Background:
Kawasaki disease (KD) is an acute vasculitis of young children. A comparison of US hospitalization rates and epidemiologic features of KD in 2020 to those of precoronavirus disease years has yet to be reported.
Methods:
Using a large, inpatient database, we conducted a retrospective cohort study and analyzed data for patients with (1) diagnosis coding for KD, (2) IV immunoglobulin treatment administered during hospitalization and (3) discharge date between January 1, 2016, and December 30, 2020. Severe cases were defined as those requiring adjunctive therapy or IV immunoglobulin–resistant therapy.
Results:
The annual number of KD hospitalizations were stable from 2016 to 2019 (n = 1652, 1796, 1748, 1692, respectively) but decreased in 2020 (n = 1383). KD hospitalizations demonstrated seasonal variation with an annual peak between December and April. A second peak of KD admissions was observed in May 2020. The proportion of KD cases classified as severe increased to 40% in 2020 from 33% during the years 2016–2019 (P < 0.01). Median age in years increased from 2.9 in subjects hospitalized from 2016 to 2019 to 3.2 in 2020 (P = 0.002).
Conclusions:
Compared with the previous 4 years, the annual number of pediatric KD admissions decreased, and children discharged with diagnostic codes for KD in 2020 were generally older and more likely to have severe morbidity possibly reflective of misdiagnosed multisystem inflammatory syndrome in children. Clinicians should be wary of a possible rise in KD rates in the postcoronavirus disease 2019 era as social distancing policies are lifted and other viruses associated with KD return.
Keywords: Kawasaki, multisystem inflammatory syndrome in children, coronavirus disease 2019
BACKGROUND
Kawasaki disease (KD) is an acute, medium- and small-sized vasculitis that primarily affects children.1 The disease typically presents in children between 6 months and 5 years of age and has increased incidence among males and children of Asian ethnicity.1,2 Despite being initially reported in 1967, the etiology and pathogenesis of the disease has yet to be fully elucidated.2,3 However, several studies suggest a seasonal pattern and implicate an association with viral etiologies.2,3 Although diagnosis is made clinically in classic presentations, supplemental laboratory evaluations help to confirm the diagnosis in cases termed incomplete KD. Coronary artery aneurysms or ectasia develop in up to 25% of untreated children and may result in myocardial infarction or sudden death.1
Over the past several decades, epidemiologic studies report the incidence of KD in the United States to be stable4–6 or with a slight, significant increase.7,8 Conversely, countries such as Japan, Korea and Australia have reported consistently increasing incidence.9–11 Countries with rapidly growing economies such as China and India also show increasing incidence, although increased disease awareness and improved access to care may be a confounding factor.12
The rapid spread of the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to a global pandemic in 2020.13 Initial case series from Italy and France reported outbreaks of children meeting criteria for KD with 30-fold and 4-fold increase, respectively, in monthly rates compared with historic rates, suggesting a possible association between the novel virus and a KD-like syndrome.14,15 In May 2020, the US Centers for Disease Control and Prevention issued a health advisory to report on cases meeting criteria for multisystem inflammatory syndrome in children (MIS-C).16 This syndrome exhibits several clinical symptoms similar to KD with initial national US case series reporting 40% of children with MIS-C also met KD criteria.13 Although initial reports show decreasing KD hospitalization rates over the first several months of the pandemic,17 the full effect of COVID-19 on KD hospitalizations in all of 2020 has yet to be reported to our knowledge. The aim of this study was to assess the number of KD hospital admissions in 2020 and compare them with that of the previous 4 years.
METHODS
A retrospective cohort study was conducted using discharge data from the Pediatric Health Information System (PHIS), an administrative database compiled by the Children’s Hospital Association in partnership with 51 US children’s hospitals. Updated on a quarterly basis, the PHIS database contains deidentified clinical, demographic and billing information for hospital discharges.
The primary outcome was defined as the annual number of KD hospitalizations across the study period. Secondary outcomes included demographic characteristics, volume of diagnostic studies and treatment modalities and reported outcomes of patients hospitalized with acute KD.
The study population consisted of children younger than 18 years old discharged from a PHIS hospital between January 1, 2016, and December 31, 2020. Subjects included were those with first admissions with International Classification of Diseases, 10th revision Clinical Modification (ICD-10-CM) code of M30.3, the diagnostic code denoting Mucocutaneous Lymph Node Syndrome or KD, as well as at least one dose of IV immunoglobulin (IVIG) during their stay. Subjects with duplicate encounters or missing data on admission date, date of birth or disposition were excluded.
Supplemental lab work up was defined as those who had all of the following labs: erythrocyte sedimentation rate, C-reactive protein, complete blood count, urine analysis, alanine transaminase and albumin obtained during admission. Adjunctive therapy was defined as steroids, infliximab or etanercept started within 1 day of initiating IVIG. IVIG-resistant therapy was defined as cases requiring adjunctive therapy, second IVIG doses, infliximab, cyclosporine, anakinra or cyclophosphamide 1 day or more after initial IVIG dose, in close alignment with 2017 American Heart Association scientific statement recommendations.1 Severe cases were defined as those requiring either adjunctive therapy, IVIG-resistant therapy or both. Subjects with coronary artery aneurysm were identified with ICD-10-CM code of I25.41. Per American Academy of Pediatrics guidance, subjects with MIS-C were defined as children younger than 18 years of age with ICD-10-CM codes of: 1) U07.1 as the principal diagnosis and M35.8 as a secondary diagnosis or 2) M35.8 as the principal diagnosis and a secondary diagnosis code of B94.8.18
To evaluate the impact of the COVID-19 pandemic, demographic characteristics, including age, sex, race, ethnicity, insurance status and US census region, as well as clinical data were compared between patients discharged during calendar year 2020 and those hospitalized from 2016 to 2019. Race and ethnicity of subjects with KD were compared with all patients discharged from PHIS hospitals during the study period. χ2 tests were employed to assess differences between observed discrete variables such as demographic characteristics and severity. t tests were applied to compare mean values for continuous variables. To compare median age, the Kruskal–Wallis test was used. Using the 2016–2019 aggregated monthly data, a seasonally adjusted autoregressive time series model was developed to predict expected values for 2020. A P value of less than 0.01 was considered significant. Statistical analyses were conducted using SAS version 9.4 and Matlab version R2019b (The MathWorks, Inc., 2019, Natick, MA).
RESULTS
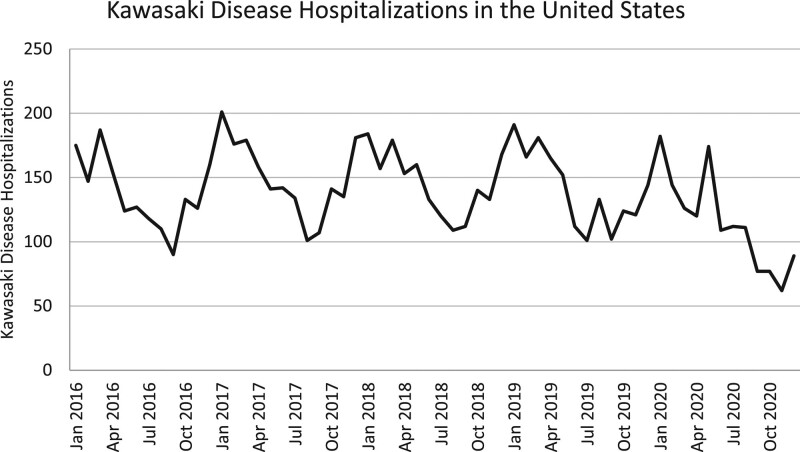
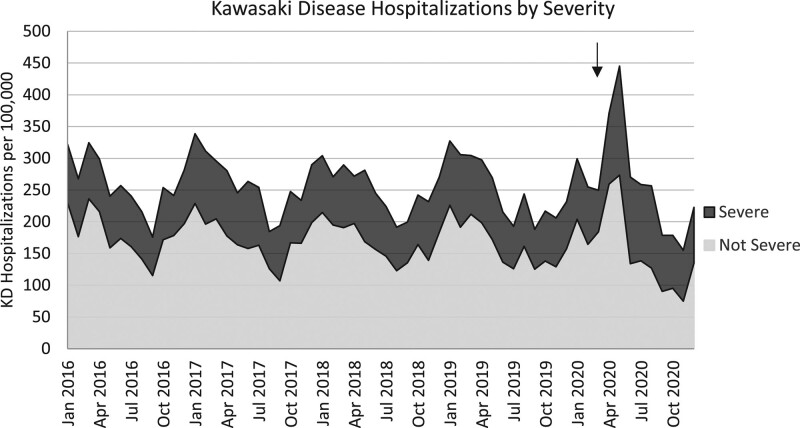
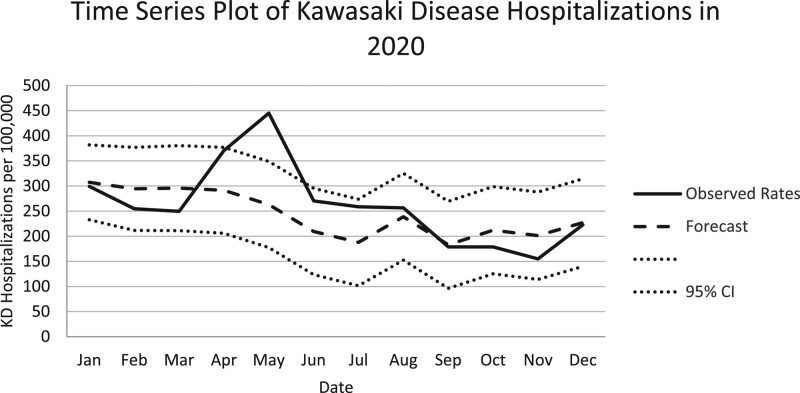
During the 5-year study period, a total of 8271 subjects were discharged from a PHIS-associated hospital, diagnosed with KD and received treatment with at least 1 dose of IVIG. The annual number of KD hospitalizations were stable from 2016 to 2019 (1652, 1796, 1748, 1692, respectively) but decreased significantly in 2020 (1383; P < 0.001; Fig. 1). In children younger than 5 years of age, there were a total of 213 KD hospitalizations per 100,000 hospitalizations over the 5-year study period. Between 2016 and 2020, KD hospitalizations demonstrated seasonal variation, with an annual peak in the winter months between December and April (Fig. 2). Two peaks were observed in 2020. The first occurred in January, which aligns with known seasonality and was within the predicted 95% CIs of our time series plot, and the second peak was in May which showed a sharp increase in KD hospitalizations per 100,000 outside of the predicted 95% CIs (Fig. 3).
FIGURE 1.
Number of KD hospitalizations by month in the United States.
FIGURE 2.
Rates of KD hospitalizations per 100,000 admissions (black solid line) in the United States by severe disease (dark gray area) and not severe disease (light gray area) cases. COVID-19 pandemic declared on March 11, 2020 (black arrow).
FIGURE 3.
Rates of KD hospitalizations per 100,000 admissions in 2020 (solid line) with expected autoregressive time series model (dashed line) and 95% confidence intervals (dotted lines). The hospitalization rate in May 2020 falls outside of the 95% CI.
Demographic characteristics of all subjects are summarized in Table 1. The median age in years at time of admission for all subjects was 2.9 [interquartile range (IQR) 1.5–5.1], 3.1 (IQR 1.4–5.9) for the 2020 cohort and 3.0 (IQR 1.5–5.1) for the 2016–2019 cohort. There was a statistically significant increase in the median age of admission in 2020 compared with the pre-COVID periods (P = 0.002), driven by an increase in hospitalizations of children 10–17 years of age. The majority of subjects were male (59%), which was similar across the study period. In regard to race, the number of subjects identified as Asian showed a significant decrease between the 2 groups from 12% in 2016–2019 to 9% in 2020 (P = 0.008). There was a significant overrepresentation in the KD population of subjects who identified as Asian across both cohorts (11%) compared with all children hospitalized in PHIS hospitals during same time periods (3.9%; P < 0.001). The remaining distribution of race and ethnicities was statistically unchanged throughout the study period. In 2020, the proportion of KD subjects who had private insurance (45%) was significantly smaller compared with the prior 4 years (49%; P = 0.005). Concurrently, rates of public insurance increased from 46% in 2016–2019 to 50% in 2020 (P = 0.004). There was no significant variation in KD cases across US census regions during the study period, with 1717 (21%) subjects admitted in the Midwest, 978 (12%) in the Northeast, 3186 (39%) in the South and 2390 (29%) in the West.
TABLE 1.
Demographics of KD Hospitalizations From 2016 to 2020
| Characteristics | Total: 2016–2020 (N = 8271, 100%), n/N (%) | 2016–2019 (N = 6888, 83%), n/N (%) | 2020 (N = 1383, 17%), n/N (%) | P |
|---|---|---|---|---|
| Median age, yr (interquartile range) | 2.9 (1.5–5.1) | 2.9 (1.5–5) | 3.1 (1.4–5.9)x | 0.002 |
| <1 | 1376 (17) | 1130 (16) | 246 (18) | 0.2 |
| 1–4 | 4780 (58) | 4060 (59) | 720 (52) | <0.001 |
| 5–9 | 1759 (21) | 1452 (21) | 307 (22) | 0.4 |
| 10–17 | 356 (4) | 246 (4) | 110 (8) | <0.001 |
| Sex | ||||
| Male | 4906 (59) | 4111 (60) | 795 (58) | 0.1 |
| Female | 3363 (41) | 2776 (40) | 587 (42) | 0.1 |
| Race | ||||
| Asian | 944 (11) | 815 (12) | 129 (9) | 0.008 |
| American Indian | 21 (0.3) | 16 (0.2) | 5 (0.4) | 0.4 |
| Black | 1777 (21) | 1486 (22) | 291 (21) | 0.7 |
| Pacific Islander | 115 (1) | 98 (1) | 17 (1) | 0.6 |
| White | 4048 (49) | 3328 (48) | 720 (52) | 0.011 |
| Other | 1126 (14) | 929 (13) | 197 (14) | 0.5 |
| Ethnicity | ||||
| Hispanic | 1787 (22) | 1454 (21) | 333 (24) | 0.014 |
| Not Hispanic | 5897 (71) | 4925 (72) | 972 (70) | 0.4 |
| Unknown | 587 (7) | 509 (7) | 78 (6) | 0.02 |
| Region | ||||
| Midwest | 1717 (21) | 1442 (21) | 275 (20) | 0.4 |
| Northeast | 978 (12) | 840 (12) | 138 (10) | 0.02 |
| South | 3186 (39) | 2625 (38) | 561 (41) | 0.09 |
| West | 2390 (29) | 1981 (29) | 409 (30) | 0.5 |
| Insurance | ||||
| Private | 3973 (48) | 3356 (49) | 617 (45) | 0.005 |
| Public | 3862 (47) | 3167 (46) | 695 (50) | 0.004 |
| Self-pay | 128 (2) | 96 (1) | 32 (2) | 0.011 |
| Other | 308 (4) | 269 (4) | 39 (3) | 0.05 |
During the 5-year study period, a total of 761 (9%) subjects were admitted to the intensive care unit (ICU), and 7510 (91%) were admitted to the inpatient unit. There was a significant increase in the rate of ICU admissions from 8% of KD admissions in 2016–2019 to 15% of KD admissions in 2020 (P < 0.001; Table 2). The percentage of subjects receiving supplemental lab testing decreased from 17% in 2016–2019 compared with 14% in 2020 (P = 0.001). Rate of echocardiogram increased significantly from 95% to 98% between the 2 time periods (P < 0.001). Eleven percent of all subjects had a diagnostic code for coronary artery aneurysms with no significant difference between the 2 cohorts.
TABLE 2.
Admission and Outcomes for KD Hospitalizations From 2016 to 2020
| Outcomes | 2016–2019, N = 6888 (83%), n/N (%) | 2020, N = 1383 (17%), n/N (%) | P |
|---|---|---|---|
| Admission | |||
| In patient | 6330 (92) | 1180 (85) | <0.001 |
| ICU | 558 (8) | 203 (15) | <0.001 |
| Work up | |||
| Supplemental labs | 1201 (17) | 191 (14) | 0.001 |
| Echocardiogram | 6524 (95) | 1360 (98) | <0.001 |
| Coronary artery aneurysm | 747 (11) | 172 (12) | 0.09 |
| Severe cases | 2285 (33) | 549 (40) | <0.001 |
| Adjunctive therapy | 727 (11) | 278 (20) | <0.001 |
| IVIG-resistant therapy | 2070 (30) | 505 (37) | <0.001 |
| Mortality | 4 (0.06) | 0 (0) | — |
| COVID | 108 (8) | — | |
| MIS-C ICD-10-CM | 52 (4) | — |
A total of 2834 (34%) subjects met criteria for severe disease during the study period. The proportion of severe cases significantly increased from 33% of cases in 2016–2019 to 40% of cases in the 2020 cohort (P < 0.001). The percentage of subjects who received adjunctive therapy increased from 11% in 2016–2019 to 20% in 2020 (P < 0.001). Rates of administration of therapy for IVIG-resistance also increased from 30% in 2016–2019 to 37% in 2020 (P < 0.001). From June to November of 2020, over 45% of KD hospitalizations were identified as severe. In 2020, zero cases were fatal compared with 4 over the preceding 4 years, yielding a mortality rate of 0.05% over the study period.
During the year 2020, 108 (8%) subject records contained diagnostic code for COVID-19, and 52 (4%) subjects were diagnosed with MIS-C. The median age of this MIS-C subset was 6 (6.0) years old (IQR 3.9–9.3). Forty-two (81%) met criteria for severe KD disease with 33 (63%) receiving adjunctive therapy and 41 (79%) receiving IVIG-resistance therapy.
DISCUSSION
The present study describes the most recent epidemiologic pattern, severity features and demographic characteristics of KD hospitalizations in the United States before and during the COVID-19 pandemic using the national PHIS database. We report that annual KD hospitalizations were stable from 2016 to 2019. Prior studies using the PHIS database reported a slight, significant increase7,8 in rates of KD hospitalizations in the United States in years before the COVID pandemic, whereas reports using other national databases suggest a stable 4–6 incidence over the past several years. Corroborating with more recent studies,17,19,20 we report a significant decrease in annual KD hospitalizations in 2020. Seasonal variation was observed similar to previous reports.21 In this dataset of PHIS hospitals, monthly KD hospitalizations per 100,000 admissions significantly increased in May 2020 compared with the expected rates based on KD hospitalization rates of the previous 4 years. This peak, accentuated by the concurrent decrease in overall hospitalizations during the time period, follows the initial wave of COVID-19 in the United States and is similar to the rise in KD-like cases after the initial COVID-19 waves in France and Italy.14,15 Given this apparent temporal delay and that other coronaviruses have been associated with KD in the past,22 our findings could suggest a potential association between SARS-CoV-2 and KD possibly as a direct trigger, an intermediate primer or cofactor or conduit for entry or exposure to a true causative agent.23 However, as discussed in depth below, the clinical overlap between MIS-C and KD is a more likely explanation of this peak. Additionally, subsequent COVID-19 waves with peaks much higher than the initial wave of the pandemic were observed in the latter half of 2020, and these were not followed by temporal increases in KD rates.
We postulate that KD hospitalizations in 2020 had increased morbidity compared with the previous 4 years as evidenced by increased proportion of ICU admissions and significant increases in rates of adjunctive therapy use and IVIG-resistance therapy administration. In 2020, 37% of subjects with KD were resistant to initial IVIG treatment, which is increased from 30% in 2016–2019 and also increased when compared with prior studies which report resistance rates approximately between 10% and 20%.1,24,25 Worsening morbidity could be because of true increase in severity incidence or emergence of a new trigger for KD, such as SARS-CoV-2, that may have a predilection for more severe phenotype.
During 2020, MIS-C became increasingly recognized as a postviral, inflammatory sequelae of COVID-19.16 There is considerable clinical and diagnostic overlap between MIS-C and KD as patients of both conditions can present with persistent fevers, rash, conjunctivitis, swollen hands and feet and lymphadenopathy.26 In several case series, approximately 20%–40% of children with MIS-C met criteria for complete or incomplete KD.13,27 Given significant overlap between these conditions and that guidance on ICD-10 diagnostic coding for MIS-C was not introduced until July 2020,18 the observed increase in KD admission rates per 100,000 hospitalizations in May 2020 could be attributed to the potential mislabeling of the new disease entity. This supposition is further supported by the following observations: an increase in median age, given that MIS-C commonly affects older children and adolescents26,27; the increase in adjunctive therapy use in 2020, as steroid therapy has increasingly become standard treatment for MIS-C28; the increased rate of ICU admissions in 2020, as myocardial dysfunction and shock occur more often in MIS-C than in classic KD28 and an observed, subsequent decrease in KD rates after the summer of 2020, after recommendations for ICD-10 diagnostic coding of MIS-C were published.
We observed that the monthly number of KD hospitalizations declined after the sharp rise in May of 2020. Given rates of influenza and other viral illnesses had declined significantly in 2020 as a result of public health measures such as mask mandates and social distancing policies,29–31 the decrease in KD admissions during the pandemic period may be because of a decrease in other circulating, non–SARS-CoV-2 viruses that have also been associated with KD.22
The majority of KD admissions in our study were children younger than 5 years of age consistent with previous reports.4–8 Median age increased in 2020 compared with the prior 4 years and may represent the overlap with MIS-C, an actual increased incidence in older children or increased provider awareness that KD can manifest in older children. Also similar to previous reports, we found a predominance of male gender with an approximate 1.5:1 male-to-female ratio.1,2 Laboratory testing for all components of the supplemental criteria decreased in 2020 consistent with reports of decreased laboratory utilization in the initial phase of the pandemic.32 Decrease in private insurance and increase in public insurance was noted which may be reflective of the loss of jobs and associated medical benefits during the COVID-19 pandemic.33
Our data stand in contrast to findings published from East Asian countries where, despite having historically higher rates of KD compared with Europe and the United States in the years before the pandemic, there was no observed significant increase in KD-like cases after their initial COVID waves.34,35 Hara et al36 reported no significant change in age or severity of KD cases in Japan. Although we found an overrepresentation of subjects who identified as Asian during the study period, the proportion of subjects admitted for KD who identified as Asian decreased in 2020 compared with the 2016–2019 cohort. This finding further suggests that the overlap between MIS-C and KD may be a contributing factor for the increase in KD rates per 100,000 hospitalizations in early 2020, given that MIS-C has been reported to have less of a predisposition for the Asian population.37 Therefore, given that our 2020 data skews in the direction of patients with MIS-C in regard to age, severity and race, patients diagnosed with KD in early 2020 may need to be reclassified as MIS-C. We acknowledge that limited testing for COVID-19 because of a paucity of diagnostic tests during this time frame may prove delineation between the 2 entities to be difficult.
Some limitations should be acknowledged. Our data rely on discharge International Classification of Diseases diagnoses which may also not always reflect a patient’s actual diagnosis, and given the nature of the database, we were unable to review subject charts for clinical data and lab results to verify the diagnoses. The American Heart Association guideline defines IVIG-resistant therapy as treatment for children who remain febrile 36 hours after initial IVIG dose. Given that PHIS does not report medication administration in hours, we defined IVIG resistance as specified medications occurring 1 day after IVIG. This may result in an overreporting of IVIG-resistant therapy rates. Additionally, guideline also lists plasma exchange as a potential option for IVIG-resistant therapy. Given that there is no separate procedure code for plasma exchange, we were not able to report the rate or changes of this therapy. Finally, a comprehensive comparison of MIS-C and its relation to KD is limited in our study given that laboratory testing for and clinician awareness of the new disease entity were not yet ubiquitous and that guidance regarding ICD-10-CM coding for COVID-19 was not available early in the course of the pandemic.
CONCLUSIONS
By using a large, pediatric inpatient database, we found that although total KD hospitalizations decreased overall in 2020 compared with annual rates of prior years, the monthly rate of KD hospitalizations per 100,000 admissions increased beyond the expected range in May 2020 based on data from the previous 4 years likely reflecting a misdiagnosis of MIS-C. Children with discharge ICD-10-CM diagnosis codes for KD in 2020 were generally older and more likely to have severe morbidity compared with those admitted in the previous 4 years. Further studies are necessary to elucidate a possible association between SARS-CoV-2 and KD as well as to delineate the similarities and differences of MIS-C and KD. Given that long-term complications of MIS-C have yet to be fully characterized, children with diagnostic coding for KD early on in the pandemic may warrant close monitoring by pediatricians. Finally, clinicians should be wary of a possible rise in KD rates in the post–COVID-19 era as mask mandates are lifted and other viruses associated with KD return.
Footnotes
K.W.H., A.H.H. and J.C.S. are funded through National Institute of Child Health and Human Development R61HD105613 “Identifying Biomarker Signatures of Prognostic Value for MIS-C.” The remaining authors have no conflicts of interest to disclose.
T.T.P. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. T.T.P., S.S., K.W.H., P.T.P., J.C.S. and J.S. contributed to concept and design. T.T.P., S.S., K.W.H., P.T.P., M.B. and J.S. contributed to acquisition, analysis and interpretation of the data. T.T.P., S.S., K.W.H., M.B. and J.S. contributed to drafting of the article. T.T.P., S.S., K.W.H., M.B., A.H.H, J.C.S. and J.S. contributed to critical revision of the article for important intellectual content. S.S., J.C.S. and J.S. contributed to supervision.
REFERENCES
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. Erratum in: Circulation. 2019;140:e181–e184. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67:1738–1749. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E, Sundel R. Kawasaki disease at 50 years. JAMA Pediatr. 2016;170:1093–1099. [DOI] [PubMed] [Google Scholar]
- 4.Holman RC, Belay ED, Christensen KY, et al. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29:483–488. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki K, Blackshear C, Hobbs CV. Deep neck space involvement of Kawasaki disease in the US: a population-based study. J Pediatr. 2019;215:118–122. [DOI] [PubMed] [Google Scholar]
- 6.Maddox RA, Person MK, Kennedy JL, et al. Kawasaki disease and Kawasaki disease shock syndrome hospitalization rates in the United States, 2006-2018. Pediatr Infect Dis J. 2021;40:284–288. [DOI] [PubMed] [Google Scholar]
- 7.Son MB, Gauvreau K, Ma L, et al. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez SR, Birkholz M, Anderson MS, et al. Diagnostic and treatment trends in children with Kawasaki disease in the United States, 2006-2015. Pediatr Infect Dis J. 2019;38:1010–1014. [DOI] [PubMed] [Google Scholar]
- 9.Ae R, Makino N, Kosami K, et al. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. 2020;225:23–29.e2. [DOI] [PubMed] [Google Scholar]
- 10.Kim GB, Eun LY, Han JW, et al. Epidemiology of Kawasaki disease in South Korea: a nationwide survey 2015-2017. Pediatr Infect Dis J. 2020;39:1012–1016. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien K. Australian hospitalisations for Kawasaki disease, 1993-1994 to 2017-2018. J Paediatr Child Health. 2020;56:1126–1133. [DOI] [PubMed] [Google Scholar]
- 12.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; Centers for Disease Control and Prevention COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Health Alert Network. Emergency preparedness and response: health alert notice 00432 [Internet]. May 14, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed April 27, 2021.
- 17.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics (AAP). Coding during the COVID-19 public health emergency [Internet]. March 15, 2021. Available at: https://downloads.aap.org/AAP/PDF/COVID%202020.pdf. Accessed March 28, 2021.
- 19.Pelletier JH, Rakkar J, Au AK, et al. Trends in US pediatric hospital admissions in 2020 compared with the decade before the COVID-19 pandemic. JAMA Netw Open. 2021;4:e2037227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman S, Geevarghese B, Kim KY, et al. The impact of social distancing for COVID-19 upon diagnosis of Kawasaki disease [published online ahead of print March 23, 2021]. J Pediatric Infect Dis Soc. doi: 10.1093/jpids/piab013. [DOI] [PMC free article] [PubMed]
- 21.Burns JC, Herzog L, Fabri O, et al. ; Kawasaki Disease Global Climate Consortium. Seasonality of Kawasaki disease: a global perspective. PLoS One. 2013;8:e74529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang LY, Lu CY, Shao PL, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCrindle BW, Manlhiot C. SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA. 2020;324:246–248. [DOI] [PubMed] [Google Scholar]
- 24.Moffett BS, Syblik D, Denfield S, et al. Epidemiology of immunoglobulin resistant Kawasaki disease: results from a large, national database. Pediatr Cardiol. 2015;36:374–378. [DOI] [PubMed] [Google Scholar]
- 25.Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory kawasaki disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2:71–76. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik A, Gupta S, Sood M, et al. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39:e340–e346. [DOI] [PubMed] [Google Scholar]
- 29.Chan KH, Lee PW, Chan CY, et al. Monitoring respiratory infections in covid-19 epidemics. BMJ. 2020;369:m1628. [DOI] [PubMed] [Google Scholar]
- 30.Antoon JW, Williams DJ, Thurm C, et al. The COVID-19 pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021;16:294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeoh DK, Foley DA, Minney-Smith CA, et al. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72:2199–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durant TJS, Peaper DR, Ferguson D, et al. Impact of COVID-19 pandemic on laboratory utilization. J Appl Lab Med. 2020;5:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173:63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki J, Abe K, Matsui T, et al. Kawasaki disease shock syndrome in Japan and comparison with multisystem inflammatory syndrome in children in European countries. Front Pediatr. 2021;9:625456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YJ, Park H, Choi YY, et al. Defining association between COVID-19 and the multisystem inflammatory syndrome in children through the pandemic. J Korean Med Sci. 2020;35:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara T, Furuno K, Yamamura K, et al. Assessment of pediatric admissions for kawasaki disease or infectious disease during the COVID-19 state of emergency in Japan. JAMA Netw Open. 2021;4:e214475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]