Supplemental Digital Content is available in the text.
Keywords: pediatric multisystem inflammatory syndrome, severe acute respiratory syndrome coronavirus 2, myocardial injury, immunoglobulins, corticosteroids
Background:
Cardiovascular complications with myocarditis in multisystem inflammatory syndrome in children (MIS-C) associated with severe acute respiratory syndrome coronavirus 2 infection have been reported, but the optimal therapeutic strategy remains unknown.
Methods:
A retrospective cohort study included 19 patients with acute left ventricular systolic dysfunction associated with MIS-C, average years of age 13.2 ± 3.8, treated from April 2020 to April 2021.
Results:
Treatment failure (TF) was observed in 8 patients (in the intravenous immunoglobulin [IVIG] group 7/10; in the corticosteroid [CS] group 1/9). The independent risk factor for TF was IVIG treatment (odds ratio [OR] 18.6, 95% confidence interval [CI] 1.6–222.93, P = 0.02). Patients initially treated with CS became afebrile during in-hospital day 1 (1.5, interquartile range [IQR] 1–2), while IVIG-treated patients became afebrile on in-hospital day 4 (IQR 2–4.25), after CS was added. The C-reactive protein (CRP) significantly declined in CS-treated patients on day 2 (P = 0.01), while in the IVIG group, CRP decreased significantly on the fourth day (P = 0.04). Sodium and albumin levels were higher on third in-hospital day in the CS group than in the IVIG group (P = 0.015, P = 0.03). A significant improvement and normalization of ejection fraction (EF) during the first 3 days was observed only in the CS group (P = 0.005). ICU stays were shorter in the CS group (4, IQR 2–5.5) than in the IVIG group (IVIG group 7, IQR 6–8.5) (P = 0.002).
Conclusions:
Among children with MIS-C with cardiovascular involvement, treatment with CS was associated with faster normalization of LV EF, fever, laboratory analysis, and shorter ICU than IVIG-treated patients.
Multisystem inflammatory syndrome in children (MIS-C) temporarily associated with novel coronavirus disease 19 (COVID-19) is a delayed immune response to a recent asymptomatic or symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MIS-C typically occurs 2–6 weeks after an acute SARS-CoV-2 infection, and most patients had a positive serology result.1 As the incidence of MIS-C was 2 per 100,000, a hypothesis has been posed that only genetically predisposed children can develop MIS-C.2,3
Patients with MIS-C predominantly had a fever (> 38°C or subjective) for at least 24 hours, with laboratory evidence of inflammation, cardiovascular, gastrointestinal, and mucocutaneous manifestations.1,2 Although MIS-C has overlapping features with Kawasaki disease (KD) and toxic shock syndrome, the inflammatory storm observed in MIS-C is much more intense.4 Specifically, MIS-C can present with multiorgan failure, including neurologic involvement, hyperferritinemia, and cardiogenic or vasoplegic shock requiring hemodynamic support in 60%–75% of cases.5,6 Cardiovascular symptoms were present in 71% of the cases, and most patients had significant systolic myocardial dysfunction.7–9
The overlapping features between these syndromes suggest that they may share similar pathophysiology, probably explaining why they respond to similar therapies.4 Early reports support the use of intravenous immunoglobulins (IVIGs)—which is the standard treatment for KD—to treat MIS-C patients. However, due to the refractory nature of the disease, many children required second-line treatment—corticosteroids (CSs) and immune modulators.2,6
This retrospective cohort study aimed to compare the outcomes in patients with myocardial damage related to MIS-C depending on the initial treatment—IVIG or CSs.
METHODS
The retrospective cohort study included all children under 18 with acute left ventricular (LV) systolic dysfunction or cardiogenic shock associated with MIS-C admitted to our Clinic from April 2020 to April 2021. A serologic examination for SARS-CoV-2 was performed using the enzyme-linked immunosorbent assay and the immunochromatography technique. The diagnosis of MIS-C temporarily associated with COVID-19 was based on the criteria of the US Centers for Disease Control and Prevention. MIS-C shock was defined as the persistence of systolic arterial hypotension, a decrease of at least 20% from a basal systolic blood pressure, or the appearance of signs of peripheral hypoperfusion.10,11
Patients were initially treated with IVIG according to the protocols for KD; IVIG-nonresponsive patients were treated with CS—three intravenous methylprednisolone pulses (IVMPs). However, we observed that most patients required CS despite the IVIG administration, so we treated patients initially with CS without IVIG. Patients with acute LV systolic dysfunction or cardiogenic shock associated with MIS-C were initially treated with IVMP without IVIG (see Figure, Supplemental Digital Content 1; http://links.lww.com/INF/E469); only 3 patients received dexamethasone initially.
We divided the patients into 2 groups depending on their initial treatment for MIS-C: IVIG and CS. The groups were compared according to clinical presentation, vital and laboratory parameters, electrocardiogram, clinical course and disease outcome.
The therapy’s impact was analyzed by reviewing the vital parameters and laboratory analyses for the first 5 in-hospital days. Treatment failure (TF) was defined as fever persistence (>38 °C or > 100.4 °F) for 48 hours after therapy initiation or the occurrence of acute LV systolic dysfunction (ejection fraction [EF] < 55%) and a need for vasoactive drugs.6
The study was approved by the local ethics committee, which waived the need for informed parental consent (authorization number: 9/13).
Statistical Analysis
The data were processed using the statistical software SPSS 25.0 for Windows 10. All statistical methods were considered significant if the P value was ≤0.05. The descriptive statistics included the mean values, median, standard deviations and the interquartile range (IQR) of the parameters monitored. The difference in the distribution of specific parameters among the groups tested was determined using the χ2 or Fisher test. The normality of the numerical variables’ distribution was tested using the Shapiro Wilk and Kolmogorov Smirnov tests. The comparison between the 2 groups was made using the Student’s t test and the Mann-Whitney U test. Paired T tests and Wilcoxon tests were used to compare 2 related samples. The correlation between vital parameters in different groups was tested by Pearson test or Spearman test. Binominal logistic regression analysis was used to explain the relationship between the dependent binary variable and the independent variables.
RESULTS
From April 2020 to April 2021, 51 patients with MIS-C were treated in our institute, 21 women and 30 men (9.3 ± 5.0 years). There were positive serologic tests for SARS-CoV-2 for 49 patients, while 2 patients had a positive PCR test for SARS-CoV-2.
Myocardial injury was observed in 22 patients, and those patients were included in our retrospective study. Patients with MIS-C older than 10 had 12 times higher probability of myocardial affection (odds ratio [OR] 10.9, 95% confidence interval [CI]: 2.8–39.6; p < 0.001). Only those patients had shock syndrome on admission (P = 0.001).
Among the 22 children, 10 received IVIG as the initial treatment, while CS was used as the initial treatment in 12 patients (IVMP—9 patients and dexamethasone—3 patients). The difference between groups in terms of age distribution and fever duration before first-line therapy was not significant (P = 0.4, P = 0.42, respectively) (Table 1). The clinical presentation of the groups is presented in Figure, Supplemental Digital Content 2A; http://links.lww.com/INF/E471. On admission, 8 of 22 patients met MIS-C shock syndrome criteria (IVIG treated 5, CS alone—3; P = 0.65). However, during IVIG infusion 1 patient experienced clinical deterioration and 3 patients developed shock syndrome (P = 0.03).
TABLE 1.
Patients Characteristics at the Admission
| IVIG 10 Patients (45.4%) | CS 12 Patients (54.5%) | P | |
|---|---|---|---|
| Male gender | 8 | 7 | 0.38 |
| Age | 13.4 ± 3.7 | 11.8 ± 4.4 | 0.38 |
| KD-like | 7 | 9 | 1.0 |
| Shock | 5 | 3 | 0.37 |
| Fever (days) | 6 (IQR 5 to 7) | 5 (IQR 4.25 to 6.75) | 0.37 |
| Heart rate (/min) | 116.5 ± 16.8 | 111.3 ±17.4 | 0.49 |
| CRP (mg/mL) | 175 (IQR 121.9 to 258.7) | 246.5 (IQR 180.3 to 293) | 0.1 |
| NT-pro BNP (pg/mL) | 4839 (IQR 2739 to >5000) | 4256 (IQR 2175 to >5000) | 1.0 |
| Elevated cTnI | 7 | 8 | 1.0 |
| Elevated SGOT | 5 | 4 | 0.7 |
| Elevated SGPT | 6 | 6 | 0.7 |
| Thrombocytopenia | 6 | 7 | 1.0 |
| Na (mmol/L) | 133.1 ± 3.75 | 132.7 ± 2.5 | 0.75 |
| Albumin (g/L) | 35.2 ± 4.6 | 31.7 ± 4.3 | 0.08 |
| Phosphate (mmol/L) | 0.93 ± 0.3 | 1.03 ± 0.2 | 0.37 |
| D-dimer (ng/mL) | 753 (IQR 494 to 1758) | 1312 (IQR 685 to 2488) | 0.26 |
| Fibrinogen (g/L) | 5.6 ± 2.5 | 5.3 ± 1.7 | 0.7 |
| Ejection fraction (%) | 47.9 ± 7.7 | 51.7 ± 5.7 | 0.27 |
| Fraction of shortening (%) | 24.5 ± 4.17 | 27.1 ± 5.7 | 0.31 |
| Left ventricle EDD (Z score) | 0.54 (IQR −0.5 to 1.06) | 0.4 (IQR 0.06 to 1.17) | 0.46 |
| Left ventricle ESD (Z score) | 2.05 (IQR 0.7 to 3.13) | 1.97 (IQR 1.55 to 2.2) | 0.72 |
| Posterior wall (Z score) | 0.9 (IQR 0.46 to 1.6) | 1.44 (IQR 1.1 to 2.34) | 0.13 |
EDD indicates end-diastolic diameter; ESD, end-systolic diameter.
TF was observed in 9 patients (in the IVIG group 7/10; in the CS group 2/9). There was a higher prevalence of TF in the IVIG group than in the CS group (P = 0.03). Specifically, treatment with IVIG as compared with CS was an independent risk factor for TF (OR 11.7, 95% CI: 1.5–89.3, P = 0.02). In 7 of 10 patients initially treated with IVIG, CS was subsequently added on day 3 on average (IQR 2–4); IVMP in 5 of 7 patients and dexamethasone in 2 of 7 patients. The clinical presentation did not influence TF (see Figure, Supplemental Digital Content 2B; http://links.lww.com/INF/E471). The difference between groups is presented in Table 2. Patients with phosphate levels lower than 1 mmol/L and fibrinogen higher than 5.5 g/L had 24 times and 10 times higher probability of TF, respectively (OR 24, 95% CI: 1.7–330.8, P = 0.02; OR 9.6, 95% CI: 0.9–105.2, P = 0.049), than children with a phosphate level higher than 1 mmol/L and fibrinogen level lower than 5.5 g/L.
TABLE 2.
The Differences Between Patients With and Without Treatment Failure
| Treatment Failure | P value | ||
|---|---|---|---|
| No | Yes | ||
| CRP (mg/L) fourth day | 65.0, IQR 38.0–80.0 | 100.0, IQR 97.5–195.0 | 0.001 |
| Na (mmol/L) second day | 136.8 ± 4.2 | 131.5 ± 2.7 | 0.005 |
| LV EF (%) third day | 60.3 ± 4.6% | 54.33 ± 7.66 | 0.004 |
CRP indicates C-reactive protein; Na, sodium; LV EF, left ventricle ejection fraction.
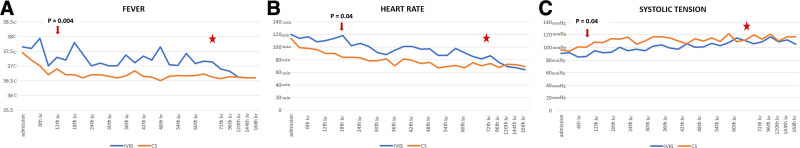
Patients initially treated with CS became afebrile during in-hospital day 1 (1.5, IQR 1–2), while patients initially treated with IVIG became afebrile averagely on in-hospital day 4 (IQR 2–4.25; P = 0.007) (Fig. 1A). A significant difference between the body temperature and heart rate of those groups was registered after 9 and 15 hours of in-hospital stay, respectively (P = 0.004, P = 0.04) (Fig. 1B).
FIGURE 1.
The vital parameters dynamics during in-hospital stay. Red star—the moment of corticosteroid administration in IVIG-nonresponders. IVIG indicates intravenous immunoglobulin.
A significant higher systolic blood pressure was registered in the CS group after 15 hours of in-hospital stay (P = 0.04) compared with IVIG-treated patients (Fig. 1C). Hypertension was observed in 5/22 patients (22.7%) during the in-hospital stay. The therapy applied did not influence hypertension (P = 0.63) or renal injury (P = 0.4), but these patients were older (16.8 ± 0.6 years) than the others (11.9 ± 3.5 years; P = 0.008).
On admission, 10 patients had electrocardiogram abnormalities. The most common abnormality was a negative T wave in the inferior leads. A prolonged corrected QT interval was observed in 13 patients, on day 7 on average (IQR 5.25–9.75).
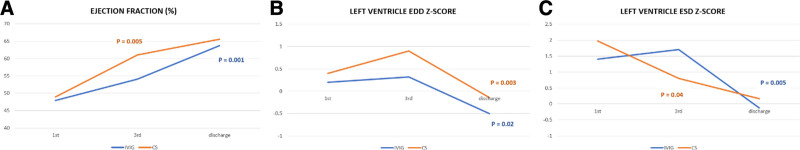
Echocardiography examination on admission identified depressed systolic function (EF 49.9 ± 7.8%), and walls edema. A moderate negative correlation was proved between the cardiac troponin I (cTnI) value and EF (ρ = −0.55, P = 0.008), and between the C-reactive protein (CRP) value and the EF on admission (r = −0.53, P = 0.03). Uniform coronary artery (CA) dilatation was observed on admission in 4 patients (LCA—3 patients; LCA and RCA—1 patient; LCA Z score 2.95 ± 0.3; RCA Z score 2.6), only in patients with KD-like clinical presentation; CA were of appropriate diameter on discharge. No difference was observed between the groups in terms of the echocardiography parameters on admission (Table 1), but CS-treated patients had normal and a significantly higher EF (EF 61.1 ± 5.1%) on in-hospital day 3 than patients treated with IVIG (EF 54.1 ± 6.5%) (P = 0.015). A significant improvement of systolic function during the first 3 days was observed in the CS group (P = 0.005) (Fig. 2).
FIGURE 2.
Recovery of the left ventricular systolic function during in-hospital stay in the different groups. (A) A significant improvement of EF during the first 3 days was observed in the CS group (P = 0.005); in the IVIG group, in the discharge (P = 0.001); (B) The left ventricle ESD Z score decreased significantly during the first 3 days in the CS group (P = 0.04); in the IVIG group on discharge (P = 0.005); (C) The left ventricle EDD Z-score reduced significantly on discharge in both groups, IVIG and CS (P = 0.003, P = 0.02). CS, corticosteroid; ESD, end-systolic diameter; EDD, end-diastolic diameter; IVIG indicates intravenous immunoglobulin.
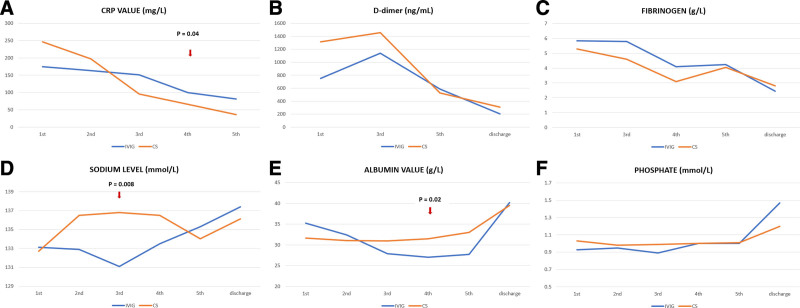
On admission, all patients had elevated inflammatory parameters in serum. The CRP significantly declined in patients initially treated with CS on days 2, 3 and 4 (P = 0.008, P = 0.003, P = 0.008 respectively), while in the IVIG group, CRP decreased significantly on day 4, immediately after CS was added (P = 0.04). A significant difference in CRP value between groups was observed on day 4 (P = 0.001) (Fig. 3A). No significant difference in D-dimers and fibrinogen levels was observed between groups (Fig. 3B and C).
FIGURE 3.
The dynamics of laboratory analysis during the in-hospital stay.
On admission hyponatremia was observed in 16 of 22 patients. The sodium level was higher on in-hospital day 3 in the CS group than in the IVIG group (P = 0.008) (Fig. 3D). In the CS group, sodium significantly increased on day 2 (P < 0.001), and on day 4 in the IVIG group after CS was added (P = 0.05). Hypoalbuminemia was registered on admission in 13/22 patients. Albumin levels were higher on day 4 in the CS group (31.5 ± 3.1 g/L) than in the IVIG group (27.0 ± 3.5 g/L) (P = 0.02). A significant reduction of the albumin level was observed in the IVIG group during the first 3 days (P = 0.003), with a subsequent gradual increase after CS was added (Fig. 3E). In the IVIG group, serum phosphate increased significantly after CS was added on day 4 (P = 0.02) (Fig. 3F).
ICU stays were shorter in the CS group (4, IQR 2–5.2) than in the IVIG group (IVIG group 7, IQR 6–8.5) (P < 0.001).
During the follow-up period (1.25 months, IQR 1–2.65), 1 patient had a 1-day fever of 38.5 °C with elevated acute phase reactants (CRP 83.9 mg/L, ESR 35 mm/h) and microscopic hematuria followed by spontaneous resolution. Two patients had systolic hypertension on the control Holter monitoring of blood pressure, despite antihypertensive therapy; one of them also had calcinuria, phosphaturia, uricosuria and proteinuria. Neither the gender, age, initially prescribed therapy, nor TF influenced the short-term sequelae of MIS-C (P = 0.17, P = 1.0, P = 0.7 and P = 0.66).
DISCUSSION
Although many similarities between MIS-C and KD were identified, it has become evident that cardiovascular involvement, shock, gastrointestinal symptoms, and coagulopathy, which are rarely seen in classic KD, are prominent features of this unique syndrome.10 The etiology of cardiovascular involvement in MIS-C is likely multifactorial. Cardiac injury was caused through multiple hypothesized mechanisms, including cardiomyocyte injury due to an acute and dysregulated inflammatory response related to a cytokine storm, microvascular dysfunction, and a viral invasion of cardiomyocytes resulting in cellular damage and ischemic injury.8,9,12,13 Almost half of all our patients with MIS-C had a myocardial injury (22/51), and those patients were significantly older than patients without myocardial damage. All patients had elevated N-terminal-pro hormone b-type natriuretic peptide, while cTnI was increased in 15 of 22 patients. The cTnI release results from increased permeability of the cardiomyocyte cell membrane and does not exclusively reflect cell necrosis.12 These findings support myocardial stunning or edema rather than myocarditis in MIS-C. We proved the moderate negative correlation between CRP and EF, which suggests that LV systolic function is conditioned by the degree of inflammation. In KD, myocardial edema is the main finding without or with limited cardiomyocytes necrosis as evidenced by the mild to moderate elevation of cTnI.14 In the autoptic hearts, the cardiac tissue’s inflammatory process seemed to permeate the vascular wall, consequently leading to arterial obliteration and ischemic tissue damage, without signs of typical lymphocytic or viral myocarditis.15,16
Although KD is presumed to be triggered by a viral infection, investigations remained inconclusive, given the lack of reproducibility of a putative viral pathogen resulting in KD.9 We assume MIS-C and KD represent a spectrum of postviral, immune-mediated myocarditis with shared similar immunopathogenesis. It is recommended that patients with immune-mediated myocarditis should be treated with immunosuppressive drugs, such as CS.17 In the last decade, CS was commonly used in KD, especially with a high Kobayashi index and myocarditis, and CS would probably help prevent IVIG resistance in KD.4,5,9–11,18 Some authors have reported IVMP as a rescue therapy in children with refractory KD who had myocarditis and symptomatic congestive heart failure during the acute stage of the disease.19–21
Some studies have shown that 51%–80% of patients with MIS-C did not respond to IVIG treatment,6,10 while 70% of our patients with MIS-C appear to be unresponsive to IVIG. CS was added to this group’s therapy on day 3 on average. Immediately after CS introduction to those patients, on day 4 of in-hospital stay, serum levels of sodium and phosphate increased significantly, CRP decreased significantly and patients became afebrile.
Patients treated with CS had a faster normalization of body temperature, heart rate and systolic pressure than patients treated with IVIG and a rapid decline in pro-inflammatory parameters in the blood. Compared with the IVIG group, CS-treated patients had a lower CRP on day 4, but higher sodium, albumin, and phosphate levels. Echocardiography evaluation on day 3 showed that CS-treated patients had a normal and significantly higher EF compared with IVIG-treated patients. Belhadjer et al concluded that adding CS to IVIG was associated with a shorter cardiac function recovery time in patients with MIS-C.13 We showed that patients treated with CS had shorter ICU stays than another group, which is similar to the results from Ouldali et al.6
IVIG-treated patients had an almost 19-times higher probability of TF than patients treated with CS. SARS-CoV-2-epithelial injury at an initial stage may provoke secondary local endotheliitis with delayed auto-inflammatory vasculitic phenotype with upregulation of IL-1β or IL-6.22 The constellation of elevated cytokines mediated an increase in vascular permeability, causing fluid leakage into the extravascular compartment.23 More than half of our patients had hypoalbuminemia, hyponatremia, hypophosphatemia and thrombocytopenia on admission. Severe hyponatremia and hypoalbuminemia are common features in previous “classic” KD myocarditis reports, and they were associated with more severe and more prolonged acute disease.24–26 This finding supports the fact that MIS-C inflammation is much more intense than in classic KD.4 More intense inflammation could explain the ineffectiveness of IVIG to block fluid leakage, leading to hypoalbuminemia, hyponatremia and hypovolemia, contributing to the newly formed MIS-C distributive shock in 4 of 10 IVIG-treated patients. Hyponatremia could be explained by third-space loss of fluid and sodium or the renal loss of sodium and increased BNP concentration or intensive inflammation, elevated plasma IL-6 and the tumor necrosis factor α (TNF-α) influenced the release of antidiuretic hormone.23,24 We showed that having a phosphate level lower than 1 mmol/L and fibrinogen higher than 5.5 g/L increased the probability of TF, resulting from more intense inflammation. The phosphate level negatively correlated with TNF-α and IL-6 in early sepsis,27,28 while cytokines regulated fibrinogen production in the liver, and the production is greatly enhanced in the acute phase of infection response and other inflammatory processes.29 The significant elevation of the phosphate level after CS introduction in IVIG-nonresponsive patients was a consequence of CS dose-dependent inhibition of cytokine (TNF-α and IL-6) release.27,28 CS acutely reduces the hyperinflammatory response, inhibits vasodilatation and the increased vascular permeability that occurs following inflammatory insult; these anti-inflammatory effects are apparent within minutes due to the expression of IL-1α and IL-1β being inhibited.30
This study has several limitations. First, it was not a randomized trial, which might provide the best level of evidence. Second, the number of patients included in the study was small. Third, the follow-up period was insufficient to draw conclusions.
CONCLUSION
Among children with a myocardial injury during MIS-C, treatment with CS only was associated with a faster normalization of fever, improved laboratory results, and a shorter ICU stay compared with IVIG-treated patients. Additionally, in CS-treated patients, normalization of LV EF was observed on in-hospital day 3. CS should also be used for refractory MIS-C because after CS was added, patients became afebrile, the CRP value dropped and the sodium and phosphate levels increased significantly. The short-term sequelae of MIS-C and CS side-effect were reversible in our study.
ACKNOWLEDGMENTS
We thank and acknowledge the following for their assistance in the treatment of patients with MIS-C: Predrag Minic, MD, PhD, Natasa Stajic, MD, and Srdjan Pasic, MD, PhD (Head of Immunology Department), Mother and Child Health Institute of Serbia. Additionally, we truly appreciate the following for performing the biochemical analysis: Ljubica Zatezalo, MD, Dragana Bojanin Mr Ph.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Elias MD, McCrindle BW, Larios G, et al. ; of the International Kawasaki Disease Registry. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the international Kawasaki disease registry. CJC Open. 2020;2:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennon TR, Penque MD, Abdul-Aziz R, et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2020;23:101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouldali N, Toubiana J, Antona D, et al. ; French Covid-19 Paediatric Inflammation Consortium. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito S, Principi N. Multisystem inflammatory syndrome in children related to SARS-CoV-2. Paediatr Drugs. 2021;23:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JC, May JW, Cunningham MW, et al. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis-a critical review of its pathogenesis and treatment. Front Pediatr. 2020;8:626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated With SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janardhanan R. Myocarditis with very high troponins: risk stratification by cardiac magnetic resonance. J Thorac Dis. 2016;8:E1333–E1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belhadjer Z, Auriau J, Méot M, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142:2282–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 15.Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–1131. [DOI] [PubMed] [Google Scholar]
- 16.Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7:2440–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caforio AL, Pankuweit S, Arbustini E, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636– 2648a.2648 [DOI] [PubMed] [Google Scholar]
- 18.Inoue T, Murakami S, Matsumoto K, et al. Functional benefits of corticosteroid and IVIG combination therapy in a coronary artery endothelial cell model of Kawasaki disease. Pediatr Rheumatol Online J. 2020;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal P, Suri D, Narula N, et al. Symptomatic myocarditis in Kawasaki disease. Indian J Pediatr. 2012;79:813–814. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Somura J, Maruo Y. Steroid pulse therapy for Kawasaki disease complicated with myocarditis. Indian Pediatr. 2016;53:1015–1016. [DOI] [PubMed] [Google Scholar]
- 21.Suga K, Inoue M, Ono A, et al. Early combined treatment with steroid and immunoglobulin is effective for serious Kawasaki disease complicated by myocarditis and encephalopathy. J Med Invest. 2016;63:140–143. [DOI] [PubMed] [Google Scholar]
- 22.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousef MS, Idris NS, Yap C, et al. Systematic review on the clinical presentation and management of the COVID-19 associated multisystem inflammatory syndrome in children (MIS-C). Allergy Immunol. 2020;5:38–55. [Google Scholar]
- 24.Kim DS. Kawasaki disease. Yonsei Med J. 2006;47:759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, Oh YS, Choi MJ, et al. Hyponatremia may reflect severe inflammation in children with febrile urinary tract infection. Pediatr Nephrol. 2012;27:2261–2267. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Abe Y, Sato S, et al. Hyponatremia in Kawasaki disease. Pediatr Nephrol. 2006;21:778–781. [DOI] [PubMed] [Google Scholar]
- 27.de Kruif MD, Lemaire LC, Giebelen IA, et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845–1851. [DOI] [PubMed] [Google Scholar]
- 28.Yamazawa K, Kodo K, Maeda J, et al. Hyponatremia, hypophosphatemia, and hypouricemia in a girl with macrophage activation syndrome. Pediatrics. 2006;118:2557–2560. [DOI] [PubMed] [Google Scholar]
- 29.Kerlin B, Cooley BC, Isermann BH, et al. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103:1728–1734. [DOI] [PubMed] [Google Scholar]
- 30.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.