Abstract
Reasons for the Study:
The Neuropsychiatric Inventory (NPI) has been used for studies of neuropsychiatric symptoms in neurodegenerative disorders for the past 25 years. This article reviews the history of the development and application of the NPI.
Main Findings:
The NPI consists of 10 (or 12) items that are assayed with questions, subquestions, and ratings of frequency and severity. The NPI has been shown to be valid and reliable. The NPI has been translated into approximately 40 languages; it has 4 of versions designed for different clinical applications. The NPI studies show contrasting profiles of behavioral symptoms in different neurologic disorders. The NPI has been used in approximately 350 clinical trials. In economic studies, the NPI captures the cost of behavioral symptoms in dementias.
Principle Conclusions:
The NPI is a useful instrument for capturing behavioral changes in Alzheimer disease and other neurodegenerative disorders.
Keywords: Neuropsychiatric Inventory (NPI), Alzheimer disease, agitation, psychosis, depression, apathy
The Neuropsychiatric Inventory (NPI) was developed to assess the neuropsychiatric syndromes that occur in Alzheimer disease (AD) and other neurodegenerative disorders (NDDs).1 It now has 4 major versions, has been translated into more than 40 languages, and has been used to characterize the neuropsychiatric features of most NDDs as well as other neurologic and psychiatric illnesses. The NPI is used in many types of interventional and noninterventional research where collecting neuropsychiatric information is important. The availability of the many translations of the NPI has facilitated the use of the NPI in global clinical trials and other types of international research. Studies of brain imaging and cerebrospinal fluid (CSF) correlates of NPI scores provide insight into the neurobiology of neuropsychiatric syndrome (NPS) in NDDs. This article reviews the history of the NPI, discusses the role of the different versions of the NPI, and describes research findings based on the NPI.
Development of the NPI
The author developed the NPI because existing instruments such as the Behavioral pathology in Alzheimer Disease Rating Scale (BEHAVE-AD),2 although capturing many aspects of the neuropsychiatry of NDD and representing pioneering efforts in scale development for NPS, did not include apathy or disinhibition considered to be important features of AD and other NDDs such as frontotemporal dementia (FTD), had different numbers of questions for different syndromes (agitation, delusions, etc), were not fully operationalized to help support reliability of data capture, and collected data only on severity and not on frequency. A tool capturing a wider range of behaviors and with more systematic scoring was needed, and these features were built into the NPI.
Prior to the development of the NPI, neuropsychiatric research in NDDs typically used tools developed for psychiatric illnesses such as schizophrenia and major depressive disorder. The Brief Psychiatric Rating Scale (BPRS) was commonly used. The types of delusions and hallucinations observed in schizophrenia differ from those of NDDs,3 and a tool more specific to NDD was needed. The NPI was developed and validated specifically for the neuropsychiatry of NDD. The NPI was developed as an informant-based interview; the tool was intended to characterize behavior changes in patients with NDD who may lack memory of or insight into their own behavior.
Rating scales used to characterize mood changes in major depressive disorder such as the Hamilton Depression Rating Scale (HDRS) include aspects of depression such as withdrawal, appetite changes, and sleep disturbances that may occur in NDD without depression. For this reason, the depression/dysphoria domain of the NPI includes only mood-related items, while apathy, sleep changes, and appetite changes are captured in other sections of the NPI.
The need to balance comprehensiveness of behavioral assessment with acceptable administration time motivated the use of screening questions. If the screen is negative, the subquestions are not asked, whereas if the screen is positive, the subquestions are then administered and the caregiver scores the frequency and severity of the behavioral domain. The instrument is highly scripted, and allowable deviations from the script are limited; this approach was chosen to support the reliability of the instrument. The final scoring of the frequency (1-4) and severity (1-3) is done by the research participant’s or patient’s partner as part of the administered interview. This strategy was also chosen to enhance the reliability of the tool. The score for each domain is based on a frequency × severity (F × S) product, and the total score of the NPI is the sum of the domain scores. The F × S approach results in asymmetric score distributions; scores of 5, 7, and 11 cannot be achieved with this calculation. Parametric and nonparametric analyses that do or do not take the asymmetric distribution into account, respectively, have come to very similar findings, and the missing scores do not seem to impact interpretation of the total or domain scores.
The universe of domains to be interrogated was determined by the author based on clinical experience and use of other tools such as the BEHAVE-AD and the BPRS. A Delphi panel reviewed the domains. The original 10-item version assesses hallucinations, delusions, depression, anxiety, disinhibition, agitation, elation, apathy, irritability, and aberrant motor behavior (Table 1). Sleep and appetite changes were added later to create the 12-item version.4
Table 1.
Domains of the Neuropsychiatric Inventory.
| Depression | Delusions |
|---|---|
| Anxiety | Hallucinations |
| Irritability | Agitation |
| Elation | Aberrant motor disturbances |
| Disinhibition | Appetite/eating changes |
| Apathy | Night time sleep disturbances |
Once constructed, the approach and questions of the NPI were reviewed and shaped by a Delphi panel of neurologists, psychiatrists, and neuropsychologists familiar with the behavioral changes in NDD.
When a final version was available, the concurrent validity of the instrument was assessed using the available instruments then in use. Concurrent validity for depression was shown using the HDRS, and concurrent validity for delusions, hallucinations, and agitation was shown for the BEHAVE-AD. Concurrent validity with the Cohen-Mansfield Agitation Inventory (CMAI) has since been shown.5,6 Since the development of the NPI, convergent validity was been demonstrated using autopsy, CSF, and brain imaging data (discussed below).
Two types of test–retest reliability were shown for the NPI—intrarater and interrater. Half of the sample in the interrater reliability assessment were interviewed by telephone for the second interview and established the reliability of phone-based interviews once a single in-person interview had been conducted.
The normative data for behavioral changes occurring in the normal elderly population were based on NPI administration to 40 cognitively normal individuals. Low rates of mood symptoms, anxiety, and apathy were shown to be present in normal older persons as characterized by the NPI.
Versions of the NPI
Table 2 summarizes the main features of the NPI versions. The original 10-item version of the NPI was expanded to the 12 item version, adding sleep and appetite change.1,4 Realization of the important role of care partners in AD and the impact of behavioral changes in patients with AD on their family and careers led to the creation of the caregiver distress scale of the NPI.7 The caregiver scores their own distress for each domain of the NPI leading to 12 distress items that can be summed for a total behavior-related distress score. This has proven to be useful in clinical trials where a reduction in the domain score accompanied by a corresponding reduction in the distress score increases confidence in the validity of the finding.8 The NPI caregiver distress predicts institutionalization when patients with dementia exhibit delusions or agitation.9 The 12-item NPI with integrated caregiver distress scale is the most widely used version of the NPI. The caregiver distress scale cannot be administered without administering the NPI, since the 2 are integrated and the caregiver distress follows characterization of the patient behavior.
Table 2.
Versions of the NPI and Their Differentiating Features.
| NPI Version | Specific Features |
|---|---|
| NPI-10 | Original version; lacks sleep and appetite domains1 |
| NPI-12 | Identical to the 4NPI-10 with sleep and appetite added4 |
| NPI-12 with caregiver distress | The standard version of the NPI used in most studies7 |
| NPI-Q | NPI Questionnaire; uses only the screening question of the original NPI without the subquestions; uses only the severity rating (not frequency); includes caregiver distress; can be self-rated by a caregiver10 |
| NPI-NH | Nursing home version of the NPI used in residential settings where the reporter is not a family member and does not know the history of the individual; questions are rephrased to reflect the profession relationship but use nearly identical descriptions; “caregiver distress” is changed to “occupational disruptiveness” to capture the impact of the behavior on the living setting16 |
| NPI-C | Clinical-based NPI allowing the clinician to participate in the rating should be used with expert raters; divides some scales (eg, agitation/aggression) into 2 scales (agitation; aggression); subscales can be used as standalone scales (eg, the agitation subscale in agitation trials)18 |
| Sleep Disturbance Inventory | Expanded version of the sleep disturbance subscale of the NPI19 |
Abbreviations: NPI, Neuropsychiatric Inventory; NPI-Q, NPI Questionnaire; NPI-NH, NPI–Nursing Home version; NPI-C, NPI–Clinician version.
The NPI is streamlined for administration through the use of screening questions but still requires approximately 15 minutes to complete with an individual demonstrating an average level of behavioral change; more time is required if more severe behavioral disturbances are present, since more subquestions must be asked. The time requirements of the NPI led to creation of the NPI Questionnaire (NPI-Q).10 The NPI-Q consists of the 12 items of the NPI; each is given only a severity score (mild, moderate, or severe). The scale is designed for unsupervised completion by the caregiver with brief review by the clinician, although it can also be administered by a clinician as an interview. The concurrent validity of the NPI-Q compared to the NPI has been established.10 Telephone administration of the NPI-Q has been concluded based on the telephone validation studies of the NPI; no explicit validation of the telephone-based NPI-Q has been performed. The NPI-Q has been adopted as the standard neuropsychiatric tool by the AD Neuroimaging Initiative11 and the US National Alzheimer Coordinating Center.12,13 Behavioral data derived from the NPI-Q are widely available in these 2 publically accessible databases. The restricted range of the NPI-Q (maximum domain score 3) compared to the NPI (maximum domain score 12) limits the power to do correlations and other types of statistical analyses.
Behavioral disturbances are among the most important reasons for patients with NDD to be admitted to residential facilities.14,15 The desire to use the NPI in residential settings led to the development of the NPI–Nursing Home version (NPI-NH).16 The tool is identical to the NPI with 2 adjustments. First, the phrasing of the questions was altered, since the reporter is not a family member and did not know the patient prior to disease onset or residential change. Second, the caregiver distress scale was changed to an occupational disruptiveness scale (with identical scoring to the caregiver distress scale) to capture the impact of the behavioral changes on daily life in the nursing home and the occupational effectiveness of the professional caregiver. Study of the validity of the NPI-NH as scored by nursing home personnel compared to research observers showed that qualified nurses were more accurate reporters than nursing assistants. Training of assistants to acquaint them with the behaviors being queried with the NPI-NH improved the validity of their reports.17 The NPI-NH is the appropriate version of the NPI for use in any inpatient setting where there are nonfamily professional caregiver reporters including emergency departments, psychiatric facilities, assisted living, and skilled nursing homes.
The NPI interview excludes contributions from the clinician unless there is a frank contradiction between the clinician and the family reporter, in which case the “not applicable” response is chosen and no score for the domain is given. The desire to expand the role of the clinician in behavioral scoring led to the development of the NPI–Clinician version (NPI-C).18 The NPI-C is intended for use by expert observers and is more comprehensive than the original NPI. It splits the agitation/aggression domain into separate agitation and aggression sections; it eliminates the screening question and requires administration of each question; and it captures the clinician’s view of the severity and frequency of the behavior. Because it asks each question of a domain, it lends itself to assigning a total score for the domain by adding the individual question responses. This summing of items is more appealing to some regulatory agencies than the F × S approach. The summary approach also allows the NPI-C domains to be used as stand-alone scales (eg, using the agitation domain as an outcome in agitation trials). The reliability of the NPI-C and the validity in comparison with the NPI have been established.18
The Sleep Disturbances Inventory (SDI) was derived from the sleep item of the NPI and retains the scoring approach of the NPI. The SDI has been validated with actigraph recordings and with Sleep Quality Ratings reported in diary form.19
Comment
The NPI has evolved to meet clinical needs as they were identified. The usefulness of the NPI has been extended to practices and projects where more succinct assessments were needed with the NPI-Q. The need for an expert version led to the NPI-C, and the desire to assess patients in residential settings with professional caregivers gave rise to the NPI-NH. Related tools have evolved for sleep and for patients with very minimal symptoms (discussed below). The forms of the various NPI versions are available at www.npitest.net.
Translations and International Applications of the NPI
The NPI has been translated into more than 40 languages, and the NPI-NH and NPI-Q are available in a similar number. Most of the translations have been done by the MAPI Research Trust (www.mapigroup.com). The translations follow the MAPI Research Trust methodology in compliance with the International Society for Pharmacoeconomics and Outcomes Research guidelines.20 The process consists of: (1) definition of the concept behind each item, (2) forward translations by more than 1 native translator, (3) reconciliation of the translations, (4) one back-translation by an English speaker fluent in the target language, and (5) final reconciliation with the NPI author.
The NPI has shown good reliability across translation into multiple languages including Chinese,21–24 Chilean Spanish,25 Korean,26,27 Turkish,28 Brazilian Portugese,6,29 European Portugese,30 Farsi,31 Icelandic,32 Japanese,33–35 Norwegian,36 Polish,37 Greek,38 Nigerian,39 Danish,40 Dutch,41 Italian,42 and European Spanish.43,44 The validity of the NPI in non-Western cultures has rarely been rigorously examined and remains an area to be researched.
The NPI has been used to assess differences in behavioral changes as reported by caregivers from different cultures. When comparing Chinese (Taiwan, Hong Kong) and US Caucasian reports, Chinese caregivers reported anxiety and delusions more frequently (58.1%) than Caucasians (37.3% and 39.6%; χ2, P < .01 and P < .05, respectively). Caucasians reported appetite changes (47.3%) and apathy (59.2%) more frequently than the Chinese samples (χ2, P < .05 and P < .01, respectively).45 Chinese caregivers had less severe responses (less distress) than Caucasian caregivers to patient depression and apathy; they had similar responses to all other domains of the NPI.46
Review of NPI total scores among patients recruited to global clinical trials by Grill and colleagues47 showed that baseline scores across 7 geographic regions had substantial heterogeneity varying from 6.6 in Japan to 11.9 Australia/South Africa and South America/Mexico. This heterogeneity was observed despite the fact that identical protocols were implemented across all regions. This regional behavioral heterogeneity may contribute to variable outcomes, since more severe behavioral changes are associated with accelerated cognitive decline.48
Comment
The NPI is a reliable tool across languages and cultures. Studies with the NPI have shown that behavioral disturbances are common in NDD across global populations and have effects on caregivers. The NPI profiles and caregiver responses show variations across world regions.
Characterizing Behavioral Changes in NDDs With the NPI
The NPI has been used to characterize the behavioral abnormalities of many types of NDD. These studies have contributed importantly to understanding the role of NPSs in disease diagnosis, the utility of assessing behavior for differential diagnosis, and the evolution of behavioral changes in the course of different NDDs.49 Different NDDs have distinct neuropsychiatric profiles captured by the NPI. By linking these profiles to the underlying neuropathology, regional vulnerability, and associated neurochemical alterations in the brain, inferences can be drawn about the neurobiology of behavior. These insights can be helpful in understanding brain-behavior relationships, planning management, and developing new treatments. Table 3 provides a summary of the common neuropsychiatric symptoms observed in NDD.
Table 3.
NPI Profiles Reported in Neurodegenerative Disorders.a
| NPI Domain | AD | MCI | FTD | PD | PSP | CBD | HD | DLB |
|---|---|---|---|---|---|---|---|---|
| Delusions | X | X | X | X | ||||
| Hallucinations | X | X | X | |||||
| Depression | X | X | X | X | X | X | ||
| Anxiety | X | X | X | |||||
| Apathy | X | X | X | X | X | |||
| Irritability | X | X | X | |||||
| Elation | X | |||||||
| Disinhibition | X | X | X | X | ||||
| Agitation | X | X | ||||||
| Aberrant motor behavior | X | X | X | |||||
| Appetite and eating disturbance | X | X | ||||||
| Nighttime behavior disturbances | X | X | X |
Abbreviations: AD, Alzheimer’s disease; CBD, corticobasal degeneration; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; HD, Huntington’s disease; MCI, mild cognitive impairment; NPI, Neuropsychiatric Inventory; PD, Parkinson disease (without dementia); PSP, progressive supranuclear palsy.
The populations are not matched on any specific features; they reflect clinical samples of patients presented for care and agreeing to provide NPI information.
“X” indicates domains where behavioral changes are particularly common across reported populations.
Alzheimer Disease and Mild Cognitive Impairment
The first disorder characterized with the NPI was AD in an outpatient setting.50 The NPI-NH has been widely used to assess behavior in more severely affected patients in residential settings.51 The studies show a high prevalence of neuropsychiatric symptoms among patients with AD; 90% show some evidence of behavioral change as characterized by the NPI during the course of the illness. Agitation, irritability, aberrant motor disturbances, depression, and anxiety are the most common syndromes observed; delusions and hallucinations are of intermediate frequency; and elation and disinhibition are less common in AD. The symptoms commonly co-occur, forming complex super-syndromes with multiple behavioral changes in the same individual. Factor structure studies of the NPI vary but usually suggest psychosis, mood, apathy, and agitation factors.52 Behaviors progress over time and are more frequent in those with more advanced dementia.51
Studies with the NPI have established that NPSs occur early in AD and that when they occur in patients with mild cognitive impairment (MCI), they are a harbinger of progression to AD dementia. Teng and colleagues,53 for example, found that patients progressing to AD had a significantly higher prevalence of psychopathology than patients who remained stable or improved (100% vs 59%). Depression (67% vs 31%) and apathy (50% vs 18%) were more common in patients with MCI who were later diagnosed with AD.
Behavioral changes have been demonstrated in the MCI syndrome of autosomal dominant AD. This is particularly important since the gene is fully penetrant, the patients uniformly progress to AD dementia, and changes occurring early in the course of the clinical evolution of the disorder can be considered early manifestations of AD. Ringman et al54 found that depression (56% vs 17%, P = .0003), apathy (40% vs 4%, P < .0001), disinhibition (16% vs 2%, P = .009), irritability (48% vs 9%, P = .0001), sleep changes (28% vs 7%, P = .003), and agitation (24% vs 6%, P = .008) were more common among mutation carriers with MCI than among those without cognitive symptoms.
The NPI has been used in epidemiologic studies of NPSs occurring in community-dwelling individuals with cognitive impairment. The Cache County Study55 demonstrated a high prevalence of behavioral changes in community-dwelling patients with dementia who were not necessarily enrolled in health-care delivery systems. The overall prevalence of NPSs in these patients is lower than that in clinical samples, suggesting that changes in behavior are among the reasons that cognitively impaired patients seek medical attention.
Frontotemporal Degeneration Spectrum Disorders
The NPI profiles have been established for FTD where they show more elation, apathy, and disinhibition and less psychosis than a typical AD population.56–58 Within FTD, disinhibition has been linked to diminished thickness of the right frontal cortex as measured on magnetic resonance imaging.59 Profiles of FTD have been shown to be influenced by the underlying pathology—tau or TAR DNA-binding protein 43 (TDP-43).60 The marked caregiver burden of FTD has been determined with the NPI caregiver distress scale.56,57
Progressive supranuclear palsy (PSP) and corticobasal degeneration, two other disorders in the frontotemporal lobar degeneration spectrum, have also been studied with the NPI. The NPI profiles in PSP have demonstrated a high prevalence of apathy.61–63 Corticobasal degeneration has a less distinctive profile but depression occurs and is sometimes severe.64
Dementia With Lewy Bodies
Dementia with Lewy bodies (DLBs) is a disorder with characteristic neuropsychiatric symptoms including visual hallucinations, rapid eye movement sleep behavior disorder, delusions, and depression. The NPI has been used to describe and quantitate this symptom complex in population of patients with DLB.57,58 Two NPI factors progressed over time (apathy, aberrant motor disturbances, sleep disturbances, agitation, euphoria, disinhibition, and irritability), whereas mood and psychotic factors did not.65
Parkinson Disease
The NPI studies have been conducted in patients with Parkinson disease (PD) without cognitive impairment and in those with PD dementia.66–68 Essentially all domains of the NPI are more abnormal in patients with dementia than in those with normal cognition. Early symptoms of depression, anxiety, and apathy progress to include hallucinations and delusions.
Huntington Disease
Huntington disease is characterized by an NPI profile featuring agitation, irritability, depression, delusions, and aberrant motor behavior.69
Use of the NPI in Nondegenerative Disorders
The NPI was designed to characterize the neuropsychiatric and behavioral symptoms of NDD, but it has been applied to non-NDD disorders. Studies of traumatic brain injury (TBI) have included the NPI to provide insight into the often-disabling behavioral consequences of brain trauma. Depression, disinhibition, and apathy are among the prevalent symptoms among patients with TBI.70
Vascular cognitive impairment (VCI) has also been studied with the NPI including patients with amyloid imaging evidence of the absence of concomitant AD.71 Depression and apathy are common in the NPI profiles of VCI.72–75
Epilepsy is a non-NDD disorder to which the NPI has been applied, revealing high rates of depression and psychotic symptoms.76 The inventory was helpful in anticipating caregiver needs and resource planning as well as characterizing patient behaviors. Gilles de la Tourette’s syndrome is a complex childhood tic and behavior disorder that has been studied with the NPI.77 The profile features depression, anxiety, and aberrant motor behavior distinctive from most other NPI profiles. Delirium has been assessed with the NPI; there were few notable differences between the NPI profile of AD and that of delirium.78
Comment
The NPI has been used to characterize the neuropsychiatric profiles of a variety of NDD and other neurological disorders. The NPI helped to link changes in behavior to specific types of pathological changes and regional brain dysfunction. The NPI profiles demonstrated that behavioral symptoms can assist in differential diagnosis of complex NDD with overlapping symptoms. The NPI studies assist in determining caregiver burden in conjunction with NDD and assist in planning for the resource needs of patients and caregivers.
Among the discoveries that emerged from the widespread use of the NPI is the unanticipated high prevalence of apathy among NDD. Apathy is common in AD, PD, FTD, and PSP.79,80 Apathy is disabling to patients and bothersome to caregivers. Preliminary trials have begun to address apathy as a treatment target whose relief may provide important benefits to patients and caregivers.81
The NPI and the Neurobiology of Behavior
Neurobiological studies and autopsy findings have provided convergent validity for several of the NPI domains. Apathy has been shown to correlate with medial frontal lobe dysfunction with cortical thickness mapping using MRI,82 cerebral perfusion mapping with single-photon emission computed tomography (SPECT),83,84 and cerebral hypometabolism with fluorodeoxyglucose (FDG) positron emission tomography (PET).85 Autopsy studies have shown a greater burden of AD pathological changes in this same medial frontal area in those with apathy during the course of their illness.86 These correlations have been consistent across several NDDs including AD and FTD.87 Studies contrasting types of apathy and the corresponding neurobiology are warranted.
Brain imaging studies of agitation have shown greater hypometabolism with FDG-PET in frontal lobes compared to non-agitated patients.88 Autopsy studies have shown a greater burden of neurofibrillary tangles but not of amyloid plaques in those with agitation during life.86
Hallucinations characterized on the NPI have been correlated with occipital lobe dysfunction,89 and disinhibition as assessed on the NPI reflects orbitofrontal dysfunction.87 Hallucinations also exhibit a characteristic histologic relationship; they are more common among patients with α-synuclein disorders (PD and DLB).
Disinhibition has been linked to dysfunction of the frontal polar and inferior orbital frontal cortex in patients with AD.59 Not all domains of the NPI have shown consistent anatomical correlates. Depression, delusions, aberrant motor disturbances, and sleep and appetite changes have not shown reproducible anatomic associations. Figure 1 shows the brain regions most reproducibly linked to specific domains of the NPI.
Figure 1.
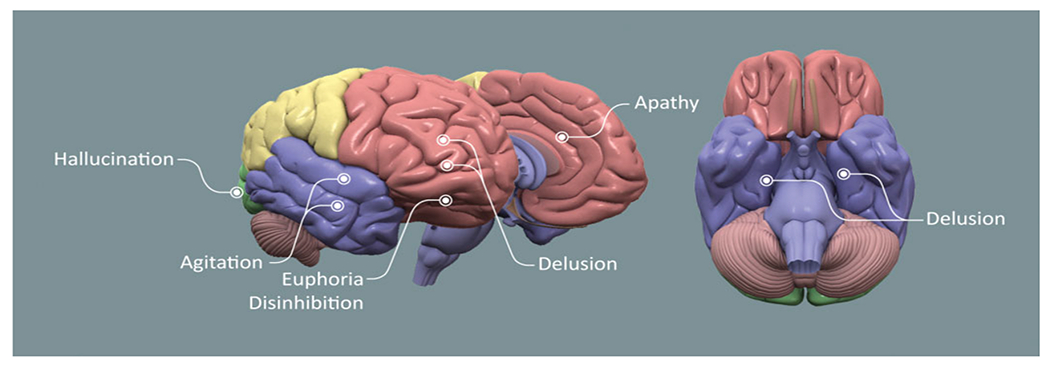
Brain diagram showing areas of dysfunction consistently correlated with abnormalities on Neuropsychiatric Inventory (NPI) domains (figure by Mike de la Flor).
Amyloid PET
Recent studies with amyloid PET show relationships between anxiety and frontal and cingulate amyloid burden and between irritability and frontal, cingulate, and parietal amyloid burden.90 Apathy correlates with prefrontal amyloid load.91
Cerebrospinal Fluid
Some studies found that higher levels of tau protein in CSF in cognitively normal persons correlate with mood abnormalities as assessed with the NPI-Q,92 whereas others identified relationships between mood changes and lower levels of CSF amyloid.93 Tau levels have been linked to agitation in patients with AD.94 Relatively few studies have examined biomarker relationships with NPI profiles.
Genetic Investigations
Genetic studies of the NPI have been pursued, and preliminary genetic relationships have emerged. Ringman and colleagues54 showed that compared to mutation non-carriers, symptomatic individuals with presenilin 1 mutations are more likely to exhibit depression, apathy, disinhibition, sleep changes, and agitation.
Comment
Studies using the NPI have been useful in establishing informative biological relationships between behavioral changes and anatomic regions of dysfunction. The regional vulnerability of NDD results in differential behavioral expressions and reveals consistent relationships between behavior and regional anatomy (Figure 1). This anatomic-level investigation is complemented by genetic research that is beginning to reveal relationships between specific patterns of gene expression and some NPI domains. Unique histological relationships have emerged such as that between hallucinations and α-synuclein pathology.
The NPI in Clinical Trials
The NPI is widely used in clinical trials of AD as a means of determining the severity levels of behavioral symptoms necessary for trial participation, as a primary outcome in trials of psychotropic agents, and as a secondary outcome in trials of cognitive enhancers and disease-modifying agents. As of 2019, the NPI has been used in more than 350 clinical trials (clinicaltrials.gov).
Clinical Trials of Cognitive Enhancing Drugs
Cholinesterase inhibitors (ChE-Is; eg, donepezil, rivastigmine, galantamine, tacrine) were developed as cognitive-enhancing agents but have consistently shown modest effects on behavioral symptoms as reflected by changes on the NPI. Anxiety, apathy, hallucinations, disinhibition, and aberrant motor behaviors are most likely to respond to treatment with ChE-Is.95 Mega and colleagues showed that decreased cerebral blood flow in frontal regions on SPECT predicted improvement on the NPI following ChE-I treatment.96
Many patients participating in trials do not exhibit behavioral changes on all domains of the NPI, and how best to handle this issue analytically was addressed in studies of ChE-I. Improvement analyses were conducted on patients who had non-zero domain scores on the NPI at baselines; “emergence analyses” were conducted for patients who had domain scores of zero at baseline, since they cannot improve but can have less emergence of new symptoms. The ChE-I’s suppress emergence of behavioral problems as well as reduce existing behavioral disturbances.97
Memantine, an N-methyl-D-aspartate receptor antagonist and cognitive enhancer approved for the treatment of AD, has behavioral effects in studies using the NPI. Reduced agitation and irritability are seen with memantine treatment.98,99
Clinical Trials of Disease-Modifying Treatments
Disease-modifying agents (small molecules and immunotherapies) are expected to delay the emergence of behavioral symptoms in AD and other NDD when treatment is successful in reducing disease progression. To capture this effect, the emergence analysis of the NPI may be most informative. The Clinical Dementia Rating (CDR), commonly used as a global outcome measure in trials of disease-modifying agents, contains no behavioral questions, making inclusion of the NPI particularly important as a means of capturing behavioral effects of these agents in trials using the CDR.
The NPI can be seen as a safety measure in clinical trials of disease-modifying agents. For example, it detected behavioral changes occurring with the Beta-site amyloid precursor protein cleaving enzyme (BACE) inhibitor verubecestat.100
Clinical Trials of Psychotropic Agents
The NPI has been extensively used in clinical trials of psychotropic agents in patients with NDD. The NPI (or NPI-NH) was used as an outcome measure in trials of olanzapine and aripiprazole for agitation and psychosis in AD.8,101 The NPI has been used to determine baseline severity of agitation in trials of dextromethorphan/quinidine,102 baseline severity of psychosis in trials of pimavanserin for delusions and hallucinations associated with PD,103 and baseline severity of agitation in the citalopram in AD (CiTAD) trial.104 The NPI was the primary outcome measure of the trial of dextromethorphan/quinidine for agitation in AD.105 It was used as a secondary outcome of a trial of methylphenidate treatment of apathy in AD81 and is the primary outcome in a follow-up trial.106 The ability of the NPI to capture secondary behavioral outcomes is useful in understanding the multiple dimensions of impact of psychotropic agents.104
The NPI has had three primary roles in clinical trials of psychotropic agents: (1) determination of the baseline severity of specific behaviors to allow entry into a trial, (2) use as a secondary outcome to capture behavioral changes when it is not the primary outcome, and (3) use as a primary outcome in trials of psychotropic agents.
Clinical Trials of Nonpharmacologic Interventions
Studies of nonpharmacologic interventions have used the NPI to show the benefit of targeted multidisciplinary interventions.107 A comprehensive dementia care management program using nurse practitioners to administer the care was associated with improved NPI-Q scores.108 Caregiver education programs have also been shown to result in improved patient behavior as measured by the NPI-Q.109
Comment
Concurrent validity of the NPI has accrued through use of the NPI in clinical trials. In most trials, results on other instruments such as the CMAI or BPRS have closely paralleled the results seen on the NPI.
Lessons learned from clinical trials using the NPI for psychotropic drug development include the challenges of including both outpatients and residential patients in the same trial, since different reporters (family vs professional) and different versions of the NPI (NPI vs NPI-NH) are used for these 2 populations; summing items on the NPI-C may produce scores that behave more regularly than the F × S approach of the original NPI, and this may be more acceptable to regulatory authorities; behaviors fluctuate over time, so power calculations must account for this spontaneous variability; and there are marked placebo or trial participation responses in those not getting active treatment.
Caregiver Burden, Cost of Care, and Other Research Areas Informed by the NPI
Neuropsychiatric syndromes increase the cost of care of NDD as shown when costs of caring for patients with and without behavioral changes are compared. The NPI has been used in several studies assessing the impact of behavior on cost of care.110 These costs are largely generated by the increased rate of institutionalization of patients with neuropsychiatric symptoms. Murman and colleagues111 showed that a 1-point worsening of the NPI score is associated with an incremental increase of between US$247 and US$409 per year in total direct costs of care based upon year 2001 US dollars. Using the NPI, Morris et al showed that the mean excess cost associated with agitation per person was £4091 a year, accounting for 12% of the health and social care costs of AD in our data and equating to £2 billion a year across all people with AD in the United Kingdom.112 Exercise has been shown to improve behavior and to have a concomitant reduction in cost of care.113 The NPI has contributed importantly to studies of health-care costs related to behavior disturbances of NDD.
Challenges With Use of the NPI
The NPI has proven to be robust as a clinical assessment instrument and is used worldwide in many types of research where neuropsychiatric information is of value. It does not meet all assessment needs, and adjustments have evolved to optimize its use. The NPI covers a wide range of behaviors, whereas clinical trials of psychotropic agents generally focus on one specific domain (eg, agitation, depression, apathy). In these trials, use of an instrument that is domain-specific such as the CMAI in studies of agitation, the Cornell Scale for Depression in Dementia for studies of depression, or the Apathy Evaluation Scale for studies of apathy is useful to enhance understanding of the therapeutic response in the domain of interest while using the other domains of the NPI to capture associated changes in other NPS.
As noted, the scoring irregularities of the NPI stemming from the F × S approach have led some to prefer tools where scores are summed. The NPI-C lends itself to this type of summation. Some definitions such as the International Psychogeriatric Association (IPA) definition of agitation in cognitive impairment114 were developed after the NPI and do not use same terms as the NPI. Instruments that more closely reflect the IPA definition may be of value. The NPI domain of agitation/aggression combines 2 types of behavior, and some clinical trials may concentrate on only one of these. The NPI-C has separate domains for agitation and aggression.
The NPI lacks some types of behavior such as changes in sexual demeanor and the impulse control disorders occurring especially frequently among patients with PD treated with dopamine agonists. These behaviors must be addressed with other tools.
The NPI has not been applied in many areas of drug development where gathering behavioral information may be valuable, especially as a measure of behavioral toxicity. This would include placing the instrument in trials of any drug with central nervous system (CNS) effects such as anticonvulsants, PD therapies, drugs for hyperactivity/attention deficit disorder, pain therapies, CNS cancer therapies, and multiple sclerosis treatments. Data on behavioral effects of neurosurgical interventions, postoperative states, delirium, and medical illnesses (such as patients with cancer undergoing chemotherapy) may also provide useful management information.
Summary
The NPI is a useful tool and has produced behavioral profiles for many NDD and other CNS disorders. It has robust psychometric properties and has proliferated versions to respond to specific clinical contingencies. It has assisted in understanding caregiver responses to behavioral changes and the cost of behavioral alterations in NDD. The NPI has bridged between clinical and neurobiological studies to contribute to understanding the biological basis of NPS of NDD. Use of the NPI in clinical trials assists in understanding behavioral effects of cognitive enhancers, disease-modifying agents, and psychotropic drugs and has played a role in developing important new therapies for patients with NDD. The NPI has provided a widely used vocabulary for describing behavioral changes in NDD and has moved the field to conceptualize NDD as complex cognitive–behavioral–motor disorders.
Funding
The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: COBRE grant # P20GM109025; TRC-PAD # R01AG053798; DIAGNOSE CTE # U01NS093334, NVeADRC grant# NIA P20AG068053, and support from Keep Memory Alive.
Footnotes
Declaration of Conflicting Interests
The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Cummings has provided consultation to Acadia, Actinogen, AgeneBio, Alkahest, Allergan, Alzheon, Avanir, Axsome, BiOasis Technologies, Bracket, Cerecin, Denali, Diadem, EIP Pharma, Eisai, Genentech, Green Valley, Grifols, Hisun, Idorsia, Merck, Otsuka, Casava Therapeutics, QR, Resverlogix, Roche, Samumed, Samus, Sunovion, Suven, Takeda, and United Neuroscience pharmaceutical and assessment companies. Dr Cummings owns stock in ADAMAS, BioAsis, MedAvante, and QR Pharma. Dr Cummings owns the copyright of the Neuropsychiatric Inventory (NPI). Dr Cummings is the Chief Scientific Officer of CNS Innovations.
References
- 1.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994; 44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 2.Reisberg B Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr. 2007;19(3):421–456. [DOI] [PubMed] [Google Scholar]
- 3.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias: diagnostic criteria for a distinct syndrome. Am J Geriat Psychiat. 2000;8(1):29–34. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–S16. [DOI] [PubMed] [Google Scholar]
- 5.De Deyn PP, Wirshing WC. Scales to assess efficacy and safety of pharmacologic agents in the treatment of behavioral and psychological symptoms of dementia. J Clin Psychiatry. 2001;62 (suppl 21):19–22. [PubMed] [Google Scholar]
- 6.Stella F, Forlenza OV, Laks J, et al. The Brazilian version of the Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity in dementia. Int Psychogeriatr. 2013; 25(9):1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the neuropsychiatric inventory caregiver distress scale. J Am Geriatr Soc. 1998;46(2):210–215. [DOI] [PubMed] [Google Scholar]
- 8.Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU study group. Arch Gen Psychiatry. 2000;57(10):968–976. [DOI] [PubMed] [Google Scholar]
- 9.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59(3):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. [DOI] [PubMed] [Google Scholar]
- 11.Weiner MW, Veitch DP, Aisen PS, et al. Alzheimer’s disease neuroimaging I. Recent publications from the Alzheimer’s Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13(4):e1–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270–277. [PubMed] [Google Scholar]
- 13.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. [DOI] [PubMed] [Google Scholar]
- 14.Roen I, Selbaek G, Kirkevold O, Engedal K, Testad I, Bergh S. Resourse Use and Disease Couse in dementia–Nursing Home (REDIC-NH), a longitudinal cohort study; design and patient characteristics at admission to Norwegian nursing homes. BMC Health Serv Res. 2017;17(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tun SM, Murman DL, Long HL, Colenda CC, von Eye A. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(4):314–327. [DOI] [PubMed] [Google Scholar]
- 16.Wood S, Cummings JL, Hsu MA, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry. 2000;8(1):75–83. [DOI] [PubMed] [Google Scholar]
- 17.Wood S, Cummings JL, Schnelle B, Stephens M. A videotape-based training method for improving the detection of depression in residents of long-term care facilities. Gerontologist. 2002;42(1):114–121. [DOI] [PubMed] [Google Scholar]
- 18.de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The sleep disorders inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J Sleep Res. 2003;12(4):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Lin K, Wang H, Yamakawa M, Makimoto K, Liao X. Reliability and structural validity of the Chinese version of the Neuropsychiatric Inventory, Nursing Home version. Psychogeriatrics. 2018;18(2):113–122. [DOI] [PubMed] [Google Scholar]
- 22.Fuh JL, Liu CK, Mega MS, Wang SJ, Cummings JL. Behavioral disorders and caregivers’ reaction in Taiwanese patients with Alzheimer’s disease. Int Psychogeriatr. 2001;13(1):121–128. [DOI] [PubMed] [Google Scholar]
- 23.Leung VP, Lam LC, Chiu HF, Cummings JL, Chen QL. Validation study of the Chinese version of the Neuropsychiatric Inventory (CNPI). Int J Geriatr Psychiatry. 2001;16(8):789–793. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Xiao S, Li X, et al. Reliability and validity of the Chinese version of the Neuropsychiatric Inventory in mainland China. Int J Geriatr Psychiatry. 2012;27(5):539–544. [DOI] [PubMed] [Google Scholar]
- 25.Musa G, Henriquez F, Munoz-Neira C, Delgado C, Lillo P, Slachevsky A. Utility of the Neuropsychiatric Inventory Questionnaire (NPI-Q) in the assessment of a sample of patients with Alzheimer’s disease in Chile. Dement Neuropsychol. 2017;11(2):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the Neuropsychiatric Inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15(6):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Choi KH, Kim SH, Cummings JL, Yang DW. Validation study of the Korean version of the brief clinical form of the Neuropsychiatric Inventory. Dement Geriatr Cogn Dis Extra. 2016;6(2):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin Cankurtaran E, Danisman M, Tutar H, Ulusoy Kaymak S. The reliability and validity of the Turkish version of the Neuropsychiatric Inventory-clinician. Turk J Med Sci. 2015;45(5):1087–1093. [DOI] [PubMed] [Google Scholar]
- 29.Camozzato AL, Godinho C, Kochhann R, Massochini G, Chaves ML. Validity of the Brazilian version of the Neuropsychiatric Inventory Questionnaire (NPI-Q). Arq Neuropsiquiatr. 2015;73(1):41–45. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira AR, Martins S, Ribeiro O, Fernandes L. Validity and reliability of the European Portuguese version of Neuropsychiatric Inventory in an institutionalized sample. J Clin Med Res. 2015;7(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malakouti SK, Panaghi L, Foroughan M, Salehi M, Zandi T. Farsi version of the Neuropsychiatric Inventory: validity and reliability study among Iranian elderly with dementia. Int Psychogeriatr. 2012;24(2):223–230. [DOI] [PubMed] [Google Scholar]
- 32.Davidsdottir SR, Snaedal J, Karlsdottir G, Atladottir I, Hannesdottir K. Validation of the Icelandic version of the Neuropsychiatric Inventory with caregiver distress (NPI-D). Nord J Psychiatry. 2012;66(1):26–32. [DOI] [PubMed] [Google Scholar]
- 33.Hirono N, Mori E, Ikejiri Y, et al. Japanese version of the Neuropsychiatric Inventory–a scoring system for neuropsychiatric disturbance in dementia patients [in Japanese]. No To Shinkei. 1997;49(3):266–271. [PubMed] [Google Scholar]
- 34.Matsumoto N, Ikeda M, Fukuhara R, et al. Validity and reliability of the Japanese version of the Neuropsychiatric Inventory Caregiver Distress Scale (NPI D) and the Neuropsychiatric Inventory Brief Questionnaire Form (NPI-Q) [in Japanese]. No To Shinkei. 2006;58(9):785–790. [PubMed] [Google Scholar]
- 35.Shigenobu K, Hirono N, Tabushi K, Ikeda M. Validity and reliability of the Japanese version of the Neuropsychiatric Inventory-nursing home version (NPI-NH) [in Japanese]. Brain Nerve. 2008;60(12):1463–1469. [PubMed] [Google Scholar]
- 36.Selbaek G, Kirkevold O, Sommer OH, Engedal K. The reliability and validity of the Norwegian version of the Neuropsychiatric Inventory, nursing home version (NPI-NH). Int Psychogeriatr. 2008;20(2):375–382. [DOI] [PubMed] [Google Scholar]
- 37.Bidzan L, Bidzan M. Reliability of the neuropsychiatric inventory-nursing homes Polish version [in Polish]. Psychiatr Pol. 2005;39(6):1219–1229. [PubMed] [Google Scholar]
- 38.Politis AM, Mayer LS, Passa M, Maillis A, Lyketsos CG. Validity and reliability of the newly translated Hellenic Neuropsychiatric Inventory (H-NPI) applied to Greek outpatients with Alzheimer’s disease: a study of disturbing behaviors among referrals to a memory clinic. Int J Geriatr Psychiatry. 2004;19(3):203–208. [DOI] [PubMed] [Google Scholar]
- 39.Baiyewu O, Smith-Gamble V, Akinbiyi A, et al. Behavioral and caregiver reaction of dementia as measured by the neuropsychiatric inventory in Nigerian community residents. Int Psychogeriatr. 2003;15(4):399–409. [DOI] [PubMed] [Google Scholar]
- 40.Korner A, Lauritzen L, Lolk A, Abelskov K, Christensen P, Nilsson FM. The Neuropsychiatric Inventory—NPI. Validation of the Danish version. Nord J Psychiatry. 2008;62(6):481–485. [DOI] [PubMed] [Google Scholar]
- 41.Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Neuropsychiatric symptoms in nursing home patients: factor structure invariance of the Dutch nursing home version of the Neuropsychiatric Inventory in different stages of dementia. Dement Geriatr Cogn Disord. 2007;24(3):169–176. [DOI] [PubMed] [Google Scholar]
- 42.Farina E, Baglio F, Caffarra P, et al. Frequency and clinical features of Lewy body dementia in Italian memory clinics. Acta Biomed . 2009;80(1):57–64. [PubMed] [Google Scholar]
- 43.Boada M, Cejudo JC, Tarraga L, Lopez OL, Kaufer D.Neuropsychiatric Inventory questionnaire (NPI-Q): Spanish validation of an abridged form of the Neuropsychiatric Inventory (NPI) [in Japanese]. Neurologia. 2002;17(6):317–323. [PubMed] [Google Scholar]
- 44.Vilalta-Franch J, Lozano-Gallego M, Hernandez-Ferrandiz M, Llinas-Regla J, Lopez-Pousa S, Lopez OL. The Neuropsychiatric Inventory. Psychometric properties of its adaptation into Spanish [in Spanish]. Rev Neurol. 1999;29(1):15–19. [PubMed] [Google Scholar]
- 45.Chow TW, Liu CK, Fuh JL, et al. Neuropsychiatric symptoms of Alzheimer’s disease differ in Chinese and American patients. Int J Geriatr Psychiatry. 2002;17(1):22–28. [DOI] [PubMed] [Google Scholar]
- 46.Pang FC, Chow TW, Cummings JL, et al. Effect of neuropsychiatric symptoms of Alzheimer’s disease on Chinese and American caregivers. Int J Geriatr Psychiatry. 2002;17(1):29–34. [DOI] [PubMed] [Google Scholar]
- 47.Grill JD, Raman R, Ernstrom K, et al. Comparing recruitment, retention, and safety reporting among geographic regions in multinational Alzheimer’s disease clinical trials. Alzheimers Res Ther. 2015;7(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23(2):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai CK. The merits and problems of Neuropsychiatric Inventory as an assessment tool in people with dementia and other neurological disorders. Clin Interv Aging. 2014;9:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. [DOI] [PubMed] [Google Scholar]
- 51.Zuidema SU, Derksen E, Verhey FR, Koopmans RT. Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. Int J Geriatr Psychiatry. 2007; 22(7):632–638. [DOI] [PubMed] [Google Scholar]
- 52.Selbaek G, Engedal K. Stability of the factor structure of the Neuropsychiatric Inventory in a 31-month follow-up study of a large sample of nursing-home patients with dementia. Int Psychogeriatr. 2012;24(1):62–73. [DOI] [PubMed] [Google Scholar]
- 53.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(4):253–259. [DOI] [PubMed] [Google Scholar]
- 54.Ringman JM, Liang LJ, Zhou Y, et al. Early behavioural changes in familial Alzheimer’s disease in the dominantly inherited Alzheimer network. Brain. 2015;138(pt 4):1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708–714. [DOI] [PubMed] [Google Scholar]
- 56.Lima-Silva TB, Bahia VS, Carvalho VA, et al. Neuropsychiatric symptoms, caregiver burden and distress in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2015;40(5-6):268–275. [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Jin Y, Shi Z, et al. The effects of behavioral and psychological symptoms on caregiver burden in frontotemporal dementia, Lewy body dementia, and Alzheimer’s disease: clinical experience in China. Aging Ment Health. 2017;21(6):651–657. [DOI] [PubMed] [Google Scholar]
- 58.Shea YF, Ha J, Chu LW. Comparisons of clinical symptoms in biomarker-confirmed Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal dementia patients in a local memory clinic. Psychogeriatrics. 2015;15(4):235–241. [DOI] [PubMed] [Google Scholar]
- 59.Finger E, Zhang J, Dickerson B, Bureau Y, Masellis M; Alzheimer’s Disease Neuroimaging Initiative. Disinhibition in Alzheimer’s disease is associated with reduced right frontal pole cortical thickness. J Alzheimers Dis. 2017;60(3):1161–1170. [DOI] [PubMed] [Google Scholar]
- 60.Leger GC, Banks SJ. Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2014;37(1-2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996;47(5):1184–1189. [DOI] [PubMed] [Google Scholar]
- 62.Yatabe Y, Hashimoto M, Kaneda K, et al. Neuropsychiatric symptoms of progressive supranuclear palsy in a dementia clinic. Psychogeriatrics. 2011;11(1):54–59. [DOI] [PubMed] [Google Scholar]
- 63.Jecmenica-Lukic M, Pekmezovic T, Petrovic IN, Tomic A, Svetel M, Kostic VS. Use of the neuropsychiatric inventory to characterize the course of neuropsychiatric symptoms in progressive supranuclear palsy. J Neuropsychiatry Clin Neurosci. 2018;30(1):38–44. [DOI] [PubMed] [Google Scholar]
- 64.Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry. 1998; 65(5):717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kazui H, Yoshiyama K, Kanemoto H, et al. Differences of behavioral and psychological symptoms of dementia in disease severity in four major dementias. PLoS One. 2016;11(8): e0161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24(15):2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paulsen JS, Ready RE, Hamilton JM, Mega MS, Cummings JL. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001;71(3):310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciurli P, Formisano R, Bivona U, Cantagallo A, Angelelli P. Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil. 2011;26(2):116–126. [DOI] [PubMed] [Google Scholar]
- 71.Jung NY, Kim HJ, Kim YJ, et al. Neuropsychiatric characteristics of PiB-negative subcortical vascular dementia versus behavioral variant frontotemporal dementia. Arch Gerontol Geriatr. 2016;67:86–91. [DOI] [PubMed] [Google Scholar]
- 72.Santos MAO, Bezerra LS, Correia CDC, Bruscky IS. Neuropsychiatric symptoms in vascular dementia: epidemiologic and clinical aspects. Dement Neuropsychol. 2018;12(1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin YL, Zhang H, Gao YZ, et al. Neuropsychiatric symptoms in patients with vascular dementia in mainland China. Transl Neurosci. 2015;6(1):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chin J, Seo SW, Kim SH, et al. Neurobehavioral dysfunction in patients with subcortical vascular mild cognitive impairment and subcortical vascular dementia. Clin Neuropsychol. 2012;26(2):224–238. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh CJ, Chang CC, Lin CC. Neuropsychiatric profiles of patients with Alzheimer’s disease and vascular dementia in Taiwan. Int J Geriatr Psychiatry. 2009;24(6):570–577. [DOI] [PubMed] [Google Scholar]
- 76.Krishnamoorthy ES, Trimble MR. Prevalence, patterns, service needs, and assessment of neuropsychiatric disorders among people with epilepsy in residential care: validation of the Neuropsychiatric Inventory as a caregiver-rated measure of neuropsychiatric functioning in epilepsy. Epilepsy Behav. 2008;13(1):223–228. [DOI] [PubMed] [Google Scholar]
- 77.Kulisevsky J, Litvan I, Berthier ML, Pascual-Sedano B, Paulsen JS, Cummings JL. Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Mov Disord. 2001;16(6):1098–1104. [DOI] [PubMed] [Google Scholar]
- 78.Holtta E, Laakkonen ML, Laurila JV, et al. The overlap of delirium with neuropsychiatric symptoms among patients with dementia. Am J Geriatr Psychiatry. 2011;19(12):1034–1041. [DOI] [PubMed] [Google Scholar]
- 79.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314–319. [DOI] [PubMed] [Google Scholar]
- 80.Kumfor F, Zhen A, Hodges JR, Piguet O, Irish M. Apathy in Alzheimer’s disease and frontotemporal dementia: distinct clinical profiles and neural correlates. Cortex. 2018;103:350–359. [DOI] [PubMed] [Google Scholar]
- 81.Rosenberg PB, Lanctot KL, Drye LT, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(2):91–97. [DOI] [PubMed] [Google Scholar]
- 83.Craig AH, Cummings JL, Fairbanks L, et al. Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol. 1996;53(11):1116–1120. [DOI] [PubMed] [Google Scholar]
- 84.Jeong H, Kang I, Im JJ, et al. Brain perfusion correlates of apathy in Alzheimer’s disease. Dement Neurocogn Disord. 2018;17(2):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum Brain Mapp. 2016;37(12):4234–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49(3):355–361. [PubMed] [Google Scholar]
- 87.Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weissberger GH, Melrose RJ, Narvaez TA, Harwood D, Mandelkern MA, Sultzer DL. (18)F-fluorodeoxyglucose positron emission tomography cortical metabolic activity associated with distinct agitation behaviors in Alzheimer disease. Am J Geriatr Psychiatry. 2017;25(6):569–579. [DOI] [PubMed] [Google Scholar]
- 89.Iaccarino L, Sala A, Caminiti SP, Santangelo R, Iannaccone S, Magnani G, Perani D. The brain metabolic signature of visual hallucinations in dementia with Lewy bodies. Cortex. 2018;108:13–24. [DOI] [PubMed] [Google Scholar]
- 90.Bensamoun D, Guignard R, Furst AJ, et al. Associations between neuropsychiatric symptoms and cerebral amyloid deposition in cognitively impaired elderly people. J Alzheimers Dis. 2016; 49(2):387–398. [DOI] [PubMed] [Google Scholar]
- 91.Mori T, Shimada H, Shinotoh H, et al. Apathy correlates with prefrontal amyloid beta deposition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85(4):449–455. [DOI] [PubMed] [Google Scholar]
- 92.Babulal GM, Ghoshal N, Head D, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24(11):1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzales MM, Insel PS, Nelson C, et al. Chronic depressive symptomatology and CSF amyloid beta and tau levels in mild cognitive impairment. Int J Geriatr Psychiatry. 2018;33(10):1305–1311. [DOI] [PubMed] [Google Scholar]
- 94.Bloniecki V, Aarsland D, Cummings J, Blennow K, Freund-Levi Y. Agitation in dementia: relation to core cerebrospinal fluid biomarker levels. Dement Geriatr Cogn Dis Extra. 2014;4(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaufer DI, Cummings JL, Christine D. Effect of tacrine on behavioral symptoms in Alzheimer’s disease: an open-label study. J Geriatr Psychiatry Neurol. 1996;9(1):1–6. [DOI] [PubMed] [Google Scholar]
- 96.Mega MS, Dinov ID, Lee L, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2000;12(2):209–218. [DOI] [PubMed] [Google Scholar]
- 97.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(3):532–538. [DOI] [PubMed] [Google Scholar]
- 98.Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry. 2008;23(5):537–545. [DOI] [PubMed] [Google Scholar]
- 99.Cummings JL, Schneider E, Tariot PN, Graham SM; Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67(1):57–63. [DOI] [PubMed] [Google Scholar]
- 100.Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2018;378(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Deyn P, Jeste DV, Swanink R, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. J Clin Psychopharmacol. 2005;25(5):463–467. [DOI] [PubMed] [Google Scholar]
- 102.Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology. 2015;84(14 suppl):S16.007. [Google Scholar]
- 103.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. [DOI] [PubMed] [Google Scholar]
- 104.Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242–1254. [DOI] [PubMed] [Google Scholar]
- 106.Scherer RW, Drye L, Mintzer J, et al. The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials. 2018;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lichtwarck B, Selbaek G, Kirkevold O, et al. Targeted interdisciplinary model for evaluation and treatment of neuropsychiatric symptoms: a cluster randomized controlled trial. Am J Geriatr Psychiatry. 2018;26(1):25–38. [DOI] [PubMed] [Google Scholar]
- 108.Reuben DB, Tan ZS, Romero T, Wenger NS, Keeler E, Jennings LA. Patient and caregiver benefit from a comprehensive dementia care program: 1-year results from the UCLA Alzheimer’s and Dementia Care Program. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang SH, Chio OI, Chang LH, et al. Caregiver active participation in psychoeducational intervention improved caregiving skills and competency. Geriatr Gerontol Int. 2018;18(5):750–757. [DOI] [PubMed] [Google Scholar]
- 110.Rattinger GB, Sanders CL, Vernon E, et al. Neuropsychiatric symptoms in patients with dementia and the longitudinal costs of informal care in the cache county population. Alzheimers Dement (N Y). 2019;5:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59(11):1721–1729. [DOI] [PubMed] [Google Scholar]
- 112.Morris S, Patel N, Baio G, Kelly L, Lewis-Holmes E, Omar RZ, Katona C, Cooper C, Livingston G. Monetary costs of agitation in older adults with Alzheimer’s disease in the UK: prospective cohort study. BMJ Open. 2015;5(3): e007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.D’Amico F, Rehill A, Knapp M, et al. Cost-effectiveness of exercise as a therapy for behavioural and psychological symptoms of dementia within the EVIDEM-E randomised controlled trial. Int J Geriatr Psychiatry. 2016;31(6):656–665. [DOI] [PubMed] [Google Scholar]
- 114.Cummings J, Mintzer J, Brodaty H, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


