Abstract
Purpose
It is unclear whether high-definition (HD) imaging improves visibility and diagnostic ability in early gastric cancer (EGC) compared with standard-definition (SD) imaging. We aimed to compare the diagnostic performance and visibility scores of HD and SD ultraslim endoscopes in EGC.
Materials and Methods
We used HD and SD ultraslim endoscopes to obtain 60 images with similar compositions of gastric environments. Of the 60 images, 30 showed EGC (15 images for each modality) and 30 showed no EGC (15 images for each modality). Seventeen endoscopists evaluated the presence and location of the lesions in each image. Diagnostic ability was compared between modalities. The color difference between a lesion and the surrounding mucosa (ΔE) was measured and compared between the modalities.
Results
The ability of HD to detect EGC was significantly higher than that of SD (accuracy: 80.8% vs. 71.6%, P=0.017; sensitivity: 94.9% vs. 76.5%, P<0.001; positive predictive value, 76.2% vs. 55.3%, P<0.001; and negative predictive value (NPV), 94.1% vs. 73.5%, P<0.001). The ability of HD to determine the horizontal extent of EGC was significantly higher than that of SD (accuracy: 71.0% vs. 57.8%, P=0.004; sensitivity: 75.3% vs. 49.0%, P<0.001; NPV, 72.9% vs. 55.9%, P<0.001; and area under the curve: 0.891 vs. 0.631, P=0.038). The mean ΔE was significantly higher for HD than for SD (10.3 vs. 5.9, P=0.011).
Conclusions
The HD ultraslim endoscope showed a higher diagnostic performance in EGC than the SD endoscope because it provided good color contrast.
Keywords: Gastric cancer, Endoscopy, Screening, Diagnosis, Diagnostic imaging
INTRODUCTION
According to the World Health Organization, gastric cancer is the third most common cause of cancer-related deaths in men and women worldwide, with almost 3-quarters of a million deaths occurring annually [1]. Mortality due to gastric cancer can be reduced more effectively by detecting gastric lesions at an early stage. In regions with a high prevalence of gastric cancer, such as the East Asian countries, endoscopic resection is widely accepted as a less invasive treatment for differentiated- and undifferentiated-type early gastric cancer (EGC) with good long-term outcomes [2,3,4,5,6]. The 5-year survival rate in stage I gastric cancer following endoscopic or surgical treatment is reportedly greater than 85% [7,8]. Furthermore, early detection of gastric cancer is important for reducing healthcare costs [9]. Therefore, diagnosis in the early stages is important for managing patients with gastric cancer.
Esophagogastroduodenoscopy is the best modality for detecting EGC and is widely used for gastric cancer screening in regions with a high prevalence of gastric cancer [10]. It has a sensitivity of 78%–84% for gastric cancer and reduces gastric cancer mortality by 47%–67% [11,12,13]. Endoscopic technology has developed dramatically with respect to the outer diameter and image quality. High-definition (HD) imaging has recently been developed for ultraslim endoscopes. The image resolution of HD is 1,280 × 720 pixels and that of standard-definition (SD) is 720 × 480 pixels. Since HD endoscopes provide higher quality images than SD endoscopes, HD imaging is likely to improve visualization and diagnostic ability in gastric cancer. However, to the best of our knowledge, no studies have investigated the effects of endoscopic image quality on the visibility and diagnostic ability in gastric cancer. As HD endoscopes were developed only in the past few decades, they are more expensive than SD endoscopes and are not used worldwide, such that SD endoscopes are still commonly used in some regions, especially in developing countries. If it could be shown that HD endoscopes have higher diagnostic ability than SD endoscopes, it may promote the widespread use of HD endoscopes in such regions. Therefore, we aimed to compare the diagnostic performance of HD and SD imaging in EGC using ultraslim endoscopes.
MATERIALS AND METHODS
Patient and endoscopic image extraction
We retrospectively reviewed the medical records and endoscopic images of 23 patients with EGC who underwent endoscopic submucosal dissection (ESD) at Nihon University Hospital between March 1, 2020, and May 1, 2020. If the gastric lesions were initially examined using an SD ultraslim endoscope (GIF-290N; Olympus Co., Tokyo, Japan), we also examined the lesions using an HD ultraslim endoscope (GIF-1200N; Olympus Co.) to evaluate lesion characteristics in detail and determine an appropriate treatment strategy. We compared the endoscopic images acquired using the HD ultraslim endoscope and those acquired using the SD ultraslim endoscope. These images were obtained using the same endoscopic processor (EVIS X1; Olympus Co.), the same structure emphasis (A5), and the same color emphasis (C0). Finally, images of 7 lesions in 7 patients who were examined using both HD and SD endoscopes before ESD were included in this study. Images from the remaining 16 patients were excluded, as these patients were examined using other endoscopes or only an HD endoscope. The study protocol was approved by the Ethics Committee of Nihon University Hospital (IRB No. 20200705).
For each patient, one investigator selected 2 to 3 endoscopic images with or without gastric cancer for each modality using the following criteria: 1) endoscopic images were obtained by white-light imaging, not narrow-band imaging or chromoendoscopy with indigo carmine; 2) in the images with gastric cancer, the entire lesion was captured in the endoscopic image; 3) a paired set of endoscopic images for each modality had a similar composition (Supplementary Fig. 1); and 4) all endoscopic images in each modality had different compositions. In total, 30 endoscopic images of gastric cancer (15 endoscopic images for each modality) and 30 endoscopic images without gastric cancer (15 endoscopic images for each modality) were prepared with similar image compositions in each set. The investigator performed the analysis and was not involved in further evaluations.
Analysis of the diagnostic ability of both modalities in gastric cancer
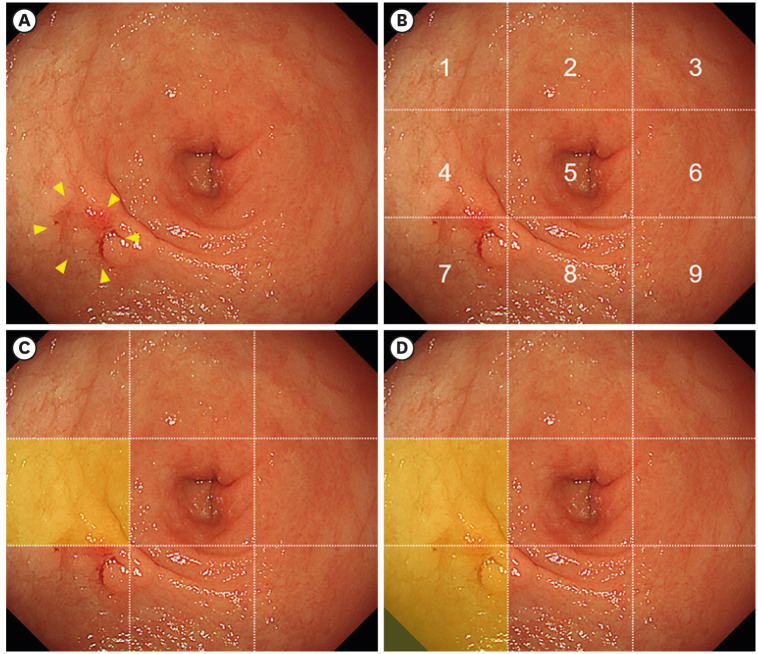
The site and horizontal extent of gastric cancer in each endoscopic image were determined by collating the pathological diagnosis with ESD. All endoscopic images were divided into 9 sections using lines to divide the length and width into 3 equal parts (Fig. 1A and B). Seventeen endoscopists at our hospital evaluated 60 endoscopic images randomly and were asked to identify the section with gastric cancer in each image. Information on the type of imaging (HD or SD) and the number of images with gastric cancer was concealed. In endoscopic images of gastric cancer, detection of gastric cancer was defined as correct when endoscopists correctly identified at least one of the sections that included gastric cancer (Fig. 1C), and determination of the horizontal extent of gastric cancer was defined as correct when they correctly identified all the sections that included gastric cancer (Fig. 1D). In endoscopic images without gastric cancer, detection of gastric cancer and determination of the horizontal extent of gastric cancer were defined as correct when they identified that no section had gastric cancer.
Fig. 1. Definition of detection of gastric cancer and determination of the horizontal extent of gastric cancer. (A) Type 0-IIc early gastric cancer is shown on the bottom left of the endoscopic image (yellow arrows). (B) Endoscopic images were divided into 9 sections using lines that divided the length and width into 3 equal parts. The lesion is present in sections 4 and 7. Detection of gastric cancer was defined as correct when any of the sections that included gastric cancer (either section 4 or 7 in this image) were identified. Determination of the horizontal extent of gastric cancer was defined as correct when all the sections that included gastric cancer (both sections 4 and 7 in this image) were identified. (C) When endoscopists selected section 4, the detection of gastric cancer was correct, but the determination of the horizontal extent of gastric cancer was incorrect. (D) When endoscopists selected both sections 4 and 7, both detection of gastric cancer and determination of the horizontal extent of gastric cancer were correct.
Assessment of the color value and color difference
When determining differences between HD and SD imaging in their ability to diagnose gastric cancer, we investigated the color difference between the lesion and surrounding mucosa in each imaging modality. We measured the color value of the lesion and surrounding mucosa in all endoscopic images of gastric cancer and calculated the color difference between the lesion and the surrounding mucosa.
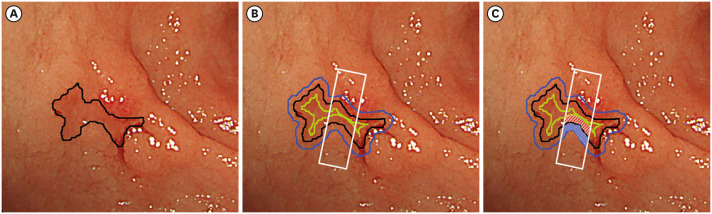
We referred to a previous study by Kanzaki et al. [14] to measure the color value of images of EGC and surrounding mucosa. This process was performed using Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA). First, a line was drawn along the border between the lesion and surrounding mucosa by collating the pathological diagnosis of ESD (Fig. 2A). Second, an outer line was drawn parallel to the border line. This outer line enclosed an area twice as large as the area enclosed by the border line. Finally, an inner line was drawn inside and parallel to the border line, such that the distance between the border line and the inner line was equal to the distance between the border line and the outer line. A rectangle, one-third of the width of the lesion, was drawn in the center of the lesion (Fig. 2B). The rectangle was set to avoid shadows, mucus, bleeding, and halation. The sections separated by this rectangle and the 3 lines were the region of interest (ROI) of the lesion and surrounding mucosa, respectively (Fig. 2C). The color value of the images of the lesion and surrounding mucosa was defined as the average color value in each ROI using CIE 1976 (L*a*b*) color space (CIELAB).[15] In CIELAB, color is expressed as 3 values: L* for perceptual lightness, and a* and b* for the 4 unique colors of human vision: red, green, blue, and yellow. The color value of the lesion (L*l, a*l, b*l) and surrounding mucosa (L*s, a*s, b*s) were converted from RGB, and the color difference between the lesion and surrounding mucosa (ΔE) was calculated using the following equation [16]:
Fig. 2. Process of measuring the color value of the lesion and the surrounding mucosa. (A) A border line between the lesion and the surrounding mucosa (black) was drawn. (B) An outer line (blue) was drawn parallel to the border line, enclosing an area twice as large as the area enclosed by the border line. An inner line (green) was drawn inside and parallel to the border line, and the width between the border line and the inner line was equal to the width between the border line and the outer line. A rectangle (white line) was drawn in the center of the lesion with a width of one-third of the width of the lesion. (C) The sections separated by this rectangle and the 3 lines were the ROI of the lesion (red shaded area) and the surrounding mucosa (blue shaded area). The color value of the lesion and the surrounding mucosa was defined as the average of the color value in each ROI.
ROI = region of interest.
| ΔE=√(L*l- L*s)2+(a*l-a*s)2+(b*l-b*s)2 |
Study outcomes and statistical analysis
The primary study outcome was the diagnostic ability of HD and SD endoscopic imaging for gastric cancer. The number of correct and incorrect identifications for each endoscopist were summated. The accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each endoscope modality were calculated. The calculated values were compared between HD and SD imaging using the Mann-Whitney U test. We also analyzed the diagnostic ability of each endoscope using the receiver operating characteristic (ROC) curve and area under the curve (AUC). The correct answers in the images with and without gastric cancer were scored as 1 and 0, respectively. The ROC curves relating to the points marked for each endoscopist and the presence of gastric cancer were plotted, and the AUCs were calculated and compared between HD and SD endoscopy.
The secondary outcome was the comparison of the color difference of the lesion and surrounding mucosa between the 2 endoscopic imaging modalities. The color differences of ΔL*, Δa*, Δb*, and ΔE were compared using the Mann-Whitney U test.
The significance level was set at 2-tailed P<0.05 in all analyses. EZR statistical analysis software (Jichi Medical University Saitama Medical Center, Saitama, Japan) was used for statistical analyses.
Sample size
Generally, the diagnostic accuracy of various inspection methods is sufficient at an AUC of ≥0.8 in. In this study, a total of 28 images (14 images with gastric cancer and 14 images without gastric cancer) were deemed necessary based on the following parameters: AUC=0.8, power=0.9, α=0.05 (2-sided), and the ratio of presence of gastric cancer to no gastric cancer=1:1. According to this hypothesis, we required a sample size of 30 images in this study (15 with gastric cancer and 15 without gastric cancer) for each endoscopic imaging modality.
RESULTS
Endoscopic and pathological characteristics of the lesions
All endoscopic images showed gastric mucosal atrophy. In the 15 endoscopic images with gastric cancer, the lesion characteristics were as follows: the locations were lower-third in 6 (40.0%), middle-third in 5 (33.3%), and upper-third in 4 (26.7%) images; the macroscopic types were 0-IIc in 8 (53.3%) and 0-IIa in 7 (46.7%) images; the histological types were well-differentiated tubular adenocarcinoma in 11 (73.3%), moderately differentiated tubular adenocarcinoma in 2 (13.3%), and non-solid type poorly differentiated adenocarcinoma in 2 (13.3%) images; the depth of invasion was T1a in 13 (86.7%) and T1b in 2 (13.3%) images; and the lesion size (mean±standard deviation [range]) was 17.7±6.2 (11–27) mm.
Diagnostic performance of HD and SD imaging in gastric cancer
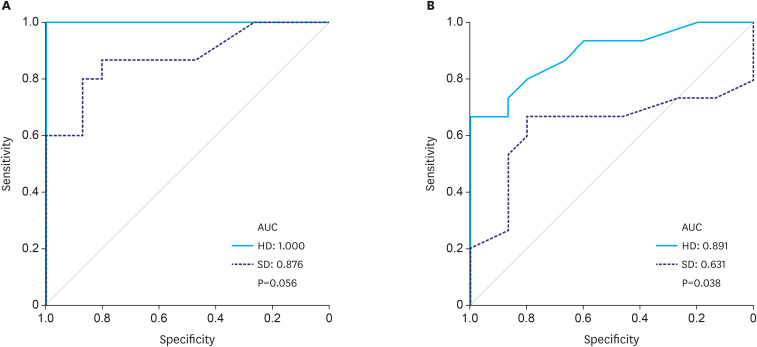
Regarding the detection of gastric cancer, Fig. 3A illustrates the ROC curves and AUCs of HD and SD images, and Table 1 summarizes the accuracy, sensitivity, specificity, PPV, and NPV of both modalities. The ability of HD imaging to detect gastric cancer was significantly higher than that of SD imaging in terms of accuracy (80.8% vs. 71.6%, P=0.017), sensitivity (94.9% vs. 76.5%, P<0.001), PPV (76.2% vs. 55.3%, P<0.001), and NPV (94.1% vs. 73.5%, P<0.001). The AUC for detection of gastric cancer was higher for HD images than for SD images, but the difference was not statistically significant (1.000 vs. 0.876, P=0.056). The Supplementary Fig. 1 shows 2 paired sets of endoscopic images comparing HD and SD.
Fig. 3. The receiver-operating characteristic curve and the AUC values of HD and SD images in the detection of gastric cancer (A) and determination of the horizontal extent of gastric cancer (B). (A) The AUC value for the detection of gastric cancer in HD images was higher than that in SD images; however, it did not reach statistical significance (1.000 vs. 0.876, P=0.056). (B) The AUC value for the determination of the horizontal extent of gastric cancer in HD images was significantly higher than that in SD images (0.891 vs. 0.631, P=0.038).
AUC = area under the curve; HD = high-definition; SD = standard-definition.
Table 1. Diagnostic performance of HD and SD images in the detection of early gastric cancer.
| Measurements | HD | SD | P-value |
|---|---|---|---|
| Accuracy | 80.8 (75.3–86.3) | 71.6 (67.0–76.2) | 0.017 |
| Sensitivity | 94.9 (90.4–99.4) | 76.5 (70.8–82.2) | <0.001 |
| Specificity | 66.7 (55.4–78.0) | 66.7 (56.6–76.7) | 0.917 |
| PPV | 76.2 (69.5–82.8) | 55.3 (48.4–62.2) | <0.001 |
| NPV | 94.1 (89.7–98.4) | 73.5 (68.4–78.5) | <0.001 |
Values are given as a percentage (95% confidence interval).
HD = high-definition; SD = standard-definition; PPV = positive predictive value; NPV = negative predictive value.
Regarding the determination of the horizontal extent of gastric cancer, Fig. 3B illustrates the ROC curves and AUCs of HD and SD images, and Table 2 summarizes the accuracy, sensitivity, specificity, PPV, and NPV of HD and SD images. The ability of HD images to determine the horizontal extent of gastric cancer was significantly higher than that of SD images in terms of accuracy (71.0% vs. 57.8%, P=0.004), sensitivity (75.3% vs. 49.0%, P<0.001), NPV (72.9% vs. 55.9%, P<0.001), and AUC (0.891 vs. 0.631, P=0.038).
Table 2. Diagnostic performance of HD and SD images in determining the horizontal extent of early gastric cancer.
| Measurements | HD | SD | P-value |
|---|---|---|---|
| Accuracy | 71.0 (65.2–76.8) | 57.8 (51.8–63.9) | 0.004 |
| Sensitivity | 75.3 (69.0–81.6) | 49.0 (42.3–55.8) | <0.001 |
| Specificity | 66.7 (55.4–78.0) | 66.7 (56.6–76.7) | 0.917 |
| PPV | 72.1 (64.5–79.8) | 61.4 (54.5–68.2) | 0.053 |
| NPV | 72.9 (66.8–79.0) | 55.9 (49.5–62.3) | <0.001 |
Values are given as a percentage (95% confidence interval).
HD = high-definition; SD = standard-definition; PPV = positive predictive value; NPV = negative predictive value.
Diagnostic performance of HD and SD imaging according to histological types
Regarding differentiated cancer, the ability of HD imaging to detect gastric cancer was significantly higher than that of SD imaging in terms of accuracy (79.4% vs. 71.0%, P=0.042), sensitivity (94.1% vs. 76.0%, P<0.001), PPV (73.6% vs. 50.1%, P<0.001), and NPV (94.1% vs. 75.4%, P<0.001), and the ability of HD images to determine the horizontal extent of gastric cancer was significantly higher than that of SD images in terms of accuracy (69.1% vs. 58.2%, P=0.015), sensitivity (71.9% vs. 48.4%, P<0.001), PPV (68.6% vs. 38.2%, P<0.001), and NPV (73.1% vs. 58.8%, P=0.001).
Regarding undifferentiated cancer, the ability of HD imaging to detect gastric cancer was significantly higher than that of SD imaging in terms of sensitivity (100% vs. 79.4%, P=0.009), PPV (38.5% vs. 21.9%, P=0.016), and NPV (100% vs. 96.7%, P=0.009), and the ability of HD images to determine the horizontal extent of gastric cancer was significantly higher than that of SD images in terms of sensitivity (97.1% vs. 52.9%, P<0.001), PPV (37.6% vs. 20.1%, P=0.009), and NPV (99.6% vs. 89.8%, P<0.001).
Diagnostic performance of HD and SD imaging according to physician's level
We defined an expert as a Board Certified Fellow of the Japan Gastroenterological Endoscopy Society and a non-expert as an endoscopist without certification. Among the 17 endoscopists who evaluated the endoscopic image sets, 7 were experts and 10 were non-experts.
In expert endoscopists, the accuracy, sensitivity, specificity, PPV, and NPV for detecting gastric cancer in HD and SD were as follows: 80.0% vs. 70.0%, P=0.053; 94.3% vs. 75.2%, P=0.003; 65.7% vs. 64.8%, P=1.000; 74.9% vs. 56.2%, P=0.041; and 93.7% vs. 70.5%, P=0.002, respectively. In non-expert endoscopists, the accuracy, sensitivity, specificity, PPV, and NPV for detecting gastric cancer in HD and SD were as follows: 81.3% vs. 72.7%, P=0.128; 95.3% vs. 77.3%, P=0.002; 67.3% vs. 68.0%, P=0.970; 77.0% vs. 54.7%, P=0.002; and 94.3% vs. 75.5%, P=0.002, respectively.
In expert endoscopists, the accuracy, sensitivity, specificity, PPV, and NPV for determining the horizontal extent of gastric cancer in HD and SD were as follows: 71.9% vs. 55.2%, P=0.017; 78.1% vs. 45.7%, P=0.007; 65.7% vs. 64.8%, P=1.000; 71.3% vs. 58.6%, P=0.055; and 76.0% vs. 53.1%, P=0.011, respectively. In non-expert endoscopists, the accuracy, sensitivity, specificity, PPV, and NPV for determining the horizontal extent of gastric cancer in HD and SD were as follows: 70.3% vs. 59.7%, P=0.102; 73.3% vs. 51.3%, P=0.001; 67.3% vs. 68.0%, P=0.970; 72.7% vs. 63.3%, P=0.226; and 70.8% vs. 57.8%, P=0.015, respectively.
Color difference between the lesion and surrounding mucosa
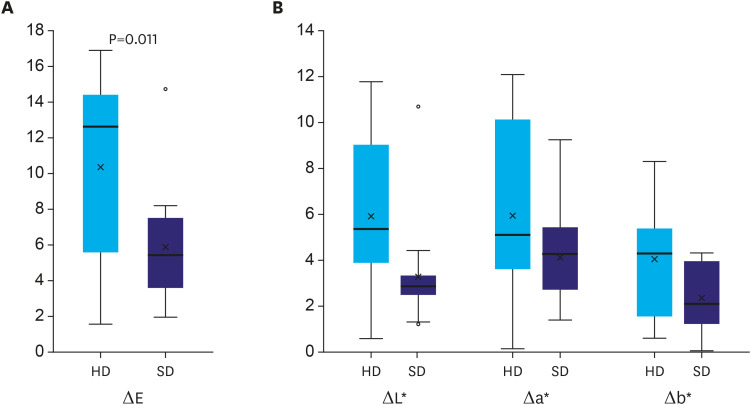
The ΔE (mean±standard deviation [range]) was 10.3±4.9 (1.6–16.9) in HD images and 5.9±3.1 (1.9–14.7) images in SD images. ΔE was significantly higher in HD images than in SD images (P=0.011) (Fig. 4A). In the subgroup analysis of ΔL*, Δa*, and Δb*, ΔL* was significantly higher in HD images than that in SD images (5.9±3.6 vs. 3.3±2.2, P=0.025). There was no significant difference in Δa* (5.9±3.5 vs. 4.1±2.0, P=0.135) and Δb* (4.1±2.4 vs. 2.3±1.4, P=0.051) (Fig. 4B).
Fig. 4. Comparison of ΔE, ΔL*, Δa*, and Δb* between HD and SD images. (A) ΔE (mean±standard deviation) was 10.3±4.9 in HD images and 5.9±3.1 in SD images. The difference was statistically significant (P=0.011). (B) ΔL* was significantly higher in HD images than in SD images (5.9±3.6 vs. 3.3±2.2, P=0.025). There was no significant difference between Δa* (5.9±3.5 vs. 4.1±2.0, P=0.135) and Δb* (4.1±2.4 vs. 2.3±1.4, P=0.051).
HD = high-definition; SD = standard-definition.
DISCUSSION
To the best of our knowledge, this is the first study to investigate and compare the diagnostic abilities of HD and SD endoscopy in gastric cancer. Our results indicated that HD imaging using an ultraslim endoscope had higher diagnostic performance in EGC than SD imaging, in particular, in terms of determining the horizontal extent of gastric cancer. Additionally, we observed a significant color difference between HD and SD imaging, which suggested that this could be the reason HD endoscopy shows a higher diagnostic ability than SD endoscopy.
Although there are no reports comparing the diagnostic ability of HD and SD imaging in gastric lesions, several studies have compared the performance of HD and SD imaging in colonoscopy. The major indicator for assessing the outcome quality of colonoscopy is the adenoma detection rate (ADR) [17,18,19]. The usefulness of HD imaging for colonoscopy ADR is debatable. Pioche et al. reported that the ADR of HD imaging was significantly higher than that of SD imaging (43.8% vs. 36.5%, P=0.030) [20]. In contrast, other studies reported that there were no significant differences in colonoscopy ADR between HD and SD imaging [21,22,23]. The difference in the impact of HD imaging between gastric and colorectal lesions could be due to the morphological differences in the lesions. More than half of EGCs are of the flat or depressed type [24], whereas most colorectal adenomas are of the protruded type, with the flat type constituting <10% of adenomas [20,21,22]. Lesions with significant morphological changes, such as protruded or pedunculated polyps, can be easily detected irrespective of the endoscopic image quality; however, lesions with few morphological changes, such as flat or depressed lesions, might be difficult to detect in poor quality endoscopy images. Some studies have provided supportive data on our hypotheses. Rastogi et al. reported that HD endoscopy improved the detection of flat colorectal adenomas compared with SD endoscopy (9.5% vs. 2.4%) [22]. The quality of HD images might help identify lesions with few morphological changes in both gastric and colorectal lesions.
Another advantage of this study was the detailed evaluation of the color difference between the lesion and the surrounding mucosa. Some reports have investigated the color difference between EGC and the surrounding mucosa and demonstrated that the average ΔE was 6.0–18.6 in white-light imaging [14,25,26]. The ΔE values obtained from HD and SD imaging in this study were within the range and comparable to those in previous reports. In this study, ΔL* in HD imaging was significantly higher than that in SD imaging. This suggests that HD imaging provides brighter and clearer images than SD imaging, which results in a higher diagnostic ability for gastric lesions. Image-enhanced endoscopic (IEE) systems that enhance the mucosal vasculature and/or architecture without using a dye have also been developed. Linked color imaging (LCI) is a newer IEE modality. LCI is intended to enhance slight color differences in the red regions of the mucosa. LCI has a higher diagnostic ability or detectability in gastritis and gastric neoplasms than white-light imaging [27,28]. Furthermore, LCI provides greater color differences in both Δa* and Δb* compared to that of white-light imaging while maintaining a high ΔL* [14,26]. These color difference data might support the clinical impact of LCI on further improving diagnostic performance in gastric diseases.
We compared HD and SD imaging using ultraslim endoscopes. HD image quality has been commonly used in standard transoral endoscopy (approximately 10 mm in diameter) or colonoscopy; however, ultraslim endoscopy with HD imaging quality is a newer development. Unsedated esophagogastroduodenoscopy using an ultraslim endoscope provides similar satisfaction for patients with reduced total procedure costs compared to sedated conventional endoscopy [29], and unsedated transnasal endoscopy using an ultraslim endoscope offers better comfort and tolerability for patients than sedated conventional transoral endoscopy [30,31]. It is therefore used to diagnose gastric cancer, Barrett’s esophagus, or esophageal varices [32,33,34]. However, transnasal endoscopy is limited by low image quality, because it does not have HD imaging capabilities. The HD ultraslim endoscope might overcome such limitations and provide better outcomes in screening patients at a higher risk of gastric cancer.
This study had some limitations. First, only a small number of still images were used in the analyses. For endoscopies in real clinical practice, lesions are diagnosed using video images and not still images. Second, the ratio of the presence to absence of gastric cancer in endoscopic images significantly differed from that in real clinical practice. The prevalence or detection rate of gastric cancer is 0.8% in clinical practice [13], and it significantly differed in half of the endoscopic images showing gastric cancer in this study. We used ROC analysis to minimize the limitations; however, the higher rate of gastric cancer might have led to overestimation of the diagnostic ability of HD imaging in this study. Finally, the validity of the evaluation method for determining the horizontal extent of gastric cancer is uncertain. We believe that identifying all included sections approximately determines the extent of the lesion. However, as other studies have reported [35], tracing or delineating the lesion border in endoscopic images is the method to precisely evaluate lesion extent. Therefore, this study result cannot be directly generalized to clinical practice due to the limitation of a small number of still images used, the significant difference in the detection rate of gastric cancer, and the method of determining the horizontal extent of the lesion.
In conclusion, HD imaging ultraslim endoscopy showed higher diagnostic performance in EGC than SD imaging because HD imaging provided a brighter contrast in color difference between the lesion and surrounding mucosa. HD was more useful than SD for detecting EGC. HD endoscopy is recommended for gastric cancer screening, especially for high-risk patients.
Footnotes
Funding: This study received technical assistance from Olympus Corporation. Olympus Corporation did not influence the data analysis and was not involved in this study.
- Conceptualization: S.S., G.T.
- Data curation: S.S.
- Formal analysis: S.T., S.S.
- Funding acquisition: G.T., M.M.
- Investigation: S.T., I.R., O.K.
- Methodology: S.S.
- Project administration: S.S.
- Supervision: K.C., I.H.
- Writing - original draft: S.T., S.S.
- Writing - review & editing: I.R., O.K., K.C., I.H., G.T., M.M.
Conflict of Interest: Takuji Gotoda received an honorarium from Olympus Corporation.
SUPPLEMENTARY MATERIAL
Two examples of a pair of endoscopic images comparing HD and SD imaging. (A, B) Depressed type lesion, 20 mm in diameter, located at the posterior wall of the gastric antrum (white arrows). This lesion was examined and captured using HD and SD endoscopy with similar composition (A; taken using HD endoscopy, B; taken using SD endoscopy). (C, D) Depressed type lesion, 10 mm in diameter, located at the anterior wall of the gastric antrum (yellow arrows). This lesion was examined and captured using HD and SD endoscopy with similar composition (C; taken using HD endoscopy, D; taken using SD endoscopy).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333–341.e1. doi: 10.1016/j.gie.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Gu L, Khadaroo PA, Chen L, Li X, Zhu H, Zhong X, et al. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for early gastric cancer: a systematic review and meta-analysis. J Gastrointest Surg. 2019;23:1493–1501. doi: 10.1007/s11605-019-04227-8. [DOI] [PubMed] [Google Scholar]
- 4.Jeon HK, Kim GH, Lee BE, Park DY, Song GA, Kim DH, et al. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric Cancer. 2018;21:133–143. doi: 10.1007/s10120-017-0719-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Chung H. Endoscopic resection of undifferentiated-type early gastric cancer. J Gastric Cancer. 2020;20:345–354. doi: 10.5230/jgc.2020.20.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490–499. doi: 10.1007/s10120-017-0772-z. [DOI] [PubMed] [Google Scholar]
- 7.Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175–184. doi: 10.1007/s00535-016-1210-4. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi S, Sakakibara Y, Sakuramoto S, Kobayashi N, Shimao H, Mieno H, et al. Recent results in the surgical treatment of gastric cancer according to the Japanese and TNM classification. Anticancer Res. 2001;21:3589–3593. [PubMed] [Google Scholar]
- 9.Kim JH, Kim SS, Lee JH, Jung DH, Cheung DY, Chung WC, et al. Early detection is important to reduce the economic burden of gastric cancer. J Gastric Cancer. 2018;18:82–89. doi: 10.5230/jgc.2018.18.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer. 2001;4:132–136. doi: 10.1007/pl00011735. [DOI] [PubMed] [Google Scholar]
- 11.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84:18–28. doi: 10.1016/j.gie.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Hamashima C, Shabana M, Okada K, Okamoto M, Osaki Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744–1749. doi: 10.1111/cas.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328.e7. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Kanzaki H, Takenaka R, Kawahara Y, Kawai D, Obayashi Y, Baba Y, et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open. 2017;5:E1005–E1013. doi: 10.1055/s-0043-117881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehni RG. Color-tolerance data and the tentative CIE 1976 L a b formula. J Opt Soc Am. 1976;66:497–500. doi: 10.1364/josa.66.000497. [DOI] [PubMed] [Google Scholar]
- 16.Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 17.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Millan MS, Gross P, Manilich E, Church JM. Adenoma detection rate: the real indicator of quality in colonoscopy. Dis Colon Rectum. 2008;51:1217–1220. doi: 10.1007/s10350-008-9315-3. [DOI] [PubMed] [Google Scholar]
- 19.Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923–1929. doi: 10.1136/gutjnl-2016-312044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pioche M, Denis A, Allescher HD, Andrisani G, Costamagna G, Dekker E, et al. Impact of 2 generational improvements in colonoscopes on adenoma miss rates: results of a prospective randomized multicenter tandem study. Gastrointest Endosc. 2018;88:107–116. doi: 10.1016/j.gie.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Pellisé M, Fernández-Esparrach G, Cárdenas A, Sendino O, Ricart E, Vaquero E, et al. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: a randomized controlled trial. Gastroenterology. 2008;135:1062–1068. doi: 10.1053/j.gastro.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann-Fraedrich K, Groth S, Sehner S, Schubert S, Aschenbeck J, Mayr M, et al. Effects of two instrument-generation changes on adenoma detection rate during screening colonoscopy: results from a prospective randomized comparative study. Endoscopy. 2018;50:878–885. doi: 10.1055/a-0607-2636. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: ‘Real-world evidence’ in Japan. Dig Endosc. 2019;31:30–39. doi: 10.1111/den.13246. [DOI] [PubMed] [Google Scholar]
- 25.Fujiyoshi T, Miyahara R, Funasaka K, Furukawa K, Sawada T, Maeda K, et al. Utility of linked color imaging for endoscopic diagnosis of early gastric cancer. World J Gastroenterol. 2019;25:1248–1258. doi: 10.3748/wjg.v25.i10.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda H, Miura Y, Osawa H, Takezawa T, Ino Y, Okada M, et al. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J Gastroenterol. 2019;54:396–406. doi: 10.1007/s00535-018-1515-6. [DOI] [PubMed] [Google Scholar]
- 27.Dohi O, Majima A, Naito Y, Yoshida T, Ishida T, Azuma Y, et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig Endosc. 2020;32:191–203. doi: 10.1111/den.13540. [DOI] [PubMed] [Google Scholar]
- 28.Ono S, Kawada K, Dohi O, Kitamura S, Koike T, Hori S, et al. Linked color imaging focused on neoplasm detection in the upper gastrointestinal tract: a randomized trial. Ann Intern Med. 2021;174:18–24. doi: 10.7326/M19-2561. [DOI] [PubMed] [Google Scholar]
- 29.Garcia RT, Cello JP, Nguyen MH, Rogers SJ, Rodas A, Trinh HN, et al. Unsedated ultrathin EGD is well accepted when compared with conventional sedated EGD: a multicenter randomized trial. Gastroenterology. 2003;125:1606–1612. doi: 10.1053/j.gastro.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Atar M, Kadayifci A. Transnasal endoscopy: Technical considerations, advantages and limitations. World J Gastrointest Endosc. 2014;6:41–48. doi: 10.4253/wjge.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroppa I, Grasso E, Paoluzi OA, Razzini C, Tosti C, Andrei F, et al. Unsedated transnasal versus transoral sedated upper gastrointestinal endoscopy: a one-series prospective study on safety and patient acceptability. Dig Liver Dis. 2008;40:767–775. doi: 10.1016/j.dld.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Honing J, Kievit W, Bookelaar J, Peters Y, Iyer PG, Siersema PD. Endosheath ultrathin transnasal endoscopy is a cost-effective method for screening for Barrett's esophagus in patients with GERD symptoms. Gastrointest Endosc. 2019;89:712–722.e3. doi: 10.1016/j.gie.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 33.McGoran J, Bennett A, Cooper J, De Caestecker J, Lovat LB, Guha N, et al. Acceptability to patients of screening disposable transnasal endoscopy: qualitative interview analysis. BMJ Open. 2019;9:e030467. doi: 10.1136/bmjopen-2019-030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanuma T, Morita Y, Doyama H. Current status of transnasal endoscopy worldwide using ultrathin videoscope for upper gastrointestinal tract. Dig Endosc. 2016;28(Suppl 1):25–31. doi: 10.1111/den.12612. [DOI] [PubMed] [Google Scholar]
- 35.Nagahama T, Yao K, Uedo N, Doyama H, Ueo T, Uchita K, et al. Delineation of the extent of early gastric cancer by magnifying narrow-band imaging and chromoendoscopy: a multicenter randomized controlled trial. Endoscopy. 2018;50:566–576. doi: 10.1055/s-0044-100790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two examples of a pair of endoscopic images comparing HD and SD imaging. (A, B) Depressed type lesion, 20 mm in diameter, located at the posterior wall of the gastric antrum (white arrows). This lesion was examined and captured using HD and SD endoscopy with similar composition (A; taken using HD endoscopy, B; taken using SD endoscopy). (C, D) Depressed type lesion, 10 mm in diameter, located at the anterior wall of the gastric antrum (yellow arrows). This lesion was examined and captured using HD and SD endoscopy with similar composition (C; taken using HD endoscopy, D; taken using SD endoscopy).