Supplemental digital content is available in the text.
Abstract
SIGNIFICANCE
Patients with glaucoma and providers recognized perceived treatment efficacy, patient-provider relationship, psychological stress, instillation skill, good quality of life, and forgetfulness as key determinants of glaucoma adherence. This shared insight could help shape the development of clinical and behavioral interventions for addressing treatment barriers and improving adherence.
PURPOSE
Despite their impact on adherence in glaucoma, sociobehavioral factors may not be adequately explored during clinical consultations. We aimed to elicit consensus between patients and providers around key determinants of adherence and hypothesized that patients would place greater emphasis on sociobehavioral factors compared with providers.
METHODS
A two-round Delphi survey was used to assess treatment beliefs, barriers, facilitators, motivators, and needs among 18 patients with glaucoma and providers. In round 1, agreement with 46 statements was scored on a 5-point Likert scale (strongly disagree to strongly agree). Statements with which 80% or more of panelists agreed reached consensus and advanced to round 2, where participants were asked to prioritize them based on their importance to treatment.
RESULTS
There was consensus regarding the influence of perceived treatment efficacy, good provider relationship, good quality of life, psychological stress, glaucoma knowledge, instillation skill, and forgetfulness on glaucoma adherence. For statements that failed to reach consensus, the Bonferroni-corrected Mann-Whitney U test revealed that the greatest differences between patients and providers pertained to regimen complexity (provider median, 4 [interquartile range {IQR}, 1]; patient median, 1.5 [IQR, 1]; P = .002), instillation skill (providers, 4 [IQR, 0.5]; patients, 2 [IQR, 1]; P = .001), and low motivation (providers, 3 [IQR, 2.25]; patients, 1 [IQR, 0]; P = .003).
CONCLUSIONS
Although patients and providers prioritized sociobehavioral factors as key determinants of adherence, disagreement between these groups was observed in other areas. Continued juxtaposition of patient and provider perspectives could spotlight underexplored areas and guide the development of successful interventions for improving adherence.
More than 60 million people worldwide are affected by primary open-angle glaucoma,1–3 a progressive optic neuropathy characterized by retinal ganglion cell death and distinctive patterns of vision loss. Although daunting, this figure is likely to be an underestimation, as only half of all persons living with glaucoma are believed to be diagnosed.4 Glaucoma is a leading cause of irreversible blindness in the United States,2,5 and eye drops that lower intraocular pressure (IOP) and delay glaucomatous progression accounted for more than 50% of Medicare part D prescribing costs in 2013.6 Despite extensive prescribing, the proportion of patients with good adherence to recommended therapy is reported to be as low as 20%.7 Although later studies have reported higher rates,8–10 adherence in glaucoma remains suboptimal. High treatment cost, low education level, forgetfulness, and regimen complexity have been identified as key sociodemographic and clinical determinants of poor adherence.11 However, many interventions based on these variables have demonstrated variable degrees of success, suggesting the possible influence of social, psychological, and behavioral factors on adherence to glaucoma therapy.
Sociobehavioral factors such as poor patient-provider relationship,12 low self-efficacy,13 and psychological stress14 have been found to affect adherence in glaucoma. A study in diabetes also reported that patients were intentionally nonadherent in social settings because of embarrassment and public perception.15 Despite their influence, providers may have a limited ability to address sociobehavioral factors because of disparate perspectives and experiences relative to patients.16 A 2005 study reported that poor communication between patients and providers led to nearly one in five patients using the wrong regimen.17 It is vital that patients and providers improve their understanding of each other, as this is the basis for shared decision making and effective treatment. We aimed to elicit consensus between patients and providers around key determinants of adherence using Delphi surveys. We used a mixed-methods approach to assess treatment perspectives and hypothesized that patients would place a greater emphasis on sociobehavioral factors compared with providers.
METHODS
Participant Selection and Recruitment
This research was reviewed by an independent ethical review board and conforms with the principles and applicable guidelines for the protection of human subjects in biomedical research. In addition, all research adhered to the Health Insurance Portability and Accountability Act, as well as the tenets of the Declaration of Helsinki. Optometrists and ophthalmologists with at least 2 years of experience treating glaucoma, and patients diagnosed with primary open-angle glaucoma for at least 2 years were recruited to participate in the Delphi survey. Patients also had to be older than 40 years, have best-corrected visual acuity better than 20/40, have been using hypotensive eye drops for at least 3 months, and have at least two reliable visual field tests (false positive rates <33% and fixation loss rates <20%). Providers were recruited from Callahan Eye Hospital and Clinics (CEHC), the University of Alabama at Birmingham School of Optometry Eye Care Clinic, and community-based practices within Jefferson County. All patients were recruited from CEHC. Visual field tests were obtained from patients' clinical charts and used to determine disease severity. Based on perimetric research, we accepted visual field tests taken within 6, 12, and 24 months of study commencement for patients with severe, moderate, and mild glaucoma, respectively.18–20 Regardless of disease severity, 90% of patients underwent visual field testing within 12 months of study commencement. Disease severity was ascertained according to the Hodapp-Parrish-Anderson criteria.21
Delphi Survey Methodology
Delphi surveys use iterative rounds of questionnaires to refine consensus around a topic of interest among diverse respondents. These respondents—referred to as panelists—may represent one or more professional groups. In our study, we used two professional groups: patients with glaucoma and glaucoma eye care providers. In Delphi surveys, panelists complete questionnaires in each round, and items that reach high levels of agreement (consensus) are identified. Responses are summarized, and items that fail to reach consensus are excluded from successive rounds of questionnaires.22 In this way, expert consensus on a specific topic is continuously refined. Delphi surveys lack the limitations of other qualitative methods such as focus groups, which provide rich qualitative data but afford little anonymity. An additional advantage of Delphi surveys is their allowance for meaningful findings using relatively few participants. Sample size determination in Delphi surveys, unlike studies that use inferential statistics, is motivated by the need to maximize the generation of ideas while minimizing cost and procedural inefficiencies. Panels with 15 to 25 members are both common and empirically sound in health care research.23 We determined the sample size for our study by referring to Delphi literature recommending 10 to 50 panelists.24 We determined the size of our patient groups (n = 10) and provider groups (n = 8) by following recommendations that advise 5 to 10 panelists per professional group.25 We used the modified, two-round Delphi survey, which is appropriate when substantial primary literature exists on the topic under study.22
We used purposive, nonrandom sampling, which in Delphi studies is primarily based on panelist expertise and experience in the research area.26–28 Consequently, Delphi panels may, by design, be unrepresentative of the larger population to ensure that panelists have expertise and experience relevant to the topic being investigated. To maximize the expertise of our panel, we oversampled for patients more likely to have difficulty maintaining good adherence, racial and ethnic minorities, patients with severe glaucoma, patients with glaucoma for more than 2 years, and patients with complex regimens. Provider panelists were selected from various backgrounds (e.g., ophthalmology, optometry, tertiary referral centers, and community-based clinics). Recruitment letters were mailed to eligible participants and followed up with up to three phone calls.
Round 1 Data Collection
Before study commencement, the interviewer (SP) was trained in qualitative data collection by completing instructional modules from the University of Minnesota29 and the University of Kansas,30 and later completed three trial interviews under the supervision of study personnel. Modules covered recommendations for conducting focus groups, in-person and telephone interviews, guidance on note-taking and recording during interviews, recommendations for transcribing and reporting qualitative research findings, and guidelines for minimizing bias. In round 1, participants completed the National Eye Institute Visual Function Questionnaire 25,31 demographic questions, and a semistructured Health Belief Model (HBM)–based questionnaire exploring several dimensions of glaucoma treatment. The HBM predicts the likelihood of a given health behavior by factoring in modifying variables called constructs.32 Documented determinants of adherence were identified via literature review and mapped onto the HBM constructs they addressed. Five groups of determinants reflecting five HBM constructs were identified: treatment beliefs, treatment barriers, treatment motivators (perceived benefits of treatment), and treatment facilitators (thoughts and actions that lead to desired behavior). The final group constituted treatment needs, which despite being recognized in glaucoma literature, are not included in the HBM. Statements addressing identified determinants were developed, and face validity of each statement was assessed by a panel of optometrists and social scientists.
Participants' level of agreement with each questionnaire statement was scored on a 5-point Likert scale (strongly disagree to strongly agree), and wording for patient and provider questionnaires was adjusted to reflect their respective perspectives. For instance, providers were asked “Do you think that the medication you prescribe is effective?” whereas patients were asked “Do you believe that the medication prescribed by your doctor is effective?” In addition to Likert scale–based responses, panelists were encouraged to provide additional context, which was audio recorded with participants' consent to allow for transcription. The National Eye Institute Visual Function Questionnaire 25 was excluded from provider questionnaires because only patients' clinical characteristics were of interest. Patient questionnaires were administered by the interviewer in private rooms at CEHC, whereas provider questionnaires were administered at CEHC (n = 2) or their practice (n = 6). All data were collected from September 2019 to November 2019 (Appendix Table A1, available at http://links.lww.com/OPX/A520).
Round 1 Analysis
Likert responses were recoded so that 1 indicated strongly disagree; 2, disagree; 3, neutral; 4, agree; and 5, strongly agree. IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY)33 was used to perform Bonferroni-corrected Mann-Whitney U tests for significant differences between patient and provider responses. Neutral scores (3) were then omitted for each statement, and remaining scores were dichotomized into two response types: agreement (4 or 5) or disagreement (1 or 2). Disagreement was indicated by negative values, whereas agreement was indicated by positive values. For example, a statement receiving scores of 4 and 5 from 9 of 18 panelists had an agreement level of 50%, whereas a statement receiving scores of 1 and 2 from 9 of 18 panelists had an agreement level of −50%. Consensus was defined as an agreement level of 80% or more, and all statements reaching consensus advanced to round 2. This threshold was selected because it was the most conservative threshold reported in similarly sized Delphi studies.34
After quantitative analysis was complete, audio recordings of the questionnaire sessions were transcribed, and qualitative analysis was performed in Nvivo Version 12 (QSR International, Victoria, Australia).35 A codebook was developed by two researchers (SLA, SP) during the preliminary review of the transcripts, and codes were assigned to the transcribed text based on content.36 Per each code, verbal responses were sorted into two groups: confirmatory (+), where panelists agreed that the factors being discussed impacted adherence, and contradictory (−), where panelists disagreed. A coding comparison between the two researchers was performed, and Cohen κ statistic was used to assess intercoder reliability.
Round 2 Data Collection and Analysis
Once round 1 data were analyzed, post-round reports containing individual questionnaire scores and median scores for the entire panel were mailed to all panelists, who were also invited to review the reports and revise their round 1 responses if desired. No panelists amended their responses after reviewing round 1 reports. In round 2, panelists were asked to prioritize the statements that reached consensus in round 1 based on their importance to glaucoma treatment. Round 2 was conducted from December 2019 to February 2020, and post-round reports were issued to panelists after analysis. No panelists were lost to attrition, and we had a 100% response rate in both Delphi rounds.
RESULTS
Demographic and Clinical Characteristics
Table 1 shows the clinical and demographic characteristics of Delphi panelists. Fifty percent of patient panelists had severe glaucoma (mean deviation worse than −12 dB), whereas 70% of patients were diagnosed with three or more chronic health conditions, the most common of which were hypertension, depression, gastroesophageal reflux disease, and diabetes. Persons of African descent constituted the largest racial group among patients (70%), followed by persons of European descent (30%). Among providers, persons of European descent constituted the largest racial group (62.5%), followed by persons of African descent (25%) and persons of Asian descent (12.5%). Men constituted 40% of patients compared with 37.5% of providers. All patients were between the ages of 50 and 70 years, compared with only 37.5% of providers.
TABLE 1.
Clinical and demographic characteristics of patient and provider panelists
| Study variables | |
|---|---|
| Patients (n = 10) | |
| Acuity (logMAR), mean (SD) | 0.24 (0.14) |
| IOP (mmHg), OD, mean (SD) | 14.5 (3.7) |
| IOP (mmHg), OS, mean (SD) | 14.5 (4.6) |
| General health score, median (IQR) | 50 (0) |
| General vision score, median (IQR) | 37.5 (25) |
| Mental health score, median (IQR) | 46.9 (51.6) |
| Glaucoma severity, n (%) | |
| Mild | 2 (20) |
| Moderate | 3 (30) |
| Severe | 5 (50) |
| No. comorbidities, n (%) | |
| 0 | 1 (10) |
| 1–2 | 2 (20) |
| 3–4 | 5 (50) |
| ≥5 | 2 (20) |
| Comorbidities (%) | |
| Diabetes | 3 (30) |
| Hypertension | 7 (70) |
| High cholesterol | 3 (30) |
| GERD | 4 (40) |
| Depression | 4 (40) |
| Medication type (%) | |
| Prostaglandin analogs | 50 |
| β-Blockers | 21.43 |
| Carbonic anhydrase inhibitors | 21.43 |
| α-Agonists | 7 |
| Sex, n (%) | |
| Male | 4 (40) |
| Female | 6 (60) |
| Age, n (%) | |
| 50–59 y | 4 (40) |
| 60–69 y | 4 (40) |
| 70–79 y | 2 (20) |
| Race, n (%) | |
| African descent | 7 (70) |
| European descent | 2 (20) |
| Multiracial (European and Native American) | 1 (10) |
| Ethnicity, n (%) | |
| Hispanic | 1 (10) |
| Income level, n (%) | |
| <$10,0000 | 1 (10) |
| $10,000–$59,000 | 6 (60) |
| $60,000–$100,000 | 1 (10) |
| $100,000–$149,000 | 1 (10) |
| >$150,000 | 1 (10) |
| Education level, n (%) | |
| Some high school | 1 (10) |
| Some college | 6 (60) |
| Bachelor's degree | 2 (20) |
| Graduate or professional degree | 1 (10) |
| Employment level, n (%) | |
| Unemployed/unable to work | 1 (10) |
| Employed full-time | 3 (30) |
| Retired | 6 (60) |
| Providers (n = 8) | |
| Sex, n (%) | |
| Male | 3 (37.5) |
| Female | 5 (62.5) |
| Age, n (%) | |
| 30–39 y | 2 (25) |
| 40–49 y | 3 (37.5) |
| 50–59 y | 2 (25) |
| 60–69 y | 1 (12.5) |
| Race, n (%) | |
| African descent | 2 (25) |
| Asian descent | 1 (12.5) |
| European descent | 5 (62.5) |
| Specialty type, n (%) | |
| Optometrists | 5 (62.5) |
| Ophthalmologists (specialists and surgeons) | 3 (37.5) |
| Weekly patient load, n (%) | |
| 25–50 | 5 (62.5) |
| 50–75 | 1 (12.5) |
| 75–100 | 1 (12.5) |
| 100–125 | 1 (12.5) |
| Method for assessing adherence, n (%) | |
| Self-report | 6 (75) |
| Self-report and prescription records | 2 (25) |
GERD = gastroesophageal reflux disease; IQR = interquartile range; SD = standard deviation.
Statements Failing to Reach Consensus
Of the 36 statements that failed to reach consensus, 19 showed opposing responses (one group agreed, whereas the other disagreed). These data are shown in Fig. 1. One statement was excluded from analysis because patient scores were evenly dichotomized into agreement and disagreement, and a majority response type could not be determined (“Reminders and alarms are helpful”). This reduced the number of statements to 46. Relative to providers, patients agreed that they could manage glaucoma without instrumental help (assistance with physical tasks such as clinic visits and instilling drops). However, patients disagreed that they could manage glaucoma without emotional support or that they could easily detect changes in their vision over time (Fig. 1A). Among treatment barriers, patients disagreed with providers that any barriers except for busy schedule negatively affected adherence (Fig. 1B). Bonferroni-corrected Mann-Whitney U tests revealed significant differences between patients and providers regarding the influence of regimen complexity (provider median, 4 [interquartile range {IQR}, 1]; patient median, 1.5 [IQR, 1]; P = .002), poor instillation skill (providers, 4 [IQR, 0.5]; patients, 2 [IQR, 1], P = .001), low motivation (providers, 3 [IQR, 2.25]; patients, 1 [IQR, 0]; P = .003), medication cost (providers, 5 [IQR, 3]; patients, 2 [IQR, 0]; P = .002), and transportation (providers, 3 [IQR, 2.25]; patients, 1 [IQR, 0]; P = .001). Patients agreed with providers that all facilitators except for making schedules positively impacted adherence (Fig. 1C) and disagreed with providers that help was needed with transportation or paying for treatment (Fig. 1D).
FIGURE 1.
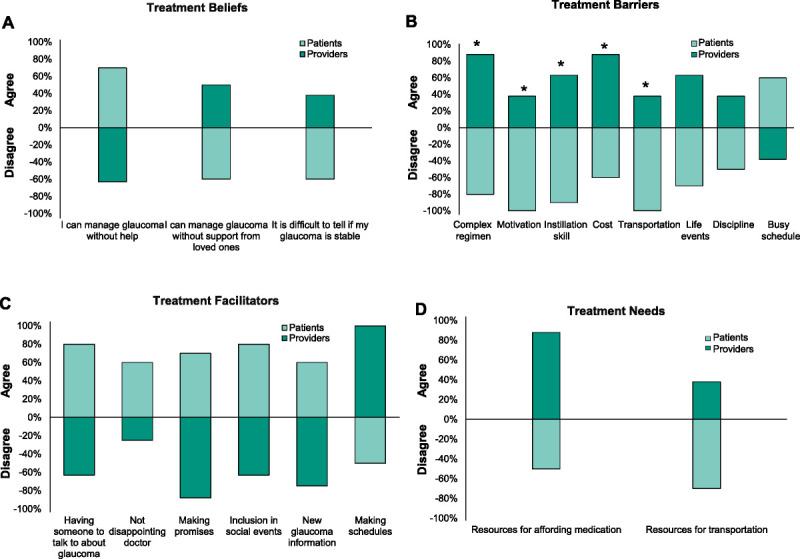
Majority response types and agreement levels for statements that did not reach consensus. Three treatment beliefs (A), eight treatment barriers (B), six treatment motivators (C), and two treatment needs (D) failed to reach consensus and showed opposing responses among patients and providers (n = 18). Consensus refers to an 80% or more overall agreement. Negative values indicate disagreement with statements, whereas positive values indicate agreement. *Statistically significant differences between patient and provider responses detected by the Bonferroni-corrected Mann-Whitney U test.
Statements Reaching Consensus
Fig. 2 depicts the agreement levels for statements that reached consensus and advanced to round 2. In round 2, perceived treatment efficacy (“Prescribed medication is effective,” “Not using eyedrops affects vision”) was prioritized as the most impactful treatment-related belief, followed by good patient-provider relationship (“I can openly discuss problems with my doctor”) and adequate glaucoma knowledge (“I have a good understanding of glaucoma”). Also in round 2, reducing worry about blindness was prioritized as the strongest motivator for good adherence, followed by being independent, being able to navigate freely, and being able to drive. Memory aides were identified as the most pressing treatment need, followed by guides for instilling drops.
FIGURE 2.
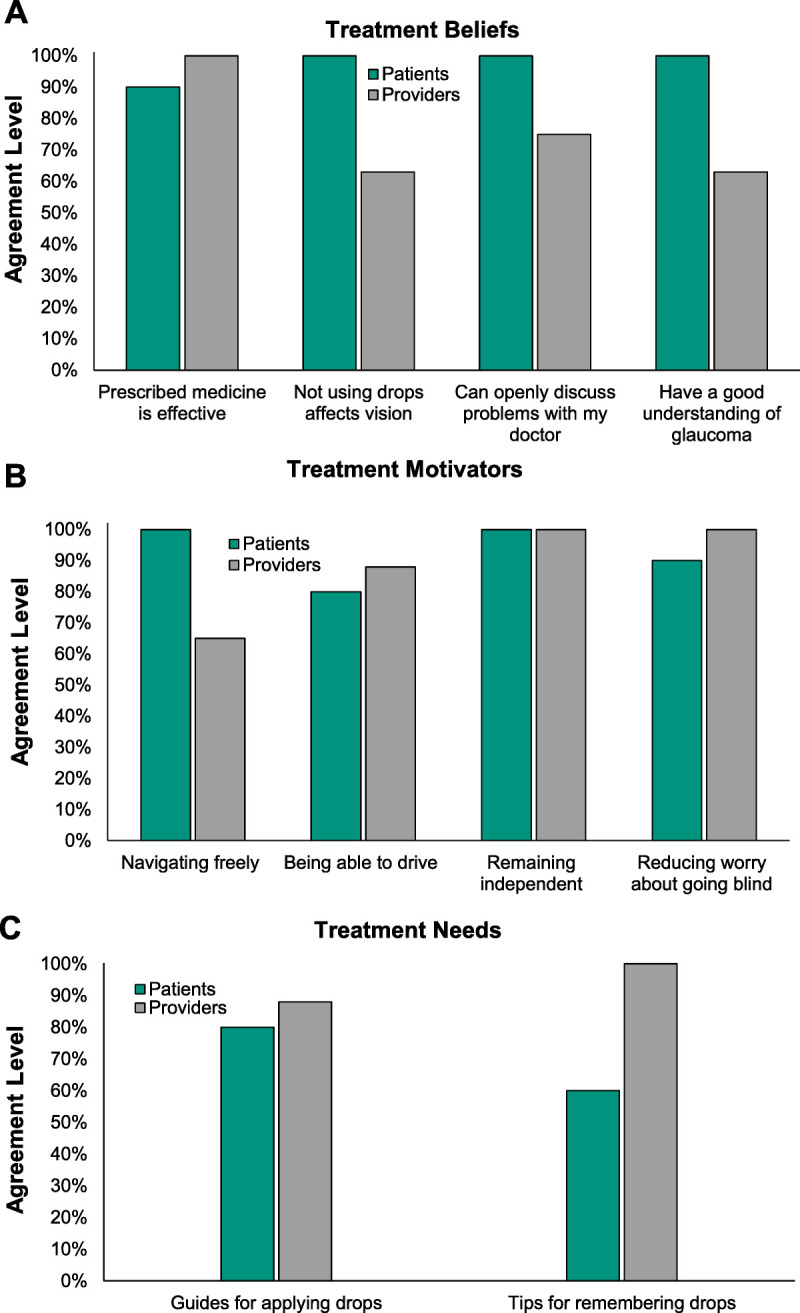
Agreement levels for statements that reached consensus. Four treatment beliefs (A), four treatment motivators (B), and two treatment needs (C) reached consensus in round 1. Consensus refers to an 80% or more agreement. No statistically significant differences between patients and providers (n = 18) were detected by the Bonferroni-corrected Mann-Whitney U test.
Qualitative Analysis
Results of our thematic analysis are presented in Table 2. For patients, prominent themes were related to good quality of life (13 comments), psychological stress (10 comments), and glaucoma knowledge (9 comments). Among providers, patient-provider relationship (40 comments), glaucoma knowledge (29 comments), and quality of life (16 comments) were the most recurrent themes. Cohen κ was calculated to be 0.62 indicating good interrater reliability.35
TABLE 2.
Major themes emerging from content analysis
| Themes | Patients | Providers | ||||
|---|---|---|---|---|---|---|
| Sample quote | (+) | (−) | Sample quote | (+) | (−) | |
| Health system, provider relationship | “I do not want to disappoint my doctor because I'm the patient that really does what they say, and they know” | 2 | 2 | “Patients want to please the physicians. If they think the physician will be disappointed if they say I'm not taking my drops” | 39 | 1 |
| Treatment cost | “This one we had to get for surgery prep was expensive” | 4 | 2 | “Cost of eye drops, that's a big deal” | 8 | 0 |
| Social/emotional support | “I only talk to my daughter about this” | 3 | 1 | “Especially for moderate-severe or those having surgery” | 10 | 0 |
| Psychological stress (worry, fear, anxiety) | “I'm embarrassed because other people can read along with subtitles and I cannot even get to it” | 7 | 3 | “They become frustrated by that and you must keep reminding them that the goal is to prevent loss of vision not to get more” | 11 | 6 |
| Instrumental support | “I cannot drive or do any of those things, I need help” | 3 | 1 | “I would say that most need some type of support system” | 14 | 0 |
| Medication side effects | “The taste, just the taste” | 2 | 1 | “Even if effective, it may not be used because of burning, stinging” | 6 | 2 |
| Transportation | “I will have to disagree with that since I cannot drive or do any of those things” | 1 | 1 | “Lack of reliable transportation—I've had a lot of no shows—IOP check, things like that” | 7 | 1 |
| Instillation skill and dexterity | “I remember when it was the child top, but now you have to squeeze and line this up.” | 2 | 0 | “It's got to be 90 percent of patients who would need help with this” | 11 | 1 |
| Glaucoma knowledge and health literacy | “Yes, I teach anatomy and I take the eyes apart in class” | 7 | 2 | “We give patients a ton of information and a lot is lost as soon as they hear the diagnosis” | 26 | 3 |
| Treatment efficacy | “I understand if I do not take my medicine, I will be blind” | 6 | 1 | “Sometimes it's hard to really tell if it's working” | 7 | 3 |
| Life events and busy schedules | “I take care of people; I take care of my husband” | 3 | 0 | “It's just hard when they are on vacation” | 4 | 1 |
| Comorbidities and complex regimens | “I take so much medicine. The drops are the last thing I do at the end of the day” | 1 | 0 | “The problem is that there is a balance. After 2, 3 medications, compliance just falls” | 11 | 0 |
| Forgetfulness and reminders | “They gonna call to remind me so I do not even keep up” | 4 | 3 | “Reminders, if patients are able to, are incredibly helpful.” | 14 | 0 |
| Self-efficacy | “I might miss some here and there” | 1 | 0 | “Many of them do need help” | 5 | 0 |
| Motivation | — | 0 | 0 | “Motivation is there, but it can wax and wane” | 3 | 1 |
| Quality of life | “It took me from being independent to being dependent again.” | 11 | 2 | “Patients want to be independent. If the VF gets tiny and central vision is affected, they will not be” | 15 | 1 |
| Surgical treatment (fear or complications) | “He has to pause from regular medication after surgery, but he is on another one” | 1 | 0 | “I'll see people that are teetering on surgery or not. I'll say let us just give it one more month, then they'll come clean” | 5 | 0 |
Positive signs (+) indicate confirmatory statements where panelists agreed that themes affected adherence. Negative signs (−) indicate contradictory statements where panelists disagreed that themes affected adherence.
DISCUSSION
Although several studies have explored patient perspectives in glaucoma, a smaller proportion have comparatively assessed patient and provider perspectives.10,14,37 Our study revealed consensus regarding the impact of perceived treatment efficacy, patient-provider relationship, forgetfulness, psychological stress, instillation skill, wanting a good quality of life, and glaucoma knowledge. Among these, perceived treatment efficacy, reduced psychological stress, and memory aides were the most highly prioritized treatment beliefs, treatment motivators, and treatment needs, respectively. Although both panelist groups identified determinants of socioeconomic and sociobehavioral origin, providers tended to recognize socioeconomic treatment barriers such as cost and transportation. Patients tended to recognize sociobehavioral treatment facilitators such as social support and close patient-provider relationships.
Other prominent differences between patients and providers pertained to the importance of day-to-day support. Relative to providers, patients minimized instrumental support while prioritizing emotional support, suggesting a need for greater emphasis on patients' level of social and emotional wellness. Social support is also closely related to good quality of life,38,39 another factor that reached consensus. Both patient and provider panelists recognized the importance of being able to navigate freely, drive, and remain independent, as well as the threat that glaucoma posed to the continuation of these activities. The patient-provider relationship was also spotlighted; patients agreed that not wanting to disappoint their doctor influenced their adherence behavior, whereas providers disagreed. Because many clinicians rely on patient-reported adherence, patient overestimation due to provider expectations could skew an assessment and misinform treatment decisions.40 Some providers commented that provider expectations were barriers to honest communication, whereas others considered them to be facilitators of good adherence if properly leveraged. One patient admitted to deliberately skipping clinic visits during periods of poor compliance because they believed that their doctor would know.
Despite differences in perspectives, several factors reported to be important in adherence literature reached consensus in this study. Both panelist groups recognized the impact of psychological stress, a finding consistent with research indicating that patients with glaucoma are up to 12 times more likely to experience depression than persons without glaucoma.41 In response to such findings, there have been increasing appeals for the adoption of interventions that manage the negative effect associated with glaucoma diagnosis.42 Panelists also expressed a need for eye-drop instillation guides. Poor instillation skill has been identified as a treatment barrier,43–45 with as few as 10% of patients correctly instilling eye drops.46 This is concerning because poor instillation may result in poor IOP control and increased treatment costs, as well as poor treatment efficacy, which was another factor that reached consensus. Unlike many chronic conditions, glaucoma has no overt symptoms that prompt patients to maintain good adherence. This suggests that positive perceptions about the effectiveness of treatment are strong determinants of adherence, as evinced by the continued use of IOP-lowering drops among patients, even when there is no immediate perceived benefit.47 Providers stated that they reinforced treatment efficacy with a variety of techniques such as simulations of progression.
Other notable themes included patient motivation and the irreversible nature of glaucoma. Because therapy delays progression rather than restoring vision, patients may experience dampened treatment expectations and lower motivation. In recent years, motivational interviewing has become a common strategy for resolving patient ambivalence and has demonstrated favorable results.48 Patients also communicated high levels of openness with providers. Research has shown that communication styles and clinical priorities vary across ethnicity, race, and culture49 and that their incorporation into clinical decision making is associated with improved outcomes.50 However, such findings stand in contrast with glaucoma research indicating that patients' views and treatment goals may not be adequately explored.51 Our results highlight the need for providers to remain vigilant for sociobehavioral determinants, particularly because less observable factors such as acute psychological stress have been associated with elevated IOP.52
In addressing the underrepresentation of complementary patient and provider perspectives in glaucoma literature, this study revealed areas of consensus regarding the impact of perceived treatment efficacy, provider relationship, psychological stress, glaucoma knowledge, wanting a good quality of life, instillation skill, and forgetfulness. Qualitative analysis revealed the patient-provider relationship to be the most discussed theme, and we believe that it is one of the most proximal and direct determinants of good adherence. Strengths of this study include qualitative analysis, which supported our findings,53 and panelists' diverse clinical and demographic backgrounds, which provided nuanced perspectives. Although unaware, several patients and their personal providers participated in the study. This imparted an added layer of granularity to the study, as these paired responses directly measured differences and similarities in perspectives. Other strengths include use of an established health model in the development of questionnaires and the issuance of post-round reports that afforded patient panelists the opportunity to appreciate research findings.
This study is not without limitations, however. The relatively small panel size may limit the generalizability of our findings, as providers' responses were based on experiences with multiple patients, whereas patients' responses were based on experience with a single provider and their unique clinical history. Lastly, all participants were aware that the Delphi panel comprised both patients and providers and that both groups would receive post-round reports. Despite the data being deidentified, this knowledge could have contributed to responder bias. To our knowledge, this is the first study to comparatively assess treatment perspectives among patients with glaucoma and providers using both qualitative and quantitative methods. Our hypothesis was partially supported, as both groups prioritized sociobehavioral factors as key treatment beliefs, barriers, motivators, facilitators, and needs. However, per Delphi studies, the external validity of our findings lies in whether they are substantiated in real-world situations. Continued juxtaposition of patient and provider perspectives could spotlight other underexplored areas and inform the development of successful interventions for improving treatment adherence in glaucoma.
Supplementary Material
Footnotes
Supplemental Digital Content: Appendix Table A1, available at http://links.lww.com/OPX/A520, depicts the median Likert score and majority response type for questionnaire statements in round 1. For each statement, median scores are reported for the entire panel (overall), as well as for patient and provider groups. Neutral scores (3) were removed, and responses were dichotomized as agreement (positive values) or disagreement (negative values). Response levels indicate the proportion of panelists who agreed or disagreed. Statement phrasing is consistent with patient questionnaires. Bolded items reached consensus and advanced to round 2.
Funding/Support: This research was supported by an unrestricted grant from Research to Prevent Blindness.
Conflict of Interest Disclosure: The authors listed (LR) have reported a financial conflict of interest. The sponsor provided financial support but had no role in study design, conduct, analysis and interpretation, or writing. Each of the authors had full access to the study data and takes full responsibility for the presentation in this article.
Author Contributions: Conceptualization: SP, MDT; Data Curation: SP; Formal Analysis: SP, MF, SLA; Investigation: SP; Methodology: SP, LR, MF, Y-MS-G, SLA; Supervision: LR, MF, MDT; Writing – Original Draft: SP; Writing – Review & Editing: SP, LR, MF, Y-MS-G, SLA.
Supplemental Digital Content: Direct URL links are provided within the text.
Contributor Information
Shervonne Poleon, Email: skpoleon@gmail.com.
Lyne Racette, Email: lracette@uabmc.edu.
Matthew Fifolt, Email: mfifolt@uab.edu.
Yu-Mei Schoenberger-Godwin, Email: yschoenberger@uabmc.edu.
Sampson Listowell Abu, Email: sabu@uabmc.edu.
Michael D. Twa, Email: mdtwa@central.uh.edu.
REFERENCES
- 1.Kapetanakis VV Chan MP Foster PJ, et al. Global Variations and Time Trends in the Prevalence of Primary Open Angle Glaucoma (POAG): A Systematic Review and Meta-analysis. Br J Ophthalmol 2016;100:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC Li X Wong TY, et al. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-analysis. Ophthalmology 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- 3.Adelson JD Bourne RRA Briant PS, et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to Vision 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob Health 2021;9:e144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer A Tielsch JM Katz J, et al. Relationship between Intraocular Pressure and Primary Open Angle Glaucoma among White and Black Americans: The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090–5. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Broman AT. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman-Casey PA Woodward MA Niziol LM, et al. Brand Medications and Medicare Part D: How Eye Care Providers' Prescribing Patterns Influence Costs. Ophthalmology 2018;125:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olthoff C Schouten J Borne B, et al. Noncompliance with Ocular Hypotensive Treatment in Patients with Glaucoma or Ocular Hypertensionan Evidence-based Review. Ophthalmology 2005;112:953–61. [DOI] [PubMed] [Google Scholar]
- 8.Cate H Bhattacharya D Clark A, et al. Patterns of Adherence Behaviour for Patients with Glaucoma. Eye 2013;27:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okeke CO Quigley HA Jampel HD, et al. Adherence with Topical Glaucoma Medication Monitored Electronically: The Travatan Dosing Aid Study. Ophthalmology 2009;116:191–9. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DS Quigley HA Gelb L, et al. Using Pharmacy Claims Data to Study Adherence to Glaucoma Medications: Methodology and Findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci 2007;48:5052–7. [DOI] [PubMed] [Google Scholar]
- 11.Dreer LE, Girkin C, Mansberger SL. Determinants of Medication Adherence to Topical Glaucoma Therapy. J Glaucoma 2012;21:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai JC. Barriers to Adherence with Glaucoma Therapy: Potential Relationships to Disease Progression. Adv Stud Ophthalmol 2007;4:72–5. [Google Scholar]
- 13.Sleath B Blalock SJ Carpenter DM, et al. Ophthalmologist-patient Communication, Self-efficacy, and Glaucoma Medication Adherence. Ophthalmology 2015;122:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietlein TS Jordan J Dinslage S, et al. What Do Glaucoma Specialists Know about Their Patients? Graefes Arch Clin Exp Ophthalmol 2006;244:859–62. [DOI] [PubMed] [Google Scholar]
- 15.Peyrot M Barnett AH Meneghini LF, et al. Insulin Adherence Behaviours and Barriers in the Multinational Global Attitudes of Patients and Physicians in Insulin Therapy Study. Diabet Med 2012;29:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Addario BJ Fadich A Fox J, et al. Patient Value: Perspectives from the Advocacy Community. Health Expect 2017;21:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buller AJ, Connell B, Spencer AF. Compliance: Clear Communication's Critical. Br J Ophthalmol 2005;89:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan BC Garway-Heath DF Goñi FJ, et al. Practical Recommendations for Measuring Rates of Visual Field Change in Glaucoma. Br J Ophthalmol 2008;92:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansonius NM. Progression Detection in Glaucoma Can Be Made More Efficient by Using a Variable Interval between Successive Visual Field Tests. Graefes Arch Clin Exp Ophthalmol 2007;245:1647–51. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z Saunders LJ Daga FB, et al. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology 2017;124:786–92. [DOI] [PubMed] [Google Scholar]
- 21.Chang TC Ramulu P Hodapp E, et al. Clinical Decisions in Glaucoma. 2nd ed. Miami, FL: Ta Chen Chang; 2016. [Google Scholar]
- 22.Hsu CC, Sandford B. The Delphi Technique: Making Sense of Consensus. Pract Assess Res Eval 2007;12:1–8. [Google Scholar]
- 23.Akins RB, Tolson H, Cole BR. Stability of Response Characteristics of a Delphi Panel: Application of Bootstrap Data Expansion. BMC Med Res Methodol 2005;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Needham RD, de Loë RC. The Policy Delphi: Purpose, Structure, and Application. Can Geogr 1990;34:133–42. [Google Scholar]
- 25.Delbecq A, Van deVen A, Gustafson D. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Processes. Glenview, IL: Scott Foresman and Company; 1975:83–107. [Google Scholar]
- 26.Hasson F, Keeney S, McKenna H. Research Guidelines for the Delphi Survey Technique. J Adv Nurs 2000;32:1008–15. [PubMed] [Google Scholar]
- 27.Brady SR. Utilizing and Adapting the Delphi Method for Use in Qualitative Research. Int J Qual Methods 2015;14. [Google Scholar]
- 28.Powell C. The Delphi Technique: Myths and Realities. J Adv Nurs 2003;41:376–82. [DOI] [PubMed] [Google Scholar]
- 29.Krueger RA. Designing and Conducting Focus Group Interviews. Available at: https://www.eiu.edu/ihec/Krueger-FocusGroupInterviews.pdf. Accessed September 30, 2019.
- 30.Vilela M. Conducting Interviews. Last Updated: 2020. Available at: https://ctb.ku.edu/en/table-of-contents/assessment/assessing-community-needs-and-resources/conduct-interviews/main. Accessed October 26, 2018.
- 31.Mangione CM Lee PP Gutierrez PR, et al. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–8. [DOI] [PubMed] [Google Scholar]
- 32.Jones CL Jensen JD Scherr CL, et al. The Health Belief Model as an Explanatory Framework in Communication Research: Exploring Parallel, Serial, and Moderated Mediation. Health Commun 2015;30:566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SPSS Statistics for Windows [Computer Program]. Version 26.0. Armonk, NY: IBM Corp.; 2019. [Google Scholar]
- 34.Green RA. The Delphi Technique in Educational Research. SAGE Open 2014;4:1–8. [Google Scholar]
- 35.Nvivo Qualitative Data Analysis Software [Computer Program]. Version 12. Victoria, Australia: QSR International; 2018. [Google Scholar]
- 36.Zhang Y, Wildemuth BM. Qualitative Analysis of Content. Hum Brain Mapp 2005;30:2197–206. [Google Scholar]
- 37.Sleath B Sayner R Vitko M, et al. Glaucoma Patient-provider Communication about Vision Quality-of-life. Patient Educ Couns 2017;100:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y Zhu L Yuan F, et al. The Relationship between Social Support and Quality of Life: Evidence from a Prospective Study in Chinese Patients with Esophageal Carcinoma. Iran J Public Health 2015;44:1603–12. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y Zhao Y Xie S, et al. Resilience Mediates the Relationship between Social Support and Quality of Life in Patients with Primary Glaucoma. Front Psychiatry 2019;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partridge AH Avorn J Wang PS, et al. Adherence to Therapy with Oral Antineoplastic Agents. J Natl Cancer Inst 2002;94:652–61. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X Olson DJ Le P, et al. The Association between Glaucoma, Anxiety, and Depression in a Large Population. Am J Ophthalmol 2017;183:37–41. [DOI] [PubMed] [Google Scholar]
- 42.Mendez-Ulrich JL, Sanz A. Psycho-ophthalmology: Contributions of Health Psychology to the Assessment and Treatment of Glaucoma. Psychol Health 2017;32:330–42. [DOI] [PubMed] [Google Scholar]
- 43.Stone JL Robin AL Novack GD, et al. An Objective Evaluation of Eyedrop Instillation in Patients with Glaucoma. Arch Ophthalmol 2009;127:732–6. [DOI] [PubMed] [Google Scholar]
- 44.Hennessy AL Katz J Covert D, et al. Videotaped Evaluation of Eyedrop Instillation in Glaucoma Patients with Visual Impairment or Moderate to Severe Visual Field Loss. Ophthalmology 2010;117:2345–52. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy AL Katz J Covert D, et al. A Video Study of Drop Instillation in Both Glaucoma and Retina Patients with Visual Impairment. Am J Ophthalmol 2011;152:982–8. [DOI] [PubMed] [Google Scholar]
- 46.Lampert A Bruckner T Haefeli WE, et al. Improving Eye-drop Administration Skills of Patients—A Multicenter Parallel-group Cluster-randomized Controlled Trial. PLoS One 2019;14:e0212007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stryker JE Beck AD Primo SA, et al. An Exploratory Study of Factors Influencing Glaucoma Treatment Adherence. J Glaucoma 2010;19:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubak S Sandbaek A Lauritzen T, et al. Motivational Interviewing: A Systematic Review and Meta-analysis. Br J Gen Pract 2005;55:305–12. [PMC free article] [PubMed] [Google Scholar]
- 49.Rees G Chong XL Cheung CY, et al. Beliefs and Adherence to Glaucoma Treatment: A Comparison of Patients from Diverse Cultures. J Glaucoma 2014;23:293–8. [DOI] [PubMed] [Google Scholar]
- 50.Kangovi S Mitra N Grande D, et al. Patient-centered Community Health Worker Intervention to Improve Posthospital Outcomes: A Randomized Clinical Trial. JAMA Intern Med 2014;174:535–43. [DOI] [PubMed] [Google Scholar]
- 51.Sleath B Slota C Blalock SJ, et al. Provider Use of Collaborative Goal Setting with Glaucoma Patients. Optom Vis Sci 2014;91:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillmann K, Hoskens K, Mansouri K. Acute Emotional Stress as a Trigger for Intraocular Pressure Elevation in Glaucoma. BMC Ophthalmol 2019;19:69–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy HP. Enhancing Delphi Research: Methods and Results. J Adv Nurs 2004;45:504–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


