Abstract
Background
Foot infection is the most common cause of non‐traumatic amputation in people with diabetes. Most diabetic foot infections (DFIs) require systemic antibiotic therapy and the initial choice is usually empirical. Although there are many antibiotics available, uncertainty exists about which is the best for treating DFIs.
Objectives
To determine the effects and safety of systemic antibiotics in the treatment of DFIs compared with other systemic antibiotics, topical foot care or placebo.
Search methods
In April 2015 we searched the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library); Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE, and EBSCO CINAHL. We also searched in the Database of Abstracts of Reviews of Effects (DARE; The Cochrane Library), the Health Technology Assessment database (HTA; The Cochrane Library), the National Health Service Economic Evaluation Database (NHS‐EED; The Cochrane Library), unpublished literature in OpenSIGLE and ProQuest Dissertations and on‐going trials registers.
Selection criteria
Randomised controlled trials (RCTs) evaluating the effects of systemic antibiotics (oral or parenteral) in people with a DFI. Primary outcomes were clinical resolution of the infection, time to its resolution, complications and adverse effects.
Data collection and analysis
Two review authors independently selected studies, assessed the risk of bias, and extracted data. Risk ratios (RR) were estimated for dichotomous data and, when sufficient numbers of comparable trials were available, trials were pooled in a meta‐analysis.
Main results
We included 20 trials with 3791 participants. Studies were heterogenous in study design, population, antibiotic regimens, and outcomes. We grouped the sixteen different antibiotic agents studied into six categories: 1) anti‐pseudomonal penicillins (three trials); 2) broad‐spectrum penicillins (one trial); 3) cephalosporins (two trials); 4) carbapenems (four trials); 5) fluoroquinolones (six trials); 6) other antibiotics (four trials).
Only 9 of the 20 trials protected against detection bias with blinded outcome assessment. Only one‐third of the trials provided enough information to enable a judgement about whether the randomisation sequence was adequately concealed. Eighteen out of 20 trials received funding from pharmaceutical industry‐sponsors.
The included studies reported the following findings for clinical resolution of infection: there is evidence from one large trial at low risk of bias that patients receiving ertapenem with or without vancomycin are more likely to have resolution of their foot infection than those receiving tigecycline (RR 0.92, 95% confidence interval (CI) 0.85 to 0.99; 955 participants). It is unclear if there is a difference in rates of clinical resolution of infection between: 1) two alternative anti‐pseudomonal penicillins (one trial); 2) an anti‐pseudomonal penicillin and a broad‐spectrum penicillin (one trial) or a carbapenem (one trial); 3) a broad‐spectrum penicillin and a second‐generation cephalosporin (one trial); 4) cephalosporins and other beta‐lactam antibiotics (two trials); 5) carbapenems and anti‐pseudomonal penicillins or broad‐spectrum penicillins (four trials); 6) fluoroquinolones and anti‐pseudomonal penicillins (four trials) or broad‐spectrum penicillins (two trials); 7) daptomycin and vancomycin (one trial); 8) linezolid and a combination of aminopenicillins and beta‐lactamase inhibitors (one trial); and 9) clindamycin and cephalexin (one trial).
Carbapenems combined with anti‐pseudomonal agents produced fewer adverse effects than anti‐pseudomonal penicillins (RR 0.27, 95% CI 0.09 to 0.84; 1 trial). An additional trial did not find significant differences in the rate of adverse events between a carbapenem alone and an anti‐pseudomonal penicillin, but the rate of diarrhoea was lower for participants treated with a carbapenem (RR 0.58, 95% CI 0.36 to 0.93; 1 trial). Daptomycin produced fewer adverse effects than vancomycin or other semi‐synthetic penicillins (RR 0.61, 95%CI 0.39 to 0.94; 1 trial). Linezolid produced more adverse effects than ampicillin‐sulbactam (RR 2.66; 95% CI 1.49 to 4.73; 1 trial), as did tigecycline compared to ertapenem with or without vancomycin (RR 1.47, 95% CI 1.34 to 1.60; 1 trial). There was no evidence of a difference in safety for the other comparisons.
Authors' conclusions
The evidence for the relative effects of different systemic antibiotics for the treatment of foot infections in diabetes is very heterogeneous and generally at unclear or high risk of bias. Consequently it is not clear if any one systemic antibiotic treatment is better than others in resolving infection or in terms of safety. One non‐inferiority trial suggested that ertapenem with or without vancomycin is more effective in achieving clinical resolution of infection than tigecycline. Otherwise the relative effects of different antibiotics are unclear. The quality of the evidence is low due to limitations in the design of the included trials and important differences between them in terms of the diversity of antibiotics assessed, duration of treatments, and time points at which outcomes were assessed. Any further studies in this area should have a blinded assessment of outcomes, use standardised criteria to classify severity of infection, define clear outcome measures, and establish the duration of treatment.
Plain language summary
Antibiotics to treat foot infections in people with diabetes
Review question
We reviewed the effects on resolution of infection and safety of antibiotics given orally or intravenously (directly into the blood system) in people with diabetes that have a foot infection.
Background
One of the most frequent complications of people with diabetes is foot disorders, specially foot ulcers or wounds. These wounds can easily become infected, and are known as a diabetic foot infections (DFIs). If they are not treated, the infection can progress rapidly, involving deeper tissues and threatening survival of the limb. Sometimes these infections conclude with the affected limb needing to be amputated.
Most DFIs require treatment with systemic antibiotics, that is, antibiotics that are taken orally, or are inserted straight into the bloodstream (intravenously), and affect the whole body. The choice of the initial antibiotic treatment depends on several factors such as the severity of the infection, whether the patient has received another antibiotic treatment for it, or whether the infection has been caused by a micro‐organism that is known to be resistant to usual antibiotics (e.g. methicillin‐resistant Staphylococcus aureus ‐ better known as MRSA). The objective of antibiotic therapy is to stop the infection and ensure it does not spread.
There are many antibiotics available, but it is not known whether one particular antibiotic ‐ or type of antibiotic ‐ is better than the others for treatment of DFIs.
The investigation
We searched through the medical literature up to March 2015 looking for randomised controlled trials (which produce the most reliable results) that compared different systemic antibiotics against each other, or against antibiotics applied only to the infected area (topical application), or against a fake medicine (placebo) in the treatment of DFIs.
Study characteristics
We identified 20 relevant randomised controlled trials, with a total of 3791 participants. Eighteen of the 20 studies were funded by pharmaceutical companies. All trials compared systemic antibiotics with other systemic antibiotics.
Key results
It is unclear whether any particular antibiotic is better than any another for curing infection or avoiding amputation. One trial suggested that ertapenem (an antibiotic) with or without vancomycin (another antibiotic) is more effective than tigecycline (another antibiotic) for resolving DFI. It is also generally unclear whether different antibiotics are associated with more or fewer adverse effects. The following differences were identified:
1. carbapenems (a class of antibiotic) combined with anti‐pseudomonal agents (antibiotics that kill Pseudomonas bacteria) produced fewer adverse effects than anti‐pseudomonal penicillins (another class of antibiotic);
2. daptomycin (an antibiotic) caused fewer adverse effects than vancomycin or other semi‐synthetic penicillins (a class of antibiotic);
3. linezolid (an antibiotic) caused more harm than ampicillin‐sulbactam (a combination of antibiotics);
4. tigecycline produced more adverse effects than the combination of ertapenem with or without vancomycin.
Quality of the evidence
There were important differences between the trials in terms of the diversity of antibiotics assessed, the duration of treatments, and the point at which the results were measured. The included studies had limitations in the way they were designed or performed, as a result of these differences and design limitations, our confidence in the findings of this review is low.
Background
Description of the condition
Among the serious complications of diabetes, disorders of the feet (ulceration, infection, gangrene and amputation) are among the most frequent causes of morbidity, and a leading cause of hospitalisation (Boulton 2005; Frykberg 2006; IDSA 2012; Nelson 2006a). Foot infection is the most common precipitating cause of non‐traumatic amputations, hospitalisation and reduction of quality of life in people with diabetes (Blanes 2011; IDSA 2012; Pecoraro 1990; Raspovic 2014; Reiber 1999). Approximately 15% of people with diabetes will have a foot ulcer during their life, with an annual incidence of 1% to 4% (Blanes 2011). Foot ulcers are clinically infected in just over half of patients at presentation (Lavery 2003; Prompers 2007). A diabetic foot infection (DFI) is defined as any type of skin, soft tissue or bone infection affecting tissues below the ankle in people with diabetes. These infections include cellulitis (in deep skin), paronychia (around nails), abscesses, myositis (in muscle), tendonitis (in tendons), necrotising fasciitis (infection that kills tissue), osteomyelitis (in bone) and septic arthritis (in joints; Lipsky 2004a). The major predisposing factor to these infections is foot ulceration, which is usually a consequence of peripheral neuropathy (nerve damage), and is often accompanied by peripheral arterial disease, or trauma. Ulcers cause disruption of the protective skin barrier, which exposes the underlying soft tissue to bacterial colonisation (i.e. proliferation of a micro‐organism that does not cause cell damage or an inflammatory host response). Once the foot wound is colonised it may become actively infected, which can cause further destruction of the tissues and, in severe cases, systemic (whole body) inflammatory responses. The sequence from uninfected to infected foot wound can progress relatively quickly, within a few days, but occasionally within even hours. When infection progresses without interruption to involve deeper tissues, it becomes potentially limb‐threatening, and even life‐threatening (Bader 2008; Lipsky 2004b; West 1995). Although rare, infection can occasionally develop without any remembered ulceration or traumatic lesion (Bader 2008). In addition to the associated morbidity, DFI is also a costly complication, with a total cost (including direct and indirect costs) that ranges from EUR 9273 to EUR 16,835, with the highest cost related to hospitalisation (Prompers 2008).
A DFI is defined clinically, not microbiologically, by the presence of systemic signs of infection apparently related to a foot lesion, purulent secretions, or at least two classic signs or symptoms of inflammation (redness, warmth, pain or tenderness, and tissue hardening; IDSA 2012). Sometimes it is difficult to decide whether a chronic ulcer (i.e. a lesion that has been present for several weeks and exhibits delayed or stalled healing) is infected (Bradley 1999; Edmonds 2005). This is especially true in people with peripheral neuropathy or vascular insufficiency (impaired blood flow), which may conceal or mimic infection. Furthermore, people with diabetes may not show the typical inflammation response to an infection (Bader 2008).
All infected diabetic foot wounds require treatment, which almost always includes antimicrobial therapy. This therapy is almost always an antibiotic agent, given through a topical (surface application) or systemic route. The available evidence does not support administration of antibiotic therapy for diabetic foot wounds that are not clinically infected, though they obviously require appropriate local care (Lipsky 2004b).
Description of the intervention
Most DFIs require systemic antibiotic therapy in addition to other treatments, such as debridement (removal of dead tissue), drainage, dead space management, dressing and correction of any metabolic abnormalities (Bader 2008; IDSA 2012). Another Cochrane review will review the effects of topical antimicrobials for infections of the foot in diabetes (Lipsky 2014).
Since DFIs can progress relatively rapidly, and infection is defined clinically rather than microbiologically, there is no reason to delay starting antibiotic therapy if infection is suspected. The selection of an antibiotic regimen should take into consideration the particular needs and comorbidities of the individual patient as well as the proven or suspected pathogens and their antibiotic susceptibilities. Then, the clinician can decide which specific drug or combination is needed, including the optimal route of administration and the treatment duration required.
The choice of initial antibiotic therapy is usually empirical (i.e. based on the best guess of the nature of the causative organism(s) and made before the results of wound cultures are available). Treatment selection should take into account the severity of the foot infection, any history of recent antimicrobial treatment, previous infection with antibiotic‐resistant organisms, recent culture results, current Gram‐stained smear findings and various patient factors (Lipsky 2007). This empirical therapy should then be reassessed and modified, when needed, on the basis of the patient’s clinical response, the cultural results, and the sensitivity of the pathogens identified (Bader 2008; IDSA 2012).
Almost all mild, and many moderately severe, infections in patients who have not recently received antibiotic therapy can be treated with an oral antibiotic regimen with a relatively narrow spectrum of activity, such as cephalexin, clindamycin or amoxicillin‐clavulanate. For more extensive moderate infections and all severe infections, treatment should usually be parenteral (typically intravenous) at least until the patient is stable, and employ relatively broad‐spectrum antibiotics such as piperacillin‐tazobactam, clindamycin plus ciprofloxacin, imipenem‐cilastatin or clindamycin plus tobramycin plus ampicillin (IDSA 2012).
The selected antibiotic therapy should always cover Staphylococcus aureus, as it is the most frequent and virulent pathogen isolated in DFI. The decision to provide coverage for metcillin‐resistantS aureus (MRSA; also known as 'methicillin‐resistant S aureus) depends on the overall local prevalence of that micro‐organism, the presence or absence of risk‐factors for MRSA infection, and the severity of the infection. Other organisms that may cause DFIs that raise concerns about antibiotic resistance are Pseudomonas aeruginosa, which is resistant to many commonly prescribed antibiotics, and various Gram‐negative isolates that produce extended spectrum beta‐lactamases or carbapenemase (Lipsky 2007).
How the intervention might work
The objective of antibiotic therapy for DFIs is to kill micro‐organisms and thus, achieve resolution of the clinical signs and symptoms of infection and avoid the consequences of infection spreading, that is, tissue destruction, lower‐extremity amputation, sepsis or death of patients (IDSA 2012). Prompt resolution of the signs of infection also reduces the need for hospitalisation with its associated financial cost and potential morbidity, and hastens healing of the wound (Prompers 2008). At the same time, optimally, systemic antibiotic therapy should avoid being associated with adverse effect. Among the more common of these are allergic reactions, renal insufficiency or the development of Clostridium difficile disease (a bacterial infection that can affect the digestive system and commonly affects people who have been treated with antibiotics). The available antibiotic agents have different propensities for causing these problems. Furthermore, deployment of antibiotic therapy should be rational in order to avoid the risk of inducing antibiotic resistance through excessive, overly broad or unnecessarily prolonged therapy (OMS 2014).
While all infected foot lesions in a person with diabetes likely require antibiotic therapy, it is often not sufficient. Appropriate surgical procedures (particularly incision and drainage, resection of deep, infected tissues) and wound care are almost always needed.
Why it is important to do this review
The systematic reviews on antibiotic treatment of DFI that are available do not support the superiority of any single drug or combination of antibiotics (Berendt 2008; Nelson 2006a; Peters 2012). However, these systematic reviews have become out of date as new randomised clinical trials are now available for consideration. This systematic review should help to determine whether any specific systemic antibiotic agents or regimens are associated with better clinical outcomes or fewer adverse effects when used to treat DFIs.
Objectives
To determine the effects and safety of systemic antibiotics in the treatment of DFIs compared with other systemic antibiotics, topical foot care or placebo.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that allocated people individually or by cluster. Non‐randomised or quasi‐randomised controlled trials were not eligible for inclusion.
Types of participants
We included studies of people with diabetes mellitus (type 1 or 2) with any type of foot wound (e.g. ulcers of neuropathic or ischaemic aetiology, or traumatic wounds) that had been diagnosed as infected (using any definition reported by the study authors), or people with diabetes mellitus with any infection located in the foot that was ‐ or was not ‐ associated with a wound. If studies included both diabetic and non‐diabetic participants, they were included only if it was possible to obtain separate data for the participants with diabetes.
We considered studies conducted in all settings (e.g. hospital, primary healthcare centre, home care) and excluded people with diabetic foot ulcers that were not infected.
Types of interventions
We included any type of systemic antibiotic regimen (either oral or parenteral, i.e. intravascular) with any number of agents, in any dose, frequency of administration or duration of therapy used for treating DFIs, that was compared with any other antibiotic control group (either oral or parenteral), placebo or topical foot care. We did not consider primary interventions of topical (non‐systemic) antimicrobials (antiseptics or antibiotics), although they were eligible as comparators to a systemic antibiotic regimen.
Types of outcome measures
In order to be included, studies needed to report at least one of the outcomes of interest to the review.
Primary outcomes
Clinical resolution of the infection, defined as the resolution of all acute signs and symptoms related to the infection, or improvement such that additional antimicrobial therapy of any kind was not required (Lipsky 2007). This outcome was not initially defined in the protocol, as it was integrated in the 'time to resolution of the infection' outcome. We later decided to separate these two outcomes in order not to lose important information (see Differences between protocol and review).
Time to resolution of the infection, defined as the time needed to reach clinical resolution of the infection (as defined above).
Adverse effects of treatment (allergic reactions, organ toxicity, intolerance, etc.).
Serious infections or complications of infection (for example, septicaemia, septic shock or amputation ‐ major amputation is defined as an amputation above the ankle and minor amputation as an amputation limited to the foot).
Secondary outcomes
Infection‐related mortality.
Health‐related quality of life, as assessed by any standardised instrument.
Length of hospitalisation.
Wound healing, evaluated by objective measures such as the change in ulcer size (area or radius), the proportion of people whose ulcer completely healed within the trial period, and the time to complete healing.
Recurrence of wound infections.
Search methods for identification of studies
Electronic searches
In April 2015 we searched the following electronic databases for potentially relevant RCTs:
The Cochrane Wounds Group Specialised Register (searched 1 April 2015);
The Cochrane Metabolic and Endocrine Disorders Group Specialised Register (latest);
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, Issue 2);
The Database of Abstracts of Reviews of Effects (DARE; The Cochrane Library 2015, Issue 2);
The Health Technology Assessment Database (HTA; The Cochrane Library 2015, Issue 2);
NHS Economic Evaluation Database (NHSEED; The Cochrane Library 2015, Issue 2);
Ovid MEDLINE (1946 to March 30 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, 30 March 2015);
Ovid EMBASE (1974 to 1 April 2015);
EBSCO CINAHL (1982 to 1 April 2015).
We used the following search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor Foot Ulcer explode all trees #2 MeSH descriptor Diabetic Foot explode all trees #3 diabet* NEAR/3 ulcer*: ti,ab,kw #4 diabet* NEAR/3 (foot or feet):ti,ab,kw #5 diabet* NEAR/3 wound*:ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Anti‐Bacterial Agents explode all trees #8 antibiotic*:ti,ab,kw #9 nafcillin or oxacillin or ampicillin or dicloxacillin or ticarcillin* or piperacillin* or amoxicillin* or clindamycin or vancomycin or tobramycin or levofloxacin or ciprofloxacin or moxifloxacin or tigecycline or doxycycline or cefazolin or ceftazidime or cephalexin or cefepime or cefotaxime or ceftriaxone or cefazolin or cefoxitin or cefotetan or imipenem* or meropenem or ertapenem or aztreonam or metronidazole or sulfamethoxazole* or trimethoprim or cilastatin*:ti,ab,kw #10 (#7 OR #8 OR #9) #11 (#6 AND #10)
This search strategy was adapted to search Ovid MEDLINE (Appendix 1), Ovid EMBASE (Appendix 2) and EBSCO CINAHL (Appendix 3). We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision; Lefebvre 2011). We combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions on the basis of date or language of publication.
We also searched the following websites to identify ongoing clinical trials (Appendix 4):
ClinicalTrials.gov (https://www.clinicaltrials.gov/);
Controlled trials (www.controlled‐trials.com).
Searching other resources
To identify additional studies we reviewed the reference lists of all selected articles. We searched the OpenSIGLE database to identify grey literature and the ProQuest Dissertations and Theses to retrieve dissertation theses related to our topic of interest. These search strategies can be found in Appendix 5 and Appendix 6.
Data collection and analysis
Selection of studies
We managed the citations using a reference management software program (ProCite). Two review authors independently assessed the titles and abstracts of citations identified by the search strategy. The review authors were not blinded to the study authors or the names of the publications. We retrieved full reports of all potentially relevant trials for further assessment of eligibility based on the inclusion criteria. We resolved any disagreements through discussion or, if required, through consultation with a third review author.
Data extraction and management
We designed a data extraction form and tested it before recording the results. For eligible studies, three review authors extracted data regarding the study objective; date of publication; country; sponsorship; patients' baseline data; type of antibiotic, route of administration and dosage compared; and outcomes of interest (Types of outcome measures). Discrepancies were resolved through discussion. We entered data into Review Manager 5.3 software (RevMan 2014), and checked the data for accuracy. When any information collected on the extraction form was missing or unclear, we attempted to contact the authors of the original reports to request further details. When we located duplicate publications for the same trial, we assessed them and extracted the maximum amount of data from them. Both publications were cited under the same study ID.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011; Appendix 7 provides details of criteria on which the judgements were based). We considered blinding and completeness of outcome data for the main outcome 'clinical resolution of the infection'. To assess selective outcome reporting, we actively sought trial protocols; when they were not available, we assessed whether all outcomes mentioned in the methods section had been reported in the results section of trial reports. Where we suspected reporting bias, we attempted to contact study authors to ask them to provide the missing outcome data.
We considered that overall risk of bias was low when allocation concealment was adequate and outcome assessors were blinded to the allocation, and high where the rest of the domains were either unclear or judged to be at a high risk of bias.
We discussed any disagreement amongst all authors to achieve a consensus.
We have presented our assessment of risk of bias using two 'Risk of bias' summary figures; one is a summary of bias for each item across all studies, and the other shows a cross‐tabulation of each trial by all of the risk of bias items.
Measures of treatment effect
We assessed dichotomous outcomes using the risk ratio (RR) with 95% confidence intervals (CI) and continuous outcomes (e.g. health‐related quality of life, length of hospitalisation) using mean difference (MD). We planned to compute hazard ratios (HR) for time‐to‐event variables (time to resolution of the infection, time to healing). If the papers did not report HR, we planned to compute these following the formula of Parmar 1998, implemented in a freely‐available spread sheet (Tierney 2007).
Dealing with missing data
We addressed missing data for dichotomous outcomes by performing an intention‐to‐treat analysis based on a 'worst‐case' scenario (Gamble 2005). We considered all randomised participants: we assumed that participants for whom there was no information about the outcome of interest had not had a positive result. We planned to perform the analysis of continuous outcomes with available data only.
Assessment of heterogeneity
To assess heterogeneity we examined the forest plot visually to see whether CIs for individual study estimates overlapped, and examined the Chi² statistic. To quantify heterogeneity we used the I² statistic (Higgins 2003), and interpreted it according to the following thresholds:
0% up to 40%: might not be important;
40% up to 60%: may represent moderate heterogeneity;
More than 60%: represents important heterogeneity.
Data synthesis
We reported data narratively by outcome and then by comparison. We considered pooling when there were sufficient studies that were clinically similar. If heterogeneity was absent or low (I² up to 40%) we used a fixed‐effect model and if it was moderate (I² of 40% to 60%) we used the random‐effects model. However we did not plan to pool the data if heterogeneity was very high (I² over 60%). If data were available for pooling, we used RR with 95% CI for dichotomous outcomes, and the pooled mean difference or the standardised mean difference (SMD) with 95% CI for continuous outcomes, depending on whether the outcomes were measured using the same scales.
Subgroup analysis and investigation of heterogeneity
We planned to investigate potential causes of heterogeneity, such as diversity in characteristics of included patients, intervention characteristics (different doses or duration) or study methods, by performing subgroup analysis. However, pooling data was only possible with very few trials (three or fewer) in only two outcomes from two different comparisons.
Sensitivity analysis
We planned to explore whether analysing studies stratified by quality (overall low risk of bias versus high risk of bias) produced similar or different results. However, included studies were very heterogeneous (with respect to their populations, design and use of different regimens), which precluded any sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
In total, we identified 941 references: 929 were found by electronic literature searching and 12 were found by reviewing the list of references of the included studies. We excluded 877 references after examining the title and abstract and obtained full text copies of the remaining 64 references for more detailed examination. We also identified two ongoing trials in ongoing trial registers (see Characteristics of ongoing studies). We finally included 24 references that provided information from 20 different studies. We have detailed our reasons for excluding the remaining 40 references in the Characteristics of excluded studies section and in a PRISMA flow diagram (Figure 1).
1.
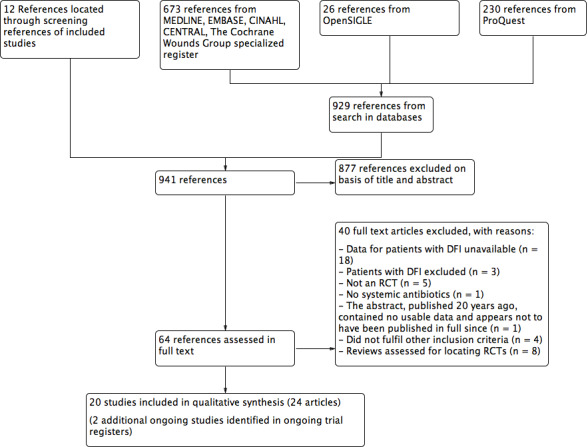
Study flow diagram
Included studies
Twenty RCTs that included 3791 patients with DFIs met our inclusion criteria. A detailed description of the included trials is provided in Characteristics of included studies. Fourteen studies were multicentred while the remaining six were single‐centred. All included studies compared a systemic antibiotic regimen against another systemic antibiotic regimen. An overview of the comparisons addressed by trials is provided in Table 1 and in more detail in Table 2.
1. Comparisons.
| Study ID | Intervention | Comparison |
| A | Anti‐pseudomonal penicillins | |
| Anti‐pseudomonal penicillins | Anti‐pseudomonal penicillins | |
| Tan 1993 | Piperacillin‐tazobactam 3 g/375 mg iv every 6 h (min 5 days) | Ticarcillin‐clavulanate 3 g/100 mg iv every 6 h (min 5 days) |
| Anti‐pseudomonal penicillins | Broad‐spectrum penicillins | |
| Harkless 2005 | Piperacillin‐tazobactam 4 g/0.5 g iv every 8 h (4‐14 days, max 21 days) | Ampicillin‐sulbactam 2 g/1 g iv every 6 h (4‐14 days, max 21 days) |
| Anti‐pseudomonal penicillins | Carbapenems | |
| Saltoglu 2010 | Piperacillin‐tazobactam 4.5 g iv every 8 h (14 days) | Imipenem‐cilastatin 500 mg iv every 6 h (14 days) |
| B | Broad‐spectrum penicillins | |
| Broad‐spectrum penicillins | Cephalosporins | |
| Erstad 1997 | Ampicillin‐sulbactam 3 g iv every 6 h (min 5 days) | Cefoxitin 2 g iv every 6 h (min 5 days) |
| C | Cephalosporins | |
| Fifth‐generation cephalosporins | Third‐generation cephalosporins + glycopeptide | |
| Noel 2008a | Ceftobiprole 500 mg iv every 8 h (7‐14 days) | Vancomycin 1000 mg iv every 12 h plus ceftazidime 1000 mg iv every 8 h (7‐14 days) |
| 2. | Third‐generation cephalosporin + nitroimidazole | Anti‐pseudomonal penicillin |
| Clay 2004 | Ceftriaxon ‐metronidazole 1 g/1 g iv every 24 h (duration not described) | Ticarcillin‐clavulanate 3.1 g iv every 6 h (duration not described) |
| D | Carbapenems | |
| Carbapenems | Anti‐pseudomonal penicillins | |
| Ertapenem | Piperacillin‐tazobactam | |
| SIDESTEP Study | Ertapenem 1 g iv every 24 h (min 5 days). Switch to amoxicillin‐clavulanate 875 mg/125 mg po every 12 h | Piperacillin‐tazobactam 3 g/375 mg iv every 6 h (min of 5 days). Switch to amoxicillin‐clavulanate 875 mg/125 mg po every 12 h |
| Graham 2002a | Ertapenem 1 g iv every 24 h (7‐14 days) | Piperacillin‐tazobactam 3 g/375 mg iv every 6 h (7‐14 days) |
| Imipenem + cilastatin | Piperacillin + clindamycin | |
| Bouter 1996 | Imipenem‐cilastatin 500 mg iv every 6 h (10 days) | Piperacillin 3 g iv every 6 h plus clindamycin 600 mg iv every 8 h (10 days) |
| Carbapenems | Broad‐spectrum penicillins | |
| Grayson 1994 | Imipenem‐cilastatin 500 mg iv every 6 h (5 days) | Ampicillin‐sulbactam 2 g/1 g iv every 6 h (5 days) |
| E | Fluoroquinolones | |
| Fourth‐generation fluoroquinolones | Anti‐pseudomonal penicillins | |
| Giordano 2005‐Lipsky 2007 | Moxifloxacin 400 mg every 24 h (iv for 3 days, then po for 7‐14 days) | Piperacillin‐tazobactam 3 g/375 mg iv every 6 h (3 days). Then amoxicillin‐clavulanate 800 mg po every 12 h (7‐14 days) |
| RELIEF Study | Moxifloxacin 400 mg every 24 h (iv for 3 days, then 400 mg every 12 h po for 7‐21 days) | Piperacillin‐tazobactam 4 g/0.5 g iv every 8 h. Then amoxicillin‐clavulanate 875 mg/125 mg po every 12 h (7‐21 days) |
| Siami 2001 | Cinafloxacin 200 mg iv every12 h (max 14 days), then 200 mg po every 12 h | Piperacillin‐tazobactam 3 g/375 mg po every 6 h. Then amoxicillin‐clavulanate 500 mg po every 8 h |
| Fourth‐generation fluoroquinolones | Broad‐spectrum penicillins | |
| STIC Study | Moxifloxacin 400 mg iv every 24 h for 3 days, then po for 7‐21 days | Amoxicillin 1000 g‐clavulanate 200 mg iv every 8 h for 3 days, then 500 mg/125 mg po every 8 h for 7‐21 days |
| Third‐generation fluoroquinolones | Antipseudomonal penicillins | |
| Graham 2002b | Levofloxacin 750 mg iv or po every 24 h (7‐14 days) | Ticarcillin‐clavulanate 3.1g every iv 4 h‐6 h, then changed to amoxicillin‐clavulanate 875 mg po every 12 h (7‐14 days) |
| Second‐generation fluoroquinolones | Broad‐spectrum penicillins | |
| Lipsky 1997 | Ofloxacin 400 mg iv every 12 h (then switch to po; 14‐28 days) | Ampicillin‐sulbactam 1‐2 g/0.5‐1 g iv every 6 h. Switch to amoxicillin‐clavulanate 500 mg/125 mg po every 8 h (14‐28 days) |
| F | Other antibiotics | |
| Arbeit 2004 | Daptomycin 4 mg/kg iv every 24 h (7‐14 days) | Vancomycin 1 g iv every 12 h (7‐14 days) |
| Lipsky 2004 | Linezolid 600 mg iv or po every 12 h (≥ 7 days ≤ 28) | Ampicillin/sulbactam 1.5 g‐3 g iv every 6 h OR amoxicillin/clavulanate 500 mg/875mg po every 12 h (≥ 7 days ≤ 28) |
| Lipsky 1990 | Clindamycin 300 mg iv every 6 h (14 days) | Cephalexin 500 mg every 6 h po (14 days) |
| Lauf 2014 | Tigecycline 150 mg iv every 24 h (28 days) | Ertapenem 1 g iv every 24 h (28 days) |
Abbreviations
h: hour(s) iv: intravenous max: maximum min: minimum po: per oral (by mouth)
2. Detailed comparisons.
| Ticarcillin‐clavulanate iv | Ampicillin‐sulbactam iv | Ceftriaxone‐metronidazol iv | Ceftazidime‐vancomicyn iv | Cefoxitin iv | Ertapenem iv | Ertapenem iv followed by amoxicillin‐clavulanate po | Imipenem‐cilastatin iv | Moxifloxacin iv followed by po |
Cinafloxacin iv followed by po |
Levofloxacin iv | Ofloxacin iv followed by po | Linezolid iv or po | Clindamycin iv | Tigecycline | Vancomycin | |
| Anti‐pseudomonal penicillins | ||||||||||||||||
| Piperacillin‐tazobactam iv | 1 Tan 1993 | 1 Harkless 2005 | 1 Graham 2002a | 1Saltoglu 2010 | ||||||||||||
| Piperacillin‐tazobactam iv followed by amoxicillin po | 1 SIDESTEP Study | 2 Giordano 2005; RELIEF Study | 1 Siami 2001 | |||||||||||||
| Ticarcillin‐clavulanate iv | 1 Clay 2004 | |||||||||||||||
| Ticarcillin‐clavulanate iv followed by amoxicillin‐clavulanate po | 1 Graham 2002b | |||||||||||||||
| Piperacillin‐clindamycin iv | 1 Bouter 1996 | |||||||||||||||
| Broad‐spectrum penicillins | ||||||||||||||||
| Amoxicillin‐clavulanate iv followed by po | 1 STIC Study | |||||||||||||||
| Ampicillin‐sulbactam iv | 1 Erstad 1997 | 1 Grayson 1994 | ||||||||||||||
| Ampicillin‐sulbactan followed by amoxicillin‐clavulanate po | 1 Lipsky 1997 | 1 Lipsky 2004 | ||||||||||||||
| Cephalosporins | ||||||||||||||||
| Ceftobiprole iv | 1 Noel 2008a | |||||||||||||||
| Ceftriaxone‐metronidazol iv | ||||||||||||||||
| Ceftazidime‐vancomicyn iv | ||||||||||||||||
| Cefoxitin iv | ||||||||||||||||
| Cephalexin iv | 1 Lipsky 1990 | |||||||||||||||
| Carbapenems | ||||||||||||||||
| Ertapenem iv | 1 Lauf 2014 | |||||||||||||||
| Ertapenem iv followed by amoxicillin‐clavulanate po | ||||||||||||||||
| Imipenem‐cilastatin iv | ||||||||||||||||
| Fluoroquinolones | ||||||||||||||||
| Moxifloxacin iv followed by po | ||||||||||||||||
| Cinafloxacin iv followed by po | ||||||||||||||||
| Levofloxacin iv | ||||||||||||||||
| Ofloxacin iv followed by po | ||||||||||||||||
| Other antibiotics | ||||||||||||||||
| Daptomycin iv | 1 Arbeit 2004 | |||||||||||||||
| Linezolid iv or po | ||||||||||||||||
| Clindamycin iv | ||||||||||||||||
| Tigecycline | ||||||||||||||||
| Vancomycin |
This table shows all the comparisons included in the review and the number of evaluations for each comparison. To simplify, only different type of antibiotics are represented, irrespective of dose. The grey shaded areas represent comparisons between the same antibiotic. The numbers in the cells shown refer to number of RCT assessing that comparison.
Population
Trials differed with respect to their populations: 12 studies included only diabetic participants with foot infections, whilst eight trials included both diabetic and non‐diabetic participants with skin or skin structure‐related infections (but including at least some with a DFI).
Only five studies reported the type of diabetes (type 1 or type 2) of their participants (Bouter 1996; Erstad 1997; Lauf 2014; Lipsky 2004; SIDESTEP Study). In all included studies the participants had either type 1 or 2 diabetes.
Sex of participants
Only 11 studies provided disaggregated data on the sex of participants with DFI (Arbeit 2004; Bouter 1996; Clay 2004; Grayson 1994; Harkless 2005; Lauf 2014; Lipsky 1990; Lipsky 1997Lipsky 2004; Saltoglu 2010; SIDESTEP Study). Only men were enrolled in the Clay 2004 and Lipsky 1990 studies. When these two studies were excluded, the remaining studies included an average of 62% men. One study did not describe the sex of participants (Erstad 1997), and the rest of the studies did not provide data separately for patients with DFI (Giordano 2005; Graham 2002a; Graham 2002b; Noel 2008a; RELIEF Study; Siami 2001; STIC Study; Tan 1993).
Age of participants
Twelve studies provided age data for participants with DFI (Arbeit 2004; Bouter 1996; Clay 2004; Erstad 1997; Grayson 1994; Harkless 2005; Lauf 2014; Lipsky 1990; Lipsky 1997; Lipsky 2004; Saltoglu 2010; SIDESTEP Study). The mean age of included participants was 61.40 years. The rest of the studies included participants with skin or soft tissue infections that were not DFIs and they did not provide disaggregated age data for participants relevant to this review (Giordano 2005; Graham 2002a; Graham 2002b; Noel 2008a; RELIEF Study; Siami 2001; STIC Study; Tan 1993).
Characteristics of the diabetic foot infection (DFI)
All but two studies provided the definition they used for diagnosing a DFI (Bouter 1996; Saltoglu 2010). However, these definitions were heterogeneous, with some using only clinical signs or symptoms (e.g. erythema, purulent discharge, pain) while others also considered various laboratory parameters (e.g. leukocytosis).
It is remarkable that 11 studies excluded patients with osteomyelitis (Arbeit 2004; Clay 2004; Graham 2002a; Graham 2002b; Harkless 2005; Lipsky 1997; Lipsky 1990; Noel 2008a; Siami 2001; SIDESTEP Study; Tan 1993). While the Lauf 2014 trial excluded patients with baseline osteomyelitis from the main study, these patients were included in a prespecified sub study. Anatomic location of the DFI was reported in only four trials (Clay 2004; Lipsky 1990; Lipsky 2004; SIDESTEP Study).
Thirteen trials reported severity of infection (using various definitions). Although the majority of studies included participants with what appeared to be moderate to severe infection (as defined by the Infectious Diseases Society of America (IDSA) guidelines; IDSA 2012), most of them did not report which classification system they used for this designation. The systems that trials reported using included the: Wagner scale (Bouter 1996; Clay 2004; Saltoglu 2010; STIC Study); University of Texas system (Harkless 2005; SIDESTEP Study); PEDIS scale (Lauf 2014), and a combination of all three that did not report the final distribution of infection severity (RELIEF Study). Erstad 1997 and Tan 1993 used their own bespoke scales. The review authors attempted to transform the definitions of severity reported by some studies into the IDSA scale (Appendix 8). According to our interpretation, two studies included patients with mild to moderate infections (Clay 2004; Lipsky 1990); seven studies included mild to severe infections (Bouter 1996; Erstad 1997; Graham 2002a; Harkless 2005; Lauf 2014; Lipsky 2004; RELIEF Study); seven studies included moderate to severe infections (Arbeit 2004; Giordano 2005; Graham 2002b; Saltoglu 2010; SIDESTEP Study; STIC Study; Tan 1993); and one included only severe infections (Siami 2001).
The presence or absence of peripheral vascular disease, using a variety of definitions, was reported by Grayson 1994 (undefined, but present in 81% of patients), Bouter 1996 (ankle/brachial index mean (standard deviation (SD)) 0.71 (0.22) classified as mild artery disease), Erstad 1997 (ankle‐brachial index range 0.83 to 0.90), Harkless 2005 (41% of participants had “peripheral vascular disease”), Lipsky 2004 (40% had critical limb ischaemia), Clay 2004 (28% had “peripheral artery disease”), Saltoglu 2010 (19% with “ischemia”), SIDESTEP Study (normal dorsalis pedis and posterior tibial pulse in 16% to 19% of patients and those “requiring vascularization” were excluded) and Noel 2008a (exclusion of patients with “critical limb ischemia”).
Setting
Six trials were conducted entirely on an inpatient basis (Bouter 1996; Erstad 1997; Giordano 2005; Grayson 1994; Saltoglu 2010; Tan 1993); one trial entirely on an outpatient basis (Lipsky 1990); seven trials started with inpatients who could be discharged later to continue to participate on an outpatient basis (Arbeit 2004; Graham 2002a; Graham 2002b; Harkless 2005; Lauf 2014; Lipsky 1997; Siami 2001); and two trials allowed participants who were inpatients or outpatients (Lipsky 2004; SIDESTEP Study). The study setting was not defined in four studies, but we assumed participants were likely to be inpatients as the antibiotic treatment was intravenous (Clay 2004; Noel 2008a; RELIEF Study; STIC Study).
Intervention
Antibiotic agents and regimens
Studies compared a variety of different antibiotic agents and regimens. Overall, there were 16 different comparisons. We have categorized comparisons by antibiotic groups or classes:
anti‐pseudomonal penicillins;
broad‐spectrum penicillins;
cephalosporins;
carbapenems;
fluoroquinolones;
In many instances a study compared drugs from two different groups; in those cases we assigned the study to the group corresponding to the antibiotic considered to be the intervention, as opposed to the control or comparator according to the trial authors' judgement. In the review text, doses are presented as stated as in the original papers.
Route of administration
Trials also differed in the route of drug administration: 10 trials used parenterally administered antibiotics (Bouter 1996; Clay 2004; Erstad 1997; Graham 2002a; Grayson 1994; Harkless 2005; Lauf 2014; Noel 2008a; Saltoglu 2010; Tan 1993); one used only oral antibiotic agents (Lipsky 1990); and, in two the intervention drug could be given either orally or intravenously (Graham 2002b; Lipsky 2004). The rest of the included trials started with a parenteral regimen that was switched to an oral one (Arbeit 2004; Giordano 2005; Lipsky 1997; RELIEF Study; Siami 2001; SIDESTEP Study; STIC Study).
Duration of antibiotic treatment
The duration of antibiotic treatment also varied across studies: in the STIC Study parenteral antibiotic was administered for a minimum of three days and then oral agents were given for seven to 21 days; in three studies antibiotics were administered for a minimum of five days (Erstad 1997; Grayson 1994; Tan 1993); the SIDESTEP Study administered parenteral antibiotics for a minimum of five days and continued with oral antibiotics until day 28. In nine studies antibiotics were administered for 14 days or less (Arbeit 2004; Bouter 1996; Giordano 2005; Graham 2002a; Graham 2002b; Harkless 2005; Lipsky 1990; Noel 2008a; Siami 2001). In Saltoglu 2010 treatment lasted for 14 days or less unless there was a diagnosis of osteomyelitis, in which case antibiotic therapy was continued for 28 days from the time of debridement, if performed. In the RELIEF Study antibiotics were administered for seven to 21 days. In three studies intravenous antibiotic therapy lasted for 28 days or less (Lauf 2014; Lipsky 1997;Lipsky 2004). The Clay 2004 study did not provide information regarding the duration of parenteral antibiotic therapy.
Co‐interventions
Some studies allowed participants to receive antibiotics other than the ones specifically being studied. Two studies allowed the addition of vancomycin to both study groups when meticillin‐resistant Staphylococcus aureus (MRSA) or meticillin‐resistant S epidermidis (MRSE) was suspected or isolated (Harkless 2005; SIDESTEP Study). Lauf 2014 also allowed investigators, at their discretion, to use adjunctive vancomycin (a placebo was given in its place in the group randomised to tigecycline and real vancomycin in the group randomised to ertapenem). Siami 2001 allowed the addition of treatment with vancomycin under the same conditions for the control group (piperacillin/tazobactam) but not the clinafloxacin group. In the RELIEF Study, participants could be treated with additional narrow‐spectrum antibiotic agents (which were not specified) if polymicrobial infection with MRSA, MRSE or vancomycin‐resistant enterococci was confirmed. In Lipsky 1997, if there was no improvement in infection, metronidazole could be added to the group treated with ofloxacin, while intravenous gentamicin or oral trimethoprim‐sulfamethoxazole could be added to the group treated with ampicillin‐sulbactam. In Noel 2008a, metronidazole could be added to the study treatment after reviewing the culture results. In Lipsky 2004, participants from both study groups could have aztreonam added to the regimen if Gram‐negative pathogens were suspected; only participants in the control group could receive additional vancomycin if there was an MRSA infection. In Arbeit 2004, aztreonam could be added to cover suspected or proven polymicrobial infection with Gram‐negative bacteria and metronidazole could also be added to the study regimen to cover obligate anaerobic bacteria. In the Saltoglu 2010 study, glycopeptides were added to the study drugs if cultures confirmed the presence of antibiotic‐resistant enterococci or MRSA. A switch of antibiotics was performed in Bouter 1996; participants from either study group (imipenem/cilastatin or piperacillin‐clindamycin) diagnosed with chronic osteomyelitis were switched to ciprofloxacin or ofloxacin and/or clindamycin. Graham 2002b excluded participants who need additional antibiotics.
In five studies other treatments, such as surgical debridement or drainage, were specifically allowed if necessary (Lauf 2014; Lipsky 1990; Siami 2001; STIC Study; Tan 1993). Saltoglu 2010 allowed vacuum‐assisted closure in both study groups, when considered necessary.
Hypothesis and sample size
In eight studies the study design was to test superiority of the intervention drug (Bouter 1996; Clay 2004; Erstad 1997; Grayson 1994; Lipsky 1990; Lipsky 1997Saltoglu 2010; Tan 1993), five studies tested equivalence between the study drugs (Graham 2002a; Graham 2002b; Harkless 2005; Lipsky 2004; Siami 2001), and seven tested non‐inferiority of the intervention drug (Arbeit 2004; Giordano 2005; Lauf 2014; Noel 2008a; RELIEF Study; SIDESTEP Study; STIC Study).
Only five studies reported a sample size calculation and also reached the sample size required (Graham 2002a; Grayson 1994; RELIEF Study; Siami 2001; SIDESTEP Study). In three studies the sample size calculation was reported, but was not reached (Arbeit 2004; Harkless 2005; Tan 1993); the remaining 12 studies did not report a sample size calculation (Bouter 1996; Clay 2004; Erstad 1997; Giordano 2005; Graham 2002b; Lauf 2014; Lipsky 1990; Lipsky 1997Lipsky 2004; Noel 2008a; Saltoglu 2010; STIC Study).
Sample sizes of participants with DFIs ranged from 36 to 955 (Erstad 1997; Lauf 2014, respectively), with a mean sample size for all studies of 189.60 participants (median of 94.50, standard deviation (SD) 220.58).
Excluded studies
Thirty‐four studies did not fulfil our inclusion criteria and so were excluded. We excluded most because they not provide disaggregated data for the subset of participants with DFIs. See Characteristics of excluded studies for a detailed account of the reasons for exclusion for each of these studies.
Risk of bias in included studies
The risk of bias of the studies is described in detail in the 'Risk of bias' tables in the Characteristics of included studies section. In general, the included studies did not report enough details for us to assess their possible limitations in the design or execution (e.g. only one‐third of studies reported enough information for us to assess patient allocation in the study groups). The main limitation of the included trials concerned the blinding procedures, especially for outcome assessment (see Figure 2; Figure 3).
2.
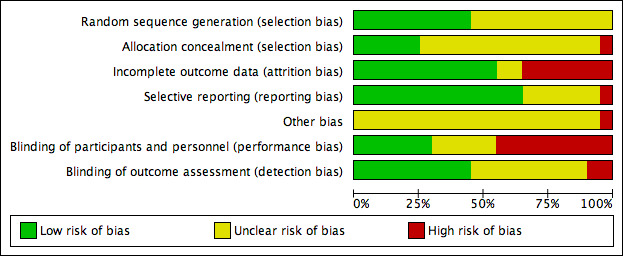
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
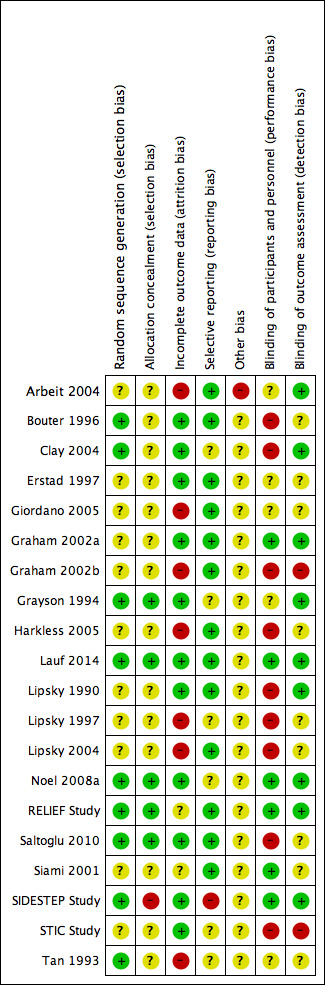
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only nine of the included trials reported an adequately generated random sequence. Noel 2008a did not provide details of the method used to generate the random sequence, but randomised participants through a central interactive voice response system. Eight studies reported computer‐generated sequences of random numbers (Bouter 1996; Clay 2004; Grayson 1994; Lauf 2014; RELIEF Study; Saltoglu 2010; SIDESTEP Study; Tan 1993). In two studies the random sequence was prepared by the study sponsor (RELIEF Study; SIDESTEP Study). The other 11 trials included in the review did not provide enough data to assess the adequacy of the randomizations sequence.
Only five studies accurately concealed the randomisation sequence by requesting the study drug to be selected by an independent unit (Grayson 1994; Saltoglu 2010), or via a remote call system (Lauf 2014; Noel 2008a; RELIEF Study). The random sequence in the SIDESTEP Study could be exposed to bias because an unblinded pharmacist randomised participants. Fourteen trials did not provide the information needed for us to assess whether allocation concealment was ensured.
Blinding
In nine of the included trials, study personnel knew the treatment allocation resulting in a high risk of performance bias (Bouter 1996; Clay 2004; Graham 2002b; Harkless 2005; Lipsky 1990; Lipsky 1997Lipsky 2004; Saltoglu 2010; STIC Study). Five studies did not provide details that allowed us to assess the risk of performance bias (Arbeit 2004; Erstad 1997; Giordano 2005; Grayson 1994; Tan 1993). Only six trials described how the intervention was blinded for both participants and healthcare personnel (Graham 2002a; Lauf 2014; Noel 2008a; RELIEF Study; Siami 2001; SIDESTEP Study), with a double‐dummy design used in two studies (RELIEF Study; SIDESTEP Study).
Since the main outcomes of this review required investigators to make judgements about the severity of infection and the presence of clinical signs and symptoms to determine whether the infection had resolved clinically, it was especially important that those involved in the outcome assessment were unaware of the treatment assigned to participants. Detection bias was avoided in nine trials that blinded outcome assessment (Arbeit 2004; Clay 2004; Graham 2002a; Grayson 1994; Lauf 2014; Lipsky 1990; Noel 2008a; RELIEF Study; SIDESTEP Study). In two trials study investigators assessed the outcomes in an unblinded fashion (Graham 2002b; STIC Study), which exposed these trials to a high risk of bias. The rest of the studies did not provide sufficient details for us to be able to assess this domain.
Incomplete outcome data
Seven included trials did not address missing outcome data correctly (Arbeit 2004; Giordano 2005; Graham 2002b; Harkless 2005; Lipsky 1997; Lipsky 2004; Tan 1993). These studies had no data that we could evaluate for a range of 15% to 30% of randomised participants (Arbeit 2004; Lipsky 1997; Lipsky 2004; and Graham 2002b; Harkless 2005, respectively). Two trials were underpowered and at very high risk of attrition bias because they had data for only 40% or 45% of participants randomised (Giordano 2005; Tan 1993, respectively). The remaining 11 studies provided sufficient data for us to assess how the investigators managed withdrawals and losses to follow‐up.
Selective reporting
Most studies reported enough information to ensure that the outcomes planned in their protocols were adequately described when their findings were published (Arbeit 2004; Bouter 1996; Erstad 1997; Giordano 2005; Graham 2002a; Graham 2002b; Harkless 2005; Lauf 2014; Lipsky 1990; Lipsky 2004; RELIEF Study; Saltoglu 2010; Siami 2001). We could not assess this domain for six studies (Clay 2004; Grayson 1994; Lipsky 1997; Noel 2008a; STIC Study; Tan 1993). The SIDESTEP Study report did not provide data for some of the secondary outcomes included in the trial protocol.
Other potential sources of bias
Whether the 'Risk of bias' assessment should include information about the funding source of a trial is a controversial area (Bero 2013; Sterne 2013); nonetheless, we collected data on funding from the included trials. Only Saltoglu 2010 explicitly reported that his group did not receive funding from private sources for developing the trial. Bouter 1996 did not provide information about this issue. Biopharmaceutical industry sponsors funded the remaining 18 trials and study authors from 12 trials were employed by the sponsor that provided funding for the study (Arbeit 2004; Clay 2004; Giordano 2005; Graham 2002a; Graham 2002b; Harkless 2005; Lauf 2014; Noel 2008a; RELIEF Study; Siami 2001; SIDESTEP Study; STIC Study).
Effects of interventions
Outcome 1: Clinical resolution of the infection
The included studies measured the outcome of clinical resolution of infection at different time points after the initiation of study antibiotic treatment. Most studies assessed this variable at a variety of times after completion of the study antibiotic regimens: two studies assessed this during the first week after completion of antibiotic treatment (Graham 2002b; Lipsky 1997); five assessed between the first and second week after completion of antibiotic treatment (Lipsky 1990; Noel 2008a; Siami 2001; SIDESTEP Study; Tan 1993); and six during the four weeks after completion of treatment (Arbeit 2004; Graham 2002a; Harkless 2005; Lipsky 2004; RELIEF Study; STIC Study). Studies that made more long‐term evaluations included Giordano 2005 (between 10 and 42 days after treatment), Grayson 1994 (at the end of therapy and 13 weeks later), and Lauf 2014 (between 12 and 92 days after the last dose of antibiotic in the main study and after 25 to 27 weeks in the sub study of participants with osteomyelitis). However, four trials measured the clinical resolution only on the final day of antibiotic therapy (Bouter 1996; Clay 2004; Erstad 1997; Saltoglu 2010).
A. Anti‐pseudomonal penicillins
Anti‐pseudomonal penicillin versus anti‐pseudomonal penicillin
Piperacillin‐tazobactam versus ticarcillin‐clavulanate
Tan 1993 compared the administration of piperacillin‐tazobactam 3 g/375 mg intravenously (iv) every six hours (h) for a minimum of five days and at least 48 h after resolution of signs and symptoms of infection with the administration of ticarcillin‐clavulanate 3 g/100 mg iv every 6 h for the same time period. At 10 to 14 days after the end of antibiotic therapy there was no difference between the groups in the proportion of participants with clinical resolution of the infection (37.50% versus 32.25%, RR 1.16, 95% CI 0.59 to 2.29; 1 trial, 63 participants, 22 events; Analysis 1.1).
1.1. Analysis.
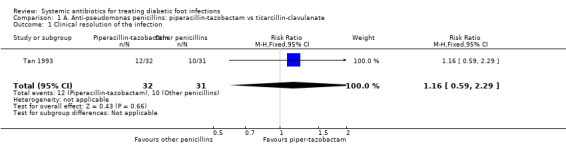
Comparison 1 A. Anti‐pseudomonas penicillins: piperacillin‐tazobactam vs ticarcillin‐clavulanate, Outcome 1 Clinical resolution of the infection.
Anti‐pseudomonal penicillin versus broad‐spectrum penicillin
Piperacillin‐tazobactam versus ampicillin‐sulbactam
Harkless 2005 compared the administration of piperacillin‐tazobactam 4 g/0.5 mg iv every 8 h with the administration of ampicillin‐sulbactam 2 g/1 g iv every 6 h. Both treatments were administered for between 4 to 14 days, up to a maximum of 21 days. At 14 to 21 days after the end of therapy there was no difference in the proportion of participants with clinical resolution of the infection (63.87% versus 62.89%, RR 1.02, 95% CI 0.86 to 1.20; 1 trial, 314 participants, 199 events; Analysis 2.1).
2.1. Analysis.
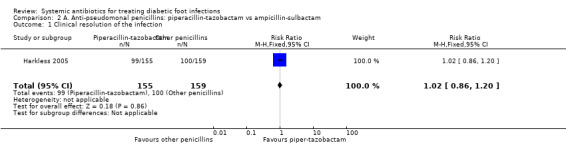
Comparison 2 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs ampicillin‐sulbactam, Outcome 1 Clinical resolution of the infection.
Anti‐pseudomonal penicillin versus carbapenems
Piperacillin‐tazobactam versus imipenem‐cilastatin
Saltoglu 2010 found no clear difference in the proportion of participants with clinical resolution of the infection between the groups treated with piperacillin‐tazobactam 4.5 g iv every 8 h and the group treated with imipenem‐cilastatin 0.5 g iv every 24 h (45.16% versus 27.27%; RR 1.66, 95% CI 0.84 to 3.26; 1 trial, 64 participants, 23 events; Analysis 3.1). Two participants in the piperacillin‐tazobactam group and one in the imipenem‐cilastatin group received a glycopeptide in addition to the study drugs because cultures confirmed the presence of a drug‐resistant Enterococcus or MRSA.
3.1. Analysis.
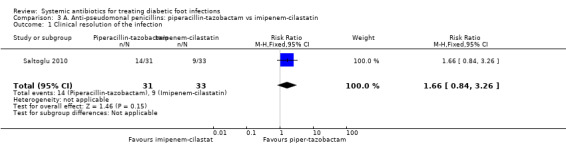
Comparison 3 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs imipenem‐cilastatin, Outcome 1 Clinical resolution of the infection.
B. Broad‐spectrum penicillins
Broad‐spectrum penicillin versus cephalosporin
Ampicillin‐sulbactam versus cefoxitin
In a small study involving only 36 participants, Erstad 1997 reported fewer participants treated with ampicillin‐sulbactam 3 g iv every 6 h (5.55%) achieved clinical resolution of the infection at the end of ≥5 days' therapy compared with those treated with cefoxitin 2 g iv every 6 h (38.88%). This difference was not statistically significant (RR 0.14, 95% CI 0.02 to 1.05; 1 trial, 36 participants, 8 events; Analysis 4.1).
4.1. Analysis.
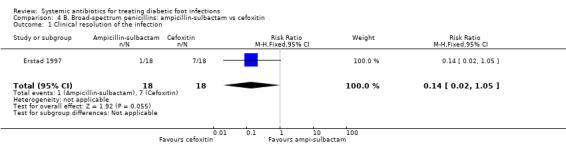
Comparison 4 B. Broad‐spectrum penicillins: ampicillin‐sulbactam vs cefoxitin, Outcome 1 Clinical resolution of the infection.
C. Cephalosporins
Fifth‐generation cephalosporin versus third generation cephalosporin plus glycopeptide
Ceftobiprole versus ceftazidime plus vancomycin
Noel 2008a compared ceftobiprole 500 mg iv every 8 h with ceftazidime 1 g iv every 8 h plus vancomycin 1 g iv every 12 h, for 7 to 14 days. Metronidazole could be added at the discretion of the investigators in either group if the culture grew obligate anaerobes. There was no clear difference in the proportion of participants with clinical resolution of the infection at 7 to 14 days after the end of therapy (74.40% versus 70.79%, RR 1.05, 95% CI 0.90 to 1.23; 1 trial, 257 participants, 188 events; Analysis 5.1). Metronidazole was administered to 22 (13%) of the participants treated with ceftobiprole and to 17 (19%) participants treated with ceftazidime plus vancomycin.
5.1. Analysis.
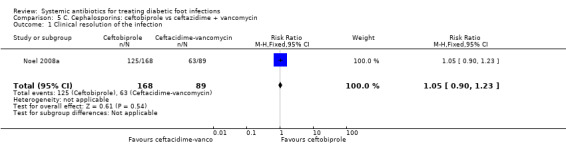
Comparison 5 C. Cephalosporins: ceftobiprole vs ceftazidime + vancomycin, Outcome 1 Clinical resolution of the infection.
Third‐generation cephalosporin plus nitroimidazole versus anti‐pseudomonal penicillin
Ceftriaxone plus metronidazole versus ticarcillin‐clavulanate
Clay 2004 compared ceftriaxone 1 g plus metronidazole 1 g, both given iv every 24 h for a mean of 6.7 days with ticarcillin‐clavulanate 3.1 g iv every 6 h for a mean of 6.7 days. There was no clear difference in the proportion of participants with clinical resolution of the infection on the final day of therapy (72.22% versus 76.47%, RR 0.94, 95% CI 0.72 to 1.24; 1 trial, 70 participants, 52 events; Analysis 6.1). There was a change in the antibiotic regimen before the final evaluation in 41.60% of participants treated with ceftriaxone plus metronidazole and in 35.29% of those treated with ticarcillin‐clavulanate; these changes were predominantly switches to oral therapeutic equivalents at the time of hospital discharge, and there was no statistically significant difference between groups.
6.1. Analysis.
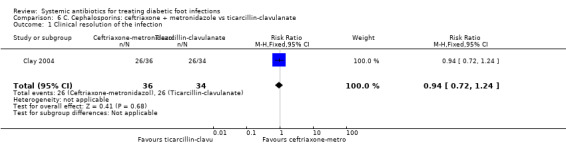
Comparison 6 C. Cephalosporins: ceftriaxone + metronidazole vs ticarcillin‐clavulanate, Outcome 1 Clinical resolution of the infection.
D. Carbapenems
Carbapenems versus anti‐pseudomonal penicillins
Ertapenem versus piperacillin‐tazobactam
Two RCTs compared ertapenem 1 g iv every 24 h with piperacillin‐tazobactam 3.4 g iv every 6 h (Graham 2002a; SIDESTEP Study). In the SIDESTEP Study participants received parenteral treatment for a minimum of five days, after which oral amoxicillin‐clavulanate 875 mg/125 mg every 12 h could be given for up to 28 days in both groups. In Graham 2002a, the parenteral antibiotic could be given for 7 to 14 days. These studies were pooled (random effects) and there was no difference in the proportion of participants with clinical resolution of the infection two to five days after the end of antibiotic treatment (65.80% versus 63.63%, RR 1.07, 95% CI 0.96 to 1.19; I² 0%; 2 trials, 684 participants, 439 events; Analysis 7.1).
7.1. Analysis.
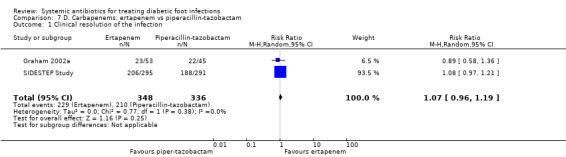
Comparison 7 D. Carbapenems: ertapenem vs piperacillin‐tazobactam, Outcome 1 Clinical resolution of the infection.
Imipenem‐cilastatin versus piperacillin plus clindamycin
Bouter 1996 compared imipenem‐cilastatin 500 mg iv every 6 h with piperacillin 3 g iv every 6 h plus clindamycin 600 mg iv every 8 h, with both regimens given for at least 10 days. There were no difference in the proportions of participants with clinical resolution of the infection after completion of antibiotic therapy (18.18% versus 25.00%, RR 0.73, 95% CI 0.24 to 2.24; 1 trial, 46 participants, 10 events; Analysis 8.1).
8.1. Analysis.
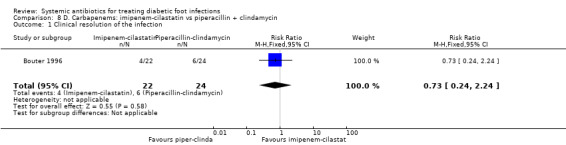
Comparison 8 D. Carbapenems: imipenem‐cilastatin vs piperacillin + clindamycin, Outcome 1 Clinical resolution of the infection.
Carbapenems versus broad‐spectrum penicillins
Imipenem‐cilastatin versus ampicillin‐sulbactam
Grayson 1994 compared imipenem‐cilastatin 500 mg iv every 6 h (given for a mean of 15 days) with ampicillin‐sulbactam 2 g/1 g iv every 6 h (given for a mean of 13 days). As a unit of analysis this trial used 'infection episodes' rather than participants. There was no difference in the proportion of infection episodes that were clinically cured, either at the completion of parenteral therapy (81.25% versus 85.41%, RR 0.95, 95% CI 0.80 to 1.14; 1 trial, 93 participants, 96 episodes; Analysis 9.1), or after 13 weeks of follow‐up (56.25% versus 68.75%, RR 0.82, 95% CI 0.60 to 1.12; 1 trial, 93 participants, 96 episodes; Analysis 9.2).
9.1. Analysis.
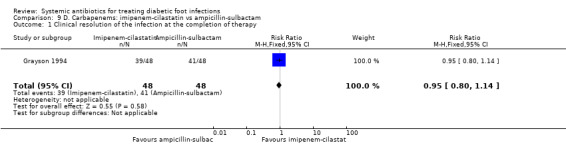
Comparison 9 D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam, Outcome 1 Clinical resolution of the infection at the completion of therapy.
9.2. Analysis.
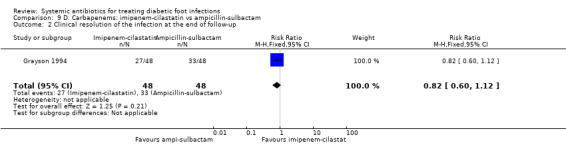
Comparison 9 D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam, Outcome 2 Clinical resolution of the infection at the end of follow‐up.
E. Fluoroquinolones
Fourth‐generation fluoroquinolones versus anti‐pseudomonal penicillins
Moxifloxacin or clinafloxacin versus piperacillin‐tazobactam
Three RCTs pooled using a fixed effect model (I2=0%) found no difference in the proportion of participants with clinical resolution of the infection when the administration of a fourth‐generation fluoroquinolone was compared with piperacillin‐tazobactam (RR 1.03, 95% CI 0.89 to 1.20; 3 RCTs, 387 participants, 239 events; Analysis 10.1; Giordano 2005; RELIEF Study; Siami 2001). Two of these studies compared the administration of 400 mg of moxifloxacin iv every 24 h for at least three days followed by the same dose orally for 7 to 19 more days versus piperacillin‐tazobactam at a total dose of 13.5 g a day for at least three days, followed by amoxicillin‐clavulanate 875 mg/125 mg orally every 12 h (Giordano 2005; RELIEF Study). The third study compared the administration of clinafloxacin 200 mg iv every 12 h for at least three days followed by the same dose orally versus piperacillin‐tazobactam 3.4 g iv every 6 h followed by amoxicillin‐clavulanate 500 mg orally every 8 h (Siami 2001).
10.1. Analysis.
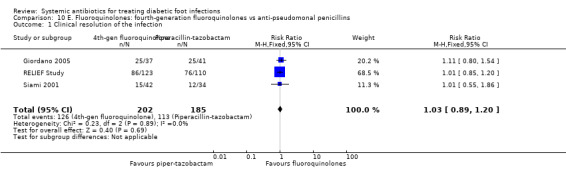
Comparison 10 E. Fluoroquinolones: fourth‐generation fluoroquinolones vs anti‐pseudomonal penicillins, Outcome 1 Clinical resolution of the infection.
Fourth‐generation fluoroquinolones versus broad‐spectrum penicillins
Moxifloxacin versus amoxicillin‐clavulanate
One trial compared moxifloxacin 400 mg iv every 24 h for at least three days, followed by the same dose orally for 7 to 21 days, versus amoxicillin 1000 mg plus clavulanate 200 mg iv every 8 h for at least three days, followed by the same antibiotic, that is, amoxicillin‐clavulanate 500 mg/125 mg orally every 8 h for 7 to 21 days (STIC Study). The difference in rates of clinical resolution of infection was not statistically significant (47.62% with moxifloxacin versus 60.56% with amoxicillin‐clavulanate, RR 0.79, 95% CI 0.57 to 1.08; 1 trial, 134 participants, 73 events; Analysis 11.1). However as with most of these studies the study is too small to exclude a potentially important treatment effect.
11.1. Analysis.
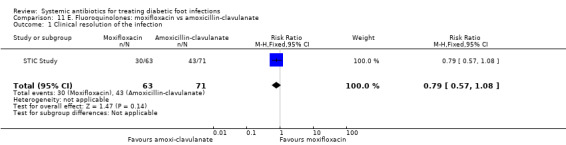
Comparison 11 E. Fluoroquinolones: moxifloxacin vs amoxicillin‐clavulanate, Outcome 1 Clinical resolution of the infection.
Third‐generation fluoroquinolones versus anti‐pseudomonal penicillins
Levofloxacin versus ticarcillin‐clavulanate
One trial compared levofloxacin 750 mg iv or orally given once daily for seven to 14 days versus ticarcillin‐clavulanate 3.1 g iv every 4 h to 6 h, followed by amoxicillin‐clavulanate 875 mg orally every 12 h (Graham 2002b). There was no evidence of a difference in the proportion of participants with clinical resolution of the infection (50.00% versus 51.61%, RR 0.97, 95% CI 0.60 to 1.55; 1 trial, 67 participants, 34 events; Analysis 12.1).
12.1. Analysis.
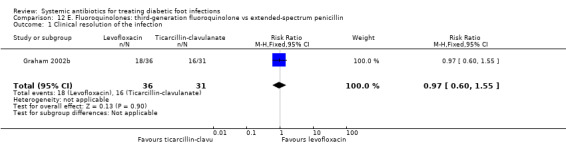
Comparison 12 E. Fluoroquinolones: third‐generation fluoroquinolone vs extended‐spectrum penicillin, Outcome 1 Clinical resolution of the infection.
Second‐generation fluoroquinolones versus broad‐spectrum penicillins
Ofloxacin versus ampicillin‐sulbactam
One trial compared ofloxacin 400 mg iv every 12 h, with a switch to oral when indicated, for a total of 14 to 28 days, versus ampicillin‐sulbactam 1 g to 2 g/0.5 g to 1 g iv every 6 h, with a switch to oral amoxicillin–clavulanate 500 mg/125 mg every 8 h (Lipsky 1997). There was no statistically significant difference in the proportion of participants with clinical resolution of the infection (72.73% versus 64.15%, RR 1.13, 95% CI 0.88 to 1.47; 1 trial, 108 participants, 74 events; Analysis 13.1). Additional antibiotics could be added in both groups if there was not sufficient clinical improvement: five (9%) participants treated with ofloxacin also received metronidazole and 42 (79%) treated with ampicillin‐sulbactam also received either gentamicin or trimethoprim‐sulfamethoxazole (Chi² 54.04 and P value < 0.001 for the difference between the two groups in the percentage of participants who received an additional drug).
13.1. Analysis.
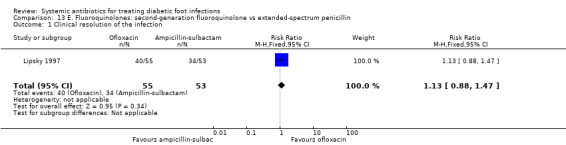
Comparison 13 E. Fluoroquinolones: second‐generation fluoroquinolone vs extended‐spectrum penicillin, Outcome 1 Clinical resolution of the infection.
F. Other antibiotics
Daptomycin versus vancomycin
Arbeit 2004 compared seven to 14 days of treatment with daptomycin 4 mg/kg iv every 24 h with a control group treated with either vancomycin 1 g iv every 12 h or a semi‐synthetic penicillin (nafcillin, oxacillin, cloxacillin or flucoxacillin) 4 g to 12 g iv every 24 h. The addition of aztreonam was allowed to cover Gram‐negative bacteria in suspected or proven polymicrobial infections and was administered to 29.50% of participants in the intervention group and 31.94% of participants in the control group. There was no evidence of a difference between the groups in the proportion of participants with clinical resolution of the infection at six to 20 days after completion of the study drug (50.81% versus 54.16%, RR 0.94, 95% CI 0.68 to 1.30; 1 RCT, 133 participants, 56 events; Analysis 14.1).
14.1. Analysis.
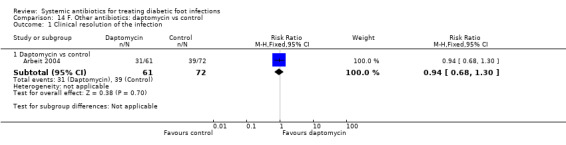
Comparison 14 F. Other antibiotics: daptomycin vs control, Outcome 1 Clinical resolution of the infection.
Linezolid versus aminopenicillin plus beta‐lactamase inhibitors
Lipsky 2004 compared linezolid 600 mg iv or orally every 12 h with a combination of aminopenicillin and beta‐lactamase inhibitors (ampicillin‐sulbactam 1.5 g/3 g iv every 6 h or amoxicillin‐clavulanate 500 mg/875 mg orally every 8 to 12 h), both given for between seven and 28 days. Participants were assessed 15 to 21 days after completing treatment; there was no evidence of a difference in the proportion of participants with clinical resolution of the infection (68.46% versus 64.16%, RR 1.07 95% CI 0.91 to 1.25; 1 trial, 361 participants, 242 events; Analysis 15.1). Participants with MRSA infection in the control group could receive vancomycin 1 g iv every 12 h; five participants in the control group received vancomycin, as did (by error) one participant in the linezolid group. Aztreonam could be added if Gram‐negative pathogens were suspected in either study group; 12 participants in the linezolid group and three participants in the control group received aztreonam.
15.1. Analysis.
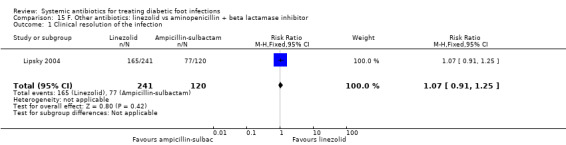
Comparison 15 F. Other antibiotics: linezolid vs aminopenicillin + beta lactamase inhibitor, Outcome 1 Clinical resolution of the infection.
Clindamycin versus cephalexin
Lipsky 1990 compared clindamycin hydrochloride 300 mg orally every 6 h with cephalexin 500 mg orally every 6 h, each regimen given for 14 days. At the end of treatment there was no evidence of a difference between groups in the proportion of participants with clinical resolution of the infection (77.77% versus 72.41%, RR 1.07, 95% CI 0.79 to 1.45; 1 trial, 56 participants, 42 events; Analysis 16.1).
16.1. Analysis.
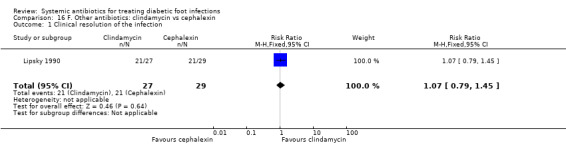
Comparison 16 F. Other antibiotics: clindamycin vs cephalexin, Outcome 1 Clinical resolution of the infection.
Tigecycline versus ertapenem with or without vancomycin
Lauf 2014 trial compared tigecycline 150 mg iv every 24 h with ertapenem 1 g iv every 24 h with or without vancomycin (dose not specified). Both drugs were given for 28 days in participants with DFI without osteomyelitis. Investigators could add adjunctive vancomycin (placebo vancomycin in the tigecycline group and real vancomycin in the ertapenem group) at their discretion for coverage of MRSA, coagulase‐negative staphylococci or enterococci. At 12 to 92 days after the end of treatment, clinical resolution of infection was more likely to have occurred in participants treated with ertapenem with or without vancomycin than in those treated with tigecycline (76.90% versus 39.00%, RR 1.09, CI 95% 1.01 to 1.18; 1 trial, 955 participants, 703 events; Analysis 17.1). In absolute terms, this means that for every 1000 participants treated with ertapenem with or without vancomycin instead of tigecycline, 65 more people would show clinical resolution of the infection (95% confidence interval 7 to 120 more assuming the risk of the control group as a baseline risk). This study was at low risk of bias.
17.1. Analysis.
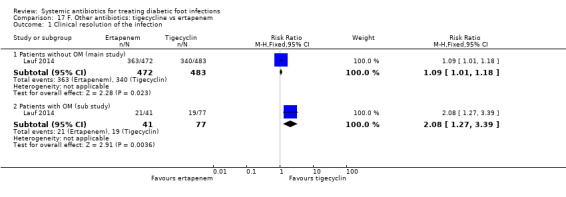
Comparison 17 F. Other antibiotics: tigecycline vs ertapenem, Outcome 1 Clinical resolution of the infection.
In a pre‐planned sub study of participants who had osteomyelitis at baseline, participants received the same antibiotic regimens but for a longer time (up to 42 days). At 25 to 27 weeks after the last dose of antibiotic, participants treated with ertapenem with or without vancomycin had higher rates of clinical resolution of the infection than participants treated with tigecycline (51.21% versus 24.67%, RR 2.08, 95% CI 1.27 to 3.39; 1 trial, 118 participants, 40 events; Analysis 17.1).
Vancomycin placebo was administered to 10.30% (84/483 participants) of the tigecycline group, while adjunctive vancomycin was given to 15.50% (73/472 participants) of the ertapenem group in the primary study. There was no statistically significant difference between groups in the proportion of participants who received non‐pharmacologic treatments or procedures in the main study (35% of participants treated with tigecycline versus 38% of participants treated with ertapenem).
Outcome 2: Time to clinical resolution of the infection
None of the studies reported on time to clinical resolution of the infection.
Outcome 3: Adverse effects of treatments
A. Anti‐pseudomonal penicillins
Anti‐pseudomonal penicillins versus anti‐pseudomonal penicillins
Piperacillin‐tazobactam versus ticarcillin‐clavulanate
Although the Tan 1993 trial investigated piperacillin‐tazobactam versus ticarcillin‐clavulanate, the trial report did not provide disaggregated adverse event data for diabetic participants.
Anti‐pseudomonal penicillins versus broad‐spectrum penicillins
Piperacillin‐tazobactam versus ampicillin‐sulbactam
Harkless 2005 found no difference between piperacillin‐tazobactam and ampicillin‐sulbactam in the rate of adverse events (75.48% versus 66.04 %, RR 1.14, 95% CI 0.99 to 1.32; 1 trial, 314 participants, 222 events), or the rate of treatment‐related adverse events (18.7% versus 13.2%, RR 1.42, 95% CI 0.85 to 2.37; 1 trial, 314 participants, 50 events; Analysis 2.2) or the rate of serious adverse events (27.09% versus 28.93%, RR 0.94, 95% CI 0.66 to 1.34; 1 trial, 314 participants, 88 events; Analysis 2.2). The most commonly reported treatment‐related adverse events were diarrhoea and nausea, and the most commonly reported serious adverse events were amputations, infection, peripheral vascular disorder, and osteomyelitis.
2.2. Analysis.
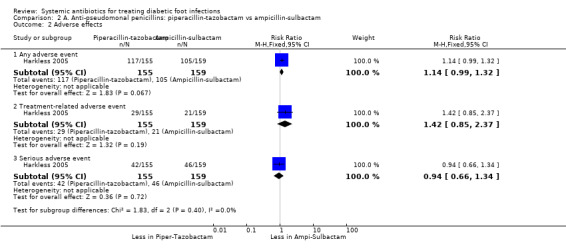
Comparison 2 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs ampicillin‐sulbactam, Outcome 2 Adverse effects.
Anti‐pseudomonal penicillins versus carbapenems
Piperacillin‐tazobactam versus imipenem‐cilastatin
Saltoglu 2010 reported more adverse events in participants treated with piperacillin‐tazobactam (29.03%)compared with imipenem‐cilastatin (9.09%), although this difference did not reach conventional levels of statistical significance (RR 3.19, 95% CI 0.95 to 10.72; 1 trial, 64 participants, 12 events; Analysis 3.2). There were more cases of hepatotoxicity and nephrotoxicity in the group treated with piperacillin‐tazobactam, although differences were not statistically significant (for hepatotoxicity:16.13% versus 3.03%, RR 5.32, 95% CI 0.66 to 43.05; 6 events; for nephrotoxicity: 19.35% versus 3.03%, RR 6.36, 95% CI 0.81 to 50.09; 7 events; Analysis 3.2). There was one case of nausea in the imipenem‐cilastatin group versus none in the piperacillin‐tazobactam group. Two participants treated with piperacillin‐tazobactam developed haematological side effects. This study was very small and therefore important differences in adverse events cannot be ruled out.
3.2. Analysis.
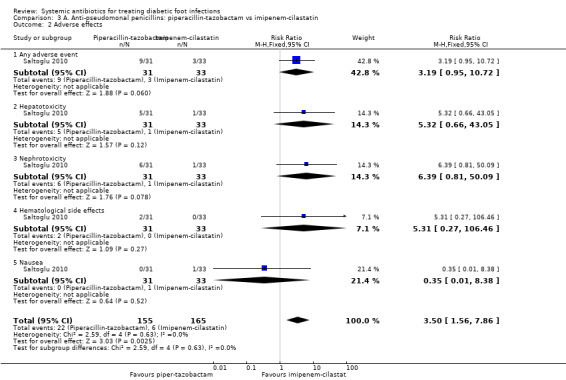
Comparison 3 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs imipenem‐cilastatin, Outcome 2 Adverse effects.
B. Broad‐spectrum penicillins
Broad‐spectrum penicillins versus cephalosporins
Ampicillin‐sulbactam versus cefoxitin
Erstad 1997 found no difference between ampicillin‐sulbactam and cefoxitin in the rate of adverse events, which were mostly gastrointestinal disturbances of minor clinical importance (39% versus 33%, RR 1.17, 95% CI 0.49 to 2.79; Analysis 4.2).
4.2. Analysis.
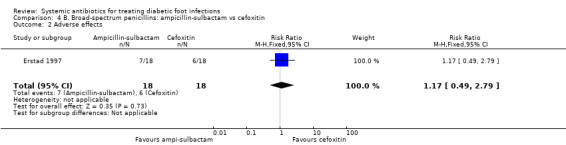
Comparison 4 B. Broad‐spectrum penicillins: ampicillin‐sulbactam vs cefoxitin, Outcome 2 Adverse effects.
C. Cephalosporins
Fifth‐generation cephalosporins versus third‐generation cephalosporins plus glycopeptide
Ceftobiprole versus ceftacidime‐vancomycin
The Noel 2008a trial did not provide disaggregated data for diabetic participants for adverse effects.
Third‐generation cephalosporin plus nitroimidazole versus anti‐pseudomonal penicillins
Ceftriaxoneplusmetronidazole versus ticarcillin‐clavulanate
The Clay 2004 trial (70 participants) reported no cases of adverse effects in any group.
D. Carbapenems
Carbapenems versus anti‐pseudomonal penicillins
Ertapenem versus piperacillin‐tazobactam
The SIDESTEP Study found no clear difference between ertapenem and piperacillin‐tazobactam in the rate of drug‐related adverse events (14.90% versus 19.58%, RR 0.76, 95% CI 0.53 to 1.09; 1 trial, 586 participants, 101 events; Analysis 7.2). There were no differences in the proportion of participants with nausea (RR 0.83, 95% CI 0.44 to 1.58; 1 trial, 586 participants, 35 events) or in the proportion of participants with headache (RR 0.62, 95% CI 0.28 to1.34; 1 trial, 586 participants, 26 events; Analysis 7.2). Treatment with ertapenem caused fewer cases of diarrhoea (RR 0.58, 95% CI 0.36 to 0.93; 1 trial, 586 participants, 65 events; Analysis 7.2). That means that for every 1000 participants treated with ertapenem instead of piperacillin‐tazobactam, 59 fewer would present with diarrhoea (from 10 to 90 participants fewer; assuming the risk of the control group as a baseline risk).
7.2. Analysis.
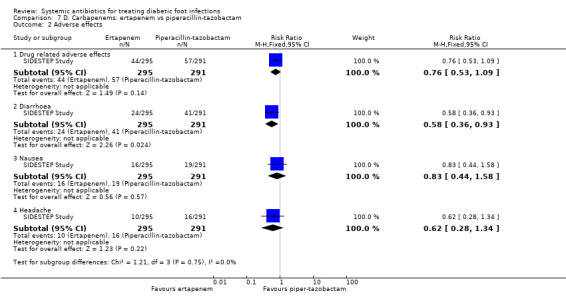
Comparison 7 D. Carbapenems: ertapenem vs piperacillin‐tazobactam, Outcome 2 Adverse effects.
Imipenem‐cilastatin versus piperacillin plus clindamycin
Bouter 1996 reported a significantly lower rate of adverse effects in participants treated with imipenem‐cilastatin than in those treated with piperacillin plus clindamycin (13.63% versus 50.00%, RR 0.27, 95% CI 0.09 to 0.84; 1 trial, 46 participants, 15 events; Analysis 8.2). Diarrhoea was the single most commonly reported side effect (4 participants in both groups; no disaggregated data reported).
8.2. Analysis.
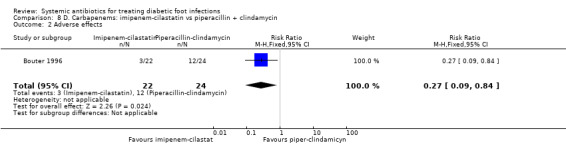
Comparison 8 D. Carbapenems: imipenem‐cilastatin vs piperacillin + clindamycin, Outcome 2 Adverse effects.
Carbapenems versus broad‐spectrum penicillins
Imipenem‐cilastatin versus ampicillin‐sulbactam
Grayson 1994 found no differences in the proportion of infection episodes associated with any type of adverse effects in the two groups (35.41% versus 33.33%, RR 1.06, 95% CI 0.61 to 1.85; 1 trial, 93 participants, 96 episodes; Analysis 9.3). There were no significant differences in the proportion of episodes with 'significant' adverse effects, defined as a severe reaction necessitating withdrawal of the study agent or specific treatment (18.75% versus 14.58%, RR 1.29, 95% CI 0.52 to 3.17; 1 trial, 96 episodes; Analysis 9.3).
9.3. Analysis.
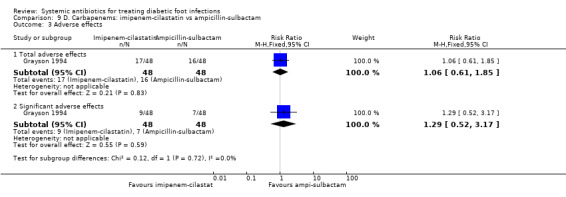
Comparison 9 D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam, Outcome 3 Adverse effects.
E. Fluoroquinolones
Fourth‐generation fluoroquinolones versus anti‐pseudomonal penicillins
Moxifloxacin or clinafloxacin versus piperacillin‐tazobactam
The included studies did not provide disaggregated data for diabetic participants for adverse events.
Fourth‐generation fluoroquinolones versus broad‐spectrum penicillins
Moxifloxacin versus amoxicillin clavulanate
The STIC Study did not provide disaggregated data for diabetic participants for adverse events.
Third‐generation fluoroquinolones versus anti‐pseudomonal penicillins
Levofloxacin versus ticarcillin‐clavulanate
Graham 2002b did not provide disaggregated data for diabetic participants for adverse events.
Second‐generation fluoroquinolone versus broad‐spectrum penicillins
Ofloxacin versus ampicillin‐sulbactam
Lipsky 1997 reported that 30.90% of participants in the ofloxacin group and 16.98% in the aminopenicillin‐sulbactam group developed an adverse effect, but this difference was not statistically significant (RR 1.82; 95% CI 0.89 to 3.72; 1 trial, 108 participants, 26 events; Analysis 13.2). No adverse effect led to discontinuation of treatment and none was rated as severe.
13.2. Analysis.
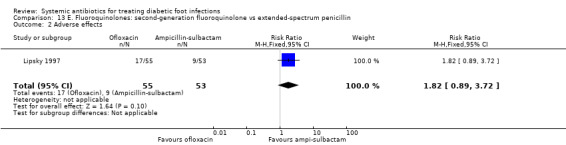
Comparison 13 E. Fluoroquinolones: second‐generation fluoroquinolone vs extended‐spectrum penicillin, Outcome 2 Adverse effects.
F. Other antibiotics
Daptomycin versus vancomycin
Arbeit 2004 reported significantly fewer adverse effects in the daptomycin group compared with the group treated either with vancomycin or a semi‐synthetic penicillin (31.4% versus 51.38%, RR 0.61, 95% CI 0.39 to 0.94; 1 RCT, 153 participants, 56 events; Analysis 14.2). This means that for every 1000 participants treated with daptomycin instead of vancomycin, 200 fewer would experience adverse effects (from 31 to 313 participants fewer; assuming the risk of the control group as a baseline risk). One participant in the daptomycin group and six participants in the control group developed a severe adverse effect.
14.2. Analysis.

Comparison 14 F. Other antibiotics: daptomycin vs control, Outcome 2 Adverse effects.
Linezolid versus aminopenicillins plus beta‐lactamase inhibitors
Lipsky 2004 reported more adverse effects with linezolid than ampicillin‐sulbactam (27% versus 10 %, RR 2.66; 95% CI 1.49 to 4.73; 1 trial, 361 participants, 76 events; Analysis 15.2). This means that for every 1000 participants treated with linezolid instead of ampicillin‐sulbactam, 166 more would experience adverse effects (from 49 to 373 participants more; assuming the risk of the control group as a baseline risk). The most frequent adverse effects were diarrhoea (18 versus 4 participants), nausea (14 versus 0), anaemia (11 versus 0), thrombocytopenia (9 versus 0), vomiting (4 versus 1), decreased appetite (3 versus 0) and dyspepsia (3 versus 1).
15.2. Analysis.
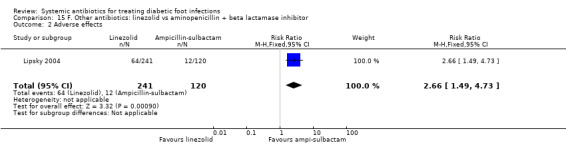
Comparison 15 F. Other antibiotics: linezolid vs aminopenicillin + beta lactamase inhibitor, Outcome 2 Adverse effects.
Clindamycin versus cephalexin
Lipsky 1990 reported a case of mild diarrhoea caused by Clostridium difficile in the clindamycin group and two cases of mild nausea and diarrhoea in the cephalexin group (RR 0.47, 95% CI 0.04 to 4.84; 1 trial, 56 participants, 3 events; Analysis 16.2), this result was not statistically significant.
16.2. Analysis.
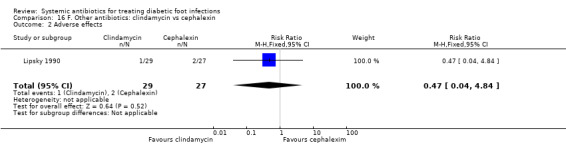
Comparison 16 F. Other antibiotics: clindamycin vs cephalexin, Outcome 2 Adverse effects.
Tigecycline versus ertapenem with or without vancomycin
Lauf 2014 reported more adverse effects with tigecycline than ertapenem with or without vancomycin (56.36% versus 82.61%, RR 1.47, 95% CI 1.34 to 1.60; 1 trial, 955 participants, 665 events; Analysis 17.2). This means that for every 1000 participants treated with tigecycline instead of ertapenem (with or without vancomycin), 265 more would experience adverse effects (from 192 to 338 participants more; assuming the risk of the control group as the baseline risk). The most frequent adverse events in the group treated with tigecycline were nausea (39.34%) and vomiting (24.43%), while those in the ertapenem group were diarrhoea (9.75%) and nausea (8.26%). Participants treated with tigecycline developed significantly higher rates of nausea (36.34% versus 8.26%, RR 4.76, 95% CI 3.46 to 6.56; 1 trial, 955 participants, 229 events; Analysis 17.2) and vomiting (24.43% versus 4.66%, RR 5.24, 95% CI 3.39 to 8.12; 1 trial, 955 participants, 140 events; Analysis 17.2) than those in the ertapenem arm. The tigecycline treated participants also developed more cases of osteomyelitis (4.26% versus 2.23%, RR 1.95, 95% CI 0.96 to 3.99; 1 trial, 955 participants, 33 events; Analysis 17.2), although this difference did not reach statistically significance. There were no statistically significant differences between groups for the following adverse events: fever, headache, pain, hypertension, anaemia, increase in serum glutamic oxaloacetic transaminase, increase in serum glutamic pyruvic transaminase and hypoglycaemia.
17.2. Analysis.
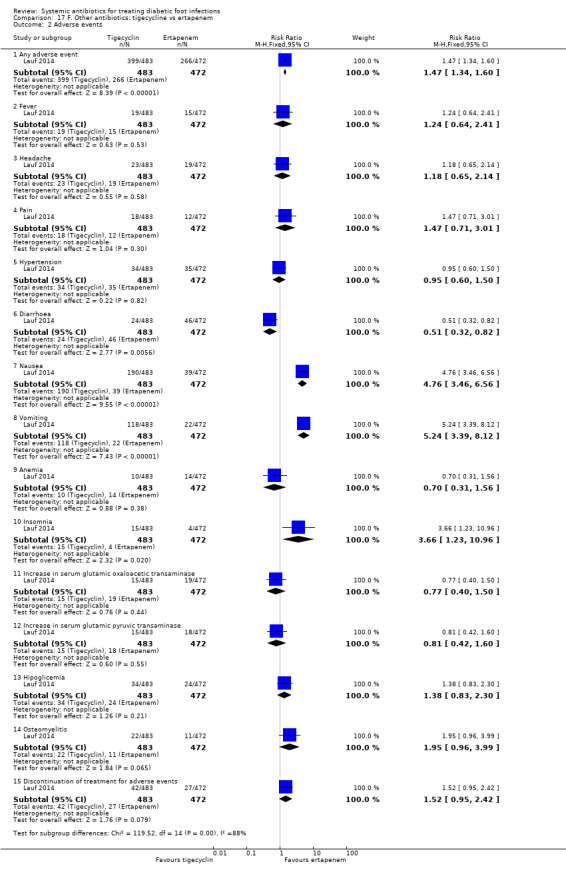
Comparison 17 F. Other antibiotics: tigecycline vs ertapenem, Outcome 2 Adverse events.
Outcome 4: Serious infections or complications of infection: lower extremity amputation
A. Anti‐pseudomonal penicillins
Anti‐pseudomonal penicillins versus anti‐pseudomonal penicillins
Piperacillin‐tazobactam versus ticarcillin‐clavulanate
Tan 1993 did not report on serious infections or complications of infection.
Anti‐pseudomonal penicillins versus broad‐spectrum penicillins
Piperacillin‐tazobactam versus ampicillin‐sulbactam
Harkless 2005 found no difference in the rate of amputations (combining amputations of toe, foot, or leg) between participants treated with piperacillin‐tazobactam versus ampicillin‐sulbactam (RR 0.97, 95% CI 0.51 to 1.84; 1 trial, 314 participants, 33 events; Analysis 2.3).
2.3. Analysis.
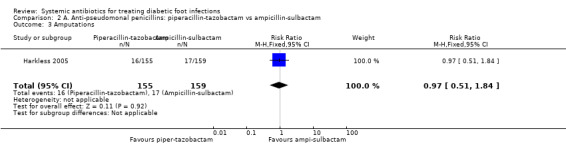
Comparison 2 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs ampicillin‐sulbactam, Outcome 3 Amputations.
Anti‐pseudomonal penicillins versus carbapenems
Piperacillin‐tazobactam versus imipenem‐cilastatin
Saltoglu 2010 found no difference in rates of lower extremity amputations between piperacillin‐tazobactam and imipenem‐cilastatin group (RR 0.87, 95% CI 0.59 to 1.28; 1 trial, 64 participants, 40 events; Analysis 3.3).
3.3. Analysis.
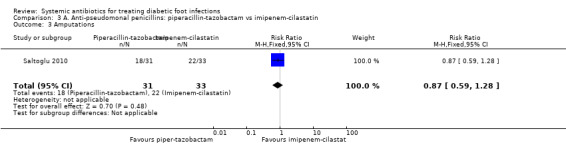
Comparison 3 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs imipenem‐cilastatin, Outcome 3 Amputations.
B. Broad‐spectrum penicillins
Ampicillin‐sulbactam versus cefoxitin
Erstad 1997 found no difference in rates of lower extremity amputations between ampicillin‐sulbactam and cefotoxitin (RR 1.00, 95% CI 0.48 to 2.08; 1 trial, 16 events; Analysis 4.3).
4.3. Analysis.

Comparison 4 B. Broad‐spectrum penicillins: ampicillin‐sulbactam vs cefoxitin, Outcome 3 Amputations.
C. Cephalosporins
Noel 2008a and Clay 2004 did not report on serious infections or complications of infection.
D. Carbapenems
Carbapenems versus antipseudomonal penicillins
Ertapenem versus piperacillin‐tazobactam
Neither the Graham 2002a study nor the SIDESTEP Study reported on serious infections or complications of infection.
Imipenem‐cilastatin versus piperacillin plus indamicin
Bouter 1996 reported that none of the participants underwent an amputation.
Cabapenems versus broad‐spectrum penicillins
Imipenem‐cilastatin versus ampicillin‐sulbactam
Grayson 1994 reported no difference in the risk of amputation from infection between imipenem‐cilastatin (28 or 58.33% amputations) compared with 33 amputations (68.75%) in the ampicillin‐sulbactam group. The difference was not statistically significant (RR 0.85, 95% CI 0.62 to 1.15; 1 trial, 96 episodes; Analysis 9.4). There was one case of major amputation in the imipenem‐cilastatin group and three cases in the ampicillin‐sulbactam group.
9.4. Analysis.
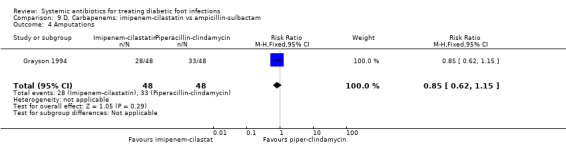
Comparison 9 D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam, Outcome 4 Amputations.
E. Fluoroquinolones
Fourth‐generation fluoroquinolones versus anti‐pseudomonal and broad‐spectrum penicillins
No trial reported on serious infections or complications of infection.
Third‐generation fluoroquinolones versus anti‐pseudomonal penicillins
No trial reported on serious infections or complications of infection.
Second‐generation fluoroquinolones versus broad‐spectrum penicillins
Ofloxacin versus ampicillin‐sulbactam
Lipsky 1997 found no clear evidence of a difference in amputation rates between ofloxacin and aminopenicillin‐sulbactam (RR 0.11, 95% CI 0.01 to 1.94; 1 trial, 108 participants, 4 events; Analysis 13.3).
13.3. Analysis.
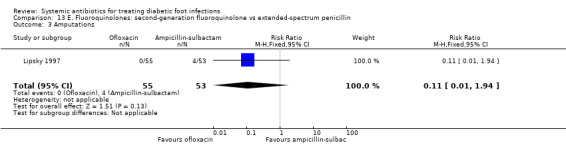
Comparison 13 E. Fluoroquinolones: second‐generation fluoroquinolone vs extended‐spectrum penicillin, Outcome 3 Amputations.
F. Other antibiotics
Daptomycin versus vancomycin
Arbeit 2004 did not report on serious infections or complications of infection.
Linezolid versus aminopenicillins plus beta‐lactamase inhibitors
Lipsky 2004 did not report on serious infections or complications of infection.
Clindamycin versus cephalexin
Lipsky 1990 reported only one case of minor amputation in the group treated with clindamycin (RR 3.21, 95% CI 0.14 to 75.68; 1 trial, 1 event).
Tigecycline versus ertapenem with or without vancomycin
Lauf 2014 did not report on serious infections or complications of infection.
Outcome 5: Serious infections or complications of infection: septicaemia
Only one study reported on this outcome. Lauf 2014 found no difference in the risk of developing septicaemia between tigecycline and ertapenem (3.93% versus 5.08%; RR 0.77, 95% CI 0.43 to 1.39; 1 trial, 955 participants, 43 events; Analysis 17.3). Similarly, in this trial’s sub study of participants with osteomyelitis at baseline there were no notable differences (2.6% versus 2.44%; RR 1.06, 95% CI 0.10 to 11.40; 1 trial, 118 participants, 3 events; Analysis 17.3).
17.3. Analysis.
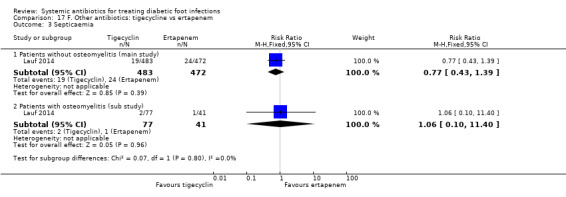
Comparison 17 F. Other antibiotics: tigecycline vs ertapenem, Outcome 3 Septicaemia.
Outcome 6: Infection‐related mortality
Four studies reported on this outcome. Harkless 2005 reported three deaths in the piperacillin‐tazobactam group, but none of them was considered to be related to study medication. Bouter 1996 reported one death due to cardiac arrest in the group treated with imipenem‐cilastatin and four deaths in the group treated with piperacillin‐clindamycin. Of these four cases, two were from heart failure and two from uncontrolled infection at the site of the leg ulcer. Lipsky 1990 reported five deaths, but all were unrelated to the foot infection. The study did not provide information regarding how many deaths occurred in each study group. Lauf 2014 reported seven deaths in the group treated with tigecycline (six in the main study and one in the sub study) and three deaths in the group treated with ertapenem (two in the main study and one in the sub study). None of these deaths was considered to be related to study drug by the investigator, most were cardiovascular in nature and all occurred after the antibiotic treatment had finished.
Outcome 7: Length of hospitalisation
Only Erstad 1997 reported on this outcome. The duration of hospitalisation was longer in the group treated with ampicillin‐sulbactam (mean of 21.10 days (minimum‐maximum: 6.00 to 58.00)) than in the group treated with cefoxitin (mean of 12.10 days (minimum‐maximum: 4.00‐39.00)), although this difference was not statistically significant at conventional levels (P value 0.06) it was very underpowered with only 36 participants.
Outcome 8: Ulcer healing
Only the Lipsky 1990 study reported this outcome; 40% of participants treated with clindamycin and 33% participants treated with cephalexin experienced wound healing (RR 1.20, 95% CI 0.59 to 2.46; 1 trial, 19 events).
Outcome 9: Recurrence of ulcer infection
Four studies reported on this outcome. Saltoglu 2010 noted that two participants (6%) treated with imipenem had a relapse at the end of two months of follow‐up, while there were no relapses in the group treated with piperacillin‐tazobactam. In Bouter 1996, three participants (13.6%) treated with imipenem‐cilastatin developed a recurrence but there were none in the group treated with piperacillin‐clindamycin. Grayson 1994 reported a recurrence rate of 31.25% in episodes treated with imipenem‐cilastatin compared with 43.75% in episodes treated with ampicillin‐sulbactam (RR 0.71, 95% CI 0.42 to 1.21; 1 RCT, 36 events; Analysis 9.5). In Lipsky 1990 there were eight cases (15.68%) of recurrence or persistence of the ulcer infection during long‐term follow‐up, but the study did not report how many were in each treatment group.
9.5. Analysis.

Comparison 9 D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam, Outcome 5 Recurrence.
Outcome 10: Health‐related quality of life
No study reported results on any measure of health‐related quality of life.
Discussion
This systematic review summarizes the available evidence on the effects of systemic antibiotic therapy for treating DFIs. The review includes 20 randomised controlled trials with a total of 3791 participants with a DFI. The studies tested various antibiotic agents, either singly or in combination, administered in a variety of regimens to evaluate their effectiveness for treating these difficult infections. The antibiotics used varied in the specific agent, the route of administration and the duration of therapy. Furthermore, the studies differed in whether, and which, co‐interventions of various types were allowed. To help organise the data, we have grouped the antibiotic agents into six large groups. We assessed their effectiveness based on the resolution of the clinical signs and symptoms of the infection, as it is the presence of these clinical findings that are used to define DFIs. It is difficult to state what overall rate of resolution of findings of infection should be expected for DFIs, as this varies considerably and depends upon the severity of infection of participants enrolled in the trial, the presence of certain complications (such as concomitant bone infection) or co‐morbidities (such as severe arterial insufficiency) and what the trialists considered constituted resolution. Our analysis of the results demonstrated that no one antibiotic treatment showed a statistically significant clinical effect over comparators, with the exception of one trial that showed better results for patients receiving ertapenem with or without vancomycin compared to tigecycline in the clinical resolution of the infection (Lauf 2014).
Foot infections in people with diabetes are common and associated with serious potential consequences, including impaired wound healing, contiguous spread to deeper tissues, necessity for lower extremity amputation, and, occasionally death. The advent of antibiotic therapy in the late 1930s with sulpha drugs (Regan 1949), and in the early 1940s with penicillin (McKittrick 1946), was associated with a marked reduction in lower extremity (especially above the knee) amputations, as well as mortality. Since then, many new antibiotic agents have been used to treat these infections. Over the past few decades microbiological studies, have revealed that aerobic Gram‐positive cocci, especially Staphylococcus aureus, are the most common pathogens in these infections (IDSA 2012). More recent studies, however, have shown that aerobic Gram‐negative bacilli are common co‐pathogens in patients who have received antibiotic therapy or who live in hot climates. Similarly, molecular microbiological techniques have shown that obligate anaerobes can frequently be isolated from polymicrobial infections. In the past decade, multidrug‐resistant bacteria, especially MRSA, but also highly resistant Gram‐negative bacilli, have become more common pathogens in DFIs. Thus, many different types of antibiotics have been investigated to see which might be most effective and safe for treating these dreaded infections.
Summary of main results
The 20 studies that met our inclusion criteria were published between 1990 and 2014. The investigations were almost exclusively conducted in North American and European countries, and the majority of enrolled patients were late middle‐aged men. Most subjects were treated as inpatients with intravenous antibiotic therapy, at least initially. Thus, any conclusions we can draw from this systematic review apply largely to patients with these characteristics.
Efficacy of antibiotic treatment
A. Anti‐pseudomonal penicillins
Although Pseudomonas aeruginosa is a relatively uncommon pathogen in uncomplicated DFI, antibiotics active against this organism are often preferred because it is isolated from selected groups of patients. These include people who have recently received antibiotic therapy (in whom P aeruginosa is selected out by its resistance to many commonly used antibiotics) or who live in hot climates in low‐income countries (where it is often the most common causative organism). Overuse of these agents is a problem, however, because P aeruginosa is also a common colonising organism, especially in patients treated with some form of hydrotherapy, which exposes them to this water‐borne agent. Newer penicillins developed to be active against P aeruginosa, as well as carbapenems, are the mainstays of parenteral anti‐pseudomonal treatment. Three trials included in the review showed no significant differences of anti‐pseudomonal penicillins in achieving a clinical resolution of the infection or avoiding amputations, when compared to piperacillin‐tazobactam, ticarcillin‐clavulanate (Tan 1993), ampicillin‐sulbactam (Harkless 2005), and, imipenem–cilastatin (Saltoglu 2010).
B. Broad‐spectrum penicillins
Broad‐spectrum penicillins have activity against the most common Gram‐positive, Gram‐negative (Enterobacteriaceae) and anaerobic pathogens that cause DFIs. Thus, they are a popular choice to use against these infections. Only one study compared a broad‐spectrum penicillin (ampicillin‐sulbactam) to another agents (cefoxitin, a second‐generation cephalosporin) and found no significant difference in clinical resolution of the infection, reduction in the rate of amputations or length of hospitalisation for DFIs (Erstad 1997).
C. Cephalosporins
Depending upon which generation is used, cephalosporins generally have a similar spectrum of activity to their beta‐lactam relatives, the broad‐spectrum penicillins. Some of the higher generation agents are active against Pseudomonas species and the newest fifth‐generation agents have also been formulated to have activity against MRSA. Again, the two included studies showed no differences between cephalosporins and other beta‐lactam antibiotics in the clinical resolution of infection (Clay 2004; Noel 2008a).
D. Carbapenems
Carbapenems generally have a very broad spectrum of activity. Group 1 agents are active against most aerobic Gram‐positive bacteria (except MRSA) and most Gram‐negative organisms (except Pseudomonas species), while group 2 agents add coverage of non‐fermentative Gram‐negatives, such as Pseudomonas species. The available studies included in this review did not demonstrate differences between carbapenems (the group 1 ertapenem or group 2 imipenem‐cilastatin) and anti‐pseudomonal penicillins (piperacillin‐tazobactam, piperacillin plus clindamycin; Bouter 1996; Graham 2002a; SIDESTEP Study), or broad‐spectrum penicillins (ampicillin‐sulbactam) in clinical resolution of DFIs (Grayson 1994).
E. Fluoroquinolones
Fluoroquinolone antibiotics, which also are classified by generation, are characterised by their broad‐spectrum of activity and good oral bioavailability. Our systematic review found that third‐ or fourth‐generation agents (levofloxacin and clinafloxacin or moxifloxacin, respectively) do not differ from anti‐pseudomonal penicillins in effectiveness in clinical resolution of DFIs (Giordano 2005; Graham 2002b; RELIEF Study; Siami 2001). There is also no evidence that fourth‐ (moxifloxacin) and second‐generation (ofloxacin) fluoroquinolones differ from broad‐spectrum penicillins on the same outcome measurements (Lipsky 1997; STIC Study). However, the study that compared ofloxacin with a broad‐spectrum penicillin (ampicillin‐sulbactam) showed a significantly higher proportion of participants treated with ampicillin‐sulbactam who required additional antibiotics because of non‐improvement (42 participants (79.40%) received gentamicin or trimetroprim‐sulfamethoxazole) than those treated with ofloxacin (5 participants (9%) received metronidazole; Lipsky 1997). This difference in the additional antibiotic requirement could have an impact in the results observed for clinical resolution in this study.
F. Other antibiotics
In a large, well‐designed study (sponsored by the manufacturers of tigecycline), ertapenem with or without vancomycin showed significantly better results than tigecycline in clinical resolution of DFIs in patients with and without osteomyelitis at baseline (Lauf 2014). However, there were no differences in the risk of developing septicaemia in the two patient groups.
Among other antibiotics with varied antimicrobial spectra, no differences in clinical resolution of infection were found in the following comparisons: daptomycin versus vancomycin (each of which is usually selected for its activity against MRSA); linezolid versus aminopenicillins combined with beta‐lactamase inhibitors; or, clindamycin versus cephalexin.
Four studies reported rates of patient mortality, but in only one was the outcome related to the DFI; Bouter 1996 reported two cases of infection‐related mortality in patients treated with piperacillin‐clindamycin.
Only one study reported on length of hospital stay and found no differences between participants treated with ampicillin‐sulbactam and those treated with cefoxitin (Erstad 1997). Similarly, only one study reported on ulcer healing and found no differences between participants treated with clindamycin and those treated with cephalexin (Lipsky 1990). In four studies that reported on recurrence of ulcer infection there were no statistically significant differences between piperacillin‐tazobactam and imipenem‐cilastatin (Saltoglu 2010), imipenem‐cilastatin and piperacillin‐clindamycin (Bouter 1996), imipenem‐cilastatin and ampicillin‐sulbactam (Grayson 1994), or clindamycin and cephalexin (Lipsky 1990). No study reported data on health‐related quality of life.
Safety of antibiotic treatment
A. Anti‐pseudomonal penicillins
In one study there was no difference in the rate of treatment‐related adverse events between an anti‐pseudomonal penicillin (piperacillin‐tazobactam) and a broad‐spectrum penicillin (ampicillin‐sulbactam; Harkless 2005). In another, more participants treated with piperacillin‐tazobactam developed adverse events (hepatotoxicity and nephrotoxicity) compared to those treated with a carbapenem (imipenem‐cilastatin; Saltoglu 2010), but this difference was based on only 12 events and was not statistically significant.
B. Broad‐spectrum penicillins
One study reported no differences between ampicillin‐sulbactam and cefoxitin (a cephalosporin) in the rate of adverse events, all of which were of minor clinical importance (Erstad 1997).
C. Cephalosporins
The one study that reported on the safety of antibiotic treatment noted no adverse events in either study group (ceftriaxone plus metronidazole versus ticarcillin‐clavulanate; Clay 2004).
D. Carbapenems
Carbapenems, either alone or combined with an anti‐pseudomonal agent, were associated with fewer cases of diarrhoea than piperacillin‐tazobactam (anti‐pseudomonal penicillin; Bouter 1996; SIDESTEP Study). However, no differences in adverse events were found when a carbapenem (imipenem‐cilastatin) was compared with a broad‐spectrum penicillin (ampicillin‐sulbactam; Grayson 1994).
E. Fluoroquinolones
No study reported data on adverse effects for third‐ or fourth‐generation fluoroquinolones. One study reported no differences in the rate of adverse events between ofloxacin (a second‐generation fluoroquinolone) and ampicillin‐sulbactam (a broad‐spectrum penicillin; Lipsky 1997).
F. Other antibiotics
Daptomycin was associated with a statistically significant 39% reduction in the rate of adverse events compared to vancomycin or a semi‐synthetic penicillin (Arbeit 2004). Linezolid was associated with a 16% increase in the rate of adverse events compared to ampicillin‐sulbactam, especially diarrhoea, nausea and anaemia (Lipsky 2004). Tigecycline was associated with a significantly higher rate of adverse events (47%), most frequently nausea and vomiting, compared to ertapenem with or without vancomycin (Lauf 2014). There were no differences in the rate of adverse events between participants treated with clindamycin versus cephalexin (Lipsky 1990).
Overall completeness and applicability of evidence
The applicability of these results is limited, as interpreting the available studies is hampered by heterogeneity in their design, the use of many different antibiotic regimens and the failure to report many key outcomes. Thus, we are not able to determine whether any one antibiotic agent or regimen for treating DFI is better or safer than any other, with the exception of tigecycline being significantly less effective than ertapenem (with or without vancomycin).
Quality of the evidence
We consider the overall quality of evidence on which this review is based to be low. While the quality of the studies varied, almost all had limitations in their design or execution, or both, that limited confidence in their effect estimates. Most importantly, a blinded outcome assessment should have been conducted for the main outcome of treatment, considering that both the diagnosis of infection and its resolution require a judgement by the investigator of the clinical signs and symptoms. Such a blinded assessment was only completed in nine of the 20 included trials (Arbeit 2004; Clay 2004; Graham 2002a; Grayson 1994; Lauf 2014; Lipsky 1990; Noel 2008a; RELIEF Study; SIDESTEP Study), while in two the assessment was by an unblinded person (Graham 2002b; STIC Study). In addition, only about one‐third of the studies accurately concealed the randomisation sequence for assigning participants to one of the study treatments (Grayson 1994; Lauf 2014; Noel 2008a; RELIEF Study; Saltoglu 2010).
Our confidence in the effect estimates obtained in the review was also diminished by the heterogeneity (inconsistency) of the body of evidence assessed. The 20 trials included had 16 different comparisons of a variety of types of antibiotics. The antibiotic regimens differed in terms of class of drugs, spectrum of antibacterial activity, dosages, routes of drug administration, and duration of treatment. Furthermore, they differed in which, if any, antibiotic co‐interventions could be administered. Finally, outcomes assessed varied and were categorised at different time points after the end of antibiotic therapy.
Most antibiotics were compared in single trials with a range of different controls. The limited sample size in the majority of included trials made the possibility of detecting clinically important differences impossible and for every comparison differences in benefits and harms cannot be ruled out.
It is remarkable that all but two of the trials received funding from pharmaceutical industry‐sponsors of at least one of the agents compared in the study. This is an important issue as a Cochrane Review found that industry sponsored drug and device studies are associated with more favourable results and positive conclusions than studies sponsored by other sources (Lundh 2012).
Potential biases in the review process
To minimize the ever‐present risk of publication bias we conducted an exhaustive search in numerous databases and tried to locate grey literature. Nevertheless, we cannot exclude the possibility that publication bias affected the papers we were able to find. The low number of studies included in the meta‐analysis precluded the realization of a funnel plot or a statistical test to explore the presence of publication bias.
Agreements and disagreements with other studies or reviews
Several other researchers and scientific organisations have investigated whether there is an optimal antibiotic treatment for DFI. A systematic review published in 2008 by members of the International Working Group on the Diabetic Foot (IWGDF) assessed the effectiveness of treatments for diabetic foot osteomyelitis and concluded that no data supported the superiority of any particular route of delivery of systemic antibiotics or informed the optimal duration of antibiotic therapy (Berendt 2008). Four years later the same organization conducted a systematic review of all interventions in the management of infection in the diabetic foot, and specifically considered antibiotic treatment (Peters 2012). The review included 11 randomised controlled trials (RCTs) with data on antibiotic therapy for skin and soft‐tissue DFIs without bone involvement and found no significant benefit for any specific antibiotic agent, route of administration or duration of treatment. Seven additional RCTs that contained data on infections with bone involvement also reported no differences between treatment regiments, except in one trial. The IWGDF review also included the study by Erstad 1997, for which the authors referred to the P value reported by the primary study, but did not report an effect estimate. In our review, we re‐calculated the effect estimate from the data provided by the primary study, and obtained a relative risk with a 95% confidence interval that included the null value 1.
A previous review of eight trials investigated antibiotics for DFI (Nelson 2006a). The authors considered that the available trials were underpowered and too dissimilar to be pooled and concluded there was no strong evidence for recommending any particular antimicrobial agent for the prevention of amputation, resolution of infection, or ulcer healing. Crouzed 2011 reported a 'critical review' of RCTs on antibiotic therapy for DFI focusing on study quality and endpoints. After reviewing 14 papers that met their inclusion criteria they concluded that “discrepancies in study design, inclusion criteria, statistical methodology, and the varying definitions of both clinical and microbiological endpoints between the published studies, make it difficult to compare them, as well as to determine which regimen may be the most appropriate for patients with diabetic foot infection.”
Our conclusions are in broad agreement with recommendations made by clinical practice guidelines on the management of diabetic foot infections. In general, these guidelines conclude that the evidence is insufficient to make recommendations on any particular antibiotic treatment (International Working Group Diabetic Foot 2011; IDSA 2012; NICE 2011). All guidelines recognize the need to start with empirical antibiotic therapy based on infection severity until the results of microbiological culture are available, which will allow a change to a more specific regimen, when needed (CDA 2013, International Working Group Diabetic Foot 2011; IDSA 2012; NICE 2011). They also suggest using antibiotics with activity against Gram‐positive organisms for mild infections (CDA 2013; International Working Group Diabetic Foot 2011; IDSA 2012; NICE 2011). The CDA 2013 suggests that for localized infections that are not limb or life‐threatening clinicians can use oral antibiotics, such as cloxacillin, cephalexin, trimetroprim‐sulfamethoxazole, clindamycin, amoxicillin‐clavulanate, linezolid or doxycycline. The IDSA 2012 guideline suggests that these antibiotics should also be used for moderate infections, while the NICE 2011 guideline, recommends also covering Gram‐negative organisms and anaerobic bacteria.
In case of severe infections, IDSA, IWGDF and NICE recommend covering Gram‐positive and Gram‐negative (including anaerobic) bacteria. IDSA suggests that it is usually unnecessary to target Pseudomonas aeruginosa except for patients with known risk factors for infection with this organism. All guidelines also suggest empirically covering MRSA only in patients with a prior history of MRSA infection (or colonisation), where there is a high local prevalence of MRSA colonisation or infection, or in cases of clinically severe infection.
The IDSA 2012, International Working Group Diabetic Foot 2011, and NICE 2011 guidelines also point that the route of therapy should depend on the severity of infection: intravenous therapy should be used for all severe and some moderate infections, switching to oral agents when the patient is systemically well and culture results available. Oral antibiotics can be used for most mild, and many moderate, infections.
With regard to the duration of antibiotic treatment, the IDSA 2012 suggests an initial antibiotic course for soft‐tissue infections of about one to two weeks for mild infections and two to three weeks for moderate to severe infections.
Authors' conclusions
Implications for practice.
While uninfected ulcers should not be treated with antibiotics, people with diabetes and infections of the foot require systemic antibiotic therapy (IDSA 2012). The initial antibiotic regimen is usually selected empirically, and it may be modified later depending on culture results and the patient’s clinical response to the selected regimen. The selection of empirical therapy should be based on several key factors, such as: the severity of the infection; information from any recent culture results; any recent previous antibiotic treatments; any history of meticillin‐ (methicillin) resistant Staphylococcus aureus (MRSA) colonisation or infection; the presence of known risk factors for Pseudomonas aeruginosa infection (high local prevalence, warm climate, chronic exudative wounds, previous antibiotic treatment); local prevalence of antibiotic‐resistant micro‐organisms; patient history of allergic reactions to antibiotic agents; and, presence of renal or hepatic impairment (IDSA 2012).
The findings of our systematic review do not show that any specific systemic antibiotic agent or regimen is associated with better results over comparators in terms of clinical resolution of infection, or other end‐points. Only one trial (at low risk of bias) identified a difference in the risk of clinical resolution of infection between two regimens. In this non‐inferiority trial the proportion of participants whose infection resolved was significantly higher with ertapenem treatment (with added vancomycin if MRSA was isolated) than with tigecycline. In addition, participants treated with tigecycline experienced higher rates of adverse events. In the absence of differences among the various agents and regimens in terms of effectiveness, clinicians should take into account the differences in the safety profile shown in some of the included studies. Unfortunately, the quality of the evidence is low, due to limitations in the design of included trials and the important differences between them in terms of the diversity of antibiotics assessed, duration of the treatments, and the time points at which the outcomes were assessed.
Implications for research.
Although we included a considerable number of trials in this review, we detected great variability in their characteristics, mainly related to the drugs and regimens compared. Any further study in this field should use standardised criteria to classify infection severity, clearly define the outcome measures, establish the duration of treatment and report both short‐ and long‐term outcomes. The outcomes assessed in these studies should prioritise clinically important issues (e.g. clinical resolution of infection, development of complications such as osteomyelitis or amputation) over other less important outcomes, for decision‐making. All these issues merit further investigation with methodologically sound trials with at least a blinded assessment of the study outcomes and a complete reporting of outcome data. Other additional relevant issues to address could include assessment of the effectiveness of the drugs in selected subgroups of patients (e.g. patients with different severities of infection, with or without bone involvement) and cost‐effectiveness of different drugs. Finally, any primary study or review of antibiotic therapy must now consider the current crisis of growing antibiotic resistance worldwide, as well as the increasing costs of health care.
History
Protocol first published: Issue 4, 2011 Review first published: Issue 9, 2015
| Date | Event | Description |
|---|---|---|
| 14 October 2008 | Amended | Converted to new review format. |
Notes
This systematic review is based on a previously published protocol (Selva 2011).
Anna Selva is a doctoral candidate in Public Health and Methodology of Biomedical Research, at the Department of pediatrics, obstetrics, gynaecology and preventive medicine at Universitat Autònoma de Barcelona, Spain.
Acknowledgements
The authors thank Marta Roqué of the Iberoamerican Cochrane Centre, Barcelona, Spain, for her advice in statistical and methodology issues, Virgina Pomar and David Rigau for their advise on antibiotic therapy. The authors would also like to thank the contribution of the peer referees (Susan O'Meara, Amanda Briant, Kristel King, Gill Worthy, Stephanie Wu, Lee Rogers, Robert Ashford and Suzanne Cunliffe) and Wounds Group Editors for their comments to improve the review.The authors would also like to acknowledge the contribution of Elizabeth Royle who copy‐edited the review and to Amanda Briant and Ruth Foxlee for developing the search strategies and running the literature searches.
Appendices
Appendix 1. MEDLINE database search strategy
Search date: 1946 to 30 March 2015
1 exp Foot Ulcer/ (6728)
2 exp Diabetic Foot/ (5646)
3 (diabet* adj3 ulcer*).tw. (2591)
4 (diabet* adj3 (foot or feet)).tw. (4907)
5 (diabet* adj3 wound*).tw. (1334)
6 or/1‐5 (9370)
7 exp Anti‐Bacterial Agents/ (519357)
8 antibiotic*.tw. (208767)
9 (nafcillin or oxacillin or ampicillin or dicloxacillin or ticarcillin* or piperacillin* or amoxicillin* or clindamycin or vancomycin or tobramycin or levofloxacin or ciprofloxacin or moxifloxacin or tigecycline or doxycycline or cefazolin or ceftazidime or cephalexin or cefepime or cefotaxime or ceftriaxone or cefazolin or cefoxitin or cefotetan or imipenem* or meropenem or ertapenem or aztreonam or metronidazole or sulfamethoxazole* or trimethopri* or cilastatin*).tw. (111293)
10 or/7‐9 (619105)
11 6 and 10 (1006)
12 randomized controlled trial.pt. (363020)
13 controlled clinical trial.pt. (87529)
14 randomi?ed.ab. (314261)
15 placebo.ab. (142471)
16 clinical trials as topic.sh. (167815)
17 randomly.ab. (187940)
18 trial.ti. (112931)
19 or/12‐18 (852829)
20 exp animals/ not humans.sh. (3882907)
21 19 not 20 (784017)
22 11 and 21 (106)
Appendix 2. EMBASE database search strategy
Search date: 1974 to 30 MArch 2015
1 exp Foot Ulcer/ (3655)
2 exp Diabetic Foot/ (9045)
3 (diabet* adj3 ulcer*).tw. (4131)
4 (diabet* adj3 (foot or feet)).tw. (7697)
5 (diabet* adj3 wound*).tw. (2167)
6 or/1‐5 (15117)
7 exp Antibiotic Agent/ (992006)
8 antibiotic*.tw. (297239)
9 (nafcillin or oxacillin or ampicillin or dicloxacillin or ticarcillin* or piperacillin* or amoxicillin* or clindamycin or vancomycin or tobramycin or levofloxacin or ciprofloxacin or moxifloxacin or tigecycline or doxycycline or cefazolin or ceftazidime or cephalexin or cefepime or cefotaxime or ceftriaxone or cefazolin or cefoxitin or cefotetan or imipenem* or meropenem or ertapenem or aztreonam or metronidazole or sulfamethoxazole* or trimethopri* or cilastatin*).tw. (159430)
10 or/7‐9 (1121204)
11 6 and 10 (2424)
12 Randomized controlled trials/ (48612)
13 Single‐Blind Method/ (19169)
14 Double‐Blind Method/ (123498)
15 Crossover Procedure/ (40214)
16 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (1363509)
17 (doubl$ adj blind$).ti,ab. (151947)
18 (singl$ adj blind$).ti,ab. (14834)
19 or/12‐18 (1431748)
20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ (21122137)
21 human/ or human cell/ (15425084)
22 and/20‐21 (15378424)
23 20 not 22 (5743713)
24 19 not 23 (1237715)
25 11 and 24 (198)
Appendix 3. CINAHL database search strategy
Search date: 1 April 2015
S1 (MH "Foot Ulcer+")
S2 TI diabet* N3 ulcer* or AB diabet* N3 ulcer*
S3 TI ( diabet* N3 foot OR diabet* N3 feet ) or AB ( diabet* N3 foot OR diabet* N3 feet )
S4 S1 or S2 or S3
S5 (MH "Antibiotics+")
S6 TI antibiotic* or nafcillin or oxacillin or ampicillin or dicloxacillin or ticarcillin* or piperacillin* or amoxicillin* or clindamycin or vancomycin or tobramycin or levofloxacin or ciprofloxacin or moxifloxacin or tigecycline or doxycycline or cefazolin or ceftazidime or cephalexin or cefepime or cefotaxime or ceftriaxone or cefazolin or cefoxitin or cefotetan or imipenem* or meropenem or ertapenem or aztreonam or metronidazole or sulfamethoxazole* or trimethopri* or cilastatin*
S7 AB antibiotic* or nafcillin or oxacillin or ampicillin or dicloxacillin or ticarcillin* or piperacillin* or amoxicillin* or clindamycin or vancomycin or tobramycin or levofloxacin or ciprofloxacin or moxifloxacin or tigecycline or doxycycline or cefazolin or ceftazidime or cephalexin or cefepime or cefotaxime or ceftriaxone or cefazolin or cefoxitin or cefotetan or imipenem* or meropenem or ertapenem or aztreonam or metronidazole or sulfamethoxazole* or trimethopri* or cilastatin*
S8 S5 or S6 or S7
S9 S4 and S8
S10 (MH "Clinical Trials+")
S11 PT Clinical trial
S12 TX clinic* n1 trial*
S13 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S14 TX randomi* control* trial*
S15 (MH "Random Assignment")
S16 TX random* allocat*
S17 TX placebo*
S18 (MH "Placebos")
S19 (MH "Quantitative Studies")
S20 TX allocat* random*
S21 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S22 S9 AND S21
Appendix 4. Search in clinical trials registries
clinicaltrials.gov
Search date: 05/08/2014
(diabetic foot OR diabetic feet OR foot ulcer OR foot infection) 103 references
controlled‐trials.com
Search date: 05/08/2014
(diabet* AND infection) 9 references
(diabet* AND foot) 24 references
(diabet* AND ulcer) 15 references
Appendix 5. ProQuest Dissertations & Theses A&I search
(1639‐present)
all((diabetic foot OR diabetic feet OR foot ulcer* OR foot infection*)) 230 references
Appendix 6. OpenSINGLE database search
Search date: 16/10/2012
(diabetic foot) 13 references
(diabetic feet) 1 reference
(foot ulcer*) 9 reference
(foot infection*) 3 reference
Appendix 7. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process available to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Yes, low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
No, high risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
No, high risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following:
Insufficient information available to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following:
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information available to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
stopped early due to some data‐dependent process (including a formal‐stopping rule); or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 8. Clinical classification of a diabetic foot infection
| Clinical manifestation of infection | IDSA Infection severity | PEDIS grade | |
| Wound without purulence or any manifestation of inflammation | Uninfected | 1 | |
| Two or more manifestations of inflammation (purulence or erythema, pain, tenderness, warmth or induration); any cellulitis or erythema extends up to 2 cm around ulcer, and infection is limited to skin or superficial subcutaneous tissues; no local complications or systemic illness | Mild | 2 | |
| Infection in a patient who is systemically well and metabolically stable but has one or more of the following: cellulitis extending for more than 2 cm; lymphangitis; spread beneath fascia; deep tissue abscess; gangrene; muscle, tendon, joint or bone involvement | Moderate | 3 | |
| Infection in a patient with systemic toxicity or metabolic instability (e.g. fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, hyperglycaemia or azotaemia) | Severe | 4 |
Footnotes
PEDIS: perfusion, extent (size, depth/tissue loss, infection and sensation).
Appendix 9. Glossary
Definitions of The Medical Dictionary of Medline Plus (MedlinePlus).
Abscess: a localized collection of pus surrounded by inflamed tissue.
Cellulitis: diffuse and especially subcutaneous inflammation of connective tissue.
Dead space: a space left in the body as the result of a surgical procedure.
Debridement: the usually surgical removal of lacerated, devitalized, or contaminated tissue.
Drainage: the act or process of drawing off fluids from a cavity or wound by means of suction or gravity.
Myositis: muscular discomfort or pain from infection or an unknown cause.
Necrotizing fasciitis: a severe soft tissue infection typically by Group A streptococci or by a mixture of aerobic and anaerobic bacteria that is marked by oedema and necrosis of subcutaneous tissues with involvement of the fascia and widespread undermining of adjacent tissue, by painful red swollen skin over affected areas, and by polymorphonuclear leucocytosis and that usually occurs as a complication of surgery, injury, or infection by extension from the initially affected site.
Osteomyelitis: an infectious usually painful inflammatory disease of bone that is often of bacterial origin and may result in death of bone tissue.
Paronychia: inflammation of the tissues adjacent to the nail of a finger or toe usually accompanied by infection and pus formation.
Septic arthritis: inflammation of joints due to infection.
Tendonitis: inflammation of a tendon.
Data and analyses
Comparison 1. A. Anti‐pseudomonas penicillins: piperacillin‐tazobactam vs ticarcillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.59, 2.29] |
Comparison 2. A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs ampicillin‐sulbactam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.86, 1.20] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any adverse event | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.99, 1.32] |
| 2.2 Treatment‐related adverse event | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.85, 2.37] |
| 2.3 Serious adverse event | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.34] |
| 3 Amputations | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.84] |
Comparison 3. A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs imipenem‐cilastatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.84, 3.26] |
| 2 Adverse effects | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [1.56, 7.86] |
| 2.1 Any adverse event | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.95, 10.72] |
| 2.2 Hepatotoxicity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.66, 43.05] |
| 2.3 Nephrotoxicity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.39 [0.81, 50.09] |
| 2.4 Hematological side effects | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.27, 106.46] |
| 2.5 Nausea | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.38] |
| 3 Amputations | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.28] |
| 4 Recurrence | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.27, 106.46] |
3.4. Analysis.
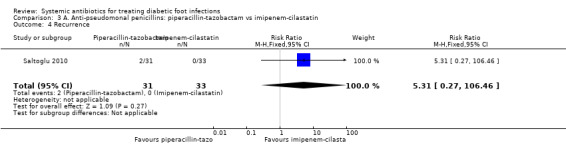
Comparison 3 A. Anti‐pseudomonal penicillins: piperacillin‐tazobactam vs imipenem‐cilastatin, Outcome 4 Recurrence.
Comparison 4. B. Broad‐spectrum penicillins: ampicillin‐sulbactam vs cefoxitin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.05] |
| 2 Adverse effects | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.49, 2.79] |
| 3 Amputations | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.48, 2.08] |
Comparison 5. C. Cephalosporins: ceftobiprole vs ceftazidime + vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.23] |
Comparison 6. C. Cephalosporins: ceftriaxone + metronidazole vs ticarcillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.72, 1.24] |
Comparison 7. D. Carbapenems: ertapenem vs piperacillin‐tazobactam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Drug related adverse effects | 1 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.09] |
| 2.2 Diarrhoea | 1 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.36, 0.93] |
| 2.3 Nausea | 1 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.44, 1.58] |
| 2.4 Headache | 1 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.28, 1.34] |
Comparison 8. D. Carbapenems: imipenem‐cilastatin vs piperacillin + clindamycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.24] |
| 2 Adverse effects | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.84] |
| 3 Recurrence | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.61 [0.42, 139.47] |
8.3. Analysis.
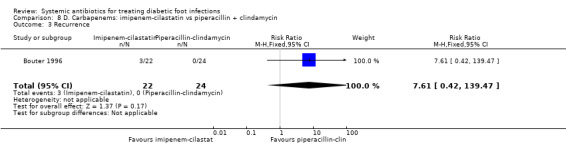
Comparison 8 D. Carbapenems: imipenem‐cilastatin vs piperacillin + clindamycin, Outcome 3 Recurrence.
Comparison 9. D. Carbapenems: imipenem‐cilastatin vs ampicillin‐sulbactam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection at the completion of therapy | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.14] |
| 2 Clinical resolution of the infection at the end of follow‐up | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 3 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Total adverse effects | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.85] |
| 3.2 Significant adverse effects | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.52, 3.17] |
| 4 Amputations | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.15] |
| 5 Recurrence | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.21] |
Comparison 10. E. Fluoroquinolones: fourth‐generation fluoroquinolones vs anti‐pseudomonal penicillins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.20] |
Comparison 11. E. Fluoroquinolones: moxifloxacin vs amoxicillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.08] |
Comparison 12. E. Fluoroquinolones: third‐generation fluoroquinolone vs extended‐spectrum penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.55] |
Comparison 13. E. Fluoroquinolones: second‐generation fluoroquinolone vs extended‐spectrum penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.47] |
| 2 Adverse effects | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.89, 3.72] |
| 3 Amputations | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.94] |
Comparison 14. F. Other antibiotics: daptomycin vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Daptomycin vs control | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Advese effects related to treatment | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.94] |
| 2.2 Severe adverse effects | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.59] |
Comparison 15. F. Other antibiotics: linezolid vs aminopenicillin + beta lactamase inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
| 2 Adverse effects | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.49, 4.73] |
Comparison 16. F. Other antibiotics: clindamycin vs cephalexin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| 2 Adverse effects | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 4.84] |
| 3 Ulcer healing | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.59, 2.46] |
16.3. Analysis.
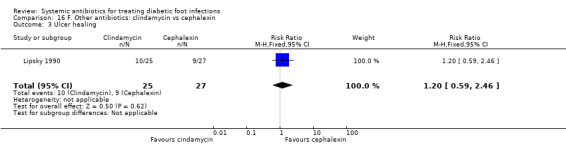
Comparison 16 F. Other antibiotics: clindamycin vs cephalexin, Outcome 3 Ulcer healing.
Comparison 17. F. Other antibiotics: tigecycline vs ertapenem.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution of the infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Patients without OM (main study) | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.18] |
| 1.2 Patients with OM (sub study) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.27, 3.39] |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any adverse event | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.34, 1.60] |
| 2.2 Fever | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.64, 2.41] |
| 2.3 Headache | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.65, 2.14] |
| 2.4 Pain | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.71, 3.01] |
| 2.5 Hypertension | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.60, 1.50] |
| 2.6 Diarrhoea | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.82] |
| 2.7 Nausea | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [3.46, 6.56] |
| 2.8 Vomiting | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [3.39, 8.12] |
| 2.9 Anemia | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.56] |
| 2.10 Insomnia | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.66 [1.23, 10.96] |
| 2.11 Increase in serum glutamic oxaloacetic transaminase | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.40, 1.50] |
| 2.12 Increase in serum glutamic pyruvic transaminase | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.60] |
| 2.13 Hipoglicemia | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.83, 2.30] |
| 2.14 Osteomyelitis | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.96, 3.99] |
| 2.15 Discontinuation of treatment for adverse events | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.95, 2.42] |
| 3 Septicaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Patients without osteomyelitis (main study) | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.39] |
| 3.2 Patients with osteomyelitis (sub study) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arbeit 2004.
| Methods |
Design: 2 identical multicentre, international, phase III randomised trials; non inferiority trial Follow‐up period: 28 days |
|
| Participants |
N: 1092 For the diabetic participants subgroup, the data came from Lipsky 2005: N:133 Sex (%male): 54% Age (mean): Intervention Group: 60 years; Control Group: 63 years Inclusion criteria: people with diabetes; between the ages of 18‐85 years; requiring hospitalisation for an infected ulcer that was known or suspected to be caused by a Gram‐positive organism (based on a Gram‐stained smear) Exclusion criteria: minor or superficial skin infections; uncomplicated cellulitis; myositis; multiple infected ulcers at distant sites; infected third‐degree burn wounds; osteomyelitis; known bacteraemic shock; hypotension, or any disorder that could interfere with the treatment evaluation; pregnancy; infection due to an organism known to be resistant to any study drug; < 40 kg in weight; hypersensitivity reaction to study drugs; haemodialysis or peritoneal dialysis; impaired renal function (creatinine clearance < 30 mL/min); immunosuppression; serum creatine phosphokinase > 50% above the upper limit of normal; statin use; systemic antibiotic treatment for more than 24 h within the previous 48 h (unless the infecting Gram‐positive organism was resistant to that therapy, or it was clinically ineffective) |
|
| Interventions |
Intervention (n = 61): daptomycin 4 mg/kg iv every 24 h over 30 minutes Control (n = 72): preselected comparator (previous to randomizations) based on the investigator’s assessment of the participant’s likelihood of infection with MRSA: either vancomycin 1 g iv every 12 h over 60 minutes or a semi‐synthetic penicillin (nafcillin, oxacillin, cloxacillin or flucloxacillin, according to the investigator’s choice) The investigator could add aztreonam to cover Gram‐negative bacteria if they were suspected or proven to be part of a polymicrobial infection, or metronidazole to cover possible or proven infection with obligate anaerobic bacteria Treatment duration: 7‐14 days |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes |
Funding source: Cubist Pharmaceuticals (makers of daptomycin); 4 authors were employees of Cubist Pharmaceuticals, and the other was an investigator for Cubist clinical trials Other: no sample size calculation. This was a subgroup analysis of another RCT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a randomised study, but no information provided about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 participants were not clinically evaluable (22.5%) with no differences between the study groups. The reasons were not explained |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were also reported in results section. |
| Other bias | High risk | This study was a subgroup analysis of another study so it was not big enough to identify differences between the comparison groups. The original study recruited 1092 participant with a power of 80% to detect non‐inferiority |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded investigator categorized the clinical outcome |
Bouter 1996.
| Methods |
Design: RCT, 1 centre (the Netherlands) Follow‐up period (mean): follow‐up until completion of antibiotic therapy (mean of 23.6‐24.3 days) |
|
| Participants |
N: 46 participants randomised Sex (%male): 43.5% Age (mean(SD)): 71.4 years (9.8) Inclusion criteria: participants hospitalised for diabetic foot lesions; Wagner stages II, III or IV; ankle/brachial index of at least 0.45; and normal renal and liver function Exclusion criteria: hypersensitivity to the study drugs; having received antimicrobial therapy effective against the infecting pathogens within the 48 h before enrolment; high probability of death within 48 h; infection with Xanthomonas maltophilia or other micro‐organisms resistant to the study drugs |
|
| Interventions |
Intervention (n = 22): imipenem/cilastatin 500 mg iv every 6 h Control (n = 24): piperacillin 3000 mg iv every 6 h plus clindamycin 600 mg iv every 8 h. In case of chronic osteomyelitis, antibiotic therapy was continued with oral quinolone (ciprofloxacin 500 mg or ofloxacin 400 mg every 12 h) and/or clindamycin 600 mg every 8 h depending on culture results Treatment duration: minimum of 10 days. Mean duration of antibiotic treatment 23.6 days for imipenem‐cilastatin group and 24.3 days for piperacillin + clindamycin group |
|
| Outcomes |
|
|
| Notes |
Funding source: not described Other: no sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All outcomes in mentioned in the methods section were also reported in results section. Primary outcome not described |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as 'open' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. Attempt to contact authors was unsuccessful |
Clay 2004.
| Methods |
Design: open‐label RCT, 1 centre (USA) Follow‐up period: not defined |
|
| Participants |
N: 70 participants randomised Sex (%male): 100% Age (mean): 63.8 years (10.8) Inclusion criteria: ≥18 years; with type 1 or 2 diabetes mellitus and clinical diagnosis of a diabetic lower extremity infection (i.e. at least 2 of the following: local heat, purulent drainage from a wound, erythema, fever and stage 1, 2 or 3 ulcer severity using the Wagner scale) Exclusion criteria: bone involvement (osteomyelitis); hypersensitivity to the study antibiotics; having received iv antibiotic for > 24 h immediately before study enrolment; uncontrolled use of oral antibiotics before hospitalisation; neutropenia (≤1000/mm³); thrombocytopenia (≤ 50.000/mm³) |
|
| Interventions |
Intervention (n = 36): metronidazole 1 g + ceftriaxone 1 g iv over 90 minutes once a day Control (n = 34): ticarcillin/clavulanate 3.1 g over 30 minutes every 6 h Treatment duration (mean, SD): Intervention Group: 6.7 days (3.3); Control Group: 6.1 days (4.3) |
|
| Outcomes |
|
|
| Notes |
Funding source: the trial received a grant from Roche Pharmaceuticals and,one of the study authors was an employee of Cubist Pharmaceutical. Other: sample size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated schedule |
| Allocation concealment (selection bias) | Unclear risk | No data provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No deviations described. |
| Selective reporting (reporting bias) | Unclear risk | Safety not clearly reported |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The final treatment outcome was based on the non‐study provider's discharge assessment of the efficacy of study treatment |
Erstad 1997.
| Methods |
Design: double blind, RCT; 1 centre (USA) Follow‐up period: 5 days |
|
| Participants |
N: 36 participants randomised Sex: not described Age (mean): Intervention Group: 60.7 years; Control Group: 57.8 years Inclusion criteria: ≥18 years; insulin or non insulin‐dependent diabetes; followed by the Vascular Surgery Service; at least Grade 1 (own classification) foot infection (cellulitis, no skin break); without antimicrobial therapy within the previous 4 days Exclusion criteria: hypersensitivity to penicillin or cephalosporins; creatinine clearance < 15 mL/minute; history of drug or alcohol abuse; concomitant infection at a site other than the foot; imminently terminal illness; neutropenia (< 1500/m³); pregnancy or breastfeeding |
|
| Interventions |
Intervention (n = 18): ampicillin/sulbactam 3 g iv every 6 h Control (n = 18): cefoxitin 2 g iv every 6 h Treatment duration: at least 5 days. The maximum duration was left to the discretion of the treating surgeon |
|
| Outcomes |
|
|
| Notes |
Funding source: Roerig Division, Pfizer Inc Other: no sample size calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described as randomised but the method used to generate the allocation sequence was not described. Attempt to contact authors unsuccessful |
| Allocation concealment (selection bias) | Unclear risk | Not described. Attempt to contact authors unsuccessful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants (8%) did not complete 5‐day treatment (1 from the intervention group and 2 from control the group). Reasons explained. Intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods were reported |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was described as double blinded but no description of the methods used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not describe who did the clinical evaluations and whether this person was blinded |
Giordano 2005.
| Methods |
Design: prospective, randomised, double‐blind, multicentre phase IIIb non‐inferiority trial Follow‐up period: 10‐42 days post therapy |
|
| Participants |
N: 617 participants, only 78 participants had diabetic foot infections For all study participants: Sex (%male): 65% Age (mean): 52.6 years Inclusion criteria: hospitalised patients ≥18 years; with cSSI of bacterial origin based on Gram‐strained smear; need for 1 week of antibiotic therapy (complicated skin and skin structure infections included: infected ischaemic ulcers, DFIs, infected decubitus ulcers, major abscesses, carbuncles, skin infections requiring significant surgical intervention in addition to antimicrobial therapy, deep soft tissue infection, infection resulting from bites); with at least 3 of the following signs and symptoms: drainage or discharge, erythema, fluctuance, heat or localised warmth, pain or tenderness, swelling or induration, fever or leucocytosis or >15% immature neutrophils. Appropriate specimens obtained prior to initiation of the study drug Exclusion criteria: necrotizing fasciitis; Fournier’s gangrene; ecthyma gangrenosum; streptococcal necrotizing fasciitis; streptococcal gangrene; clostridial necrotizing fasciitis or synergistic necrotizing fasciitis; folliculitis or furunculosis; osteomyelitis if the infected bone was not resected; secondary infections of a chronic skin disease; infections of prosthetic materials; when a surgical procedure alone was considered the definitive therapy; uncomplicated infections; infected burns. Pregnancy or nursing; immunological compromise; hypersensitivity to the study drugs; renal insufficiency; need for haemodialysis or peritoneal dialysis; severe hepatic insufficiency; electrocardiographic QTc prolongation; uncorrected hypokalaemia; seizure disorder; fluoroquinolone‐associated tendinopathy. Having received antibiotics within 3 days of study enrolment for a dosing duration of > 24 h or if concomitant systemic antibiotic therapy was needed for treatment of another infection |
|
| Interventions |
Intervention (n = 37): moxifloxacin 400 mg iv once daily for 3 days followed by oral moxifloxacin 400 mg once daily Control (n = 41) : piperacillin‐tazobactam 3.0 g/0.375 g iv every 6 h for 3 days, followed by amoxicillin‐clavulanate 800 mg every 12 h Treatment duration: 7‐14 days |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: supported by Bayer Pharmaceuticals Corporation, 4 authors were employees of Bayer Other: sample size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but the methods used were not detailed |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The efficacy‐valid populations consisted only of 40% of the randomised participants. The most common reasons for excluding subjects were: use of prohibited therapy; concomitant or post‐therapy antibiotics; insufficient treatment duration; essential data missing; and, lost to follow‐up. The proportion of participants for each reason was similar in both groups. No sample size calculation |
| Selective reporting (reporting bias) | Low risk | The trial report presented results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was double‐blinded and double‐dummy but the method used to assure the blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Graham 2002a.
| Methods |
Design: randomised double‐blind, multicentre, clinical trial of equivalence Follow‐up period: 10‐21 days after completing antibiotic therapy |
|
| Participants |
N: 540 participants randomised, 98 of whom had a diabetic foot infection For all study participants: Sex (%male): 351 (65%) Age (mean, SD): Intervention Group 48.7 years (16.5); Control Group: 48 years (17.4) Inclusion criteria: men and women ≥18 years; with cSSSIs requiring parenteral antimicrobial therapy; signs and symptoms of acute (in the absence of chronic) infection or indwelling foreign material. Surgical drainage had to have been completed ≤ 48 h after initiation of therapy Patients recently treated with antimicrobial therapy for > 24 h could be enrolled if there was clinical evidence of treatment failure after ≥ 3 days of therapy and if the pathogen was susceptible to both study drugs. Polymicrobial infections with resistant pathogens could remain in the study at investigator’s discretion if ≥ 1 of the isolated organisms was susceptible to both study drugs Exclusion criteria: pregnancy; lactation; allergy or intolerance to any beta‐lactam (except to mild rash); progressive or terminal illness; immunosuppressive therapy; AIDS; infected burn wounds; necrotizing fasciitis; osteomyelitis; septic arthritis; gangrene or need for amputation; deep‐vein thrombosis; current treatment with other systemic antimicrobials; other concurrent infection; undergoing renal dialysis; infection with a pathogen known to be resistant to either of the study medications; > 24 h of systemic antimicrobial therapy during the 72 h before study; specified abnormal laboratory test results |
|
| Interventions |
Intervention (n = 53): ertapenem 1 g iv once daily Control (n = 45): piperacillin‐tazobactam 3.4 g iv every 6 h Treatment duration: 7‐14 days |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: supported by Merck & Co, 4 study authors were employees of Merk and Co Other: sample size calculation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but the sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 32% and 34% of randomised participants in each group were clinically evaluable. Reasons for exclusions were not clearly reported. The results of analyses of 'modified intention to treat' and 'only clinically evaluable patients' groups did not show many differences |
| Selective reporting (reporting bias) | Low risk | All the outcomes described in the methods sections are reported in the results section |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The ertapenem group also received placebo infusions of saline every 6 h |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were the investigators |
Graham 2002b.
| Methods |
Design: Multicentre, international, RCT. Designed as a non‐inferiority study Follow‐up period: 3–4 weeks after completion of therapy |
|
| Participants |
N: 400 participants randomised, only 67 of whom had a diabetic foot infection For all study participants: Sex (%men): 243 (61%) Age (mean): Intervention Group: 51.9 years; Control Group: 49.8 years Inclusion criteria: men and women ≥18 years; with bacterial SSSI in the presence of a complicating factor (pre‐existing skin lesions or underlying conditions that adversely affected the immunologic or tissue‐healing response or the delivery of drug to the infected area); with at least 2 of the following signs and symptoms: pain, swelling, erythema or induration. Multiple skin sites of infection permitted unless multiple infected ulcers at distant sites (the most severely affected side was chosen for the study) Exclusion criteria: furuncles; simple abscesses or minor superficial infections not warranting systemic antimicrobial therapy; cellulitis not associated with complicating factors; multiple infected ulcers at distant sites; conditions for which surgery alone is curative or removal of the infected site (amputation); conditions that required emergent surgical intervention; perirectal abscess or hidradenitis suppurativa; myositis not associated with SSSI; osteomyelitis; burn wound infections; micro‐organism resistance; use of non‐study antimicrobial regimen or topical antimicrobial; prior systemic antimicrobial therapy > 24 h in the 48 h prior to admission; shock; hypotension or oliguria; neutropaenia; HIV infection with ≤ 200 CD4; creatinine clearance < 30 mL/minute; pregnancy or lactation; seizure disorders; unstable psychiatric disorder; underweight (≤ 40 kg); allergy; prior treatment with any investigational agent in the 30 days before |
|
| Interventions |
Intervention (n = 36): levofloxacin 750 mg iv or po every 24 h Control (n = 31): ticarcillin‐clavulanate 3.1 g iv every 4‐6 h. Switched to oral amoxicillin‐clavulanate 875 mg every 12 h at the investigator’s discretion Treatment duration: 7‐14 days |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: supported by Johnson & Johnson Research and Development, 2 authors were employees of Johnson & Johnson Other: sample size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised. No details provided. Stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised but no specific details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33% of total sample not evaluable. The reasons varied between the treatment groups: twice as many participants were not evaluable as lost to follow‐up in Control group. Twice as many participants not evaluable for insufficient course of therapy in the Intervention group. More than 3 times as many non‐evaluable participants for effective concomitant therapy in the Control group. Withdrawals with no data available: 7 participants in Intervention group and 20 in the Control |
| Selective reporting (reporting bias) | Low risk | The outcomes described in methods section were the same as those reported in results section |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators assessed outcomes |
Grayson 1994.
| Methods |
Design: double‐blind, single‐centre (USA), randomised, non‐inferiority trial Follow‐up period: 13 weeks |
|
| Participants |
N: 93 participants with 96 infection episodes randomised Sex (%male): 72% Age (mean): 60 years Inclusion criteria: adults with diabetes mellitus and limb‐threatening infection involving the lower extremity, who required hospitalisation Exclusion criteria: hypersensitivity to beta‐lactam antibiotics; requirement for other antibiotic treatment; plasmatic creatinine ≥ 3.5 mg/dL; pregnancy; severe illness with probability of death within 48 h; immune depression; participation in a clinical study of an investigational drug; recently received antibiotic therapy with a similar antimicrobial spectrum |
|
| Interventions |
Intervention (n = 47 participants, 48 infection episodes): ampicillin‐sulbactam 2 g/1 g iv every 6 h Control (n = 46 participants, 48 infection episodes): imipenem‐cilastatin 500 mg iv every 6 h Treatment duration (mean (SD)): Intervention Group: 13 (6.5) days; Control Group: 15 (8.6) days |
|
| Outcomes |
|
|
| Notes |
Financial support: supported by Pfizer Pharmaceuticals Other: sample size calculation performed and reached |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by the pharmacy to receive either intervention or control on the basis of a computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | The pharmacy assigned participants to receive one or other treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by episode unit and not by participant unit. 92 participants were randomised (the trial publication reported 47 intervention vs 46 control). 5 participants were randomised twice because they experience more than one infection during the study period. 9 protocol violations during the study (3 Intervention Group vs 6 Control Group; non‐significant difference) |
| Selective reporting (reporting bias) | Unclear risk | Main variables were not described in the methods section |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blinded but no specific details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinical assessment was made in a blinded fashion by the study physicians |
Harkless 2005.
| Methods |
Design: randomised, open‐label, multicentre study Follow‐up period: 14‐21 days |
|
| Participants |
N: 314 participants randomised Age (median (min‐max)): Intervention A: 62 years (26‐101); Intervention B: 60.1 years (26‐92) Inclusion criteria: adults with diabetes mellitus; open infected foot ulcer that met the University of Texas Grade IB, ID, IIB or IID classification of foot ulcers; at least 1 full‐or partial‐thickness infected ulcer at or below the ankle and purulent drainage or 2 of the following: erythema, local oedema, fluctuance, induration, increased local warmth or fever Exclusion criteria: pregnancy or lactation; anticipated amputation within 2 months; need for topical antibiotics at the ulcer site or other systemic antibacterials; creatine clearance < 40 ml/min; renal replacement therapy; requiring immunosuppressive drugs; hypersensitivity to penicillins; beta‐lactamase inhibitors or vancomycin; organism known or suspected to be resistant to study drugs; osteomyelitis; thrombocytopenia |
|
| Interventions |
Intervention A (n = 155): piperacillin‐tazobactam 4 g/0.5 g iv every 8 h Intervention B (n = 159): ampicillin‐sulbactam 2 g/1 g iv every 6 h Treatment duration: 4‐14 days, maximum of 21 days. Vancomycin 1 g every 12 h to participants for whom MRSA or MR‐Staphylococcus epidermidis had been identified as an organism involved in the infection |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Wyeth Pharmaceuticals; 3 authors were employee of Wyeth Pharmaceuticals Other: sample size calculation performed but not reached |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe how the allocation sequence was generated. Attempt to contact authors was unsuccessful |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this domain. Attempt to contact authors unsuccessful |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size estimated at 150 participants per group, 314 participants were randomised (155 vs 159) to the safety population. 294 participants were evaluable (139 vs 150) with some non‐significant differences that could be unbalanced between groups (e.g. resistance to study drug: 6 (4.3%) vs 19 (12.7%); 29.7% and 31.4% in each group dropped out |
| Selective reporting (reporting bias) | Low risk | The main outcome described in the methods section was described in the results |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess this domain. Attempt to contact authors was unsuccessful |
Lauf 2014.
| Methods |
Design: multicentre, international, phase III randomised non inferiority trial. The study contained a sub study Follow‐up period: 25‐27 weeks after last dose of antibiotic |
|
| Participants |
N of the main study: 955 N of the sub study: 118 Sex (main study; %male): 62.9 (tigecycline group); 67.5% (ertapenem group) Age (mean; main study): 59 years Inclusion criteria: hospitalised men and women; ≥ 18 years with diabetes mellitus; with a foot infection that did not extend above the knee. Infections of a PEDIS infection grade from 2 to 4 and a perfusion grade from 1 to 2, of acute onset or a worsening within 14 days prior; participants with osteomyelitis at baseline were not evaluable in the primary study but were included in the secondary study Exclusion criteria: participants receiving more than 48 h of a prior antibiotic treatment; necrotizing fasciitis; crepitant cellulitis; wet gangrene; gas gangrene; ecthyma gangrenosum; or implanted prosthetic material or devices that were not to be removed; infection known or suspected to be caused by a pathogen resistant to study drugs. Severely impaired arterial supply of the foot or requiring amputation within 1 month. Renal replacement therapy; plasmapheresis; hypersensitivity to study drugs; neutropenia or immunosuppressive treatment; creatinine clearance < 30 mL/min; hepatic disease; lactating women or fertile women not using contraception |
|
| Interventions |
Intervention (n = 483): tigecycline 150 mg iv every 24 h ± placebo vancomycin Control (n = 472): ertapenem 1 g iv every 24 h ± vancomycin Treatment duration: 28 days for the main study and 42 days for sub study in participants with osteomyelitis |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: sponsored by Pfizer Inc, 7 authors were employees of Pfizer Other: sample size calculation not reported; the sub study was underpowered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. participants stratified by the presence or absence of osteomyelitis and by infection severity (grade 2‐3 vs 4) |
| Allocation concealment (selection bias) | Low risk | Trans‐telephonic randomizations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not ITT analysis: 11 participants did not receive the study drug and were not considered in the analysis (MITT: Modified ITT) but there were no differences between groups Withdrawals were similar between groups and reasons reported |
| Selective reporting (reporting bias) | Low risk | Available protocol: NCT00366249. Same outcomes as those reported in the protocol |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded. Dose adjustment was done by an unblinded dispenser at the request of the investigator at the investigational site’s standard of care |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded investigator assessed the outcome |
Lipsky 1990.
| Methods |
Design: single‐centred RCT, conducted in USA Follow‐up period: 3 months |
|
| Participants |
N: 56 participants randomised (intervention: 27; control: 29) Sex (%male): 100% Age (SE): Intervention Group: 59.4 years 2.3); Control Group: 62.7 years (2.4) Inclusion criteria: people with diabetes mellitus; lower extremity infections not threatening to life; not received systemic or topical antimicrobials within the preceding 2 weeks; all type of lesions Exclusion criteria: systemic toxicity (high fever, hypotension, metabolic decompensation); infection threatening to life or limb (extensive cellulitis, lymphangitis, or necrosis; gangrene; crepitus or gas in tissues; presumed deep‐space infection or osteomyelitis); unable to perform daily wound care; non‐compliance; unwilling to visit; allergy to study drugs. |
|
| Interventions |
Intervention (n = 27): clindamycin hydrochloride 300 mg po every 6 h Control (n = 29): cephalexin 500 mg po every 6 h Treatment duration: 2 weeks Co‐interventions: wound care with debridement and toenail removal if necessary |
|
| Outcomes |
|
|
| Notes |
Funding source: Department of Veterans Affairs and a grant‐in‐aid from the Upjohn Company, Kalamazoo, Michigan, USA Other: sample size calculation not performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A printed randomizations scheme was provided from the sponsor (Upjohn), but no information was provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants randomised (29 Control Group vs 27 Intervention Group). 4 participants excluded (distribution between groups not reported) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in the results section. Primary outcome not identified |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physician investigators, who were blinded to the antibiotic regimen, determined the outcome of the infection |
Lipsky 1997.
| Methods |
Design: randomised controlled trial, multicentre (12 centres), national (USA) Follow‐up period: not described |
|
| Participants |
N: 180 participants randomised Age (mean (range)): 61.5 years (31‐90) Sex (%men): 84% Inclusion criteria: ≥18 years; diabetes mellitus; foot infection requiring antibiotic therapy; purulent drainage or erythema or swelling Exclusion criteria: osteomyelitis if all of the infected bone was not to be removed soon; presence of micro‐organisms resistant to the study drugs; underweight; seizures; major psychiatric disorder; pregnancy or lactation; renal replacement therapy; poor prognosis; antibiotic 48 h before study; antibiotic for any other reason; allergy to study drugs |
|
| Interventions |
Intervention (n = 55): ofloxacin 400 mg iv every 12 h. Changed when appropriate to ofloxacin 400 mg po every 12 h Control (n = 53): ampicillin‐sulbactam 1‐2 g/0.5‐1 g iv every 6 h (initial dose depended on severity). Switched to amoxicillin‐clavulanate 500 mg/125 mg po every 8 h when appropriate Co‐interventions: wound care; additional antibiotic could be added if participant did not respond: in Intervention Group metronidazole added (n = 5); in Control Group, gentamicin, trimethoprim‐sulfamethoxazole or another (n = 42) Treatment duration: 14‐28 days |
|
| Outcomes |
|
|
| Notes |
Funding source: Department of Veterans Affairs and the Robert Wood Johnson Pharmaceutical Research Institute Other: no sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but the sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% of participants excluded with no balanced reasons. No ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | No data on superinfection. Author contacted and no data available |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Initial dose of ampicillin‐ofloxacin chosen by investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported |
Lipsky 2004.
| Methods |
Design: RCT, multicentre (45 centres), international Follow‐up period: 15 to 21 days after treatment completed |
|
| Participants |
N: 371 participants randomised Sex (%male): 69% Age (mean (SD)): Intervention Group: 63 years (12); Control Group: 62 years (13) Inclusion criteria: men and women ≥ 18 years; with diabetes mellitus (American Diabetes Association definition) with food infection (≥1 of the following: cellulitis, paronychia, infected ulcer, deep soft‐tissue infection, septic arthritis, abscess, osteomyelitis). Could have been debrided Exclusion criteria: amputation of all infected tissue; critical ischaemia in the affected limb unless revision from vascular surgeon; wound with prosthesis infection requiring > 28 days of antibiotics; extensive gangrene; antibiotics > 72 h in the week before; need of additional antibiotic; renal failure; neutropenia; pregnancy‐lactation; allergy to study drugs. |
|
| Interventions |
Intervention (n = 241): linezolid 600 mg iv or po every 12 h Control (n = 120): ampicillin‐sulbactam 1.5‐3 g iv or po every 6 h or amoxicillin‐clavulanate 500mg‐875 mg iv or po every 8‐12 h. Addition of vancomycin (1 g iv every 12 h) if MRSA suspected Treatment duration:≥ 7 days and ≤ 28 days Co‐interventions: switch to aztreonam (1‐2 g iv every 8‐12 h) if Gram‐negative pathogens suspected (5% Intervention Group vs 2.5% Control Group) |
|
| Outcomes |
|
|
| Notes |
Funding source: Pharmacia Corporations and Department of Veterans Affairs; Pfizer Other: sample size calculation not performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised 2:1 ratio, but no details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. 16% vs 10% participants withdrew, reasons no reported |
| Selective reporting (reporting bias) | Low risk | The outcomes reported in the methods were the same reported in results |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data reported |
Noel 2008a.
| Methods |
Design: multicentre (129 sites), international, RCT,non‐inferiority trial Follow‐up period: 7‐14 days after the end of therapy |
|
| Participants |
N: 828 participants randomised, 257 with DFI For all study participants: Sex (male): Intervention Group: 63%; Control Group 64% Age (mean): Intervention Group: 52.9 years; Control Group: 51.9 years Inclusion criteria: ≥18 years with complicated SSSI. SSSI was defined as an infection involving subcutaneous tissues or requiring surgery that required iv therapy, along with one or more of the following:
Exclusion criteria: foreign body infection; osteomyelitis; critical limb ischaemia; septic arthritis |
|
| Interventions |
Intervention (n = 168): ceftobiprole 500 mg for 120 minutes iv every 8 h Control (n = 89): vancomycin 1000 mg iv every 12 h and ceftazidime 1000 mg iv every 8 h Empirical metronidazole for 48 h at discretion depending on culture results: used in 22 and 17 participants in the Intervention and Control Groups respectively Treatment duration: 7‐14 days |
|
| Outcomes |
Principal outcome
Secondary outcomes
|
|
| Notes |
Funding source: Johnson & Johnson Pharmaceutical Research and Development. All study authors were employees of the funding company Other: sample size calculation not performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done via a central interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Randomization was done via a central interactive voice response system |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% and 10% of participants of each group did not complete the trial. The most common reason for not completing the trial was loss to follow‐up, which occurred for 3% of participants in both groups. The results of the evaluable participants' analysis were not different from the ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | 2 secondary outcomes described in the study protocol (NCT00210899) were not reported in the study: clinical cure rate and microbiological relapse rate at the late follow‐up visit |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. To ensure this, participants in the ceftobiprole group also received placebo in a manner that matched the vancomycin regimen. An unblinded pharmacist used coloured sleeves to blind the appearance of infusions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Microbiological assessment was made centrally. An unblinded independent monitor checked the blinded staff at each site |
RELIEF Study.
| Methods |
Design: multicentre (61 centres), international (Belgium, Bulgaria, Germany, Greece, Hungary, Israel, Latvia, Lithuania, Poland, Romania, Russia, South Africa, Spain, Ukraine and the UK), non‐inferiority RCT Follow‐up period: 14‐28 days after last dose of study drug |
|
| Participants |
N: 813 participants randomised N participants with diabetes: 223 For all study participants Sex (% male): 64% Age (mean): Intervention Group: 53.4 years; Control Group: 52.8 years Inclusion criteria: men and women ≥ 18 years; with cSSI of 21 days’ duration and at least 3 of the following: purulent drainage or discharge; erythema of 1 cm from the wound edge; fluctuance, pain or tenderness to palpation; swelling or induration; fever; elevated WBC count > 12000 mm³ or > 15% immature neutrophils or C‐reactive protein > 20 mg/L Exclusion criteria: necrotizing fasciitis; burn wound infections; secondary infections of a chronic skin disease; infection of a prosthesis; if a surgical procedure alone was definitive, contraindications to the study drugs; poor prognosis; severe hepatic insufficiency (Child–Pugh C); or creatinine clearance < 0.40 mL/min; lactation or pregnancy; neutropenia; lymphocytopenia; an AIDS defining event; antiretroviral therapy; chronic immunosuppressant therapy; antibacterial treatment for > 24 h in the 7 days before; infection due to MRSA; MR‐S epidermidis or vancomycin‐resistant enterococci |
|
| Interventions |
Intervention (n = 123): moxifloxacin 400 mg iv every 24 h + piperacillin‐tazobactam placebo iv every 8 h switched to* moxifloxacin 400 mg po every 24 h + amoxicillin‐clavulanic placebo po every 12 h Control (n = 110): piperacillin/tazobactam 4.0 g/0.5 g iv every 8 h + moxifloxacin placebo iv every 24 h switched to* amoxicillin/clavulanate 875 mg/125 mg po every 12 h + moxifloxacin placebo po every 24 h * Switch from iv to oral therapy was decided by the investigator but the participant had to be afebrile for ≥ 24 h and to have received iv therapy for ≥ 3 days Treatment duration: 7‐21 days |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: Bayer Healthcare AG; 4 authors were employees of Bayer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code generated by the sponsor |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size calculation that was reached No ITT analysis. Excluded from ITT population: 16% moxifloxacin group and 19% piperacillin‐tazobactam group |
| Selective reporting (reporting bias) | Low risk | The study protocol reported 2 assessment time points for the clinical efficacy endpoint (during, and end of treatment) apart from the TOC visit |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
Saltoglu 2010.
| Methods |
Design: open label RCT, single centre Follow‐up period: 2 months |
|
| Participants |
N: 68 randomised Sex (%male): 47% Age (median): Intervention Group: 58.3 years; Control Group: 58.5 years Inclusion criteria: hospitalised adults (≥ 18 years), clinical diagnosis of moderate to severe diabetic lower extremity infection (Wagner scale 2‐4), caused by bacteria known or suspected to be susceptible to the antibiotics tested Exclusion criteria: antibiotic treatment in the previous 48 h; hypersensitivity to study drugs; epilepsy; psychiatric illness; pregnancy or lactation |
|
| Interventions |
Intervention (n = 31): piperacillin‐tazobactam 4.5 g iv every 8 h Control (n = 33): imipenem‐cilastatin 500 mg iv every 6 h Treatment duration: 14 days (28 days if osteomyelitis present) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: The study reported that it was not funded by any pharmaceutical company or other source Other: the trial was not registered, no sample size calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table prepared by the university statistics department |
| Allocation concealment (selection bias) | Low risk | Independent randomisation. Phone call to randomise |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were only 2 participants (one in each group) excluded as a result of an allergic reaction. No ITT analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes defined in the methods section were the same as those reported in the results section. The study was not registered |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data provided |
Siami 2001.
| Methods |
Design: multicentre (3 centres), international RCT Follow‐up period: 21 to 35 days post‐therapy |
|
| Participants |
N: 409; 76 participants with DFI: Intervention Group: 42; Control Group: 34 For all included participants Sex (%men): 294 (71.8%) Age (median): Intervention Group: 52 years; Control Group: 54 years Inclusion criteria: adults with severe or limb‐threatening SSTIs that required hospitalisation and iv therapy; acute (< 5 days) physical findings of complicated SSTI of bacterial etiology and a diagnosis of spontaneous infection (phlegmon, cellulitis, lymphangitis), wound infections (trauma wound, surgical wound), or DFI; having material available for culture Exclusion criteria: pregnancy or breastfeeding; hepatobiliary or renal dysfunction; immunodeficiency; risk of convulsive disorders; hypersensitivity to study drugs; septic shock; infected burns or decubitus ulcers; osteomyelitis; major amputation; more than a single dose of systemic antibacterial for the current SSTI; topical antibiotic the 24 h before baseline culture collection or actually requiring it; treatment with study medication 7 days prior or other investigational drug during the 4 weeks prior to the trial; corticosteroids or other antibacterial therapy for concomitant infections |
|
| Interventions |
Intervention (n = 213): clinafloxacin 200 mg iv every 12 h changed to po every 12 h Control (n = 196): piperacillin‐tazobactam 3.4 g in 30 min iv every 6 h changed to amoxicillin‐clavulanate 500 mg po every 8 h. Could also receive vancomycin iv if methicillin‐resistant staphylococci or enterococci were suspected or isolated Duration of treatment: < 14 days unless approved by sponsor. Change to po after minimum of 3 days of iv therapy |
|
| Outcomes |
Primary efficacy outcomes
Secondary outcomes
|
|
| Notes |
Funding source: Parke‐Davis Pharmaceutical Research; 2 authors were employees of Parke‐Davis Pharmaceutical Research Other: sample size calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was defined as randomised, but no details of sequence generation were provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size accomplished. Similar percentages of participants completed the study treatment in both groups (69.5% and 74%), but more participants discontinued clinafloxacin prematurely because of adverse events (11.4 vs 6.3%, P value 0.05) and more discontinued piperacillin‐tazobactam due to treatment failure (8.7 vs 5.6, P value 0.25). 32.4% and 31.1% of participants of each group were not clinically evaluable, and the reasons were explained. No ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | The outcomes defined in the methods section were all reported in the results section |
| Other bias | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure blinding, participants in the clinafloxacin group also received placebo infusions every 12 h. Defined as investigator‐blind study. To maintain investigator blinding during outpatient therapy, a third‐party member, not involved in assessing participant medical status, dispensed study medication and retrieved participant diaries |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
SIDESTEP Study.
| Methods |
Design: randomised, double‐blinded, non‐inferiority trial, multicentred Follow‐up period: 10 days after the end of antibiotic therapy |
|
| Participants |
N: 586 participants randomised Sex (%male): 63.14% Age (median (min‐max)): Intervention Group: 59 years (25‐90); Control Group: 57 years (22‐94) Inclusion criteria: adults with diabetes mellitus (type 1 or 2) with foot infection that did not extend above the knee Exclusion criteria: mild infections that did not require parenteral antibiotic therapy; infection caused by resistant pathogens; thermal burn; necrotizing fasciitis; osteomyelitis unless all the infected bone was removed within 48 h after initiating study therapy; infection complicated by prosthetic material; gangrenous tissue not removed; pregnancy; nursing; fertile women not using contraception; reaction to beta‐lactam antibiotic; need for additional antibacterial agents; secondary diabetes mellitus or impaired glucose tolerance; insufficient arterial perfusion requiring revascularization; rapidly progressive or terminal illness; dialysis; immunosuppression; corticosteroid therapy; abnormal liver function; haematocrit < 25%; haemoglobin < 8; platelet count < 75000; coagulation test altered; more than 24 h of systemic antibiotic therapy within the 72 h before study screening |
|
| Interventions |
Intervention (n = 295): ertapenem 1 g iv once daily Control (n = 291): piperacillin/tazobactam 3.4 g iv every 6 h Treatment duration: minimum of 5 days, then oral amoxicillin‐clavulanate (875 mg/125 mg every 12 h) could be given until day 28 |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: study funded by Merck and Co; the sponsor participated in study design, data collection, data analysis, data interpretation, and writing of the report; 2 study authors were employees of the sponsor Other: participants with mild infections and with possible osteomyelitis were excluded Sample size calculation performed and reached Most of the participants received some oral antibiotic therapy after parenteral study medication Authors commented that the trial did not assess long‐term outcomes and this could have had an impact on the durability of infection resolution |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of computer‐generated allocation numbers provided by the manufacturer |
| Allocation concealment (selection bias) | High risk | An unblinded pharmacist randomised participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data, and their reasons, well reported and presented in a CONSORT flowchart. No differences between groups |
| Selective reporting (reporting bias) | High risk | Trial report did not report a secondary variable described in the protocol |
| Other bias | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded. The intervention group was given a saline placebo every 6 h (double‐dummy). Quote: "The pharmacist was responsible for randomising participants (1:1 ratio), and prepared intravenous therapy to be administered by clinical personnel (unaware of treatment allocation)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinical personnel, who were unaware of treatment allocation, assessed the study's outcomes |
STIC Study.
| Methods |
Design: Multicentre, multinational, non‐inferiority RCT (74 centres, 12 countries) Follow‐up period: 14‐28 days |
|
| Participants |
N: 804 participants randomised; 134 participants with DFI For all included participants: Sex (%male): 60.6% Age (mean): Intervention Group: 52.1 years; Control Group: 51.0 years Inclusion criteria: ≥ 18 years with a cSSSI or SSSI (DFI, necrotizing fasciitis, post‐surgical wound infection, complicated cellulitis, complicated erysipelas, major abscess of the skin, infection of traumatic lesion, and infected ischaemic ulcer) at one site only; requiring systemic antimicrobial therapy; sample culture taken within 24 h prior to inclusion Exclusion criteria: uncomplicated mild‐to‐moderate SSSIs; secondary infected burns; atopic dermatitis; eczema; pregnancy; nursing; life expectancy < 2 months; end‐stage liver cirrhosis; dialysis; septic shock; chronic immunosuppressant treatment; neutropenia ≤ 1000 cells/ml; AIDS with CD4 < 200 cells/μl; HIV with highly activated antiretroviral treatment; syndromes of QTc prolongation or medication that increases the QTc; hypersensitivity to study drugs; tendinopathy with quinolones; SSSI secondary to prosthetic materials; > 18% of the skin and soft tissue affected; osteomyelitis not related to DFI; requirement for systemic concomitant antibacterial agents; failure to respond to previous antibacterial treatment if it contained a fluoroquinolone, amoxicillin or a beta‐lactam/beta‐lactamase inhibitor combination; systemic antibacterial treatment for > 24 h within the 24 h before enrolment |
|
| Interventions |
Intervention (n = 63)::moxifloxacin 400 mg once daily iv followed by moxifloxacin 400 mg once daily po. Control (n = 71): amoxicillin‐clavulanate 1000 mg/200 mg iv every 8 h followed by amoxicillin‐clavulanate 500 mg/125 mg po every 8 h Switch from iv to po therapy was decided by the investigator based on clinical response Treatment duration: iv for at least 3 days; po for 7‐21 days Co‐interventions: surgery at investigator's discretion |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes |
Financial source: Bayer; 4 authors were employees of Bayer Other: sample size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was defined as randomised. No additional data described |
| Allocation concealment (selection bias) | Unclear risk | The study was defined as randomised. No additional data described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No sample size calculation described. Reasons for withdrawal were described and there were no differences between groups (P value > 0.1) Failures were carried forward for the efficacy analysis; ITT and per protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | Only data on TOC assessment (14‐28 days) reported. Other assessments were made but not reported: prior to therapy, during treatment (days 1‐3), and day of switch to po |
| Other bias | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Evaluations were performed by investigators (unblinded) |
Tan 1993.
| Methods |
Design: multicentred RCT Follow‐up period: 10‐14 days after the completion of therapy |
|
| Participants |
N: 251participants randomised; 63 participants with DFIs For all included participants (not only participants with DFIs): Sex (%male): 75% (piperacillin‐tazobactam group); 70% (ticarcillin‐clavulante group)‐ Age (mean): 52‐53 years Inclusion criteria: ≥ 16 years; cSSSI or SSSI (cellulitis with drainage or fluid collection, cutaneous abscess, wound infection, ischaemic or DFI and acute infections of decubitus ulcers); purulent drainage or collection, and at least 2 of the following: fever, > 10,000/mm³ leukocyte count with > 5% immature neutrophils, erythema, swelling, tenderness, pain, or fluctuance Exclusion criteria: hypersensitivity to beta‐lactam antibiotics or beta‐lactamase inhibitors; renal dysfunction; active liver disease; leukopenia or thrombocytopenia; > 2 doses of another antibacterial agent in the last 72 h; another investigational drug in the last month; leukaemia; AIDS; dialysis; plasmapheresis; haemoperfusion; osteomyelitis; requirement for amputation; pressure ulcer infections with duration > 2 weeks; concomitant infections |
|
| Interventions |
Intervention (n = 32): piperacillin‐tazobactam 3 g/375 mg iv every 6 h Control (n = 31): ticarcillin‐clavulanate 3 g/100 mg iv every 6 h Treatment duration: minimum of 5 days and for at least 48 h after the resolution of signs and symptoms |
|
| Outcomes |
|
|
| Notes |
Funding source: supported by Lederle/American Cyanamid Other: sample size calculation performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule 3:2 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size calculation not reached. 55.5% of randomised participants were considered not evaluable. The reasons were comparable between the 2 treatment groups. ITT analysis only for the general population but not for subgroups |
| Selective reporting (reporting bias) | Unclear risk | The outcomes reported in the methods section were reported in the results section too, but results at early follow‐up were not reported |
| Other bias | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded but no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
Abbreviations
CD4: cluster of differentiation 4 (glycoprotein) cSSSI: complicated skin and skin structure infection DFI: diabetic foot infection h: hour(s) ITT: intention‐to‐treat (analysis) iv: intravascular max: maximum min: minimum MR: meticillin‐resistant MRSA: meticillin‐resistant Staphylococcus aureus po: per oral (by mouth) QTc: corrected QT interval (measurement of heartbeat) SSSI: skin and skin structure infection SSTI: skin and soft tissue infection TOC: test‐of‐cure WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acevedo 1990 | Not an RCT |
| Akova 1996 | Not an RCT |
| Al‐Ebous 2005 | Data for participants with DFI unavailable |
| Beam 1989 | Not an RCT |
| Bradsher 1984 | Data for participants with DFI unavailable for the main outcome (clinical evaluation). |
| Cenizal 2007 | People with DFIs excluded |
| Chantelau 1996 | The study did not evaluate any of the systematic review's main outcomes |
| Chen 2013 | Review assessed for locating RCT |
| Crouzet 2011 | Review assessed for locating RCT |
| Daniel 1999 | Data for participants with DFI unavailable |
| Darwish 1993 | The study did not evaluate systemic antibiotics |
| Deresinski 2008 | The study described 2 trials, one included in the review, and the other did not fill the inclusion criteria |
| Edmonds 2004 | Review assessed for locating RCT |
| Embil 2006 | Data for participants with DFI unavailable |
| Fernández Montequín 1991 | Study with participants post‐amputation |
| File 1983 | Data for participants with DFI unavailable |
| File 1994 | Review assessed for locating RCT |
| Foster 1998 | The study was in people with foot ulcers but no infection |
| Gentry 1989a | Data for participants with DFI unavailable |
| Gentry 1989b | Data for participants with DFI unavailable |
| Hughes 1987 | Data for participants with DFI unavailable |
| Itani 2010 | Data for participants with DFI unavailable |
| Joshi 2003 | Not an RCT |
| Lipksy 2011 | Review assessed for locating RCT |
| Lipsky 1999a | Data for participants with DFI unavailable |
| Nelson 2006b | Review assessed for locating RCT |
| Noel 2008b | Data for participants with DFI unavailable |
| Peters 2012 | Review assessed for locating RCT |
| Peterson 1989 | Data for participants with DFI unavailable |
| Pérez‐Ruvalcaba 1987 | Data for participants with DFI unavailable |
| Siami 2002 | Data for participants with DFI unavailable |
| Siebert 1985 | Only 2 participants with DFI and both treated with the same antimicrobial |
| Smith 1992 | The abstract was published approximately 20 years ago; it contained no usable data and appears not to have been published in full subsequently |
| Smith 1993 | Data for participants with DFI unavailable |
| Stevens 1999 | Data for participants with DFI unavailable |
| Stevens 2000 | People with diabetic foot ulcer excluded |
| Stevens 2002 | People with diabetic foot ulcer excluded |
| Stupin 2014 | Open clinical trial without randomisation |
| Vardakas 2008 | Review assessed for locating RCT |
| Weigelt 2005 | Data for participants with DFI unavailable |
Abbreviations
DFI: diabetic foot infection RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01199783.
| Trial name or title | Application of daptomycin in MRSA infected diabetic foot in comparison to vancomycin treatment |
| Methods | Phase III RCT, parallel assignment, open‐label |
| Participants | Men and women (age 18‐80 years) with type 1 or type 2 diabetes mellitus and MRSA infected foot ulcers Wagner grades 1‐2 without primary surgical intervention |
| Interventions |
Intervention: daptomycin infusion 6 mg/kg/bodyweight once daily Control: vancomycin once daily (effective blood‐plasma concentration of 15 mg/L) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | July 2011 |
| Contact information | Diethelm Tschoepe, Prof Dr Dr Ruhr University of Bochum |
| Notes | Estimated study completion date: December 2013. No publication available |
NCT01370616.
| Trial name or title | A phase III, randomized, double‐blind, active comparator‐controlled clinical trial to study the efficacy and safety of ertapenem sodium (MK‐0826) versus piperacillin/tazobactam sodium in the treatment of diabetic foot infections in Chinese adults |
| Methods | Phase III RCT, parallel assignment, double‐blind |
| Participants | Chinese participants with type I or II diabetes mellitus with clinically ‐ or bacteriologically ‐ documented moderate‐to‐severe (non‐life‐threatening) DFI that requires treatment with iv antibiotics |
| Interventions |
Intervention: ertapenem sodium 1.0 g iv daily and piperacillin‐tazobactam‐matching placebo for 5 to 28 days Control: piperacillin‐tazobactam sodium 4.5 g iv every 6 h for 5 to 28 days Participants in both groups could be switched to oral antibiotics beginning on day 6 if clinically indicated |
| Outcomes |
Primary outcomes
|
| Starting date | September 2011 |
| Contact information | Not provided. Responsible party and study sponsor: Merck Sharp & Dohme Corp |
| Notes | Primary completion date: December 2013. No publication available |
Abbreviations
DFI: diabetic foot infection iv: intravenous MRSA: meticillin‐resistant Staphylococcus aureus TOC: test‐of‐cure
Differences between protocol and review
In the protocol, under the outcome 'Time to resolution of the infection', we considered the clinical resolution of the infection and the time needed for that. After further discussions we realized that considering these two outcomes together was misleading and did not allow us to include the majority of studies that presented data on the clinical resolution of the infection, but not the time needed for this process. For this reason, we decided to split this outcome into two: i) clinical resolution of the infection; and, ii) time to resolution of the infection.
Contributions of authors
All the authors participated in the protocol development. Anna Selva: conceived and designed the systematic review, and led all the process of the development of the systematic review (screening, data abstraction, assessing risk of bias, data analysis, synthesis and interpretation of results). She wrote the initial draft of the review and co‐ordinated the writing of its final version and provided answers to peer referee comments. She acts as the guarantor of the review. Ivan Solà: substantially contributed to the methodological content, participated in data abstraction, risk of bias assessment, quality of evidence rating, and interpreting results. He commented the different drafts of the review and provided answers to peer referee comments. Leticia Barajas: participated in the screening, data abstraction and data analysis. Provided a methodological perspective, and commented the different drafts of the review and provided answers to peer referee comments. Oscar Gianneo: participated in the screening and data abstraction process, and commented the final version of the review. Xavier Bonfill Cosp: contributed to the methodological content and reviewed the draft, and commented on the final version of the review. Benjamin A. Lipsky: substantially contributed to the clinical content, and provided relevant style advice to the review draft. He commented on the different drafts of the review and provided answers to peer referee comments.
Contributions of editorial base
Editor: Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Approved the final review prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review. Ruth Foxlee: designed the search strategy and edited the search methods section. Amanda Briant: updated and ran the literature searches.
Sources of support
Internal sources
Iberoamerican Cochrane Centre, Spain.
Universitat Autònoma de Barcelona, Spain.
External sources
Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico.
-
NIHR, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health UK
Declarations of interest
Anna Selva: none known. Leticia Andrea Barajas Nava: none known. Oscar Gianneo: none known. Xavier Bonfill Cosp: none known. Ivan Solà Arnau: none known. Benjamin A. Lipsky: consulting with Pfizer, Merck, Wyeth‐Ayerst, Cubist, Ortho‐McNeil and Novartis in an advisory capacity, I do not believe that these consultations have impaired my ability to work on this review. Also, I am a full‐time employee of the US Federal Government (Department of Veterans Affairs). I am an investigator in some trials included in this review, but had no involvement in the selection of studies, or the assessment of risk of bias, analysis and interpretation of the included trials. I provided expertise content, and contributed substantial clinical content to the review.
New
References
References to studies included in this review
Arbeit 2004 {published data only}
- Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI. The safety and efficacy of daptomycin for the treatment of complicated skin and skin‐structure infections. Clinical Infectious Diseases 2004;38:1673‐81. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi‐synthetic penicillins for complicated skin and skin‐structure infections. Journal of Antimicrobial Chemotherapy 2005;55:240‐5. [DOI] [PubMed] [Google Scholar]
Bouter 1996 {published data only}
- Bouter KP, Visseren FL, Loenhout RM, Bartelink AK, Erkelens DW, Diepersloot RJ. Treatment of diabetic foot infection: an open randomised comparison of imipenem/cilastatin and piperacillin/clindamycin combination therapy. International Journal of Antimicrobial Agents 1996;7:143‐7. [DOI] [PubMed] [Google Scholar]
Clay 2004 {published data only}
- Clay PG, Graham MR, Lindsey CC, Lamp KC, Freeman C, Glaros A. Clinical efficacy, tolerability, and cost savings associated with the use of open‐label metronidazole plus ceftriaxone once daily compared with ticarcillin/clavulanate every 6 hours as empiric treatment for diabetic lower‐extremity Infections in older males. The American Journal of Geriatric Pharmacotherapy 2004;2(3):181‐9. [DOI] [PubMed] [Google Scholar]
Erstad 1997 {published data only}
- Erstad BL, McIntyre KE. Prospective, randomized comparison of ampicillin/sulbactam and cefoxitin for diabetic foot infections. Vascular Surgery 1997;31(4):419‐26. [Google Scholar]
Giordano 2005 {published data only}
- Giordano P, Song J, Pertel P, Herrington J, Kowalsky S. Sequential intravenous/oral moxifloxacin versus intravenous pipreacillin‐tazobactam followed by oral amoxicillin‐clavulanate for the treatment of complicated skin and skin structure infection. International Journal of Antimicrobial Agents 2005;26:357‐65. [DOI] [PubMed] [Google Scholar]
Graham 2002a {published data only}
- Graham DR, Lucasti C, Malafaia O, Nichols RL, Holtom P, Perez NQ, et al. Ertapenem once daily versus piperacillin‐tazobactam 4 times per day for treatment of complicated skin and skin‐structure infections in adults: results of a prospective, randomized, double‐blind multicenter study. Clinical Infectious Disease 2002;34:1460‐8. [DOI] [PubMed] [Google Scholar]
Graham 2002b {published data only}
- Graham DR, Talan DA, Nichols RL, Lucasti C, Corrado M, Morgan N, et al. Once‐daily, high‐dose levofloxacin versus ticarcillin‐clavulanate alone or followed by amoxicillin‐clavulanate for complicated skin and skin‐structure infections: a randomized, open‐label trial. Clinical Infectious Diseases 2002;35:381‐9. [DOI] [PubMed] [Google Scholar]
Grayson 1994 {published data only}
- Grayson ML, Gibbons GW, Habershaw GM, Freeman DV, Pomposelli FB, Rosenblum BI, et al. Use of ampicillin/sulbactam versus imipenem/cilastatin in the treatment of limb‐threatening foot infections in diabetic patients. Clinical Infectious Disease 1994;18:683‐93. [DOI] [PubMed] [Google Scholar]
- McKinnon PS, Paladino JA, Grayson ML, Gibbons GW, Karchmer AW. Cost‐effectiveness ofaAmpicillin/sulbactam versus imipenem/cilastatin in the treatment of limb‐threatening foot infections in diabetic patients. Clinical Infectious Disease 1997;24:57‐63. [DOI] [PubMed] [Google Scholar]
Harkless 2005 {published data only}
- Harkless L, Boghossian J, Pollak R, Caputo W, Dona A, Gray S, et al. An open‐label, randomized study comparing efficacy and safety of intravenous piperacillin/tazobactam and ampicillin/sulbactam for infected diabetic foot ulcers. Surgical Infections 2005;6:27‐40. [DOI] [PubMed] [Google Scholar]
Lauf 2014 {published data only}
- Lauf L, Ozsvár Z, Mitha I, Regöly‐Mérei J, Embil JM, Cooper A, et al. Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagnostic Microbiology and Infectious Disease 2014;78:469‐80. [DOI] [PubMed] [Google Scholar]
Lipsky 1990 {published data only}
- Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JE. Outpatient management of uncomplicated lower‐extremity infections in diabetic patients. Archives of Internal Medicine 1990;150:790‐7. [PubMed] [Google Scholar]
Lipsky 1997 {published data only}
- Lipsky BA, Baker PD, Landon GC, Fernau R. Antibiotic therapy for diabetic foot infections: comparison of two parenteral‐to‐oral regimens. Clinical Infectious Diseases 1997;24:643‐8. [DOI] [PubMed] [Google Scholar]
Lipsky 2004 {published data only}
- Lipsky BA, Itani K, Norden C and the Linezolid Diabetic Foot Infections Study Group. Treating foot infections in diabetic patients: a randomized, multicenter, open‐label trial of linezolid versus ampicillin‐sulbactam/amoxicillin‐clavulanate. Clinical Infectious Disease 2004;38:17‐34. [DOI] [PubMed] [Google Scholar]
Noel 2008a {published data only}
- Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. A randomized, double‐blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin‐structure infections. Clinical Infectious Diseases 2008;46:647‐55. [DOI] [PubMed] [Google Scholar]
RELIEF Study {published data only}
- Gynssens IC, Dryden M, Kujath P, Nathwani D, Schaper N, Hampel B, et al. A randomized trial of the efficacy and safety of sequential intravenous/oral moxifloxacin monotherapy versus intravenous piperacillin/tazobactam followed by oral amoxicillin/clavulanate for complicated skin and skin structure infections. Journal of Antimicrobial Chemotherapy 2011;66:2632‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper N, Dryden M, Kujath P, Nathwani D, Arvis P, Reimnitz P, et al. Efficacy of moxifloxacin in the treatment of diabetic foot infections: results of the RELIEF study. Diabetologia 2010;53 Suppl1:462‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saltoglu 2010 {published data only}
- Saltoglu N, Dalkiran A, Tetiker T, Bayram H, Tasova Y, Dalay C, et al. Piperacillin/tazobactam versus imipenem/cilastatin for severe diabetic foot infections: a prospective, randomized clinical trial in a university hospital. Infectious Diseases 2010;16:1252‐7. [DOI] [PubMed] [Google Scholar]
Siami 2001 {published data only}
- Siami G, Christou N, Eiseman I, Tack KJ and the Clinafloxacin Severe Skin and Soft Tissue Infections Study Group. Clinafloxacin versus piperacillin‐tazobactam in treatment of patients with severe skin and soft tissue infections. Antimicrobial Agents and Chemotherapy 2001;45(2):525‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIDESTEP Study {published data only}
- Abramson MA. The SIDESTEP study of diabetic foot infections (DFI): a multicenter,double‐blinded, randomized, controlled trial (RCT) of ertapenem (E)v s. piperacillin/tazobactam (P/T). Diabetologia. 2005; Vol. 48 Suppl 1:A81‐2.
- Lipsky BA, Armstrong D, Citron D, Halperin GJ, Sheehan P, Tice A, et al. The SIDESTEP study of diabetic foot infections (DFI): a multicenter, double‐blinded, randomized, controlled trial (RCT) of ertapenem (E) vs. piperacillin/tazobactam (P/T). Society of Hospital Medicine. 2005 Annual Meeting Abstracts:28.
- Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double‐blinded, multicentre trial. Lancet 2005;366:1695‐703. [DOI] [PubMed] [Google Scholar]
STIC Study {published data only}
- Vick‐Fragoso R, Hernández‐Oliva G, Cruz‐Alcázar J, Amábile‐Cuevas CF, Arvis P, Reimnitz P, et al. Efficacy and safety of sequential intravenous/oral moxifloxacin vs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection 2009;37(5):407‐17. [DOI] [PubMed] [Google Scholar]
Tan 1993 {published data only}
- Tan JS, Wishnow M, Talan DA, Duncanson FP, Norden CW and the Piperacillin/Tazobactam Skin And Skin Strucutre Study Group. Treatment of hospitalized patients with complicated skin and skin structure infections: double‐blind, randomized, multicenter study of piperacillin‐tazobactam versus ticarcillin‐clavulanate. Antimicrobial Agents and Chemotherapy 1993;37(8):1580‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Acevedo 1990 {published data only}
- Acevedo A, Schoop W, Shnell A, Toledo L. Antibiotic treatment for diabetic foot. Advantages of intravenous regional route as alternative for systemic route [Tratamiento antibiótico del pie diabético. Ventajas de la vía regional intravenosa como alternativa a la vía sistémica]. Revista Médica de Chile 1990;118(8):881‐8. [PubMed] [Google Scholar]
Akova 1996 {published data only}
- Akova M, Ozcebe O, Gullu I, Unal S, Gur D, Akalin S, et al. Efficacy of sulbactam‐ampicillin for the treatment of severe diabetic foot infections. Journal of Chemotherapy (Florence, Italy) 1996;8:284‐9. [DOI] [PubMed] [Google Scholar]
Al‐Ebous 2005 {published data only}
- Al‐Ebous AD, Hiasat B, Sarayrah M, Al‐Jahmi M, Al‐Zuriqat AN. Management of diabetic foot in a Jordanian hospital. La Revue de Santé de la Méditerranée Orientale 2005;11(3):490‐3. [PubMed] [Google Scholar]
Beam 1989 {published data only}
- Beam TR Jr, Gutierrez I, Powell S, Hewitt R, Hocko M, Brackett M, et al. Prospective study of the efficacy and safety or oral and intravenous ciprofloxacin in the treatment of diabetic foot infections. Reviews of Infectious Diseases 1989;11 Suppl 5:1163. [Google Scholar]
Bradsher 1984 {published data only}
- Bradsher RW, Snow RM. Ceftriaxone treatment of skin and soft tissue infections in a once daily regimen. The American Journal of Medicine 1984;19:63‐7. [PubMed] [Google Scholar]
Cenizal 2007 {published data only}
- Cenizal MJ, Skiest D, Luber S, Bedimo R, Davis P, Fox P, et al. Prospective randomized trial of empiric therapy with trimethoprim‐sulfamethoxazole or doxycycline for outpatient skin and soft tissue infections in an area of high prevalence of methicillin‐resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 2007;51(7):2628‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chantelau 1996 {published data only}
- Chantelau E, Tanudjaja T, Altenhöfer F, Ersanli Z, Lacigova S, Metzger C. Antibiotic treatment for uncomplicated neuropathic forefoot ulcers in diabetes: a controlled trial. Diabetic Medicine 1996;13:156‐9. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen F, Zheng N, Wang Y, Wen JL, Tu WF, Du YQ, et al. Sequential intravenous/oral moxifloxacin monotherapy for complicated skin and skin structure infections: A meta‐analysis of randomised controlled trials. International Journal of Clinical Practice 2013;67:934‐42. [DOI] [PubMed] [Google Scholar]
Crouzet 2011 {published data only}
- Crouzet J, Lavigne JP, Richard JL, Sotto A, Nimes University Hospital Working Group on the Diabetic Foot (GP30). Diabetic foot infection: a critical review of recent randomized clinical trials on antibiotic therapy. International Journal of Infectious Diseases 2011;15:601‐10. [DOI] [PubMed] [Google Scholar]
Daniel 1999 {published data only}
- Daniel R, and the Trovafloxacin Study Group. Comparison of the efficacy and safety of once‐daily oral trovafloxacin and 3‐times‐daily amoxicillin/clavulanic acid for the treatment of complicated skin and soft‐tissue infections. Drugs 1999;58 Suppl 2:288‐90. [Google Scholar]
Darwish 1993 {published data only}
- Darwish AA, Reddy A, Kamal M, Yaneza A, Al‐Teheawy MM. Management of diabetic foot lesions with limited use of antibiotics. Annals of Saudi Medicine 1993;13(1):101‐3. [DOI] [PubMed] [Google Scholar]
Deresinski 2008 {published data only}
- Deresinski SC. The efficacy and safety of ceftobiprole in the treatment of complicated skin and skin structure infections: evidence from 2 clinical trials. Diagnostic Microbiology and Infectious Disease 2008;61:103‐9. [DOI] [PubMed] [Google Scholar]
Edmonds 2004 {published data only}
- Edmonds M, Foster A. The use of antibiotics in the diabetic foot. American Journal of Surgery 2004;187 Suppl 5A:25‐8. [DOI] [PubMed] [Google Scholar]
Embil 2006 {published data only}
- Embil JM, Soto NE, Melnick DA. A post hoc subgroup analysis of meropenem versus imipenem/cilastatin in a multicenter, double‐blind, randomized study of complicated skin and skin‐structure infections in patients with diabetes mellitus. Clinical Therapeutics 2006;28(8):1164‐74. [DOI] [PubMed] [Google Scholar]
Fernández Montequín 1991 {published data only}
- Fernández JI, McCook J, Lima B, Velasco N, Montalvo J, Mahía M. Antibiotic treatment in patients amputated for ischemic diabetic foot [Antibioticoterapia en pacientes amputados por pie diabético isquémico]. Angiologia 1991;43(5):200‐3. [PubMed] [Google Scholar]
File 1983 {published data only}
- File TM, Tan JS. Amdinocillin plus cefoxitin versus cefoxitin alone in therapy of mixed soft tissue infections (including diabetic foot infections). The American Journal of Medicine 1983;29:100‐5. [DOI] [PubMed] [Google Scholar]
File 1994 {published data only}
- File TM, Tan JS. Efficacy and safety of piperacillin/tazobactam in skin and soft tissue infections. European Journal of Surgery 1994;Suppl 573:51‐5. [PubMed] [Google Scholar]
Foster 1998 {published data only}
- Foster A, McColgan M, Edmonds M. Should oral antibiotics be given to "clean" foot ulcers with no cellulitis?. Diabetic Medicine 1998;15 Suppl 2:10. [Google Scholar]
Gentry 1989a {published data only}
- Gentry L, Ramírez‐Ronda CH, Rodríguez‐Noriega E, Thadepalli H, Leal del Rosal P, Ramirez C. Oral ciprofloxacin vs parenteral cefotaxime in the treatment of difficult skin and skin structure infections. Archives of Internal Medicine 1989;149:2579‐83. [PubMed] [Google Scholar]
Gentry 1989b {published data only}
- Gentry LO, Rodriguez‐Gomez G, Zeluff BJ, Khoshdel A, Price M. A comparative evaluation of oral ofloxacin versus intravenous cefotaxime therapy for serious skin and skin structure infections. The American Journal of Medicine 1989;87 Suppl 6C:57‐60. [PubMed] [Google Scholar]
Hughes 1987 {published data only}
- Hughes CE, Johnson CC, Bamberger DM, Reinhardt JF, Peterson LR, Mulligan ME, et al. Treatment and long‐term follow‐up of foot infections in patients with diabetes or ischemia: a randomized, prospective, double‐blind comparison of cefoxitin and ceftizoxime. Clinical Therapeutics 1987;10 Suppl A:36‐49. [PubMed] [Google Scholar]
Itani 2010 {published data only}
- Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft‐tissue infections proven to be caused by methicillin resistant Staphylococcus aureus. American Journal of Surgery 2010;199:804‐16. [DOI] [PubMed] [Google Scholar]
Joshi 2003 {published data only}
- Joshi S, Maroli S, Moulick ND, Badhane S, Joshi S, Sthalekar N, et al. Efficacy and tolerability of a combination of ofloxacin and tinidazole in the management of infectious diabetic foot ulcer. Journal of the Indian Medical Association 2003;101:329‐32. [PubMed] [Google Scholar]
Lipksy 2011 {published data only}
- Lipsky BA, Itani KM, Weigelt JA, Joseph W, Paap CM, Reisman A, et al. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin‐resistant Staphylococcus aureus: results from three randomized controlled trials. International Journal of Infectious Diseases 2011;15:140‐6. [DOI] [PubMed] [Google Scholar]
Lipsky 1999a {published data only}
- Lipsky BA, Miller B, Schwartz R, Henry DC, Nolan T, McCabe A, et al. Sparfloxacin versus ciprofloxacin for the treatment of community‐acquired, complicated skin and skin‐structure infections. Clinical Therapeutics 1999;21(4):675‐90. [DOI] [PubMed] [Google Scholar]
Nelson 2006b {published data only}
- Nelson EA, O'Meara S, Golder S, Dalton J, Craig D, Iglesias C. Systematic review of antimicrobial treatments for diabetic foot ulcers. Diabetic Medicine 2006;23(4):348‐59. [DOI] [PubMed] [Google Scholar]
Noel 2008b {published data only}
- Noel GJ, Strauss RS, Amsler K, Heep M, Pypstra R, Solomkin JS. Results of a double‐blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by Gram‐positive bacteria. Antimicrobial Agents and Chemotherapy 2008;52(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez‐Ruvalcaba 1987 {published data only}
- Pérez‐Ruvalcaba JA, Quintero‐Pérez NP, Morales‐Reyes JJ, Huitrón‐Ramírez JA, Rodríguez‐Chagollán JJ, Rodríguez‐Noriega E. Double‐blind comparison of ciprofloxacin with cefotaxime in the treatment of skin and skin structure infections. The American Journal of Medicine 1987;82 Suppl 4A:242‐6. [PubMed] [Google Scholar]
Peters 2012 {published data only}
- Peters EJG, Lipsky BA, Berendt AR, Embil JM, Lavery LA, Senneville E, et al. A systematic review of the effectiveness of interventions in the management of infection in the diabetic foot. Diabetes/Metabolism Research and Reviews 2012;28 Suppl 1:142‐62. [DOI] [PubMed] [Google Scholar]
Peterson 1989 {published data only}
- Peterson LR, Lissack LM, Canter K, Fasching CE, Clabots C, Gerding D. Therapy of lower extremity infections with ciprofloxacin in patients with diabetes mellitus, peripheral vascular disease, or both. The American Journal of Medicine 1989;86:801‐8. [DOI] [PubMed] [Google Scholar]
Siami 2002 {published data only}
- Siami FS, LaFleur BJ, Siami GA. Cinafloxacin versus piperacillin/tazobactam in the treatment of severe skin and soft‐tissue infections in adults at a veterans affairs medical center. Clinical Therapeutics 2002;24:59‐72. [DOI] [PubMed] [Google Scholar]
Siebert 1985 {published data only}
- Siebert T, Evans P. Ticarcillin plus clavulanic acid versus moxalactam therapy of osteomyelitis, septic arthritis, and skin and soft tissue infections. The American Journal of Medicine 1985;79 Suppl 5B:141‐5. [DOI] [PubMed] [Google Scholar]
Smith 1992 {published data only}
- Smith OB, Richards CS, Taylor AT, Nesbit RR, Fisher JF, Willis EB. Prospective, controlled, randomized, evaluator‐blind, comparative study of oral ciprofloxacin with and without oral metronidazole versus intravenous ticarcillin/clavulanic acid followed by oral amoxicillin/clavulanic acid in the treatment of diabetic foot infections. ASHP Midyear Clinical Meeting 1992;27:P‐159R. [Google Scholar]
Smith 1993 {published data only}
- Smith JW, Nichols RL. Comparison of oral fleroxacin with oral amoxicillin/clavulanate for treatment of skin and soft tissue infections. The American Journal of Medicine 1993;94 Suppl 3A:150‐3. [PubMed] [Google Scholar]
Stevens 1999 {published data only}
- Stevens DL. Teicoplanin for skin and soft tissue infections: an open study and a randomized, comparative trial versus cefazolin. Journal of Infection and Chemotherapy 1999;5:40‐5. [DOI] [PubMed] [Google Scholar]
Stevens 2000 {published data only}
- Stevens DL, Smith LG, Bruss JB, McConnell‐Martin MA, Duvall SE, Todd WM, et al. Randomized comparison of linezolid (PNU‐100766) versus oxacillin‐dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrobial Agents and Chemotherapy 2000;44(12):3408‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2002 {published data only}
- Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin‐resistant Staphylococcus aureus infections. Clinical Infectious Diseases 2002;34:1481‐90. [DOI] [PubMed] [Google Scholar]
Stupin 2014 {published data only}
- Stupin VA, Prividentsev AI, Goriunov SV, Zhilina SV, Vasin VI. [Vasin new fluoroquinolones in treatment of patients with infectious lesions in diabetic foot syndrome]. Khirurgiia 2014;10:102‐8. [PUBMED: 25484159] [PubMed] [Google Scholar]
Vardakas 2008 {published data only}
- Vardakas KZ, Horianopoulou M, Falagas ME. Factors associated with treatment failure in patients with diabetic foot infections: an analysis of data from randomized controlled trials. Journal Diabetes Research and Clinical Practice 2008;80:344‐51. [DOI] [PubMed] [Google Scholar]
Weigelt 2005 {published data only}
- Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C, Linezolid CSSTI Study Group. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrobial Agents Chemotherapy 2005;49:2260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01199783 {published data only}
- NCT01199783. Application of daptomycin in MRSA infected diabetic foot in comparison to vancomycin treatment. clinicaltrials.gov/show/ NCT01199783 (accessed September 2014) 29 April 2010.
NCT01370616 {published data only}
- NCT01370616. A phase III, randomized, double‐blind, active comparator‐controlled clinical trial to study the efficacy and safety of ertapenem sodium (MK‐0826) versus piperacillin/tazobactam sodium in the treatment of diabetic foot infections in Chinese adults. clinicaltrials.gov/show/ NCT01370616 (accessed September 2014) 2011.
Additional references
Bader 2008
- Bader MS. Diabetic foot infection. American Family Physician 2008;78(1):71‐9. [PubMed] [Google Scholar]
Berendt 2008
- Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes/Metabolism Research and Reviews 2008;24 Suppl 1:145‐61. [DOI] [PubMed] [Google Scholar]
Bero 2013
- Bero LA. Why the Cochrane risk of bias tool should include funding source as a standard item [editorial]. Cochrane Database of Systematic Reviews 2013; Vol. 10.1002/14651858.ED000075. [DOI] [PMC free article] [PubMed]
Blanes 2011
- Blanes JI, Representatives of Spanish Society of Surgeons (ACS), Representatives of Spanish Society of Angiology and Vascular Surgery (SEACV), Representatives of Spanish Society of Emergency Medicine (SEMES), Spanish Internal Medicine Society (SEMI), Representatives of Spanish Society of Critical Care Medicine and Coronary Units (SEMICYUC), Representatives of Spanish Society of Chemotherapy (SEQ). Consensus document on treatment of infections in diabetic foot [Documento de consenso sobre el tratamiento de las infecciones en el pie del diabético]. Revista Española de Quimioterapia 2011;24(4):233‐62. [PubMed] [Google Scholar]
Boulton 2005
- Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719‐24. [DOI] [PubMed] [Google Scholar]
Bradley 1999
- Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds. Health Technology Assessment 1999; Vol. 3, issue 17. [PubMed]
CDA 2013
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Bowering K, Embil JM. Foot Care. Canadian Journal of Diabetes 2013;37 Suppl 1:145‐9. [DOI] [PubMed] [Google Scholar]
Crouzed 2011
- Crouzet J, Lavigne JP, Richard JL, Sotto A. Diabetic foot infection: a critical review of recent randomized clinical trials on antibiotic therapy. International Journal of Infectious Diseases 2011;15:e601‐10. [DOI] [PubMed] [Google Scholar]
Edmonds 2005
- Edmondes M. Infection in the neuroischemic foot. International Journal of Low Extremity Wounds 2005;4(3):145‐53. [DOI] [PubMed] [Google Scholar]
Frykberg 2006
- Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). Journal of Foot and Ankle Surgery 2006;45 Suppl 5:1‐66. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson, SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
IDSA 2012
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, et al. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clinical Infectious Diseases 2012;54:(12):1679‐84. [DOI] [PubMed] [Google Scholar]
International Working Group Diabetic Foot 2011
- Lipsky BA, Peters EJG, Berendt AR, Senneville E, Bakker K, Embil JM, et al. Specific guidelines for the treatment of diabetic foot infections 2011. Diabetes/Metabolism Research and Reviews 2012;28 Suppl 1:234‐5. [DOI] [PubMed] [Google Scholar]
Lavery 2003
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non‐Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26(5):1435‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lipsky 2004a
- Lipsky BA. Medical treatment of diabetic foot infections. Clinical Infectious Diseases 2004;39 Suppl 2:104‐14. [DOI] [PubMed] [Google Scholar]
Lipsky 2004b
- Lipsky BA, Berebdt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clinical Infectious Diseases 2004;39(7):885‐910. [DOI] [PubMed] [Google Scholar]
Lipsky 2007
- Lipsky BA, Giordano P, Choudhri S, Song J. Treating diabetic foot infections with sequential intravenous to oral moxifloxacin compared with piperacillin‐tazobactam/amoxicillin‐clavulanate. Journal of Antimicrobial Chemotherapy 2007;60(2):370‐6. [DOI] [PubMed] [Google Scholar]
Lipsky 2014
- Lipsky BA, Hoey C, Cruciani M, Mengoli C. Topical antimicrobial agents for preventing and treating foot infections in people with diabetes (Protocol). Cochrane Database of Systematic Reviews 2014, Issue Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012;12:Art. No.: MR000033. DOI: 10.1002/14651858.MR000033.pub2. [DOI] [PubMed] [Google Scholar]
McKittrick 1946
- McKittrick LS. Recent advances in the management of gangrene and infections in patients with diabetes mellitus. Journal of Digestive Diseases 1946;13:142‐8. [PubMed] [Google Scholar]
MedlinePlus
- MedlinePlus. US National Library of Medicine. National Institutes of Health Page last updated on 29 January 2015; Vol. Bethesda, US:http://www.nlm.nih.gov/medlineplus/.
Nelson 2006a
- Nelson EA, O'Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technology Assessment 2006;10(12):iii‐iv, ix‐x, 1‐221. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. NICE clinical guideline 119. Diabetic foot problems. Inpatient management of diabetic foot problems. NHS March 2011. [PubMed]
OMS 2014
- World Health Organization. Antimicrobial resistance: global report on surveillance. Vol. ISBN 978 92 4 156474 8, France: World Health Organization, 2014. [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pecoraro 1990
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513‐21. [DOI] [PubMed] [Google Scholar]
Prompers 2007
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study [Diabetologia]. 2007 50;1:18‐25. [DOI] [PubMed] [Google Scholar]
Prompers 2008
- Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 2008;10:1826‐34. [DOI] [PubMed] [Google Scholar]
Raspovic 2014
- Raspovic KM, Wukick DK. Self‐reported quality of life and diabetic foot infections. Journal of Foot and Ankle Surgery 2014;53(6):716‐9. [DOI] [PubMed] [Google Scholar]
Regan 1949
- Regan JS, Bowen BD, Fernbach PA. Reduction in mortality and loss of limbs in diabetic gangrene and infection. Archives of Surgery 1949;59(3):594‐600. [DOI] [PubMed] [Google Scholar]
Reiber 1999
- Reiber GE, Vileikyte L, Boyko EJ, Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower‐extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Selva 2011
- Selva A, Barajas‐Nava LA, Gianneo OD, Solà I, Bonfill X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database of Systematic Reviews 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 28 January 2011).
Sterne 2013
- Sterne JAC. Why the Cochrane risk of bias tool should not include funding source as a standard item [editorial]. Cochrane Database of Systematic Reviews 2013; Vol. 10.1002/14651858.ED000076. [DOI] [PMC free article] [PubMed]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 1995
- West NJ. Systemic antimicrobial treatment of foot infections in diabetic patients. American Journal of Health‐System Pharmacy 1995;52(11):1199‐207. [DOI] [PubMed] [Google Scholar]


