Abstract
Neurological immune-related adverse events are complications of programmed-cell death 1 or programmed-cell death 1 ligand immunotherapies that can be life threatening and often lead to anticancer immunotherapy withdrawal. Scant clinical data are available that integrate the clinical presentation, therapeutic management and long-term outcome. All consecutive adult patients treated by programmed-cell death 1 or programmed-cell death 1 ligand immunotherapies, given alone or in combination with other treatment, who experienced a neurological immune-related adverse event with a severity grade ≥2 in Paris Saclay-University hospitals were investigated from June 2014 to February 2019. The frequency of neurological immune-related adverse events was calculated from the prospective Registre des Effets Indésirables Sévères des Anticorps Monoclonaux Immunomodulateurs en Cancérologie cohort. Forty patients presenting with 51 distinct neurological immune-related adverse events were included. The prevalence of grade ≥2 neurological immune-related adverse events was estimated to be 1.22% in the Registre des Effets Indésirables Sévères des Anticorps Monoclonaux Immunomodulateurs en Cancérologie cohort. Among 40 patients with neurological immune-related adverse events, 65% received programmed-cell death 1 or programmed-cell death 1 ligand monotherapy and 35% received a combination of programmed-cell death 1 plus anti-CTLA4 (Common Terminology Criteria for Adverse Events). Clinical neurological presentations were peripheral (48%), central (35%), or mixed (18%). The severity of neurological immune-related adverse events was grade 2 for 14 (35%) and ≥grade 3 for 26 patients (65%). The mortality rate related to neurological immune-related adverse events was 8%. Corticosteroid treatment led to neurological recovery in 74%. Long-term follow-up highlighted that 53% of patients experienced long-term neurological sequelae. Five patients were rechallenged by programmed-cell death 1 monotherapy without recurrence of their neurological immune-related adverse event(s). Neurological immune-related adverse events induced by programmed-cell death 1 or programmed-cell death 1 ligand are rare but are severe with a mortality rate of 8% and long-term sequelae for 53% of patients. Corticosteroids should be started when neurological immunological complications are identified to avoid long-term sequelae.
Keywords: immune-related adverse events, neurological toxicity, immune checkpoint inhibitors, paraneoplastic syndrome, rechallenge
Plaçais et al. report a large case-series of 40 patients presenting with 51 neurological adverse events of immune checkpoint inhibitors, wherein corticosteroid therapy was associated with a 74% rate of neurological recovery. Neurological adverse events were severe, as 7.5% of the included patients died and 53% kept long-term neurological sequelae.
Graphical Abstract
Graphical Abstract.
Introduction
Anti-programmed cell death 1 (PD-1) and anti-programmed cell death ligand 1 (PD-L1) antibodies are the most highly prescribed immune-checkpoint inhibitors (ICIs) to treat cancer.1 Since the first trial involving anti-PD-1 agents to treat metastatic melanoma in 2006, their indications have broadened and now include many types of solid tumours and haematological malignancies.2
Immune checkpoint inhibitors act on anti-cancer immunity by blocking the PD-1/PD-L1 axis, reversing the lymphocyte exhaustion found in the tumour microenvironment. As the anti-PD-1/PD-L1 axis is a physiological pathway involved in immunological tolerance, healthy tissue can be affected when patients are treated with ICIs.2 Anti-PD-1 and anti-PD-L1 agents are associated with various immune-related adverse events (irAEs), mainly involving the skin, endocrine glands, gastro-intestinal tract, lungs and joints.3 Theoretically, every organ in the body can be affected by such irAEs and the neurological system is not spared.4,5 The most severe—and fortunately rare—irAEs can involve the heart, haematopoietic tissue and neurological system.6
A review of 59 clinical trials estimated the frequency of neurological immune-related adverse events (n-irAEs) in patients treated with PD-1 inhibitors to be 6.1%.7 Such n-irAEs can be life-threatening,8 as they can represent up to 15% of the lethality related to the use of anti-PD-1 agents.9 The most severe n-irAEs are encephalitis, meningo-radiculopathies, acute inflammatory demyelinating polyneuropathy and myasthenia gravis.10–13 The management of patients with a n-irAE requires halting immunotherapy, even if it is of grade 1 severity, and treating them with systemic corticosteroid therapy, with the eventual addition of immunosuppressive drugs in corticosteroid-refractory patients.14
Given the rarity of n-irAEs, no comprehensive studies are available, including clinical data efficacy of steroid therapy and long-term outcome. We aimed to investigate in this study neurological complications related to anti-PD-1 or PD-L1 immunotherapy, reporting the frequency, clinical presentation, including central or peripheral involvement, efficacy of corticosteroid therapy, and long-term outcome.
Patients and methods
Study design and participants
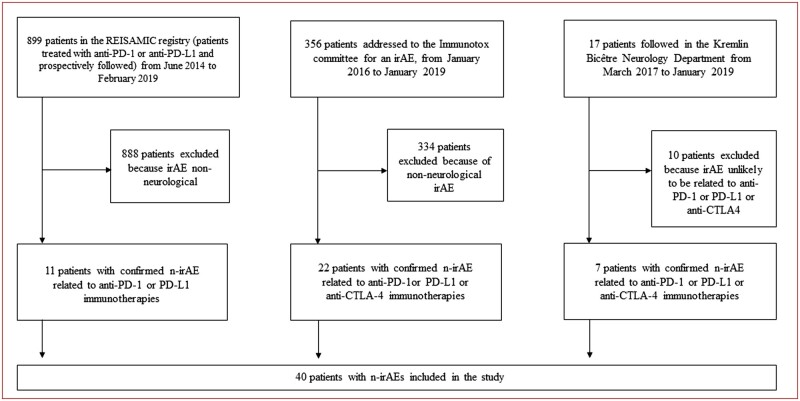
This is a retrospective, non-interventional study based on a descriptive case-series. All patients gave their oral informed consent for the study. The study was approved by the Institutional Review Board of the Institute Gustave Roussy and the REISAMIC registry was declared at the Commission Nationale de l’Informatique et des Libertés (N°2098694v0). All consecutive adult patients (18 years and older) who experienced n-irAEs related to anti-PD-1 or anti-PD-L1 immunotherapy and registered in the databases of Paris-Saclay Hospitals were included in this study. Patients could have received anti-PD-1 or anti-PD-L1 monotherapy, or a combination of anti-PD-1 and anti-CTLA-4 immunotherapies. Patient sources included the three following databases of Paris-Saclay Hospitals: prospective REISAMIC pharmacovigilance registry,15 the ImmunoTOX assessment board of the Institute Gustave Roussy, Villejuif France14 and the Paris-Saclay University Hospital Neurology Department of Kremlin-Bicêtre (Fig. 1). The study period for all patients was June 2014 to February 2019. The n-irAEs were registered in the pharmacovigilance database of Paris-Saclay Hospitals by the REISAMIC registry if they were of severity 2 or higher.15 For this study, all patients were included if they had neurological irAEs of grade 2 severity or higher. The severity of n-irAEs was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version v4.03.13 The cut-off date for analysis was 1 April 2019.
Figure 1.
Flow chart of the study. CTLA-4 = cytotoxic T-lymphocyte associated protein 4; IrAE = immune-related adverse event; n-irAE = neurological immune-related adverse event; PD-1= programmed cell death 1; PD-L1 = programmed cell death ligand 1; REISAMIC = Registre des Effets Indésirables Sévères des Anticorps Monoclonaux Immunomodulateurs en Cancérologie.
Procedures
All cases of patients with n-irAEs induced by anti-PD-1 or PD-L1 were centrally reviewed by an expert board of physicians that included neurologists and internal medicine practitioners (CC, JMM, OL, NN and LP). The causality relationship of n-irAEs with the immunotherapy was assessed according to the World Health Organization recommendations using the Uppsala Monitoring Center causality scale.16 Patients were retained for analysis in the study if their n-irAEs were of certain, likely, or possible causality. Patients with an unlikely, unclassified, or unclassifiable causality link were excluded from the analysis (Fig. 1).
Outcomes
Outcomes included the frequency of n-irAEs among patients treated with anti-PD-1 or anti-PD-L1, clinical presentation of patients with n-irAEs, treatment given for the management of the n-irAEs, and long-term outcome. Clinical characteristics, treatment and outcome were investigated at the time of the n-irAE, three months later, and at the last news available. Clinical characteristics included age, demographics, tumour characteristics, immunotherapy regimen, neurological clinical examination, severity of the n-irAE, according to the CTCAE, and the resulting disability according to the modified-Rankin scale (mRS).17
The prevalence of the n-irAEs was calculated solely from the prospective data of the REISAMIC registry to avoid selection bias.15
The responses to n-irAE treatment were evaluated three months following the occurrence of the n-irAE(s). Patients were classified as favourable responders to treatment if they achieved neurological recovery following treatment. Neurological recovery was defined as a decrease in grade of ≥1 point on the CTCAE severity scale and a decrease of mRS of ≥1 point relative to that at baseline (beginning of treatment). Neurological recovery without sequelae was defined as a decrease of both the mRS and CTCAE back to their value before the occurrence of the n-irAE(s).
Long-term outcome
Long-term outcome was assessed based on the last news available. Patients were evaluated to have sequelae or not resulting from their n-irAE(s) at this timepoint. Neurological recovery without sequelae was defined by full resolution of the n-irAE symptoms. The follow-up of patients was defined as the time elapsed from the first occurrence of n-irAE symptoms to the last clinical evaluation.
Neurological immune-related definitions
n-irAEs were defined as a neurological clinical symptom, confirmed or not by a laboratory, imaging, or other examination, and considered to be linked to the immunotherapy by the treating physician and after central review. One patient could have several different n-irAEs at the same time or any time during the follow-up. All n-irAEs were recorded and separately investigated in the study. The n-irAEs were classified into central nervous system or peripheral nervous system n-irAEs based on their neurological topography. Patients were distributed into three clinical presentation groups depending on their n-irAE(s): central nervous system, peripheral nervous system or both (mixed involvement). Differential diagnosis such as infections or tumoural infiltration were thoroughly ruled out. Patients diagnosed with pre-existing paraneoplastic neurological syndrome (PNS) were excluded from the study.
Statistical analysis
The time to n-irAE onset was defined as the time elapsed from the first infusion of anti-PD-1 or anti-PD-L1 and the beginning of the first n-irAE symptoms. Clinical characteristics, immunotherapy regimens and n-irAE characteristics were compared between patients belonging to the peripheral, central, and mixed neurological presentation groups and the treatment received (anti PD-1 or PD-L1 monotherapy or anti-PD-1 plus anti-CTLA4). Qualitative data are presented as n (%) and quantitative data as the median with interquartile range (IQR), unless otherwise stated. Data were compared using the non-parametric Kruskal–Wallis test or Mann–Whitney–Wilcoxon test. The threshold for statistical significance was set to P < 0.05. All statistical tests were performed using R Studio software v3.6.2.
Data availability statement
Detailed informations are provided in the Supplementary Table 1. Further data can be shared upon request by the authors.
Results
Selection of patients with n-irAEs
During the study period, 50 patients with suspected n-irAEs were screened in the study: 11 from the REISAMIC registry, 22 from the ImmunoTOX assessment board and 17 from the Kremlin-Bicêtre Paris-Saclay Hospital Neurology Department (Fig. 1). After a centralized review of the causality relationship of all cases, 10 patients were excluded from the analysis, as they had neurological symptoms not related to immunotherapy.
Prevalence and distribution of n-irAEs
Between 27 June 2014 and 1 February 2019, 899 patients treated with anti-PD-1 or anti-PD-L1 were prospectively included in the REISAMIC registry. Among them, 11 had confirmed n-irAEs, leading to a prevalence of 1.22% for n-irAEs ≥ grade 2 in patients receiving anti-PD-1 or anti-PD-L1.
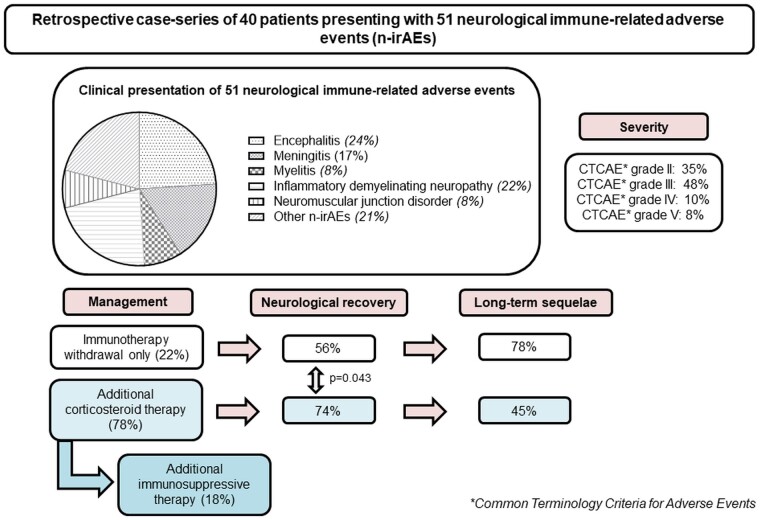
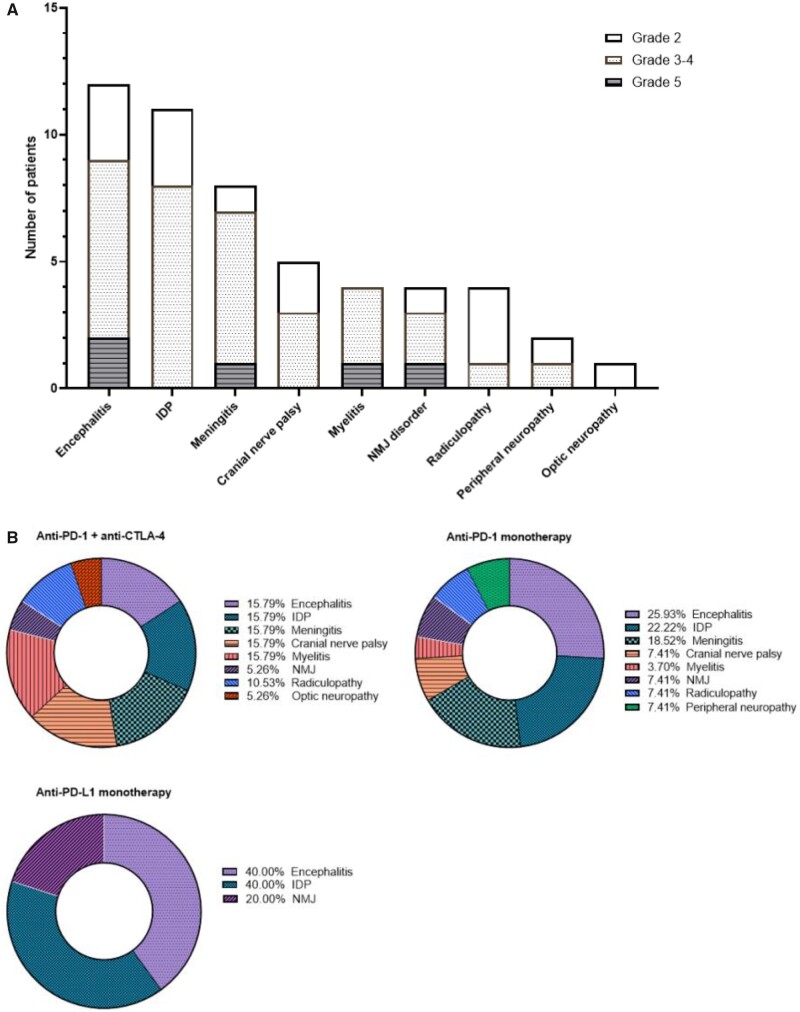
Overall, the study retained 40 patients for the analysis. Altogether, they experienced 51 n-irAEs, corresponding to a mean of 1.3 n-irAEs per patient (range: 1–3). The most frequent n-irAEs were encephalitis (n = 12, 24%), inflammatory demyelinating polyneuropathy (n = 11, 22%), meningitis (n = 8, 16%), cranial nerve palsy (n = 5, 10%) and myelitis (n = 4.8%) (Fig. 2A). Four patients experimented neuromuscular junction disorders: 3 developed myasthenia-like symptoms, of whom 1 had a flare of a previously-known seronegative myasthenia gravis and the latter developed Lambert-Eaton syndrome with diaphragmatic palsy. A detailed narrative patient description including clinical, biological and radiological features of n-irAEs in all 40 patients, is presented in the Supplementary Table 1.
Figure 2.
Clinical distribution of the 51 n-irAEs that occurred in the 40 patients included in the study. aPD1 = anti-programmed cell death 1 antibodies; aPD-L1 = anti-programmed cell death ligand 1 antibodies; aCTLA-4 = anti-cytotoxic T-lymphocyte associated protein 4 antibodies; CTCAE = Common Terminology Criteria for Adverse Events; IDP = inflammatory demyelinating neuropathies; NMJ = neuromuscular junction disorders.
Patient characteristics
Among the 40 patients with n-irAEs, the median age was 66 years [52–73] and the male/female ratio was 1.5. Twenty-six patients (65%) were treated with anti-PD-1 or anti-PD-L1 agents as monotherapy, and the other 14 (35%) received a combination of anti-PD-1 and anti-CTLA4 antibodies. Overall, patients were treated for melanoma (n = 18, 45%), lung cancer (n = 6, 15%), Merkel cell carcinoma (n = 3, 8%), renal cancer (n = 3, 8%) or other tumour types (n = 10, 25%). The clinical neurological presentation was peripheral for 19 (48%) patients, central for 14 (35%), and mixed for seven (18%). The median time to n-irAE onset was 74 days [26–167] and did not differ significantly between patients with mixed (36 days), peripheral (67 days), or central presentation (170 days) (P = 0.063) (Table 1). There was no significant difference in the distribution of the clinical central, peripheral or mixed presentation of n-irAEs depending on the immunotherapy regimen administered (Fig. 2B, Table 2). Twenty-one patients were tested for serum autoantibodies (onco-neuronal antibodies and/or anti-ganglioside antibodies), of whom 7 patients were positive and these patients have PNS unmasked or triggered by ICIs (Supplementary Table 2).
Table 1.
Clinical characteristics of patients with n-irAEs according to their clinical presentation (peripheral, central or mixed)
| All patients | Peripheral clinical presentation | Central clinical presentation | Mixed clinical presentation | P a | |
|---|---|---|---|---|---|
| (n = 40) | (n = 19) | (n = 14) | (n = 7) | ||
| Age-year (median, IQR) | 66 [52–73] | 66 [54–70] | 69 [57–75] | 66 [43–75] | 0.713 |
| Sex ratio (male/female) | 1.5 | 2.8 | 1 | 0.75 | 0.014 |
| Tumour type n (%) | |||||
| Melanoma | N = 18 (45%) | N = 10 (53%) | N = 7 (50%) | N = 1 (14%) | 0.242 |
| Lung cancer | N = 6 (15%) | N = 2 (11%) | N = 3 (21%) | N = 1 (14%) | 0.523 |
| Merkel cell carcinoma | N = 3 (8%) | N = 1 (5%) | N = 0 | N = 2 (29%) | NA |
| Renal cancer | N = 3 (8%) | N = 2 (11%) | N = 1 (7%) | N = 0 | NA |
| Other | N = 10 (25%) | N = 4 (21%) | N = 3 (21%) | N = 3 (43%) | NA |
| History of neurotoxic chemotherapy | |||||
| Platinum agents | N = 8 (20%) | N = 4 (21%) | N = 4 (29%) | N = 0 | NA |
| Immunotherapy regimen n (%) | |||||
| Monotherapy anti-PD1 or PD-L1 | N = 26 (65%) | N = 12 (63%) | N = 10 (71%) | N = 4 (57%) | 0.865 |
| Combination therapy anti-PD1 plus anti-CTLA4 | N = 14 (35%) | N = 7 (37%) | N = 4 (29%) | N = 3 (43%) | |
| Time between D1C1 and first n-irAE, in days (median, IQR) | 74 [26–167] | 67 [32–118] | 170 [74–210] | 36 [20–55] | 0.063 |
| Severity of n-irAE (max grade of severity according to CTCAE) n (%) | |||||
| Grade 2 | N = 14 (35%) | N = 9 (47%) | N = 4 (29%) | N = 1 (14%) | 0.249 |
| Grade 3 | N = 19 (48%) | N = 8 (42%) | N = 6 (43%) | N = 5 (71%) | 0.387 |
| Grade 4 | N = 4 (10%) | N = 1 (5%) | N = 2 (14%) | N = 1 (14%) | 0.644 |
| Grade 5 (death) | N = 3 (8%) | N = 1 (5%) | N = 2 (14%) | N = 0 | 0.451 |
| Treatment of n-irAE | |||||
| Corticosteroid n (%) | N = 31 (78%) | N = 14 (74%) | N = 10 (71%) | N = 7 (100%) | 0.297 |
| Immunosuppressive or immunomodulatory therapy n (%) | N = 7 (18%) | N = 3 (16%) | N = 2 (14%) | N = 2 (29%) | 0.699 |
| Outcome at last evaluation n (%) | |||||
| Deaths | N = 18 (45%) | N = 10 (53%) | N = 6 (43%) | N = 2 (29%) | 0.629 |
| Related to n-irAE | N = 14 (35%) | N = 8 (42%) | N = 4 (29%) | N = 2 (29%) | 0.676 |
| Related to cancer | N = 3 (8%) | N = 1 (5%) | N = 2 (14%) | N = 0 | 0.451 |
| Other causes of deaths | N = 1 (3%) | N = 1 (5%) | N = 0 | N = 0 | 0.395 |
| Sequelae of n-irAEs n (%) | |||||
| Yes | N = 21 (53%) | N = 10 (53%) | N = 6 (43%) | N = 5 (71%) | NA |
| No | N = 19 (47%) | N = 9 (47%) | N = 8 (57%) | N = 2 (29%) | 0.475 |
| Time to follow-up (median, IQR) | 234 [92–1062] | 381 [125–754] | 109 [75–971] | 527 [175–1062] | NA |
Kruskal–Wallis test.
D1C1 = Day 1 of cycle 1 of immunotherapy.
Table 2.
Characteristics of the 40 patients included in the study depending of their regimen of immunotherapy
| All patients (n = 40) | Monotherapy PD-1 or PD-L1 (n = 26) | Combination immunotherapy anti-PD-1 plus anti-CTLA4 (n = 14) | P b | |
|---|---|---|---|---|
| Age (median, IQR) | 66 [52–73] | 66 [58–76] | 63 [42–68] | 0.074 |
| Sex ratio (M/W) | 1.5 | 1.16 | 1.8 | 0.844 |
| Type of ICI n (%) | NA | |||
| Pembrolizumab | N = 12 (30%) | N = 12 (46%) | N = 0 | |
| Nivolumab | N = 9 (23%) | N = 9 (35%) | N = 0 | |
| Avelumab | N = 3 (8%) | N = 3 (12%) | N = 0 | |
| Atezolizumab | N = 1 (3%) | N = 1 (4%) | N = 0 | |
| Nivolumab + anti-LAG3 exp. Therapy | N = 1 (3%) | N = 1 (4%) | N = 0 | |
| Ipilimumab + Nivolumab | N = 13 (33%) | N = 0 | N = 13 (93%) | |
| Tremelimumab + Durvalumab | N = 1 (3%) | N = 0 | N = 1 (7%) | |
| Neoplasm n (%) | NA | |||
| Melanoma | N = 18 (45%) | N = 10 (39%) | N = 8 (57%) | |
| NSCLC | N = 6 (15%) | N = 6 (23%) | N = 0 | |
| Merkel cell carcinoma | N = 3 (8%) | N = 3 (12%) | N = 0 | |
| Renal cancer | N = 3 (8%) | N = 2 (8%) | N = 1 (7%) | |
| Head and neck cancer | N = 2 (5%) | N = 0 | N = 2 (14%) | |
| Pleural mesothelioma | N = 2 (5%) | N = 0 | N = 2 (14%) | |
| Others | N = 6 (15%) | N = 5 (19%) | N = 1 (7%) | |
| Maximal CTCAEv4.03 grade | 0.207 | |||
| Median (IQR) | 3 [2–3] | 3 [2–3] | 3 [2–3] | |
| Grade 2 | N = 14 (35%) | N = 11 (42%) | N = 3 (21%) | |
| Grade 3 | N = 19 (48%) | N = 11 (42%) | N = 8 (57%) | |
| Grade 4 | N = 4 (10%) | N = 3 (12%) | N = 1 (7%) | |
| Grade 5 | N = 3 (8%) | N = 1 (4%) | N = 2 (14%) | |
| Time to onset of n-irAEs from first infusion of immunotherapy (days: median, IQR) | 80 [28–170] | 74 [34–167] | 99 [26–163] | 0.486 |
| Clinical neurological presentation n (%) | ||||
| Central nervous system | 21 (35%) | 9 (35%) | 5 (36%) | 0.945 |
| Peripheral nervous system | 26 (48%) | 13 (50%) | 6 (43%) | 0.670 |
| Mixed | 7 (18%) | 4 (15%) | 3 (21%) | 0.635 |
| N-irAEs management, n (%) | ||||
| Corticosteroids only | N = 24 (78%) | N = 15 (58%) | N = 9 (64%) | 0.367 |
| Corticosteroids plus IgIV | N = 4 (10%) | N = 3 (12%) | N = 1 (7%) | |
| Corticosteroids plus TNF-α inhibitors | N = 1 (2.5%) | N = 0 | N = 1 (7%) | |
| Corticosteroids plus IgIV plus TNF-α inhibitors | N = 1 (2.5%) | N = 1 (4%) | N = 0 | |
| Corticosteroids plus anti-CD20 abs | N = 1 (3%) | N = 0 | N = 1 (7%) | |
| Untreated | N = 9 (23%) | N = 7 (27%) | N = 2 (14%) | |
| Rechallenge with immunotherapy n | N = 5 (13%) | N = 3 (8%) | N = 2 (5%) | NA |
| History of AI disease n (%) | 6 (15%) | 2 (15%) | 4 (29%) | 0.562 |
| Thyroiditis | N = 3 (8%) | N = 1 (4%) | N = 2 (14%) | |
| Psoriasis | N = 1 (3%) | N = 0 | N = 1 (7%) | |
| Myasthenia gravis | N = 1 (3%) | N = 1 (4%) | N = 0 | |
| Rhizomelic pseudopolyarthritis | N = 1 (3%) | N = 0 | N = 1 (7%) | |
| BOR according to RECISTa | NA | |||
| Complete response | N = 6 (16%) | N = 5 (19%) | N = 1 (8%) | |
| Partial response | N = 19 (50%) | N = 12 (46%) | N = 7 (58%) | |
| Stability | N = 7 (18%) | N = 4 (15%) | N = 3 (25%) | |
| Progression | N = 6 (16%) | N = 5 (19%) | N = 1 (8%) | |
| Progression and time to progression (n = 38, 2 adjuvants) (days; median, IQR) | N = 22 (55%) | N = 17 (65%) | N = 5 (36%) | NA |
| 178 [135–250] | 172 [131–209] | 261 [188–345] | ||
| Outcome at last evaluation n (%) | ||||
| Deaths | ||||
| Related to n-irAE | N = 3 (8%) | N = 1 (4%) | N = 2 (14%) | |
| Related to cancer | N = 14 (35%) | N = 11 (42%) | N = 3 (21%) | |
| Other causes of deaths | N = 1 (3%) | N = 1 (4%) | N = 0 | |
| Overall survival (days; median, IQR) | OS = 312 [170–649] | OS = 312 [206–698] | OS = 198 [54–531] | 0.292 |
| Sequelae of n-irAEs at last evaluation n (%) | ||||
| Yes | N = 21 (52%) | N = 14 (54%) | N = 7 (50%) | 0.529 |
| No | N = 19 (48%) | N = 12 (46%) | N = 7 (50%) | 0.818 |
Data available for 38/40 patients. Abs = antibodies.
Kruskal–Wallis non-parametric test, TNF-α inhibitors: Infliximab, anti-CD20 abs: Rituximab.
Severity of n-irAEs
Among the 40 patients who experienced n-irAEs, the overall maximum CTCAE grade of severity of the n-irAEs was grade 2 for 14 (35%) patients and grade ≥ 3 for 26 (65%). Three patients (8%) had a grade 5 n-irAE and subsequently died. The n-irAEs for these three patients were encephalitis for patient #1, neuromuscular junction disorder type Lambert Eaton syndrome for patient #10, and encephalitis for patient #40. A detailed narrative patient description is presented in the Supplementary Table 1.
Therapeutic management of patients with neurological irAEs and outcome corticosteroids
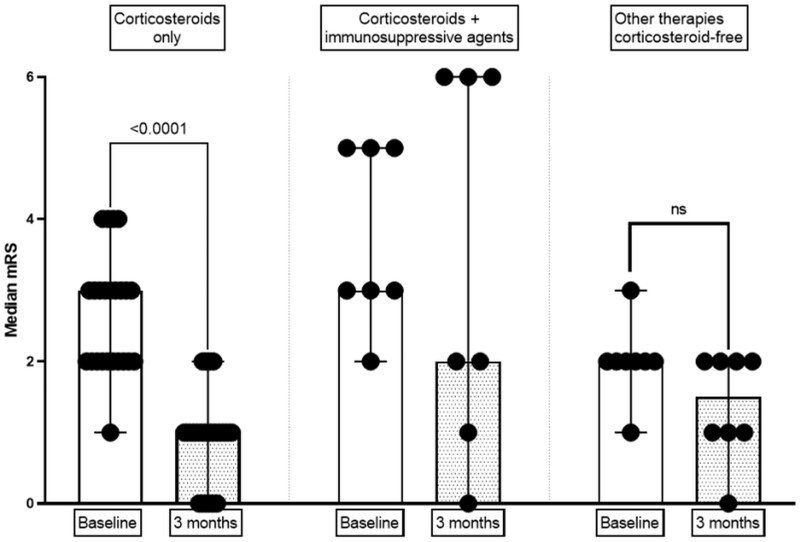
Among the 40 patients, the n-irAEs of 31 were treated with corticosteroids and those of nine with non-corticosteroid therapies. Among the 31 patients treated with corticosteroids, seven required the addition of immunosuppressive or immunomodulatory therapy (Supplementary Table 3). Treatment with systemic corticosteroids led to efficient neurological recovery for 23 (74%) patients after three months across all n-irAE clinical subtypes (Fig. 3). At the three-month evaluation, 16 (52%) of the 31 patients treated with corticosteroids achieved neurological recovery without sequelae (Supplementary Table 3). Corticosteroid treatment significantly reduced the score on the mRS between the baseline and three-month evaluation (P < 0.001, Fig. 4). The mean baseline level on the mRS was higher for patients treated with corticosteroids than those not treated with corticosteroids (Supplementary Table 3). The main reasons for not treating the patients with corticosteroids were the mild severity of the n-irAE(s) or spontaneous resolution for some patients (Supplementary Table 1). Patients not treated with corticosteroids did not significantly reduce their score on the mRS at the three-month evaluation relative to baseline, with a mean baseline mRS score of 2.00 (SD 1.01) versus 1.65 (SD 1.58) (P = 0.063) (Fig. 4). The probability of achieving neurological recovery was higher in patients treated with corticosteroids (P = 0.043). We did not find any association between the time from symptoms onset to the initiation of corticosteroid therapy and the neurological recovery rate, nor with the recovery without sequelae rate (P = 0.325 and P = 0.468, respectively).
Figure 3.
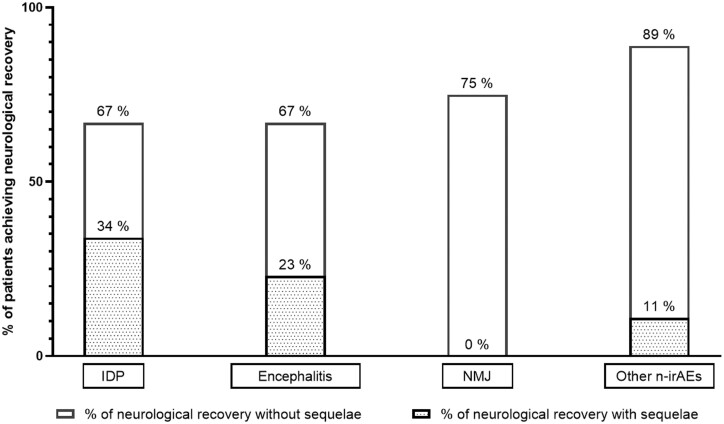
Neurological recovery after corticosteroid treatment according to the type of n-irAE. IDP, inflammatory demyelinating polyneuropathy; NMJ, neuromuscular junction; Others: meningitis, cranial nerve palsy, myelitis, radiculopathy. Neurological recovery was defined as a decrease in grade of ≥1 point on the CTCAE severity scale and a decrease of mRS of ≥1 point relative to that at baseline (beginning of treatment). Neurological recovery without sequelae was defined as a decrease of both the mRS and CTCAE back to their value before the occurrence of the n-irAE(s). The percentage of neurological recovery after corticosteroid treatment was calculated based on the number of patients showing neurological recovery after corticosteroid therapy relative to the number of patients with n-irAEs evaluable for the efficacy of corticosteroid therapy.
Figure 4.
Outcome of n-irAEs among patients treated with corticosteroids, corticosteroids plus immunosuppressants, or immunomodulatory drugs and patients with n-irAEs that were not treated. mRS, modified Rankin-Scale. A non-parametric paired Wilcoxon test compared the values at baseline (red points and bar) versus those at 6 months (blue points and bar) for the mRS (Sum of signed ranks: −276). Results are shown as the medians and 95% confidence interval. mRS: modified Rankin scale, Immunosuppressive or immunomodulatory therapies: four patients received intravenous immunoglobulin, two received anti-TNF-α antibodies (Infliximab), and one received anti-CD20 antibody (Rituximab). ****: P < 0.0001. Ns = non-significant (P = 0.063).
Immunosuppressive or immunomodulatory therapy
Among the 31 patients treated with corticosteroids, seven (23%) required the addition of immunosuppressive or immunomodulatory therapy. The additional immunosuppressive or immunomodulatory therapy consisted of intravenous immunoglobulins (given to 5 patients), anti-TNF-alpha antibodies (intravenous infliximab, given to 2 patients) and anti-CD20 antibody (intravenous rituximab, given to 1 patient). The mean baseline mRS score was 3.71 (SD 1.25) for patients treated with immunosuppressive or immunomodulatory therapy 3.43 (SD 2.44) at the three-month evaluation, with the reduction not being significantly different (P = 0.224) (Supplementary Table 3, Fig. 4).
Discontinuation of immunotherapy
Immunotherapy was withdrawn for all 40 patients at the time of n-irAE diagnosis. Overall, the n-irAE led to the permanent discontinuation of immunotherapy for 35 patients (88%) and temporary discontinuation for five (22%), who were subsequently re-challenged with immunotherapy.
Re-challenge with immunotherapy after temporary discontinuation due to neurological irAEs
Immunotherapy was temporarily discontinued for five patients (patients #14, #21, #24, #27 and #39) due to the occurrence of n-irAEs. After resolution of the n-irAE(s), these five patients were re-challenged with immunotherapy. The decision to rechallenge was taken by the referent oncologist after a thorough evaluation of the individual risk/benefit imbalance and pluridisciplinary concentration in the setting of the assessment board iTOX.18 Importantly, all patients had initial CTCAE grade II-III n-irAEs which improved either spontaneously (patients #14 and patient # 39) or after corticosteroid therapy (patients #21, #24 and #27), and with only mild sequelae at the time of rechallenge for 3/5 (patients #14, #27 and #39). Four of the five patients resumed their initial immunotherapy (i.e. Pembrolizumab), and patient #27 treatment was changed from Ipilimumab + Nivolumab to Pembrolizumab. The median time from halting immunotherapy to re-challenge was 60 days (IQR [30–150]). With a median of 510 days (IQR [450–870]) of follow-up after resuming immunotherapy, none of the five patients who were re-challenged had a recurrence of their n-irAEs. The clinical details of the patients re-challenged with immunotherapy are presented in the Supplementary Table 1.
Outcome of n-irAEs associated with auto-antibodies
Seven over 21 patients tested had positive onconeuronal or anti-ganglioside auto-antibodies in their serum concomitantly of their n-irAEs. Importantly, none of these patients had neurological symptoms before treatment by ICI. Immunotherapy was withdrawn for all the 7 patients tested auto-antibodies positive and none was rechallenged. When compared to the 14 patients who tested negative for auto-antibodies, patients with PNS-irAEs were all treated by corticosteroids (100% versus 79%), seemed to frequently require additional immunomodulative or immunosuppressive therapy (57% versus 21%), to have high rates of long-term sequelae (71% versus 43%) and mortality due to n-irAEs (29% versus 3%) (Supplementary Table 2). However, none of these differences reached statistical significance.
Neurological sequelae
The median follow-up of patients after the occurrence of their n-irAE(s) was 234 days [92–1062]. At the last evaluation timepoint, 21 of the 40 patients (53%) retained neurological sequelae from their n-irAE(s). Neurological sequelae at last evaluation were less frequent among patients treated by steroids (45% versus 78%). Patients with encephalitis and IDP recovered respectively without sequelae only in 44% and 33% (Fig. 3). The mean score on the mRS scale for these patients at the three-month evaluation was 2 (SD 1.79). There was no significant difference between the number of patients with sequelae depending on whether they had a central, peripheral, or mixed neurological clinical presentation (Table 1, P = 0.475).
Discussion
Here, we report an observational clinical study assessing the long-term outcome of patients with n-irAEs induced by anti-PD-1 or PD-L1. The neurological clinical manifestations were diverse (central, peripheral or mixed), severe and potentially life-threatening, with a mortality rate of 8%. Long-term-follow-up showed 53% of patients to experience sequelae of their n-irAEs.
Our study estimates the prevalence of patients treated by anti-PD-1 or PD-L1 with ≥grade 2 n-iRAEs to be 1.22%, based on the prospective register REISAMIC, which has consecutively included and prospectively followed nearly 1000 patients since 2016.14 This frequency of grade ≥ 2 n-iRAEs is close to that found by Mancone et al.19 with 0.95%. Of note, the frequency of n-irAEs for all grades of severity induced by anti-PD-1 or PD-L1 ranges from 2.8%20 to 7.7%.11
Our study pointed that 53% of patients experience long-term sequelae of their n-irAE. We believe that this high rate of long-term sequelae is significant and should require better understanding of n-irAEs and probably improve therapeutic strategies. A more intensive approach in the n-irAEs treatment management of patients, for example with earlier treatment or higher doses of corticosteroids, could possibly reduce the rate of long-term neurological sequelae.
In our study, the most frequent n-irAEs were encephalitis (24%) and inflammatory demyelinating polyneuropathy (22%), followed by meningitis (16%). This distribution is in line with those reported by Cuzzubo et al.7 and Dubey et al.12 We also report in our study, for the first time to the best of our knowledge, radiculopathies as n-irAEs, presenting with features of root inflammation (hypertrophy and gadolinium intake). The median time to onset of all n-irAEs in our study was 74 days, which is consistent with Johnson et al.21 series, who reported a median time to onset of within 90 days. We did not find any significant differences in the time to onset of any clinical presentation of n-irAE, neither among patients receiving anti-PD-1 or PD-L1 monotherapy nor in combination treatment with anti-CTLA4.
International guidelines recommend treating patients with ≥grade 2 n-irAEs with systemic corticosteroids.22,23 Guidelines also recommend intravenous corticosteroid bolus rather than oral treatment for high-severity grades.22 In our study, corticosteroids were associated with a 74% rate of neurological recovery. Importantly, all types of n-irAEs were improved with corticosteroid therapy, including inflammatory demyelinating neuropathies (IDPs), which are—outside the context of immunotherapy—generally deemed not to be very sensitive to corticosteroid therapy.24 These results should encourage physicians to treat systemic corticosteroid therapy promptly and as soon as patients are diagnosed with a n-irAE.
In our study, corticosteroid therapy was effective in 74% patients and not sufficiently effective for 26% of patients with a n-irAE. Patients who do not respond favourably to corticosteroid therapy may require additional immunosuppressive or immunomodulatory treatments.22 There is currently no consensus for the use of one immunosuppressive or immunomodulatory treatment over another for n-irAEs refractory to corticosteroids. Of note, two patients in our study received anti-TNF-α drugs (infliximab), without favourable effect. Despite limited data in our study, and even though anti-TNF-α antibodies may be useful for managing gastrointestinal irAE, such as colitis,25 our results do not support efficacy of anti-TNF-α drugs for the treatment of n-irAEs.
None of the 40 patients included were diagnosed with or had clinical symptoms of paraneoplastic neurological syndrome (PNS) prior to immunotherapy. However, 7 of the 21 (33%) patients tested had detectable PNS-associated autoantibodies at the n-irAE(s) onset. We cannot exclude that these patients had pre-existing auto-antibodies without related clinical symptoms. Indeed, treatment with immunotherapy can either reveal or exacerbate PNS, sometimes despite antitumoural response.26 Further studies are required to determine whether asymptomatic patients with detectable antibodies associated with PNS are at risk of n-irAEs, and whether screening for such antibodies before immunotherapy in patients with cancers associated with PNS, such as small-cell lung cancer and breast cancer, should be proposed. In our study, patients with PNS-associated auto-antibodies seemed to have high rates of long-term sequelae and mortality despite corticosteroid therapy. Others have suggested PNS with antibodies targeting intracellular onconeuronal antigens could benefit from immunomodulative therapies such as anti-CD20 antibodies (Rituximab) and/or anti-integrin antibodies (Natalizumab),27 yet one patient with anti-Ma2 antibodies (patient #1) received Rituximab without significant efficacy. However, other studies have reported encouraging results with anti-CD20 as treatment for paraneoplastic syndrome with antineuronal antibodies.28,29 Anti-CD20 could be a potential candidate treatment for n-irAEs in patients seropositive for autoantibodies and further studies are needed to investigate this therapeutic approach.
Finally, long-term follow-up of the patients showed that the five patients with CTCAE grades 2–3 n-irAEs who were re-challenged by immunotherapy after the resolution of their n-irAE had no further recurrence of n-irAEs, with a median post-resumption surveillance period of 18 months. Although guidelines generally recommend against resuming immunotherapy for patients with n-irAEs,22 our data suggest that the door to re-challenging patients with immunotherapy should not be permanently and systematically closed. In their recent report, Dubey et al.12 shared a similar experience concerning the feasibility of re-challenge. Dolladille et al.30 reported only a low rate of recurrence after re-challenge (6%, 1 case for 17 re-challenged patients) in a large study to examine immunotherapy re-challenge and n-irAEs. On the other hand, rechallenge should be considered with caution, as recently pointed out in Simonaggio et al.31 study in which 55% of 40 rechallenged patients experimented an irAE (similar to the initial one for 42.5% of them) (and even if only 4 patients with peripheral n-irAEs were included). Overall, these data suggest that a case-by-case approach should be considered in selected patients. The management of functionally-limiting or potential life-threatening neurological irAEs should be discussed on a collaborative basis to decide whether or not the ICI can be rechallenged, in line with the individual risk/benefit ratio towards the cancer evolution.18 We believe that if both the patient and physicians are willing to take an acceptable risk and the oncological situation requires it, resuming immunotherapy should be worth considering, albeit with caution.
Our study had several limitations. The monocentric nature of our study may explain some of the differences in the clinical presentation of n-irAEs from those of other studies. Because our study was retrospective, bias in the analysis and interpretation may limit the strength of evidence of the results. It was also monocentric, which may not allow the conclusions to be generalized. Finally, the size of the patient group was small, limiting the power of the analysis and the ability to search for significant differences. Larger studies, with bigger patient populations, are needed and ideally, specific prospective studies for diagnostic and therapeutic management should be set up to better detect and treat patients with n-irAEs.
Conclusion
n-irAEs of grade ≥ 2 are rare but can be life threatening, with a mortality rate we estimate to be 8%. The response rate for corticosteroids was 74% and long-term monitoring of patients found neurological sequelae in 53% of patients. Earlier recognition and prompt introduction of corticosteroids could lead to reduced neurological sequelae. A subset of patients with positive onconeuronal or anti-ganglioside auto-antibodies seemed to have high rates of sequelae despite treatment by corticosteroids, and should be further studied.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This study was funded by the Institut Gustave Roussy and belongs to the GRIP (Gustave Roussy Immunotherapy Program) launched in 2015. GRIP is a dedicated institutional program that aims to accelerate the clinical development of immunotherapy and strengthen translational research on these new treatments to benefit the largest number of patients. Data collection and writing of the report were performed by the authors independently of the funding source. Access to raw data was provided to A.L.V., A.M., J.M.M., L.P., C.C. and O.L., and will be available upon request. All authors have approved the final manuscript. The corresponding author had full access to all the data and the final responsibility to submit the manuscript for publication.
Competing interests
O.L. paid expert testimony and consultancy fees from BMS France, MSD, and Astra Zeneca; consultancy fees from Incyte; and expert testimony for Janssen. S.C. has received honoraria from AstraZeneca, BMS, Janssen, MSD, Novartis, and Roche. A.M.: Scientific Advisory Boards of Merck Serono, eTheRNA, Lytix pharma, Kyowa Kirin Pharma, Bayer, Novartis, BMS, Symphogen, Genmab, Amgen, Biothera, Nektar, GSK, Oncovir, Pfizer, Seattle Genetics, Flexus Bio, Roche/Genentech, OSE pharma, Transgene, and Gritstone. J.-M.M.: Advisory boards of Bristol-Myers Squibb, Pfizer, Roche, Novartis, Janssen, Astra-Zeneca, Cellgene, and Gilead. N.N.: speaker fees from BMS and Janssen outside the submitted work. C.C. has received honoraria from BMS. A.M. has received fees from Abbvie, Actelion, CSL Behring, Experf, Novartis, and Shire and speaking fees from Astra-Zeneca and BMS in the last 5 years.
Supplementary Material
Glossary
- CTCAE =
Common Terminology Criteria for Adverse Events
- CTLA-4 =
Cytotoxic T-lymphocyte-associated protein 4
- ICI =
Immune-checkpoint inhibitors
- IDP =
Inflammatory demyelinating neuropathies
- irAEs =
immune-related adverse events
- mRS =
Modified-Rankin scale
- n-irAEs =
Neurological immune-related adverse events
- PD-1 =
Programmed cell death 1
- PD-L1 =
Programmed cell death ligand 1
- REISAMIC =
Registre des Effets Indésirables Sévères des Anticorps Immunomodulateurs en Cancérologie
References
- 1. Haslam A, Prasad V.. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kong Y-CM, Flynn JC.. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and anti-PD-1. Front Immunol. 2014;5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. [DOI] [PubMed] [Google Scholar]
- 4. Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer Oxf Engl 1990. 2016;60:210–225. [DOI] [PubMed] [Google Scholar]
- 5. Yshii LM, Hohlfeld R, Liblau RS.. Inflammatory CNS disease caused by immune checkpoint inhibitors: Status and perspectives. Nat Rev Neurol. 2017;13(12):755–763. [DOI] [PubMed] [Google Scholar]
- 6. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. [DOI] [PubMed] [Google Scholar]
- 7. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer Oxf Engl 1990. 2017;73:1–8. [DOI] [PubMed] [Google Scholar]
- 8. Leitinger M, Varosanec MV, Pikija S, et al. Fatal necrotizing encephalopathy after treatment with nivolumab for squamous non-small cell lung cancer: Case report and review of the literature. Front Immunol. 2018;9:108.[CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Touat M, Talmasov D, Ricard D, Psimaras D.. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol. 2017;30(6):659–668. [DOI] [PubMed] [Google Scholar]
- 11. Sato K, Mano T, Iwata A, Toda T.. Neurological and related adverse events in immune checkpoint inhibitors: A pharmacovigilance study from the Japanese Adverse Drug Event Report database. J Neurooncol. 2019;145(1):1–9. [DOI] [PubMed] [Google Scholar]
- 12. Dubey D, David WS, Reynolds KL, et al. Severe neurological toxicity of immune checkpoint inhibitors: Growing spectrum. Ann Neurol. 2020;87(5):659–669. [DOI] [PubMed] [Google Scholar]
- 13. Vogrig A, Muñiz-Castrillo S, Joubert B, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry. 2020;91(7):772–778. [DOI] [PubMed] [Google Scholar]
- 14. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol. 2016;27(4):559–574. [DOI] [PubMed] [Google Scholar]
- 15. Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer Oxf Engl 1990. 2017;82:34–44. [DOI] [PubMed] [Google Scholar]
- 16. Edwards IR, Biriell C.. Harmonisation in pharmacovigilance. Drug Saf. 1994;10(2):93–102. [DOI] [PubMed] [Google Scholar]
- 17. Banks JL, Marotta CA.. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38(3):1091–1096. [DOI] [PubMed] [Google Scholar]
- 18. Michot J-M, Lappara A, Le Pavec J, et al. The 2016-2019 ImmunoTOX assessment board report of collaborative management of immune-related adverse events, an observational clinical study. Eur J Cancer Oxf Engl 1990. 2020;130:39–50. [DOI] [PubMed] [Google Scholar]
- 19. Mancone S, Lycan T, Ahmed T, et al. Severe neurologic complications of immune checkpoint inhibitors: A single-center review. J Neurol. 2018;265(7):1636–1642. [DOI] [PubMed] [Google Scholar]
- 20. Spain L, Walls G, Julve M, et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: A single centre experience and review of the literature. Ann Oncol. 2017;28(2):377–385. [DOI] [PubMed] [Google Scholar]
- 21. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J Immunother Cancer. 2019;7(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brahmer JR, Lacchetti C, Schneider BJ, et al. ; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haanen J. A G, Carbonnel F, Robert C, et al. ; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264–iv266. [DOI] [PubMed] [Google Scholar]
- 24. Hughes RA, Mehndiratta MM, Rajabally YA.. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2017;11:CD002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lesage C, Longvert C, Prey S, et al. French Group of Onco-Dermatology. Incidence and clinical impact of anti-TNFα treatment of severe immune checkpoint inhibitor-induced colitis in advanced melanoma: The Mecolit Survey. J Immunother Hagerstown Md 1997. 2019;42(5):175–179. [DOI] [PubMed] [Google Scholar]
- 26. Manson G, Maria ATJ, Poizeau F, et al. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer. 2019;7(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graus F, Dalmau J.. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2019;16(9):535–548. [DOI] [PubMed] [Google Scholar]
- 28. Shams'ili S, de Beukelaar J, Gratama JW, et al. An uncontrolled trial of rituximab for antibody associated paraneoplastic neurological syndromes. J Neurol. 2006;253(1):16–20. [DOI] [PubMed] [Google Scholar]
- 29. Sadeghian H, Vernino S.. Progress in the management of paraneoplastic neurological disorders. Ther Adv Neurol Disord. 2010;3(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5(9):1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed informations are provided in the Supplementary Table 1. Further data can be shared upon request by the authors.