Abstract
Recent advances in multiple myeloma include numerous approvals of novel therapies with unprecedented efficacy, a rapid and sustained tempo of new drug development, and further refinements to prognostication to include minimal residual disease (MRD) testing and improved risk stratification. The upfront use of immunomodulatory drug and proteasome inhibitor combinations followed by maintenance has resulted in transformative clinical benefit. Four-drug regimens incorporating monoclonal antibodies are reporting unprecedented rates of complete response and MRD negativity in the absence of intensification. In the context of these advances, the added value of high-dose melphalan with autologous stem-cell transplant (HDM-ASCT) is a key question. From a safety standpoint, HDM-ASCT is associated with both acute toxicities that reduce quality of life and long-term toxicities that may limit life expectancy for some patients. The present review discusses the recent advances in induction therapy, the impact of these advances on HDM-ASCT, the evolving role of MRD testing and the short- and long-term risks of HDM-ASCT. Recognising that prospective data remains limited, we suggest that HDM-ASCT not be considered mandatory for eligible newly diagnosed patients who are treated with highly efficacious regimens and achieve deep responses, but rather be held in reserve without early exposure to the clinical and genomic toxicity inherent to this approach.
Keywords: high-dose melphalan, multiple myeloma, autologous stem cell transplant
The impact of novel drug combinations
Significant advances in the development of new drugs, technologies for diagnosis and response assessment, risk stratification and treatment strategies for the management of multiple myeloma (MM) but most importantly, the increasing availability of novel therapeutics have translated into improvements in long-term clinical benefit and doubling of survival.1 After half a century of conventional chemotherapeutics, each of which has conferred some benefit but at the expense of significant toxicity, these more recent and dramatic improvements have largely been attributed to the widespread use of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs), as well as the introduction of monoclonal antibodies (mAbs). In the coming decade, it is reasonable to expect further improvements in overall survival (OS) as next-generation novel agents, and especially mAbs, are added to the IMiD-PI platform and evaluated as part of quadruplet combinations in the front-line. To this end, several combinations are already well developed or in process, registrational studies are ongoing, and the first quadruplet regimen was approved by the USA Food and Drug Administration (FDA) in 2019.2,3
In light of these remarkable developments, the conventional role of high-dose melphalan with autologous stem-cell transplant (HDM-ASCT) as standard of care for every eligible patient early in their treatment course can be justifiably challenged, as it continues to be studied rigorously in clinical trials. This is not only due to the immediate toxicities of HDM-ASCT, but also the long-term risks such as an approximate 10-fold increase in myelodysplastic syndrome (MDS) and acute myelogenous leukaemia (AML) when compared to non-HDM-ASCT-based therapies.4 Acknowledging geographical disparities in access to novel drugs, it is also important to note that the available therapeutic regimens for first-line treatment in many countries do not consistently deliver deep and durable treatment responses to the degree achieved by IMiD-PI combinations, and so HDM-ASCT will continue to play an important role in this regard.5 In the USA, however, both IMiD and PI in the first-line setting are widely available and adopted by treatment guidelines and the Centres for Medicare and Medicaid Services (CMS).6
IMiD-PI combination regimens have transformed the treatment landscape for newly diagnosed MM
Novel IMiD-PI-based combinations have unequivocally improved outcomes in newly diagnosed MM (NDMM) and are now considered standard of care in the USA. The road to this standard began over a decade ago when the seminal Phase 1/2 multicentre study of lenalidomide-bortezomib-dexamethasone (RVd) in NDMM reported an unprecedented 100% overall response rate (ORR), with a 52% rate of complete response (CR)/near-CR in 66 patients, most of whom were transplant eligible.7 This was followed by the EVOLUTION trial (ClinicalTrials.gov Identifier: NCT00507442), which randomised patients with NDMM to bortezomib-dexamethasone in combination with lenalidomide (VDR, using weekly dexamethasone), cyclophosphamide (VDC), or both (VDCR), and found a threefold increase in ≥very good partial response (VGPR) rate in favour of the lenalidomide-containing regimens [32% (VDR) vs. 13% (VDC)] after four cycles of induction with VDCR demonstrating no advantage compared to VDR.8 Subsequently, the Intergroupe Francophone du Myelome (IFM) 2013–04 trial (NCT01971658), which randomised patients to bortezomib, dexamethasone, and either thalidomide (VTD) or cyclophosphamide (VCD) as induction therapy, found that the ORR was significantly higher in the VTD arm (92·3% vs. 83·4%, P = 0.01), further confirming the superiority of IMiD-PI over alkylator-PI strategies.9 Finally, in the landmark Southwest Oncology Group (SWOG) S0777 study (NCT00644228) of RVd versus Rd, newly diagnosed patients who were randomly assigned to the former achieved a higher response rate (82% vs. 72% ORR) and a 29% reduction in all-cause mortality.10 Taken together, these studies established RVd as a safe and generally well-tolerated regimen with an unprecedented degree of efficacy and this has led to its widespread adoption as a standard regimen for patients with NDMM, in the USA initially and now across the globe.
In an effort to improve on the efficacy observed with RVd, the combination of carfilzomib, lenalidomide and dexamethasone (KRd) has been developed and approved by the FDA in 2015 for the treatment of relapsed and relapsed/refractory MM (RRMM),11 and is now being evaluated in earlier lines.12 Unlike bortezomib, carfilzomib does not cause peripheral neuropathy, which is a dose-limiting toxicity in the former.13,14
Carfilzomib is a potent and efficacious PI, especially when used in combination. In Phase 2 studies of KRd in NDMM incorporating lenalidomide maintenance without early HDM-ASCT, for example, rapid and deep responses were observed, with sustained MRD negativity seen in up to 62% of patients and median time to progression (TTP) of >5.5 years.15,12 Similar efficacy results, as well as evidence that carfilzomib may overcome poor cytogenetic risk, have also been reported in other studies of KRd.16,17,18,19 Despite these impressive measures of response, randomised prospective data confirming the superior efficacy of KRd over RVd in NDMM are not yet available, and in this context the results of the ongoing Eastern Cooperative Oncology Group (ECOG) sponsored Phase 3 ENDURANCE study (NCT01863550) are anticipated with great interest. Taken together, these studies have led to the consensus adoption of KRd as an acceptable option for first-line therapy, especially for patients with high-risk disease and younger patients without cardiovascular disease or the presence of known risk factors,6 although real-world practice to date has shown a preference for RVd as the regimen of choice in community practice, based upon overall efficacy and other considerations.20 Table I10,15,17,21,23 summarises efficacy results of select RVd and KRd studies without the use of HDM-ASCT.
Table I.
Selected studies supporting the use of novel immunomodulatory and proteasome-based combinations without early high-dose melphalan in newly diagnosed multiple myeloma.
| Study | Novel combination | ORR, % | mPFS/mTTP | mOS |
|---|---|---|---|---|
| SWOG S0777 ([9]; NCT00644228) | RVd arm (N = 242) | 82; CR: 16 | 43 months (95% CI 39–52) | 75 months (95% CI 65 months–NE); 4-year: 70% |
| IFM2009 ([21]; NCT01191060) | RVd arm (N = 350) | 97; CR: 48 | 36 months | Not reached; 4-year: 82% |
| NCI KRd ([15]; NCT01402284) | KRd (N = 45) | 98 (95% CI 88–100); CR: 67 | 67 months (95% CI 51 months–NE); 4-year: 78% (95% CI 62–87%) | Not reached; 5-year: 90% (95% CI 75–96%) |
| University of Chicago ([19]; NCT01029054) | KRd (N = 53) | 98; sCR: 55 | 4-year: 69% | 4-year: 93% |
| FORTE ([11]; NCT02203643) | KRd arm (N = 157) | ≥VGPR: 87; CR: 61 | n/a | n/a |
CI, confidence interval; (s)CR, (stringent) complete response rate; KRd, carfilzomib, lenalidomide, dexamethasone; mPFS, median progression free survival; mOS, median overall survival; mTTP, median time to progression; NCI, National Cancer Institute; NE, not estimable; ORR, overall response rate; SWOG, Southwest Oncology Group; VGPR, very good partial response; VRd, bortezomib, lenalidomide, dexamethasone.
For patients with cardiovascular disease or risk thereof, caution with carfilzomib is warranted, because while peripheral neuropathy is rare, significant cardiovascular toxicity has emerged as an uncommon but serious adverse event associated with its use.24 Importantly, reports of clinically relevant cardiovascular, pulmonary, and renal events consistently come from studies in the RRMM setting, and the majority have been reversible, although cases of incomplete cardiac recovery and fatal events have been documented. Additionally, there is some evidence that the risk of cardiac toxicity may be further enhanced by concomitant IMiD use,25 with thromboembolic events also being more common with KRd.16 More reassuringly, a recent pooled analysis of the ASPIRE (NCT01080391), ENDEAVOR (NCT01568866) and FOCUS (NCT01302392) trials found the absolute risk of Grade ≥3 cardiac failure with carfilzomib-containing therapy to be relatively low (at c. 5%), with the rate of fatal cardiac events being comparable to that of the non-carfilzomib control regimens (at ≤1%) in these carefully controlled clinical trials.26 Based on clinical experience with carfilzomib at both standard and higher (e.g. 56 mg/m2) doses, clinical guidelines in collaboration with onco-cardiologists have been developed, and serial assessment of patients with electrocardiograms, echocardiograms and serum assays of brain natriuretic peptide (BNP) and troponin, along with effective fluid management, thromboprophylaxis and careful monitoring as well as treatment of hypertension, have helped to mitigate cardiovascular toxicity in clinical practice.24
The combination of ixazomib with lenalidomide and dexamethasone (IRd) has been similarly successful in the upfront setting, affirming the synergy of the IMiD/PI platform.27,28 However, the activity of weekly ixazomib as part of this regimen has been less impressive than with the other triplets for transplant-eligible patients, and so at least for the present this combination appears more applicable to transplant-ineligible patients.
Anti-CD38 mAb-containing quadruplet regimens are demonstrating an unprecedented degree of efficacy in NDMM
The recent introduction of anti-CD38 mAbs into IMiD-PI combination regimens has produced impressive results. In the CASSIOPEIA trial (NCT02541383), for example, patients with NDMM who received daratumumab in combination with bortezomib-thalidomide-dexamethasone (Dara-VTd) as part of an upfront transplant-containing approach had stringent CR (sCR) and MRD negativity rates (10−5 sensitivity) after transplant of 29% and 64%; respectively, compared to 20% and 44% for those who received VTd and transplant alone.2 These results recently led to the FDA approval of Dara-VTd for treatment of transplant-eligible patients with NDMM in September 2019, effectively ushering in the era of anti-CD38-containing quadruplet regimens as a standard of care for first-line treatment of transplant-eligible patients. It should be emphasised that the VTd-backbone combination therapy is infrequently used in the USA due to the high rates of peripheral neuropathy and other dose-limiting toxicities.
Furthermore, in the recently reported GRIFFIN study (NCT02874742) of RVd plus upfront HDM-ASCT with or without daratumumab, the primary endpoint of sCR after completion of transplant and consolidation was higher in the Dara-RVd arm (42·4% vs. 32·0%; P = 0·068).29 An updated analysis of GRIFFIN presented at the American Society of Hematology Annual Meeting 2019 (ASH 2019) revealed a continued deepening of response beyond consolidation (on maintenance) in both arms of the study, but especially in the Dara-RVd arm (62·6% vs. 45·4% sCR, P = 0·05).3 Likewise, MRD negativity rates [measured by next-generation sequencing (NGS) at a sensitivity of 10−5] continued to increase in both arms after completion of transplant and beyond consolidation, with the most pronounced increase occurring in patients on the Dara-RVd/Dara-R maintenance arm (69% vs. 32% of evaluable patients in the RVd arm receiving R-only maintenance).
Similarly, a recent Phase 2 study of KRd plus daratumumab (Dara-KRd) with once weekly dosing of carfilzomib for patients with NDMM, also presented at ASH 2019, reported a 97% ≥VGPR rate as well as an unprecedented 77% (23 of 30 patients) MRD-negativity rate as the best response after up to eight cycles of Dara-KRd therapy.30 The median time to MRD negativity was after six cycles of therapy. Importantly, this high degree of MRD negativity (77%) was achieved without the use of HDM-ASCT, and is comparable to the 82% MRD negativity seen in the MASTER study (NCT03224507) of six cycles of Dara-KRd plus HDM-ASCT in patients with NDMM.31 Taken together, these studies serve to not only underscore the impressive degree of efficacy of quadruplet induction regimens, but also raise the critical question regarding the extent to which HDM-ASCT adds to this efficacy, acknowledging that cross-trial comparisons are hypothesis-generating and so warrant caution.
Finally, the ICARIA-MM study (NCT02990338) of pomalidomide-dexamethasone with or without isatuximab (Isa; a potent anti-CD38 mAb with direct tumoricidal activity) in RRMM met its primary endpoint in 2019, demonstrating a near-doubling of PFS in the Isa-Pd arm (11·5 vs. 6·5 months).32 As a result of this trial, on 2 March 2020, the FDA approved isatuximab, in combination with pomalidomide and dexamethasone, for the treatment of adult patients with MM who have received at least two prior therapies including lenalidomide and a PI. Meanwhile, the addition of isatuximab to both RVd and KRd is also being studied in NDMM, and although these trials are ongoing, Isa-KRd was just reported to have a 100% ORR in the safety run-in cohort of the GMMG-CONCEPT study (NCT03104842), which is enrolling only patients with high-risk disease.33 Similarly, the combination of Isa-RVd was recently reported to have a 100% ORR, 92% ≥VGPR and 50% MRD negativity and proved well tolerated in a smaller transplant-ineligible NDMM population.34 Table II lists USA standard of care triplet combinations and future quadruplet combinations currently in clinical trials.
Table II.
USA standard of care three-drug combination therapies and future four-drug combinations in clinical trials for newly diagnosed multiple myeloma.
| Novel combination | PI | IMiD | Steroid | 4th Drug | Select supportive trials, NCT# |
|---|---|---|---|---|---|
| RVd | Bortezomib | Lenalidomide | Dexamethasone | n/a | SWOG 0777, NCT00644228; IFM2009, NCT01191060 |
| KRd | Carfilzomib | Lenalidomide | Dexamethasone | n/a | NCI, NCT01402284; Chicago, NCT01029054; MSKCC, NCT02937571 |
| IRd* | Ixazomib | Lenalidomide | Dexamethasone | n/a | Millennium, NCT01850524, NCT01217957, NCT01383928; IFM, NCT02897830; Nordic, NCT03376672, NCT01936532 |
| D-VRd | Bortezomib | Lenalidomide | Dexamethasone | Daratumumab (anti-CD38 mAb) | Janssen, NCT03652064, NCT03412565, NCT02874742; EMN, NCT03710603 |
| D-KRd | Carfilzomib | Lenalidomide | Dexamethasone | Daratumumab (anti-CD38 mAb) | MMY1001, NCT01998971; MSKCC, NCT03290950, Chicago, NCT03500445 |
| D-IRd | Ixazomib | Lenalidomide | Dexamethasone | Daratumumab (anti-CD38 mAb) | Toulouse, NCT03669445; Mayo, NCT03012880 |
| Isa-VRd | Bortezomib | Lenalidomide | Dexamethasone | Isatuximab (anti-CD38 mAb) | Heidelberg, NCT03617731; IMROZ, NCT03319667 |
| Isa-KRd | Carfilzomib | Lenalidomide | Dexamethasone | Isatuximab (anti-CD38 mAb) | Tuebingen, NCT03104842 |
| E-RVd | Bortezomib | Lenalidomide | Dexamethasone | Elotuzumab (anti-SLAMF7 mAb) | DFCI, NCT02375555; Heidelberg, NCT02495922 |
| E-KRd | Carfilzomib | Lenalidomide | Dexamethasone | Elotuzumab (anti-SLAMF7 mAb) | Chicago, NCT02969837 |
| Pano-RVd | Bortezomib | Lenalidomide | Dexamethasone | Panobinostat (HDACi) | MDACC, NCT01440582; PANORAMA4, NCT02720510 |
IMiD, immunomodulatory drug; PI, proteasome inhibitor; n/a, not applicable; HDACi, histone deacetylase inhibitor.
Reserved for patients requiring all oral treatment or contraindication to bortezomib (neuropathy) and carfilzomib (cardiac).
Although the role and extent of adoption of mAb-containing quadruplet regimens into the NDMM space have yet to be determined, it is reasonable to assume that their use will increase and that the ability to achieve deep and sustained remissions without HDM-ASCT over the coming years is going to improve as these combination approaches are further developed.
Recent data do not convincingly demonstrate an OS advantage to HDM-ASCT in NDMM
It is important to note that the results and conclusions of prior randomised trials developed to address the role of upfront HDM-ASCT in MM cannot be broadly extrapolated to today’s patient population within the USA, where RVd and KRd are now considered standard. These otherwise well-designed trials have already lost some degree of applicability due to the ever-advancing degree of efficacy of novel agent-based induction regimens. In 2014, for example, Palumbo et al. 35 reported a remarkable 45% reduction in risk of mortality at 4 years in favour of HDM-ASCT in Rd-treated patients randomised to high-dose consolidation versus standard-dose melphalan-prednisone-lenalidomide. Just 2 years after this study was published, the SWOG S077 study reported an 11-month improvement in median OS with RVd induction compared to Rd, with a doubling of the CR rate, and critically reflected the favourable impact of incorporating a PI into an IMiD-based platform 10. Likewise, in 2017, the EMN02/HO95 study (NCT01208766) reported both PFS and OS advantage to performing double/tandem over single HDM-ASCT in transplant-eligible patients with NDMM.36 However, this study incorporated only a short course of VCd induction, which is significantly inferior to RVd and, by extrapolation, is likely to be similarly inferior to KRd. Table III35,37–45 shows the key results of the early studies evaluating HDM-ASCT without the use of IMiD-PI combination therapy.
Table III.
Efficacy results of selected randomised studies evaluating high-dose melphalan with stem cell support.
| Study (year) | Induction regimen | N | Median EFS, months | Median PFS, months | Median OS, months |
|---|---|---|---|---|---|
| IFM90 Attal et al., 1996, [37] | VMCP/BVAP | 100 | 18 | –* | 37 |
| MAG90 Fermand et al., 1998 [36] | VAMP or VMCP | 94 | 13 | –* | 64 |
| MRC7 Child et al., 2003 [38] | VAMPC or ABCM | 200 | –* | 20 | 42 |
| HOVON Segeren et al., 2003 [39] | VAD/Mel70 | 129 | 21 | –* | 50 |
| M97G Palumbo et al., 2004 [40] | VAD, MP | 99 | 16 | –* | 43 |
| MAG91 Fermand et al., 2005 [41] | VAMP or VMCP | 96 | 19 | –* | 48 |
| PETHEMA Bladé et al., 2005 [42] | VBMCP/VBAD | 83 | –* | 33 | 58 |
| S9321 Barlogie et al., 2006 [43] | VAD or VAD/VBMCP | 255 | –* | 20 | 57 |
| GIMEMA Palumbo et al., 2014 [34] | Rd | 104 | –* | 22 | 5-year OS: 59% |
| GIMEMA Gay et al., 2015 [44] | Rd/CyRd | 129 | –* | 29 | 4-year OS: 73% |
PFS, progression-free survival; EFS, event-free survival; OS, overall survival; VMCP, vincristine, melphalan, cyclophosphamide, prednisone; BVAP, carmustine, vincristine, doxorubicin, prednisone; VAMP, vincristine, doxorubicin, methylprednisolone; VAMPC, vincristine, doxorubicin, methylprednisolone, cyclophosphamide; ABCM, doxorubicin, BCNU, cyclophosphamide and melphalan; VAD, vincristine, doxorubicin, dexamethasone; Mel70, melphalan 70 mg/m2; MP, melphalan, prednisone; VBMCP, vincristine, carmustine, melphalan, cyclophosphamide, prednisone; VBAD, vincristine, carmustine, doxorubicin, dexamethasone; Rd, lenalidomide, dexamethasone; CyRd, cyclophosphamide, lenalidomide, dexamethasone.
Not reported.
To date, there have been two randomised Phase 3 studies with results reported that have evaluated the benefit of HDM-ASCT in the setting of modern-day IMiD-PI induction. The first, IFM/DFCI 2009 (NCT01191060), randomly assigned patients to eight cycles of RVd without HDM-ASCT versus three cycles of RVd followed by HDM-ASCT followed by two additional cycles (five cycles total), with all patients then proceeding to lenalidomide maintenance for 1 year. Although the HDM-ASCT arm demonstrated a significant advantage in terms of median PFS (50 vs. 36 months) and CR rate (59% vs. 48%), OS at 4 years was identical (81% vs. 82%).21 It remains to be seen whether any OS benefit will emerge for either arm with extended follow-up. Importantly, it also remains to be seen whether the ongoing pivotal DETERMINATION study (NCT01208662), which is identical in design to IFM/DFCI 2009 except for the use of lenalidomide maintenance until progression instead of 1 year, will reveal an abrogation of the HDM-ASCT arm’s PFS advantage and/or a survival benefit in either direction.22 The fact that this trial has yet to meet its primary endpoint as of December 2019 (9 years after its start and after at least two-thirds of events having occurred, with a median follow-up now of >4 years) suggests that the study population is performing well on long-term maintenance regardless of the presence or absence of HDM-ASCT.
The second reported Phase 3 trial evaluating the role of transplant in the setting of IMiD-PI induction, FORTE (NCT02203643), is a three-arm study with patients randomly assigned to 12 cycles of KRd without HDM-ASCT versus four cycles of carfilzomib-cyclophosphamide-dexamethasone (KCd) followed by HDM-ASCT followed by four additional cycles (for a total of eight cycles), versus the same HDM-ASCT-containing strategy using KRd, with all patients then randomly assigned to either K or KR maintenance. Early results presented at the 2018 ASH Annual Meeting were remarkable for significant advantages in depth of response (≥VGPR, sCR, and MRD negativity) in favour of KRd over KCd, with both sCR and MRD negativity rates being essentially identical between the KRd12 and the KRd-HDM-ASCT arms.23 In a sub-analysis of patients with Revised International Staging System (R-ISS) Stage 2/3 disease that was presented at the 2019 American Society of Clinical Oncology (ASCO) meeting, there was a similar rate of MRD negative responses (49% vs. 51%, P = 0·883) and a trend towards more frequent early relapse (≤18-month relapse-free interval) in the KRd12 arm (23% vs. 12% in the KRd-HDM-ASCT arm, P = 0·054), as well as loss of 1-year MRD negativity (28% vs. 10%, P = 0·067).46 However, there was a high rate of MRD data missing from both study arms (25% for KRd12 and 23% for KRd-HDM-ASCT) and there are no data yet on the interaction between the maintenance arms (R versus KR) and these endpoints. In this context, the PFS analysis of FORTE is awaited with great interest.
It can be argued that studies such as IFM/DFCI 2009, DETERMINATION and FORTE are inherently imbalanced in terms of design insofar as PFS is concerned, as they essentially compare three drugs (RVd or KRd) to four (plus HDM). A longer median PFS in the transplant arm, as was seen in IFM/DFCI 2009, may have some value as a surrogate for OS as it does in other settings, but this is not yet seen and it may in fact have little or even inverse correlation with long-term survival by way of preserved efficacy of salvage transplant, new salvage options, or more worryingly, enhanced genomic instability of any surviving HDM-exposed MM. To this end, a recent meta-analysis comparing early versus delayed HDM-ASCT showed no difference in OS.47 Likewise, a second meta-analysis evaluating various transplant-containing strategies versus standard-dose therapy (to include RVd) also did not demonstrate any OS benefit to the former, despite a substantial PFS advantage.48 Considering these analyses and the results of IFM/DFCI 2009, it is possible that the PFS before second progression (PFS2) may be an important endpoint to assess in ongoing and future NDMM trials that randomly assign patients to upfront versus delayed (or no) HDM-ASCT. Additionally, it may be reasonable to evaluate OS as the more valid clinical endpoint when PFS may favour the more toxic approach and so include long-term toxicity that cannot be adequately captured after several years (or even a decade) of follow-up.
The role of MRD in tailoring therapy is evolving
In addition to novel therapies, and concordant with their impact on outcome, an important development in the field has been the ability to detect MRD with a high degree of sensitivity (≤10−5). This is important because newer therapies achieve not only higher response rates but deeper responses, which are inadequately captured by traditional response criteria alone, and which may more accurately reflect the true activity of these therapies.49,50 MRD negativity as a prognostic marker has been confirmed by two independent meta-analyses, and MRD assessment at ≤10−5 sensitivity in particular may play an important role in determining the role of early HDM-ASCT.51,50 Moreover, the sustainability of MRD negativity over time, rather than merely the achievement of MRD negativity at a single time-point, may carry even greater prognostic significance.52
In IFM/DFCI 2009, MRD negativity at the start of lenalidomide maintenance (measured by next-generation sequencing (NGS) at a sensitivity of 10−6) was associated with a marked improvement in both PFS [ hazard ratio (HR) 0·22, 95% CI 0·15–0·34; P < 0·001] and OS (HR 0·24, 95% CI: 0·11–0·54; P = 0·001) irrespective of treatment arm, cytogenetic risk profile, or disease stage at diagnosis.53 Importantly, a second MRD analysis performed after 12 months of lenalidomide maintenance revealed a further decline in HR for PFS (0·18) and an increase in MRD negativity, of the 127 total MRD-negative patients, 37 achieved MRD negativity for the first time after 12 months of maintenance. While it is clearly true that a higher percentage of patients in the transplant arm achieved MRD negativity compared to the RVD-only arm (29·8% vs. 20·5%, P = 0.01), the nature of the correlation between MRD negativity over time and long-term outcome regardless of treatment modality serves as a caution against using this higher percentage early in the treatment course as the primary basis for recommending transplant for eligible patients. Specifically, the time-dependent achievement of MRD negativity in this analysis suggests that escalating treatment intensity based on early MRD assessments, given the evolving duration and nature of maintenance, may be premature. Despite these caveats, the MRD data from IFM/DFCI 2009 underscore the prognostic significance of MRD negativity and suggest a higher likelihood of achieving it with upfront transplant and limited maintenance using single-agent lenalidomide. However, the impact of long-term maintenance may substantially change this paradigm and the outcome of the DETERMINATION trial will be absolutely critical in this respect.
It must also be acknowledged that no study to date has prospectively evaluated the benefit of HDM-ASCT in patients based on MRD positivity during or after induction. Such trials are needed, but until we have direct evidence that MRD-based escalation of therapy improves long-term outcome, the decision of whether to consolidate MRD-positive patients with HDM-ASCT to reach MRD negativity is a challenge. In this context, the first large randomised multi-centre trial designed to investigate MRD-driven HDM-ASCT in NDMM (‘ADVANCE’, NCT04268498) was launched in early 2020, with other similar studies planned or in process.
HDM-ASCT is a potentially toxic therapy
High-dose therapy is associated with significant morbidity and very rarely treatment-related mortality (TRM), which is typically <5% and at expert centres <1%. Acutely, HDM-ASCT leads to high-grade toxicities such as prolonged bone marrow suppression, mucositis and infection, and much less commonly to other significant complications such as veno-occlusive disease (0·4%), interstitial pneumonitis (0·5–8%), autologous graft-versus-host disease (18%) and graft failure (0·6%).37 Additionally, TRM has been reported to occur in up to 10% of patients, although generally <1% at leading transplant centres, and older studies have likely overestimated this incidence. As a Cochrane Database review highlighted, the true incidence of TRM may also be difficult to assess due to substantial differences between trials and variable levels of quality reporting.54
Quality of life (QoL) must also be considered, especially in the absence of a proven survival advantage to intensive first-line therapy. Although most patients are able to recover to their baseline QoL or better after transplant, virtually all patients experience a significant decline in QoL, with a median duration of approximately 3 months that may be more prolonged.55 Specifically, there does appear to be an important subset of patients who continue to experience severe QoL-limiting symptoms such as fatigue, neuropathic pain and profound asthenia during the first year post-HDM-ASCT despite good control of their disease and irrespective of the use of maintenance therapy.56
The long-term potential sequelae of HDM-ASCT are now better understood and include fatigue, cytopenias, recurrent infections, cataract formation, infertility, pulmonary fibrosis, and most importantly, a significantly increased risk of secondary malignancies. Exposure to HDM increases the risk of AML and MDS in any individual, with patients with MM being particularly susceptible. For example, in a population-based study evaluating 8740 patients with MM, there was an 11·5-fold increased risk of AML/MDS in patients with MM compared to the general population.57 Moreover, patients with MM had a 1·3-fold increased risk of developing any malignancy and a twofold increased risk of developing any haematological malignancy, representing a substantial vulnerability to secondary cancer that may be further exacerbated by the genotoxic effects of HDM-ASCT, with longer follow-up time with this cohort, we will be able to evaluate the long-term differences in risk when comparing before/after 1995 (HDM-ASCT) and 2000 (IMiD) rates. To this end, a recent analysis of all of the initial autologous transplants performed for MM and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) between 1995 and 2010 found that the risks of developing MDS and AML were 100-fold and 50-fold higher, respectively, among patients treated with HDM-ASCT compared to the general population, with degrees of risk that were approximately 10-times that of non-transplanted patients within the Surveillance, Epidemiology, and End Results (SEER) database4 (Fig 1). These numbers must be interpreted with some caution as they are derived from retrospective comparisons across different databases; nevertheless, it is clear that HDM-ASCT adds to the risk of MDS/AML, likely due to genetic damage engendered by exposure of haematopoietic stem cells to phenylalanine mustard. If such damage can be avoided early in the disease course without compromising myeloma-specific survival, then patients may benefit significantly and increasingly over time.
Fig 1.
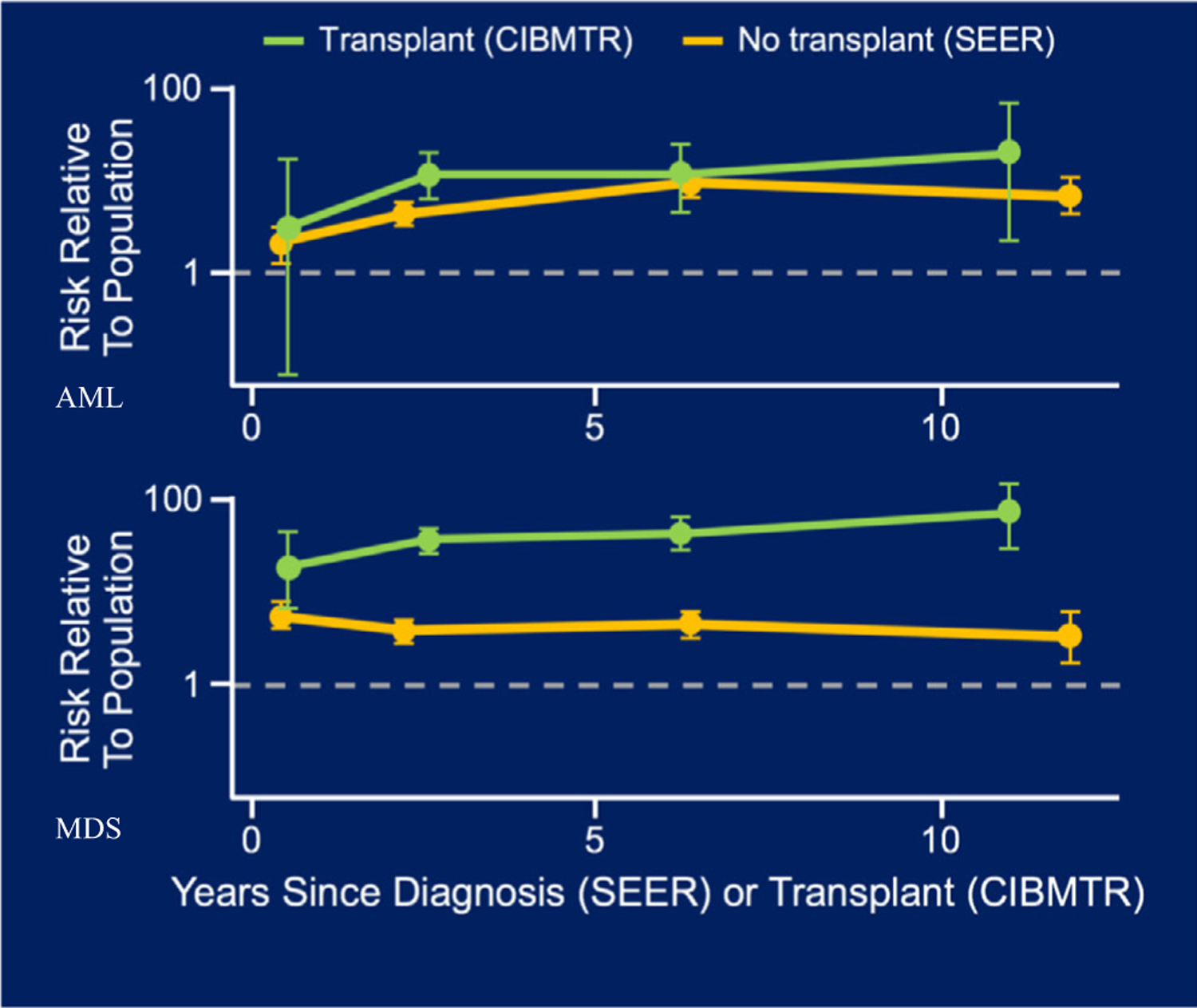
Relative risks of AML and MDS after autologous stem-cell transplant (CIBMTR data) or diagnoses (SEER data) for patients with multiple myeloma; adapted from Radivoyevitch et al.4 Patients receiving autologous transplant are at up to a 50-fold increased risk of developing AML compared to 10-fold and up to 100-fold risk of developing MDS compared to fivefold. CIBMTR, Center for International Blood and Marrow Transplant Research; SEER, Surveillance, Epidemiology and End Results Programme; AML, acute myelogenous leukaemia; MDS, myelodysplastic syndrome.
Importantly, a recent study designed to investigate the genomic landscape and timing of mutational processes shaping MM evolution in a large cohort (N = 1062) of patients evaluated genomic patterns generated by whole genome sequencing and exome sequencing.58 In this study, a new mutational signature (representing c. 20% increased mutational burden) was identified in patients previously exposed to HDM, this new signature was not undetectable in patients never exposed to melphalan. Using longitudinal samples collected from the same patients over time, the study further shows that the mutations caused by HDM persist over time, and can be tracked at every relapse among patients ever exposed to HDM/ASCT. These observations may provide clues as to the biological underpinnings of the aforementioned data indicating that patients with MM previously treated with HDM-ASCT have a 50- to 100-fold higher risk of developing MDS/AML compared to the general population.4
Moreover, as novel agents continue to increase in number and improve outcome for patients, it is possible that a higher incidence of secondary leukaemia in those exposed to HDM will be analogous to the current situation in Hodgkin lymphoma (HL), where patient survival over the past half-century has improved dramatically. As cured HL patients are now living for decades after their diagnosis, an increase in the incidence of secondary malignancies, most notably breast cancer from radiation therapy, has come to light, with breast cancer risk increasing by up to eightfold over that of the general population and persisting for 25 years.59 In one study of HL, the cumulative incidence of secondary cancer at 40 years after treatment was 48·5%.60 Although MM is not yet a curable malignancy, the prognosis has improved dramatically in recent years and will continue to improve as highly efficacious immunotherapies and next-generation novel agents are introduced, which suggests that the principle of avoiding highly mutagenic agents early in the disease course and unless such agents are necessary should be applied.
There may be a subset of patients with MM who are especially predisposed to secondary malignancy (including MDS and AML) and as such, it may be that they should not receive HDM-ASCT. Although this subset remains to be well-defined, apart from patients with pre-existing MDS, this is an area of important research. Interestingly, recent studies have identified the presence of clonal haematopoiesis of indeterminate potential (CHIP) prior to ASCT as a risk factor for various adverse outcomes to include the development of therapy-associated myeloid neoplasms, death from cardiovascular disease, overall non-relapse mortality and inferior survival.61,62,63 It is therefore possible that with additional study in this area, the incorporation of CHIP and/or other measures of genotoxic risk when evaluating patients for ASCT may further help refine practice and improve outcome.
It must be noted that novel agent-based therapies are not without their own risks and toxicities. In addition to the neurotoxicity of bortezomib and the cardiovascular and endothelial risks of carfilzomib previously described, there are risks of thrombosis and infection, among others, which demand rational preventive strategies, patient education and clinical attention on the part of the treating oncologist to minimise serious complications. Additionally, there is also real concern regarding increased risk of second primary malignancy (SPM) with lenalidomide use, albeit it is still associated with an improvement in all-cause mortality. In the updated analysis of the CALGB (Alliance) 100104 study (NCT00114101), for example, patients randomised to maintenance lenalidomide after HDM-ASCT had a 14% incidence of SPM prior to disease progression compared to 5% for patients in the placebo group, with an 8% (vs. 1%) incidence of haematological malignancies, offset by the significant OS advantage in the lenalidomide arm, as well as a doubling of median TTP.64 Importantly, a recent pooled analysis of seven Phase 3 trials that incorporated lenalidomide use in various settings found that it was not lenalidomide per se that was strongly associated with haematological SPM, but rather the interaction between lenalidomide and melphalan.65 Given that lenalidomide-based induction regimens are achieving unprecedented degrees of efficacy and that lenalidomide-based maintenance clearly improves OS, while upfront HDM-ASCT does not, and also that secondary MDS/AML will continue to increase in incidence as myeloma-specific survival continues to improve, it follows if melphalan and lenalidomide together raise the risk of SPM, then the former and not the latter should be appropriately minimised to diminish this risk. Lastly, other contributing factors that may play some synergistic role include baseline clonal haematopoiesis, duration of MM therapy and other prior therapies that patients may have been exposed to.
Lastly, some have suggested that early HDM-ASCT might be more cost effective than a delayed approach. For example, Pandya et al.66 developed a decision analytic model to perform cost-effectiveness analysis to compare early and late HDM-ASCT and found that early HDM-ASCT had a benefit of 1·96 quality-adjusted life years. However, this study evaluated patients receiving HDM-ASCT between the years 2001–2008 when novel therapies were not broadly used and retains limited relevance to the current era.
A tailored approach to HDM/ASCT may be ideal
The introduction of IMiD-PI combination therapies, such as RVd and KRd, has changed the paradigm of treatment for NDMM and challenged the conventional ‘one size fits all’ approach to HDM-ASCT. Clearly, HDM-ASCT has an important place in MM treatment and will continue to serve a role in terms of benefiting those who need it. However, in the current era of novel combination regimens that are leading to deep and lasting remissions, coupled with highly sensitive MRD detection platforms that will hopefully help guide clinical decision-making in the future, it is reasonable to suggest that HDM-ASCT is an approach we can turn to as a treatment option instead of a therapeutic requirement in the immediate future.
Based on the IFM/DFCI 2009 data described previously, MRD negativity appears to be a stronger predictor of favourable outcome than ISS stage, cytogenetic risk, age, or treatment strategy. Accordingly, MRD assessment and its surrogate of high-quality response may have the potential to further optimise choice of therapy. There are limitations of post hoc analyses in this regard, and there is need to validate MRD-based treatment decisions in prospective studies, but the option of keeping transplant in reserve for MRD-negative patients, while also supporting its use in eligible MRD-positive patients if appropriate, appears reasonable.67 Lastly, patient–physician discussion of the known advantages and drawbacks of both approaches is essential, with patient preference and choice a primary driver in decision-making.
With these considerations in mind, based on published data in combination with clinical experience, we suggest that in countries and healthcare systems with access to IMiD-PI combination therapies for NDMM, as well as next-generation options in the salvage setting, the following approach to the NDMM transplant-eligible patient can be proposed:
Transplant-eligible patients with NDMM may be treated with approximately six cycles of IMiD-PI-based therapy such as RVd or KRd, followed by stem cell mobilisation and collection for responding patients, followed by resumption of the treatment regimen for up to 12 cycles in total to maximise depth of response. Clinicians may consider assessing for MRD status prior to stem cell collection. Patients who are MRD negative at stem cell collection can be offered storage of their stem cells for potential future use (delayed HDM-ASCT). Patients who have not yet achieved VGPR or better may be considered for early HDM-ASCT, versus additional cycles of therapy after collection followed by repeat assessment and consideration of HDM-ASCT for suboptimal response. Patients who are MRD-positive at the time of collection, especially those with R-ISS Stage 3 disease at diagnosis, may be considered for early HDM-ASCT, versus additional therapy after collection followed by repeat assessment and consideration of HDM-ASCT for those who remain MRD positive. For patients who do not undergo upfront transplant and based on currently available treatment options, maintenance therapy with lenalidomide at a minimum should be offered and continued until progression or intolerance. It is also reasonable to consider maintenance with IMiD-PI combinations (e.g. RV, KR, or IR), particularly for patients at higher risk.
Importantly, the integration of continued maintenance therapy and sustained MRD negativity for several years has already set the stage for recently developed cessation studies, which have been designed to define optimal long-term strategies (NCT04221178) and are now underway.
Inherently, expert opinion will differ regarding the role of HDM-ASCT in MM. In our opinion, delaying HDM-ASCT is a reasonable and defendable alternative to upfront HDM-ASCT in certain scenarios, although we accept that others may disagree until additional data are reported. Regardless, multiple consensus and institutional guidelines have been published that support the use of delayed HDM-ASCT as an option in the treatment of MM.68 Acknowledging that prospective Phase 3 data remain limited and important randomised data are anticipated within the next several years, we suggest the best data currently available provide a rationale for a tailored approach to HDM-ASCT, recognising also that the treatment paradigm will continue to evolve.
Lastly and most unfortunately, we must evaluate the role of early HDM-ASCT in the context of the current coronavirus disease 2019 (COVID-19) global crisis, which is unprecedented in its scale, extremely high infectious risk, and associated mortality.69 As of 19 May 2020, there have been 1 520 029 confirmed COVID-19 cases and 91 187 deaths in the USA alone, with 4 876 906 cases and 321 593 deaths globally and still climbing as of the time of this writing. HDM-ASCT leads to both immediate and sustained immunosuppression that significantly increases the risk of viral and other infections. Additionally, HDM-ASCT requires prolonged hospitalisation at most transplant centres, which in the current crisis may place the patient at considerable risk of nosocomial exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as challenges to optimal care by the additional demands placed on hospital staff. For these reasons, it is our opinion that the large majority of early HDM-ASCTs should be delayed until abatement of the current crisis at the very earliest. Given the high degree of uncertainty regarding the durability and timeline of the SARS-CoV-2 threat, uncertainty which obviously was not present during the execution of MM clinical trials published to date but which must now be taken into account, we suggest that any decision to take a patient to HDM-ASCT after initial relaxing of social distancing measures must be made with caution and careful consideration of all treatment options, as well as the possibility of an undulating and variable COVID-19 incidence over the coming months to years.
These concerns are further reflected in the current ASH recommendations regarding COVID-19, which state that for transplant-eligible patients, ‘delaying the stem cell transplant (including HSPC [haematopoietic stem/progenitor cells] collection and storage) till the pandemic abates is recommended’.70 Likewise, the International Myeloma Society (IMS) advises that early HDM-ASCT ‘should be postponed, if possible’ and that patients who do proceed with HDM-ASCT be tested for COVID-19 prior to HDM-ASCT.71 Lastly, current re-immunisation programmes for melphalan-induced inactivation of prior vaccines recommend that vaccination (including pertussis, diphtheria, tetanus, haemophilus influenzae, pneumococcal, and hepatitis A and B) begin 12 months after HDM-ASCT.72 When a safe and efficacious SARS-CoV-2 vaccine becomes available, it will need to be added to these re-immunisation programmes accordingly.
Conclusion
In the modern era of MM treatment, increasing numbers of patients are entering deep and sustained remissions without the need for high-dose chemotherapy. IMiD-PI-based triplet regimens followed by maintenance have become a new standard of care for NDMM, and IMiD-PI-mAb-based quadruplet regimens may further advance this standard in the near future. Similarly, as part of clinical research in this setting, MRD negativity appears to predict for good outcome regardless of treatment modality. Highly sensitive MRD detection methods are becoming available for clinical use and may augment other important advances in risk stratification, including the continued refinement of disease-related genetics, although the real-time utility of MRD has yet to be validated by prospective randomised studies. As the results from MRD-enhanced clinical trials and similar studies identifying best treatments for individual HDM-ASCT-eligible patients with NDMM are reported, a dynamic and adaptive approach to therapy that embodies a long-term and strategic view of their disease is now justified, and not least in the context of the current COVID-19 global pandemic.
Acknowledgements
We sincerely appreciate academic discussion and feedback from Drs Neha Korde and Sham Mailankody with the original draft (Memorial Sloan Kettering, Myeloma Service). Funding support for this publication was provided by the Memorial Sloan Kettering Core Grant (P30 CA008748) and the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the editorial assistance of Jack Sparacino, in part supplemented by the RJ Corman Multiple Myeloma Research Fund.
Footnotes
Conflict of interests
Ola Landgren has received research funding from: National Institutes of Health (NIH), USA Food and Drug Administration (FDA), Multiple Myeloma Research Foundation (MMRF), International Myeloma Foundation (IMF), Leukemia and Lymphoma Society (LLS), Perelman Family Foundation, Rising Tides Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; Honoraria/ad boards: Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno, Pfizer; and serves on Independent Data Monitoring Committees (IDMCs) for clinical trials lead by Takeda, Merck, Janssen, Theradex. Paul G. Richardson has received research support from Oncopeptides, Celgene/BMS, Takeda, and serves on advisory committees for Karyopharm, Oncopeptides, Celgene/BMS, Takeda, Sanofi and Janssen. Clifton C. Mo serves on advisory boards for Celgene/BMS, Karyopharm and GSK.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019;394:29–38. [DOI] [PubMed] [Google Scholar]
- 3.Voorhees PM, Kaufman JL, Laubach JP, Sborov DW, Reeves B, Rodriguez C, et al. Depth of response to daratumumab (DARA), lenalidomide, bortezomib, and dexamethasone (RVd) improves over time in patients (pts) with transplant-eligible newly diagnosed multiple myeloma (NDMM): Griffin Study update. Blood 2019;134(Suppl. 1):691. [Google Scholar]
- 4.Radivoyevitch T, Dean RM, Shaw BE, Brazauskas R, Tecca HR, Molenaar RJ, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome after autotransplants for lymphomas and plasma cell myeloma. Leukemia Res 2018;74:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay F, Engelhardt M, Terpos E, Wasch R, Giaccone L, Auner HW, et al. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica 2018;103:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN). Multiple Myeloma, Version 2.2020 Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed May 2020.
- 7.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010;116:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119:4375–82. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013–04 trial. Blood 2016;127:2569–74. [DOI] [PubMed] [Google Scholar]
- 10.Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017;389:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival With carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol 2018;36:728–34. [DOI] [PubMed] [Google Scholar]
- 12.Korde N, Mailankody S, Smith EL, Lendvai N, Hassoun H, Lesokhin A, et al. MRD response-driven phase I/II study for newly diagnosed multiple myeloma patients using higher doses of twice-weekly carfilzomib (45 and 56 mg/m2) in combination with lenalidomide and dexamethasone. Blood 2017;130:3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration Approval Summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 2006;12:2955–60. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia 2011;26:595. [DOI] [PubMed] [Google Scholar]
- 15.Kazandjian D, Korde N, Mailankody S, Hill E, Figg WD, Roschewski M, et al. Remission and progression-free survival in patients with newly diagnosed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone: Five-year follow-up of a phase 2 clinical trial. JAMA Oncol 2018;4:1781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avet-Loiseau H, Fonseca R, Siegel D, Dimopoulos MA, Špička I, Masszi T, et al. Carfilzomib significantly improves the progression-free survival of high-risk patients in multiple myeloma. Blood 2016;128:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 2012;120:1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roussel M, Lauwers-Cances V, Robillard N, Belhadj K, Facon T, Garderet L, et al. Frontline therapy with carfilzomib, lenalidomide, and dexamethasone (KRd) induction followed by autologous stem cell transplantation, KRd consolidation and lenalidomide maintenance in newly diagnosed multiple myeloma (NDMM) patients: primary results of the Intergroupe Francophone Du MyéLome (IFM) KRd phase II study. Blood 2016;128:1142. [Google Scholar]
- 19.Zimmerman T, Raje NS, Vij R, Reece D, Berdeja JG, Stephens LA, et al. Final results of a phase 2 trial of extended treatment (tx) with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (KRd) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM). Blood 2016;128:675. [Google Scholar]
- 20.Richardson PG, San Miguel JF, Moreau P, Hajek R, Dimopolous MA, Laubach JP, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-word setting. Blood Cancer J 2018;8:109. 10.1038/s41408-018-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017;376:1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. (2012) Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med, 366, 1770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gay F, Cerrato C, Rota Scalabrini D, Galli M, Belotti A, Zamagni E, et al. Carfilzomib-lenalidomide-dexamethasone (KRd) induction-autologous transplant (ASCT)-KRd consolidation vs KRd 12 cycles vs carfilzomib-cyclophosphamide-dexamethasone (KCd) induction-ASCT-KCd consolidation: analysis of the randomized FORTE trial in newly diagnosed multiple myeloma (NDMM). Blood 2018;132(Suppl.):121. [Google Scholar]
- 24.Lendvai N, Tsakos I, Devlin SM, Schaffer WL, Hassoun H, Lesokhin AM, et al. Predictive biomarkers and practical considerations in the management of carfilzomib-associated cardiotoxicity. Leukemia Lymphoma 2018;59:1981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah C, Bishnoi R, Jain A, Bejjanki H, Xiong S, Wang Y, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leukemia Lymphoma 2018;59:2557–69. [DOI] [PubMed] [Google Scholar]
- 26.Chari A, Stewart AK, Russell SD, Moreau P, Herrmann J, Banchs J, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv 2018;2:1633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Ixazomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma: long-term follow-up including ixazomib maintenance. Leukemia 2019;33:1736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson PG, Hofmeister CC, Rosenbaum CA, Htut M, Vesole DH, Berdeja JG, et al. Twice-weekly ixazomib in combination with lenalidomide-dexamethasone in patients with newly diagnosed multiple myeloma. Br J Haematol 2018;182:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voorhees PM, Kaufman JL, Laubach JP, Sborov DW, Reeves B & Rodriguez C, et al. Daratumumab + lenalidomide, bortezomib & dexamethasone improves depth of response in transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Clin Lymphoma Myeloma Leuk 2019;19(Suppl.):E353–4. [Google Scholar]
- 30.Landgren O, Hultcrantz M, Lesokhin A, Mailankody S, Hassoun H, Smith EL, et al. Weekly carfilzomib, lenalidomide, dexamethasone and daratumumab (wKRd-D) combination therapy provides unprecedented MRD negativity rates in newly diagnosed multiple myeloma: a clinical and correlative phase 2 study. Blood 2019;134(Suppl. 1):862. [Google Scholar]
- 31.Costa LJ, Chhabra S, Godby KN, Medvedova E, Cornell RF, Hall AC, et al. Daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd) induction, autologous transplantation and post-transplant, response-adapted, measurable residual disease (MRD)-based Dara-KRd consolidation in patients with newly diagnosed multiple myeloma (NDMM). Blood 2019;134(Suppl.):860.31320380 [Google Scholar]
- 32.Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet 2019;394:2096–107. [DOI] [PubMed] [Google Scholar]
- 33.Weisel K, Asemissen AM, Schieferdecker A, Besemer B, Zago M, Mann C, et al. Isatuximab, carfilzomib, lenalidomide and dexamethasone (I-KRd) in front-line treatment of high-risk multiple myeloma: results of the safety run-in cohort in the phase II, multicenter GMMG-CONCEPT trial. Clin Lymphoma Myeloma Leuk 2019;19(Suppl.):e17. [Google Scholar]
- 34.Ocio EM, Rodriguez Otero P, Bringhen S, Oliva S, Nogai A, Attal M, et al. Preliminary results from a phase I study of isatuximab (ISA) in combination with bortezomib, lenalidomide, dexamethasone (VRd), and in patients with newly diagnosed multiple myeloma (NDMM) non-eligible for transplant. Blood 2018;132(Suppl. 1):595. [Google Scholar]
- 35.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014;371:895–905. [DOI] [PubMed] [Google Scholar]
- 36.Cavo M, Gay FM, Patriarca F, Zamagni E, Montefusco V, Dozza L, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood 2017;130:401. [Google Scholar]
- 37.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 1998;92:3131–6. [PubMed] [Google Scholar]
- 38.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996;335:91–7. [DOI] [PubMed] [Google Scholar]
- 39.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003;348:1875–83. [DOI] [PubMed] [Google Scholar]
- 40.Segeren CM, Sonneveld P, van der Holt B, Vellenga E, Croockewit AJ, Verhoef GE, et al. Overall and event-free survival are not improved by the use of myeloablative therapy following intensified chemotherapy in previously untreated patients with multiple myeloma: a prospective randomized phase 3 study. Blood 2003;101:2144–51. [DOI] [PubMed] [Google Scholar]
- 41.Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 2004;104:3052–7. [DOI] [PubMed] [Google Scholar]
- 42.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol 2005;23:9227–33. [DOI] [PubMed] [Google Scholar]
- 43.Bladé J, Rosiñol L, Sureda A, Ribera JM, Díaz-Mediavilla J, García-Laraña J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood 2005;106:3755–9. [DOI] [PubMed] [Google Scholar]
- 44.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol 2006;24:929–36. [DOI] [PubMed] [Google Scholar]
- 45.Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 2015;16:1617–29. [DOI] [PubMed] [Google Scholar]
- 46.Gay F, Cerrato C, Petrucci MT, Zambello R, Gamberi B, Ballanti S, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol 2019;37(Suppl.):8002. [Google Scholar]
- 47.Jain T, Sonbol MB, Firwana B, Kolla KR, Almader-Douglas D, Palmer J, et al. High dose chemotherapy with early autologous stem cell transplantation compared to standard dose chemotherapy or delayed transplantation in patients with newly diagnosed multiple myeloma: a meta-analysis. Biol Blood Marrow Transplant 2018;24:S48. [DOI] [PubMed] [Google Scholar]
- 48.Dhakal B, Szabo A, Chhabra S, Hamadani M, D’Souza A, Usmani SZ, et al. Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: a systematic review and meta-analysis. JAMA Oncol 2018;4:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landgren O, Owen RG. Better therapy requires better response evaluation: paving the way for minimal residual disease testing for every myeloma patient. Cytometry B Clin Cytom 2016;90:14–20. [DOI] [PubMed] [Google Scholar]
- 50.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol 2017;3:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant 2016;51:1565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn TE, Wallace PK, Fraser R, Fei M, Tario JD, Howard A, et al. Minimal residual disease (MRD) assessment before and after autologous hematopoietic stem cell transplantation (autoHCT) and maintenance for multiple myeloma (MM): results of the prognostic immunophenotyping for myeloma response (PRIMeR) study. Biol Blood Marrow Transplant 2019;25:S4–S6. [Google Scholar]
- 53.Perrot A, Cances-Lauwers V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018;132:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naumann-Winter F, Greb A, Borchmann P, Bohlius J, Engert A, Schnell R. First-line tandem high-dose chemotherapy and autologous stem cell transplantation versus single high-dose chemotherapy and autologous stem cell transplantation in multiple myeloma, a systematic review of controlled studies. Cochrane Database Syst Rev 2012;10:Cd004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roussel M, Hebraud B, Hulin C, Perrot A, Caillot D, Macro M, et al. The impact of lenalidomide, bortezomib, and dexamethasone treatment on health-related quality of life in transplant-eligible patients with newly-diagnosed multiple myeloma: results from the IFM/DFCI 2009 trial. Blood 2018;132:716. [DOI] [PubMed] [Google Scholar]
- 56.Wang XS, Shi Q, Williams LA, Shah ND, Mendoza TR, Cohen EN, et al. Longitudinal analysis of patient-reported symptoms post-autologous stem cell transplant and their relationship to inflammation in patients with multiple myeloma. Leukemia Lymphoma 2015;56:1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mailankody S, Pfeiffer RM, Kristinsson SY, Korde N, Bjorkholm M, Goldin LR, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011;118:4086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rustad EH, Yellapantula V, Leongamornlert D, Bolli N, Ledergor G, Nadeu F, et al. Timing the initiation of multiple myeloma. Nat Commun 2020;11:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, et al. Breast cancer following radiotherapy and chemotherapy among young women with hodgkin disease. JAMA 2003;290:465–75. [DOI] [PubMed] [Google Scholar]
- 60.Schaapveld M, Aleman BMP, van Eggermond AM, Janus CPM, Krol ADG, van der Maazen RWM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s Lymphoma. N Engl J Med 2015;373:2499–511. [DOI] [PubMed] [Google Scholar]
- 61.Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol 2017;35:1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mouhieddine TH, Park J, Redd RA, Gibson CJ, Manier S, Nassar AH, et al. The role of clonal hematopoiesis of indeterminate potential (CHIP) in multiple myeloma: immunomodulator maintenance post autologous stem cell transplant (ASCT) predicts better outcome. Blood 2018;132:749. [Google Scholar]
- 63.Slavin TP, Teh JB, Weitzel JN, Peng K, Wong FL, Qin H, et al. Association between clonal hematopoiesis and late nonrelapse mortality after autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019;25:2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holstein SA, Jung SH, Richardson PG, Hofmeister CC, Hurd DD, Hassoun H, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol 2017;4:e431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol 2014;15:333–42. [DOI] [PubMed] [Google Scholar]
- 66.Pandya C, Hashmi S, Khera N, Gertz MA, Dispenzieri A, Hogan W, et al. Cost-effectiveness analysis of early vs. late autologous stem cell transplantation in multiple myeloma. Clin Transplant 2014;28:1084–91. [DOI] [PubMed] [Google Scholar]
- 67.Landgren O, Giralt S. MRD-driven treatment paradigm for newly diagnosed transplant eligible multiple myeloma patients. Bone Marrow Transplant 2016;51:913–4. [DOI] [PubMed] [Google Scholar]
- 68.Kazandjian D, Dew A, Hill E. The changing role of high dose melphalan with stem cell rescue in the treatment of newly diagnosed multiple myeloma in the era of modern therapies—back to the future! Best Pract Res Clin Hematol 2020;33:101150. [DOI] [PubMed] [Google Scholar]
- 69.Siddiqui HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical—therapeutic staging proposal. J Heart Lung Transplant 2020;39:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American Society of Hematology. COVID-19 Resources, 2020 Available at: https://www.hematology.org/covid-19/covid-19-and-multiple-myeloma Accessed 24 April 2020.
- 71.International Myeloma Society. Recommendations for the management of myeloma patients during the COVID-19 pandemic, 2020 Available at: http://cms.cws.net/content/beta.myelomasociety.org/files/IMS%20recommendations%20for%20Physicians%20Final.pdf Accessed 24 April 2020.
- 72.Palazzo M, Shah GL, Copelan O, Seier K, Devlin SM, Maloy M, et al. Revaccination after autologous hematopoietic stem cell transplantation is safe and effective in patients with multiple myeloma receiving lenalidomide maintenance. Biol Blood Marrow Transplant 2018;24:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


