Abstract
Multiple isoforms of the protein tyrosine phosphatase CD45 are expressed on the surface of human T cells. Interestingly, the expression of these isoforms has been shown to vary significantly upon T-cell activation. In this report, we describe a novel cell line-based model system in which we can mimic the activation-induced alternative splicing of CD45 observed in primary T cells. Of the many proximal signaling events induced by T-cell stimulation, we show that activation of protein kinase C and activation of Ras are important for the switch toward the exclusion of CD45 variable exons, whereas events related to Ca2+ flux are not. In addition, the ability of cycloheximide to block the activation-induced alternative splicing of CD45 suggests a requirement for de novo protein synthesis. We further demonstrate that sequences which have previously been implicated in the tissue-specific regulation of CD45 variable exons are likewise necessary and sufficient for activation-induced splicing. These results provide an initial understanding of the requirements for CD45 alternative splicing upon T-cell activation, and they confirm the importance of this novel cell line in facilitating a more detailed analysis of the activation-induced regulation of CD45 than has been previously possible.
Alternative pre-mRNA splicing is a process by which multiple functionally distinct proteins may be encoded from a single gene through the variable inclusion of individual exons. In general, variable exon inclusion is regulated by proteins which bind to sequences within the regulated gene and influence the recognition of splice sites by the basic splicing machinery, or spliceosome (for recent reviews, see references 1, 4, 6, and 18). Frequently, alternative splicing is regulated in a tissue- or developmental stage-specific fashion through the activity of tissue-specific factors (17, 60). In addition, there have been several documented examples of exon inclusion being regulated in response to extracellular stimuli, including stimulation of the T-cell receptor (TCR) (10, 21, 47, 51, 61, 65). However, the mechanisms by which such signaling events can influence pre-mRNA splicing are largely unexplored.
Engagement of the TCR by ligand initiates a signal transduction cascade within the T cell which ultimately results in a number of morphological and functional changes in the cell (11, 62). One such change is a dramatic alteration in the cell surface expression of isoforms of the transmembrane protein tyrosine phosphatase CD45 (for reviews see references 57 and 58). The gene encoding CD45 encompasses 33 exons. Most of these exons, including those which encode the intracellular phosphatase domains, are constitutively included in the mature mRNA. However, three of the exons which encode part of the extracellular domain (exons 4, 5, and 6) are variably excluded from the mRNA. The peptide sequences encoded by the CD45 variable exons are rich in O-linked glycosylation sites; thus, a change in inclusion of these exons from the mRNA results in a dramatic change in the size and structure of the resulting CD45 protein (33).
No ligand has yet been identified for CD45; however, the use of chimeric molecules has demonstrated that activity of the phosphatase domain of CD45 is influenced by homodimerization, suggesting that ligand binding might regulate CD45 phosphatase activity (14, 31). Moreover, the largest and smallest CD45 isoforms have been shown to differ in the ability to associate with the TCR (26, 27). In T cells, CD45 functions to maintain Lck in an active conformation by removing an inhibitory phosphate from this kinase (34, 36, 37, 48, 50). Because Lck function is critical for early events in T-cell development and for activation, CD45 phosphatase activity is likewise required for both activation and development (7, 20, 22–24, 52). Thus, although the functional consequence of alternative isoform expression of CD45 is not yet clear, it is likely that the various isoforms differentially influence T-cell function due to a difference in their abilities to interact with ligand, with one another, or with the TCR.
CD45 is expressed in all nucleated hematopoietic cell types. Whereas CD45 surface expression changes frequently in T cells during development and upon activation, B cells only ever express the largest CD45 isoforms (58). The difference in CD45 surface expression between B cells and thymocytes (which express predominantly the smallest CD45 isoforms) has clearly been shown to be a result of regulated alternative splicing. These two cell types, and cell lines derived from each, show a marked difference in their expression of CD45 mRNA variants (39, 53, 54). Furthermore, this difference in mRNA expression requires sequences within and flanking the variable exons and is mediated by some, yet undefined, cell-specific factors (39, 42, 54, 59). An additional conclusion from these studies is that although exons 4, 5, and 6 are all variably included in CD45 mRNA, only the inclusion of exons 4 and 6 appears to be tightly regulated. Inclusion of exon 5, by contrast, is most likely a stochastic event.
Despite the progress in understanding the tissue-specific regulation of CD45 expression, characterization of activation-mediated changes in CD45 isoform expression in T cells has been significantly more limited. One reason for the limited understanding of activation-induced changes in CD45 expression is that all previous studies have analyzed primary T cells, which are not easily propagated or transfected and do not represent a homogeneous population (2, 5, 28, 40). In this report we describe a cell line-based assay which faithfully reproduces the activation-induced alternative splicing of CD45 which is observed in primary T cells. Using this cell line, we show that activation-induced exclusion of the CD45 variable exons is mediated via a protein kinase C (PKC)-dependent signaling pathway, which can be mimicked by constitutive activation of Ras. We rule out the possibility that CD45 alternative splicing is a general result of stimulation of PKC by demonstrating that treatment of several B cell lines with phorbol myristate acetate (PMA) has no effect on CD45 splicing, indicating some level of specificity to the response in T cells. In addition, synthesis of an activation-specific factor(s) is required for CD45 regulation, as indicated by the inhibition of the activation-induced alternative splicing of CD45 by treatment with cycloheximide. Last, we demonstrate that sequences within and flanking exon 4, which have previously been shown to be important for the cell-type-specific regulation of CD45 splicing, are similarly sufficient for mediating activation-induced splicing. In the future, this cell line should allow for a more detailed mechanistic study of CD45 splicing than is possible with primary cells, thereby leading to a greater understanding of how T-cell activation, and signaling events in general, may regulate alternative splicing.
MATERIALS AND METHODS
PBL isolation.
Primary blood lymphocytes (PBLs) enriched for CD4+ CD3+ T cells were isolated from 100 ml of human blood as previously described (55). Total PBLs were first isolated by diluting the blood with an equal volume of phosphate-buffered saline (PBS) and spinning through a Ficoll gradient. After two washes with PBS, the PBLs were incubated for 30 min at 4°C in PBS plus antibodies specific for CD16, CD19, CD11b, CD45R0, CD8 (all purchased from Becton Dickinson, Mountain View, Calif.), CD14 (3C10 hybridoma from the American Type Culture Collection [ATCC]), MHCII (IVA12 hybridoma from ATCC), and erythrocytes (10F7 hybridoma from ATCC). Cells were pelleted, washed once in PBS, and then incubated again for 30 min at 4°C in PBS plus 1 ml of magnetic beads coated with sheep anti-mouse immunoglobulin G (IgG) antibodies (Dynal Inc., Great Neck, N.Y.). Following incubation, the sample was exposed to a magnet for several minutes to isolate the beads. The remaining cells were removed and saved for further use. Flow cytometry of the resulting cell population indicated that approximately 80% of the remaining cells expressed CD3 and CD4.
JSL1 isolation.
Jurkat cells were plated in 96-well plates at concentrations of 1, 5, and 15 cells/ml and allowed to grow for 2 weeks. Wells which contained single colonies were expanded, and cells were analyzed by flow cytometry for expression of CD3 and CD45RA. All clones exhibited good expression of CD3. Four clones which expressed the highest levels of CD45RA were grown in medium with or without PMA plus ionomycin (100 ng/ml and 1 μM, respectively), and RNA was harvested and analyzed by reverse transcription (RT)-PCR. At least two of these clones (JSL1 and JSL4) showed a change in CD45 isoform expression in response to stimulation. Flow cytometry was performed as described previously (31), using indicated antibodies from Becton Dickinson.
Cell culture and stimulations.
JSL1, Raji, Daudi, and Ramos cells were maintained in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 2 mM glutamine, penicillin, and streptomycin. The PBLs were cultured in similar medium except that fetal calf serum was added to 10%. All cells were grown at 37°C in the presence of 5% CO2. For stimulations, cells were diluted to 3 × 105 cells/ml and incubated in medium alone or with the specified additions for the times indicated.
RT-PCR assay.
Total RNA from PBLs and transfected JSL1 cells was harvested by using an RNeasy kit (Qiagen, Valencia, Calif.) following the standard protocol. Total RNA from all other cells was isolated by using RNAzol (Tel-Test, Friendswood, Tex.) according to the included protocol. For RT-PCR analysis of endogenous CD45, 1.5 μg of total RNA was heated to 90°C in the presence of 1 ng of RT primer (Fig. 1A), 300 mM NaCl, 10 mM Tris (pH 7.5), and 2 mM EDTA and allowed to cool to 43°C. This annealed reaction was diluted into an RT mix containing final concentrations of 10 mM Tris (pH 7.5), 6 mM MgCl2, 10 mM dithiothreitol, 50 mM NaCl, and 1 mM deoxynucleoside triphosphates, and incubation was continued at 43°C for 30 min. The RT reaction was stopped by boiling samples for 5 min and then placing them on ice. For PCR, a third of the RT reaction mixture was diluted into a PCR mix containing final concentrations of 1.5 mM MgCl2, 10 mM Tris (pH 8), 50 mM KCl, 0.2 mM deoxynucleoside triphosphates, 20 ng of RT primer, and 10 ng each of primers V and C (Fig. 1A) and overlaid with mineral oil. PCR was done by heating samples to 94°C for 2 min followed by 20 cycles of 1 min at 94°C, 1 min at 70°C, and 2 min at 72°C. RT-PCR analysis of minigene RNA was done similarly except that primers MG5 and MG3, which anneal in expressed vector sequences which flank the 5′ and 3′ ends of the minigene, respectively, replaced primers V and RT, and primer MT, which anneals to exon 7, replaced primer C. Also, PCR was limited to 16 cycles. All of the above conditions were determined empirically to give a signal which was linear with respect to input RNA. Following completion of PCR, the reaction products were extracted with phenol-chloroform-isoamyl alcohol and ethanol precipitated in the presence of glycogen as carrier. The resulting pellets were resuspended in formamide loading buffer and resolved on a 6% denaturing polyacrylamide gel.
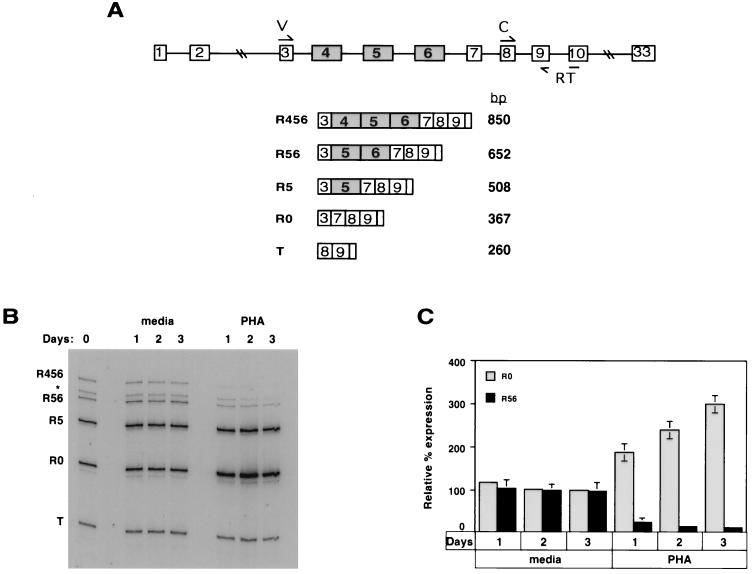
FIG. 1.
Detection of CD45 mRNA in resting and stimulated PBLs reveals an activation-dependent change in mRNA isoform expression. (A) Schematic of the CD45 gene (top) and predicted RT-PCR products (bottom). Exons are designated by boxes, and introns are designated by lines. The annealing sites for the primers used for RT-PCR are indicated in the CD45 gene. Primer RT was used in the RT step, and all three primers (V, C, and RT) were used for PCR. (B) Representative autoradiogram of an RT-PCR assay. Total RNA was harvested from CD4+ CD3+-enriched PBLs which either were freshly isolated (day 0) or had been cultured for the time indicated in either medium alone or medium supplemented with PHA (1 mg/ml). Primers V and C were both 5′ end labeled with 32P, such that following PCR the products could be separated on a denaturing polyacrylamide gel and visualized by autoradiography. The identity of each product was confirmed by sequencing. The asterisk indicates a product we believe to be R45, but we have been unable to confirm this inference due to contamination with R56. (C) Quantitation of three experiments with T-cell-enriched PBLs. The R56/T and R0/T ratios are calculated for each condition and compared to these ratios at day 0, set as 100%. Quantitation of all RT-PCR assays was done with a PhosphorImager.
Plasmids and reagents.
MG4 was synthesized by ligating three fragments containing (i) exons 2 and 3 fused together with 130 bp of downstream intron sequence, (ii) exon 4 and flanking sequence, and (iii) exon 7 and flanking sequence and then inserting into pGem7Z. The above fragments were generated by PCR using pSV-MiLCA2 (a kind gift of H. Saito) (54) as a template. The full minigene was then removed from pGem7Z and inserted into the neomycin-resistant expression vector pAWneo3 (14). MG4M was made from MG4 by using a QuickChange kit (Stratagene, La Jolla, Calif.) to engineer the point mutation. pEF-TacT was made by insertion of a truncated version of CD25 (TT-ɛT [29]) into the expression plasmid pEFBos (35). AP1-Luc and RasV12 constructs were described previously (12, 49). The inhibitor R0-31-8220 was purchased from Calbiochem (La Jolla, Calif.).
Transfections and luciferase assay.
Transfections were done as previously described (64). For stable cell lines expressing minigenes, cells were recovered for 3 days in RPMI 1640–10% serum, then serially diluted into medium containing 2 mg of geneticin per ml, and grown for an additional 2 to 3 weeks; 24 geneticin-resistant clones were then expanded further and analyzed for minigene expression by RT-PCR. For RasV12 transfections and purification, 100 × 106 cells distributed in five cuvettes were transfected for each sample. Each cuvette also contained 20 μg of pEF-TacT, 10 μg of AP1-Luc, and given amounts of RasV12. Following transfection, each sample was pooled and recovered in RPMI 1640–10% serum. For purification, transfected cells were harvested, washed in PBS, and incubated with unconjugated CD25-specific antibody (Becton Dickinson). Cells were then washed again and incubated with magnetic beads coated with sheep anti-mouse IgG antibodies (Dynal). Following incubation, the sample was exposed to a magnet for several minutes to isolate the beads, and bound cells were lysed and RNA was harvested as described above. Flow cytometry indicated lysed cells contained over 90% CD25+ cells. The luciferase assay was done 12 h posttransfection as described previously (64).
Western blotting.
Erk blotting was done as described previously (66), using rabbit polyclonal anti-Erk (25).
RESULTS
CD45 mRNA expression is altered by activation of primary lymphocytes.
We first wanted to analyze the CD45 mRNA profile from resting and stimulated primary T cells, in order to confirm that we could detect an activation-dependent change in CD45 splicing. To directly assay the expression of the endogenous CD45 spliced variants, we used a sensitive RT-PCR assay in which the RT reaction was followed by a limiting number of PCR cycles, such that the resulting signal was linear with respect to input RNA (see the legend to Fig. 1 and Materials and Methods). Consistent with previous reports (38, 39), we reproducibly detect four or five distinct CD45 mRNA isoforms in human cells (Fig. 1A and B). The identity of these isoforms has been confirmed by subcloning and sequencing the individual RT-PCR products. To normalize the expression levels of each isoform, we used an internal control (T) which is representative of the level of total spliced CD45 mRNA. Although we are unable to quantitate the absolute amount of each splice variant by this assay, we are able to quantitate the variation of a given mRNA species, relative to product T, under different conditions.
To determine the effect of stimulation on CD45 mRNA expression, we first isolated PBLs which were enriched for CD4+ CD3+ T cells (see Materials and Methods). After isolation, these cells were cultured for several days in medium alone or in the presence of the T-cell-specific mitogen phytohemagglutinin (PHA). The relative levels of the CD45 mRNA isoforms showed little variation over time when cultured in medium alone. However, stimulation of T cells with PHA for up to 3 days resulted in a threefold increase in the relative levels of the R0 isoform, with a concomitant decrease in the largest (R56 and R456) isoforms (Fig. 1B and C). Previous studies have documented that cell surface expression of the largest CD45 protein isoform is restricted to B cells (58). We cannot distinguish between the possibilities that our detection of the corresponding RNA isoform (R456) is truly indicative of the presence of this mRNA variant in T cells, as opposed to a result of B cell contamination of our T-cell-enriched PBLs. Therefore, for the purposes of quantitation of experiments done with PBLs, we have focused on the relative change in the R0 and R56 isoforms. The results presented in Fig. 1B and C are highly similar to those reported recently (28) and are consistent with the well-established activation-induced changes in CD45 protein expression (2, 5). Thus, we conclude that the activation-dependent change in CD45 isoform expression is indeed mediated, at least in large part, by alternative splicing.
A novel system for studying activation-induced CD45 alternative splicing.
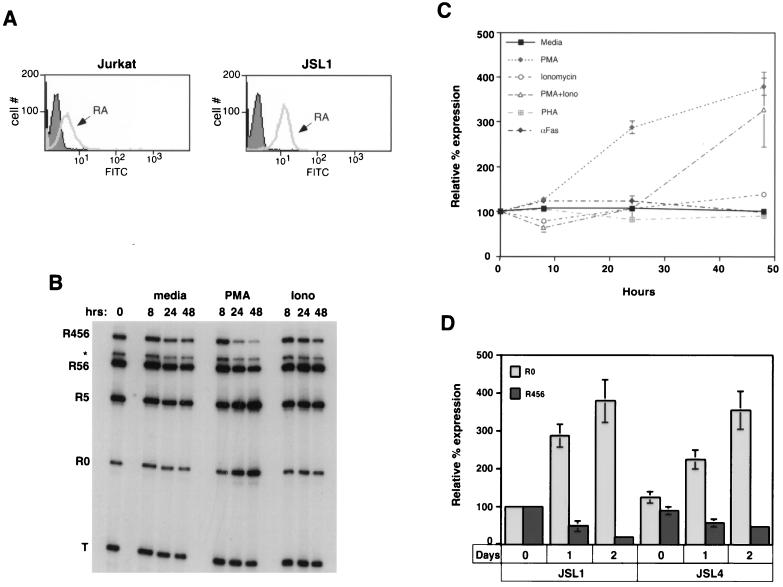
Understanding the mechanisms involved in the activation-induced change in CD45 splicing ideally requires the ability to observe and study CD45 alternative splicing in a homogeneous population of cells which are easily maintained in culture, expanded readily, are capable of being transfected. However, no T-cell line has been reported to show a change in CD45 splice variants upon stimulation. This is in part because most T-cell lines constitutively express a CD45 mRNA profile which resembles an immature thymocyte or activated primary cell (i.e., they express primarily the R0 isoform, with little or no detectable R56 and R456 isoforms) (41, 53). To isolate a cell line which could be used to study the activation-dependent alternative splicing of CD45, we performed a limiting dilution of T lymphoma-derived Jurkat cells to isolate individual clones. Jurkat cells are an attractive basis for a model system because they are well characterized and resemble naive primary T cells in their response to stimulation (63). As a population, Jurkat cells primarily express the smaller isoforms of CD45; however, studies have indicated that in vivo some T cells undergo spontaneous interconversion from expression of smaller to larger CD45 isoforms (3, 40). Therefore, we reasoned that a subpopulation of Jurkat cells may express the larger isoforms of CD45. Following limiting dilution, we screened 30 individual clones by flow cytometry for surface expression of CD45RA isoforms (proteins encoded by mRNAs containing exon 4). Six clones showed a marked increase in RA expression over that of the starting population of Jurkat cells (Fig. 2A and data not shown). We then assayed for CD45 mRNA expression in four of these six RA+ clones by RT-PCR. Figure 2B shows the CD45 mRNA profile of one of the clones that we identified, designated JSL1 (for Jurkat splicing line 1), which expressed relatively high levels of the larger CD45 isoforms relative to R0. This cell line has now undergone multiple freeze-thaw cycles and been maintained in culture up to several months at a time without any significant change in CD45 isoform expression or response to stimulation (data not shown).
FIG. 2.
CD45 pre-mRNA undergoes alternative splicing upon treatment of the JSL1 cell line with PMA. (A) Fluorescence histogram following staining of total Jurkat or JSL1 cells with either IgG-fluorescein isothiocyanate FITC (filled area) or anti-CD45RA-FITC (gray line) antibodies. The IgG-FITC provides a negative control for nonspecific fluorescence. Ten thousand events of each cell type were analyzed by flow cytometry. (B) Representative RT-PCR assay of JSL1 cells which were treated for the indicated time with medium alone or medium supplemented with PMA (100 ng/ml) or ionomycin (Iono; 1 μM). (C) Effect of multiple treatments on R0 expression in JSL1 cells. For these experiments, the concentrations used were 100 ng/ml for PMA, 1 μM for ionomycin, 1 mg/ml for PHA, and 1 ng/ml for αFas. Quantitation was done as described for Fig. 1, and results are summarized from at least three independent experiments for each condition. The effect of these treatments on the expression of the larger isoforms (namely R456) was reciprocal to their effect on R0 expression but is not shown for simplicity. (D) Quantitation of isoform expression (as described in the legend to Fig. 1) upon stimulation of JSL1 or JSL4 cells with PMA for 0, 1, or 2 days. Isoform expression is presented relative to that in JSL1 cells at time zero and is a summary of at least three independent experiments.
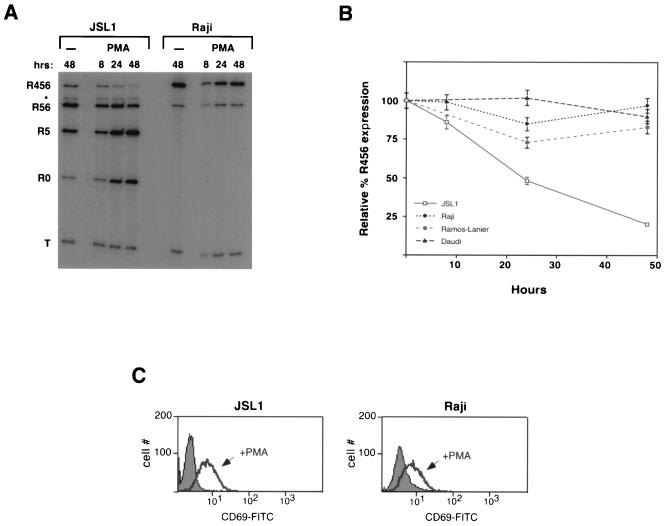
To determine whether the JSL1 cells showed an activation-dependent change in CD45 isoform expression similar to that observed in primary T cells, we treated these cells with a number of stimuli. When one is working with the JSL1 cells there is no possibility for B-cell contamination; therefore, since we are particularly interested in the regulation of exon 4, we focused on loss of expression of R456 instead of R56. However, we note that in both primary cells and JSL1 cells, the R456 and R56 isoforms follow similar patterns of regulation. Treatment of T cells with PMA and ionomycin, which stimulate PKC isoforms and Ca2+ flux, respectively, can be used to mimic many of the signaling events which are stimulated upon TCR activation. As shown in Fig. 2B and C, treatment of the JSL1 cells with PMA for up to 2 days resulted in a fourfold increase in the expression of R0 and a fivefold decrease in the relative level of R456. In contrast, incubation of the JSL1 cells with ionomycin had no effect on isoform expression, even though the levels of ionomycin used were sufficient to upregulate expression of the activation marker CD69 (resulting in a twofold increase in mean fluorescence over background). Moreover, the use of ionomycin in addition to PMA had no synergistic effect on CD45 splicing over that of PMA alone (Fig. 2C), suggesting that the PKC-dependent pathway is the predominant stimulator of CD45 alternative splicing, with little or no contribution from Ca2+-dependent factors. Although we focused on the JSL1 line for further analysis, the limiting dilution produced at least one other clone (JSL4) which behaves similarly to the JSL1 cells with regard to CD45 mRNA expression and regulation (Fig. 2D).
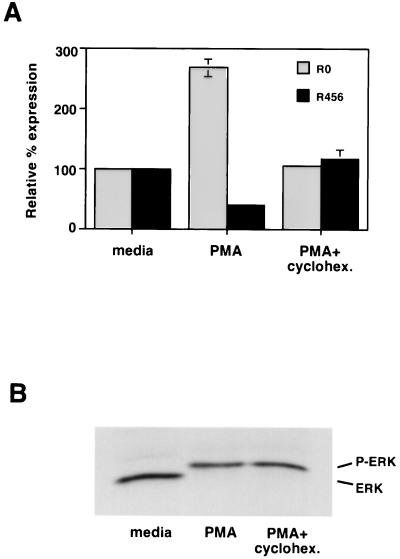
In both PBLs and JSL1 cells, the activation-dependent alternative splicing of CD45 was not readily observed until approximately 24 h poststimulation. Since stimulation of T cells can ultimately lead to Fas-mediated apoptosis of activated cells, we wanted to determine whether the splicing changes might be induced as a result of apoptosis and not directly by activation. Treatment of JSL1 cells with 1 ng/ml of anti-Fas antibody per ml induced apoptosis at a rate similar to that observed upon treatment with PMA (data not shown). However, as shown in Fig. 2C, the anti-Fas antibody had no effect on CD45 isoform expression. Therefore, it is unlikely that the activation-dependent switch in CD45 splicing is a secondary effect mediated by apoptosis. An alternative explanation for the relatively long time course required to induce CD45 alternative splicing is that protein synthesis is a necessary step in the pathway between activation and splicing. Consistent with this possibility, we observed that treatment of JSL1 cells with the protein synthesis inhibitor cycloheximide during the first 12 h of PMA stimulation was sufficient to block any alterations in CD45 splicing (Fig. 3A), although this treatment did not block PMA-induced Erk phosphorylation (Fig. 3B).
FIG. 3.
Protein synthesis is required for the PMA-induced alternative splicing of CD45. (A) CD45 isoform expression following treatment of JSL1 cells with PMA (2 ng/ml) for 24 h in the presence or absence of cycloheximide (cyclohex. 50 μM) during the first 12 h of stimulation. Quantitation was done as described for Fig. 1, and results represent two independent experiments. (B) Erk phosphorylation following PMA induction of JSL1 cells in the presence or absence of cycloheximide. Cells were preincubated with cycloheximide for 15 min, and then PMA was added as for panel A. An aliquot of cells was harvested at 10 min and lysed in the presence of phosphatase inhibitors. Total lysate was then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a membrane, and probed with anti-Erk antibody.
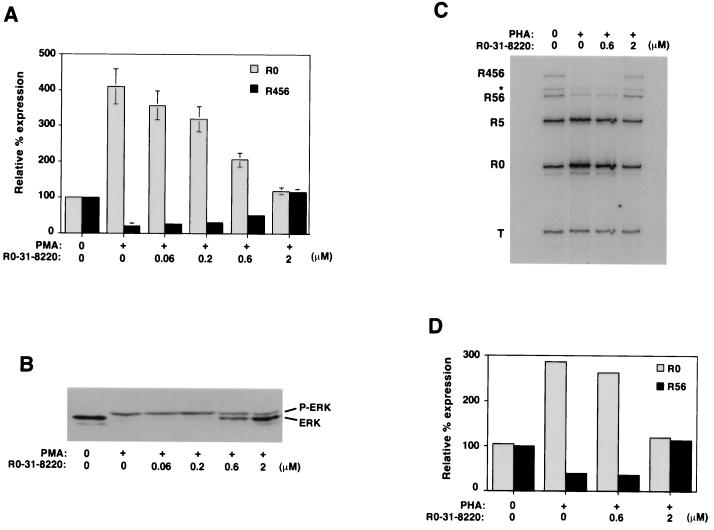
In contrast to PBLs (Fig. 1), we could not induce changes in CD45 splicing in JSL1 cells by stimulation with PHA (Fig. 2C). Given the similarity in the time course and extent of the activation-induced RNA isoform changes observed in the PBLs and JSL1 cells (compare Fig. 1C and 2C), the inability of JSL1 cells to regulate CD45 splicing in response to PHA likely reflects a difference in the susceptibility of these cells to activation, rather than a difference in the overall regulation of CD45 in the two cell types. To further confirm that CD45 splicing is regulated through similar pathways in PBLs and JSL1 cells, we tested the ability of the general PKC inhibitor R0-31-8220 (Calbiochem) to block the activation-induced alternative splicing of CD45 in both cell types. Various PKC isoforms have been shown to be activated directly by PMA and indirectly by PHA via the increase in diacylglycerides produced by the activation of phospholipase Cγ following TCR stimulation (8). As predicted by these observations, addition of R0-31-8220 to JSL1 cells blocked the PMA-induced changes in CD45 splicing at concentrations similar to that required to block the known PKC-mediated induction of Erk phosphorylation (Fig. 4A and B). Importantly, incubation of the T-cell-enriched PBLs with R0-31-8220 also significantly decreased the PHA-induced switch in CD45 splicing (Fig. 4C and D). Thus, regulation of CD45 splicing in both PBLs and JSL1 cells is mediated, at least in part, via a PKC-dependent signaling pathway.
FIG. 4.
Inhibition of PKC blocks stimulation-induced alternative splicing of CD45 in both PBLs and JSL1 cells. (A) Quantitation of the relative expression of R0 and R456 in JSL1 cells following stimulation with PMA (2 ng/ml) for 48 h in the absence or presence of an increasing concentration of the general PKC inhibitor R0-31-8220. Quantitation is as described in the legend to Fig. 1 and is representative of four independent experiments. (B) Erk phosphorylation in JSL1 cells upon stimulation in the absence or presence of R0-31-8220. Following stimulation of JSL1 cells for eventual analysis of RNA, an aliquot of cells was analyzed as described for Fig. 3B. PKC is known to be required for PMA induction of Erk phosphorylation; therefore, this blot serves as a positive control for R0-31-8220 activity. (C) RT-PCR assay of RNA harvested from CD4+ CD3+-enriched PBLs which were treated for 48 h with PHA (1 mg/ml) in the absence or presence of R0-31-8220. (D) Quantitation of the experiment shown in panel C.
Activated Ras is sufficient to mediate CD45 alternative splicing.
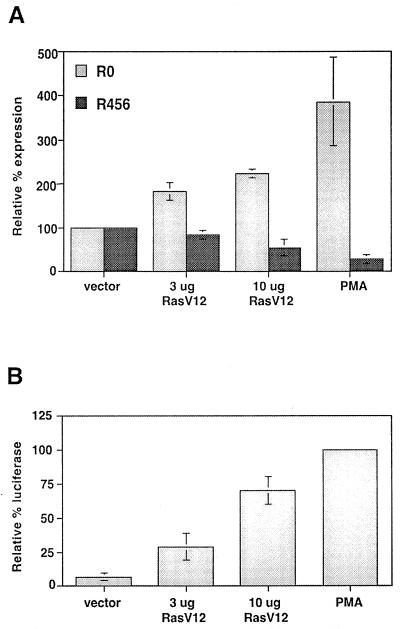
One of the best-characterized effectors of PKC isoforms in T cells is the small GTPase Ras (8, 15). Therefore, we were interested in studying more directly the potential involvement of Ras in the induction of CD45 alternative splicing. Ras is active when bound to GTP, and thus constitutive activation of Ras can be conferred by mutations which dramatically reduce its GTPase activity. One such mutation is a substitution at residue 12 of valine for glycine (RasV12) (44). To determine whether Ras activation is sufficient to induce a switch in CD45 splicing in JSL1 cells, we transfected unstimulated JSL1 cells with a RasV12 expression construct. Because only a small percentage of cells take up the DNA upon transfection, we purified those cells which expressed RasV12 before analyzing the RNA. To accomplish this, we took advantage of the observation that when cells are transfected simultaneously with multiple plasmids, there is a direct correlation between expression of each construct in any given cell. Therefore, we cotransfected the RasV12 plasmid with a construct expressing the extracellular and transmembrane domains of CD25 (TacT). Two days posttransfection, those cells which express CD25 on their surface are assumed to also express RasV12 and were readily purified with anti-CD25 antibody and magnetic beads prior to lysing and RNA harvest (see Materials and Methods). Additionally, the cells were cotransfected with a reporter construct in which the luciferase gene is driven by an AP1-dependent promoter (AP1-Luc). This reporter construct has previously been demonstrated to express the luciferase enzyme in Jurkat cells in response to PMA treatment or Ras activation (49). As shown in Fig. 5B, luciferase expression in the transfected cells was indeed induced by both PMA and cotransfection with RasV12, thus confirming that RasV12 was expressed and active.
FIG. 5.
Expression of constitutively active Ras is sufficient to induce alternative splicing of CD45. (A) Quantitation of the relative expression of R0 and R456 in JSL1 cells 50 h after transfection with RasV12. Amount of RasV12 expression plasmid included in each transfection per 20 × 106 cells is indicated. PMA was added 6 h after transfection to cells transfected with empty vector. At 50 h posttransfection, cells were harvested and RNA was isolated from purified transfected cells (see Materials and Methods). Quantitation is as described for Fig. 1 and is representative of three independent experiments. (B) Activation of an AP1-luciferase reporter construct by PMA or cotransfected RasV12. The luciferase assay was performed as described in Materials and Methods. The relative expression of luciferase is given as a percentage of that induced by treatment of the cells with PMA.
Transfection of the JSL1 cells with TacT, AP1-Luc, and vector alone had no influence on the relative expression of CD45 isoforms and no influence on the ability of PMA to induce alternative splicing (Fig. 5A and data not shown). However, when RasV12 was included in the transfection, we observed a dose-dependent switch in CD45 splicing consistent with that seen following treatment with PMA (Fig. 5A). Since 10 μg of transfected RasV12 influences both AP1-Luc activation and CD45 splicing at a level only about 60 to 70% of that of PMA, we believe it is unlikely that the effect of RasV12 on CD45 splicing is an artifact of overexpression and saturation of the cellular machinery. Instead, the data indicate that Ras activation is sufficient to stimulate alternative splicing of CD45 and suggest that Ras is a component of the splicing regulatory pathway normally stimulated by PMA.
PMA does not induce CD45 alternative splicing in B-cell lines.
The activation of PKC and/or Ras potentially has many effects on cellular metabolism and gene regulation. Therefore, we wanted to examine the specificity of the PMA-induced alternative splicing of CD45 and exclude the possibility that treatment with PMA was affecting CD45 splicing due to a change in the activity of some component of the general splicing machinery. Primary B cells are known to express predominantly the largest CD45 protein isoform (encoded by R456), and this expression is unaffected by developmental or activation state (58). Therefore, it is likely that B cells differ from T cells in their expression of some CD45 regulatory factor(s). Consistent with the in vivo observations, at least three cultured B-cell lines express solely the R456 and R56 isoforms (Fig. 6A and data not shown). In marked contrast to JSL1 cells, treatment of each of these cells lines with PMA did not cause any downregulation of R456 or R56, nor could the R0 isoform be detected (Fig. 6A and B). This was despite the ability of PMA to upregulate expression of the activation marker CD69 in all of the cell lines tested (Fig. 6C and data not shown). Thus, although we do not yet know the nature of the critical differences between the B cells and JSL1 cells, these results indicate that PMA does not alter CD45 splicing as a result of a nonspecific change in the general splicing machinery.
FIG. 6.
A cell-specific factor(s) is required for the PMA-induced alternative splicing of CD45. (A) Representative RT-PCR assay comparing CD45 expression in JSL1 cells and Raji cells upon stimulation with PMA (100 ng/ml) or in medium alone (−). (B) Quantitation of R456 expression in JSL1 cells and three B-cell-derived cell lines (Raji, Daudi, and Ramos-Lanier) following treatment with PMA (100 ng/ml). Quantitation is as described for Fig. 1 and represents two to three independent experiments. Expression of R0 was not quantitated because no R0 mRNA could be detected in any of the B-cell lines under either unstimulated or stimulated conditions. (C) Fluorescence histogram following staining of JSL1 or Raji cells grown for 5 h in the absence (filled area) or presence (black line) of PMA. Cells were stained with anti-CD69-fluorescein isothiocyanate (FITC) antibodies. Ten thousand events of each cell type were analyzed by flow cytometry. CD69 upregulation is a marker for activation of both B and T cells.
Sequences involved in regulating the splicing of CD45 variable exon 4.
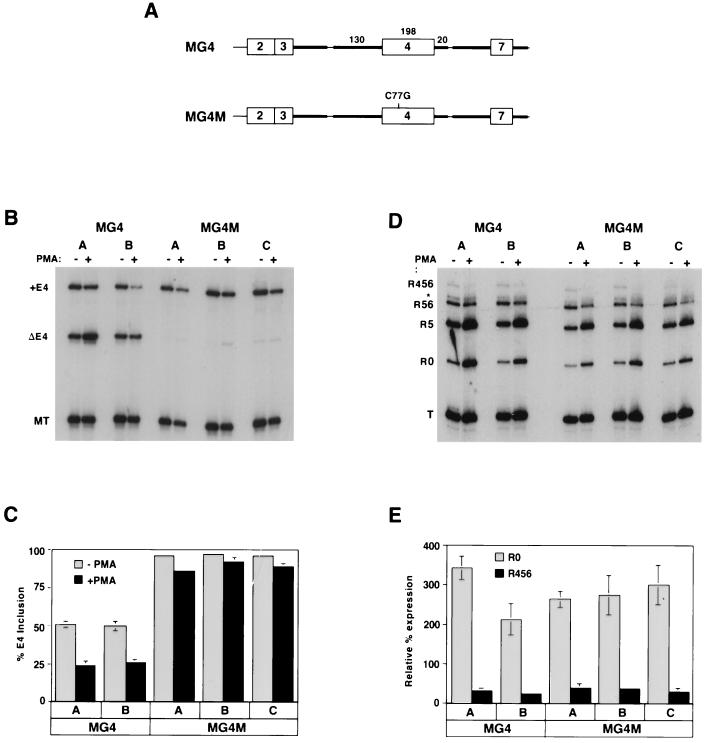
Previous studies have demonstrated that sequences within and immediately flanking regulated exons 4 and 6 are necessary and sufficient to mediate their tissue-specific isoform expression (inclusion in B cells and exclusion in thymocytes) (54, 59). To examine if these same sequences were involved in activation-dependent splicing regulation, we made a minigene in which exon 4 was flanked by exons 3 and 7. We included in this construct the intron sequences flanking exon 4 which were previously determined to be minimally required for tissue-specific regulation (Fig. 7A and reference 54). When this minigene was stably integrated into JSL1 cells, we observed that in the majority of the clones isolated, approximately 50% of the minigene mRNA contained exon 4. More importantly, upon treatment of these clones with PMA, we observed a significant shift toward exon exclusion (Fig. 7B [first four lanes] and C). When we tested minigenes which contained more of the intron sequences which endogenously flank exon 4, isoform expression and regulation were essentially the same as observed in MG4 (data not shown). Therefore, we conclude that the sequences contained in MG4 are sufficient to mediate both tissue-specific and activation-induced alternative splicing of exon 4. Moreover, since the minigene used here is expressed from a heterologous promoter, these results demonstrate that activation-induced splicing regulation does not require transcription from the endogenous CD45 promoter.
FIG. 7.
Sequences within and flanking exon 4 are necessary and sufficient for activation-induced splicing regulation. (A) A schematic of the minigene used. Exons are designated by boxes, and introns are designated by lines. Bold lines represent intron sequences which are identical to the endogenous gene; thin lines represent heterologous sequence added in the construction of the minigene. Numbers above intron and exon sequences indicate nucleotide length. MG4 and MG4M are identical in all respects except the C77G mutation. (B) RT-PCR analysis of stable integrants of minigenes MG4 and MG4M in JSL1 cells. Two clones harboring MG4 (A and B) were isolated in parallel with three MG4M clones (A, B, and C), and all were grown in the absence (−) or presence (+) of PMA before harvesting for RNA. Overall expression of the minigene is monitored by product MT and appears to decrease somewhat upon stimulation. (C) Quantitation of minigene expression from three independent experiments. (D) RT-PCR analysis of the endogenous CD45 expressed in the same RNA samples as used for panel B. (E) Quantitation of the endogenous CD45 isoforms from two independent experiments.
Finally, a naturally occurring point mutation in humans which correlates with a dramatic increase in the percentage of primary T cells expressing CD45 isoforms encoded by exon 4-containing mRNAs has been described (45, 56). A causal relationship between this mutation (a C-to-G transversion at position 77 in exon 4) and inclusion of exon 4 has been demonstrated by stable transfection of a minigene containing this mutation into COS cells, which do not normally express CD45 (68). As a further test of whether use of our JSL1 system and MG4 minigene faithfully mimics CD45 regulation in primary T cells, we wanted to determine the influence of the C77G mutation. We engineered this single point mutation into MG4 and isolated 11 stable lines which expressed this minigene (MG4M). Strikingly, in all of these clones over 95% of the minigene mRNA included exon 4 (Fig. 7B and C and data not shown). Stimulation of these clones with PMA resulted in only a minor increase in ΔE4 isoform expression (Fig. 7B and C). In all of the clones expressing MG4M, isoform expression and regulation of the endogenous CD45 mRNA was similar to that in JSL1 cells (Fig. 7D and E); therefore, the lack of exon 4 exclusion in MG4M mRNA is not due to a change in CD45 regulatory factors in these clones. Rather, as previously suggested for primary T cells, the presence of the C77G mutation results in a dramatic change in the regulation of exon 4 splicing.
DISCUSSION
Upon stimulation, naive T cells undergo numerous changes in gene expression that allow the activated cell to function in an immune response. Among these changes is the activation-induced switch in the expression of CD45 mRNA isoforms. To begin to understand the mechanisms responsible for this regulated splicing event, we have developed a cell line-based model system which mimics the alternative splicing of CD45 which we and others (28) observe in primary T cells. There are several lines of evidence that JSL1 cells represent a good model for the activation-dependent alternative splicing of CD45. First, the time course and extent of isoform change upon stimulation are highly similar between JSL1 cells and primary T cells. Second, the PKC inhibitor R0-31-8220 blocked CD45 alternative splicing at similar concentrations in both cell types. Finally, a point mutation which has been shown to increase CD45 exon 4 inclusion in primary T cells has a similar effect in JSL1 cells. Therefore, at least some components of both the initial signaling events, and the direct regulation of CD45, are conserved between primary T cells and JSL1 cells.
Requirements for activation-induced alternative splicing of CD45.
One of the surprising findings in this study is that at least 24 h are needed from the time of activation to the detection of a significant change in CD45 mRNA expression. This is consistent with the observation that a significant change in CD45 surface expression first occurs 2 to 3 days following stimulation (2, 5, 40) and with the time course observed for at least one other signaling-induced alternative splicing event (51). However, many other changes in gene expression, such as induction of interleukin-2 transcription and alternative splicing of CD44 (see below for further discussion), are observed within 6 to 7 h of T-cell activation. Since the half-life of each of the CD45 mRNA variants is on the order of 3 to 4 h (reference 13 and data not shown), the lag time observed for the CD45 isoform change is not due to slow turnover of RNA. Rather, it appears that multiple events are likely required to initiate alternative splicing. Consistent with this interpretation, the addition of cycloheximide to JSL1 cells during the first 12 h of exposure to PMA blocks alternative splicing almost entirely. Thus, we propose a model in which an initial PKC/Ras-dependent pathway results in the induction of some activation-specific protein that in turn either directly or indirectly regulates CD45 pre-mRNA.
There are several lines of evidence that PKC and Ras are involved in the initial cascade which ultimately regulates CD45 splicing. PMA directly activates PKC (9), and a 4- to 6-h pulse with PMA followed by continued incubation in PMA-free medium is sufficient to induce CD45 alternative splicing (data not shown). Furthermore, although preincubation with an inhibitor of PKC blocks PMA-mediated alternative splicing, addition of the PKC inhibitor following PMA stimulation has little to no effect. Rapid activation of Ras also occurs upon treatment of JSL1 cells with PMA, as assayed by Erk phosphorylation (Fig. 4B), and transfection with constitutively active Ras results in CD45 alternative splicing with kinetics similar to those observed with PMA. Therefore, Ras is likely to be a critical component of the initial PMA-induced pathway.
The effect of PMA treatment on CD45 expression in primary T cells has been investigated previously (67). In that study, syntheses of both the RA and R0 protein isoforms were stimulated by PMA. However, since the study relied on a mixed population of T cells and did not assay for RNA expression, it is unclear how these previous findings relate to the results described here. Our data do not enable us to rule out a requirement for additional, non-PMA-activated pathways in the activation of CD45 alternative splicing in primary T cells. However, we do observe that the induction of Ca2+-dependent signaling pathways in JSL1 cells has no effect on CD45 alternative splicing. Therefore, at least for this cell line, we can narrow down the TCR-induced signaling pathways which are required for the regulation of CD45 splicing to those containing PKC and/or Ras. A requirement for Ras activation has been demonstrated in the nerve growth factor-induced alternative splicing of agrin, an extracellular matrix protein (51), and in the T-cell activation-dependent alternative splicing of CD44 (21) (see below). This may reflect the presence of a single Ras-responsive splicing factor which regulates multiple pre-mRNAs. Alternatively, Ras may play a distinct role in each of these signaling-induced splicing events, perhaps through the activation of different downstream effectors (8, 19).
We have made no attempt in this study to identify potential activation-induced CD45 regulatory factors. However, previous studies have demonstrated the increased expression of several members of the SR family of general splicing factors upon T-cell activation (28, 46). SR proteins have been implicated in the regulation of many alternative splicing events (16, 32) and have been shown to influence CD45 alternative splicing in a heterologous system (28, 43). Therefore, it is possible that increased synthesis of SR proteins is a required step in the pathway between T-cell activation and CD45 alternative splicing.
In addition to the requirement for certain signaling pathways, our data indicate a requirement for cis-acting sequences in the regulation of at least one of the CD45 alternative exons, namely, exon 4. The insertion of a fragment of approximately 450 bp encompassing exon 4 into a minigene containing flanking exons 3 and 7 was sufficient to mediate a switch toward exon 4 exclusion upon activation of JSL1 cells. Thus, these sequences likely include activation-sensitive regulatory elements. Moreover, since these same sequences are necessary and sufficient to mediate the tissue-specific alternative splicing of CD45 pre-mRNA (54), these results suggest significant overlap between the activation-dependent and tissue-specific regulation of CD45. Strikingly, a point mutation 77 nucleotides downstream of the 3′ splice site of exon 4 (C77G) results in almost complete inclusion of exon 4 in both resting and stimulated cells. This mutation does not disrupt any splicing consensus sequences but may alter the activity of an exonic regulatory element. We are currently investigating the binding of proteins to sequences within exon 4 to better understand the functional consequence of this C77G mutation.
Comparison with other examples of activation-induced splicing in T cells.
Several genes besides CD45 have been described to undergo alternative splicing upon T-cell activation. These include Fas, a novel death domain-containing protein of unknown function called Lard, and the cell surface molecule CD44, which is involved in cell adhesion and trafficking (21, 30, 46, 47). Similar to what we have observed for CD45, alternative splicing of endogenous CD44, and/or a minigene containing a single CD44 variable exon, can be induced in a murine T-lymphoma cell line by treatment with a phorbol ester or by activated Ras but not by ionomycin (21). However, there are several notable differences in the induction of the two splicing events. First, activation of T cells induces inclusion of the CD44 variable exons, in contrast to the induced exclusion of CD45 variable exons (21, 46). Moreover, alternative splicing of the CD44 minigene occurs within 7 h of treatment with PMA and protein synthesis is not required, as indicated by the lack of a block in alternative splicing upon addition of cycloheximide (21). Likewise, alternative splicing of Fas in PBLs is initiated during the first 6 to 12 h of activation (30). Therefore, although there may be some overlap in the pathways which mediate alternative splicing of CD45, CD44, and Fas, the difference in the kinetics of these events suggests that there are at least two pathways by which T-cell activation can induce changes in splicing regulatory factors.
Ultimately a more comprehensive understanding of the activation-induced regulation of CD45 alternative splicing will require the identification of regulatory factors which directly influence CD45 splicing and an understanding of how these factors are affected by activation. The model system that we describe here is a significant step toward this goal since it will enhance the feasibility of a detailed analysis of the requirements for activation-induced alternative splicing of CD45. Beyond understanding the regulation of CD45, such a characterization is likely to have broader implications since CD45 alternative splicing is only one of many examples of signaling-induced splicing events.
ACKNOWLEDGMENTS
We thank Haruo Saito for the gift of plasmid pSV-MiLCA2 and thank Christine Guthrie and members of the Weiss and Guthrie laboratories for valuable discussions and suggestions. We also thank Ravi Majeti, Xiang-Dong Fu, Brenton Graveley, Amy Kistler, and Jon Staley for critical reading of the manuscript.
This work was supported by NIH grant GM-39553. K.W.L. was supported by a Cancer Research Institute fellowship.
REFERENCES
- 1.Adams M D, Rudner D Z, Rio D C. Biochemistry and regulation of pre-mRNA splicing. Curr Opin Cell Biol. 1996;8:331–339. doi: 10.1016/s0955-0674(96)80006-8. [DOI] [PubMed] [Google Scholar]
- 2.Akbar A N, Terry L, Timms A, Beverley P C L, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 3.Bell E B, Sparshott S M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 4.Berget S M. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 5.Birkeland M L, Johnson P, Trowbridge I S, Pure E. Changes in CD45 isoform expression accompany antigen-induced murine T cell activation. Proc Natl Acad Sci USA. 1989;86:6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black D L. Finding splice sites within a wilderness of RNA. RNA. 1995;1:763–771. [PMC free article] [PubMed] [Google Scholar]
- 7.Byth K F, Conroy L A, Howlett S, Smith A J H, May J, Alexander D R, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and in B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 9.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated phospholipid-dependent protein kinase by tumor-promoting phorbol ester. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 10.Chalfant C E, Watson J E, Bisnauth L D, Kang J B, Patel N, Obeid L M, Eichler D C, Cooper D R. Insulin regulates protein kinase CbII expression through enhanced exon inclusion in L6 skeletal muscle cells. J Biol Chem. 1998;273:910–916. doi: 10.1074/jbc.273.2.910. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 12.D'Ambrosio D, Cantrell D A, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur J Immunol. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 13.Deans J P, Serra H M, Shaw J, Shen Y J, Torres R M, Pilarski L. Transient accumulation and subsequent rapid loss of messenger RNA encoding high molecular mass CD45 isoforms after T cell activation. J Immunol. 1992;148:1898–1905. [PubMed] [Google Scholar]
- 14.Desai D M, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 15.Downward J, Graves J D, Warne P H, Rayter S, Cantrell D A. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 16.Fu X-D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 17.Grabowski P J. Splicing regulation in neurons: tinkering with cell-specific control. Cell. 1998;92:709–712. doi: 10.1016/s0092-8674(00)81399-9. [DOI] [PubMed] [Google Scholar]
- 18.Hertel K J, Lynch K W, Maniatis T. Common themes in the function of transcription and splicing enhancers. Curr Opin Cell Biol. 1997;9:350–357. doi: 10.1016/s0955-0674(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 19.Katz M E, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genes Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 20.Kishihara K, Penninger J, Wallace V A, Kundig T M, Kawai K, Wakenham A, Timms E, Pfeffer K, Ohashi P S, Thomas M L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon 6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 21.Konig H, Ponta H, Herrlich P. Coupling of signal transduction to alternative pre-mRNA splicing by a composite splice regulator. EMBO J. 1998;17:2904–2913. doi: 10.1093/emboj/17.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koretzky G A, Picus J, Thomas M L, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T cell antigen receptor to the phosphatidylinositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 23.Koretzky G, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for both T cell antigen receptor and CD2 mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koretzky G A, Kohmetscher M A, Kadlecek T, Weiss A. Restoration of T cell receptor mediated signal transduction by transfection of CD45 cDNA into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992;149:1138–1142. [PubMed] [Google Scholar]
- 25.Leevers S J, Marshall C J. Activation of extracellular signal-regulated kinase, Erk2, by p21ras oncoprotein. EMBO J. 1992;11:569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitenberg D, Boutin Y, Lu D D, Bottomly K. Biochemical association of CD45 with the T cell receptor complex: regulation by Cd45 isoform and during T cell activation. Immunity. 1999;10:701–711. doi: 10.1016/s1074-7613(00)80069-2. [DOI] [PubMed] [Google Scholar]
- 27.Leitenberg D, Novak T J, Farber D, Smith B R, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaire R, Winne A, Sarkissian M, Lafyatis R. SF2 and SRp55 regulation of CD45 exon 4 skipping during T cell activation. Eur J Immunol. 1999;29:823–837. doi: 10.1002/(SICI)1521-4141(199903)29:03<823::AID-IMMU823>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Lowin-Kropf B, Shapiro V S, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lui C, Cheng J, Mountz J D. Differential expression of human Fas mRNA species upon peripheral blood mononuclear cell activation. Biochem J. 1995;310:957–963. doi: 10.1042/bj3100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majeti R, Bilwes A M, Noel J P, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 32.Manley J L, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 33.McCall M N, Shotton D M, Barclay A N. Expression of soluble isoforms of rat CD45. Analysis by electron microscopy and use in epitope mapping of anti-Cd45R monoclonal antibodies. Immunology. 1992;76:310–317. [PMC free article] [PubMed] [Google Scholar]
- 34.McFarland E D, Hurley T R, Pingel J T, Sefton B M, Shaw A, Thomas M L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci USA. 1993;90:1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostergaard H L, Shackelford D A, Hurley T R, Johnson P, Hyman R, Sefton B M, Trowbridge I S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostergaard H L, Trowbridge I S. Coclustering CD45 with CD4 and CD8 alters the phosphorylation and kinase activity of p56lck. J Exp Med. 1990;172:347–350. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratech H, Denning S, Kaufman R E. An analysis of alternatively spliced CD45 mRNA transcripts during T cell maturation in humans. Cell Immunol. 1997;177:109–118. doi: 10.1006/cimm.1997.1111. [DOI] [PubMed] [Google Scholar]
- 39.Rothstein D M, Saito H, Streuli M, Schlossman S F, Morimoto C. The alternative splicing of the CD45 tyrosine phosphatase is controlled by negative regulatory trans-acting splicing factors. J Biol Chem. 1992;267:7139–7147. [PubMed] [Google Scholar]
- 40.Rothstein D M, Yamada A, Schlossman S F, Morimoto C. Cyclic regulation of CD45 isoform expression in a long term human CD4+CD45RA+ T cell line. J Immunol. 1991;146:1175–1183. [PubMed] [Google Scholar]
- 41.Saga Y, Furukawa K, Rogers P, Tung J S, Parker D, Boyse E A. Further data on the selective expression of Ly-5 isoform. Immunogenetics. 1990;31:296–306. doi: 10.1007/BF02115003. [DOI] [PubMed] [Google Scholar]
- 42.Saga Y, Lee J S, Saraiya C, Boyse E A. Regulation of alternative splicing in the generation of isoforms of the mouse Ly-5 (CD45) glycoprotein. Proc Natl Acad Sci USA. 1990;87:3728–3732. doi: 10.1073/pnas.87.10.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkissian M, Winne A, Lafyatis R. The mammalian homolog of suppressor-of-white-apricot regulates alternative mRNA splicing of CD45 exon 4 and fibronectin IIICS. J Biol Chem. 1996;271:31106–31114. doi: 10.1074/jbc.271.49.31106. [DOI] [PubMed] [Google Scholar]
- 44.Satoh T, Nakafuku M, Kaziro Y. Function of Ras as a molecular switch in signal transduction. J Biol Chem. 1992;267:24149–24152. [PubMed] [Google Scholar]
- 45.Schwinzer R, Schraven B, Kyas U, Meuer S C, Wonigeit K. Phenotypical and biochemical characterization of a variant CD45R expression pattern in human leukocytes. Eur J Immunol. 1992;22:1095–1098. doi: 10.1002/eji.1830220433. [DOI] [PubMed] [Google Scholar]
- 46.Screaton G R, Caceres J F, Mayeda A, Bell M V, Plebanski M, Jackson D G, Bell J I, Krainer A R. Identification and characterization of three members of the human SR family of pre-mRNA splicing factors. EMBO J. 1995;14:4336–4349. doi: 10.1002/j.1460-2075.1995.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Screaton G R, Xu X N, Olsen A L, Cowper A E, Tan R, McMichael A J, Bell J I. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci USA. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seavitt J R, White L S, Murphy K M, Loh D Y, Perlmutter R M, Thomas M L. Expression of the p56Lck Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol Cell Biol. 1999;19:4200–4208. doi: 10.1128/mcb.19.6.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro V S, Mollenauer M N, Greene W C, Weiss A. c-rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sieh M, Bolen J B, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993;12:315–322. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith M A, Fanger G R, O'Connor L T, Bridle P, Maue R A. Selective regulation of agrin mRNA induction and alternative splicing in PC12 cells by Ras-dependent actions of nerve growth factor. J Biol Chem. 1997;272:15675–15681. doi: 10.1074/jbc.272.25.15675. [DOI] [PubMed] [Google Scholar]
- 52.Stone J D, Conroy L A, Byth K F, Hederer R A, Howlett S, Takemoto Y, Holmes N, Alexander D R. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ, and ZAP-70. J Immunol. 1997;158:5773–5782. [PubMed] [Google Scholar]
- 53.Streuli M, Hall L R, Saga Y, Schlossman S F, Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987;166:1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Streuli M, Saito H. Regulation of tissue-specific alternative splicing: exon-specific cis-elements govern the splicing of leukocyte common antigen pre-mRNA. EMBO J. 1989;8:787–796. doi: 10.1002/j.1460-2075.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strober W, Kanof M E, Smith P D. Preparation of human mononuclear cell populations and subpopulations. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. pp. 7.1.1–7.4.5. [Google Scholar]
- 56.Thude H, Hundrieser J, Wonigeit K, Schwinzer R. A point mutation in the human CD45 gene associated with defective splicing of exon A. Eur J Immunol. 1995;25:2101–2106. doi: 10.1002/eji.1830250745. [DOI] [PubMed] [Google Scholar]
- 57.Trowbridge I S. CD45: a prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991;266:23517–23520. [PubMed] [Google Scholar]
- 58.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 59.Tsai A Y M, Streuli M, Saito H. Integrity of the exon 6 sequence is essential for tissue-specific alternative splicing of human leukocyte common antigen pre-mRNA. Mol Cell Biol. 1989;9:4550–4555. doi: 10.1128/mcb.9.10.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Manley J L. Regulation of pre-mRNA splicing in metazoa. Curr Opin Genet Dev. 1997;7:205–211. doi: 10.1016/s0959-437x(97)80130-x. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Shen L, Najafi H, Kolberg J, Matschinsky F M, Urdea M, German M. Regulation of insulin preRNA splicing by glucose. Proc Natl Acad Sci USA. 1997;94:4360–4365. doi: 10.1073/pnas.94.9.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 63.Wiskocil R, Weiss A, Imboden J, Kamin-Lewis R, Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985;134:1599–1603. [PubMed] [Google Scholar]
- 64.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie J, McCobb D P. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 66.Yablonski D, Kane L P, Qian D, Weiss A. A Nck-Pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 1998;17:5647–5657. doi: 10.1093/emboj/17.19.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada A, Streuli M, Saito H, Rothstein D M, Schlossman S F, Morimoto C. Effect of activation of protein kinase C on CD45 isoform expression and CD45 protein tyrosine phosphatase activity in T cells. Eur J Immunol. 1990;20:1655–1660. doi: 10.1002/eji.1830200806. [DOI] [PubMed] [Google Scholar]
- 68.Zilch C F, Walker A M, Timon M, Goff L K, Wallace D L, Beverley P C L. A point mutation within CD45 exon A is the cause of variant CD45RA splicing in humans. Eur J Immunol. 1998;28:22–29. doi: 10.1002/(SICI)1521-4141(199801)28:01<22::AID-IMMU22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]