Abstract
Background
It remains unclear whether additional left atrial posterior wall isolation (LAPWI) beyond pulmonary vein reisolation (PVRI) is beneficial in atrial fibrillation (AF) patients undergoing repeat ablation.
Objective
We sought to assess impact of LAPWI on arrhythmia outcomes in patients undergoing repeat AF ablation.
Methods
All AF patients that underwent repeat ablation between January 2016 and December 2018 were included. Those undergoing PVRI only served as control, whereas those undergoing LAPWI (with or without PVRI) were the study group. Primary endpoint was freedom from atrial arrhythmias (AA) off antiarrhythmic drugs (AADs) at 1 year follow-up. Secondary endpoint was freedom from AA on/off AADs at 1 year follow-up.
Results
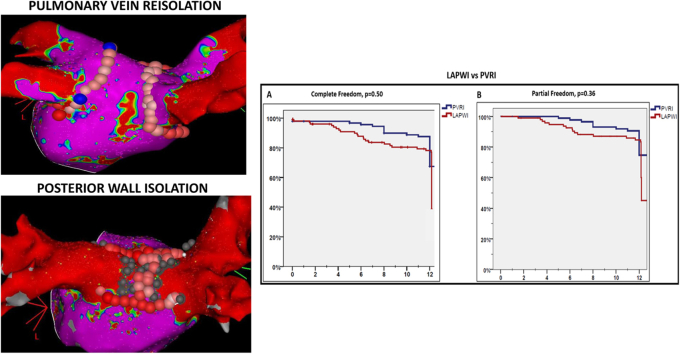
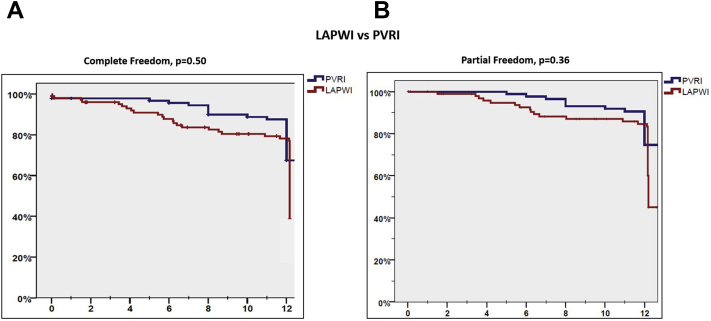
One hundred ninety-six patients (61% paroxysmal AF, 39% persistent AF) participated; 93 underwent PVRI and 103 underwent LAPWI±PVRI. Patients in the LAPWI group were older, had more hypertension and persistent AF, and had lower rates of PV reconnection (52.4% vs 100%, P < .001). LAPWI was performed empirically in 79.6% and to target triggers in 20.4%. It was accomplished by linear lesions across the LA floor and roof alone in 65% and additional LAPW lesions in 35%. The primary and secondary endpoints were similar between patients undergoing LAPWI and those undergoing PVRI (43.7% vs 69.9%, P = .50 and 66% vs 77.4%, P = .36, respectively). There was no difference in adverse events between the 2 groups.
Conclusion
LAPWI did not improve freedom from atrial arrhythmias on or off AADs at 1 year beyond PVRI in AF patients undergoing repeat ablation. Differences in patient demographics and AF type may underlie the observed lack of benefit of LAPWI, and further study is warranted.
Keywords: Atrial fibrillation, Catheter ablation, Posterior wall, Repeat ablation, Outcomes
Graphical abstract
Outcomes of left atrial posterior wall isolation during repeat atrial fibrillation ablation
Key Findings.
-
▪
Pulmonary vein reconnection is seen in majority of patients undergoing repeat atrial fibrillation ablation.
-
▪
Left atrial posterior wall isolation (PWI) does not improve long-term arrhythmia outcomes beyond pulmonary vein isolation in this patient cohort undergoing repeat catheter ablation procedures for atrial fibrillation; however, further study is required to define the value of PWI in specific patient groups.
-
▪
Left atrial PWI can be achieved in a majority of patients with endocardial ablation alone.
Introduction
Since the seminal observations demonstrating pulmonary veins as the most common sites of atrial fibrillation (AF) triggers,1 pulmonary vein isolation (PVI) has become standard of care for catheter ablation of this arrhythmia.2, 3, 4, 5, 6, 7 Arrhythmia recurrences following initial ablation are not uncommon and up to one-third of these patients may require repeat ablation.8,9 However, optimal strategies at the time of repeat AF ablation remain unclear. In patients undergoing repeat ablation, PV reconnection is commonly observed and is thought to underlie the majority of arrhythmia recurrences.10 Hence, PV reisolation (PVRI) is universally performed during repeat procedure(s).11 However, there is ongoing debate on whether that alone is sufficient to improve subsequent arrhythmia outcomes. Thus, many operators perform additional ablation beyond PVRI in these patients. Among the different extra-PV ablation strategies that are currently being utilized, left atrial posterior wall isolation (LAPWI) is frequently used. There are several potential reasons for this, including a common embryological origin of the LAPW and PVs and ease of targeting this well-defined region with a limited lesion set. However, despite its widespread use, the incremental benefit of LAPWI beyond PVI in patients undergoing repeat procedures has not been consistently proven. The objective of this study, therefore, was to assess whether LAPWI improved freedom from arrhythmia recurrences in AF patients undergoing repeat ablation beyond PVRI. We also wanted to investigate how this impacted outcomes when it was done empirically vs for documented arrhythmia triggers from this region. Finally, we also wanted to characterize the lesion distribution required to achieve LAPWI.
Methods
Patient selection
The study population comprised all AF patients that underwent their first repeat ablation at our institution from January 2016 to December 2018. Patients who had undergone >1 prior AF ablation were excluded. We also excluded patients who had undergone the initial ablation using cryoballoon or who had LAPWI performed during their first AF ablation. Eligible patients were identified from the AF ablation registry (Figure 1), which was approved by the University of Pennsylvania Health System’s Institutional Review Board. All participants provided written informed consent for both the ablation procedure and inclusion in the registry. Data collected included baseline demographics, imaging parameters such as left ventricular ejection fraction and left atrial size, ablation characteristics, and long-term outcomes as described below. The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
Figure 1.
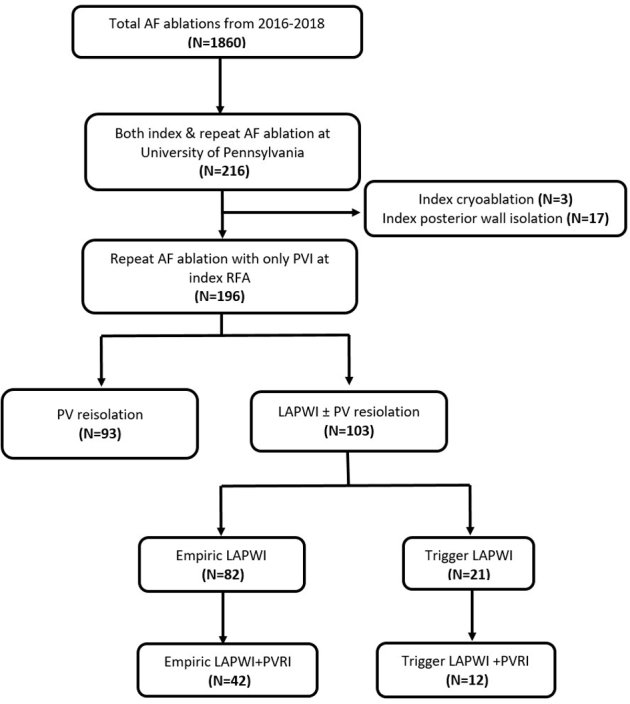
Flow diagram depicting patient selection for the study. AF = atrial fibrillation; LAPWI = left atrial posterior wall isolation; PVI = pulmonary vein isolation; PVRI = pulmonary vein reisolation; RFA = radiofrequency ablation.
AF ablation strategy
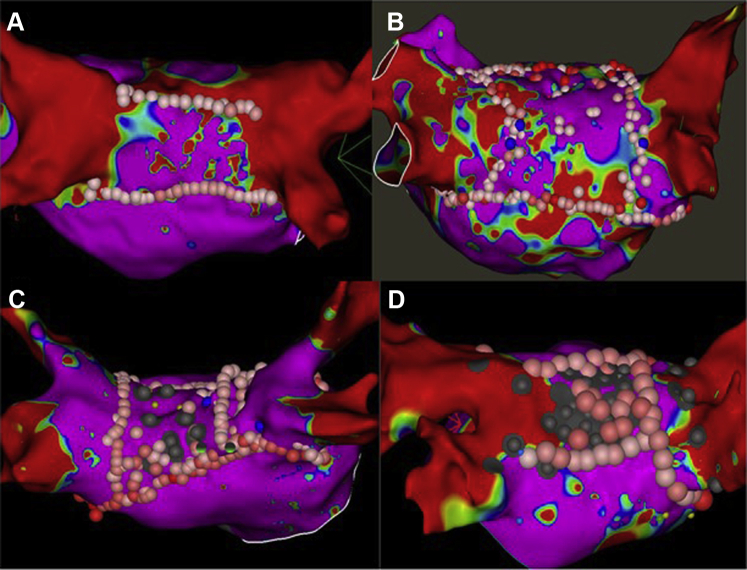
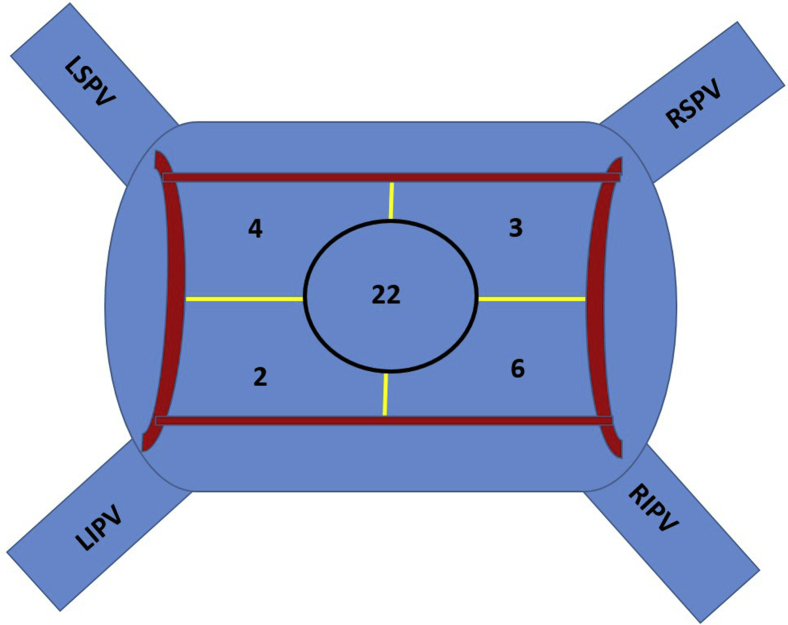
Our AF ablation approach has been previously described. All antiarrhythmic medications except amiodarone were usually discontinued >5 half-lives before the ablation and all procedures were done under general anesthesia using jet ventilation. Intracardiac ultrasound was used in all patients. Decapolar catheters were placed in the coronary sinus and posterior right atrium. After adequate heparinization (activated clotting time ≥350 seconds), 2 transseptal punctures were performed through which ablation and multielectrode mapping catheters were introduced into the LA. A voltage map of the LA was then created, preferably during sinus rhythm, to identify scar from the previous ablation and also identify potential areas of PV reconnection. To do this we used a bipolar voltage cut-off range of 0.25–0.40 mV, which we have previously shown to be useful in characterizing ablation-related LA scar.12 All PVs were mapped with multipolar catheters to identify entrance and exit. When PV reconnection was noted, additional mapping was performed with the multipolar and contact force–sensing irrigated-tip catheter (SmartTouch; Biosense Webster, Irvine, CA; TactiCath; Abbott, St. Paul, MN) to identify focal areas of reconnection, which were defined as locations along the PV antrum that demonstrated earliest activation (relative to the potentials within the vein) and/or areas with relatively preserved voltages within the dense scar. These sites were then targeted with serial lesions using power of 20–40 W for 10–30 seconds, achieving 10%–15% impedance drop from baseline. The endpoint was PV entrance and exit block, which was validated over a 20-minute waiting period, during which adenosine bolus and isoproterenol infusion were also used at the discretion of the operator. Following successful PVRI, all patients underwent a standardized stimulation protocol to identify non-PV triggers. Our stimulation protocol, which has been previously described,13 comprised incremental isoproterenol infusion (up to 20–30 mcg/min) with maintenance of maximal tolerated dose for 2–3 minutes, followed by decremental pacing from coronary sinus and/or right atrium (15-beat drive trains from 250 ms to 180 ms or failure to capture). All non-PV triggers (atrial premature depolarizations inducing sustained atrial arrhythmias and/or AF) were targeted, following which any additional ablation including LAPWI was performed at the discretion of the operator. The typical approach for LAPWI was to create linear lesions across the LA roof and floor connecting the previous circumferential lesion sets that were used for left and right PVI (Figure 2a). If this failed to achieve LAPWI, additional lesions were delivered at sites of earliest activation within the “box” to achieve entrance/exit block into this region (Figure 2b–2d). The endpoint when ablating within the box included attenuation/abolition of electrograms at the targeted site and loss of local capture at high output (10 mA @ 2 ms) together with demonstration of exit block. To characterize lesion distribution during focal ablation in the LAPW, we arbitrarily divided this region into 5 segments (right superior, right inferior, left superior, left inferior, and central) (Figure 3). During LAPWI we used power setting of 20–30 W and lesion duration of 5–20 seconds. Esophageal temperature was constantly monitored during energy delivery.
Figure 2.
A: Bipolar voltage map of the left atrium showing left atrial posterior wall (LAPW) ablation lesion set with roof and floor line. B: LAPW ablation lesion set with LAPW box and additional ablation within the box at the center. C: LAPW ablation lesions with LAPW box and additional ablation in the right inferior quadrant of the box to achieve isolation. D: LAPW ablation set with LAPW box and extensive ablation all over the LAPW targeting noncapture. Gray dots represents areas of no capture at high output pacing.
Figure 3.
Schematic of the left atrial posterior wall (LAPW) in posteroanterior view showing sites of additional ablation within the LAPW box required to achieve successful LAPW isolation. LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Postablation follow-up
After the ablation procedure, patients were discharged on antiarrhythmic drugs (AAD) at the discretion of the operator, together with either warfarin or a direct oral anticoagulant. All patients were taught to perform twice-daily pulse checks to assess for asymptomatic arrhythmia recurrence. Additionally, each patient was sent home with a mobile cardiac outpatient telemetry (MCOT) device (Lifewatch, Rosemont, IL; CardioNet, Malvern, PA) for continuous monitoring for 30 days postablation. Patients were subsequently seen for routine follow-up in the outpatient clinic at around 6 weeks, 6 months, and 1 year. Beyond this period, yearly clinic visits were advised but not mandated. Routine practice at our institution is to perform a 30-day MCOT around the 6-month and 1-year follow-up appointments, and additional MCOTs were prescribed if the patients reported symptoms suggestive of arrhythmia recurrences. Patients with cardiac implantable electronic devices and insertable cardiac monitors were also remotely followed every 3 months for device-detected arrhythmias. In the absence of documented arrhythmia recurrence, AADs were typically discontinued between 3 and 6 months postablation.
Outcomes of interest
The primary outcome of interest was freedom from atrial arrhythmias off AADs, which was defined as the absence of any AF and/or organized atrial tachycardia lasting >30 seconds at the 1-year follow-up. Secondary outcome of interest was freedom from atrial arrhythmias on or off AADs at the 1-year follow-up. All outcomes were assessed beyond a 3-month blanking period following the ablation procedure.
Statistical analysis
Clinical and demographic characteristics at the time of ablation were aggregated for comparison. The χ2 test was used to compare categorical variables and t test for continuous variables. Categorical variables were represented as frequencies and proportions; continuous variables were summarized by mean ± standard deviation. A P value ≤ .05 was considered statistically significant. Kaplan-Meier survival analysis plots were used to assess long-term arrhythmia control and complete freedom from AF. All analyses were done using SPSS (IBM SPSS Statistics for Windows, Version 25.0; IBM Corp, Armonk, NY).
Results
We included 196 AF patients who underwent their first repeat catheter ablation procedure for recurrent AF at our institution during the study period. Mean age of the population was 63.8 ± 10.4 years and 70% were male. The median time between the index and first repeat ablation was 24.7 months, with an interquartile range of 53.7 months. At the time of the repeat ablation, AF categorization was paroxysmal in 61% and persistent in 39%. Ninety-three patients (47%) underwent only PVRI for ≥1 reconnected vein, whereas 103 patients (53%) had LAPWI. In the latter group, PV reconnection was present in 52.4% (n = 54), for which PVRI was also performed.
Acute ablation outcomes
PVRI was performed successfully in all patients with evidence of PV reconnection. Out of 103 patients in the LAPWI group, successful isolation of the LAPW with demonstration of entrance and exit block was achieved in 102 (99%) patients. In 1 patient in whom LAPWI could not be achieved, radiofrequency energy delivery was limited by rapid, frequent, and persistent esophageal temperature rises. LAPW roof and floor lines were created in 95 patients. In 3 patients, only a roof line was created, as the floor of the LAPW had dense scar. Similarly, in 3 patients only a floor line was created owing to the presence of dense scar in the LAPW roof. Creation of an LAPW “box” lesion set with roof and/or floor lines led to LAPWI in 65% of patients (n = 67). Additional focal ablation within the “box” targeting sites of earliest activation, fractionated electrograms, or areas of local capture was required to achieve LAPWI in 36 patients (35%). Distribution of lesion sets within the LAPW box that led to LAPWI are shown in Figure 3. The mean number of focal lesions within the box was 9.5 ± 7.3. A total of 37 sites were targeted in 33 patients, with the majority (59.5%) localized to the center of the LAPW. In the remaining 3 patients, focal areas could not be identified and ablation across the entire LAPW was required to achieve isolation. Esophageal temperature rises >38°C were documented in only 6 cases. Additional ablation targets included cavotricuspid isthmus ablation, superior vena cava isolation, and mitral isthmus ablation, with only the latter more frequently performed in patients undergoing LAPWI. Distribution of non-PV triggers (excluding LAPW triggers) is shown in Supplemental Table A.
Long-term ablation outcomes
PVRI with or without additional LAPWI
Clinical and procedural characteristics of these 2 groups are shown in Table 1. Patients undergoing additional LAPWI were older (66 ± 9.9 years vs 62 ± 11 years for PVRI group, P = .02) and more likely to have hypertension (61.2% vs 40.9% in PVRI group, P = .06) and persistent AF (53.4% vs 22.6% in PVRI group, P = .001). The PV reconnection group had a significantly higher number of reconnected PVs (2.5 ± 1.1 vs 1.8 ± 0.8 in the LAPWI group; P < .001). Following repeat ablation at 1-year follow-up, there was no difference in the primary and secondary outcomes freedom from atrial arrhythmias off or on AADs between patients undergoing PVRI alone vs additional LAPWI (freedom from atrial arrhythmias off AADs: 69.9% vs 43.7%, P = .50; freedom from atrial arrhythmias on or off AADs: 77.4% vs 66%, P = .36). Kaplan-Meier curves comparing primary and secondary outcomes between the 2 groups are shown in Figure 4. Rates of organized atrial tachycardia were also similar in the LAPWI and PVRI groups (20% vs 26%, P = .16).
Table 1.
Comparisons of patients with and without left atrial posterior wall isolation
| PWI (n = 103) | No PWI (n = 93) | P value | |
|---|---|---|---|
| Age (years) | 66 ± 9.9 | 62 ± 11 | .02 |
| Male, n (%) | 68 (66) | 69 (74.1) | .21 |
| BMI (kg/m2) | 30.4 ± 5.7 | 30 ± 5.6 | .62 |
| Hypertension, n (%) | 63 (61.2) | 38 (40.8) | .005 |
| Diabetes mellitus, n (%) | 15 (14.6) | 14 (15) | .92 |
| Sleep apnea, n (%) | 23 (22.3) | 25 (26.8) | .46 |
| LVEF (%) | 54.3 ± 10.3 | 55.7 ± 10.6 | .62 |
| LA size (cm) | 4.5 ± 0.6 | 4.3 ± 0.7 | .20 |
| Coronary disease, n (%) | 17 (16.5) | 16 (17.2) | .90 |
| Stroke/TIA, n (%) | 6 (5.8) | 9 (9.6) | .31 |
| CHA2DS2-VASc | 2.3 ± 1.3 | 1.9 ± 1.5 | .06 |
| Paroxysmal AF, n (%) | 48 (46.6) | 72 (77.4) | <.001 |
| Persistent AF, n (%) | 55 (53.4) | 21 (22.6) | <.001 |
| AAD on discharge, n (%) | 76 (73.7) | 40 (43) | <.001 |
| AAD discontinued, n (%) | 29 (28.2) | 23 (24.7) | .028 |
| CIED/ICM, n (%) | 34 (33) | 22 (23.7) | .16 |
| Follow-up (months) | 18 ± 11 | 22.4 ± 13 | .01 |
| PROCEDURAL CHARACTERISTICS | |||
| PV reconnection, n (%) | 54 (52.4) | 93 (100) | <.001 |
| No. of PVs reconnected | 1.8 ± 0.8 | 2.5 ± 1 | <.001 |
| Cavotricuspid isthmus ablation, n (%) | 30 (29.1) | 23 (24.7) | .52 |
| Mitral annular ablation, n (%) | 26 (25.2) | 1 (1.1) | <.001 |
| Coronary sinus ablation, n (%) | 14 (13.6) | 1 (11) | .008 |
| SVC/crista ablation, n (%) | 8 (7.7) | 5 (5.4) | .57 |
| Procedure time (min) | 221 ± 67 | 202 ± 74 | .06 |
| AMBULATORY MONITORING | |||
| CIED, n (%) | 6 (5.8) | 8 (8.6%) | .65 |
| Event monitor, n (%) | 61 (59.2%) | 58 (62.4%) | .14 |
| ICM, n (%) | 32 (31.1%) | 22 (23.7%) | .65 |
| OUTCOMES | |||
| Freedom from AA at 1 year off AAD, n (%) | 45 (43.7%) | 65 (69.9%) | .50 |
| Freedom from AA at 1 year off or on AADs, n (%) | 68 (66%) | 72 (77.4%) | .36 |
AA = atrial arrhythmias; AAD = antiarrhythmia drug; CIED = cardiac implantable electronic device; ICM = insertable cardiac monitor; LVEF = left ventricular ejection fraction; PV = pulmonary vein; SVC = superior vena cava; TIA = transient ischemic attack.
Figure 4.
Kaplan-Meier curves comparing primary (A) and secondary outcomes (B) in patients undergoing left atrial posterior wall isolation (LAPWI) vs only pulmonary vein reisolation (PVRI).
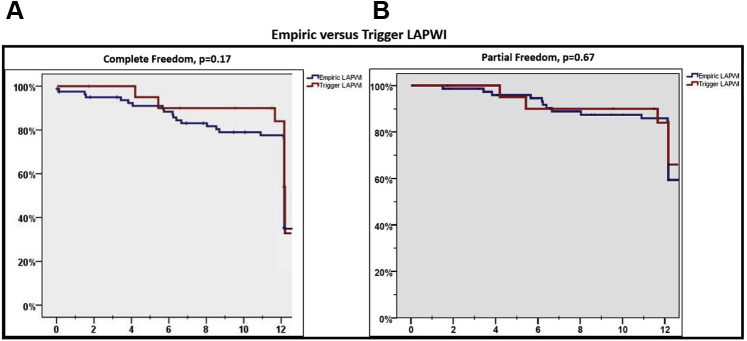
Empiric vs trigger-based LAPWI
Among patients who underwent LAPWI, in 79.6% (n = 82) this was done empirically, whereas in 20.4% of patients (n = 21) this was performed after documentation of arrhythmia triggers from the region. Characteristics of patients undergoing empiric vs trigger-based LAPWI are described in Table 2. There was no difference in baseline characteristics between these patients. Also, a comparable number of subjects in each group required additional PVRI (51.2% vs 57.1%, P = .63). Following ablation, there was no difference in primary and secondary endpoints among patients undergoing empiric vs trigger-based LAPWI (freedom from atrial arrhythmias off AADs: 40.7% vs 57.1%, P = .17; freedom from atrial arrhythmias off or on AADs: 66.7% vs 71.4%, P = .67) (Figure 5).
Table 2.
Comparison of patients undergoing empiric vs trigger guided left atrial posterior wall isolation
| Empiric (n = 82) | Trigger (n = 21) | P value | |
|---|---|---|---|
| Age (years) | 65.54 ± 10.1 | 65.29 ± 9.8 | .92 |
| Male, n (%) | 53 (64.6) | 15 (71.4) | .56 |
| Hypertension, n (%) | 47 (47.3) | 16 (76.2) | .11 |
| Diabetes mellitus, n (%) | 10 (12.2) | 5 (23.8) | .18 |
| Sleep apnea, n (%) | 19 (23.2) | 4 (19) | .78 |
| Heart failure, n (%) | 7 (8.5) | 1 (4.7) | 1.00 |
| BMI (kg/m2) | 30 ± 5.5 | 32 ± 6.6 | .20 |
| CHA2DS2-VASc | 2.29 ± 1.2 | 2.24 ± 1.3 | .86 |
| LVEF (%) | 54 ± 11 | 55 ± 8 | .63 |
| PV reconnection, n (%) | 42 (51.2) | 12 (57.1) | .63 |
| Paroxysmal AF, n (%) | 37 (45.1) | 11 (52.4) | .55 |
| Persistent AF, n (%) | 45 (54.9) | 10 (4.8) | .55 |
| Freedom from AA at 1 year off AADs, n (%) | 33 (40.7) | 12 (57.1) | .17 |
| Freedom from AA at 1 year off or on AADs, n (%) | 54 (66.7) | 15 (71.4) | .67 |
Abbreviations as in Table 1.
Figure 5.
Kaplan-Meier curves comparing primary (A) and secondary outcomes (B) in patients undergoing empiric vs trigger-based left atrial posterior wall isolation (LAPWI).
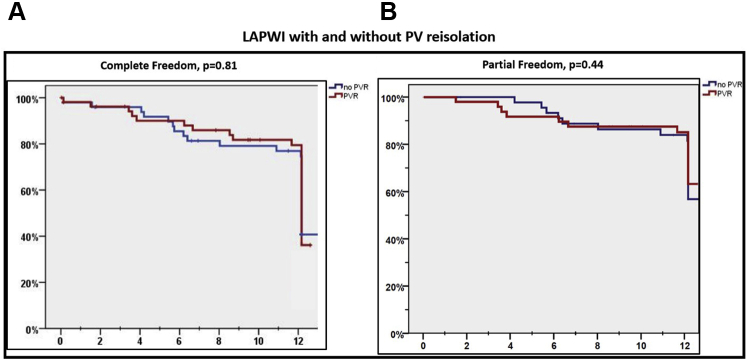
Benefit of LAPWI in patients with or without PV reconnection
Of the 103 patients who underwent LAPWI, 54 had evidence of ≥1 PV reconnection. A comparison of baseline characteristics of these patients who had PV reconnection and underwent LAPWI+PVRI with those that underwent LAPWI alone (n = 49) is presented as Supplemental Table B. Patients that underwent LAPWI+PVRI had a higher prevalence of hypertension than those that underwent LAPWI alone (70.3% vs 51%, P = .04). However, there was no difference in primary and secondary endpoints among patients that underwent LAPWI vs LAPWI+PVRI (freedom from atrial arrhythmias off AADs: 42.6% vs 44.9%, P = .81; freedom from atrial arrhythmias off or on AADs: 63.3% vs 70.4%, P = .44) (Figure 6). Additional analyses evaluating outcomes of LAPWI in patients with PV reconnection also did not reveal any statistically significant differences (Supplemental Table C).
Figure 6.
Kaplan-Meier curves comparing primary (A) and secondary outcomes (B) in patients undergoing left atrial posterior wall isolation (LAPWI) with and without additional pulmonary vein (PV) reisolation.
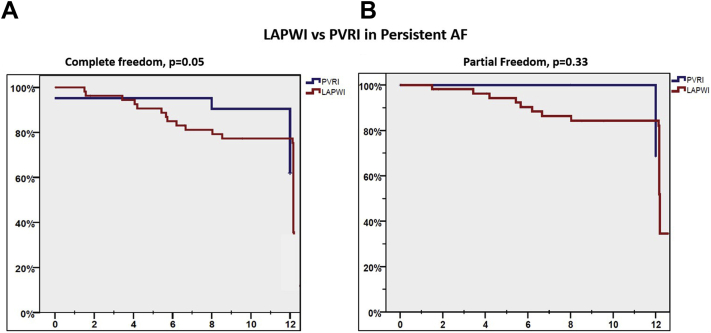
LAPWI outcomes in persistent AF
The nature of AF at repeat ablation was persistent in 76 patients. In these subjects, additional LAPWI was performed in 55 (72%), whereas 21 (28%) patients underwent only PVRI. There was higher prevalence of diabetes and prior history of stroke in patients with persistent AF that did not undergo additional LAPWI. Following repeat ablation, freedom from atrial arrhythmias off AADs (primary endpoint) was lower in persistent AF patients that underwent LAPWI vs PVRI (36.4% vs 61.9%; P = .05), but freedom from atrial arrhythmias off or on AAD (secondary endpoint) was similar between the 2 groups (56.4% vs 71.4%, P = .33) (Figure 7).
Figure 7.
Kaplan-Meier curves comparing primary (A) and secondary outcomes (B) in persistent atrial fibrillation (AF) patients undergoing left atrial posterior wall isolation (LAPWI) vs pulmonary vein reisolation (PVRI).
Predictors of recurrence
On multivariate logistic regression analysis, which included age, sex, nature of AF, presence of PV reconnection, LAPWI, and empiric vs trigger-based LAPWI, none of these variables were independent predictors of the primary or secondary outcome (Supplemental Table D).
Serious adverse events
A total of 3 procedural complications were observed in our series and all of them occurred in the PVRI group. These included 1 case of pericardial effusion requiring pericardiocentesis, 1 case of left atrial appendage perforation requiring surgical repair, and 1 hemorrhagic stroke in the immediate postoperative period.
Discussion
We report our center’s experience with performing LAPWI in patients experiencing arrhythmia recurrence after their initial AF ablation. The salient findings of our study are as follows: (1) LAPWI can be accomplished by targeting this region endocardially, (2) about one-third of patients undergoing LAPWI will require additional focal lesions beyond LA roof and floor lines to achieve isolation, (3) PV reconnection was observed in 75% of patients presenting for repeat AF ablation, and (4) performing LAPWI beyond PVRI did not significantly improve long-term arrhythmia free survival.
Role of left atrial posterior wall in AF
AF recurrence following ablation can be the result of PV reconnection, progressive left atrial pathology, systemic risk factors, or development of non-PV triggers.10 The LAPW has gained considerable interest in the pathogenesis of AF, since this region shares its embryological origin with the PVs. LAPW has also been shown to have distinct electrophysiological properties and ion channel characteristics that are potentially arrhythmogenic,14 which is the basis for targeting this region during AF ablation.
Data on left atrial posterior wall isolation
Studies in patients with paroxysmal AF have shown that inclusion of a greater part of the LAPW within the circumferential PVI lesion set may be associated with improved outcomes of ablation.15 However, Sutter and colleagues16 found that performing LAPWI beyond PVI in patients with persistent AF resulted in higher recurrence of sustained atrial arrhythmias, including AF. Other studies have reported significantly lower arrhythmia recurrence rate with adjunctive LAPWI than PVI alone (33% vs 61%, P < .001).17 In our study, LAPWI did not improve arrhythmia control beyond PVRI at 1 year in patients undergoing repeat ablation. We do, however, acknowledge that patients who had LAPWI performed in our study were older, had more HTN, and had a higher percentage of persistent AF. It has been argued that lack of benefit from adjunctive LAPWI may be explained by the inability to achieve effective and/or persistent isolation of this region. In support of this hypothesis, Bai and colleagues18 found that after extensive LA ablation involving the posterior wall, left atrial septum, and coronary sinus region, persistent isolation of the LAPW was associated with improved arrhythmia-free survival. However, in a subgroup of these patients where repeat LA mapping was performed 3 months after, there was evidence of posterior wall reconnection in about a third of patients irrespective of arrhythmia recurrences. It is interesting to note that no significant benefit of LAPWI was reported by a study employing hybrid endocardial-epicardial approach to achieve durable LAPWI.19 A meta-analysis that was limited to pooling the results of only prospective randomized trials that compared the outcomes of adjunctive LAPWI beyond PVI also did not find any difference in the long-term arrhythmia-free survival between the 2 groups.20 The findings of our study are consistent with the observations of this meta-analysis as well as the recent randomized trial by Lee and colleagues,21 which did not show any beneficial impact of empiric posterior wall box isolation beyond PVI on long-term arrhythmia-free survival in patients with persistent AF. An important distinction is that most prior studies evaluating the role of LAPWI included patients undergoing a first AF ablation procedure. Our study is the first to evaluate the role of adjunctive LAPWI during repeat AF ablation.
We also report a high success rate of achieving LAPWI with endocardial ablation alone. This is in contrast with the results of the CONVERGE (Convergence Of Epicardial And Endocardial Radiofrequency [RF] Ablation For The Treatment Of Symptomatic Persistent AF) trial,22 which showed improved arrhythmia-free outcomes when a combined endocardial-epicardial approach was used for LAPWI in patients with persistent AF. The epicardial lesion set in this trial included bilateral PV antral encircling lesions and parallel lines connecting LAPW lesions spanning the entire epicardial surface of the LAPW. The endocardial lesion set included routine PVI and a roof line only for LAPW isolation without a floor line or additional ablation of fractionated electrograms within the LAPW. This could represent inadequate endocardial LAPWI in the control arm, which could be a significant confounder in assessing long-term outcomes. It is interesting to note that 36% of our patients required additional ablation beyond LA roof and floor lines to achieve LAPWI. These lesions were delivered most commonly in the center of the LAPW. These observations suggest that LAPWI may have epicardial connections that need to be identified and targeted specifically in order to achieve durable isolation. However, doing so may sometimes involve ablating extensively over this region, and that may potentially increase the risk of adverse complications, especially injury to the esophagus. In that context, it is interesting to note that we observed esophageal temperature rise of >38°C in only 6 patients (6%). Importantly, none of the patients undergoing LAPWI developed clinical evidence of esophageal injury. Some studies have shown a high incidence of esophageal damage and even development of atrioesophageal fistula in patients undergoing LAPWI.23 Since performing LAPWI potentially exposes patients to the risk of esophageal injury, it raises the question whether it should be performed empirically in all patients undergoing repeat AF ablation. The observations of our study would suggest otherwise. We found a high incidence of PV reconnection (75%) in our study patients that experienced arrhythmia recurrences, and performing LAPWI beyond PVI did not improve long-term ablation outcomes. Also, in those patients whose PVs remained isolated at the time of the repeat ablation, performing LAPWI did not lead to significant improvement in outcomes. This would suggest that the LAPWI may not have been the source of non-PV triggers in these patients. To explore this further, we looked at arrhythmia outcomes in patients where LAPWI was performed empirically vs in the setting of documented non-PV triggers originating from this region. Once again, we did not find any difference in long-term arrhythmia control in patients who underwent LAPWI empirically vs for documented triggers originating from this region. However, our study did show comparable arrhythmia outcomes in patients with chronic PVI that underwent LAPWI alone compared to those that had PVRI for documented reconnection. Prior studies have shown that box isolation of fibrotic areas in the LAPW could lead to similar long-term outcomes between paroxysmal and persistent AF patients during repeat ablation.24 In our study, despite a higher prevalence of hypertension and persistent AF in the LAWPI group, overall long-term outcomes were similar in the LAPWI vs PVRI groups. These data could potentially point towards a benefit of LAPWI in patients with nonparoxysmal AF requiring repeat ablation.
Study limitations
We acknowledge several limitations imposed by the retrospective nature of our study, including some important population differences. Patients that underwent LAPWI were older and had a higher proportion of persistent AF which would have led to lower rates of arrhythmia-free survival in this group. Perhaps propensity matching would have allowed better comparison, but we did not perform it owing to the low proportion of persistent AF patients in the PVRI group. The choice of antiarrhythmic medication and the duration of their use was at the discretion of the treating electrophysiologist. There was also variation in type of ambulatory monitoring employed for postablation follow-up between operators. Despite this, an equal proportion of patients in both groups had continuous rhythm monitoring performed. The sample size of our study is relatively small owing to the stringent inclusion/exclusion criteria and the narrow study duration period. However, we deliberately chose to restrict our study duration from January 2016 to December 2018 so that the outcomes were reflective of our current ablation practices. Finally, the numbers of patients in the subgroups analyzed were relatively small, and that could have impacted the outcome results.
Conclusion
Our study showed a lack of benefit for adjunctive LAPWI beyond PVI in an overall unmatched cohort of AF patients undergoing repeat ablation for arrhythmia recurrence (Graphical Abstract). However, subgroup analyses of patients with persistent AF at the time of repeat ablation raises the possibility of potential beneficial effect of LAPWI. We found a high rate of PV reconnection in these patients, confirming prior reports that reconnected PVs remain a major driver for AF recurrences. We also found that endocardial ablation alone was able to achieve LAPWI in all but 1 patient. However, achieving this required additional focal ablation beyond LA roof and floor line in as many as 35% of patients. The center of the LAPW was the most common location for additional ablation to achieve LAPWI, which suggests the presence of epicardial connections in this location. However, doing so did not increase the occurrence of adverse events, including esophageal damage, which was conspicuous by its absence in our study. Further randomized studies are needed to better understand the role of LAPWI in AF patients.
Funding Sources
This work was supported by the Richard T. & Angela Clark Innovation Fund in Cardiovascular Medicine.
Disclosures
All authors have no relevant conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All participants provided written informed consent for both the ablation procedure and inclusion in the registry.
Ethics Statement
The AF ablation registry was approved by the University of Pennsylvania Health System’s Institutional Review Board. The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.07.004.
Appendix. Supplementary data
References
- 1.Haïssaguerre M., Jaïs P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Jaïs P., Haïssaguerre M., Shah D.C. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 3.Oral H., Pappone C., Chugh A. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 4.Pappone C., Augello G., Sala S. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF study. J Am Coll Cardiol. 2006;48:2340–2347. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Jaïs P., Cauchemez B., Macle L. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 6.Wilber D.J., Pappone C., Neuzil P. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 7.Jones D.G., Haldar S.K., Hussain W. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 8.Packer D.L., Mark D.B., Robb R.A. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morillo C.A., Verma A., Connolly S.J. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 10.Callans D.J., Gerstenfeld E.P., Dixit S. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15(9):1050–1055. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 11.Nanthakumar K., Plumb V.J., Epstein A.E., Veenhuyzen G.D., Link D., Kay G.N. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226–1229. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 12.Squara F., Frankel D.S., Schaller R. Voltage mapping for delineating inexcitable dense scar in patients undergoing atrial fibrillation ablation: a new end point for enhancing pulmonary vein isolation. Heart Rhythm. 2014;11:1904–1911. doi: 10.1016/j.hrthm.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Santangeli P., Zado E.S., Hutchinson M.D. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13:374–382. doi: 10.1016/j.hrthm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Suenari K., Chen Y.C., Kao Y.H. Discrepant electrophysiological characteristics and calcium homeostasis of left atrial anterior and posterior myocytes. Basic Res Cardiol. 2011;106:65–74. doi: 10.1007/s00395-010-0132-1. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi K., Kircher S., Watanabe N. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol. 2012;5:667–675. doi: 10.1161/CIRCEP.111.969923. [DOI] [PubMed] [Google Scholar]
- 16.Sutter J.S., Lokhnygina Y., Daubert J.P. Safety and efficacy outcomes of left atrial posterior wall isolation compared to pulmonary vein isolation and pulmonary vein isolation with linear ablation for the treatment of persistent atrial fibrillation. Am Heart J. 2020;220:89–96. doi: 10.1016/j.ahj.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Elbatran A.I., Gallagher M.M., Li A. Isolating the entire pulmonary venous component versus isolating the pulmonary veins for persistent atrial fibrillation: A propensity-matched analysis. Pacing Clin Electrophysiol. 2020;43:68–77. doi: 10.1111/pace.13852. [DOI] [PubMed] [Google Scholar]
- 18.Bai R., Di Biase L., Mohanty P. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016;13:132–140. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P., Bamimore A.M., Schwartz J.D. Challenges and outcomes of posterior wall isolation for ablation of atrial fibrillation. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiyagarajah A., Kadhim K., Lau D.H. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.M., Shim J., Park J. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–1261. doi: 10.1016/j.jacep.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 22.DeLurgio D.B., Crossen K.J., Gill J. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.009288. [DOI] [PubMed] [Google Scholar]
- 23.Nair K.K., Shurrab M., Skanes A. The prevalence and risk factors for atrioesophageal fistula after percutaneous radiofrequency catheter ablation for atrial fibrillation: the Canadian experience. J Interv Card Electrophysiol. 2014;39:139–144. doi: 10.1007/s10840-013-9853-z. [DOI] [PubMed] [Google Scholar]
- 24.Kottkamp H., Berg J., Bender R., Rieger A., Schreiber D. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.