Abstract
Purpose
The numeric N stage has replaced the topographic N stage in the current tumor node metastasis (TNM) staging in gastric carcinoma. However, the usefulness of the topographic N stage in the current TNM staging system is uncertain. We aimed to investigate the prognostic value of the topographic N stage in the current TNM staging system.
Materials and Methods
We reviewed the data of 3350 patients with gastric cancer who underwent curative gastrectomy. The anatomic regions of the metastatic lymph nodes (MLNs) were classified into 2 groups: perigastric and extra-perigastric. The prognostic value of the anatomic region was analyzed using a multivariate prognostic model with adjustments for the TNM stage.
Results
In patients with lymph node metastasis, extra-perigastric metastasis demonstrated significantly worse survival than perigastric metastasis alone (5-year survival rate, 39.6% vs. 73.1%, respectively, P<0.001). Extra-perigastric metastasis demonstrated significantly worse survival within the same pN stage; the multivariate analysis indicated that extra-perigastric metastasis was an independent poor prognostic factor (hazard ratio=1.33; 95% confidence interval=1.01–1.75). The anatomic region of the MLNs improved the goodness-of-fit (likelihood ratio statistics, 4.57; P=0.033) of the prognostic model using the TNM stage.
Conclusions
The anatomic region of MLNs has an independent prognostic value in the numeric N stage in the current TNM staging of gastric carcinoma.
Keywords: Stomach neoplasms, Lymph node metastasis, Neoplasm staging, Prognosis
INTRODUCTION
Gastric cancer is one of the commonest malignancies and the leading cause of cancer-related mortality in East Asia despite a decline in its global incidence [1,2]. With advances in the diagnosis and management of gastric cancer, the available treatment options include endoscopic treatment, preoperative chemotherapy or chemoradiation therapy, molecular targeting agents, and immunotherapy [3]. Curative surgery, including gastric resection and regional lymph node dissection, is the mainstay of treatment in gastric cancer. Despite the controversy between the East and West, D2 lymphadenectomy is recommended as a standard procedure to ensure adequate local disease control and accurate tumor staging [4].
Lymph node metastasis is the most relevant prognostic indicator in gastric cancer. Meticulous examination of lymph node metastasis is essential in accurately predicting the prognosis in patients with gastric cancer. Over the last few decades, there have been many changes in the classification of lymph node metastasis in gastric cancer. The topographic N staging based on the anatomic region of metastatic lymph nodes (MLNs) was introduced in Japan; it demonstrated excellent discriminative prognostic value in gastric cancer [5]. However, the non-quantitative characteristics of the topographic N stage have led to inconsistent results between different extents of lymph node dissection. The numeric N stage based on the number of MLNs was adopted in the fifth edition of the Union for International Cancer Control (UICC) tumor node metastasis (TNM) system (1997) [6] and the third English edition of the Japanese Gastric Cancer Classification (2011) [7]. The simplicity and objectivity of the numeric N stage improved the efficiency of the TNM staging system. However, the best cut-offs for the optimal N stage remain uncertain in the current TNM system.
Several studies have demonstrated the superior prognostic performance of the numeric N stage over the topographic N stage in gastric cancer [8,9,10,11]. However, the numeric N stage has limitations, including the lack of information regarding the anatomical extent of MLNs and the surgical extent of lymph node dissection. Therefore, it can be speculated that integrating the number and anatomic region of MLNs may improve the prognostic performance of the TNM stage. However, there is little information regarding the prognostic value of the anatomic region of MLNs in the current TNM staging system for gastric cancer. Therefore, in this study, we aimed to investigate the prognostic value of the anatomic region of MLNs in the current TNM staging of gastric carcinoma.
MATERIALS AND METHODS
Patients and data
We retrospectively reviewed the data of patients who underwent surgery for gastric cancer between 2009 and 2013 at our institution, from our gastric cancer database. The inclusion criteria were patients who had undergone curative gastrectomy for gastric cancer. We identified 3,481 eligible patients and excluded 131 patients who had received preoperative chemotherapy (n=29), other malignancies (n=32), remnant gastric cancer (n=42), and incomplete medical records (n=28). A total of 3,350 patients were included in the study. The clinicopathological data were retrieved from our prospectively constructed gastric cancer database and medical records. We collected data regarding patient demographics, preoperative examination findings, operative results, and pathologic outcomes. The pathologic stage was based on the 8th edition of the UICC TNM classification of gastric cancer [12].
The primary outcome was overall survival. Survival time was defined as the time from surgery to death due to any cause. We ascertained the survival status of the patients using the Korean National Cancer Registry and medical records. The last follow-up of survival was in December 2017, and the median follow-up duration was 64 months (range: 36–112 months). The institutional review board of our institution approved this study, and the requirement for informed consent was waived.
Operative procedures and postoperative follow-up
Patients underwent preoperative staging using endoscopy with biopsy and multi-detector abdominal computed tomography (CT). Endoscopic ultrasonography, liver magnetic resonance imaging, chest CT, or positron emission tomography/CT were performed as needed in selected patients. The operative techniques, including gastric resection and lymphadenectomy, were performed according to the gastric cancer treatment guidelines [13,14]. D2 lymphadenectomy was performed as the standard procedure for tumors of stage ≥ T2 or N+; otherwise, D1+ lymphadenectomy was performed. Laparoscopic surgery was indicated for clinically T1-2N0 tumors.
After the surgery, patients with tumors of pathologic stage ≥II received adjuvant chemotherapy with oral fluoropyrimidine (S-1) or capecitabine plus oxaliplatin regimen. Patients were routinely followed up at 1, 3, 6, and 12 months postoperatively. Subsequently, patients were followed up every 6 months up to 5 years postoperatively. During the follow-up, patients underwent abdominal CT every 6 months and endoscopy every 12 months.
Classification of the anatomic region of MLNs
Three experienced gastric surgeons performed all the surgeries. Postoperatively, the operating surgeon routinely performed lymph node retrieval from the gastric specimen. Lymph nodes were classified into their respective lymph node stations, which were defined by the Japanese classification of gastric carcinoma [7]. Routine pathological examination reports included the number of harvested lymph nodes and MLNs in each lymph node station.
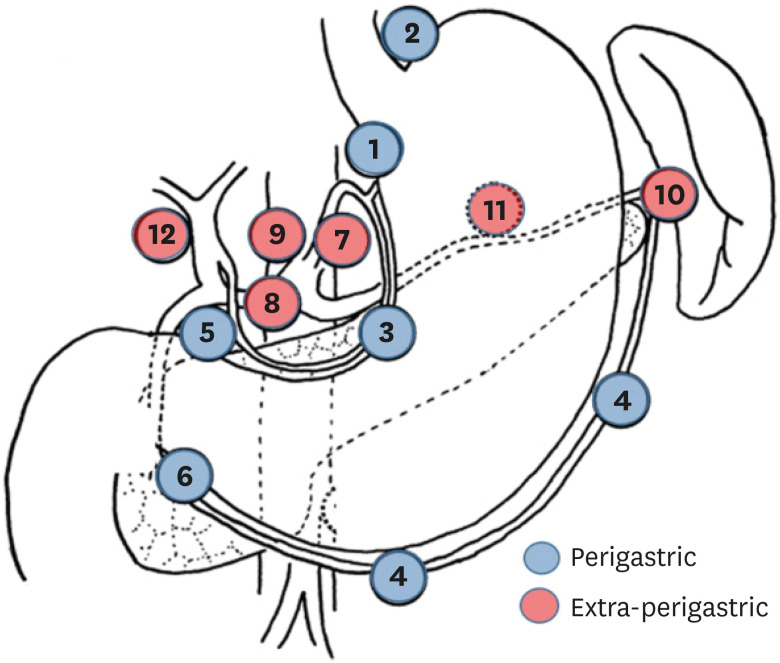
We classified the anatomic regions of MLNs into two groups: perigastric and extra-perigastric nodes (Fig. 1). Perigastric metastasis included lymph node metastasis in the perigastric regions (No. 1, 2, 3, 4, 5, and 6). Extra-perigastric metastasis included lymph node metastasis in the extra-perigastric regions (No. 7, 8a, 9, 10, 11, and 12a). Cases of skipped metastasis into the extra-perigastric region were classified as extra-perigastric metastasis.
Fig. 1. Classification of the anatomic region of metastatic lymph nodes.
Statistics
Continuous variables were compared using Student's t-test, and categorical variables were compared using the χ2 test or Fisher's exact test. The Kaplan-Meier method was used to construct survival curves, and the log-rank test was used to compare survival between the groups. Cox proportional hazards model was used for the univariate and multivariate analyses of prognostic factors. In the multivariate analysis, we used a backward stepwise selection method to identify the best predictive prognostic factors. The proportional hazards assumption was verified using a log-minus-log survival plot.
To examine the performance of the prognostic models, we calculated the likelihood ratio statistics (–2 log-likelihood) and Harrell C-index. Briefly, a lower value of –2 log-likelihood indicates a better goodness-of-fit of a model, and a higher Harrell C-index indicates a better discriminative ability. All statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). Differences were considered statistically significant based on two-sided P-values <0.05.
RESULTS
Patient characteristics
The clinicopathological characteristics of the patients are presented in Table 1. The cohort included 2,221 men and 1,129 women, with a mean age of 61.3±11.9 years. The mean body mass index was 23.6±3.2 kg/m2. Open and laparoscopic surgeries were performed in 1,399 (41.8%) and 1,951 (58.2%) patients, respectively. Overall, 1,777 (53.0%) patients underwent D2 lymphadenectomy. The mean number of harvested lymph nodes was 42±15 (range, 9–121). The final pathologic examination revealed stage I tumors in 2,374 (70.9%) patients, stage II tumors in 444 (13.3%) patients, and stage III tumors in 532 (18.9%) patients.
Table 1. Clinicopathological characteristics.
| Variables | Patients (n=3,350) | |
|---|---|---|
| Age (yr) | 61.3±11.9 | |
| Body mass index (kg/m2) | 23.6±3.2 | |
| Sex | ||
| Male | 2,221 (66.3) | |
| Female | 1,129 (33.7) | |
| Comorbidity | 1,941 (57.9) | |
| Operative approach | ||
| Open | 1,399 (41.8) | |
| Laparoscopy | 1,951 (58.2) | |
| Gastric resection | ||
| Distal gastrectomy | 2,720 (81.2) | |
| Total gastrectomy | 608 (18.1) | |
| Others | 22 (0.7) | |
| Lymph node dissection | ||
| D1+ | 1,573 (47.0) | |
| D2 | 1,777 (53.0) | |
| Tumor location | ||
| Lower third | 1,770 (52.8) | |
| Middle third | 1,085 (32.4) | |
| Upper third | 458 (13.7) | |
| Whole stomach | 37 (1.1) | |
| Tumor size (cm) | 3.5±2.5 | |
| Tumor number | ||
| 1 | 3,177 (94.8) | |
| 2 | 146 (4.4) | |
| ≥3 | 27 (0.8) | |
| Histologic differentiation | ||
| Differentiated | 1,639 (48.9) | |
| Undifferentiated | 1,711 (51.0) | |
| Lymphovascular invasion | 832 (24.8) | |
| Tumor invasion (pT)* | ||
| pT1 | 2,222 (66.3) | |
| pT2 | 332 (9.9) | |
| pT3 | 389 (11.6) | |
| pT4a | 378 (11.3) | |
| pT4b | 29 (0.9) | |
| Lymph node metastasis (pN)* | ||
| pN0 | 2,473 (73.8) | |
| pN1 | 347 (10.4) | |
| pN2 | 247 (7.4) | |
| pN3a | 174 (5.2) | |
| pN3b | 109 (3.3) | |
| Region of metastatic lymph nodes | ||
| Perigastric | 567 (16.9) | |
| Extra-perigastric | 310 (9.3) | |
| TNM stage* | ||
| Stage I | 2,374 (70.9) | |
| Stage II | 444 (13.3) | |
| Stage III | 532 (18.9) | |
Data are expressed as mean±standard deviation or number (%).
TMN = tumor node metastasis.
*According to the 8th edition of the Union for International Cancer Control tumor node metastasis classification of gastric cancer.
Survival according to the anatomic location of MLNs
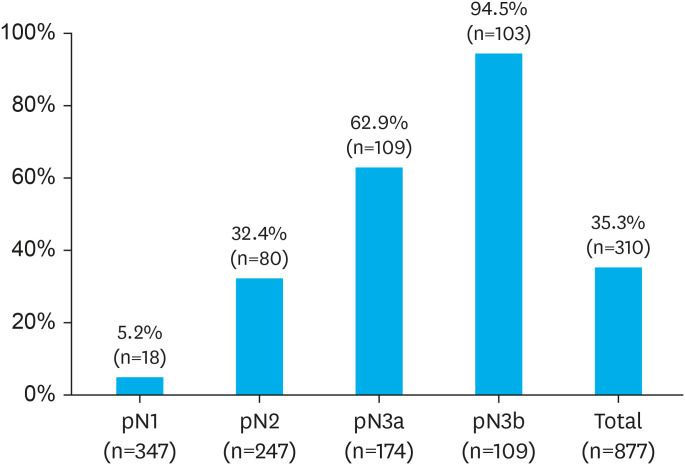
Overall, 877 (26.2%) patients had lymph node metastases. According to the anatomic regions of these MLNs, 567 and 310 patients had perigastric and extra-perigastric metastases, respectively. Fig. 2 illustrates the incidence of extra-perigastric metastasis at each pN stage. The incidence of extra-perigastric metastasis in pN1, pN2, pN3a, and pN3b was 5.2%, 32.4%, 62.6%, and 94.5%, respectively.
Fig. 2. The proportion of extra-perigastric metastases in the pN stage.
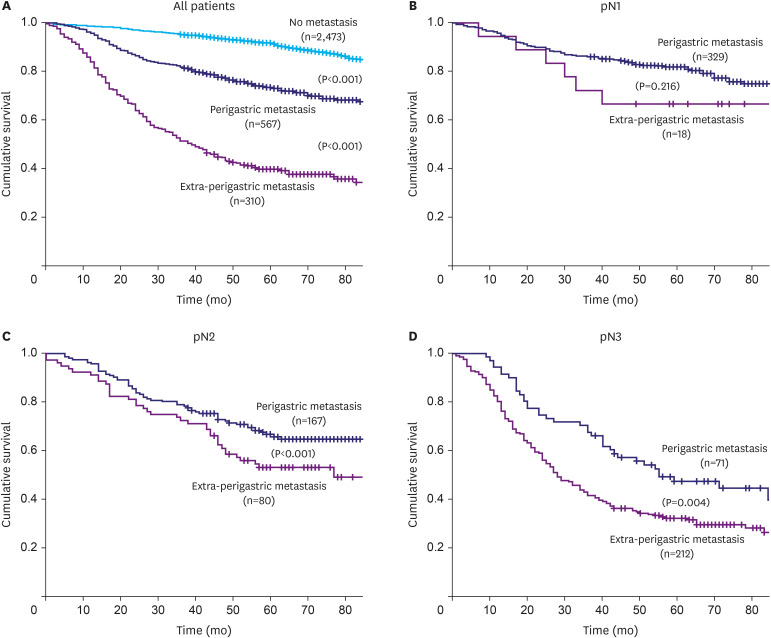
Fig. 3 illustrates the survival curves according to the anatomic region of the MLNs. Patients with extra-perigastric metastasis demonstrated significantly worse survival than those with perigastric metastasis (Fig. 3A). The 5-year survival rate (5-YSR) in the perigastric and extra-perigastric groups was 73.1% and 39.6%, respectively (log-rank P<0.001). When the survival in these groups was compared within the same pN stage, the extra-perigastric metastasis group demonstrated significantly worse survival than the perigastric metastasis group for pN2 (5-YSR, 55.7% vs. 76.8%, respectively; P<0.001) and pN3 groups (5-YSR, 33.2% vs. 47.4%, respectively; P=0.004) (Fig. 3C and D). The extra-perigastric metastasis group also demonstrated worse survival in the pN1 group (5-YSR, 66.7% vs. 81.9%, respectively); however, the difference was not statistically significant (Fig. 3B).
Fig. 3. Survival curves according to the anatomic region of metastatic lymph nodes. (A) All patients, (B) the pN1 group, (C) the pN2 group, and (D) the pN3 group.
Prognostic value of the anatomic region of MLNs
Table 2 summarizes the univariate and multivariate analyses of various prognostic factors. We examined the prognostic significance of the anatomic region of MLNs and other pathological characteristics, such as histologic differentiation, tumor size, lymphovascular invasion, tumor number, tumor location, pT stage, and pN stage. Among these factors, the anatomic region of MLNs, tumor size, tumor location, lymphovascular invasion, tumor number, pT stage, and pN stage were significantly associated with survival in univariate analysis. To identify the best predictive prognostic factors, we performed multivariate analysis with all the variables, using the backward stepwise selection method. Multivariate analysis revealed that the anatomic region of MLNs was an independent prognostic factor along with tumor size, pT stage, and pN stage.
Table 2. Univariate and multivariate analysis of prognostic factors.
| Variables | Univariate | Multivariate* | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Anatomic region of MLNs | |||||
| Perigastric | 2.87 (2.37–3.47) | < 0.001 | 5.65 (3.71–8.59) | < 0.001 | |
| Extra-perigastric | 8.45 (7.03–10.14) | < 0.001 | 7.42 (5.36–10.27) | < 0.001 | |
| Undifferentiated tumor | 1.14 (0.97–1.32) | 0.108 | |||
| Tumor size (cm) | 1.22 (1.99–1.25) | < 0.001 | 1.05 (1.01–1.08) | 0.001 | |
| Lymphovascular invasion | 2.87 (2.46–3.35) | < 0.001 | |||
| Tumor number (vs. 1) | |||||
| 2 | 0.93 (0.63–1.37) | 0.717 | |||
| ≥3 | 2.55 (1.44–4.51) | 0.001 | |||
| Tumor location (vs. lower third) | |||||
| Middle third | 0.79 (0.66–0.95) | 0.012 | |||
| Upper third | 1.46 (1.19–1.80) | < 0.001 | |||
| Whole stomach | 5.04 (3.30–7.70) | < 0.001 | |||
| pT stage (vs. pT1) | |||||
| pT2 | 1.78 (1.35–2.36) | < 0.001 | 1.37 (1.02–1.85) | 0.034 | |
| pT3 | 3.53 (2.85–4.37) | < 0.001 | 1.91 (1.45–2.50) | < 0.001 | |
| pT4a | 6.99 (5.79–8.46) | < 0.001 | 2.60 (1.95–3.46) | < 0.001 | |
| pT4b | 15.44 (9.96–23.93) | < 0.001 | 5.92 (3.63–9.63) | < 0.001 | |
| pN stage (vs. pN0) | |||||
| pN1 | 2.03 (1.58–2.62) | < 0.001 | 1.40 (1.07–1.85) | 0.014 | |
| pN2 | 4.03 (3.20–5.08) | < 0.001 | 2.29 (1.74–3.02) | < 0.001 | |
| pN3a | 6.60 (5.24–8.32) | < 0.001 | 3.24 (2.42–4.33) | < 0.001 | |
| pN3b | 18.16 (14.30–23.06) | < 0.001 | 7.78 (5.72–10.57) | < 0.001 | |
MLN = metastatic lymph node; HR = hazard ratio; CI = confidence interval.
*Multivariate model using the backward stepwise selection method.
Table 3 summarizes the prognostic indexes of the TNM stage combined with the anatomic region of the MLNs. The conventional prognostic model included pT and pN stages, and the extended model included the anatomic region of MLNs and pT and pN stages. Integrating the anatomic region of MLNs into the TNM stage increased the likelihood ratio statistics (4.57; P=0.033), thus indicating an improvement in the prognostic model's goodness-of-fit. Harrell's C-index values also increased in the model after integrating the anatomic region of MLNs (from 0.7414 to 0.7419) but did not reach statistical significance.
Table 3. Performance of the prognostic models.
| Variables | Conventional model | Extended model | P |
|---|---|---|---|
| Variables in the model | pT stage | pT stage | |
| pN stage | pN stage | ||
| Anatomic region of MLNs | |||
| Harrell's C-index | 0.7414 | 0.7419 | 0.912 |
| −2 log-likelihood | 9,516.19 | 9,511.62 | 0.033 |
MLNs = metastatic lymph nodes.
DISCUSSION
The current TNM staging system does not consider the anatomical extent of lymph node metastasis in predicting the prognosis in gastric cancer. In this study, we investigated whether the anatomic region of MLNs has any prognostic value in the current TNM staging system. We found that survival within the same pN stage varied significantly between the groups with different anatomic regions of the MLNs. Multivariate analysis adjusted for TNM stage revealed that the anatomic region of MLNs was an independent prognostic factor. Furthermore, the anatomic region of the MLNs improved the prognostic performance of the TNM stage. Our results suggest that the anatomic region of MLNs could be used as a reliable prognostic indicator in the current TNM staging of gastric cancer.
The topographic N stage emerged in Japan in the 1970s and was widely used to stage gastric cancer until the late 1990s. It determines the status of lymph node metastasis based on the anatomic location of the MLNs relative to the primary tumor [5]. The topographic N stage has an excellent discriminative ability; however, its non-quantitative characteristics result in an inefficient evaluation of lymph node metastasis depending on the extent of lymph node dissection. Since the 5th edition of the UICC TNM staging in 1997 [6], the numeric N stage—based on the number of MLNs—has replaced the topographic N stage in gastric cancer staging. The numeric N stage improved the nodal staging's simplicity and objectivity, which increased the strength of prognostic stratification over the topographic N stage [8,9,10,11]. However, the lack of information regarding the anatomical extent of the MLNs and the surgical extent of lymph node dissection is a problem with the numeric N stage.
More recently, several studies have reappraised the anatomic region of MLNs in the evaluation of the prognosis of gastric cancer. Choi et al. [15] examined the prognostic value of topographic N stage in 6,025 patients with gastric cancer. They classified the N stage according to three anatomic regions (lesser curvature, greater curvature, and extra-perigastric regions). The new TNM staging with this topographic N stage demonstrated similar prognostic performance as that of the current TNM system. The authors insisted that the topographic N stage would be efficient because of its convenience while maintaining comparable prognostic performance. Subsequent studies that validated this new topographic TNM staging system demonstrated better predictive ability with the use of the topographic N stage in gastric cancer [16,17]. Lauricella et al. [18] also demonstrated that the TNM staging with the topographic N stage had better homogeneity and discriminative value when compared with the 8th UICC TNM system. The determination of the superiority of one staging system over another remains controversial. We believe that it would be wise to develop a prognostic model that can adequately integrate the number and anatomic extent of lymph node metastasis.
It is well-known that gastric cancer metastasizes to groups of lymph nodes arranged radially around the stomach in tiers [19]. Therefore, the anatomic region of MLNs reflects the metastatic potential of the primary tumor. Our findings revealed that patients with extra-perigastric metastases had substantially worse survival than those with perigastric metastases within the same pN stage. Multivariate analysis of the prognostic factors revealed that the anatomic region of MLNs was an independent prognostic factor in combination with the TNM stage, which indicates that patients with extra-perigastric metastasis may require more attention regarding disease recurrence and postoperative surveillance. It would be reasonable to consider both the number and anatomic regions of MLNs to improve the prognostic stratification and devise an appropriate treatment plan.
Many researchers have suggested different classifications of lymph node metastasis than the numeric or topographic N stage in gastric cancer. Some researchers have suggested that the ratio of metastatic lymph nodes to harvested lymph nodes is a good alternative as it reduces the stage migration and provides useful prognostic stratification [20,21]. However, its prognostic superiority remains controversial, and there is no universally accepted cut-off ratio for adequate staging. The logarithmic odds of positive lymph nodes to negative lymph nodes was recently introduced as a new useful classification of lymph node metastasis [22]. These methods are fundamentally based on the number of MLNs. However, there is no proper N staging system that integrates the number and anatomic regions of MLNs.
Unfortunately, the present study could not introduce a new N stage that included the anatomic region of MLNs. This was because our sample size was not large enough to develop and validate a new N stage. Some may criticize that the N stage, which combines the number and anatomic region of MLNs, would make nodal staging more complicated for clinical use. Recently, various prognostic models, such as a nomogram or scoring system, have been used to obtain more practical information about patient survival [23,24]. The advantage of these models is that they estimate the actual survival rather than merely stratifying prognostic groups. The existing models include various prognostic factors in addition to the TNM stage, such as the age, sex, histologic type, tumor location, extent of gastric resection, and number of retrieved lymph nodes. No previous models have used the anatomical regions of MLNs. We believe that the anatomic region of MLNs can be an important prognostic indicator that can improve the performance of prognostic models in gastric cancer.
This study had some limitations. First, it was performed at a single high-volume institution in Korea; large multi-institutional studies are required to validate our results. Second, this study did not include patients who received preoperative chemotherapy. The applicability of the anatomic region of MLNs needs to be further investigated in such patients. Lastly, the accuracy of the anatomic regions of MLNs may rely on the operative quality of dissection and retrieval of lymph nodes, which may limit the generalizability of our results. Despite these limitations, our study demonstrated the prognostic value of the anatomic region of MLNs in a large cohort of patients with meticulous examinations of lymph node metastasis.
In conclusion, this study demonstrated that the anatomic region of MLNs has independent prognostic value in the current TNM staging of gastric cancer. Additionally, patients with extra-perigastric metastasis may require more attention regarding disease recurrence and postoperative surveillance. In future studies, a prognostic model that integrates the number and anatomic regions of MLNs may need to be developed.
Footnotes
- Conceptualization: J.O.
- Data curation: K.J.H.
- Formal analysis: J.O., J.M.R.
- Methodology: J.O.
- Supervision: J.M.R.
- Validation: J.O.
- Writing - original draft: J.O.
- Writing - review & editing: K.J.H., J.M.R.
Conflict of Interest: No potential conflicts of interest relevant to this article are reported.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–315. doi: 10.4143/crt.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilson DH. Advances in the treatment of gastric cancer. Curr Opin Gastroenterol. 2018;34:465–468. doi: 10.1097/MOG.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 4.Batista TP, Martins MR. Lymph node dissection for gastric cancer: a critical review. Oncol Rev. 2012;6:e12. doi: 10.4081/oncol.2012.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami T Japanese Research Society for Gastric Cancer. The general rules for The gastric cancer study in surgery. Jpn J Surg. 1973;3:61–71. doi: 10.1007/BF02469463. [DOI] [PubMed] [Google Scholar]
- 6.Sobin LH, Wittekind CH. TNM Classification of Malignant Tumors. 5th ed. New York (NY): Wiley; 1997. pp. 59–62. [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 8.Yoo CH, Noh SH, Kim YI, Min JS. Comparison of prognostic significance of nodal staging between old (4th edition) and new (5th edition) UICC TNM classification for gastric carcinoma. International Union Against Cancer. World J Surg. 1999;23:492–498. doi: 10.1007/pl00012337. [DOI] [PubMed] [Google Scholar]
- 9.Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, et al. Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86:553–558. doi: 10.1002/(sici)1097-0142(19990815)86:4<553::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Fujii K, Isozaki H, Okajima K, Nomura E, Niki M, Sako S, et al. Clinical evaluation of lymph node metastasis in gastric cancer defined by the fifth edition of the TNM classification in comparison with the Japanese system. Br J Surg. 1999;86:685–689. doi: 10.1046/j.1365-2168.1999.01115.x. [DOI] [PubMed] [Google Scholar]
- 11.Nio Y, Tsubono M, Kawabata K, Masai Y, Hayashi H, Meyer C, et al. Comparison of survival curves of gastric cancer patients after surgery according to the UICC stage classification and the General Rules for Gastric Cancer Study by the Japanese Research Society for gastric cancer. Ann Surg. 1993;218:47–53. doi: 10.1097/00000658-199307000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. New York (NY): Wiley; 2016. pp. 63–66. [Google Scholar]
- 13.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 14.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YY, An JY, Katai H, Seto Y, Fukagawa T, Okumura Y, et al. A lymph node staging system for gastric cancer: a hybrid type based on topographic and numeric systems. PLoS One. 2016;11:e0149555. doi: 10.1371/journal.pone.0149555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MW, Chan CP, Lin YJ, Yen HH. Anatomical location-based nodal staging system is superior to the 7th edition of the American Joint Committee on Cancer staging system among patients with surgically resected, histologically low-grade gastric cancer: a single institutional experience. PLoS One. 2019;14:e0211836. doi: 10.1371/journal.pone.0211836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Diana A, et al. Comparison of the current AJCC-TNM numeric-based with a new anatomical location-based lymph node staging system for gastric cancer: a western experience. PLoS One. 2017;12:e0173619. doi: 10.1371/journal.pone.0173619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauricella S, Caricato M, Mascianà G, Carannante F, Carnazza M, Bonaccorso A, et al. Topographic lymph node staging system shows prognostic superiority compared to the 8th edition of AJCC TNM in gastric cancer. A western monocentric experience. Surg Oncol. 2020;34:223–233. doi: 10.1016/j.suronc.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Lirosi MC, Biondi A, Ricci R. Surgical anatomy of gastric lymphatic drainage. Transl Gastroenterol Hepatol. 2017;2:14. doi: 10.21037/tgh.2016.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong SH, Lee HJ, Ahn HS, Kim JW, Kim WH, Lee KU, et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg. 2012;255:50–58. doi: 10.1097/SLA.0b013e31821d4d75. [DOI] [PubMed] [Google Scholar]
- 22.Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS, Zhang DS, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One. 2012;7:e31736. doi: 10.1371/journal.pone.0031736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo Y, Son T, Song K, Okumura N, Hu Y, Cho GS, et al. A novel prediction model of prognosis after gastrectomy for gastric carcinoma: development and validation using Asian databases. Ann Surg. 2016;264:114–120. doi: 10.1097/SLA.0000000000001523. [DOI] [PubMed] [Google Scholar]
- 24.Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after D2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–3840. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]