD ‐4.81, 95% CI ‐45.82 to 36.20; participants = 79; I2 = 0%). Viska 2008, a conference abstract, randomised adult participants to higher‐ versus lower‐dose prednisolone and reported PEFR at four weeks but did not reveal total 'n' for each group and reported no variance, so we were unable to include this study in the quantitative synthesis. Mean PEFR at four weeks (two weeks post treatment) for the higher‐dose group was 272.89 L/min, and for the lower‐dose group 296.11 L/min.
1.6. Analysis.
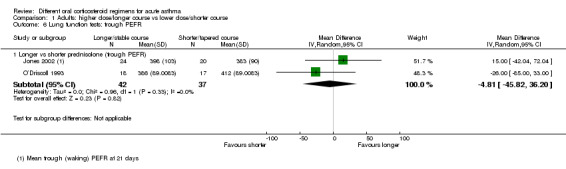
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 6 Lung function tests: trough PEFR.
FEV1: adults
Two small studies of stable (higher total dose) versus tapered (lower total dose) prednisolone, given for the same duration (Cydulka 1998; Karan 2002), reported FEV1% predicted at 21 days (exact timing of the test not specified). Again, although investigators detected no differences between treatment regimens, we cannot conclude that the regimens are equivalent because data provided were insufficient (Analysis 1.7; MD ‐1.02, 95% CI ‐4.62 to 2.58; participants = 41; I2 = 0%); our confidence in this result is further reduced by the indirectness of treatment regimens used in these studies and by the unusually small standard deviations reported.
1.7. Analysis.
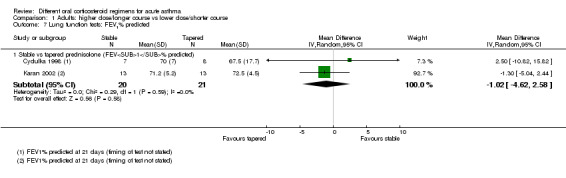
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 7 Lung function tests: FEV1% predicted.
PEFR and FEV1: children
In children, only one study, which compared high‐, medium‐ and low‐dose prednisolone (Langton Hewer 1998), measured FEV1% predicted (Analysis 3.8) and PEFR% predicted (Analysis 3.9) at discharge in a small subgroup of participants who were able to perform these tests. Results were inconsistent (i.e. did not demonstrate a dose‐response relationship) and confidence intervals were overlapping for all three comparisons (high vs medium, high vs low and medium vs low) for both outcomes.
3.8. Analysis.
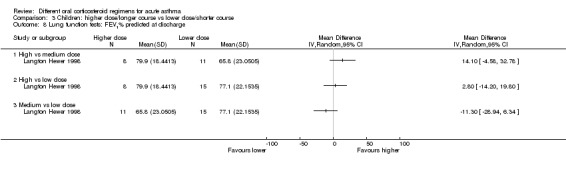
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: FEV1% predicted at discharge.
3.9. Analysis.
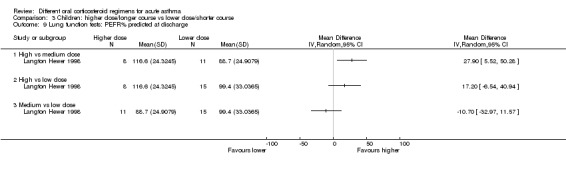
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 9 Lung function tests: PEFR% predicted at discharge.
All adverse events/side effects
Similarly to serious adverse events, all adverse events were not frequently reported by the included studies, and when they were reported, benefit of one regimen over another was not generally shown.
Adults
Lederle 1987, a study of long tapering (seven weeks) versus short tapering (seven days) of prednisolone, was the only study including adults that reported adverse events. These were defined as 'steroid side effects', including weight gain, oedema, acne and easy bruising. Findings favoured a shorter taper but with very wide confidence intervals, which did not exclude the possibility of no differences (Analysis 1.9; OR 4.15, 95% CI 0.94 to 18.41; participants = 43). Of note, many participants likely had COPD with reversibility and may represent a distinctly different group from participants in the other included studies. Our confidence in this result is very low.
1.9. Analysis.
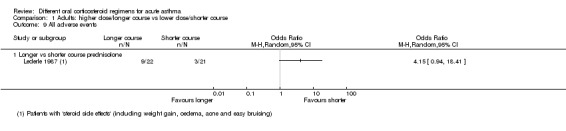
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 9 All adverse events.
Children
In children, only one study of a five‐ versus three‐day course of prednisolone (Chang 2008) reported all adverse events. Events were too infrequent to permit conclusions about the relative safety of a longer course versus a shorter course (Analysis 3.10; OR 0.67, 95% CI 0.11 to 4.08; participants = 201). Two studies of higher‐dose versus lower‐dose prednisolone (Kayani 2002; NCT00257933) specifically reported recognised steroid side effects (facial fullness, facial erythema, change in appetite, abdominal pain, diarrhoea, anxiety, euphoria, depression, quiet and reserved manner, hyperactivity and aggressive behaviour). Langton Hewer 1998 also specifically reported 'hyperactivity related to beta‐agonist use', which we combined with findings of the two aforementioned studies in a meta‐analysis. None of the meta‐analyses showed clear benefit of one regimen over another. Of note, analyses of anxiety, hyperactivity and aggressive behaviour demonstrated high levels of heterogeneity, and many showed substantial imprecision (Analysis 3.11).
3.10. Analysis.
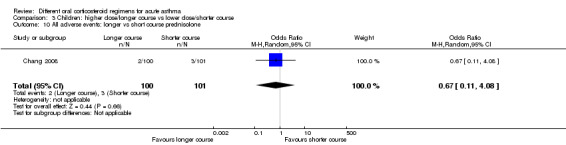
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 10 All adverse events: longer vs short course prednisolone.
3.11. Analysis.
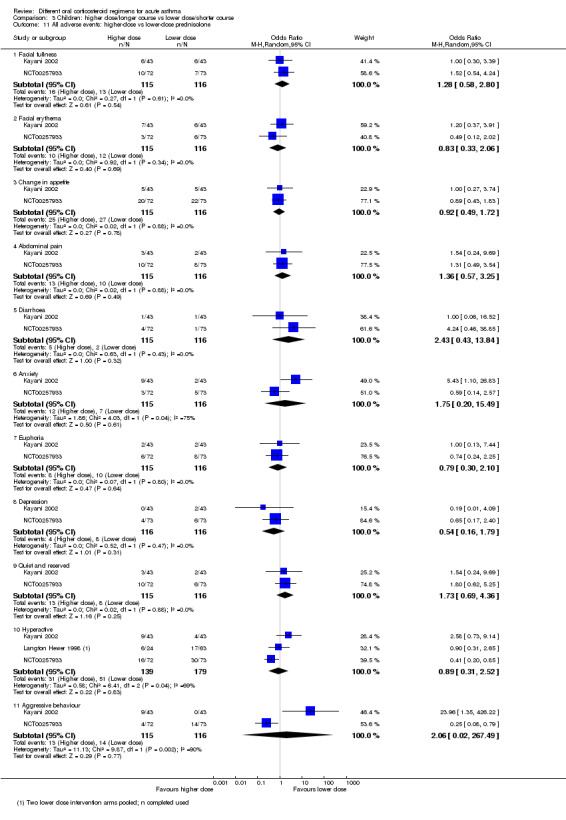
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 11 All adverse events: higher‐dose vs lower‐dose prednisolone.
Finally, Cronin 2015, Greenberg 2008 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ specifically reported the adverse event of vomiting. Findings favoured dexamethasone, but with moderate heterogeneity, and the confidence interval did not exclude the possibility of no difference or modest benefit with use of prednisolone (Analysis 4.10; OR 3.05, 95% CI 0.88 to 10.55; participants = 867; I2 = 53%).
4.10. Analysis.
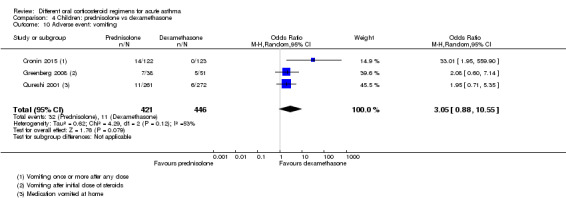
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 10 Adverse event: vomiting.
Discussion
Summary of main results
This review includes 18 studies that randomised a total of 2438 participants to comparisons of interest. Nine studies recruited only children, and seven only adults. Two studies did not report the age range of participants; we assumed one to be a study in adults, as the steroid doses described were consistent with treatment of adults (Aboeed 2014); the other was presented as a conference abstract, which did not contribute to the quantitative synthesis (Viska 2008). The included studies assessed higher versus lower doses of prednisolone (n = 4); longer versus shorter courses of prednisolone (n = 3) or dexamethasone (n = 1); tapered versus non‐tapered courses of prednisolone (n = 4); and prednisolone versus dexamethasone (n = 6). The varied interventions and outcomes reported limited the number of meaningful meta‐analyses that we could perform.
Overall, we did not find convincing evidence of a difference in outcomes between a higher dose or a longer course and a lower dose or a shorter course prednisolone or dexamethasone, or between prednisolone and dexamethasone. For two of our primary outcomes ‐ hospital admission and serious adverse events ‐ events were too infrequent to allow a conclusion about the superiority of one treatment over the other, or about their equivalence. Included studies reported asthma symptoms several different ways and rarely used validated scales, again limiting the conclusions that we could reach. Secondary outcome meta‐analysis was similarly hampered by heterogeneity among the interventions and outcomes measures used.
Included studies generally were of reasonable methodological quality, but generation of the randomisation sequence, allocation procedures and blinding of outcome assessors were frequently inadequately described. In six studies, participants were not blinded to their group allocation (Figure 2). Most outcomes in the review were assessed to be of low or very low quality, meaning that we are not confident in the effect estimates. The predominant reason for downgrading was imprecision, but indirectness and risk of bias also reduced our confidence in some estimates (Table 1; Table 2; Table 3; Table 4).
Overall completeness and applicability of evidence
Although oral steroids are commonly used for asthma exacerbations worldwide, we identified only 18 studies of variable methodological quality that met our inclusion criteria.
Management of asthma exacerbations differs internationally, affecting the definition of a 'high‐' or 'low‐' dose regimen, or a 'short' or 'long' course. Some guidelines define a recommended range for the course of steroids (GINA 2015; NAEPP 2007); others emphasise that courses should be no less than five days but keep the length otherwise open‐ended (BTS/SIGN 2014). Regimens recommended by guidelines are likely to differ in cost and possibly in adherence, leaving practitioners in doubt about the preferred plan.
The recommendation to use a low or high dose or a short or long course might be understood differently in different countries if attention is not paid to the individual studies from which the evidence has been drawn. Also, practice has changed over time. The dates of studies included in this review range from 1987 (Lederle 1987) to 2015 (Cronin 2015), and what was considered a 'shorter regimen' in an earlier study might be considered a 'longer regimen' today. Indeed, although many of the included studies compared currently used regimens, others used uncommon doses or lengths of treatment in one or both trial arms that are not recommended by current guidelines and are not commonly used in practice today (Cydulka 1998; Hasegawa 2000; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), limiting the applicability of evidence derived from these trials.
In terms of choice of steroid, prednisolone is recommended as first‐line in all guidelines, whether for adults or for paediatric patients, and the evidence presented in this review is not strong enough to indicate whether the usual second‐line option, dexamethasone, is better or worse than prednisolone. Of note, this review did not consider other head‐to‐head steroid comparisons, as the primary objective was to assess the evidence for different doses and durations. Indeed, several included studies, which compared dexamethasone versus prednisolone, gave very similar total steroid doses within each intervention arm (e.g. Aboeed 2014; Greenberg 2008; Kravitz 2011; Table 5) and so addressed a slightly different question. This may explain why researchers detected little difference between arms. We did not combine these studies with any that assessed a different total dose or duration of the same steroid.
An important question that is addressed by some of the included studies (Aboeed 2014; Altamimi 2006; Cronin 2015; Cydulka 1998; Karan 2002; Lederle 1987; Qureshi 2001) is whether duration or complexity of the regimen affects participant adherence. Potential benefits of a longer treatment course risk may be underestimated if adherence is suboptimal compared with a shorter or less complex course. In clinical practice, this may be a factor that affects an individual clinician's choice, depending on the behaviour and needs of a particular patient. For example, a clinician might choose a shorter course or the option with the fewest daily doses for patients who have trouble adhering to medications. This may be particularly true for the head‐to‐head comparison of dexamethasone versus prednisolone described in this review, wherein factors such as palatability may have resulted in differential adherence to treatment regimens. This review did not seek to address this question, but it may be an important topic for future research.
In addition, almost all of the included studies recruited participants from an emergency department setting, which limits the applicability of our findings to people with asthma exacerbations who present to a primary care provider.
We had planned to perform subgroup analyses to explore whether background asthma severity or severity of the exacerbation was an important effect modifier. However, this was not possible, as this information was not consistently reported by the included studies, and heterogeneity of the studies limited meta‐analysis.
Quality of the evidence
We assessed the quality of the evidence presented in this review according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) criteria (Higgins 2011) using GRADEpro software (GRADEpro GDT) and presented these assessments in the 'Summary of findings' tables.
Table 1 presents a higher dose/longer course versus a lower dose/shorter course of oral steroids in adults; Table 2 presents prednisolone versus dexamethasone in adults; Table 3; presents a higher dose/longer course versus a lower dose/shorter course of oral steroids in children; and Table 4 presents prednisolone versus dexamethasone in children. We assessed most outcomes to be of low or very low quality, meaning that we have limited confidence in the estimates.
We downgraded all outcomes at least once for imprecision, reflecting the small size of most of the included studies and the limited pooling that we were able to perform. Many effect estimates included a potentially important harm or benefit from either intervention, particularly for outcomes in which events were rare, such as admission to hospital or new exacerbations during the follow‐up period.
We also downgraded several outcomes because of concerns about possible performance and detection bias in the contributing studies (Cronin 2015; Ghafouri 2010; Karan 2002; Qureshi 2001), uncertainty about allocation procedures (Qureshi 2001) or attrition bias (Ghafouri 2010; Kravitz 2011; Langton Hewer 1998). Indirectness was a concern for outcomes contributed to by studies that used an intervention not currently used in common practice (Lederle 1987; Viska 2008) or that recruited a study sample likely to include many participants with co‐morbid COPD (Lederle 1987). We downgraded other outcomes for indirectness, as we had concerns about the rigorous validation of the measurement scales used (Altamimi 2006).
We did not suspect publication bias for any of the outcomes assessed. Pooled results appeared consistent, with low heterogeneity for almost all outcomes, likely reflecting our circumspect approach to combining data for which treatments, participants and underlying clinical questions were not similar enough for pooling to make sense.
Potential biases in the review process
We followed standard procedure according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to minimise bias in the review process. We performed a comprehensive search and think it unlikely that we failed to identify relevant studies. Two review authors independently screened the search, extracted study characteristics, and spot‐checked them for accuracy; and independently extracted all outcomes data, then checked them against the original report. Two or more review authors independently assessed risk of bias , showing a high level of agreement. These review authors resolved a few discrepancies by discussion, carried out GRADE assessments and achieved consensus by discussion.
However, our approach to the analysis required some flexibility, as we were unable to fully anticipate the nature of the outcome data that we would find. The precise comparisons used were inevitably performed post hoc as a result, and this introduced the risk of a data‐led analysis. We believe we mitigated for this risk by extensively discussing different approaches to the analysis and by seeking the independent opinion of the contact editor for the review.
Agreements and disagreements with other studies or reviews
Several systematic reviews have been published that address the question of the most effective dose of oral steroids for exacerbations of asthma. An 'umbrella review' (Krishnan 2009) concluded that doses of corticosteroids in excess of 50 to 100 mg per day offer no advantage over lower doses, and that a non‐tapering course given over five to 10 days is adequate for most patients. Although we did not consider the evidence presented in this review to be of sufficient quality to suggest that giving a dose over 50 to 100 mcg per day confers an advantage, we would agree that high doses have not generally proved more effective than lower doses. Furthermore, a daily dose of 50 to 100 mg exceeds the dose recommended by some current guidelines (BTS/SIGN 2014), and a five‐ to 10‐day range still leaves uncertainty for practitioners, which is especially important as many patients report that they experience unpleasant side effects while taking steroids.
An earlier Cochrane review (Manser 2001) assessed the evidence for the optimal dose of steroids, given by any route, for patients with severe asthma exacerbations requiring hospitalisation. This review also concluded that no evidence indicated that higher doses were associated with better outcomes or indeed with more adverse events. However, Manser 2001 included a cohort of patients with much more severe disease, and the doses given in the included studies far exceeded those assessed in this review, for example, high dose was considered greater than 360 mg per day methylprednisolone‐equivalent, medium dose between 80 and 360 mg and low dose 80 mg or less.
A meta‐analysis conducted to address the question of whether dexamethasone is an equivalent alternative treatment to prednisolone in children with acute asthma (Keeney 2014) included six studies, three of which are included in the current review. The additional three studies included in Keeney 2014 used dexamethasone given intramuscularly; therefore we excluded them. We also included Cronin 2015, published after Keeney 2014. However, the overall conclusions of Keeney 2014 are similar to ours; in terms of efficacy, one drug does not appear to be superior to the other. Study authors also note that dexamethasone may be associated with fewer episodes of vomiting and better adherence to prescribed therapy, but this is perhaps to be expected in a review that includes studies that used the intramuscular route for dexamethasone administration. We were unable to locate a systematic review that addressed this question in adult patients.
Authors' conclusions
Implications for practice.
The evidence is not strong enough for review authors to conclude that shorter or lower‐dose regimens are generally less effective than longer or higher‐dose regimens, or indeed that the latter are associated with more adverse events. In particular, important outcomes, such as serious adverse events and hospitalisations, occurred too infrequently for us to be certain whether one steroid regimen is superior to another. Changes to current practice should be supported by larger, well‐designed trials, and clinicians should continue to consider an individual patient's circumstances when choosing an oral corticosteroid regimen. Varied study design and outcome measures limited the number of meta‐analyses that we could perform. Some studies provided steroid regimens that are not recommended by major national or international guidelines, limiting the applicability of study findings to current practice.
Implications for research.
We were somewhat surprised by the relative paucity of evidence addressing this question. Larger studies will be required to determine whether differences between regimens can be found for rare, but important, outcomes such as serious adverse events and hospitalisations. Adherence to the prescribed regimen and palatability may also be important outcomes to include, to allow clinicians to continue to tailor treatment to individual patient circumstances. Triallists should aim to use validated measurement scales and should ensure that treatment regimens are relevant to current practice. In addition, we found few studies in which participants were recruited in a community setting ‐ where many prescriptions for oral steroids are supplied. Therefore, it is unclear how applicable our findings would be in this setting. We suggest that future trials could be conducted in this setting to improve the generalisability of findings of future reviews.
Acknowledgements
Chris Cates was the Editor for this review and commented critically on the protocol and the review.
Thank you to our user representative, Sarah Hymas, for her valuable input and advice.
Thank you to Chris Cates and Emma Welsh for editorial base support and advice.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
We thank Dr. Joel Kravitz for confirming methodological details of his trial (Kravitz 2011) and Professor Joseph Zorc (NCT00257933) for providing unpublished details of his trial. We are also very grateful for translations provided by Goto Yoshihito and Keiji Hayashi.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 (systemic* OR oral*) NEAR (steroid* or corticosteroid* or glucocorticoid)
#6 dexamethasone or decadron or prednisolone or pediapred or prednisone or sterapred or hydrocortisone or methylprenisolone or solucortef or solu‐cortef or solumedrol or solu‐medrol or betamethasone
#7 #5 or #6
#8 emergenc* or acute* or status or sever* or attack or crisis OR exacerbat* or critical
#9 #4 and #7 and #8
[Note: in search line #1, MISC1 denotes the field in the record in which the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Adults: higher dose/longer course vs lower dose/shorter course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐admission during follow‐up period | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Longer vs shorter course prednisolone | 4 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.38, 4.79] |
| 2 Asthma symptoms: asthma severity score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Longer vs shorter course prednisolone | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: complete resolution | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 New exacerbation during follow‐up period: requiring visit to healthcare provider | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Longer vs shorter course prednisolone | 2 | 55 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.14, 0.14] |
| 4.2 Stable vs tapered prednisolone | 2 | 41 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [‐0.07, 0.26] |
| 5 New exacerbation during follow‐up period: oral corticosteroids prescribed | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 5.1 Longer vs shorter course prednisolone | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 6 Lung function tests: trough PEFR | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Longer vs shorter prednisolone (trough PEFR) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐4.81 [‐45.82, 36.20] |
| 7 Lung function tests: FEV1% predicted | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Stable vs tapered prednisolone (FEV1% predicted) | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐4.62, 2.58] |
| 8 Lung function tests: number of participants achieving personal best at 4 weeks | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 All adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.8. Analysis.
Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: number of participants achieving personal best at 4 weeks.
Comparison 2. Adults: prednisolone vs dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐admission during follow‐up period | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Asthma symptoms: returned to normal activities within 3 days | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.01] |
| 3 New exacerbation during follow‐up period: any ED visit after discharge | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Children: higher dose/longer course vs lower dose/shorter course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission at initial presentation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Re‐admission during follow‐up period | 5 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Higher‐dose vs lower‐dose prednisolone | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: clinical asthma score at discharge | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Asthma symptoms: symptom free by 7 days | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 4.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 5 Serious adverse events | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 New exacerbation during follow‐up period: oral corticosteroids prescribed | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Higher‐dose vs lower‐dose prednisolone | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.25, 7.47] |
| 6.2 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.19, 1.94] |
| 6.3 Longer vs shorter course dexamethasone | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.19] |
| 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Lung function tests: FEV1% predicted at discharge | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lung function tests: PEFR% predicted at discharge | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 All adverse events: longer vs short course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.08] |
| 11 All adverse events: higher‐dose vs lower‐dose prednisolone | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Facial fullness | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.58, 2.80] |
| 11.2 Facial erythema | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.06] |
| 11.3 Change in appetite | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.72] |
| 11.4 Abdominal pain | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.57, 3.25] |
| 11.5 Diarrhoea | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.43, 13.84] |
| 11.6 Anxiety | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.20, 15.49] |
| 11.7 Euphoria | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.10] |
| 11.8 Depression | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.16, 1.79] |
| 11.9 Quiet and reserved | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.69, 4.36] |
| 11.10 Hyperactive | 3 | 318 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.52] |
| 11.11 Aggressive behaviour | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.02, 267.49] |
3.5. Analysis.
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 5 Serious adverse events.
Comparison 4. Children: prednisolone vs dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission at initial presentation | 3 | 1007 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 2 Re‐admission during follow‐up period | 3 | 985 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.33] |
| 3 Asthma symptoms: PIS | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.45, 0.25] |
| 4 Asthma symptoms: PSAS | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.67, 0.69] |
| 5 Asthma symptoms: PRAM | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 6 Asthma symptoms | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Persistent cough | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Wheeze | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Tightness of chest | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Night wakening | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Difficulty maintaining normal activities | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse events | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider | 4 | 981 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.34] |
| 9 New exacerbation during follow‐up period: oral corticosteroids prescribed | 1 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.81] |
| 10 Adverse event: vomiting | 3 | 867 | Odds Ratio (M‐H, Random, 95% CI) | 3.05 [0.88, 10.55] |
4.7. Analysis.
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 7 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboeed 2014.
| Methods |
Design: randomised trial; blinding not described Duration: corticosteroid treatment continued for 2‐5 days depending on allocation; participants followed up to 30 days (trial still ongoing) Setting: treatment initiated in the ED and completed at home; trial carried out in USA |
|
| Participants |
Population: 58 individuals with acute exacerbation of asthma randomised to receive prednisolone or dexamethasone (total number allocated to each group not reported) Age: not reported Inclusion criteria: participants with an acute asthma exacerbation Exclusion criteria: not reported Percentage withdrawn: not reported Allowed medication: "both arms received the same medical/pharmacologic interventions" Disallowed medication: not reported |
|
| Interventions |
Prednisolone group: 40 mg prednisolone once daily for 5 days (200 mg total dose prednisolone equivalent) Dexamethasone group: 16 mg dexamethasone once daily for 2 days (213 mg total dose prednisolone equivalent) |
|
| Outcomes | ED revisit rates, symptom resolution (defined as participant return to baseline or no limitation in daily activities), compliance with therapy | |
| Notes |
Type of publication: conference abstract; interim report of an ongoing study. Study authors contacted on 21 September 2015 for further information; at time of publication, no response received Funding: St. Joseph's Regional Medical Center |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details to make judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details to make judgement |
| Selective reporting (reporting bias) | High risk | Conference abstract, study incomplete, results not presented in a way that would allow inclusion in meta‐analysis. Unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Altamimi 2006.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 1‐5 days depending on allocation; followed up at 5 days and discharged from the study if fully recovered. Follow‐up for those not fully recovered continued for 3 weeks maximum Setting: treatment initiated in the ED and completed at home; trial carried out in Canada |
|
| Participants |
Population: 134 children with acute exacerbations of asthma randomised to receive prednisolone (n = 67) or dexamethasone (n = 67) Age: 2‐16 years; median age in the prednisolone group was 5 years and in the dexamethasone group 4 years Inclusion criteria: children presenting to the ED with a mild to moderate exacerbation of asthma with a history of ≥ 1 prior episode of wheezing or shortness of breath requiring treatment with salbutamol, mild to moderate exacerbation defined as PIS score < 9 and PEFR > 60% predicted Exclusion criteria: signs of severe asthma on presentation (PEFR < 60%, PIS ≥ 10); complete recovery after first dose of salbutamol; use of oral steroids in preceding 2 weeks; history of severe asthma exacerbation, including intubation or ICU admission for asthma, chronic lung disease, heart disease or neurological disorder; psychiatric disorder; history of acute allergic reaction, active chicken pox or herpes simplex infection Percentage withdrawn: withdrawal from the prednisolone group was 19.9% and from the dexamethasone group 16.4% Allowed medication: salbutamol Disallowed medication: inhaled corticosteroids |
|
| Interventions |
Prednisolone group: 1 mg/kg prednisolone twice daily for 5 days (maximum 30 mg per dose; total dose based on a 20 kg child 200 mg prednisolone equivalent) Dexamethasone group: 0.6 mg/kg dexamethasone as a single dose (maximum 18 mg; total dose based on a 20 kg child 80 mg prednisolone equivalent) |
|
| Outcomes | Number of days required for modified patient self assessment sheet (PSAS) score to return to baseline/PEFR to return to ≥ 80% predicted, adverse events, rescue medication use, unscheduled ED or family doctor visits, oxygen saturation, vital signs, PIS, participant compliance | |
| Notes |
Type of publication: peer‐reviewed Funding: Trudell Medical contributed peak flow meters; funding otherwise not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consenting participants were assigned via prepared, sealed, computer‐generated randomisation cards to receive dexamethasone or prednisolone |
| Allocation concealment (selection bias) | Low risk | Consenting participants were assigned via prepared, sealed, computer‐generated randomisation cards to receive dexamethasone or prednisolone |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy, with no involvement of study investigators, prepared randomisation cards and blended study medications to look and taste identical. Placebo medication was blended to mimic study medications. Participants receiving the single dose of dexamethasone were given placebo medication to complete a 5‐day course, as per prednisolone regimen |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, personnel and investigators were blinded to assignment and contents of study medication bottles |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out balanced and < 20% in both intervention arms; all withdrawals accounted for in study flow diagram |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported numerically apart from number of salbutamol administrations at home, which is reported narratively in the study report. However, unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Chang 2008.
| Methods |
Design: randomised (stratified by age and site of enrolment), double‐blind trial Duration: corticosteroid treatment continued for 3‐5 days depending on allocation; follow‐up continued to 28 days, or re‐admission to hospital, whichever occurred first Setting: treatment initiated in ED and completed at home; trial carried out in ED of 3 hospitals in Queensland, Australia |
|
| Participants |
Population: 201 children with acute exacerbation of asthma randomised to receive a longer course (n = 100) or a shorter course (n = 101) of prednisolone Age: 2‐15 years; mean age (SD) in prednisolone longer course group was 4.7 (3.1) years and in shorter course group 4.8 (2.8) years Inclusion criteria: children presenting with an acute exacerbation of asthma during ordinary hours (07:30–17:00) to the ED of 3 Queensland hospitals, but not hospitalised. Asthma was defined as recurrent (> 2) episodes of wheeze and/or dyspnoea with a clinical response (decreased respiratory rate and work of breathing) to salbutamol. Asthma exacerbation was defined as acute deterioration of asthma control requiring treatment with more than a single dose (> 600 μg via metered dose inhaler and spacer or > 2.5 mg nebulised) of salbutamol in an hour Exclusion criteria: underlying respiratory disease (e.g. bronchiectasis), cerebral palsy or severe neurodevelopmental abnormality, immunodeficiency, previous enrolment in the study, receiving maintenance oral corticosteroids, receiving > 1 dose of oral corticosteroids before presentation,very severe asthma (status asthmaticus; requiring hospitalisation, continuous nebulisation and/or intravenous salbutamol) Percentage withdrawn: withdrawal from longer course group was 15% and from shorter course group 20.1% Allowed medication: salbutamol Disallowed medication: additional course of oral corticosteroids |
|
| Interventions |
Prednisolone longer course group: 1 mg/kg prednisolone daily for 5 days (maximum dose 50 mg; total dose based on 20 kg child 100 mg prednisolone equivalent) Prednisolone shorter course group: 1 mg/kg prednisolone daily for 3 days (maximum dose 50 mg; total dose based on 20 kg child 60 mg prednisolone equivalent) |
|
| Outcomes | Proportion of children without asthma symptoms, as scored on validated diary cards on day 7 (children were considered still symptomatic if their average asthma score for the day was 0.2), PACQLQ scores on days 7 and 14, average asthma scores as provided on asthma and cough diary cards on days 5, 10 and 14, recurrence of exacerbation, unscheduled re‐presentation to a health facility | |
| Notes |
Type of publication: peer‐reviewed Funding: Asthma Foundation of Queensland and the Royal Children’s Hospital Foundation. All placebo and some active medication were donated by Aspen Pharmacare Study identifier: Australian Clinical Trials Registry; ACTRN012605000305628 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomised within strata of age (< 6 or 6–15 years) and site of enrolment. On recruitment, children were allocated to the next treatment regimen on a list (randomised by permutated block design at a remote site) |
| Allocation concealment (selection bias) | Low risk | A sticker obscured the next treatment group and was removed only after enrolment (concealed treatment allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Children received oral prednisolone for 5 days or prednisolone for 3 days, followed by placebo (a liquid with similar taste) for 2 days. Trial medications were stored in identical bottles and were labelled A and B. The study team (other than the pharmacist, who was not involved in data collection), children and parents were blinded to trial medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study team (other than the pharmacist, who was not involved in data collection), children and parents were blinded to trial medications. Code was revealed only after study and statistical analysis were completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out balanced and 15%‐20% in both intervention arms; all withdrawals accounted for and ITT data analysis performed for primary outcomes |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported; trial prospectively registered |
| Other bias | Low risk | None noted |
Cronin 2015.
| Methods |
Design: randomised, open‐label trial Duration: corticosteroid treatment continued for 1‐3 days with follow‐up for 2 weeks Setting: treatment initiated in the ED and completed at home; trial carried out in Ireland |
|
| Participants |
Population: 250 children with an acute exacerbation of asthma presenting to the ED at a hospital in Dublin were randomised to a 3‐day course of prednisolone (n = 123) or a 1‐day (single dose) course of dexamethasone (n = 127). NB: 19 participants were enrolled more than once during the course of the study; in total, 226 individual children participated Age: 2‐16 years; mean age (SD) in prednisolone group was 5.8 (3.22) years and in dexamethasone group 5.7 (3.52) years Inclusion criteria: children aged 2‐16 years with a history of asthma who presented to the ED with an acute asthma exacerbation. A history of asthma was defined as ≥ 1 previous episode of beta‐2‐agonist–responsive wheeze or previous diagnosis of asthma, made by a paediatrician or clinician of comparable experience. An exacerbation of asthma was defined as acute asthma that prompts ED assessment, with any or all of the following clinical features: dyspnoea, wheeze, acute cough, increased work of breathing, increased requirement for beta‐2‐agonist from baseline use or SaO2 < 95% Exclusion criteria: children with critical or life‐threatening asthma exacerbation, active varicella or herpes simplex infection; documented concurrent infection with respiratory syncytial virus; temperature > 39.5°C; use of oral or intravenous corticosteroids in previous 4 weeks; concurrent stridor, galactose intolerance, Lapp‐lactase deficiency or glucose galactose malabsorption, history of tuberculosis exposure or significant co‐morbid disease Percentage withdrawn: withdrawal from prednisolone group 3.2% and from dexamethasone group 0.8% Allowed medication: standard therapy according to guidelines and at the discretion of the treatment physician, including inhaled beta‐2‐agonist and ICS (if participant was already taking this at baseline) Disallowed medication: not reported |
|
| Interventions |
Prednisolone group: 1 mg/kg once daily for 3 days (maximum dose 40 mg; total dose based on 20 kg child 60 mg prednisolone equivalent) Dexamethasone group: 0.3 mg/kg once daily for 1 day (maximum dose 12 mg; total dose based on 20 kg child 40 mg prednisolone) |
|
| Outcomes |
Primary outcome: Pediatric Respiratory Assessment Measure (PRAM) score at day 4 Secondary outcomes: change in PRAM score from ED arrival to follow‐up, PRAM score at ED discharge, hospital admission from ED on day 1, ED length of stay, unscheduled visits to healthcare provider for asthma or respiratory symptoms within 14 days of study enrolment, re‐admission to hospital after discharge and within 14 days of study enrolment, administration of additional systemic corticosteroids within 14 days of study enrolment, number of salbutamol therapies administered after enrolment, incidence of vomiting within 30 minutes of study medication, school days and parental workdays missed and days of restricted activity |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a randomization design achieved by generating numeric codes in random permuted blocks of 12 subjects. The randomization process was designed by the study statistician and was kept in a locked storage cupboard in the hospital’s pharmacy department" |
| Allocation concealment (selection bias) | Low risk | "The recruiting clinician took the next available numbered envelope from the prerandomized pack of study envelopes contained in a locked storage cupboard in the ED. This envelope contained the subject identification number of each enrolled patient and stated to which treatment arm they were assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study; participants and personnel were aware of assignment status; this may have affected their performance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was an open‐label study. The PRAM outcome "was performed by a senior physician blinded to treatment allocation. Patients and families were instructed not to reveal treatment allocation to the clinician measuring the PRAM score on day 4." For other outcomes, such as additional courses of steroids or visits to HCP, the study is at higher risk. Overall, we rated this risk as unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low and balanced drop‐out, and all participants accounted for. Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial and published protocol. Some planned outcome measures were not clearly reported (e.g. compliance, costs), but these were not of interest in this review |
| Other bias | Unclear risk | 19 participants were enrolled more than once during the course of the study. With the exception of the 4‐day PRAM score, it is unclear from the report whether some participants contributed more than once to secondary outcomes |
Cydulka 1998.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 8 days, with follow‐up continuing to 3 weeks Setting: treatment initiated in the ED and completed at home; trial carried out in USA |
|
| Participants |
Population: 15 adults with an acute exacerbation of asthma were randomised to an 8‐day non‐tapering (n = 7) or an 8‐day tapering course (n = 8) of prednisolone Age: 19‐50 years; mean age (SD) in non‐tapering group was 24.1 (5.0) years and in tapering group 32.0 (8.5) years Inclusion criteria: Participants 19‐50 years of age with acute asthma exacerbation presenting to the ED but judged well enough to be discharged from the ED were recruited. Participants were judged suitable for discharge by the attending physician if they exhibited complete relief of wheezing or improvement in FEV1 to ≥ 70% predicted, or if they reported significant subjective improvement to near baseline Exclusion criteria: participants with history of chronic obstructive pulmonary disease, acute congestive heart failure, pneumonia, pneumothorax or any other acute pulmonary disease, such as lung cancer, tuberculosis or sarcoidosis, that might confound the results; patients already using inhaled or oral steroids, those requiring long‐term steroid use, as defined by daily steroid use, those who had required steroids within 2 weeks of admission to the ED, patients with a history of diabetes or severe hypertension Percentage withdrawn: withdrawal 0% in both treatment arms Allowed medication: standard therapy with aerosolised albuterol for a total of 3 doses Disallowed medication: not reported |
|
| Interventions |
Prednisolone non‐taper group: 40 mg/d prednisolone for 8 days (total dose 320 mg prednisolone equivalent) Prednisolone taper group: 40 mg/d prednisolone tapering by 5 mg/d over 8 days (total dose 180 mg prednisolone equivalent) |
|
| Outcomes | Relapse (defined as return of wheezing or dyspnoea requiring the participant to seek medical attention within 21 days of initial visit), pulmonary function tests, cosyntropin stimulation test, compliance with medication, symptoms | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients…were randomised via a computer‐generated randomisation table to 1 of 2 treatment regimens |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants in the taper group were given 8 tablets to take each day: 5 mg prednisone tablets, up to the daily dose of prednisone, plus placebo look‐alike tablets constituting the remainder of the 8 tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial described as double‐blind, although blinding procedure for outcome assessors not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out not specifically reported, but endpoint outcome data available for all 15 participants |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported numerically or narratively. However, unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Ghafouri 2010.
| Methods |
Design: randomised, open‐label trial Duration: corticosteroid treatment continued for 1‐2 days depending on allocation; follow‐up continued until 7 days Setting: treatment initiated in the ED and completed at home; trial carried out in USA |
|
| Participants |
Population: 125 children presenting with a mild to moderate exacerbation of asthma were randomised to 2 doses (n = 63) or a single dose (n = 62) of dexamethasone Age: 2‐17 years. Mean age (SD) in longer course group was 5.9 (4.3) years and in shorter course group 6.0 (3.6) years Inclusion criteria: children aged 2‐17 years who presented to the ED with a mild to moderate exacerbation of asthma Exclusion criteria: not reported Percentage withdrawn: withdrawal from longer course was 23.8% and from shorter course 16% Allowed medication: not reported Disallowed medication: not reported |
|
| Interventions |
Dexamethasone longer course group: 0.6 mg/kg of dexamethasone daily. First dose on day 1 and second dose on day 3 (maximum dose 16 mg; total dose based on 20 kg child 160 mg prednisolone equivalent) Dexamethasone shorter course group: 0.6 mg/kg of dexamethasone as a single dose (maximum dose 16 mg; total dose based on 20 kg child 80 mg prednisolone equivalent) |
|
| Outcomes | Time to resolution of symptoms, relapse rate (defined as hospital admission after ED discharge), unscheduled follow‐up visits, additional corticosteroids prescribed within 7 days of ED discharge | |
| Notes |
Type of publication: conference abstract Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unbalanced attrition in intervention groups (23.8% in longer course group and 16% in shorter course group). Reasons for drop‐out not stated |
| Selective reporting (reporting bias) | Low risk | Conference abstract so study details minimal, but prospectively registered. All outcomes listed in clinical trials record reported |
| Other bias | Low risk | None noted |
Greenberg 2008.
| Methods |
Design: block‐randomised, double‐blind trial Duration: corticosteroid treatment continued for 2‐5 days depending on allocation; follow‐up continued until 10 days Setting: treatment initiated in the ED and completed at home |
|
| Participants |
Population: 167 children presenting to the ED with an acute exacerbation of asthma were enrolled. Numbers randomised to each treatment arm not given. 38 completed in the prednisolone arm and 51 in the dexamethasone arm. Age: 2‐18 years. Median age in prednisolone group 6.2 years and in dexamethasone group 4.5 years (range 2‐18 years for both groups) Inclusion criteria: children 2‐18 years old with a history of asthma (≥ 2 episodes of wheezing treated with beta‐2‐adrenergic agonists) who presented to the ED with an acute exacerbation of their asthma Exclusion criteria: use of oral steroids in the past month; history of intubation for a previous asthma exacerbation; varicella exposure in the past 3 weeks; possible foreign body aspiration; any chronic lung disease (e.g. cystic fibrosis) that would affect the participant’s treatment; chronic heart, liver or kidney disease; significant respiratory distress necessitating airway intervention (e.g. intubation); previous enrolment in this study; no telephone for follow‐up; ≥ 2 episodes of emesis after steroid administration in the ED Percentage withdrawn: total exclusion after enrolment 46.7%; numbers excluded from each arm not reported Allowed medication: all participants with an acute asthma exacerbation were treated according to the institution’s asthma clinical care guideline. Children received 3 consecutive nebulisers with albuterol and ipratropium bromide. At the time of discharge, participants received instructions to use their albuterol every 4 hours for 24 hours, then as needed for symptom relief Disallowed medication: not reported |
|
| Interventions |
Prednisolone group: 1 mg/kg prednisolone twice daily for 5 days (maximum dose 30 mg; total dose based on 20 kg child 200 mg prednisolone equivalent) Dexamethasone group: 0.6 mg/kg once daily for 2 days (maximum dose 16 mg; total dose based on 20 kg child 160 mg prednisolone equivalent) |
|
| Outcomes | Relapse within 10 days (defined as need for subsequent hospitalisation or unscheduled visit with a medical provider as the result of continued or worsening asthma symptoms), emesis with steroid administration in the ED | |
| Notes |
Type of publication: peer‐reviewed Funding: supported by Grant Number MO1‐RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐randomisation (< 7 years and ≥ 7 years) was performed in the hospital pharmacy |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure double‐blinding, the pharmacy prepared both drugs to look identical as a white powder in a clear capsule. Older participants swallowed the capsule, and younger participants had the powder mixed with applesauce or pudding for ease of administration. All capsules, including placebo, were identical in appearance and were placed in capsule bubble packets labelled dose 1 through 10 to ensure that participants in the dexamethasone group received the second dose of dexamethasone as their next dose and then started placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial described as double‐blind, although blinding procedure for personnel not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Approximately half of participants randomised to each treatment did not complete the trial. The most frequent reason was related to hospital admission. Although details are given of baseline characteristics of those who completed and those who did not, it is unclear how this high level of attrition may have affected the findings |
| Selective reporting (reporting bias) | Low risk | All started outcomes reported numerically or narratively, but unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Hasegawa 2000.
| Methods |
Design: randomised trial; blinding not reported Duration: oral corticosteroid treatment continued for 1‐2 weeks depending on allocation; follow‐up continued until 6 months after initiation of oral steroids Setting: inpatient; trial carried out in Japan |
|
| Participants |
Population: 20 individuals with an acute exacerbation of asthma were randomised to 2‐week course (n = 10) or 1‐week course (n = 10) of prednisolone. Oral therapy was commenced after all participants had received 3 days of intravenous methylprednisolone (80 mg every 8 hours) Age: age range not reported; mean age (SD) in the longer course group was 49 (4.5) years and in the shorter course group 52 (6) years Inclusion criteria: "asthmatics who were admitted to our hospital due to acute exacerbation" Exclusion criteria: "near fatal attacks, serious complicated disease, pregnancy" Percentage withdrawn: withdrawal 0% in both treatment arms Allowed medication: intravenous methylprednisolone, 80 mg every 8 hours for 3 days after admission, antibiotics, theophylline Disallowed medication: not reported |
|
| Interventions |
Prednisolone longer course group: 0.5 mg/kg prednisolone once daily for 2 weeks (maximum doses not given but based on a 70 kg adult total dose would be 490 mg prednisolone equivalent) Prednisolone shorter course group: 0.5 mg/kg prednisolone once daily for 1 week (maximum doses not given but based on a 70 kg adult total dose would be 245 mg prednisolone equivalent) |
|
| Outcomes | Morning PEFR, unscheduled hospital visits due to asthma in the 3 months after discharge | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were then randomly allocated into 2 group"; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial not reported as blinded, so assume open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial not reported as blinded, so assume open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all 20 enrolled participants |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported, but unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Jones 2002.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 5‐10 days depending on allocation; follow‐up continued until 4‐6 weeks Setting: treatment initiated on an inpatient ward and completed at home; trial carried out in the UK |
|
| Participants |
Population: 47 adults with an acute exacerbation of asthma were randomised to a longer course (n = 25) or a shorter course (n = 22) of prednisolone Age: 16‐60 years; mean age (SD) in the longer course group was 29.8 (11.3) years and in the shorter course group 32.0 (11.0) years Inclusion criteria: acute adult asthma (peak expiratory flow (PEF) < 65% predicted), admission to hospital under the care of designated adult physicians, age 16‐60 years, ability to give informed consent and to maintain a PEF diary for 21 days, use of inhaled steroid on discharge Exclusion criteria: major medical illness (such as pneumonia, heart failure, lung cancer and bronchiectasis), chronic pulmonary disease other than asthma, requirement for mechanical ventilation before randomisation, long‐term use of oral corticosteroids, use of nebulised corticosteroids, any recent use of oral corticosteroids before admission Percentage withdrawn: withdrawal from longer course group 4% and from shorter course group 9.1% Allowed medication: All participants were issued a supply of open‐label prednisolone (40 mg for 5 days) for emergency use and were instructed that this should be taken in the event of deteriorating asthma and recommended to self refer to hospital under these circumstances. All other asthma treatment was provided at the discretion of the participant’s personal physician subject to a requirement for all participants to receive inhaled steroid treatment equivalent to ≥ 400 mcg of beclomethasone dipropionate per day Disallowed medication: not reported |
|
| Interventions |
Prednisolone longer course group: 40 mg prednisolone once daily for 10 days (total dose 400 mg prednisolone equivalent) Prednisolone shorter course group: 40 mg prednisolone once daily for 5 days (total dose 200 mg prednisolone equivalent) |
|
| Outcomes | Waking PEF, asthma exacerbations, post‐bronchodilator morning PEF, evening PEF, worst PEF on each day, symptom scores (overall asthma severity, wheeze severity, cough severity, nocturnal asthma symptoms), beta‐agonist use | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were entered in a double‐blind fashion. Randomisation codes (5‐ or 10‐day course) were sealed in opaque brown envelopes and shuffled into random order, then numbered sequentially |
| Allocation concealment (selection bias) | Low risk | Randomisation codes (5‐ or 10‐day course) were sealed in opaque brown envelopes...the investigator selected the next numbered envelope for each patient and sent it unopened to a non‐blinded hospital pharmacist with a prescription for ‘‘steroid trial tablets’’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were provided 40 mg prednisolone daily for the first 5 days, supplied as 5 mg prednisolone enteric‐coated tablets. For days 6‐10, each patient received 8 tablets per day of enteric‐coated prednisolone or an identical placebo tablet |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial described as double‐blind but specific details of blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low, balanced drop‐out in both groups; 3 participants in total did not complete their diary cards, but all 3 were reported to have made a satisfactory recovery and did not require further course of oral steroids or admission to hospital |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported, but unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Karan 2002.
| Methods |
Design: randomised, open‐label trial Duration: corticosteroid treatment continued for 8 days; follow‐up continued until 3 weeks Setting: treatment initiated at outpatient clinic and completed at home; trial carried out in India |
|
| Participants |
Population: 26 adults with an acute exacerbation of asthma were randomised to a non‐tapering course (n = 13) or a tapering course (n = 13) of prednisolone Age: 17‐70 years; mean age (SD) in non‐tapering group 43.9 (12.4) years and in tapering group 49.2 (12.1) years Inclusion criteria: aged 16‐70 years with an acute asthma exacerbation presenting to the chest clinic but judged well enough to be discharged (i.e. complete relief of wheezing or improvement in FEV1 to ≥ 70% predicted, or reporting subjective significant improvement in symptoms to near baseline) Exclusion criteria: history of chronic obstructive pulmonary disease, acute congestive heart failure, pneumonia, pneumothorax or any other acute pulmonary disease, such as lung cancer, tuberculosis or sarcoidosis, etc, that might confound results; asthmatic patients already using inhaled or oral steroids; long‐term steroid use, as defined by daily steroid use; steroids required within 2 weeks of admission to the chest clinic; history of diabetes or severe hypertension Percentage withdrawn: not reported Allowed medication: other asthma treatments given to both groups as per hospital policy including beta‐2‐agonists, sustained release theophylline and inhaled steroids Disallowed medication: not reported |
|
| Interventions |
Prednisolone non‐taper group: 40 mg prednisolone once daily for 8 days (total dose 320 mg prednisolone equivalent) Prednisolone taper group: 40 mg prednisolone once daily tapering by 5 mg/d over 8 days (total dose 180 mg prednisolone equivalent) |
|
| Outcomes | Relapse (defined as return of wheezing or dyspnoea requiring participant to seek medical attention within 21 days of initial visit), pulmonary function tests, adrenal suppression as assessed by low‐dose ACTH test, compliance with medication | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomised' but insufficient detail to make judgement about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All but 2 participants completed the trial; outcomes analysed as per ITT principles (last observation carried forward) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported, but unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Kayani 2002.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 5 days with follow‐up until 2 weeks (additional 1‐month follow‐up in children with reported behavioural symptoms) Setting: treatment initiated in outpatient clinic or ED and completed at home; trial carried out in USA |
|
| Participants |
Population: 88 children with an acute exacerbation of asthma were randomised to receive a higher dose (n = 44) or a lower dose (n = 44) of prednisolone Age: 2‐18 years; mean age (SD) in higher‐dose group 6.3 (0.5) years and in lower‐dose group 7.1 (0.6) years Inclusion criteria: participants aged 2‐18 years with mild persistent asthma at baseline based on National Institutes of Health guidelines (cough, shortness of breath or wheeze more than twice a week but less than once a day and similar nighttime symptoms more than twice a month but less than once a week), those receiving inhaled steroids (fluticasone 44 mcg (2 puffs) bid) daily and using an albuterol metered dose inhaler (MDI) as needed. Indications for therapy with oral steroids were an incomplete response to therapy for acute symptoms with agonists and inhaled steroids. Incomplete response to therapy was defined as persistence of cough, shortness of breath or wheeze after 3‐agonist treatment via nebuliser over a 1‐hour period or lack of response to 3‐agonist treatment of 2 to 4 puffs by MDI over 1 hour Exclusion criteria: history of chronic lung disease other than asthma; cardiac, liver or renal disease; attention deficit disorder; previous or current history of psychiatric illness; use of oral steroids within previous 2 weeks Percentage withdrawn: withdrawal from both groups 2.2% Allowed medication: doubled‐dose inhaled corticosteroids, as required short‐acting beta‐2‐agonists Disallowed medication: oral corticosteroids within preceding 2 weeks |
|
| Interventions |
Prednisolone higher‐dose group: 2 mg/kg daily prednisolone (given in 2 divided doses) for 5 days (total maximum daily dose 60 mg; total dose for 20 kg child 200 mg prednisolone equivalent) Prednisolone lower‐dose group: 1 mg/kg daily prednisolone (given in 2 divided doses) for 5 days (total maximum daily dose 60 mg; total dose for 20 kg child 100 mg prednisolone equivalent) |
|
| Outcomes | At 5 days: questionnaire asking about most common side effects of steroids, including facial fullness, facial redness, changes in appetite, abdominal pain, diarrhoea, quiet and reserved manner, euphoria (excessive happiness), depression, anxiety, hyperactivity with or without short attention span, aggressive behaviour (responses considered positive only if symptoms were absent before initiation of steroid therapy); any associated systemic symptoms; asthma symptom resolution (cough, shortness of breath and wheeze). Two weeks later: use of additional medications since oral steroid treatment, resolution of symptoms (cough, shortness of breath and wheeze), relapse (defined as presence or worsening of cough, wheezing, visits to physician’s office or emergency department or admission to hospital). One month later: further follow‐up of participants with behavioural symptoms |
|
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants enrolled in the study were given 1 of 2 different doses of oral steroids according to a random allocation chart based on a table of random numbers. Randomisation code was held by nursing staff at the asthma centre |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, principal investigator and primary care physician were not told which dose of oral steroids the child was receiving |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parents, principal investigator and primary care physician were not told which dose of oral steroids the child was receiving, but outcome assessment may not have been blinded: "It would have been ideal to have the interviewer blinded to the study questions, but every effort was made to avoid any appearance of bias during the telephone interview" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 child excluded from analysis in each arm for protocol violations (1 patient in group 1 excluded because albuterol dosage was increased to every 4 hours; 1 patient in group 2 excluded because inhaled steroid (fluticasone) dose was increased to 110 mcg and albuterol was used every 4 hours) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported, although unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Kravitz 2011.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 2‐5 days depending on allocation; follow‐up continued until 2 weeks Setting: treatment initiated in the ED and completed at home; trial carried out in USA |
|
| Participants |
Population: 257 adults with an acute exacerbation of asthma were randomised and included to receive prednisolone (n = 128) or dexamethasone (n = 129). A total of 28 participants were excluded after randomisation as the result of admission to hospital Age: 18‐45 years; median age (IQR) in prednisolone group 30 (23‐38) years and in dexamethasone group 28 (22‐37) years Inclusion criteria: participants aged 18‐45 years, with a diagnosis of asthma for ≥ 6 months and peak expiratory flow rate < 80% predicted Exclusion criteria: those had received oral corticosteroids in the previous 4 weeks; patients who experienced chronic obstructive pulmonary disease, congestive heart failure, pneumonia or sarcoidosis; those who were pregnant or breastfeeding. Age limit of 45 years was chosen to try to avoid enrolling people with concurrent diagnosis of chronic obstructive pulmonary disease. Participants were also excluded if they gave a history of corticosteroid allergy, tuberculosis, systemic fungal disease, gastritis or diabetes, or if they were unable to consent to the study or to be available for follow‐up. Participants admitted to the hospital for asthma exacerbation were also excluded from the analysis Percentage withdrawn: withdrawal from prednisolone group was 25% and from the dexamethasone group 19% Allowed medication: nebulised albuterol and ipratropium bromide. Other asthma treatments were provided at the discretion of the treating physician Disallowed medication: oral corticosteroids within preceding 4 weeks |
|
| Interventions |
Prednisolone group: 50 mg once daily for 5 days (total maximum dose 250 mg prednisolone equivalent) Dexamethasone group: 16 mg daily for 2 days (total maximum dose 213 mg prednisolone equivalent) |
|
| Outcomes | Number of days required before return to normal daily activities, number of times albuterol was used per day in the week after ED visit, relapse (defined as repeated ED or primary care provider visits or admission to hospital for worsening of asthma exacerbation within 2‐week follow‐up period) | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation table maintained by the pharmacy department was used to assign participants to 1 of 2 treatment arms |
| Allocation concealment (selection bias) | Unclear risk | No specific details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients in the prednisone group received 5 medication packets labelled 1 through 5, each containing 60 mg of prednisone. Patients in the dexamethasone group received 5 identical medication packets; the first 2 contained 16 mg of oral dexamethasone in packets 1 and 2, with placebo doses in packets 3 through 5. Medications and placebo doses were prepared in identical capsules by the hospital’s pharmacy department, so that neither the treating emergency physician nor the enrolling research staff could discern which study medication was administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as double‐blind but only blinding of enrolling staff specifically described; blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% (85 out of 285) of all randomised participants did not complete the trial; 28 of these were admitted to hospital during initial ED presentation, after they had been randomised. Outcomes are unknown. A further 19% (dexamethasone group) and 25% (prednisolone group) were lost to follow‐up, so again, outcomes are unknown |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported numerically or narratively but unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Langton Hewer 1998.
| Methods |
Design: randomised (stratified by age and gender), double‐blind trial Duration: corticosteroid treatment continued while participants admitted, then for a maximum of 3 days post discharge depending on symptoms. Follow‐up continued until 2 weeks after discharge Setting: treatment initiated on an inpatient basis and completed at home; trial carried out in UK |
|
| Participants |
Population: 98 children with an acute exacerbation of asthma were randomised to receive a high dose (n = 30), medium dose (n = 33) or low dose (n = 35) of prednisolone Age: 1‐15 years; mean age (SE) in the high‐dose group was 5.00 (0.71) years, in the medium‐dose group 5.64 (0.60) years and in the low‐dose group 5.39 (0.61) years Inclusion criteria: aged 1‐15 with diagnosis of acute asthma requiring admission Exclusion criteria: already receiving oral corticosteroids or prescribed oral corticosteroids within previous 14 days, significant underlying cardiac or pulmonary disease, unavailable investigating team, required IV therapy at the time of admission. Children could be enrolled only once. Children were withdrawn if they required IV therapy, failed to respond adequately to nebulisers or had oxygen saturation persistently < 91% in air or had response to therapy that was considered too slow Percentage withdrawn: withdrawal from high‐dose group was 20%, from medium‐dose group 9.1% and from low‐dose group 5.7% Allowed medication: following standard hospital asthma protocols (e.g. nebulised salbutamol 0.5‐4 hourly according to need) Disallowed medication: oral corticosteroids within 14 days of admission |
|
| Interventions |
Prednisolone high‐dose group: 2 mg/kg prednisolone once daily while an inpatient and up to 3 days post discharge (maximum daily dose 60 mg; total dose for 20 kg child receiving 5‐day course 200 mg prednisolone equivalent) Predisolone medium‐dose group: 1 mg/kg prednisolone once daily while an inpatient and up to 3 days post discharge (maximum daily dose 60 mg; total dose for 20 kg child receiving 5‐day course 100 mg prednisolone equivalent) Prednisolone low‐dose group: 0.5 mg/kg prednisolone once daily while an inpatient and up to 3 days post discharge (maximum daily dose 60 mg; total dose for 20 kg child receiving 5‐day course 50 mg prednisolone equivalent) |
|
| Outcomes | Asthma severity score while an inpatient (comprising clinical asthma score (based on respiratory effort, auscultation findings and patient distress, each measured on a 0‐6 scale, giving a maximum score of 18; the higher the score, the worse the symptoms), oxygen saturations, pulse rate and, when possible, FEV1 and PEFR), duration of admission, number of nebulisers given. Once home, participants/parents were asked to complete asthma diaries for 2 weeks including night‐time symptoms, SABA use and, when possible, morning and evening PEFR and cough and wheeze score | |
| Notes |
Type of publication: peer‐reviewed Funding: trial authors supported by the Royal Alexander Rockinghorse Appeal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation had been previously performed by the hospital pharmacist.." "stratification of randomisation was undertaken.." |
| Allocation concealment (selection bias) | Low risk | Randomisation had been previously performed by the hospital pharmacist, who used sealed envelopes disclosing the required dose |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dose of prednisolone was prepared on a different hospital ward and was unknown to investigating team and ward staff where the child had been admitted |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code for dosages of prednisolone given to each patient was broken only once; all patients had been discharged from the hospital and their 2‐week follow‐up completed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unbalanced attrition in the intervention groups; < 10% in medium‐ and low‐dose groups and 20% in higher‐dose group; 3 (1 from each group) were considered to be responding too slowly to treatment, so were switched to standard hospital protocol; 5 received IV therapy (2 from 2 mg group, 2 from 1 mg group and 1 from 0.5 mg group); 1 had already received oral steroids from GP (1 mg group); 3 additional participants, all from 2 mg group, withdrew because of vomiting, diagnosis of pneumonia or parent withdrawal of consent |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported, although unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Lederle 1987.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 7 weeks post discharge; follow‐up continued until 12 weeks after initial admission Setting: treatment initiated while inpatient and completed at home |
|
| Participants |
Population: 43 adults with an acute exacerbation of asthma were randomised to a long taper course (n = 22) or a short taper course (n = 21) of prednisolone Age: 30‐78 years; mean age in long taper group 62.6 years and in short taper group 63 years Inclusion criteria: men admitted to medicine services with exacerbation of asthma requiring systemic steroids; exacerbation defined as worsening dyspnoea due to airways obstruction with no other cause identified, and evidence of a reversible component to obstruction Exclusion criteria: already receiving oral corticosteroids; evidence of pneumonia, pulmonary oedema or cardiomegaly on chest x‐ray; other significant lung disease such as bronchiectasis, fibrosis, cancer; renal failure, hepatic failure and inability to comply with study protocol Percentage withdrawn: withdrawal 0% in both treatment arms Allowed medication: beta‐agonists and theophylline allowed at treating physician's discretion. Inhaled beclomethasone given throughout study period Disallowed medication: antibiotics not allowed once tapering period had begun |
|
| Interventions |
Prednisolone long taper group: 45 mg prednisolone once daily reducing by 5 mg weekly to 0 mg daily over 7 weeks (total dose 1575 mg prednisolone equivalent) Prednisolone short taper group: 45 mg prednisolone once daily reducing by 5 mg daily to 0 mg daily over 7 days (total dose 225 mg prednisolone equivalent) |
|
| Outcomes | Failure of tapering regimen (defined as re‐exacerbation of asthma requiring further corticosteroid administration during 12‐week follow‐up period); symptom diary with 10‐point VAS to evaluate breathing each day from 'best' to 'worst'; physical examination, spirometry, symptoms, adverse events and compliance assessed at 4, 8 and 12 weeks post admission | |
| Notes |
Type of publication: peer‐reviewed Funding: placebo tablets provided by Rowell Laboratories Inc., Baudette, Minn Other: mean age in both groups over 60, all but 5 included participants with > 10 pack‐year smoking history, mean of 49 years of age in long‐taper group and 56 in short‐taper group. Baseline spirometry results also suggest that many participants may have had a diagnosis of COPD (mean FEV1/FVC in both groups < 0.7). Study authors acknowledge that many participants may have had COPD with a reversible component |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned.."; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants received identical calender blister packs that contained the tapering regimen |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome of failure of tapering regimen (i.e. re‐exacerbation requiring additional oral steroids) decision made by physician blinded to participant allocation, as was decision to admit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 43 enrolled and randomised participants followed up to 12 weeks as planned (2 withdrew before starting taper; results not included) |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported in a way allowing for meta‐analysis. FEV1 outcome reported as percentage of baseline value without variance. Diary measures narratively reported in text with minimal supporting data |
| Other bias | Low risk | None noted |
NCT00257933.
| Methods |
Design: randomised, double‐blind trial Duration: randomised corticosteroid treatment continued for first 48 hours of admission; all participants still admitted after 48 hours switched to standard hospital protocol dose of steroids until discharge. Participants continued standard steroid treatment for a total of 5‐10 days at the discretion of the treating physician. Follow‐up continued until 7‐14 days after discharge Setting: treatment initiated in the ED with a loading dose of prednisolone and randomised treatment continued on inpatient basis for up to 48 hours; trial carried out in USA |
|
| Participants |
Population: 152 children with an acute exacerbation of asthma were randomised to a high‐dose (n = 74) or a low‐dose (n = 78) course of prednisolone Age: 2‐18 years; mean age (SD) in the high‐dose group was 7.9 (4.4) years and in the low‐dose group 7.0 (3.8) years Inclusion criteria: aged 2‐18 years with physician‐diagnosed asthma and ≥ 2 previous visits to ED or primary care provider for asthma care, at which time a beta‐2‐agonist was prescribed for acute symptoms; treated in the ED with a standardised asthma protocol based on NAEPP Guidelines. After initial therapy, participants were assessed by an attending physician; those determined to require admission to the hospital were eligible for enrolment Exclusion criteria: clinical decision to begin continuous intravenous beta‐agonist infusion; clinical decision to begin intravenous methylprednisolone therapy; clinical decision to admit to the Pediatric Intensive Care Unit; other concurrent disease such as sickle cell disease, cystic fibrosis or cardiac disease; any contraindication to corticosteroid administration; any systemic corticosteroid treatment within 2 weeks of presentation to the ED; potential participants excluded if informed consent not obtained Percentage withdrawn: withdrawal 0% in both treatment arms Allowed medication: albuterol on an inpatient basis. Study participants were allowed to continue other medications previously prescribed, including antihistamines, leukotriene inhibitors and inhaled corticosteroids. Both groups received a loading dose of prednisolone (2 mg/kg up to maximum 60 mg) in the ED followed by randomised treatment Disallowed medication: intravenous beta‐2‐agonist or corticosteroid, systemic corticosteroid within 2 weeks of presentation to the ED |
|
| Interventions |
Prednisolone high‐dose group: 4 mg/kg/d (1 mg/kg qds) for 48 hours, then 2 mg/kg/d (1 mg/kg bd) until discharge (maximum 30 mg per dose; total dose for 20 kg child receiving 5‐day course 400 mg prednisolone equivalent) Prednisolone low‐dose group: 2 mg/kg/d (1 mg/kg bd) for ≥ 48 hours and continuing duration of hospital admission (maximum 30 mg per dose; total dose for 20 kg child receiving 5‐day course 200 mg prednisolone equivalent) |
|
| Outcomes | Time measured from administration of loading dose of prednisolone in the ED until home dose of albuterol administered; time measured from writing of the admission order until writing of the discharge order; time spent at each severity level of the asthma care pathway; rate and degree of change in FEV1 and PEFR between treatment groups; differences in clinical asthma symptom scores during hospitalisation between treatment groups; rate of relapse between treatment groups | |
| Notes |
Type of publication: trial registration only on www.clinicaltrials.gov; unpublished data provided by trial author Funding: Children’s Hospital of Philadelphia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After informed consent was obtained, participants were randomised by the pharmacy in blocks of 6 and were stratified by severity level in the asthma pathway |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; "2 mg/kg/day orally divided 12 hourly (maximum 30mg/dose) alternating with placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind ("Subject, Caregiver, Investigator, Outcomes Assessor") |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low drop‐out overall; 16 (10.5%) participants withdrawn from study (13.5% from high‐dose arm and 7.7% from low‐dose arm) and 145/152 (95.4%) participants followed up by phone |
| Selective reporting (reporting bias) | Unclear risk | Paper has not yet been published. Some results are posted on clinicaltrials.gov; study authors kindly provided us with an unpublished manuscript. Some listed outcomes as yet are not fully reported (peak flow, clinical asthma score) |
| Other bias | Low risk | None noted |
O’Driscoll 1993.
| Methods |
Design: randomised, double‐blind trial Duration: corticosteroid treatment continued for 10‐17 days depending on allocation; follow‐up continued until 4‐6 weeks after discharge Setting: treatment initiated on inpatient basis and completed at home; trial carried out in the UK |
|
| Participants |
Population: 39 adults with an acute exacerbation of asthma were randomised to a tapering (n = 18 completed) or non‐tapering (n = 17 completed) course of prednisolone Age: 16‐55 years; mean age (range) in tapering group was 28 (18‐55) years and in non‐tapering group 37 (20‐53) years Inclusion criteria: 16‐55 years of age presenting with an acute asthma attack with PEFR < 65% predicted, admission under care of designated chest physician, ability to give informed consent and maintain PEFR diary for 28 days, use of inhaled corticosteroid (400‐2000 mcg daily) on discharge Exclusion criteria: major medical illnesses (especially pneumonia, heart failure, bronchiectasis and lung cancer), COPD, long‐term use of oral steroids, nebulisation at home, unable to comply with trial protocol, receiving IV hydrocortisone for > 2 days, requiring mechanical ventilation, had taken part in the trial during preceding 2 months Percentage withdrawn: 4 participants (10.3%) withdrawn overall but number from each group not reported Allowed medication: all other asthma treatments allowed at the discretion of the participant's personal physician, provided they were allowed under trial criteria. All participants received short‐acting beta‐2‐agonists and inhaled corticosteroids Disallowed medication: not reported |
|
| Interventions |
Prednisolone taper group: 40 mg prednisolone once daily for 10 days, then tapering by 5 mg/d for 7 days (total dose 540 mg prednisolone equivalent) Prednisolone non‐taper group: 40 mg prednisolone once daily for 10 days, followed by placebo taper (total dose 400 mg prednisolone equivalent) |
|
| Outcomes | PEFR, asthma symptoms on a numerical scale (1‐5) | |
| Notes |
Type of publication: peer‐reviewed Funding: placebo tablets provided by Pfizer UK Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prescriptions were sealed in a plain brown envelope and shuffled into a random order |
| Allocation concealment (selection bias) | Low risk | Prescriptions were sealed in a plain brown envelope and shuffled into a random order.. whenever an eligible patient entered the trial, one of the investigators would open the next envelope and dispatch the enclosed coded prescription to the pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received oral prednisolone for the active tapering arm or identical placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only the pharmacist was unblinded to allocation and prepared study medications according to the coded prescription; however, it is not clear whether outcome assessments were performed blinded throughout the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4 of 39 participants were enrolled and randomised but did not complete the trial. Two were lost to follow‐up and 2 were withdrawn for protocol violation and incorrect enrolment (PEFR did not meet inclusion criteria) |
| Selective reporting (reporting bias) | High risk | Many diary outcomes are not reported numerically so cannot be included in the meta‐analysis. Data displayed graphically in many cases with no variance. Not clear whether study was prospectively registered |
| Other bias | Low risk | None noted |
Qureshi 2001.
| Methods |
Design: randomised, open‐label trial Duration: corticosteroid treatment continued for 2‐5 days depending on allocation; follow‐up continued until 11‐14 days after ED discharge Setting: treatment initiated in the ED and completed at home; trial carried out in the USA |
|
| Participants |
Population: 628 children with an acute exacerbation of asthma were randomised to receive prednisolone (n = 319) or dexamethasone (n = 309) Age: 2‐18 years; median age (95% CIs) in prednisolone group 6 (6‐7) years and in dexamethasone group 6 (5‐7) years Inclusion criteria: 2‐18 years old with known history of asthma (≥ 2 episodes of wheezing treated with β‐adrenergic agonists with or without steroids) and presenting to paediatric ED with an acute exacerbation, defined as worsening of asthmatic symptoms or increased difficulty in breathing with worsening of peak expiratory flow rates. Children were considered for the study if they required ≥ 2 albuterol nebuliser treatments in the ED Exclusion criteria: reported use of oral corticosteroids in the 4 weeks before the current episode, history of intubation, varicella exposure in preceding 3 weeks, concurrent stridor, possible presence of an intrathoracic foreign body, chronic respiratory disease (e.g. cystic fibrosis), cardiac disease, need for immediate airway intervention Percentage withdrawn: withdrawal from the prednisolone group was 18.2% and from the dexamethasone group was 12% Allowed medication: All children were treated according to the standard ED asthma treatment protocol (nebulised albuterol and ipratropium according to asthma severity). Albuterol inhalations were recommended on a 4‐ to 6‐hour basis for the first 2 days after discharge, then as needed Disallowed medication: No other asthma medications were to be used during the next 10 days, apart from those detailed above |
|
| Interventions |
Prednisolone group: 2 mg/kg prednisolone initial dose, then 1 mg/kg daily for 5 days (maximum daily dose 60 mg; total dose for 20 kg child 120 mg prednisolone equivalent) Dexamethasone group: 0.6 mg/kg once daily for 2 days (maximum daily dose 16 mg; total dose for 20 kg child 160 mg prednisolone equivalent) |
|
| Outcomes | Rate of relapse (defined as an unscheduled visit to a medical facility resulting from participant’s or parent’s perception of persistent, worsening or recurrent asthma symptoms in the 10 days after discharge from the ED), rate of hospitalisation (initially from the ED and after relapse), frequency of vomiting, reported medication compliance, persistence of symptoms, school days or workdays missed | |
| Notes |
Type of publication: peer‐reviewed Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised trial; children allocated to treatment group depending on the day on which they attended the ED (odd days prednisolone, even days dexamethasone) |
| Allocation concealment (selection bias) | High risk | Allocation unconcealed because of the nature of sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label trial; however, primary outcome (decision to seek medical care for deteriorating symptoms) made independently of study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced drop‐out. However, significantly more children excluded from prednisolone group because of vomiting of study medication. Intention‐to‐treat analysis performed for primary outcome, assuming that all children excluded because of vomiting and those lost to follow‐up had a relapse; result favoured dexamethasone but not significantly |
| Selective reporting (reporting bias) | Low risk | All outcomes reported numerically, although unclear whether trial was prospectively registered |
| Other bias | Low risk | None noted |
Viska 2008.
| Methods |
Design: randomised trial; blinding not described Duration: corticosteroid treatment continued for 2 weeks with follow‐up continuing until 6 weeks Setting: treatment with initiated 'in hospital' and completed at home; trial carried out in Indonesia |
|
| Participants |
Population: 86 adults with an acute exacerbation of asthma were randomised to a high‐dose or low‐dose course of prednisolone (n for each group not given) Age: adults; age range not given Inclusion criteria: adults with acute exacerbation of asthma presenting to hospital Exclusion criteria: not reported Percentage withdrawn: 76 out of 86 participants were 'eligible to be included until the end of the study'; 11.6% were withdrawn overall Allowed medication: not reported Disallowed medication: not reported |
|
| Interventions |
Prednisolone high‐dose group: 36 mg prednisolone once daily for 2 weeks (total dose 504 mg prednisolone equivalent) Prednisolone low‐dose group: 12 mg prednisolone once daily for 2 weeks (total dose 168 mg prednisolone equivalent) |
|
| Outcomes | Relapse (unscheduled visit to healthcare provider), peak flow, asthma control test | |
| Notes |
Type of publication: conference abstract; study authors contacted for further information on 21 September 2015; at time of publication, no response received Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Enrolled and randomly divided"; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described, so assume open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not described, so assume open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 86 participants enrolled; 76 completed the trial. Not clear which treatment arms they dropped out of as total n for each group not given |
| Selective reporting (reporting bias) | High risk | Conference abstract, so study details minimal and not clear if prospectively registered. Unable to extract data for inclusion in review, as number randomised to each treatment arm not provided |
| Other bias | Low risk | None noted |
ACTH = adrenocorticotropic hormone
CI = confidence interval
COPD = chronic obstructive pulmonary disease
ED = emergency department
FEV1 = forced expiratory volume in 1 second
FVC = forced vital capacity
HCP = healthcare provider
ICS = inhaled corticosteroid
ICU = intensive care unit
IQR = interquartile range
ITT = intention‐to‐treat
IV = intravenous
MDI = metered dose inhaler
NAEPP = National Asthma Education and Prevention Program
PACQLQ = Paediatric Asthma Caregiver's Quality of Life Questionnaire
PEF = peak expiratory flow
PEFR = peak expiratory flow rate
PIS = pulmonary index score
PRAM = paediatric respiratory assessment measure
PSAS = patient self assessment sheet
SABA = short‐acting beta‐agonist
SaO2 = oxygen saturated as measured by blood analysis
SD = standard deviation
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2014 | Review article |
| Bowler 1990 | Different IV regimens part of randomised treatment |
| Bowler 1992 | Different IV regimens part of randomised treatment |
| Brand 2000 | Comparison of prednisolone solution vs crushed tablets |
| Brand 2001 | Not a randomised controlled trial |
| Britton 1976 | Different intravenous steroid regimens part of randomised treatment |
| Castilla Barrios 1994 | Comparison of intravenous steroids |
| Chanez 1996 | Cross‐over trial; comparison of different steroids for long‐term use |
| Chapela 1995 | Comparison of equivalent dose and duration of deflazacort and prednisolone |
| Dahlen 2007 | Mixed population of participants with asthma and COPD; comparison with placebo |
| Dawson 1993 | Comparison of prednisolone solution vs crushed tablets |
| Dente 2006 | Comparison of oral steroids vs placebo; not in acute asthma |
| Ebrahimi 2007 | Comparison of intravenous steroids |
| Figueira 1996 | Comparison of intravenous steroids |
| Gartner 2004 | Comparison of equivalent dose and duration of deflazacort and prednisolone |
| Gonzalez 1994 | Wrong population and wrong comparator; children with acute wheezy bronchitis |
| Guerot 1971 | Trial of inhaled, not oral, steroids |
| Hasegawa 1998 | Not a randomised controlled trial |
| Hatton 1995 | Comparison of oral steroids vs placebo |
| Ho 1994 | Single‐dose oral steroids vs placebo |
| Innes 2002 | Comparison of US and UK guidelines for management of asthma exacerbations. Different doses of oral steroids not the only variable |
| Kato 2004 | Trial of theophylline in addition to systemic steroids for acute asthma |
| Lucas‐Bouwman 2001 | Comparison of prednisolone solution vs crushed tablets |
| Marquette 1995 | Comparison of intravenous steroids |
| Mathew 2015 | Not a randomised controlled trial |
| Matsumoto 1994 | Not a randomised controlled trial |
| Micheletto 1997 | Not acute asthma; comparison of equivalent doses of prednisolone and deflazacort |
| Middelveld 2009 | Mixed population of participants with asthma and COPD; placebo‐controlled |
| Pierson 1971 | Trial of aminophylline in status asthmaticus |
| Pierson 1974 | Trial of intravenous steroids |
| Schwarz 2015 | Not a randomised controlled trial; review article |
| Silva 2007 | Acute wheezing rather than asthma, oral steroids vs nebulised steroids and placebo |
| Silva 2008 | Acute wheezing rather than asthma, oral steroids vs nebulised steroids and placebo |
| Skinner 1993 | Not an RCT; commentary on O’Driscoll 1993 |
| Webb 1986 | 3‐way cross‐over study |
COPD = chronic obstructive pulmonary disease
IV = intravenous
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Tanifuji 2001.
| Methods | Unclear |
| Participants | 33 participants with asthma with PEF 40‐60% of best/predicted value, requiring hospitalisation |
| Interventions | High‐dose (120 mg/d) vs low‐dose (60 mg/d) prednisolone |
| Outcomes | Number of days taken to reach 70‐80% of best/predicted PEF, duration of hospitalisation, adverse events, recurrence of asthma symptoms 1 month post discharge |
| Notes | Published as abstract only. Study authors contacted by post on 14 July 2015 to clarify trial design and route of steroid administration and to assess whether study meets inclusion criteria. At time of publication, no response received |
Characteristics of ongoing studies [ordered by study ID]
NCT01241006.
| Trial name or title | Single oral dose of dexamethasone vs 5 days of prednisone in adult asthma |
| Methods | Parallel, randomised, double‐blind trial |
| Participants | Adults with mild to moderate asthma exacerbations |
| Interventions | Single dose of oral dexamethasone 12 mg vs oral prednisone 60 mg/d for 5 days |
| Outcomes |
Primary outcome: relapse for worsening asthma within 14 days of emergency department visit Secondary outcomes: compliance, side effects, symptoms (including rescue inhaler use, wheezing, cough, shortness of breath and difficulty with activities of daily living) |
| Starting date | January 2011 |
| Contact information | Matthew Rehrer Alameda County Medical Center Oakland California United States 94602 matthewrehrer@gmail.com |
| Notes | Estimated trial completion date May 2015; no study results available at this time |
NCT02192827.
| Trial name or title | Use of dexamethasone in paediatric asthma exacerbations |
| Methods | Parallel, randomised, open‐label trial |
| Participants | Participants aged 2‐20 years presenting to the emergency department (ED) with a mild to moderate exacerbation of asthma |
| Interventions | Single‐dose 0.6 mg/kg of dexamethasone given in the ED vs 2 doses of 0.6 mg/kg of dexamethasone; first dose given in ED and second at home |
| Outcomes |
Primary outcome: peak flow at 5 days Secondary outcomes: relapse requiring medical attention, side effects (including vomiting, mood swings, behaviour changes, appetite changes, sweating or headache) |
| Starting date | April 2015 |
| Contact information | Meghan E. Martin Women and Children's Hospital of Buffalo Buffalo New York United States, 14222 MegMartinMD@hotmaill.com |
| Notes | Estimated trial completion date April 2017 |
NCT02725008.
| Trial name or title | Trial of 1 vs 2 doses of dexamethasone for paediatric asthma exacerbation (R2D2) |
| Methods | Paralell, randomised, double‐blind |
| Participants | Males and females aged 18 months‐20 years with a history of asthma defined as ≥ 2 prior episodes of respiratory illness characterised by wheezing treated with inhaled beta‐agonists. Estimated enrolment 220 participants |
| Interventions | 1 dose of oral dexamethasone 0.6 mg/kg (maximum dose 16 mg) in the ED and a second dose to take 24 hours after ED visit vs 1 dose in the ED plus a placebo dose 24 hours after ED visit |
| Outcomes | Primary outcomes: treatment failure; number of participants who experienced any of the following outcomes ‐ unplanned hospital admission for asthma symptoms, unplanned ED visit for asthma symptoms, unplanned urgent care visit for asthma symptoms, unplanned primary care physician visit for asthma symptoms, prescription of a course of steroids Secondary outcome: patient self assessment score (PSAS) |
| Starting date | July 2015 |
| Contact information | Geoffrey W. Jara‐Almonte, MD New York Methodist Hospital Brooklyn, New York United States 11215 gjaraalmonte@gmail.com |
| Notes | Estimated trial completion date July 2017 |
Differences between protocol and review
In a change to our protocol, we did not search manufacturers' websites, as the intervention medication is made generically by a large number of manufacturers worldwide. In addition, only one review author (RN) extracted study characteristics from included studies, and another review author (KMK) independently spot‐checked the extracted information for accuracy.
We stated that we would contact study authors to ask for more information when a trial was reported as an abstract only. In a change to our protocol, we did not contact the authors of Ghafouri 2010, as the trial was prospectively registered and all outcomes were clearly reported in tables that accompanied the abstract. We contacted the authors of Aboeed 2014, NCT00257933 and Viska 2008 to ask for additional details.
Contributions of authors
RN drafted the protocol and the review with substantial input, advice and revisions from KMK. RN, GM and KMK screened the search and extracted data from the included studies. RN entered the data into the review, and KMK performed cross‐checks. RN and KMK contributed to interpretation of the data, and all three authors contributed to the Discussion.
Sources of support
Internal sources
-
Rebecca Normansell and Kayleigh Kew, Cochrane Airways Group, UK.
Cochrane Airways Group is hosted by the Population Health Research Institute, St George's, University of London.
External sources
-
National Institute of Health Research, UK.
Evidence to guide care in adults and children with asthma, 13/89/14. This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
None known.
New
References
References to studies included in this review
Aboeed 2014 {published data only}
- Aboeed A, Mathew JJ, Manickavel S, Riss A, McNamee J, Debari V, et al. Dexamethasone versus prednisone in the treatment of acute asthma in adults: can an easier regimen provide the same results?. American Journal of Respiratory and Critical Care Medicine 2014;189:A1360. [Google Scholar]
Altamimi 2006 {published data only}
- Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani N, Chen R, et al. Single‐dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. Pediatric Emergency Care 2006;22(12):786‐93. [DOI] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang AB, Clark R, Sloots TP, Stone DG, Petsky HL, Thearle D, et al. A 5‐ versus 3‐day course of oral corticosteroids for children with asthma exacerbations who are not hospitalised: a randomised controlled trial. Medical Journal of Australia 2008;189(6):306‐10. [DOI] [PubMed] [Google Scholar]
- Chang AC, Clark R, Thearle D, Stone G, Petsky H, Champion A. Longer better than shorter? A multicentre randomised control trial (RCT) of 5 vs 3 days of oral prednisolone for acute asthma in children. Respirology 2007;12(Suppl 1):TP150. [Google Scholar]
- Chang AC, Clark R, Thearle D, Stone G, Petsky H, Champion A, et al. Longer better than shorter. A multicenter randomised control trial of 5 vs 3 days of oral prednisolone for acute asthma in children. Respirology 2007;12(Suppl 4):A190. [Google Scholar]
Cronin 2015 {published data only}
- Cronin J, Kennedy U, McCoy S, An Fhailí SN, Crispino‐O’Connell G, Hayden J, et al. Single dose oral dexamethasone versus multi‐dose prednisolone in the treatment of acute exacerbations of asthma in children who attend the emergency department: study protocol for a randomized controlled trial. Trials 2012;13(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, McCoy S, Nally S, Kennedy U, Crispino‐O'Connell G, Walsh S. A randomised trial of dexamethasone versus prednisolone in the treatment of acute paediatric asthma exacerbations. Archives of Disease in Childhood 2012;97:A109. [Google Scholar]
- Cronin JJ, McCoy S, Kennedy U, An Fhailí SN, Wakai A, Hayden J, et al. A randomized trial of single‐dose oral dexamethasone versus multidose prednisolone for acute exacerbations of asthma in children who attend the emergency department. Annals of Emergency Medicine 2015;67(5):593‐601. [DOI] [PubMed] [Google Scholar]
Cydulka 1998 {published data only}
- Cydulka RK, Emerman CL. A pilot study of steroid therapy after emergency department treatment of acute asthma: is a taper needed?. Journal of Emergency Medicine 1998;16(1):15‐9. [DOI] [PubMed] [Google Scholar]
Ghafouri 2010 {published data only}
- Ghafouri N, Sharieff GQ, Rajasingham A, Kanegaye J. Comparison of one‐dose and two‐dose regimes of oral dexamethasone in the management of acute asthma exacerbations in the pediatric emergency department. American Academy of Pediatrics National Conference & Exhibition; 2010 Oct 2‐5; San Francisco. 2010:P11632.
Greenberg 2008 {published data only}
- Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. Clinical Paediatrics 2008;47(8):817‐23. [DOI] [PubMed] [Google Scholar]
Hasegawa 2000 {published data only}
- Hasegawa T, Ishihara K, Takakura S, Fujii H, Nishimura T, Okazaki M. Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Internal Medicine (Tokyo, Japan) 2000;39(10):794‐7. [DOI] [PubMed] [Google Scholar]
Jones 2002 {published data only}
- Jones AM, Munavvar M, Aldridge R, Hopkinson L, Rayner C, O'Driscoll BR. A randomised controlled comparison of five days versus ten days of oral steroid therapy in acute adult asthma. Thorax 2000;55(Suppl 3):A30. [Google Scholar]
- Jones AM, Munavvar M, Vail A, Aldridge RE, Hopkinson L, Rayner C. Prospective, placebo‐controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respiratory Medicine 2002;96(11):950‐4. [DOI] [PubMed] [Google Scholar]
Karan 2002 {published data only}
- Karan RS, Pandhi P, Behera D, Saily R, Bhargava VK. A comparison of non‐tapering vs. tapering prednisolone in acute exacerbation of asthma involving use of the low‐dose ACTH test. International Journal of Clinical Pharmacology and Therapeutics 2002;40(6):256‐62. [DOI] [PubMed] [Google Scholar]
Kayani 2002 {published data only}
- Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest 2002;122(2):624‐8. [DOI] [PubMed] [Google Scholar]
Kravitz 2011 {published data only}
- Kravitz J, Dominici P, Ufberg J, Fisher J, Giraldo P. Two days of dexamethasone versus 5 days of prednisone in the treatment of acute asthma: a randomized controlled trial. Annals of Emergency Medicine 2011;58(2):200‐4. [DOI] [PubMed] [Google Scholar]
Langton Hewer 1998 {published data only}
- Langton Hewer S, Hobbs J, Reid F, Lenney W. Prednisolone in acute childhood asthma: clinical responses to three dosages. Respiratory Medicine 1998;92(3):541‐6. [DOI] [PubMed] [Google Scholar]
Lederle 1987 {published data only}
- Lederle FA, Pluhar RE, Joseph AM, Niewoehner DE. Tapering of corticosteroid therapy following exacerbation of asthma. A randomized, double‐blind, placebo‐controlled trial. Archives of Internal Medicine 1987;147(12):2201‐3. [PubMed] [Google Scholar]
NCT00257933 {published data only}
- Oral prednisolone dosing in children hospitalized with asthma. https://clinicaltrials.gov/show/NCT00257933 (accessed 27 January 2016).
O’Driscoll 1993 {published data only}
- O'Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock A. Double‐blind trial of steroid tapering in acute asthma. Lancet 1993;341(8841):324‐7. [DOI] [PubMed] [Google Scholar]
Qureshi 2001 {published data only}
- Qureshi F, Poirier MP, Zaritsky A. Oral dexamethasone versus oral prednisone: effect on relapse in acute asthma. Pediatric Research 2000;47(4):116A. [Google Scholar]
- Qureshi F, Zaritsky A, Poirier MP, Saritsky A, Poirier M. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. Journal of Paediatrics 2001;139(1):20‐6. [DOI] [PubMed] [Google Scholar]
Viska 2008 {published data only}
- Viska O, Yunus F, Wiyono WH. Comparison of short courses of oral prednisolone dose 36 mg/day 12 mg/day in moderate persistent asthma following acute exacerbation. Respirology 2008;13(Suppl 5):A146. [Google Scholar]
References to studies excluded from this review
Andrews 2014 {published data only}
- Andrews AL, Simpson AN. Dexamethasone may be a viable alternative to prednisone/prednisolone for the treatment of acute asthma exacerbation in the paediatric emergency department. Evidence‐Based Medicine 2014;19(5):175. [DOI] [PubMed] [Google Scholar]
Bowler 1990 {published data only}
- Bowler S, Mitchell C, Armstrong J. Hydrocortisone and acute severe asthma: low dose is as effective as high dose. Australian and New Zealand Journal of Medicine 1990;20:540. [Google Scholar]
Bowler 1992 {published data only}
- Bowler SD, Mitchell CA, Armstrong JG. Corticosteroids in acute severe asthma: effectiveness of low doses. Thorax 1992;47(8):584‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2000 {published data only}
- Brand PLP, Lucas Bouwman ME, Jansman FGA, Roorda RJ. Comparison of prednisolone powder and syrup for acute severe asthma in children. European Respiratory Journal 2000;16(Suppl 31):305s. [Google Scholar]
Brand 2001 {published data only}
- Brand PLP, Lucas Bouwan ME, Jansman FGA, Roorda RJ, Lucas‐Bouwan ME. Patterns of resolution of acute severe asthma in children when treated with oral prednisolone. European Respiratory Journal 2001;18(Suppl 33):122s. [Google Scholar]
Britton 1976 {published data only}
- Britton MG, Collins JV, Brown D, Fairhurst NP, Lambert RG. High‐dose corticosteroids in severe acute asthma. BMJ 1976;2(6027):73‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castilla Barrios 1994 {published data only}
- Castilla Barrios G, Saldaña Gastelo C, Aragon Graneros G. A comparative study of the methylprednisolone and hydrocortisone in child asthmatic crisis [Estudio comparativo de la metilprednisolona e hidrocortisona en la crisis asmática infantil]. Revista Médica del Instituto Peruano de Seguridad Social 1994;3(1):15‐9. [Google Scholar]
Chanez 1996 {published data only}
- Chanez P, Paradis L, Roches A, Paganin F, Bashir M, Godard P, et al. Comparison of three different oral corticosteroids in steroid‐dependent asthma patients. Allergy 1996;51(11):850‐1. [PubMed] [Google Scholar]
Chapela 1995 {published data only}
- Chapela R. Comparative study of the effectiveness of 2 oral corticoids in the control of severe crisis of bronchial asthma: deflazacort and prednisone. Revista Alergia Mexico 1995;42(4):64‐8. [PubMed] [Google Scholar]
- Chapela R. Efficacy of two corticosteroids for control of bronchial asthma crisis: deflazacort and prednisone. Investigacion Medica Internacional 1995;22(3):101‐5. [Google Scholar]
Dahlen 2007 {published data only}
- Dahlen SE. The BIOAIR oral steroid trial in severe asthma and COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #421.
Dawson 1993 {published data only}
- Dawson KP, Sharpe C. A comparison of the acceptability of prednisolone tablets and prednisolone sodium phosphate solution in childhood acute asthma. Australian Journal of Hospital Pharmacy 1993;23(5):320‐3. [Google Scholar]
Dente 2006 {published data only}
- Dente FL, Bartoli ML, Cianchetti S, Costa F, Bacci E, DiFranco A, et al. Oral prednisone induces further functional and biological improvements in severe refractory asthmatics. American Thoracic Society International Conference; 2006 May 19‐24; San Diego. 2006:A457 [Poster H42].
Ebrahimi 2007 {published data only}
- Ebrahimi S, Sarkari B. Comparative efficacy of dexamethasone versus hydrocortisone in severe acute pediatric asthma. Iranian Journal of Allergy, Asthma, and Immunology 2007;6(3):159‐60. [PubMed] [Google Scholar]
Figueira 1996 {published data only}
- Figueira M, Cova M, Isturis G. Asthma: dexamethasone vs acute crisis hydrocortisone. Centro Medico 1996;41(2):15‐23. [Google Scholar]
Gartner 2004 {published data only}
- Gartner S, Cobos N, Pérez‐Yarza EG, Moreno A, Frutos C, Liñan S, et al. Comparative efficacy of oral deflazacort versus oral prednisolone in children with moderate acute asthma. Anales de Pediatria 2004;61(3):207‐12. [DOI] [PubMed] [Google Scholar]
Gonzalez 1994 {published data only}
- Gonzalez PYE, Ruiz BA, Garate AJ, Romero IC, Bone CJ, Arranz AL. A multicenter randomized, open, parallel group study comparing different treatments for hospitalized infants with acute wheezy bronchitis. Anales Espanoles de Pediatria 1994;41(5):315‐9. [Google Scholar]
Guerot 1971 {published data only}
- Guerot C, Amsel M, Turiaf J. Corticotherapy by aerosol in the treatment of severe asthma. Therapeutique (La Semaine des hopitaux) 1971;47(9):805‐10. [PubMed] [Google Scholar]
Hasegawa 1998 {published data only}
- Hasegawa T, Ishihara K, Fujii H, Nishimura T, Umeda B. A comparison of a 3 day course with a 2 week course of oral prednisolone in patients with chronic asthma. Arerugi [Allergy] 1998;47(5):543‐9. [PubMed] [Google Scholar]
Hatton 1995 {published data only}
- Hatton MQF, Vathenen AS, Allen MJ, Davies S, Cooke NJ. A comparison of 'abruptly stopping' with 'tailing off' oral corticostercoids in acute asthma. Respiratory Medicine 1995;89(2):101‐4. [DOI] [PubMed] [Google Scholar]
Ho 1994 {published data only}
- Ho L, Landau LI, Souëf PN. Lack of efficacy of single‐dose prednisolone in moderately severe asthma. Medical Journal of Australia 1994;160(11):701‐4. [PubMed] [Google Scholar]
Innes 2002 {published data only}
- Innes NJ, Stocking J A, Harrison BDW. Prednisolone forty milligrams per day is sufficient in the treatment of acute asthma presenting to hospital. Thorax 2000;55(Suppl 3):A30. [Google Scholar]
- Innes NJ, Stocking JA, Daynes TJ, Harrison BD. Randomised pragmatic comparison of UK and US treatment of acute asthma presenting to hospital. Thorax 2002;57(12):1040‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2004 {published data only}
- Kato M, Masago K. Add on therapy of pure theophylline solution with systemic corticosteroid is more effective for the treatment of asthma attack. European Respiratory Journal 2004;24(Suppl 48):334s. [Google Scholar]
Lucas‐Bouwman 2001 {published data only}
- Lucas‐Bouwman ME, Roorda RJ, Jansman FG, Brand PL. Crushed prednisolone tablets or oral solution for acute asthma?. Archives of Disease in Childhood 2001;84(4):347‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas‐Bouwman ME, Roorda RJ, Jansman FGA, Brand PLP. Prednisolone oral solution better tolerated and more efficacious than prednisolone powder for the treatment of acute severe asthma in children. Nederlands Tijdschrift Voor Geneeskunde 2001;145(44):2130‐4. [Google Scholar]
Marquette 1995 {published data only}
- Marquette CH, Stach B, Cardot E, Bervar JF, Saulnier F, Lafitte JJ. High‐dose and low‐dose systemic corticosteroids are equally efficient in acute severe asthma. European Respiratory Journal 1995;8(1):22‐7. [DOI] [PubMed] [Google Scholar]
Mathew 2015 {published data only}
- Mathew JL. Enhancing the management of acute asthma in children: do we have the evidence?. Indian Journal of Pediatrics 2015;82(1):306‐8. [DOI] [PubMed] [Google Scholar]
Matsumoto 1994 {published data only}
- Matsumoto H, Ishihara K, Hasegawa T, Sakamoto H, Umeda B. Effects on bone metabolism of asthma treatment with beclomethasone dipropionate (BDP) inhalation and short term burst of oral steroids. Nihon Kyobu Shikkan Gakkai zasshi 1994;32(10):970‐6. [PubMed] [Google Scholar]
Micheletto 1997 {published data only}
- Micheletto C, Turco P, Mauroner L, Burti E, Dal Negro R. Oral deflazacort 30 mg and prednisone 25 mg affect serum ECP and plasma eosinophyls according to different kinetics in moderate‐to‐severe asthma. European Respiratory Journal 1997;10(Suppl 25):443S. [Google Scholar]
Middelveld 2009 {published data only}
- Middelveld R, Kupczyk M, Dahlen SE. Oral steroid intervention in severe asthma: focus on phenotypes. Report from the Bioair study. European Respiratory Society 19th Annual Congress. 2009 Sep 12‐15; Vienna:[375].
Pierson 1971 {published data only}
- Pierson WE, Bierman CW, Stamm SJ, Arsdel PP, Arsdel PP Jr. Double‐blind trial of aminophylline in status asthmaticus. Pediatrics 1971;48(4):642‐6. [PubMed] [Google Scholar]
Pierson 1974 {published data only}
- Pierson WE, Bierman CW, Kelley VC. A double‐blind trial of corticosteroid therapy in status asthmaticus. Pediatrics 1974;54(3):282‐8. [PubMed] [Google Scholar]
Schwarz 2015 {published data only}
- Schwarz ES, Cohn BG. Is dexamethasone as effective as prednisone or prednisolone in the management of pediatric asthma exacerbations?. Annals of Emergency Medicine 2015;65(1):81‐2. [DOI] [PubMed] [Google Scholar]
Silva 2007 {published data only}
- Silva M, Ferrari G. Comparison of prednisone and budesonide in the acute exacerbations of wheezing. European Respiratory Journal 2007;30(Suppl 51):776s [E4557]. [Google Scholar]
Silva 2008 {published data only}
- Silva ML, Ferrari GF. Budesonide versus prednisone in acute wheezing: randomized double blinded controlled placebo study. European Respiratory Society 18th Annual Congress. 2008 Oct 3‐7; Berlin:[4594].
Skinner 1993 {published data only}
- Skinner J, Siddiqui R, Gribbin H, Sinclair D. Steroid tapering in acute asthma. Lancet 1993;341(8847):772. [DOI] [PubMed] [Google Scholar]
Webb 1986 {published data only}
- Webb JR. Dose response of patients to oral corticosteroid treatment during exacerbations of asthma. BMJ 1986;292(6527):1045‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Tanifuji 2001 {published data only}
- Tanifuji Y, Yoshida K, Kobayashi H, Yamauchi K, Inoue H. Efficacy of short‐term high dose predonisolone therapy in acute exacerbations of bronchial asthma. European Respiratory Journal 2001;18(Suppl 33):71s. [Google Scholar]
References to ongoing studies
NCT01241006 {published data only}
- Single oral dose of dexamethasone versus five days of prednisone in adult asthma. https://clinicaltrials.gov/show/NCT01241006. 2010 Vol. (accessed 27 January 2016).
NCT02192827 {published data only}
- Use of dexamethasone in pediatric asthma exacerbations. https://clinicaltrials.gov/show/NCT02192827. 2014 Vol. (accessed 27 January 2016).
NCT02725008 {published data only}
- Trial of one versus two doses of dexamethasone for pediatric asthma exacerbation (R2D2). https://clinicaltrials.gov/ct2/show/NCT02725008 (accessed 22 April 2016).
Additional references
Alangari 2014
- Alangari AA. Corticosteroids in the treatment of acute asthma. Annals of Thoracic Medicine 2014;9(4):187‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1992
- Barnes PJ. Effects of corticosteroids in acute severe asthma. Thorax 1992;47(8):582‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2003
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma?. Annals of Internal Medicine 2003;139(5 Pt 1):359‐70. [DOI] [PubMed] [Google Scholar]
BNF
- Joint Formulary Committee. British National Formulary. http://www.bnf.org/bnf/index.htm (accessed 14 May 2015).
BTS/SIGN 2014
- Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. http://sign.ac.uk/pdf/SIGN141.pdf (accessed 14 May 2015).
CDC 2006
- Centers for Disease Control and Prevention. You can control your asthma: a guide to understanding asthma and its triggers. http://www.cdc.gov/asthma/pdfs/asthma_brochure.pdf (accessed 14 May 2015).
CDC 2011
- Centers for Disease Control and Prevention. CDC Vitalsigns Asthma ‐ Asthma in the US. http://www.cdc.gov/VitalSigns/Asthma/index.html (accessed 14 May 2015).
CDC 2012
- Centers for Disease Control and Prevention. Asthma. http://www.cdc.gov/asthma/default.htm (accessed 15 May 2015).
Fuhlbrigge 2012
- Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Gern J, et al. Asthma outcomes: exacerbations. Clinical Immunology 2012;129(3 Suppl):S34‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2015
- Global Initiative for Asthma. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015. http://www.ginasthma.org/local/uploads/files/GINA_Report_2015.pdf (accessed 15 May 2015).
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 5 February 2016. Hamilton ON, Canada: GRADE Working Group, McMaster University, 2014.
Hewer 1998
- Langton‐Hewer L, Hobbs J, Reid F, Lenney W. Prednisolone in acute childhood asthma: clinical responses to three dosages. Respiratory Medicine 1998;92(3):541‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Keeney 2014
- Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GE, et al. Dexamethasone for acute asthma exacerbations in children: a meta‐analysis. Pediatrics 2014;133(3):493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishnan 2009
- Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: corticosteroid therapy for adults with acute asthma. The American Journal of Medicine 2009;122(11):977‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lahn 2004
- Lahn M, Bijur P, Gallagher EJ. Randomized clinical trial of intramuscular vs oral methylprednisolone in the treatment of asthma exacerbations following discharge from an emergency department. Chest 2004;126(2):362‐8. [DOI] [PubMed] [Google Scholar]
Manser 2001
- Manser R, Reid D, Abramson MJ. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001740] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NAEPP 2007
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf (accessed 15 May 2015).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowe 2001
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002178] [DOI] [PubMed] [Google Scholar]
Rowe 2007
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000195.pub2] [DOI] [PubMed] [Google Scholar]
van der Velden 1998
- Velden VH. Glucocorticoids: mechanisms of action and anti‐inflammatory potential in asthma. Mediators of Inflammation 1998;7(4):229‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. http://www.who.int/gard/publications/GARD_Manual/en/ (accessed 15 May 2015).
Zhang 2014
- Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009471.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


