Supplemental digital content is available in the text.
Key words/Abbreviations: mindfulness; hair cortisol; glucocorticoids; objective and subjective stress; DHEA = dehydroepiandrosterone; HC = hair cortisol; HE = hair cortisone; HPA = hypothalamic-pituitary-adrenal; LMM = linear mixed model; MBSR = mindfulness-based stress reduction; PSS = Perceived Stress Scale, TICS = Trier Inventory for Chronic Stress; RCC = retest control cohort; TC1–3 = training cohorts 1–3
ABSTRACT
Objective
This study aimed to investigate the effect of regular contemplative mental training on endocrine and psychological indices of long-term stress.
Methods
An open-label efficacy trial that comprised three distinct 3-month long modules targeting attention and interoception, socioaffective, or sociocognitive abilities through dyadic exercises and secularized meditation practices was conducted with healthy adults. Participants underwent the training for 3 or 9 months, or were assigned to a retest control cohort. Chronic stress indices were assayed at four time points: pretraining and after 3, 6, and 9 months. The main outcome measures were cortisol (HC) and cortisone (HE) concentration in hair and self-reported long-term stress.
Results
Of 362 initially randomized individuals, 30 dropped out before study initiation (n = 332; mean [SD] age = 40.7 [9.2] years; 197 women). Hair-based glucocorticoid assays were available from n = 227, and questionnaire data from n = 326. Results from three separate training cohorts (TC1–3) revealed consistent decreases in HC and HE levels over the first three (TC3) to 6 months (TC1 and TC2) of training, with no further reduction at the final 9-month mark (baseline to end of training differences, HC, TC1: t(355) = 2.59, p = .010, contrast estimate (est.) [SE] = 0.35 [0.14]; HC, TC2: t(363) = 4.06, p < .001, est. = 0.48 [0.12]; HC, TC3: t(368) = 3.18, p = .002, est. = 0.41 [0.13]; HE, TC1: t(435) = 3.23, p = .001, est. = 0.45 [0.14]; HE, TC2: t(442) = 2.60, p = .010, est. = 0.33 [0.13]; HE, TC3: t(446) = 4.18, p < .001, est. = 0.57 [0.14]). Training effects on HC increased with individual compliance (practice frequency), and effects on both HC and HE were independent of training content and unrelated to change in self-reported chronic stress. Self-reported stress, and cortisol-to-dehydroepiandrosterone ratios as an exploratory endpoint, were also reduced, albeit less consistently.
Conclusions
Our results point to the reduction of long-term cortisol exposure as a mechanism through which meditation-based mental training may exert positive effects on practitioners’ health.
Trial Registration: ClinicalTrials.gov identifier: NCT01833104.
INTRODUCTION
Rising prevalence of stress-related mental and physical disorders (1,2) has led to the recognition of chronic stress as one of the 21st century’s major health risks (3). The health outcomes of exposure to psychosocial stress are mediated by prolonged activation of our main neuroendocrine stress systems, the sympathetic-adrenal-medullary and the hypothalamic-pituitary-adrenal (HPA) axes. Both systems exert complex effects on immune and metabolic processes and are causally involved in the development of cardiovascular, metabolic, and autoimmune disorders, among others (4). In striving to reduce stress and promote health and well-being, secular meditation-based mental training interventions, such as the mindfulness-based stress reduction (MBSR) program (5), have gained popularity. Various health-related benefits have been associated with engagement in such training interventions (see, e.g., (6,7) for meta-analyses). Findings from our own 9-month mental training study, the ReSource Project (8), show differential positive changes in subjective well-being, cognition, peripheral physiology, and brain plasticity after distinct types of contemplative mental training (9).
Of particular interest for application in healthcare are the downstream health benefits of contemplative training, such as mitigation or prevention of stress-related disorders. Current theory suggests that these outcomes are mediated by dampened activity of physiological stress systems, above all the HPA axis (10). In line with this theory, reduced subjective-psychological stress load is one of the most widely reported training outcomes (7). At the same time, self-report measures of contemplative training effects may be particularly susceptible to confounds such as demand effects and expectancy bias because the training trials are inevitably conducted without blinding. Researchers are thus increasingly relying on physiological measures as more reliable and objective health outcomes. For healthy adults, results show that even though correspondence between psychological and physiological measures of stress is often assumed, evidence for training-related endocrine stress reduction currently remains mixed and inconclusive. Studies of mental training effects on stress-related biomarkers predominantly focus on the secretion of the HPA axis output hormone cortisol, either in response to acute stress or during basal activity, measured in blood or saliva. First evidence for reduced cortisol output after psychosocial stress induction was found immediately after a single mindfulness-based meditation session preceded by 5 days of practice (11). In comparing different practice types, we identified reduced cortisol secretion in response to an acute psychosocial laboratory stressor after the 3-month-long training of either socioaffective or sociocognitive practices, but not after the training of present-moment attention and interoception (12). Several other studies of psychosocial stress induction found no effects of mindfulness- or compassion-based training on acute cortisol release (e.g., (13,14); see also (15) for a review). Similarly heterogeneous results emerge at the level of basal HPA axis activity. Reports of lower diurnal cortisol output mainly stem from interventions using the MBSR program, where reductions in the cortisol awakening response and afternoon/evening cortisol levels were found in healthy and diseased individuals (16–18). Again, these findings are contrasted by several null results (19,20).
The aformentioned mixed outcomes do not sufficiently corroborate the hypothesis that reduced HPA axis activity can mediate long-term training-related health benefits in healthy adults (see also (21) for a recent review). Notably, however, although acute and diurnal cortisol indices provide a window to an individual’s long-term cortisol exposure, both bear shortcomings as measures of chronic stress. Cortisol levels collected after acute challenge reflect stress responses in a highly specific setting, and indices of diurnal cortisol measured in saliva, blood, or urine fluctuate considerably from day-to-day (22,23). Because it is the long-term, cumulative HPA axis activation that is particularly maladaptive and related to ill health (4,24), methodological limitations in capturing chronic physiological stress may account for some of the heterogeneity in the contemplative training literature.
The present study aimed to investigate whether contemplative mental training affects patterns of long-term cortisol secretion as a potential mediator of downstream health benefits in 227 healthy adults. Instead of acute or diurnal cortisol secretion, we assessed hair cortisol (HC) and cortisone (HE) levels as indices of long-term physiological stress load. HC and HE concentrations are assumed to capture systemic (i.e., whole body) cortisol exposure and have been linked to the experience of psychosocial stress (25). HC concentration is also positively correlated with diurnal cortisol output (25,26) but less prone to state-related variance, which may allow for a particularly stable prediction of whether mental training has a long-term impact on HPA axis activity. Alongside cortisol, it has been suggested that levels of the inactive cortisol metabolite and precursor molecule cortisone yield a complementary, potentially more stable glucocorticoid signal (27). We thus assayed cortisol and cortisone levels in 3-cm proximal hair segments, corresponding to approximately 3 months of exposure. In light of slowly increasing evidence for hair glucocorticoid levels as indicators of long-term cortisol exposure upon planning of the study in 2011, HC and HE measures were registered as secondary outcomes to this clinical trial. To capture psychological stress load, self-reported chronic stress was measured using the Perceived Stress Scale (PSS; (28)) and the Trier Inventory for Chronic Stress (TICS; (29)).
As an exploratory endpoint, we additionally assayed dehydroepiandrosterone (DHEA) concentration in hair and assessed potential training effects on the ratio of HC relative to DHEA levels (HC/DHEA). The anabolic functions of DHEA complement the metabolic effects of cortisol in a coregulatory framework, in which DHEA buffers the detrimental influences of cortisol signaling through neuroprotective, anti-inflammatory, antioxidative, and antiglucocorticoid effects (30–32). The ratio of cortisol to DHEA levels can be used as an indicator for the balance between anabolic and catabolic processes (33). Although HPA axis dysregulation may be reflected in elevated levels of either hormone, high DHEA levels are generally associated with protective and stress resilience related processes (34–36) and high cortisol/DHEA ratios with psychiatric disorders including depression, posttraumatic stress disorder, and schizophrenia (37–39) or with chronic stress in healthy adults (40). Accordingly, we here explored whether patterns of change in HC/DHEA ratios may mirror HC, which would support the proposition that HC reduction reflects improved regulation of HPA axis activity.
The training regimen of the ReSource Project was designed to disentangle the specific effects of three different types of mental practice. This differentiated approach is especially valuable given the multifaceted nature of many mindfulness-based programs, which typically combine diverse practice types (41). In three separate modules termed Presence, Affect, and Perspective, participants trained attention-, socioemotional-, or sociocognitive-based practices for 3 months each (Figure 1A). Participants were assigned either to one of two 9-month training cohorts that completed all three training modules in partially counterbalaned order (TC1 and TC2), a 3-month Affect-only training cohort (TC3) or a retest control cohort (RCC; Figure 1B). During each module, participants completed a standardized training routine involving weekly 2-hour group sessions and daily practice of core exercises.
FIGURE 1.
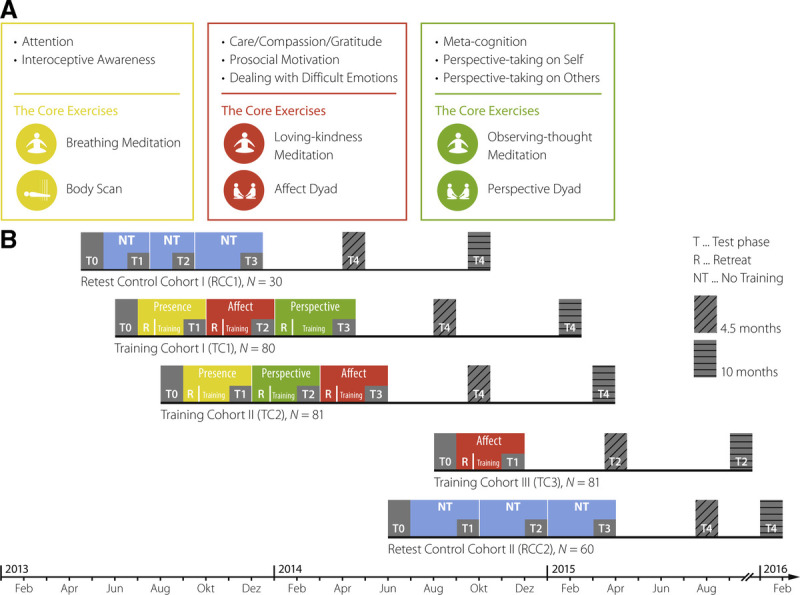
Study protocol and design. A, Core processes and practices of the ReSource training. The Presence module aims to train attention and interoceptive body awareness; its two core practices are breathing meditation and body scan. The Affect module targets social emotions such as compassion, loving kindness, and gratitude; core practices are loving-kindness meditation and the Affect dyad. In the Perspective module, metacognition and perspective taking on the self and others are trained through the core practices observing-thoughts meditation and Perspective dyad. B, Design and timeline of the Resource Project. Two training cohorts, TC1 and TC2, started their training with the mindful attention–based Presence module. They then underwent the social Affect and Perspective modules in different orders. The total training time for TC1 and TC2 was 39 weeks (13 weeks per module). TC3 only trained the Affect module for 13 weeks and the two RCC underwent all testing but no training (for more detailed information, see (42,43)). Figure reproduced and adapted from (8,44). RCC = retest control cohort; TC1–3, training cohorts 1–3. Color image is available online only at www.psychosomaticmedicine.org.
Previous studies investigated the potential effects of mindfulness-based training on HC after 7 to 10 weeks of group training (45–48) and 12 weeks of online interventions (49,50). Among these, only one pilot study with 18 at-risk participants detected decreased HC levels (45). Extending on theses preliminary findings, the large-scale ReSource Project can produce conclusive results about the more longitudinal effects of a 9-month-long intervention, as well as potential differential outcomes of distinct types of contemplative practice. In light of the aforementioned evidence for changes in diurnal cortisol after mindfulness-based training (16–18), we primarily expected to find decreased HC and HE levels after the attention-based Presence module, which included classic mindfulness practices that are also central to the MBSR program. We hypothesized that training-related reduction would be observable relative to the study baseline and to the RCC. Because basal and stress-induced cortisol levels are not reliably associated (51), it remained an open question whether the acute stress-reducing properties of the social Affect and Perspective modules identified in our previous study (12) would translate to reduced cortisol levels in hair. Finally, we expected a decrease in self-reported long-term stress in parallel to change in physiological stress load, aligning also with consistent reports of stress reduction after mindfulness-based training (7) and, to a lesser extent, after compassion-based training (52).
MATERIALS AND METHODS
Participants
All participants underwent comprehensive face-to-face mental health diagnostic interviews with a trained clinical psychologist and completed additional mental health questionnaires. Volunteers were excluded if they fulfilled the criteria for an Axis I disorder within the past 2 years, or for schizophrenia, psychotic disorder, bipolar disorder, substance dependency, or any Axis II disorder at any time in their life. Volunteers who had prior meditation experience or were taking medication known to influence the HPA axis were also excluded (for further details on the screening procedure, see (53)). The ReSource Project was registered with the Protocol Registration System of ClinicalTrial.gov (Identifier NCT01833104) and approved by the Research Ethics Boards of Leipzig University (ethic number: 376/12-ff) and Humboldt University Berlin (ethic numbers: 2013-20, 2013-29, 2014-10). The study was conducted in accordance with the Declaration of Helsinki. Participants gave written informed consent, could withdraw from the study at any time and were financially compensated.
To avoid straining participants through excessive testing in the context of the multimeasure ReSource Project, sampling of hair was presented to participants as an optional rather than a core testing procedure, leading to lower adherence rates. Of 332 initial ReSource participants (197 women; mean [SD] age = 40.74 [9.24] years; age range 20–55 years), 217 provided hair samples at baseline (T0), of which 179 could be reassayed for the present change analysis; 157 provided samples at T1, 136 at T2, and 150 at T3 (see Figure 2 and Supplemental Material, Tables S1–S3 for sample sizes of all measures per cohort and reasons for missing cases, http://links.lww.com/PSYMED/A759). Twenty-four participants (18 women) were light smokers (≤10 cigarettes/d; mean [SD]: 16.01 [16.09] cigarettes/wk).
FIGURE 2.
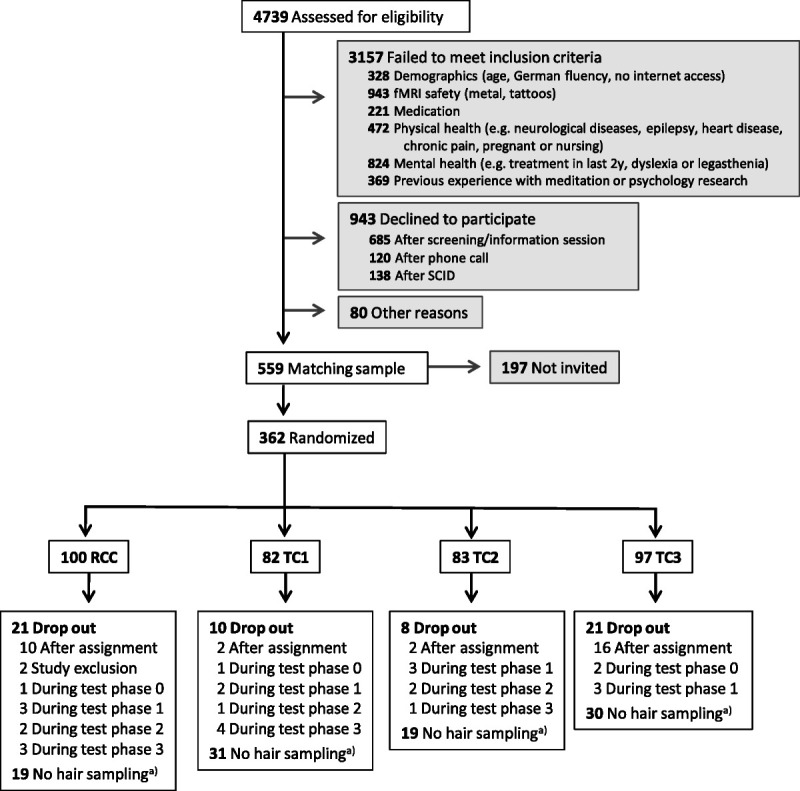
Participant flowchart for the analysis of HC and HE. This figure combines numbers from two recruitment periods in 2012/2013 and 2013/2014. Adapted from (8). Further details on the sex distribution in dropouts and final analysis samples are shown in the Supplemental Material, Table S2, http://links.lww.com/PSYMED/A759. HC = hair cortisol; HE = hair cortisone; fMRI = functional magnetic resonance imaging; SCID = Structural Clinical Interview for DSM-IV Disorders (Axis I and Axis II); RCC = retest control cohort; TC = training cohort. a) Reasons for no hair sampling throughout were baldness or opting-out.
Training Program
The ReSource Project examined the specific effects of three commonly practiced types of mental training, namely attention-, socioemotional-, or sociocognitive-based techniques. For this purpose, the training program was parceled into three separate modules (Presence, Affect, and Perspective), each of which cultivated distinct contemplative capacities for 3 months (Figure 1A; (8)). Every module began with a 3-day retreat during which professional teachers introduced participants to the conceptual core and the relevant practices of a given module. Afterward, participants attended weekly 2-hour group sessions and were asked to exercise the respective module’s two core practices for 30 minutes daily on 5 days per week using a tailor-made app and online platform.
The psychological processes targeted in the Presence module are attention and interoceptive awareness. Its core practices are breathing meditation and body scan, both of which are classical mindfulness-based exercises also implemented in the MBSR program. The Affect module targets social emotions such as compassion, loving kindness, and gratitude and aims to enhance prosocial motivation and dealing with difficult emotions. These skills are targeted through the core practices loving-kindness meditation, which is also featured in MBSR-type programs, and the novel Affect dyad. Together, the Presence and Affect modules target and disentangle the two main components of the MBSR program. In the Perspective module, participants train metacognition and perspective taking on the self and others through the core practices observing-thoughts meditation and Perspective dyad.
The two contemplative dyads are partner exercises that were developed for the ReSource training (54). They address different skills such as perspective taking on the self and others (perspective dyad) or gratitude, acceptance of difficult emotions, and empathic listening (affect dyad), but are similar in structure (for details, see also (8)). In each 10-minute dyadic practice, two randomly paired participants share their experiences with alternating roles of speaker and listener, using the app or telephone to connect to their dyad partner. The dyadic format is designed to foster interconnectedness by providing opportunities for self-disclosure and nonjudgmental listening (8,54).
The distinction between Affect and Perspective modules reflects research identifying distinct neural routes to social understanding: one socioaffective route including emotions such as empathy and compassion, and one sociocognitive route including the capacity to mentalize and take perspective on the self and others (55,56).
Study Design
Participants were assigned either to one of two 9-month training cohorts that completed all three training modules in partially counterbalanced order (TC1, initial n = 80, n for the present study = 48; and TC2, initial n = 81, present n = 62), a 3-month Affect-only training cohort (TC3, initial n = 81, present n = 49) or a RCC (initial n = 90, present n = 68; Figure 1B; (54)). Cohort assignment was completed using bootstrapping without replacement to ensure the formation of demographically homogeneous groups. TC1 and TC2 began their training with the attention-based Presence module. Subsequently, they underwent Affect and Perspective training in different orders, thus controlling for sequence effects. TC3 was conducted to isolate the specific effects of the Presence module from the Affect module. The study followed a mixed design, in which most, but not all, participants received all types of training. Training and data collection took place between April 2013 and February 2016.
Assay of Steroid Hormone Concentration in Hair
HC and HE concentrations are indicative of systemic cortisol exposure and markers of chronic stress (25). Levels of the inactive cortisol metabolite and precursor molecule cortisone have been suggested to yield a complementary, potentially more stable glucocorticoid signal alongside cortisol itself (27). Although the precise mechanism behind hormone accumulation in hair is incompletely understood, it is assumed that during hair growth, free hormone molecules are continuously incorporated into follicles, proportional to their overall concentration in the body. HC and HE concentrations in a 1-cm hair segment are thus assumed to indicate the cumulative systemic cortisol or cortisone exposure over an approximately 1-month period (25). The same applies to accumulation of DHEA in hair, which we assayed in an exploratory approach.
For their assessment, hair strands were taken as close as possible to the scalp from a posterior vertex position at T0 and after each training module (at T1, T2, and T3). Hair samples were wrapped in aluminum foil and stored in the dark at room temperature until assay at the Department of Psychology, TU Dresden, Germany. Based on the assumption of an average hair growth rate of 1 cm/month (57), we analyzed the proximal 3-cm segment of hair to assess accumulation of cortisol, cortisone, and DHEA over each 3-month period. Hormone concentrations were measured using liquid chromatography–tandem mass spectrometry, the current criterion standard approach for hair steroid analysis (58), following our previously published protocol with a limit of quantification for cortisol and cortisone less than 0.09 pg/mg and intra- and interassay coefficients of variance between 3.7% and 8.8% (59). All hormone concentrations were reported in picograms per milligram.
A first assay of samples collected at baseline was conducted in 2015, allowing researchers to address cross-sectional research questions (26) before termination of the longitudinal data collection. Thirty-eight samples were used up in this analysis. For the current longitudinal research aim, the remaining baseline samples were reassayed jointly with all additional samples (assessed at T1, T2, and T3) to avoid potential systematic effects of storage time and minimize reagent batch effects. Specifically, all samples of one participant were always run with the same reagent batch to avoid intraindividual variance due to batch effects.
Subjective Stress Measures
Self-reported chronic stress was measured using the summary score of the PSS (28) and the global stress score of the TICS (29). The 10-item PSS is the most widely used psychological instrument for measuring the perception of stress. It focuses on the degree to which situations in the past month are appraised as unpredictable, uncontrollable, and overloaded, and produces one summary stress score. The 39-item TICS captures a time span of 1 to 3 months and measures six aspects of chronic stress (work overload, worries, social stress, lack of social recognition, work discontent, and intrusive memories) and one global stress score. Both questionnaires have satisfactory reliability and validity (28,29).
Measures of Training Engagement
To examine causes of individual variability in training effects, we assessed two measures of training engagement: practice frequency, objectively traced via our online training platform, and self-reported liking of the different training modules. Details on the measurement and analysis of both metrics are provided in the Supplementary Methods, http://links.lww.com/PSYMED/A759. Practice frequency is a particularly interesting metric as it provides insights into the impact of training compliance and dosage.
Statistical Analysis
Data Processing
Raw HC and HE data were each treated with a natural log transformation to remedy skewed distributions. Ratios of cortisol to DHEA (HC/DHEA) as an exploratory outcome were computed by dividing raw HC measures by raw DHEA measures, and subsequently also treated with a natural log transformation. Across the full sample of each dependent measure, any values diverging more than 3 SD from the mean were labeled outliers and winsorized to the respective upper or lower 3 SD boundary to avoid influential cases. In previous ReSource publications, data have been analyzed as change scores (e.g., (60)). However, change scores can only be computed if a set of consecutive measures is available. Because the number of missing HC and HE samples (Table 1) was larger than for other variables assessed in the ReSource Project we chose to analyze the data as simple scores to be able to use all available samples.
TABLE 1.
Raw Data and Demographic Characteristics of Samples
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| HC (pg/mg), mean (SD) | 7.46 (8.97) | 5.81 (6.85) | 4.59 (5.18) | 4.66 (3.51) |
| HE (pg/mg), mean (SD) | 11.6 (8.84) | 9.89 (8.52) | 9.03 (6.32) | 9.59 (6.84) |
| HC/DHEA (ratio), mean (SD) | 7.85 (13.21) | 6.07 (7.83) | 4.77 (5.28) | 5.49 (6.27) |
| PSS (summary score), mean (SD) | 14.1 (5.9) | 13.4 (5.9) | 13.2 (6.0) | 12.5 (6.1) |
| TICS (global stress score), mean (SD) | 15.0 (6.9) | 13.8 (7.4) | 13.8 (7.8) | 13.1 (7.7) |
| HC/HE sample | ||||
| n (% female) [comparison to sample without HC/HE measures] | 177 (68.4) [χ2 = 26.5, df = 1, p < .001] | 155 (63.2) [χ2 = 3.35, df = 1; p = .07] | 131 (59.5) [χ2 = 0.08, df = 1, p > .5] | 146 (64.4) [χ2 = 9.8, df = 1, p = .002] |
| Age (yrs), mean (SD)[comparison to sample without HC/HE measures] | 39.6 (9.35) [t(330) = 2.42, p = .016] | 39.1 (9.49) [t(330) = 3.13, p = .002] | 39.4 (9.71) [t(249) = 2.64, p = .009] | 39.7 (9.74) [t(249) = 2.39, p = .018] |
| Smoker, n (%) | 21 (11.9) | 12 (7.7) | 11 (8.4) | 15 (10.3) |
| PSS/TICS sample | ||||
| n (% female) | 322 (59.6) | 311 (59.5) | 233 (59.2) | 226 (58.4) |
| Age (yrs), mean (SD) | 40.7 (9.22) | 40.7 (9.27) | 40.6 (9.35) | 40.6 (9.45) |
| Smoker, n (%) | 38 (11.8) | 37 (11.9) | 30 (12.9) | 28 (12.4) |
| HC/DHEA subsample | ||||
| n (% female) | 143 (65.0) | 126 (59.5) | 108 (53.7) | 121 (62.0) |
| Age (yrs), mean (SD) | 39.4 (9.30) | 38.4 (9.53) | 38.3 (9.91) | 39.1 (9.81) |
| Smoker, n (%) | 18 (12.6) | 9 (7.1) | 9 (8.3) | 12 (9.9) |
HC = hair cortisol; HE = hair cortisone; HC/DHEA = hair cortisol to dehydroepiandrosterone ratio; PSS = Perceived Stress Scale; TICS = Trier Inventory for Chronic Stress; T0–3, time points 0–3; SD = standard deviation.
“HC/HE sample” refers to participants with at least one usable sample of either HC or HE at the given time point; “TICS/PSS sample” refers to participants with at least one self-report rating; “HC/DHEA subsample” refers to the subsample of participants with both HC and DHEA data. More older men than women had short hair or were bald, presumably leading to the higher percent of women in the HC/HE sample. Statistical analysis confirmed that participants providing hair samples were younger and more female compared with those who did not; however, they did not differ on PSS or TICS scores at any time point. For further details on the demographic characteristics of the sample, see (53). Baseline associations are described in the Supplemental Material, Supplementary Results A, http://links.lww.com/PSYMED/A759.
Significance Testing
All statistical analyses were conducted in the statistical software R (version 3.5.1, (61)) and with an α threshold of ≤.05. Hypotheses were tested by means of multivariate linear mixed models (LMMs), which are robust to unbalanced and incomplete data in longitudinal designs. Models were fit using the function “lmer” of the r package “lme4” (62). In models predicting HC or HE, age and sex were included as covariates to account for their potential influence on hormone concentrations (25). The full model included the following terms:
where DV = dependent variable (HC, HE, or subjective stress scores assessed via PSS and TICS), ß0 = intercept, i = subject ID, j = measurement time point (T0, T1, T2, T3), and rand(ID) = random intercept per subject.
In an omnibus test, we first evaluated whether the respective dependent variable differed as a function of training routine or of time, by testing for an interaction of training by time. Full models with the aforementioned terms were compared with reduced models lacking the interaction term via likelihood ratio tests (63). If TCs differed from the RCC over time, the interaction model provided a significantly better fit. To ensure accurate model comparisons, models were fit with the maximum likelihood method. Effect sizes of significant interactions were calculated as omega squared (ω2) by dividing the variance of the residuals of the full model by the variance of the residuals of the reduced model and subtracting the outcome from 1 (64). The resulting effect sizes were classified as small (ω2 ≥ 0.010), medium (ω2 ≥ 0.059), or large (ω2 ≥ 0.138) (65). Given a significantly better fit of an interaction model, potential differences between training modules and individual measurement time points were evaluated in detail by contrasting model estimates through follow-up t-tests, computed through the function “lsmeans” of the package “lsmeans” (66). To this end, models were refit with the restricted maximum likelihood method to obtain unbiased model estimates. Follow-up contrasts were thus conducted within the LMM framework and not corrected for multiple comparisons. To assess the general efficacy of a training module, measures of stress-load following that module were compared within subjects to the pretraining baseline, and between subjects to the same testing interval in the RCC (3, 6, or 9 months into the study). Within-subject contrasts provide a particularly sensitive assessment of change while controlling for implicit covariates, whereas between-subject comparisons are crucial to evaluate training-related change in measures with potential retest effects, which in the present study applies to the self-report measures. Assessment of differential training effects was conducted following the same procedure but within and across training cohorts. The results of model residual checks are reported in the Supplemental Material (Supplementary Results B, http://links.lww.com/PSYMED/A759).
Power Analysis
Because the present study is part of a large-scale investigation (the ReSource Project) with numerous subprojects, the sample sizes of the cohorts could not be tailored to this study. To determine whether the analyses planned here were sufficiently powered to be meaningful, we used the function “powerSim” from the package simr (67) to simulate what effect sizes they were sensitive to, given our sample size. Power analyses were based on 1000 runs and conducted according to our hypotheses, meaning effects were simulated after Presence, Affect, and/or Perspective modules. Depending on the exact pattern of effects, sufficient power (80% or more) was given to detect a minimum of 19% to 34% change in HC, 11% to 22% in HE, 11% to 21% in PSS, and 13% to 21% in TICS as a function of training (Supplemental Material, Table S4, http://links.lww.com/PSYMED/A759). While there are no previous studies that may serve as guidelines for reasonable effect sizes regarding HE or HC reduction after mental training, we had previously detected large relative decreases in acute cortisol reactivity of 32% to 59% following the same training as used here (12). Our analyses were adequately powered to detect effects even at the lower end of that spectrum.
Baseline-Matched Analysis
In randomized clinical trials, baseline differences are by definition the product of chance rather than representing a latent confound (68). It is, however, possible that participants with higher baseline values are disproportionally assigned to the training cohorts by chance, leading to an overestimation of training effects through conflation with regression to the mean. Following up on our planned analyses, we examined to what extent such a pattern may have influenced study outcomes. To this end, we selected a subsample of participants with matched baseline characteristics and tested whether our results would hold in these data. Similar to clinical studies in which patients are matched to control participants based on their baseline characteristics, we here matched TC participants to RCC participants with respect to their baseline glucocorticoid levels and sex, using the function ‘matchit’ of the R package “Match.It” with replacement (69). Each TC was matched separately, with the respective cohort serving as the subject pool from which participants could be drawn multiple times. Participant samples were not artificially duplicated in this process, but instead, the relative matching frequency of each participant was recorded as a weight (higher weights representing multiple matching). Weights for RCC participants were set to 1. Unmatched participants were excluded from the analysis; participants who had missing samples at baseline but provided data at a later time point were included. The main analysis of HC and HE was then repeated in this generated sample with the addition of a weighting parameter based on the frequency of matching.
RESULTS
Of 332 participants recruited for the ReSource Project, 227 provided samples of HC or HE, and 326 provided subjective stress ratings at one or more of the four measurement time points (see Table 1 for samples and demographic characteristics; Figure 2 and Supplemental Material, Table S1 for sample size and reasons for missingness, http://links.lww.com/PSYMED/A759). Participants providing hair samples were more likely to be young and female than those who did not (see Table 1 for comparisons by time point), and a χ2 test of equivalence of distributions indicated that, compared with the full ReSource sample, there were marginally more women in the HC/HE subsample (χ2 = 3.74, df = 1, p = .053). Baseline associations between dependent variables and covariates are described in Supplementary Results A, http://links.lww.com/PSYMED/A759.
Over the 9 months of training, HC and HE levels showed high consistency in their pattern of change (Figure 3). A significant cohort by time interaction was detected for both HC (χ2 = 30.87, df = 7, p < .001, ω2 = 0.104) and HE (χ2 = 19.14, df = 7, p = .008, ω2 = 0.036). Follow-up contrasts (Tables S5, S6, http://links.lww.com/PSYMED/A759) showed that HC and HE levels remained stable in the no-training RCC. With mental training, HC and HE levels decreased steadily until 6 months into the training regimen, regardless of practice content (Figure 3). After three (TC3 and TC2) to 6 months (TC1), hair glucocorticoid levels in all training cohorts were significantly reduced compared with the respective pretraining baseline. HC concentrations at 6 months and HE concentrations at 3 to 6 months were also lower in the TCs than in the no-training RCC at the corresponding time points. Only HE in TC2 never dropped below the corresponding RCC level. At the final 9-month measurement, HC and HE levels stabilized at this lowered level or regressed slightly toward baseline, but always remained significantly below baseline. Change in HC but not HE concentration was significantly and negatively associated with practice frequency (χ2 = 4.46, p = .035, beta estimate (est.) [SE] = −0.140 [0.066], ω2 = 0.025), suggesting that greater training compliance led to stronger HC reduction. Neither HC nor HE change were associated with self-reported liking of the modules.
FIGURE 3.
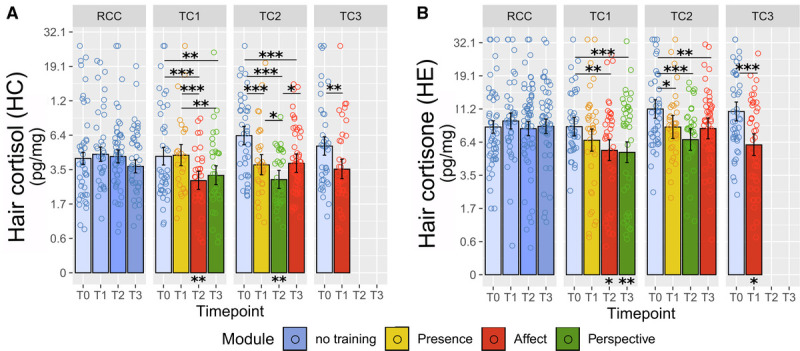
Training effects on HC and HE. Estimated HC (A) and HE (B) levels were derived from the linear mixed-model analysis as a function of training cohort and time point. Note the natural log scale on the y-axis. Error bars represent ±1 SE; each circle represents one raw data point with outliers winsorized as described in the Methods section. Asterisks below bars indicate comparison with the RCC at the matched time point. *Significant at p ≤ .05. **Significant at p ≤ .01. ***Significant at p ≤ .001. See Tables S5 and S6, http://links.lww.com/PSYMED/A759 for a full list of contrast outcomes. HC = hair cortisol; HE, hair cortisone; SE, standard error; RCC, retest control cohort; TC, training cohort. Color image is available online only at www.psychosomaticmedicine.org.
Visualization in Figure 3 suggests that HC and HE baseline (T0) values differed somewhat across cohorts, with TC2 and TC3 displaying numerically higher values than TC1 and RCC. In randomized controlled trials, testing for significance of baseline differences is redundant because random subject assignment ensures that any observed baseline differences must arise by chance (68). Instead, an illustrative baseline-matched weighted LMM analysis suggested that results would be comparable in a sample with matched baseline levels (Figure 4; omnibus test HC: χ2 = 13.4, df = 7, p = .062, ω2 = 0.082; omnibus test HE: χ2 = 13.11, df = 7, p = .069, ω2 = 0.032). Baseline-matched post hoc contrasts revealed a similar pattern as in the main analysis (Figure 4). Reduced overall significance indicates a potential overestimation of training effects due to skewed baselines. Notably, however, omnibus effect sizes remained comparable with the main results (HC, main ω2 = 0.104, matched: ω2 = 0.082; HE, main: ω2 = 0.036, matched: ω2 = 0.032. This suggests that the pattern of lower significance may be partially attributable to the reduced sample size of the baseline-matched analysis, in which several TC participants were excluded because of their relatively higher hormone levels at baseline.
FIGURE 4.
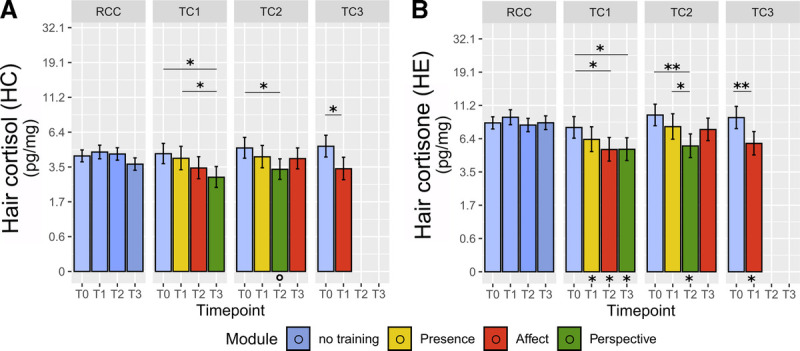
Training effects on HC and HE in baseline-matched analysis. Estimated HC (A) and HE (B) levels were derived from LMM analysis in a sample of participants with matched baseline HC and HE levels across cohorts, generated based on the study participant pool. Participants from each TC were matched to RCC participants with replacement depending on their baseline glucocorticoid levels and sex. Note the natural log scale on the y-axis. Error bars represent ±1 SE. Asterisks below bars indicate comparison with RCC at the matched time point. *Significant at p ≤ .05. **Significant at p ≤ .01. ***Significant at p ≤ .001. HC = hair cortisol; HE = hair cortisone; SE = standard error; RCC = retest control cohort; TC = training cohort. Color image is available online only at www.psychosomaticmedicine.org.
In another analysis of potential bias, baseline HC and HE levels did not differ between TC participants who dropped out from hair sampling throughout the study and those who did not (HC: t(112) = 0.5, p = .62; HE: t(125) = −0.7, p = .49), demonstrating that there was no selective dropout.
As an exploratory outcome, potential effects of training on HC/DHEA ratios were evaluated using the same statistical approach as for the main analyses. Full to reduced model comparison showed a significant effect of the cohort by time interaction term (χ2 = 23.17, df = 7, p = .002, ω2 = 0.080). Like the pattern observed for HC and HE, HC/DHEA ratios seemed stable in the RCC and showed decreases in TC2 and TC3 (Figure 5). HC/DHEA ratios of TC1, however, did not decrease. Results of post hoc comparisons are shown in Figure 5 and the Supplemental Material, Table S7, http://links.lww.com/PSYMED/A759. Notably, a follow-up analysis of log-transformed and winzorized DHEA values independent of HC showed no significant change as a function of training (χ2 = 9.10, df = 7, p > .2), suggesting that the observed change in HC/DHEA ratios may be predominantly driven by training effects on HC levels.
FIGURE 5.
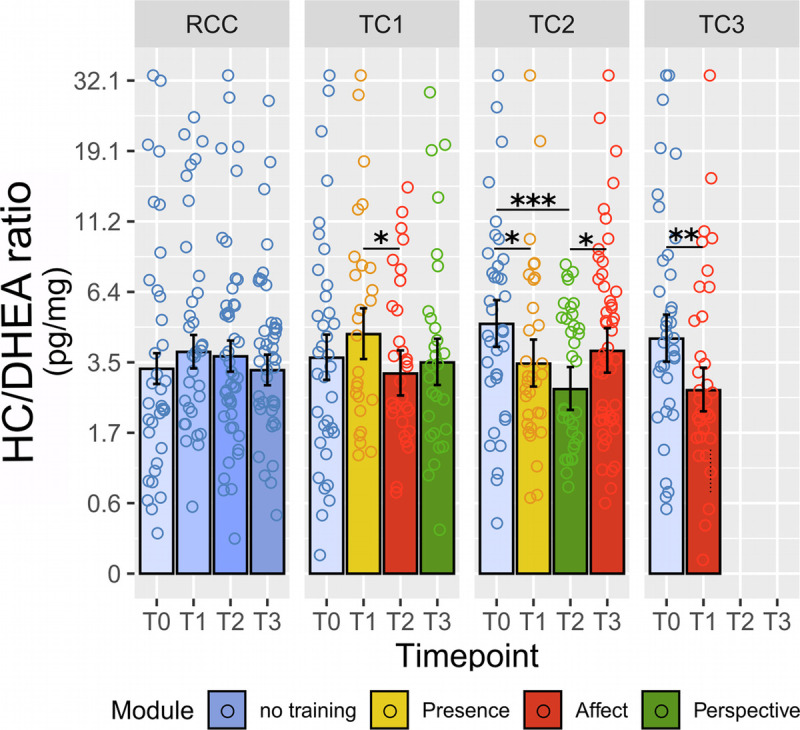
Training effects on HC/DHEA ratios in hair. Estimated HC/DHEA ratios were derived from the linear mixed-model analysis as a function of training cohort and time point. Note the natural log scale on the y-axis. Error bars represent ±1 SE; each circle represents one raw data point with outliers winsorized as described in the Methods section. Asterisks below bars indicate comparison with RCC at the matched time point. *Significant at p ≤ .05. **Significant at p ≤ .01. ***Significant at p ≤ .001. See Supplemental Material, Table S7, http://links.lww.com/PSYMED/A759 for a full list of contrast outcomes. HC = hair cortisol; DHEA = dehydroepiandrosterone; SE = standard error; RCC = retest control cohort; TC = training cohort. Color image is available online only at www.psychosomaticmedicine.org.
In the analysis of subjective-psychological stress reduction, the cohort by time interaction was significant for PSS (χ2 = 22.20, df = 7, p = .002, ω2 = 0.030), but only marginal for TICS values (χ2 = 13.66, df = 7, p = .058, ω2 = 0.018; Figure 6). Follow-up contrasts of PSS scores suggested that participants reported lowest subjective stress experience following the Perspective module, but only in TC1. Exploratory LMM analyses showed no significant association of PSS or TICS scores with HC or HE concentrations throughout the study.
FIGURE 6.
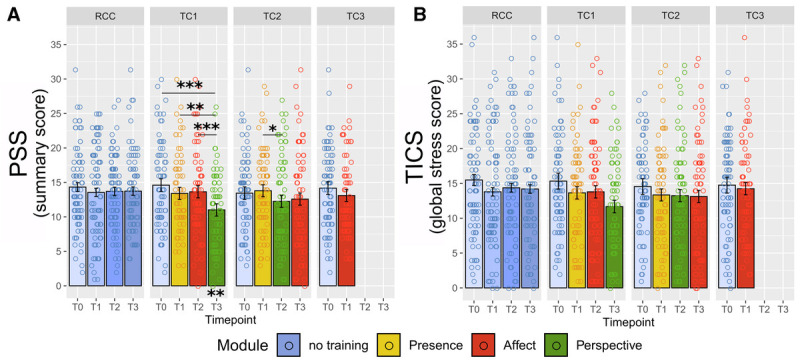
Training effects on self-reported long-term stress. Estimated scores of (A) Perceived Stress Scale (PSS; (28)) and (B) Trier Inventory for Chronic Stress (TICS; (29)) derived from the linear mixed-model analysis as a function of training cohort and time point. Error bars represent ±1 SE; each circle represents one data point. *Significant at p ≤ .05. **Significant at p ≤ .01. ***Significant at p ≤ .001. Color image is available online only at www.psychosomaticmedicine.org.
Similar to HC, PSS change was negatively associated with practice frequency (χ2 = 4.99, p = .025, est. = −0.591 [0.264], ω2 = 0.010) and additionally with liking of the modules (χ2 = 9.34, p = .002, est. = −0.975 [0.318], ω2 = 0.019; see also Supplementary Methods, http://links.lww.com/PSYMED/A759). However, the association with practice frequency disappeared when controlling for module liking, suggesting that module enjoyment was the latent driver of the practice association. The effect of liking contrarily persisted even when controlling for practice frequency (χ2 = 6.07, p = .014, est. = −0.815 [0.330], ω2 = 0.012). Considering that change in HC was not associated with self-reported liking, subjective measures of stress and training engagement seem to cluster, perhaps reflecting the lack of psychoendocrine covariance that is commonly reported in the stress literature (26,70).
DISCUSSION
The present investigation examined whether up to 9-month-long training of different types of contemplative mental practice affects physiological indices of chronic stress. Our results show that daily mental training for 3 to 6 months can buffer the long-term systemic stress load of healthy adults. This was reflected in a reduction of cortisol (HC) and cortisone (HE) accumulation in hair, while levels of self-reported chronic stress were less consistently decreased. Effects on HC and HE were independent of specific training content, positively associated with practice frequency for HC, and reached a ceiling after 6 months of training. It equally took 6 months until significant differences to baseline were achieved in all training cohorts, suggesting that reliable long-term benefits for HPA axis activity emerge only after a relatively long period of intense training. This may explain why previous studies found no HC reduction after the typical 8 to 12 weeks of mindfulness-based training (21,47,48). Exploration of HC/DHEA ratios revealed a similar, albeit less consistent pattern of change. Because DHEA alone did not change as a function of training, effects on HC/DHEA ratios were likely driven by change in HC. These results provide supporting evidence that the training specifically affected glucocorticoid steroid hormones.
In an earlier ReSource Project publication with the same participant sample (12), we found that Affect and Perspective training selectively reduced acute salivary cortisol release in response to a stressful psychosocial laboratory challenge, the Trier Social Stress Test (71). The diverging pattern of results between indices of acute compared with chronic HPA axis activity suggests that distinct processes may underlie change in either type of activity. It is conceivable that stress “immunization” to a psychosocial challenge is best achieved with a training that targets social processes, such as the dyadic partner exercises implemented in the Affect and Perspective modules. In contrast, the cumulative HPA axis load as monitored in hair may reflect the more low-grade and continuous strain inherent to various daily hassles (72–74), which seems to be equally buffered by all three mental training techniques. Even though, in the ReSource Project, we find differential training effects of the three realized practice types on many levels of observation (9), some changes seemingly need time to develop, irrespective of practice type (see (75) also).
Changes in self-reported measures of chronic stress were unrelated to changes in HC and HE concentration. This lack of psychoendocrine covariance is a recurring phenomenon in stress research (26,70) and may be particularly emphasized through biases in retrospective self-assessments (76). Moreover, a substantial proportion of variance in hair glucocorticoids is attributable to other variables than subjective stress, such as an individuals’ general propensity to release glucocorticoids (77), as is the case for most physiological correlates of stress. Although psychoendocrine covariance can generally be improved with time-sensitive analysis techniques (78), physiological and self-report measures in the present study were both retrospective in nature, precluding such an approach. More generally, the fact that integrative markers like HC do not capture time-sensitive dynamics may contribute to the overall observed pattern of poor correspondence with subjective stress indices (25,79) despite relatively consistent reports of elevated HC in highly stressed or burdened groups (25,80–82). As a promising remedy, one recent study was able to predict HC in healthy adults through a combination of more objective self-report data, namely, counts of daily hassles, and statistical modeling of latent time courses in subjective stress (77).
Although we expected to see a training-related decrease in subjective stress, perhaps even exaggerated through biases, change in self-report measures was inconsistent and did not show the robust reductions reported in previous studies (7). The discrepancy between TC1 and TC2 in particular suggests that the specific pattern of change in this study should not be overinterpreted. In general, is possible that participants experienced the uniquely large-scale testing of the ReSource Project as straining, leading to our diverging results. To this effect, we previously found that the realized training practices can also be experienced as effortful (83).
Despite our large sample size, the number of dropouts from the hair glucocorticoid assessment—partly attributable to the optional nature of this assessment—is a limitation of the current work. Importantly, however, its negative impact on within-cohort comparisons is limited because participants dropped out already at baseline and subsequent dropouts were unrelated to participants’ HC and HE levels. Nonetheless, results should be interpreted in the context of the specific subsamples, given that participants providing hair samples were systematically younger and more female than those who did not, presumably because older men were more likely to have short hair or be bald. For future studies and the interpretation of this work, it should also be noted that cumulative indices of HPA axis regulation like HC and HE do not allow for specific conclusions about the physiological mechanisms leading to cortisol or cortisone levels in hair. Changes in diurnal cortisol dynamics, and cortisol release under acute stress or under more low-level strain may all contribute to lower HC or HE levels. Crucially, the influence of cortisol release during eustress, such as exercise, on HC and HE also remains poorly understood (84). Future studies will need to develop time-sensitive models of how psychosocial stress and different forms of daily cortisol secretion relate to glucocorticoid accumulation in hair.
In sum, the present investigation provides evidence that mental training has a beneficial effect on individuals’ long-term physiological stress load, irrespective of specific practice type. With HC and HE concentration, we targeted the cumulative burden of frequent HPA axis activation, which is particularly maladaptive and related to ill health. Our results thus point to one mechanism via which mental training can exert positive effects on practitioners’ health status in general: by lowering systemic cortisol exposure, regular practice of about 30 minutes daily for 3 to 6 months may reduce vulnerability to stress-associated disease. We conclude that to achieve chronic stress reduction at the level of HPA axis activation, it is worth to practice more and to carry on mental practice beyond the typical 8-week training period of mindfulness-based stress reduction programs currently offered in Western societies.
Supplementary Material
Acknowledgments
This study forms part of the ReSource Project headed by Tania Singer. Data for this project were collected between 2013 and 2016 at the former Department of Social Neuroscience at the Max Planck Institute for Human Cognitive and Brain Sciences (MPI CBS), Leipzig, Germany. We are thankful to the members of the former Social Neuroscience Department involved in the ReSource Project over many years, in particular to Astrid Ackermann, Christina Bochow, Matthias Bolz, and Sandra Zurborg for managing the large-scale longitudinal study; to Elisabeth Murzik, Nadine Otto, Sylvia Tydecks, and Kerstin Träger for help with recruiting and data archiving; to Henrik Grunert for technical assistance; and to Hannes Niederhausen and Torsten Kästner for data management.
Source of Funding and Conflicts of Interest: Dr. Singer, as the principal investigator, received funding for the ReSource Project from the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013; ERC grant agreement number 205557) and from the Max Planck Society. The authors declare that they have no competing interests. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Availability: The present work is based on personal and sensitive physiological data that could be matched to individuals. Participants did not consent to data sharing with parties outside the MPI CBS, such that in line with the General Data Protection Regulation, data cannot be made publicly available. Data are available upon reasonable request (contact via puhlmann@cbs.mpg.de).
Author Contributions: T.S. initiated and developed the ReSource Project and secured all funding except for the hair glucocorticoid analysis. T.S. and V.E. designed the experiment. V.E., L.M.C.P., P.V., and R.L. were involved in data curation. L.M.C.P. and P.V. analyzed the data. C.K. funded the hair analyses. C.K. and T.S. were responsible for planning, performing, and interpreting the hair glucocorticoid analysis. V.E. and L.M.C.P. drafted the manuscript, and all authors critically revised the manuscript.
L.M.C.P. and PV. share the first authorship, and V.E. and T.S. share the last authorship.
Footnotes
Supplemental Digital Content
Contributor Information
Pascal Vrtička, Email: p.vrticka@essex.ac.uk.
Roman Linz, Email: linz@cbs.mpg.de.
Tobias Stalder, Email: Tobias.Stalder@psychologie.uni-siegen.de.
Clemens Kirschbaum, Email: clemens.kirschbaum@tu-dresden.de.
Veronika Engert, Email: veronika.engert@med.uni-jena.de.
Tania Singer, Email: singer@social.mpg.de.
REFERENCES
- 1.Habib SH, Saha S. Burden of non-communicable disease: global overview. Diabetes Metab Syndr Clin Res Rev 2010;4:41–7. [Google Scholar]
- 2.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Al E. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosch PJ. The quandry of job stress compensation. Heal Stress 2001;3:1–4. [Google Scholar]
- 4.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009;5:374–81. [DOI] [PubMed] [Google Scholar]
- 5.Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Every-Day Life. New York, NY: Hyperion; 1994. [Google Scholar]
- 6.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 7.Khoury B, Sharma M, Rush SE, Fournier C. Mindfulness-based stress reduction for healthy individuals: a meta-analysis. J Psychosom Res 2015;78:519–28. [DOI] [PubMed] [Google Scholar]
- 8.Singer T, Kok BE, Bornemann B, Zurborg S, Bolz M, Bochow CA. The ReSource Project: Background, Design, Samples, and Measurements. 2nd ed. Leipzig, Germany: Max Planck Institute for Human Cognitive and Brain Sciences; 2016. [Google Scholar]
- 9.Singer T, Engert V. It matters what you practice: differential training effects on subjective experience, behavior, brain and body in the ReSource Project. Curr Opin Psychol 2019;28:151–8. [DOI] [PubMed] [Google Scholar]
- 10.Creswell JD, Lindsay EK. How does mindfulness training affect health? A mindfulness stress buffering account. Curr Dir Psychol Sci 2014;23:401–7. [Google Scholar]
- 11.Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A 2007;104:17152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engert V, Kok BE, Papassotiriou I, Chrousos GP, Singer T. Specific reduction in cortisol stress reactivity after social but not attention-based mental training. Sci Adv 2017;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arch JJ, Brown KW, Dean DJ, Landy LN, Brown KD, Laudenslager ML. Self-compassion training modulates alpha-amylase, heart rate variability, and subjective responses to social evaluative threat in women. Psychoneuroendocrinology 2014;42:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenkranz MA, Davidson RJ, MacCoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 2013;27:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton ML, Helminen EC, Felver JC. A systematic review of mindfulness interventions on psychophysiological responses to acute stress. Mindfulness (N Y) 2020;11:2039–54. [Google Scholar]
- 16.Brand S, Holsboer-Trachsler E, Naranjo JR, Schmidt S. Influence of mindfulness practice on cortisol and sleep in long-term and short-term meditators. Neuropsychobiology 2012;65:109–18. [DOI] [PubMed] [Google Scholar]
- 17.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology 2004;29:448–74. [DOI] [PubMed] [Google Scholar]
- 18.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun 2007;21:1038–49. [DOI] [PubMed] [Google Scholar]
- 19.Sanada K, Montero-Marin J, Alda Díez M, Salas-Valero M, Pérez-Yus MC, Morillo H, Demarzo MM, García-Toro M, García-Campayo J. Effects of mindfulness-based interventions on salivary cortisol in healthy adults: a meta-analytical review. Front Physiol 2016;7:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascoe MC, Thompson DR, Jenkins ZM, Ski CF. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J Psychiatr Res 2017;95:156–78. [DOI] [PubMed] [Google Scholar]
- 21.Koncz A, Demetrovics Z, Takacs ZK. Meditation interventions efficiently reduce cortisol levels of at-risk samples: a meta-analysis. Health Psychol Rev 2020;1–29. [DOI] [PubMed] [Google Scholar]
- 22.Law R, Hucklebridge F, Thorn L, Evans P, Clow A. State variation in the cortisol awakening response. Stress 2013;16:483–92. [DOI] [PubMed] [Google Scholar]
- 23.Ross KM, Murphy MLM, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology 2014;39:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Sci Ment Heal Stress Brain 2000;9:2–18. [DOI] [PubMed] [Google Scholar]
- 25.Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 2017;77:261–74. [DOI] [PubMed] [Google Scholar]
- 26.Engert V, Kok BE, Puhlmann LMC, Stalder T, Kirschbaum C, Apostolakou F, Papanastasopoulou C, Papassotiriou I, Pervanidou P, Chrousos GP, Singer T. Exploring the multidimensional complex systems structure of the stress response and its relation to health and sleep outcomes. Brain Behav Immun 2018;73:390–402. [DOI] [PubMed] [Google Scholar]
- 27.Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, Stark S, Bosch JA, Fischer JE. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab 2013;98:2573–80. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 29.Schulz P, Schlotz W. Trierer Inventar zur Erfassung von Chronischem Stress (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. Diagnostica 1999;45:8–19. [Google Scholar]
- 30.Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem 1994;131:99–104. [DOI] [PubMed] [Google Scholar]
- 31.Lam JCW, Shields GS, Trainor BC, Slavich GM, Yonelinas AP. Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress Health 2019;35:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendörfer RA, Pirke KM, Kirschbaum C. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab 1997;82:2363–7. [DOI] [PubMed] [Google Scholar]
- 33.Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Horm Behav 2017;89:69–85. [DOI] [PubMed] [Google Scholar]
- 34.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 2009;30:65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman N, Glei DA. Sex differences in the relationship between DHEAS and health. Exp Gerontol 2007;42:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert K, Hunter RG, Bartlett AA, Lapp HE, Kent M. In search of optimal resilience ratios: differential influences of neurobehavioral factors contributing to stress-resilience spectra. Front Neuroendocrinol 2020;56:100802. [DOI] [PubMed] [Google Scholar]
- 37.Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol 2004;14:267–73. [DOI] [PubMed] [Google Scholar]
- 38.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand 2006;114:187–93. [DOI] [PubMed] [Google Scholar]
- 39.Young AH, Gallagher P, Porter RJ. Elevation of cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry 2002;159:1237–9. [DOI] [PubMed] [Google Scholar]
- 40.Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. Neuroendocrine and immunological correlates of chronic stress in ‘strictly healthy’ populations. Neuroimmunomodulation 2009;17:9–18. [DOI] [PubMed] [Google Scholar]
- 41.Dahl CJ, Lutz A, Davidson RJ. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cogn Sci 2015;19:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer T, Kok BE, Bornemann B, Zurborg S, Bolz M, Bochow CA. The Resource Protocol. In: The ReSource Project: Background, Design, Samples, and Measurements. 2nd ed. Leipzig, Germany: Max Planck Institute for Human Cognitive and Brain Sciences; 2016:25–30. [Google Scholar]
- 43.Singer T, Kok BE, Bornemann B, Zurborg S, Bolz M, Bochow CA. Design, timeline, and training setting. In: The ReSource Project: Background, Design, Samples, and Measurements. 2nd ed. Leipzig, Germany: Max Planck Institute for Human Cognitive and Brain Sciences; 2016:33–5. [Google Scholar]
- 44.Puhlmann LMC, Valk SL, Engert V, Bernhardt BC, Lin J, Epel ES, Vrticka P, Singer T. Association of short-term change in leukocyte telomere length with cortical thickness and outcomes of mental training among healthy adults: a randomized clinical trial. JAMA Netw Open 2019;2:e199687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, Koren G, Davis JM. Hair cortisol as a biomarker of stress in mindfulness training for smokers. J Altern Complement Med 2014;20:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen P, Dahmen-Zimmer K, Kudielka BM, Schulz A. Effects of karate training versus mindfulness training on emotional well-being and cognitive performance in later life. Res Aging 2017;39:1118–44. [DOI] [PubMed] [Google Scholar]
- 47.Nery SF, Paiva SPC, Vieira ÉL, Barbosa AB, Sant’Anna EM, Casalechi M, Dela Cruz C, Teixeira AL, Reis FM. Mindfulness-based program for stress reduction in infertile women: randomized controlled trial. Stress Health 2019;35:49–58. [DOI] [PubMed] [Google Scholar]
- 48.Wynne B, McHugh L, Gao W, Keegan D, Byrne K, Rowan C, Hartery K, Kirschbaum C, Doherty G, Cullen G, Dooley B, Mulcahy HE. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory bowel diseases. Gastroenterology 2019;156:935–945.e1. [DOI] [PubMed] [Google Scholar]
- 49.Gotink RA, Younge JO, Wery MF, Utens EMWJ, Michels M, Rizopoulos D, Van Rossum LFC, Roos-Hesselink JW, Hunink MMG. Online mindfulness as a promising method to improve exercise capacity in heart disease: 12-month follow-up of a randomized controlled trial. PLoS One 2017;12:e0175923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younge JO, Wester VL, Van Rossum EFC, Gotink RA, Wery MF, Utens EMWJ, Hunink MGM, Roos-Hesselink JW. Cortisol levels in scalp hair of patients with structural heart disease. Int J Cardiol 2015;184:71–8. [DOI] [PubMed] [Google Scholar]
- 51.Kidd T, Carvalho LA, Steptoe A. The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol 2014;99:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galante J, Galante I, Bekkers MJ, Gallacher J. Effect of kindness-based meditation on health and well-being: a systematic review and meta-analysis. J Consult Clin Psychol 2014;82:1101–14. [DOI] [PubMed] [Google Scholar]
- 53.Singer T, Kok BE, Bornemann B, Zurborg S, Bolz M, Bochow CA. Recruitment, Sample Description, and Dropout. In: The ReSource Project: Background, Design, Samples, and Measurements. 2nd ed. Leipzig, Germany: Max Planck Institute for Human Cognitive and Brain Sciences; 2016:44–51. [Google Scholar]
- 54.Kok BE, Singer T. Effects of contemplative dyads on engagement and perceived social connectedness over 9 months of mental training a randomized clinical trial. JAMA Psychiat 2017;74:126–34. [DOI] [PubMed] [Google Scholar]
- 55.Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev 2006;30:855–63. [DOI] [PubMed] [Google Scholar]
- 56.Singer T. The past, present and future of social neuroscience: a European perspective. Neuroimage 2012;61:437–49. [DOI] [PubMed] [Google Scholar]
- 57.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int 2000:107;5–12. [DOI] [PubMed] [Google Scholar]
- 58.Gao W, Kirschbaum C, Grass J, Stalder T. LC-MS based analysis of endogenous steroid hormones in human hair. J Steroid Biochem Mol Biol 2016;162:92–9. [DOI] [PubMed] [Google Scholar]
- 59.Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci 2013;928:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Valk SL, Bernhardt BC, Trautwein FM, Böckler A, Kanske P, Guizard N, Louis Collins D, Singer T. Structural plasticity of the social brain: differential change after socio-affective and cognitive mental training. Sci Adv 2017;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Team RC. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 62.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 63.Dobson AJ. An Introduction to Generalized Linear Models. Boca Raton, FL: Chapman & Hall/CRC; 2002. [Google Scholar]
- 64.Xu R. Measuring explained variation in linear mixed effects models. Stat Med 2003;22:3527–41. [DOI] [PubMed] [Google Scholar]
- 65.Kirk RE. Practical significance: a concept whose time has come. Educ Psychol Meas 1996;56:746–59. [Google Scholar]
- 66.Lenth RV. Least-Squares Means: The R Package lsmeans. Journal of Statistical Software 2016;69:1–33. [Google Scholar]
- 67.Green P, Macleod CJ. SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol 2016;7:493–8. [Google Scholar]
- 68.de Boer MR, Waterlander WE, Kuijper LD, Steenhuis IH, Twisk JW. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 70.Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 2012;37:1111–34. [DOI] [PubMed] [Google Scholar]
- 71.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76–81. [DOI] [PubMed] [Google Scholar]
- 72.Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Curr Direct Psychol Sci 2005;14. [Google Scholar]
- 73.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company; 1984. [Google Scholar]
- 74.DeLongis A, Coyne JC, Dakof G, Folkman S, Lazarus RS. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychol 1982;1:119–36. [Google Scholar]
- 75.Bornemann B, Singer T. Taking time to feel our body: Steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 2017;54:469–82. [DOI] [PubMed] [Google Scholar]
- 76.Conner TS, Feldman Barret L. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom Med 2012;274:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weckesser LJ, Dietz F, Schmidt K, Grass J, Kirschbaum C, Miller R. The psychometric properties and temporal dynamics of subjective stress, retrospectively assessed by different informants and questionnaires, and hair cortisol concentrations. Sci Rep 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wüst S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: a question of timing. Psychosom Med 2008;70:787–96. [DOI] [PubMed] [Google Scholar]
- 79.Prado-Gascó V, de la Barrera U, Sancho-Castillo S, de la Rubia-Ortí JE, Montoya-Castilla I. Perceived stress and reference ranges of hair cortisol in healthy adolescents. PLoS One 2019;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer SE, Lopez-Duran NL, Sen S, Abelson JL. Chronic stress, hair cortisol and depression: a prospective and longitudinal study of medical internship. Psychoneuroendocrinology 2018;92:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 2012;37:589–601. [DOI] [PubMed] [Google Scholar]
- 82.van der Meij L, Gubbels N, Schaveling J, Almela M, van Vugt M. Hair cortisol and work stress: importance of workload and stress model (JDCS or ERI). Psychoneuroendocrinology 2018;89:78–85. [DOI] [PubMed] [Google Scholar]
- 83.Lumma AL, Kok BE, Singer T. Is meditation always relaxing? Investigating heart rate, heart rate variability, experienced effort and likeability during training of three types of meditation. Int J Psychophysiol 2015;97:38–45. [DOI] [PubMed] [Google Scholar]
- 84.Gerber M, Brand S, Lindwall M, Elliot C, Kalak N, Christian H, Pühse U, Jonsdottir IH. Concerns regarding hair cortisol as a biomarker of chronic stress in exercise and sport science. J Sports Sci Med 2012;11:571–81. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


