Abstract
Tumor heterogeneity may impact immunohistochemical (IHC) interpretation, thus potentially affecting decision making by treating oncologists for cancer patient management. Programmed cell death ligand-1 (PD-L1) IHC 22C3 pharmDx is a companion diagnostic used as an aid in identifying patient eligibility for treatment with pembrolizumab (KEYTRUDA). This study aims to investigate tumor heterogeneity impact on IHC staining when evaluating PD-L1 expression using PD-L1 IHC 22C3 pharmDx. The effect of tumor heterogeneity was evaluated based on the PD-L1 diagnostic status of PD-L1 IHC 22C3 pharmDx stained tumor tissue sections at relevant diagnostic cutoffs for non–small cell lung carcinoma, gastric or gastroesophageal junction adenocarcinoma, urothelial carcinoma, head and neck squamous cell carcinoma, esophageal cancer and triple negative breast cancer. Overall agreement for the PD-L1 diagnostic status was assessed for each tumor type within a given specimen block (Intra-Block), between specimen blocks from the same surgical resection (Intra-Case), and between intrapatient primary and metastatic specimens. Intrablock and intracase point estimates were above 75%, and primary versus metastatic point estimates were above 50%. The results suggest that PD-L1 expression is consistent across cut sections through a minimum of 150 µm within a tissue block and between blocks from the same surgical resection and is significantly maintained across primary and metastatic blocks from the same patient despite changes to the tissue microenvironment. These data provide confidence for histopathologists and oncologists that evaluation of PD-L1 expression at clinically relevant cutoffs is reproducible among different assessments (or samplings) of a single tumor specimen.
Key Words: pembrolizumab, TNBC, patients, programmed cell death protein, therapeutic
Tumor heterogeneity, as defined by the presence of cancer cells in different functional states, has increasingly become an important aspect of cancer diagnosis that plays a significant role in therapeutic treatment, therapeutic resistance, cancer recurrence, and metastasis.1,2 Tumor heterogeneity may impact immunohistochemical (IHC) interpretation, thus potentially affecting decision making by treating oncologists for cancer patient management. If tissue heterogeneity affects programmed cell death ligand-1 (PD-L1) expression, then it will be detected when the samples are stained with PD-L1 IHC 22C3 pharmDx.
Over the last century, researchers have attempted to utilize the immune system response to diagnose and treat cancer. Programmed cell death protein-1 (PD-1) and PD-L1 have been successful drug targets for the treatment of different tumor types.3–5 PD-L1 protein is the most commonly used biomarker for predicting response to treatment with pembrolizumab (KEYTRUDA), an anti-PD-1 therapy. Clinical data shows elevated levels of PD-L1 in a wide range of malignant tumors, including non–small cell lung cancer (NSCLC), urothelial carcinoma (UC), head and neck squamous cell carcinoma (HNSCC) and triple negative breast cancer (TNBC).5–18 Evaluation of PD-L1 expression in formalin-fixed paraffin-embedded (FFPE) human cancer tissues using PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying patients for treatment with pembrolizumab in NSCLC, gastric or gastroesophageal junction (GEJ) adenocarcinoma (GC/GEJ), esophageal cancer or squamous cell carcinoma (EC/ESCC), cervical cancer, UC, TNBC, and HNSCC. Regulatory approvals vary by country and region.
The effect of tumor heterogeneity on IHC staining results and corresponding clinical interpretation are relevant when determining patient eligibility using a companion diagnostic assay for treatment with pembrolizumab.19–21 The development of companion and complementary diagnostic products for use with Autostainer Link 48 have involved studies assessing tissue heterogeneity in tissue sections from FFPE tumor blocks. Deidentified blocks were procured by Agilent Technologies Inc. (also known as Dako) for analytical validation studies to assemble a specimen cohort demonstrating a wide distribution of cases that closely mimic real-world scenarios of variable tissue histologies.
The impact on PD-L1 expression by tumor heterogeneity was evaluated by assessing overall agreement (OA) in diagnostic PD-L1 status based on scoring of PD-L1 IHC 22C3 pharmDx stained specimen sections. Three studies per tumor indication were conducted to assess heterogeneity: (1) multiple sections within a specimen (Intra-Block); (2) between specimens from the same patient case, defined as sister blocks from the same surgical resection (Intra-Case); and (3) between matched pairs of primary and metastatic specimens from the same patient case (primary vs. metastatic). All studies focused only on the comparison of intrapatient tissue specimens. Representative studies conducted with NSCLC, GC/GEJ, UC, HNSCC, EC, and TNBC are described herein.
MATERIALS AND METHODS
The Advarra Institutional Review Board (IRB) has reviewed and approved the Protocol for Reagent Optimization and In Vitro Diagnostic Assay Development at Dako (Pro00026911, Approval Notice MOD00587074, February 7, 2020). The tissues used in this study were collected from suppliers and include tissue banks such as Cooperative Human Tissue Network and left-over tissue specimens from hospitals that were no longer needed for diagnostic purposes (either excess samples or held over 10+ years). Information received with these samples was diagnostic in nature with occasionally the age, sex, and race noted. All Health Insurance Portability and Accountability Act (HIPAA) protected information is never shared. If such information is accidentally shared, Dako has instructions in place to actively remove the information upon arrival to our laboratory.
Tissue Specimen Preparation
A specimen is defined as an FFPE tumor tissue block. Sections were cut at 4 µm thickness, placed on Dako FLEX IHC Microscope Slides (Code K8020; Dako North America Inc., Carpinteria, CA) or Superfrost Plus charged glass slides, and oven-dried at 58±2°C for ~1 hour. To preserve the PD-L1 antigen on mounted tissue sections, the cut sections were stored in the dark at 2 to 8°C before immunostaining with PD-L1 IHC 22C3 pharmDx (code SK006; Dako North America Inc.). The slides were stained within 1 month of sectioning or within the timeframe described in the PD-L1 IHC 22C3 pharmDx package insert22 for the relevant tumor types.
Staining Procedure
Specimens were pretreated using a 3-in-1 procedure [deparaffinization, rehydration, and target retrieval in the PT Link (code PT100/PT101/PT200)] using a low pH (code K8005; Dako North America Inc.). Subsequently, they were stained on the Autostainer Link 48 (code AS480), an automated IHC testing platform with a staining protocol validated for PD-L1 IHC 22C3 pharmDx, using the PD-L1 IHC 22C3 pharmDx reagents and protocol (package insert22). The stained specimens were counterstained with hematoxylin (code K8008; Dako North America Inc.) and coverslipped.
Scoring Interpretation
All specimens were qualified by a histopathologist to confirm specific requirements such as histologic diagnosis, presence of a minimum of 100 viable tumor cells per slide, and satisfactory tissue preparation for IHC analysis before study initiation.
The evaluation of PD-L1 expression in Intra-Block, Intra-Case, and primary versus metastatic heterogeneity employed indication-specific scoring algorithms. Scoring of PD-L1 expression according to indication-specific scoring guidelines for PD-L1 IHC 22C3 pharmDx was performed by trained, tested, and certified observers. The same observer evaluated all specimens, per study and indication, for all applicable cutoffs. Observers were blinded to the testing parameters and slides were randomized before scoring of each individual study.
All scoring was performed according to the PD-L1 IHC 22C3 pharmDx package insert.22 PD-L1 expression in NSCLC was determined by using the tumor proportion score (TPS). TPS is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity (≥1+) relative to all viable tumor cells present in the specimen section.
PD-L1 expression in GC/GEJ, UC, HNSCC, EC, and TNBC was determined by using the Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. Although the result of the calculation can exceed 100, the maximum score is defined as CPS 100.
PD-L1 expression on stained slides was evaluated with a light microscope equipped with 4×, 10×, and 20× magnification objective lenses. Two tissue sections were needed to support PD-L1 IHC 22C3 pharmDx staining and evaluation per specimen: 1 section stained with the negative control reagent, for evaluation of nonspecific staining, and 1 section stained with the primary monoclonal mouse antibody to PD-L1. The inclusion of a hematoxylin and eosin stained section to evaluate specimen adequacy is also standard practice, to confirm the required minimum of 100 viable tumor cells and that the specimen has been properly fixed and prepared before scoring.
Additional details regarding sample sectioning, storage, staining, reagents and scoring are present in the SK006 PD-L1 IHC 22C3 pharmDx package insert.22
Statistical Analysis
A specimen’s PD-L1 diagnostic status is based on a diagnostic cutoff. For each diagnostic cutoff, specimens are considered to have PD-L1 expression for PD-L1 scores at or above the cutoff and no PD-L1 expression for scores below the cutoff.
For statistical analysis purposes, designations of “positive” and “negative,” as they relate to PD-L1 expression level and agreement, were utilized. For the scope of these studies, a sample having PD-L1 expression equal to or above the specific cutoff was considered to have a diagnostic status of “positive” and a sample having no PD-L1 expression or PD-L1 expression below the specific cutoff was considered to have a diagnostic status of “negative.”
Agreement in diagnostic status between test conditions was evaluated by OA. All possible pair-wise comparisons were made between the observations within each specimen, case or matched pair. Pair-wise comparison methods for calculation of OA can be seen in the 2×2 contingency table (Table 1). Discordant pair-wise comparisons were pooled as there was no reference among test conditions.
TABLE 1.
Contingency Table for Calculation of Overall Agreement
| Level 1 (Front Section, Block A, Primary) | |||
|---|---|---|---|
| Negative | Positive | Total | |
| Level 2 (ie, back section, block B, metastatic) | |||
| Negative | CN | PN | CN+PN |
| Positive | NP | CP | NP+CP |
| Total | CN+NP | PN+CP | CN+PN+NP+CP |
CN+PN+NP+CP is the number of total pair-wise comparisons; CN is the number of concordant negative pair-wise comparisons; CP is the number of concordant positive pair-wise comparisons; NP+PN=Disc (the total number of discordant pair-wise comparisons).
OA was calculated as follows23:
Intra-Block
These studies evaluated the performance of PD-L1 IHC 22C3 pharmDx across multiple sections within a specimen. The anterior and posterior sections spanned a minimum of 150 µm for all studies. Where noted in Table 2, sections from the approximate anterior, middle, and posterior of the block were used, roughly defined as 0 to 40; 90 to 130; ≥180 µm, respectively, and positioning of the tested sections within each portion was dependent on tissue availability and block size. Minimally, sections were selected from the anterior and posterior portions of the tumor tissue block; however, some studies included sections from the approximate middle of the specimen for additional assessment. The specimens selected for this study represented the dynamic range of PD-L1 expression for each scoring algorithm in the relevant indications: TPS 0% to 100% and CPS 0 to 100.
TABLE 2.
Detailed Parameters by Indication for Heterogeneity Studies
| Indication | NSCLC | GC/GEJ | UC | HNSCC | EC | TNBC |
|---|---|---|---|---|---|---|
| Cutoff(s) | TPS≥1%, TPS≥50% | CPS≥1 | CPS≥1, CPS≥10 | CPS≥1, CPS≥20 | CPS≥10 | CPS≥1, CPS≥10 |
| Intra-Block sample size (specimens/locations sampled) | 20/* | 34/† | 36/† | 34/* | 53/† | 44/† |
| Intra-Case sample size (cases/tissue blocks) | 20/61 | 21/42 | 20/40 | 18/36 | 21/42 | 18/36 |
| Primary vs. metastatic sample size (specimen pairs) | 23 | 19 | 11 | 18 | 19 | 19 |
Studies performed only on anterior and posterior portions of samples.
Studies performed on anterior, middle, and posterior samples.
CPS indicates Combined Positive Score; EC, esophageal cancer; GC/GEJ, gastric or gastroesophageal junction; HNSCC, head and neck squamous cell carcinoma; NSCLC, non–small cell lung carcinoma; OA, overall percent agreement; TNBC, triple negative breast cancer; TPS, tumor proportion score; UC, urothelial carcinoma.
Intra-Case
These studies evaluated the performance of PD-L1 IHC 22C3 pharmDx on sister blocks from the same patient case. Sister blocks were defined as specimens from the same surgical resection; each case represented 1 patient. For NSCLC only, due to its status as the initial study in the series, >2 sister blocks per case were analyzed for many specimens. For these additional sister block sections comparisons were made individually between sections, resulting in more comparisons for the given case. No criteria for the range of PD-L1 expression were applied to specimen selection to maximize the number of included cases.
Primary Versus Metastatic
These studies evaluated the performance of PD-L1 IHC 22C3 pharmDx in matched tumor specimens from primary malignant neoplasms and distant metastases from the same patient. The number of specimen pairs used for each study can be found in Table 2. No criteria for the range of PD-L1 expression were applied to specimen selection to maximize the number of included matched pairs.
RESULTS
Studies were conducted independently at different points in time and product development resulting in discrepant study sample counts. Sample size and specimen selection was driven by the ability to procure specimens from vendors, which, depending on the indication and/or study, could be challenging. Matched primary and metastatic cases were especially difficult to obtain for UC.
Intra-Block
Diagnostic concordances and discordances were evaluated, OA was calculated, and specimen distribution by indication and cutoff were noted (Table 3). A depiction of OAs by indication can be found in Figure 1. For each specimen, the sections were individually compared with the other(s) with respect to diagnostic status for a specific cutoff. For indications that assessed 3 sections per sample comparisons were made individually, resulting in 3 times as many comparisons used to calculate OA.
TABLE 3.
OA Outcomes for Intra-Block, Intra-Case, and Primary Versus Metastatic in All Studies
| Indication | Cutoff | Intra-Block OA (nC/nT)* | Intra-Case OA (nC/nT)* | Primary vs. Metastatic OA (nC/nT)* |
|---|---|---|---|---|
| NSCLC | TPS≥1% | 95% (19/20) | 86.3% (63/73) | 87% (20/23) |
| TPS≥50% | 100% (20/20) | 100% (73/73) | 87% (20/23) | |
| GC/GEJ | CPS≥1 | 94.1% (96/102) | 90.5% (19/21) | 89.5% (17/19) |
| UC | CPS≥1 | 98.1% (106/108) | 94.4% (17/18) | 54.5% (6/11) |
| CPS≥10 | 98.1% (106/108) | 94.4% (17/18) | 81.8% (9/11) | |
| HNSCC | CPS≥1 | 97.1% (33/34) | 100% (20/20) | 88.9% (16/18) |
| CPS≥20 | 94.1% (32/34) | 90% (18/20) | 77.8% (14/18) | |
| EC | CPS≥10 | 93.0% (146/157) | 77.8% (14/18) | 68.4% (13/19) |
| TNBC | CPS≥1 | 94.4% (102/108) | 89.5% (17/19) | 68.4% (13/19) |
| CPS≥10 | 96.1% (98/102) | 89.5% (17/19) | 89.5% (17/19) |
nC and nT are respectively defined as the number of concordant comparisons and total comparisons in the study.
CPS indicates Combined Positive Score; EC, esophageal cancer; GC/GEJ, gastric or gastroesophageal junction; HNSCC, head and neck squamous cell carcinoma; NSCLC, non–small cell lung carcinoma; OA, overall percent agreement; TNBC, triple negative breast cancer; TPS, tumor proportion score; UC, urothelial carcinoma.
FIGURE 1.
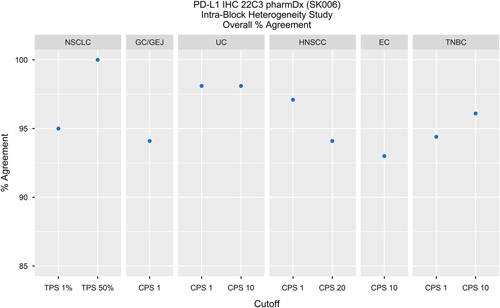
Intra-Block studies for all indications evaluated demonstrated >90% OA. In addition, NSCLC, TNBC CPS≥10, HNSCC CPS≥1, and UC demonstrated≥95% OA. CPS indicates Combined Positive Score; EC, esophageal cancer; GC/GEJ, gastric or gastroesophageal junction; HNSCC, head and neck squamous cell carcinoma; IHC, immunohistochemical; NSCLC, non–small cell lung carcinoma; OA, overall percent agreement; PD-L1, programmed cell death ligand-1; TNBC, triple negative breast cancer; TPS, tumor proportion score; UC, urothelial carcinoma.
Intra-Case
Diagnostic concordances and discordances were evaluated, OA was calculated and is noted (Table 3) along with specimen distribution by indication and cutoff. A depiction of OAs by indication can be found in Figure 2. For each specimen set, the sections were individually compared with the other(s) with respect to diagnostic status for a specific cutoff.
FIGURE 2.
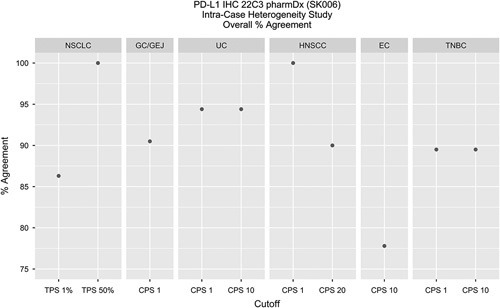
Intra-Case studies for all indications evaluated demonstrated >75% OA. In addition, NSCLC, TNBC, HNSCC, GC/GEJ, and UC demonstrated ≥85% OA. CPS indicates Combined Positive Score; EC, esophageal cancer; GC/GEJ, gastric or gastroesophageal junction; HNSCC, head and neck squamous cell carcinoma; IHC, immunohistochemical; NSCLC, non–small cell lung carcinoma; OA, overall percent agreement; PD-L1, programmed cell death ligand-1; TNBC, triple negative breast cancer; TPS, tumor proportion score; UC, urothelial carcinoma.
Primary Versus Metastatic
Diagnostic concordances and discordances were evaluated, OA was calculated and is noted (Table 3) along with specimen distribution by indication and cutoff. A depiction of OAs by indication can be found in Figure 3. For each matched pair, the sections were individually compared with the other with respect to diagnostic status for a specific cutoff.
FIGURE 3.
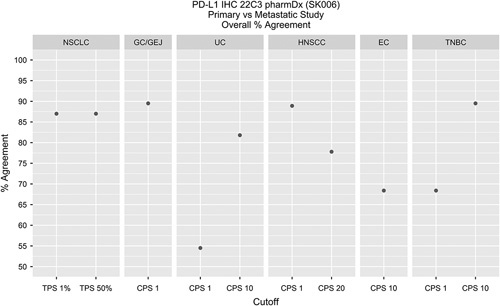
Primary versus metastatic studies for all indications evaluated demonstrated >50% OA. In addition, NSCLC, TNBC CPS≥10, HNSCC CPS ≥1, and GC/GEJ, demonstrated >85% OA. CPS indicates Combined Positive Score; EC, esophageal cancer; GC/GEJ, gastric or gastroesophageal junction; HNSCC, head and neck squamous cell carcinoma; IHC, immunohistochemical; NSCLC, non–small cell lung carcinoma; OA, overall percent agreement; PD-L1, programmed cell death ligand-1; TNBC, triple negative breast cancer; TPS, tumor proportion score; UC, urothelial carcinoma.
Results Summary
Moderate-to-high OA was observed between specimens for Intra-Block, Intra-Case, and matched primary versus metastatic studies when PD-L1 IHC 22C3 pharmDx stained tissue sections were assessed by qualified observers using indication-specific scoring algorithms and diagnostic cutoffs (Table 3).
DISCUSSION
The relationship between PD-L1 expression of tumor cells and response to anti-PD-1 therapy has been well documented.24 Due to the dynamic nature of the tumor microenvironment and the cancer immunity cycle, intrapatient tumor heterogeneity may influence IHC-based diagnostic decisions and potentially impact treatment selection for a given patient.20,21,25 Each tumor specimen is unique, and the degree of heterogeneity may be affected by the tumor type and sampling method. The relationship between the tumor microenvironment, heterogeneity and cancer immunity cycle is diverse and intricate, making it necessary for the modes of study and potential treatment to be likewise multifaceted and complex.
FFPE tumor tissues stained with PD-L1 IHC 22C3 pharmDx demonstrated high OA in the Intra-Block studies across all evaluated indications. In addition, moderate-to-high agreement was observed overall for the Intra-Case studies. This data suggests that Intra-Block and Intra-Case heterogeneity do not greatly impact diagnostic status of PD-L1 for the tested indications when tissues are stained with PD-L1 IHC 22C3 pharmDx. These findings provide relevant information for histopathologists and oncologists to consider when conducting PD-L1 diagnostic status evaluation and making treatment decisions. Even if a histopathologist observes high tumor heterogeneity, there is data to support that additional sections within a block that are tested with PD-L1 IHC 22C3 pharmDx will have consistent PD-L1 diagnostic status through a minimum of 150 µm within a block and between FFPE blocks of the same surgical resection. Histopathologists can therefore have increased confidence in the diagnostic information that is sent to the oncologist.
As noted by Li and colleagues, with special respect to the lymph node metastases associated with advanced TNBC,2 paired primary and metastatic specimens from the same patient should be assessed to ensure PD-L1 diagnostic accuracy. In the studies presented herein, PD-L1 expression was maintained in a noteworthy portion patient-paired primary and metastatic test specimens, providing additional relevant information for histopathologists and oncologists when considering the appropriate tissue sections to test for PD-L1 diagnostic status and treatment eligibility. This data suggests that primary versus metastatic heterogeneity may or may not greatly impact diagnostic status of PD-L1 for the tested indications when tissues are stained with PD-L1 IHC 22C3 pharmDx. With many indications demonstrating OA below 85%, due diligence is prudent when testing patient samples until more in-depth studies can be executed. Limiting factors in the execution of these studies include sample size, metastasis distribution and sample quality, all of which are due to the availability of and difficulties involved in matched pair procurement.
As these data sets were not subjected to acceptance criteria, the studies were not statistically powered. One limitation of the Intra-Case and Primary versus Metastatic studies was the low number of patient-matched specimens (Table 2). Further studies assessing the effect of tumor heterogeneity on PD-L1 diagnostic status are needed to address relative population size and diversity associated with clinical outcomes.
Moderate-to-high OA was observed in the heterogeneity studies conducted on NSCLC (using TPS), GC/GEJ, UC, HNSCC, EC, and TNBC (using CPS). Although tumor heterogeneity may introduce variable PD-L1 protein expression, as detected through the broad range of TPS and CPS specimen evaluations performed in these studies, PD-L1 IHC 22C3 pharmDx will reliably inform on PD-L1 diagnostic status at the clinically and analytically validated cutoffs for the 6 unique tumor indications studied here. This data provides confidence for histopathologists and oncologists that evaluation of PD-L1 expression at clinically relevant cutoffs is reproducible among different assessments (or samplings) of a single tumor.
ACKNOWLEDGMENTS
The authors acknowledge Merck & Co (Kenilworth, NJ) for their long-term partnership and funding for these studies and the development of class III companion diagnostics with special emphasis on PD-L1 IHC 22C3 pharmDx.
Footnotes
S.H. and K.K. are authors on the patent #10613092.
S.H., J.M., C.R., A.A., M.V., L.P., B.W., and C.L.: designed and executed the studies described and participated in editorial review of the article. S.T-.F. is the biostatistician who performed the data analysis, created tables and figures, and participated in editorial review of the article. M.D. is the lead histopathologist who participated in specimen evaluation, slide scoring, and editorial review of the article. R.W. and K.K. enabled these studies by directing resources and giving guidance on study design. Both participated in editorial review of the article. Grant Toland and Sara Alvarez helped perform the research and design the research study. Gary Ponto helped perform the research. Tissue samples were provided by the Cooperative Human Tissue Network which is funded by the National Cancer Institute. Other investigators may have received specimens from the same subjects. The authors thank the IUSCC Cancer Center at Indiana University School of Medicine, for the use of the Tissue Procurement and Distribution Core, which provided Dako North America, Inc. service. Samples/tissue supplied by Conversant. Tissue samples supplied by BioIVT (Hicksville, NY). The data and biospecimens used in this project was provided by Centre Hospitalier Universitaire (CHU) de Nice, US Biolab, Contract Research Ltd, DRCRO, Pakistan, SageBio LLC, Sapien Biosciences Pvt. Ltd, Hospices Civils de Lyon CRB, Lyon, France, with appropriate ethics approval and through Trans-Hit Biomarkers Inc. Biological materials were provided by the Ontario Tumour Bank, which is supported by the Ontario Institute for Cancer Research through funding provided by the Government of Ontario. The data and biospecimens used in this project was provided by Centre Antoine Lacassagne (CAL) with appropriate ethics approval and through Trans-Hit Biomarkers Inc.
Supported by Merck & Co (Kenilworth, NJ).
M.K., S.H., J.M., C.R., A.A., M.V., L.P., B.W., C.L., M.A.D., S.T-.F., R.W., and K.K. are employees of Agilent Technologies Inc. M.K., S.H., K.K., J.M., C.R., C.L., S.T-.F., and L.P. own Agilent stock.
Contributor Information
Megan Kalpakoff, Email: megan.kalpakoff@agilent.com.
Stephanie Hund, Email: Stephanie.hund@agilent.com.
Jeanette Musser, Email: Jeanette.musser@agilent.com.
Charlotte Roach, Email: Charlotte.roach@agilent.com.
Angeliki Apostolaki, Email: angeliki.apostolaki@agilent.com.
Monika Vilardo, Email: Monika.vilardo@agilent.com.
Lindsay Peltz, Email: Lindsay.guerrero@agilent.com.
Brittany Watts, Email: Brittany.watts@agilent.com.
Chris LaPlaca, Email: Christopher.laplaca@agilent.com.
Siena Tabuena-Frolli, Email: Siena.tabuena-frolli@agilent.com.
Michael A. DiMaio, Email: michael.dimaio2@q2labsolutions.com.
Rosanne Welcher, Email: rosannewelcher@sbcglobal.net.
Karina Kulangara, Email: Karina.kulangara@agilent.com.
REFERENCES
- 1. Simmons A, Lau K. Deciphering tumor heterogeneity from FFPE tissues: Its promise and challenges. Mol Cell Oncol. 2016;4:e1260191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 3. Li M, Li A, Zhou S, et al. Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer. 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swaika A, Hammond W, Joseph R. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67:4–17. [DOI] [PubMed] [Google Scholar]
- 5. Dong H, Strome S, Salomao D, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 6. Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. [DOI] [PubMed] [Google Scholar]
- 9. Velcheti V, Schalper K, Carvajal D, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbst R, Soria J, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nanda R, Chow L, Dees E, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang H, Qiao J, Fu Y. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taube J, Klein A, Brahmer J, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karasar P, Esendagli G. T helper responses are maintained by basal-like breast cancer cells and confer to immune modulation via upregulation of PD-1 ligands. Breast Cancer Res Treat. 2014;145:605–614. [DOI] [PubMed] [Google Scholar]
- 16. Cimino-Mathews A, Thompson E, Taube J, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLaughlin J, Han G, Schalper K, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA Oncol. 2016;2:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coates A, Winer E, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips T, Millett M, Zhang X, et al. Development of a diagnostic programmed cell death 1-ligand 1 Immunohistochemistry assay for nivolumab therapy in melanoma. Appl Immunohistochem Mol Morphol. 2018;26:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. [DOI] [PubMed] [Google Scholar]
- 22. Agilent Technologies. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako North America Inc., Agilent Pathology Solutions; 2020. [Google Scholar]
- 23. CLSI Quality Assurance for Design Control and Implementation of Immunohistochemistry Assays: Approved Guideline, 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 24. Cho J, Sorensen S, Choi Y, et al. Programmed death ligand 1 expression in paired non–small cell lung cancer tumor samples. Clin Lung Cancer. 2017;18:e473–e479. [DOI] [PubMed] [Google Scholar]
- 25. Zou W, Chen L. Inhibitory B7-family molecules in the tumor microenvironment. Nat Rev Immunol. 2008;8:467–477. [DOI] [PubMed] [Google Scholar]


