Background:
The coronavirus disease 2019 pandemic was caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although the predominant clinical presentation is a respiratory disease, neurologic manifestations are being recognized increasingly.
Case Report:
We report 2 children 9 years of age who developed acute disseminated encephalomyelitis-like disease associated with SARS-CoV-2. Seizures and encephalopathy were the main central nervous system symptoms. The cerebrospinal fluid analysis performed within the first week of disease onset showed elevated protein in both children with normal cell count and no evidence of infection including negative SARS-CoV-2 by antibody and polymerase chain reaction. Brain magnetic resonance imaging revealed T2A, fluid-attenuated inversion recovery cortical and subcortical hyperintensity without restricted diffusion consistent with acute disseminated encephalomyelitis–like disease. They received methylprednisolone followed by therapeutic plasma exchange. One of them showed complete clinical improvement and resolution in magnetic resonance imaging findings. The other developed laminar necrosis in brain magnetic resonance imaging and showed significant clinical improvement after therapeutic plasma exchange. He was positive for positive SARS-CoV-2 antibody in cerebrospinal fluid on day 55 of admission. They were both positive for SARS-CoV-2 antibodies in serum after 2 weeks.
Conclusions:
Our two cases highlight the occurrence of acute disseminated encephalomyelitis–like disease as a postinfectious/immune-mediated complication of SARS-CoV-2 infection.
Keywords: ADEM-like disease, COVID-19, encephalopathy, TPE
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in patients presenting with pneumonia in China in December 2019.1 Although the predominant clinical presentation is a respiratory disease, neurologic manifestations are being recognized increasingly. The pathophysiologic mechanisms including direct viral effects upon the nervous system, endothelial injury, and downstream effects of para- and postinfectious inflammation have been suggested as the potential cause of neurologic involvement in COVID-19.2 Postinfectious inflammation induced by SARS-CoV-2 may trigger autoimmune phenomena, such as demyelinating disease and encephalopathy.2 Acute disseminated encephalomyelitis (ADEM) is a rare, immune-mediated demyelinating central nervous system (CNS) disorder with a predilection to early childhood. ADEM is characterized by acute encephalopathy with neurologic deficits and brain magnetic resonance imaging (MRI) findings consistent with multifocal demyelination. ADEM often occurs with postinfectious inflammation.3 Postinfectious encephalomyelitis is caused by viral pathogens such as varicella zoster virus, rubella virus, herpes simplex virus, human herpes virus-6, Epstein-Barr virus, HIV, mumps virus, influenza virus, coxsackie B virus, and cytomegalovirus. It has also been described in association with bacterial pathogens, including infections with Legionella cincinnatiensis, Mycoplasma pneumonia and other atypical pneumonic infections.4 We present 2 pediatric patients who developed neurologic involvement with MRI changes indicative of SARS-CoV-2–associated ADEM-like disease.
CASE REPORT
The written informed consent to publication has been obtained from the parents on behalf of the patient.
Case 1
A 9-year-old previously healthy boy presented with status epilepticus after 3 days of fever, headache and vomiting. He had recurrent epileptic seizures. Brain MRI and his clinical course were consistent with ADEM. Methylprednisolone 30 mg/kg intravenous daily for 5 days and intravenous immunoglobulin 1 g/kg daily for 2 days were administered for presumed ADEM. His immunization status was appropriate to his age. His parents had an SARS-CoV-2 infection 1.5 months ago. He was admitted to our pediatric intensive care unit (PICU) on day 5 since he neurologically deteriorated and his demyelinating lesions on MRI aggravated (Fig. 1). On physical examination, he was unconscious and unoriented. His Glasgow Coma Scale was 6 (E 2, V 2, M 2), the pupillary light reflex was positive in both eyes. He had no signs of meningeal irritation. He was intubated due to lower Glasgow Coma Scale and mechanical ventilation commenced. SARS-CoV-2 real-time polymerase chain reaction (PCR) on nasopharnygeal swab and antibodies both in serum and cerebrospinal fluid (CSF) were negative on admission. Clinical, laboratory and imaging findings are shown in Table 1. In PICU, 10 consecutive therapeutic plasma exchange (TPE) sessions with 5% albumin replacement were performed daily using the Prismaflex TPE 1000 filter set (Gambro Lundia AB, Lund, Sweden), methylprednisolone (30 mg/kg/day for 2 days) was administered for 2 more days. Levetiracetam, midazolam, and ketamine treatments were initiated after he sustained several episodes of generalized tonic-clonic convulsions. After 10 consecutive TPE sessions, brain MRI showed cortical linear hyperintense lesion, consistent with laminar necrosis (Fig. 2). In terms of the etiology of laminar necrosis, rheumatologic examinations (erythrocyte sedimentation rate, antinuclear antibodies, C3-C4, lupus anticoagulant, anticardiolipin antibodies, anti-ds DNA, c-ANCA, p-ANCA, pathergy test), other infection examinations (varicella zoster virus, rubella virus, herpes simplex virus, human herpes virus-6, Epstein-Barr virus, HIV, mumps virus, influenza virus, coxsackie B virus, and cytomegalovirus), and MRG angiography tests were performed, and it was found to be normal. Four TPE sessions on alternate days were performed after the initial 10 consecutive TPEs. On day 20, brain MRI showed prominent areas of necrosis in both frontoparietal regions (Fig. 3). On day 25, because of the inadequate response to treatment, second cure of intravenous immunoglobulin (2 g/kg) was given and a tracheostomy was performed while in the PICU. Following 14 TPE sessions and second-dose intravenous immunoglobulin, his mental and general condition improved. On day 30, he was separated from the mechanical ventilator. On day 33, a brain MRI showed chronic changes (Fig. 4). On day 55, SARS-CoV-2–specific antibodies were positive in CSF. On day 60, he was discharged from the hospital. He was conscious and tracheostomized. He had eye contact, object tracking, and head control. He was able to sit unsupported and walk short distances with one-hand support. His muscle strength was reduced with the upper limbs and the lower limbs of 4/5. He was executing commands.
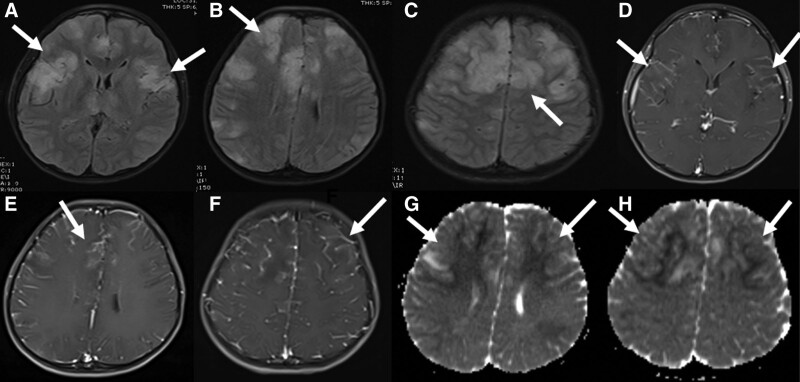
FIGURE 1.
Brain MRI of a 9-year-old boy at presentation. In the fluid-attenuated inversion recovery sequences, pathologic signal changes were observed in cortical and subcortical areas in bilateral frontoparietal regions (A–C). These lesions showed contrast enhancement after contrast enhancement (D–F) and marked diffusion restriction in apparent diffusion coefficient mapping in diffusion-weighted sequences (G and H).
TABLE 1.
Clinical Characteristics, MRI Findings, Laboratory Findings and Outcome in 2 Patients With COVID-19 Infection–Related Acute Disseminated Encephalomyelitis
| Case 1 | Case 2 | |
|---|---|---|
| Age/gender | 9/male | 9/female |
| Concomitant illness | No | No |
| Symptoms | Fever, headache, vomiting | Fever, vomiting, diarrhea |
| Presentations | Status epilepticus | Hallucinations, afebrile seizure |
| Vital signs (PICU adminission) | ||
| SpO2 (%) | 97 | 100 |
| Pulse rate (per minute) | 128 | 113 |
| Blood pressure (mm Hg) | 113/76 | 130/93 |
| Respiratory rate (per minute) | 38 | 29 |
| Body temperature (°C) | 36.4 | 39 |
| Laboratory findings (PICU adminission) | ||
| White blood cell (per μL) | 3290 | 9420 |
| Lymphocyte (per μL) | 820 | 990 |
| Neutrophil (per μL) | 2200 | 7560 |
| Platelet (per μL) | 175,000 | 317,000 |
| Hemoglobin (g/dL) | 10.6 | 10.8 |
| CRP (mg/L) | 2 | 10 |
| Procalcitonin (ng/mL) | 0.18 | 0.16 |
| Urea (mg/dL) | 31 | 16 |
| Creatinine (mg/dL) | 0.32 | 0.4 |
| AST (U/L) | 40 | 35 |
| ALT (U/L) | 14 | 17 |
| CK (U/L) | 271 | 808 |
| CSF analysis | ||
| Glucose (mg/dL) | 75 | 88 |
| Protein (mg/dL) | 117 | 108 |
| Cell count (per mm3) | No | No |
| Oligoclonal band | Negative | Negative |
| Autoimmune encephalitis panel | Negative | Negative |
| Anti-MOG antibody | Negative | Negative |
| Antiaquaporin-4 antibody | Negative | Negative |
| Chest radiograph | Prominent bronchovascular markings | Prominent bronchovascular markings |
| EEG | Diffuse slowing | Diffuse slowing |
| Treatment | MP (30 mg/kg/day) 7 days | MP (30 mg/kg/day) 5 days |
| IVIG (1 g/kg/day) 2 days | TPE | |
| TPE 14 | ||
| SARS-CoV-2 antibody | Positive (serum and CSF) | Positive (serum) |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CRP, C-reactive protein; EEG, electroencephalography; IVIG, intravenous immunoglobulin; MOG, myelin oligodendrocyte glycoprotein; MP, methylprednisolone.
Autoimmune encephalitis panel, including anti–NMDA-R-Ab, AMPA-R1 Ab, AMPA-R2 Ab, CASPR2 Ab. (VGKC), lg11 Ab. (VGKC) and GABA-R-Ab.
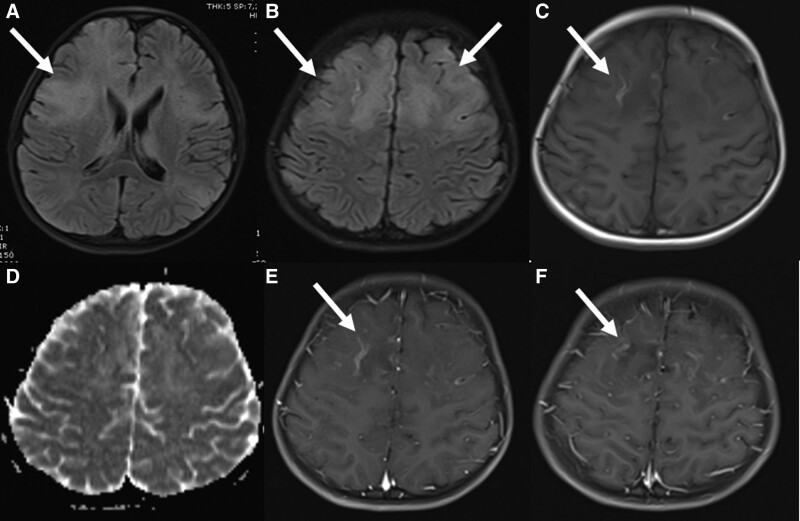
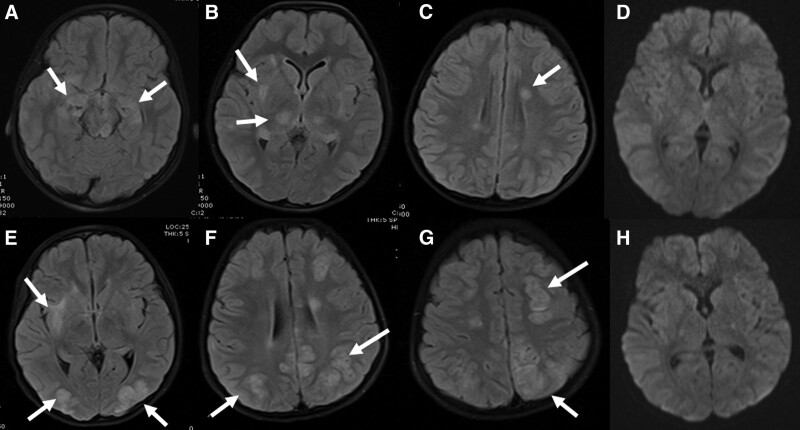
FIGURE 2.
Brain MRI of a 9-year-old boy on day 9. The lesions continued in fluid-attenuated inversion recovery sequences (A and B) and cortical linear hyperintensities compatible with laminar necrosis occurred in T1A sequences (C). It was found that the restriction disappeared in the diffusion-weighted sequences (D) and contrast enhancement continued in the frontoparietal regions after contrast agent administration (E and F) and necrotic areas began to occur in these regions.
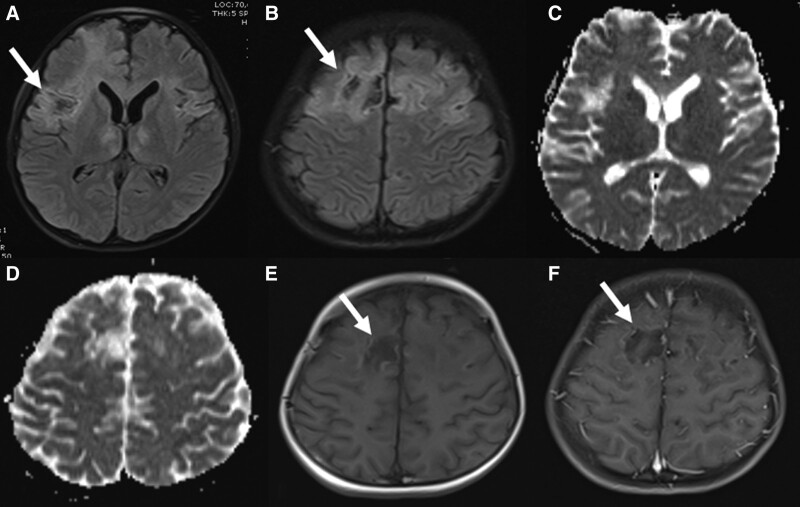
FIGURE 3.
Brain MRI of a 9-year-old boy on day 20. Prominent areas of necrosis in both frontoparietal regions (A–F).
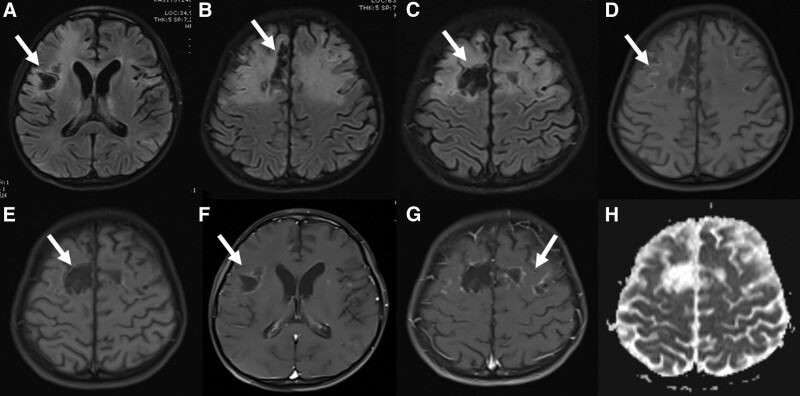
FIGURE 4.
Brain MRI of a 9-year-old boy on day 33. Pathologic signal changes, contrast enhancements, and laminar necrosis continued in both frontoparietal regions, areas of cystic necrosis became prominent, and atrophy occurred in the brain (A–H).
Case 2
A 9-year-old previously healthy girl presented with hallucinations, lethargy and afebrile seizure after 5 days of fever, vomiting and diarrhea. Her immunization status was appropriate to her age. She was admitted to PICU with a prediagnosis of encephalitis. She had no history of contact with a symptomatic COVID-19 patient. On physical examination, she was unconscious and unoriented. Her Glasgow Coma Scale was 8 (E 2, V 2, M 4), the pupillary light reflex was positive in both eyes. She had no signs of meningeal irritation. The nasopharyngeal swab for SARS-CoV-2 with real-time PCR and antibody was negative. She was intubated due to lower Glasgow Coma Scale and mechanical ventilation commenced. Clinical, laboratory and imaging findings are shown in Table 1. Brain MRI and her clinical course were consistent with ADEM (Fig. 5). Methylprednisolone 30 mg/kg intravenous daily for 5 days was administered for presumed ADEM. On day 5, she was seizure free under treatment. Sedation and mechanical ventilator parameters were weaned, and she was extubated. On day 9, she became hypertensive despite antihypertensive treatment, and on day 10, her seizures returned. In PICU, 5 consecutive TPE sessions with 5% albumin replacement were performed daily using Prismaflex TPE 1000 filter set (Gambro Lundia AB). On day 12, brain MRI revealed posterior reversible encephalopathy. Anti–SARS-CoV-2 antibody was detected in her serum before discharge on day 17, and she was diagnosed with encephalitis associated with SARS-CoV-2. The patient was discharged on day 17, recovering completely. During her routine follow-up visit, she had no symptoms and signs; MRI findings regressed totally within 3 months, and no new lesions were seen in follow-up MRI scans.
FIGURE 5.
Brain MRI of a 9-year-old girl at presentation. In the fluid-attenuated inversion recovery (FLAIR) sequences, pathologic signal increases were observed in the bilateral temporal region in the hippocampal areas, bilateral thalamus, putamen and deep white matter. Diffusion restriction was not observed in these lesions in diffusion-weighted sequences. These lesions were evaluated as acute disseminated encephalomyelitis–like diseases (A–D). MRI 12 days later (E–H) showed the lesions previously detected in FLAIR sequences disappeared but new lesions developed in the right temporal lobe inferior, bilateral occipital localization, cortical and subcortical areas extending toward the corona radiata and showing no diffusion restriction. These newly formed lesions were considered as posterior reversible encephalopathy syndrome.
DISCUSSION
We, herein, describe 2 children with ADEM-like disease associated with COVID-19. Both cases were diagnosed with SARS-CoV-2 infection based on the detection of anti–SARS-CoV-2 antibody. In a multinational and multicenter study focusing on neuroimaging manifestations in children with SARS-CoV-2 infection, the presence of ADEM-like lesions was notified in 16 children. The most common neurologic manifestation of SARS-CoV-2 infection was ADEM. In this study, acute-phase and delayed-phase, SARS-CoV-2–related ADEM-like disease was reported in children. Two of the patients with ADEM-like neuroimaging had CNS-directed antibodies (anti-N-methyl-D-aspartate receptor and anti-myelin oligodendrocyte glycoprotein antibodies).5 In our patients, T2-weighted hyperintensity was present in the white matter. In our laboratory examination CNS-directed antibodies, SARS-CoV-2 PCR, and antibody were determined negative, but in our delayed laboratory examination, SARS-CoV-2 antibodies were determined positive. We confirmed delayed-phase SARS-CoV-2–related ADEM-like disease. In a study, antibody responses to SARS-CoV-2 in serum and CSF specimens of COVID-19 patients with neurologic symptoms were assessed. This study showed IgG specific for the virus spike protein was detected in serum (81%) and CSF (56%) of the patients. Also, 2 patients who were negative for SARS-CoV-2 IgG in serum had positive levels of IgG in CSF.6 ADEM is characterized by encephalopathy in association with polyfocal neurologic deficits, sometimes preceded by prodromal symptoms. Neurologic manifestations in ADEM include pyramidal signs, ataxia, acute hemiparesis, optic neuritis or other cranial nerve involvement, seizures, spinal cord syndrome, and impairment of speech. Seizures with fever are described more frequently in ADEM compared with other acute demyelinating syndromes. Seizures may develop into status epilepticus.3 Seizures and encephalopathy were the main CNS symptoms in our patients, also both of them developed status epilepticus. ADEM incidence reported of 0.1–0.3 per 100 000 children per year also severe presentation resulting in admission to a PICU has been reported in 15%–25% of children with ADEM.3 Our patients were followed up in the PICU due to severe presentation. Further one of them was tracheostomized.
In children, cortical laminar necrosis is caused by hypoxia and metabolic disorders, like hypoglycemia, intoxication and hypoxic-ischemic encephalopathy, renal and hepatic dysfunction.7 It may also be seen in patients with infections disease like influenza A and dengue encephalitis.8,9 One of our patients had cortical laminar necrosis. This is the first laminar necrosis reported in SARS-CoV-2–related ADEM-like disease. We do not know why laminar necrosis developed in one of our patients and the other fully resolved. Probably, genetic factors are responsible for this.10
Our case series have limitations. Both cases were diagnosed with SARS-CoV-2 infection based on the detection of SARS-CoV-2 antibody. SARS-CoV-2 antibody in CSF of one of the children was found to be positive, but SARS-CoV-2 antibody in CSF of the other patient was not detected.
In conclusion, we described the clinical course of children with ADEM-like disease associated with SARS-CoV-2 infection. While the initial SARS-CoV-2 antibody of the patients was negative, the SARS-CoV-2 antibody sent afterward was found to be positive in the blood.
Footnotes
The authors have no funding or conflicts of interest to disclose.
References
- 1.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JE, Asfour A, Sewell TB, et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743:135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 suppl 2):S38–S45. [DOI] [PubMed] [Google Scholar]
- 4.Mihai C, Jubelt B. Post-infectious encephalomyelitis. Curr Neurol Neurosci Rep. 2005;5:440–445. [DOI] [PubMed] [Google Scholar]
- 5.Lindan CE, Mankad K, Ram D, et al. ; ASPNR PECOBIG Collaborator Group. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham JL, Virhammar J, Rönnberg B, et al. Anti-SARS-CoV2 antibody responses in serum and cerebrospinal fluid of COVID-19 patients with neurological symptoms [published online ahead of print March 21, 2021]. J Infect Dis. jiab153. doi: 10.1093/infdis/jiab15333744954 [Google Scholar]
- 7.Niwa T, Aida N, Shishikura A, et al. Susceptibility-weighted imaging findings of cortical laminar necrosis in pediatric patients. AJNR Am J Neuroradiol. 2008;29:1795–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaginuma K, Watanabe M, Suzuki Y, et al. Cortical laminar necrosis in an infant with influenza A virus infection. Clin Case Rep. 2020;8:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg RK, Rizvi I, Ingole R, et al. Cortical laminar necrosis in dengue encephalitis-a case report. BMC Neurol. 2017;17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debnath M, Banerjee M, Berk M. Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes. FASEB J. 2020;34:8787–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]