Highlights
-
•
Cerebral blood flow in ME/CFS patients remains abnormal 5 min post-tilt test.
-
•
Post cerebral blood flow abnormalities do not depend on hemodynamic results and on end-tidal carbon dioxide pressures during the tilt-test.
-
•
Post cerebral blood flow abnormalities are most severe in more severely diseased ME/CFS patients.
Abbreviations: BMI, Body Mass Index; BSA, Body Surface Area; CBF, Cerebral blood flow; CI, Cardiac Index; DBP, Diastolic Blood pressure; dOH, delayed orthostatic hypotension; HR, Heart rate; ICC, International Consensus Criteria; ME/CFS, Myalgic encephalomyelitis/chronic fatigue syndrome; NormHRBP, normal heart rate and blood pressure response; PET, end-tidal pressure; POTS, Postural orthostatic tachycardia syndrome; SBP, Systolic Blood pressure; VTI, Time velocity integral
Keywords: Orthostatic intolerance, Cerebral blood flow, Post exertional malaise, Tilt table testing, ME/CFS, Recovery, Extracranial Doppler echography, Cardiac Index, Postural Orthostatic Tachycardia Syndrome, Normal heart rate and blood pressure response
Abstract
Objective
Orthostatic symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may be caused by an abnormal reduction in cerebral blood flow. An abnormal cerebral blood flow reduction was shown in previous studies, without information on the recovery pace of cerebral blood flow. This study examined the prevalence and risk factors for delayed recovery of cerebral blood flow in ME/CFS patients.
Methods
60 ME/CFS adults were studied: 30 patients had a normal heart rate and blood pressure response during the tilt test, 4 developed delayed orthostatic hypotension, and 26 developed postural orthostatic tachycardia syndrome (POTS) during the tilt. Cerebral blood flow measurements, using extracranial Doppler, were made in the supine position pre-tilt, at end-tilt, and in the supine position at 5 min post-tilt. Also, cardiac index measurements were performed, using suprasternal Doppler imaging, as well as end-tidal PCO2 measurements. The change in cerebral blood flow from supine to end-tilt was expressed as a percent reduction with mean and (SD). Disease severity was scored as mild (approximately 50% reduction in activity), moderate (mostly housebound), or severe (mostly bedbound).
Results
End-tilt cerebral blood flow reduction was −29 (6)%, improving to −16 (7)% at post-tilt. No differences in either end-tilt or post-tilt measurements were found when patients with a normal heart rate and blood pressure were compared to those with POTS, or between patients with normocapnia (end-tidal PCO2 ≥ 30 mmHg) versus hypocapnia (end-tidal PCO2 < 30 mmHg) at end-tilt. A significant difference was found in the degree of abnormal cerebral blood flow reduction in the supine post-test in mild, moderate, and severe ME/CFS: mild: cerebral blood flow: −7 (2)%, moderate: −16 (3)%, and severe :-25 (4)% (p all < 0.0001). Cardiac index declined significantly during the tilt test in all 3 severity groups, with no significant differences between the groups. In the supine post-test cardiac index returned to normal in all patients.
Conclusions
During tilt testing, extracranial Doppler measurements show that cerebral blood flow is reduced in ME/CFS patients and recovery to normal supine values is incomplete, despite cardiac index returning to pre-tilt values. The delayed recovery of cerebral blood flow was independent of the hemodynamic findings of the tilt test (normal heart rate and blood pressure response, POTS, or delayed orthostatic hypotension), or the presence/absence of hypocapnia, and was only related to clinical ME/CFS severity grading. We observed a significantly slower recovery in cerebral blood flow in the most severely ill ME/CFS patients.
Significance
The finding that orthostatic stress elicits a post-stress cerebral blood flow reduction and that disease severity greatly influences the cerebral blood flow reduction may have implications on the advice of energy management after a stressor and on the advice of lying down after a stressor in these ME/CFS patients.
1. Introduction
Using extracranial Doppler imaging of the carotid and vertebral arteries, taking both flow velocity and vessel diameters into account, we have demonstrated that cerebral blood flow is significantly reduced in 90% of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients during a 70 degree tilt test (van Campen et al., 2020c). Importantly, the degree of reduction in cerebral blood flow is linearly related to the number of orthostatic symptoms, as assessed during the tilt test: the greater the reduction in cerebral blood flow, the larger the number of different orthostatic intolerance symptoms. Moreover, during a low-grade tilt test of 20 degrees, a similar reduction in cerebral blood flow was observed in ME/CFS patients with a severe form of the disease (van Campen et al., 2020a). An abnormal cerebral blood flow reduction was also demonstrated during a sitting test in ME/CFS patients with a severe form of the disease (van Campen et al., 2020b). Our findings, together with earlier studies on orthostatic intolerance in ME/CFS patients (Bou-Holaigah et al., 1995, De Lorenzo et al., 1997, Duprez et al., 1998, Hollingsworth et al., 2010, Jones et al., 2005, LaManca et al., 1999, Naschitz et al., 2000, Naschitz et al., 2002, Poole et al., 2000, Razumovsky et al., 2003, Schondorf et al., 1999, Streeten et al., 2000), clearly show that orthostatic intolerance is an important contributor to ME/CFS symptoms and is associated with a reduction of cerebral blood flow during tilt testing. The importance of orthostatic intolerance symptomatology in ME/CFS was recognized in the Institute of Medicine case definition of ME/CFS, which included orthostatic intolerance as a cardinal symptom of the disease (Institute Of Medicine (IOM), 2015).
We have observed that ME/CFS patients often report symptoms of dizziness/lightheadedness directly after completion of the tilt test while resuming an upright posture after the post-test supine recovery phase. These symptoms have a variable duration, from seconds to 15 min or even longer. Because of the persistence of these orthostatic intolerance symptoms, we hypothesized that cerebral blood flow may not immediately return to the pre-test supine values in ME/CFS patients. Although the mechanism of the abnormal cerebral blood flow reduction in ME/CFS patients is not fully understood, one of the hypothesized mechanisms is that the abnormal cerebral blood flow reduction is related to an abnormal cardiac output reduction due to increased venous pooling and lack of venous return during the tilt test (Timmers et al., 2002, van Campen and Visser, 2018a). It has previously been shown in healthy controls that cardiac output returns to normal pre-tilt test values within 1 min of the supine recovery phase (Barde and Deepak, 2012, Miyake et al., 2014, Toska and Walløe, 2002). In ME/CFS patients the cardiac output in the post-test supine recovery phase has not been studied previously.
Therefore, the aim of the current study was to investigate whether cerebral blood flow and cardiac output normalize immediately after head-up tilt testing.
2. Methods
2.1. Participants
A pilot study was performed at the outpatient clinic of the Stichting CardioZorg from November 2015 to March 2016 in 30 patients with an established diagnosis of ME/CFS and with orthostatic intolerance complaints. The diagnosis of ME/CFS was established according to both the ME and CFS criteria (Carruthers et al., 2011, Fukuda et al., 1994), taking exclusion criteria for ME/CFS and any other illness that could explain symptoms into account. All patients were evaluated by the same clinician (FVC). Disease severity was graded using the International Consensus Criteria (ICC) with severity scored as mild (approximately 50% reduction in activity), moderate (mostly housebound), or severe (mostly bedbound), very severe (bedbound and dependent on help for physical functions) (Carruthers et al., 2011). Very severe patients were not included because the tilt test was too taxing. Moreover, the clinician ascertained for the presence of orthostatic intolerance symptoms in daily life like dizziness/light-headedness, prior (near)-syncope, nausea, etc., as well as provoking events like standing in a line (Institute Of Medicine (IOM), 2015). Because of the suspicion of orthostatic intolerance in these patients, a tilt test was performed to ascertain the presence of orthostatic hypotension, POTS or an abnormal cerebral flow reduction. The use of heart rate and blood pressure lowering drugs was noted. After a preliminary analysis of the results, we decided to additionally study 40 consecutive ME/CFS patients who visited the outpatient clinic between August and December 2020.
The study was carried out in accordance with the Declaration of Helsinki. All ME/CFS participants gave informed, written consent. The use of descriptive clinical data of patients was approved by the medical ethics committee of the Slotervaart Hospital, Amsterdam, the Netherlands, P1450.
2.2. Head-up tilt test with cerebral blood flow measurements
Measurements were performed as described previously (van Campen et al., 2020c, van Campen et al., 2018). Briefly, all participants were positioned for 20 min supine before being tilted head-up to 70 degrees for a maximum of 30 min. The process of raising the tilt table to 70 degrees took approximately 30 s. Heart rate, systolic, and diastolic blood pressures were continuously recorded by finger plethysmography. Heart rate, blood pressure and cardiac index data were extracted from the device and imported into an Excel spreadsheet. To measure the time interval for the cardiac index by finger plethysmography in the post-tilt recovery phase to return to the pre-tilt cardiac index values, the time of the tilt-back was noted as well as the time point at which the mean of 1 min consecutive cardiac index values were starting to be equal or higher than the mean cardiac index values of the last minute pre-tilt. Internal carotid artery and vertebral artery Doppler flow velocity frames were acquired by one operator (FCV), using a Vivid-I system (GE Healthcare, Hoevelaken, the Netherlands) equipped with a 6–13 MHz linear transducer. High resolution B mode images, color Doppler images and the Doppler velocity spectrum (pulsed wave mode) were recorded in one frame. At least two consecutive series of six frames per artery were recorded.
Frames were recorded in the supine position approximately 8 min before the onset of the tilt period, while upright at the end of the tilt period, and starting at 5 min in the supine recovery phase of the test. Image acquisition for all 4 vessels at each time point (supine, end-tilt and post-tilt) lasted 3 ± 1 min.
Blood flow of the internal carotid and vertebral arteries was calculated offline by an investigator (CMCvC) who was unaware of the patient severity status. Blood flow in each vessel was calculated from the mean blood flow velocities × the vessel surface area and expressed in mL/minute. Flow in the individual arteries was calculated in 3–6 cardiac cycles and data were averaged. Total cerebral blood flow was calculated by adding the flow of the four arteries. We previously demonstrated that this methodology had good intra- and inter-observer variability (van Campen et al., 2018). Heart rate and blood pressure of the echo recording time intervals were averaged. End-tidal PCO2 was monitored using a Lifesense device (Nonin Medical, Minneapolis USA).
2.3. Doppler measurements for determination of cardiac index
The cardiac index is the cardiac output corrected for body surface area (BSA; Dubois formula), reported in L/min/m2. Measurements were performed as described previously (van Campen and Visser, 2018b). Briefly, the time-velocity integral (VTI) of the aorta was measured using a continuous wave Doppler pencil probe connected to a Vivid I machine (GE, Hoevelaken, NL) with the transducer positioned in the suprasternal notch. A maximal Doppler signal was assumed to be the optimal flow alignment. At least 2 frames of 6 s were obtained. Echo Doppler recordings were stored digitally.
VTI frames were obtained in the resting supine position after acquisition of the cerebral blood flow Doppler frames, while upright at the end of tilt testing, and immediately after measuring the post-tilt supine cerebral blood flow. In the post-test recovery phase VTI imaging started at 9 ± 1 min. VTI frame acquisition lasted <1 min.
We measured the diameter of the outflow tract at an earlier point using echocardiography. The aortic VTI was measured by manual tracing of at least 6 cardiac cycles, using the GE EchoPac post-processing software. This was done by one operator (CMCvC). Stroke volumes indices (stroke volume/BSA) were calculated from the VTI and the outflow tract area, corrected for the aortic valve area (Kusumoto et al., 1995, van Campen et al., 2006). Stroke volume index of separate cycles were averaged. The cardiac index was calculated from the heart rate and stroke volume index. We have previously validated this methodology by a direct comparison with cardiac index measurements using transthoracic VTI images from the apical 4-chamber view (van Campen and Visser, 2018b).
2.4. Classification of the hemodynamic changes during tilt testing
The changes in heart rate and blood pressure during tilt test were classified according to the consensus statement and guidelines (Freeman et al., 2011, Sheldon et al., 2015, Shen et al., 2017): a) normal heart rate and blood pressure response, b) Initial orthostatic hypotension, defined by a transient blood pressure decrease (40 mmHg systolic blood pressure and/or 20 mmHg diastolic blood pressure) within 15 s of standing, c) classic orthostatic hypotension, defined as a >20 mmHg reduction in systolic blood pressure and/or >10 mmHg reduction in diastolic blood pressure within 3 min of the start of standing. In the event of a baseline systolic blood pressure over 160 mmHg, a blood pressure reduction of >30 mmHg was required (Fedorowski et al., 2009). d) Delayed orthostatic hypotension defined using the same criteria as for classic orthostatic hypotension, but with an onset after 3 min of the start of the upright position. e) Postural orthostatic tachycardia syndrome (POTS), defined by a sustained increase in heart rate of 30 bpm or more within 10 min of standing, without an abnormal blood pressure response, and f) syncope or near-syncope.
2.5. Statistical analysis
Data were analyzed using Graphpad Prism version 8.4.2 (Graphpad software, La Jolla, California, USA) and using SPSS version 21 (IBM, USA). All continuous data were tested for normal distribution using the D’Agostino-Pearson omnibus normality test, and presented as mean (SD) or as median (IQR), where appropriate. Nominal data were compared using the Chi-square test (up to a 3×3 table). Groups were compared using the unpaired t-test or the Mann-Whitney U test where appropriate. Within group comparison was performed using the paired t-test or the Wilcoxon signed ranks test where appropriate. Within group comparison was done by the ordinary one way variance of analysis (ANOVA) or Kruskal-Wallis test where appropriate. Where significant, results were then explored further using the post-hoc Tukey’s test or post-hoc Dunn’s correction where appropriate. A standard multiple regression analysis was performed according to Laerd Statistics (Statistics, 2015) with creation of dummy variables for the severity scales and hemodynamic abnormalities. Initially, the dependent variable: the ratio of post-tilt cerebral blood flow/supine cerebral blood flow was tested against gender, age, disease duration, fibromyalgia, hemodynamic abnormalities, severity scales, heart rate in the recovery phase, the ratio: end-tidal PCO2 recovery phase end-tidal PCO2 supine, and the ratio: cardiac index in the recovery phase/cardiac index supine. We assessed the independence of residuals, linearity, homoscedasticity, multicollinearity, outliers, high leverage points, influential points and normality of standardized residuals using P-P plots. As disease severity was highly correlated with the ratio post-tilt cerebral blood flow/supine cerebral blood flow, we next performed an hierarchical multiple regression to determine whether factors other than disease severity could significantly add to the model. To model 1: disease severity (mild, moderate, and severe) the following models were added, model 2: adding the ratio: cardiac index in the recovery phase/cardiac index supine to model 1, model 3: adding normal heart rate and blood pressure, delayed orthostatic hypotension and POTS to model 2, model 4: adding the ratio: end-tidal PCO2 recovery phase/ end-tidal PCO2 supine to model 3, model 5: adding age, disease duration, gender and fibromyalgia to model 4, model 6: adding heart rate post-tilt and the difference between heart rate post-tilt and heart rate supine to model 5. Because of the large number of comparisons, we considered a p-value of <0.01 to be statistically significant.
3. Results
Of the 40 individuals evaluated in the second study period, 38 patients were diagnosed as having ME/CFS. Two had unexplained chronic fatigue, but not ME/CFS, and were excluded. Of the 38 with ME/CFS, two patients had no orthostatic intolerance and three were under the age of 18, and were also excluded. One patient had a very severe form of ME/CFS and orthostatic intolerance, but a tilt test was not performed because it was deemed too taxing. A tilt test was performed in the remaining 32 patients. Two patients had no cerebral blood flow measurements at the end of the study because the sudden development of syncope did not allow sufficient time for image acquisition. This left 30 new patients meeting study eligibility criteria and with complete data. None of the patients were using heart rate or blood pressure lowering drugs.
As patient characteristics of the pilot study and of the prospective study were not different (data not shown), we combined the two groups, leading to 60 patients with ME/CFS for analysis. Baseline characteristics of the complete study group were as follows: mean age 39 (12) year, duration of disease 8 (4–12) years, height 172 (9) cm, weight 72 (17) kg, BSA 1.84 (0.21) m2 and body mass index (BMI) 24.6 (5.7) kg/m2. Daily-life orthostatic intolerance symptoms in this patient group were reported by all 60 (100%) ME/CFS patients. Tilt-test results showed that 30/60 (50%) had a normal heart rate and blood pressure response, 4/60 (7%) had delayed orthostatic hypotension, and 26/60 (43%) had POTS. None of the patients showed an initial or classic orthostatic hypotension. Tilt duration was median 10 min, interquartile range (IQR) 7–14 min. Sixteen patients were graded according to the ICC as having mild disease, 28 as having moderate disease, and 16 as having severe disease.
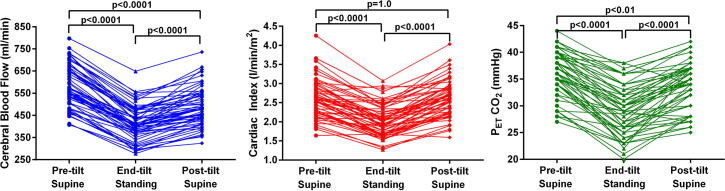
Fig. 1 shows the supine, end-tilt, and the supine recovery phase of cerebral blood flow, cardiac index and PETCO2 for all patients. Supine cerebral blood flow was 584 (96) ml/min, at end-tilt 415 (76) ml/min, and at post-tilt 489 (86) ml/min. All three time points were statistically significantly different (p-values < 0.0001), indicating that the cerebral blood flow in the supine recovery phase was still significantly lower than the supine pre-tilt cerebral blood flow values. Supine cardiac index was 2.61 (0.49) L/min/m2, at end-tilt 2.02 (0.42) L/min/m2 and at the supine post-tilt recovery phase 2.61 (0.48) L/min/m2. Both supine pre-tilt and supine post-tilt cardiac index were significantly different from the upright end-tilt measurements (both p-values <0.0001); the supine cardiac index in the recovery phase had returned to the pre-tilt supine cardiac index values. Supine PETCO2 was 36 (4) mmHg, at end-tilt 29 (5) mmHg and at post-tilt 34 (4) mmHg. All three time points were statistically significantly different with p-values ranging between <0.01 and <0.0001, indicating that the supine PETCO2 in the recovery phase was still significantly lower than the pre-tilt supine PETCO2 values.
Fig. 1.
Cerebral blood flow, cardiac index, and end-tidal PCO2 results for all patients, as measured in the supine position pre-tilt, at the end of tilt phase, and post-tilt recovery phase. Cerebral blood flow, cardiac index, and end-tidal PCO2 results for all patients, as measured in the supine position pre-tilt, at the end of tilt phase, and post-tilt recovery phase. PETCO2: end-tidal PCO2; blue points and lines: cerebral blood flow data; red points and lines: cardiac index data; green points and lines: end-tidal PCO2 data. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
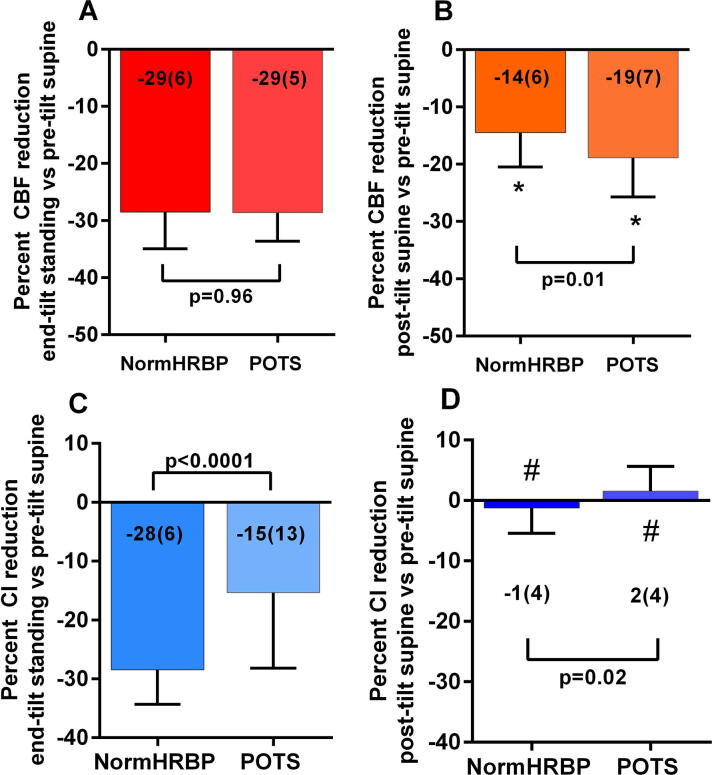
A further division was made based on the different hemodynamic profiles (normal heart rate and blood pressure response vs POTS), the PETCO2 at the end of the tilt phase (<30 mmHg vs ≥30 mmHg) and the disease severity (mild, moderate and severe). Due to the low numbers, patients having delayed orthostatic hypotension were not analyzed. Table 1a, Table 1b shows the demographic (a) and tilt table test characteristics (b) in the group with normal heart rate and blood pressure response compared to the patients showing POTS. By definition, a significantly higher heart rate at end-tilt was found in patients with POTS. A significant difference with a higher heart rate in the patients with POTS compared to patients with normal heart rate and blood pressure response was also found in the supine recovery phase post-test (p = 0.009). No other variables were significantly different. Fig. 2 shows the percent cerebral blood flow and cardiac index reduction. ME/CFS patients with a normal heart rate and blood pressure response and those with POTS had similar reductions in cerebral blood flow from their supine pre-tilt values while upright at end-tilt (Fig. 2a). The groups did not differ significantly in the degree of cerebral blood flow reduction in the supine recovery phase post-tilt (Fig. 2b). The cerebral blood flow reduction of the post-tilt recovery phase for both groups was significantly lower than zero: both p < 0.0001). The cardiac index reduction at end-tilt was significantly higher in patients with a normal heart rate and blood pressure response compared to patients with POTS (p < 0.0001) (Fig. 2c), but the cardiac index reduction at the supine post-tilt recovery phase did not differ between groups and was not significantly different from zero (Fig. 2d).
Table 1a.
Demographic data of ME/CFS patients with a normal heart rate and blood pressure response during tilt testing compared to ME/CFS patients with POTS.
| NormHRBP (n = 30) |
POTS (n = 26) |
p-value | |
|---|---|---|---|
| Demographic characteristics: | |||
| Age (years) | 42 (14) | 36 (10) | 0.05 |
| Male/Female | 4/26 | 4/22 | 0.83 * |
| Mild/moderate/severe disease | 10/16/4 | 3/12/11 | 0.03 * |
| Height (cm) | 169 (8) | 173 (8) | 0.06 |
| Weight (kg) | 71 (15) | 72 (20) | 0.95 |
| BMI (kg/m2) | 25.0 (5.4) | 24.1 (7.0) | 0.46 |
| BSA (duBois; m2) | 1.81 (0.19) | 1.84 (0.23) | 0.61 |
| Disease duration (years) | 8 (4–15) | 8 (3–10) | 0.18 † |
BMI: body mass index; BSA: body surface area; formula of Dubois; normHRBP: normal heart rate and blood pressure response; POTS: postural orthostatic tachycardia syndrome; * Chi-square; † Mann-Whitney U test.
Table 1b.
Tilt-test data of ME/CFS patients with a normal heart rate and blood pressure response during tilt testing compared to ME/CFS patients with POTS.
| NormHRBP (n = 30) |
POTS (n = 26) |
p-value | |
|---|---|---|---|
| Tilt test results: | |||
| HR supine (bpm) | 73 (13) | 80 (11) | 0.04 |
| HR end-tilt (bpm) | 89 (13) | 117 (15) | <0.0001 |
| HR post-tilt (bpm) | 69 (10) | 77 (11) | 0.009 |
| SBP supine (mmHg) | 142 (24) | 135 (10) | 0.21 |
| SBP end-tilt (mmHg) | 139 (27) | 131 (15) | 0.18 |
| SBP post-tilt (mmHg) | 138 (20) | 132 (12) | 0.20 |
| DBP supine (mmHg) | 86 (12) | 83 (9) | 0.36 |
| DBP end-tilt (mmHg) | 92 (16) | 91 (12) | 0.68 |
| DBP post-tilt (mmHg) | 83 (13) | 84 (9) | 0.80 |
| PETCO2 supine (mmHg) | 37 (4) | 35 (4) | 0.16 |
| PETCO2 end-tilt (mmHg) | 30 (5) | 27 (5) | 0.02 |
| PETCO2 post-tilt (mmHg) | 35 (4) | 32 (4) | 0.02 |
| CI supine (L/min/m2) | 2.64 (0.47) | 2.61 (0.52) | 0.83 |
| CI end-tilt (L/min/m2) | 1.90 (0.39) | 2.18 (0.40) | 0.01 |
| CI post-tilt (L/min/m2) | 2.61 (0.49) | 2.64 (0.50) | 0.81 |
| CBF supine (ml/min) | 592 (101) | 578 (95) | 0.59 |
| CBF end-tilt (ml/min) | 422 (79) | 412 (76) | 0.63 |
| CBF post-tilt (ml/min) | 506 (92) | 467 (70) | 0.08 |
CBF: cerebral blood flow; CI: cardiac index; DBP: diastolic blood pressure; HR: Heart rate; normHRBP: normal heart rate and blood pressure response; PET: end-tidal pressure; POTS: postural orthostatic tachycardia syndrome; SBP: systolic blood pressure.
Fig. 2.
Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the supine pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a normal HR and BP response and in patients with POTS. Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the supine pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a normal heart rate and blood pressure response and in patients with POTS. CBF: cerebral blood flow; normHRBP: normal heart rate and blood pressure response; CI: cardiac index; POTS: postural orthostatic tachycardia syndrome; *: columns significantly different from zero (P < 0.0001); #: columns not significantly different from zero.
Table 2a, Table 2b shows the demographic (a) and tilt table test characteristics (b) in the patient group with an PETCO2 at the end of the head-up tilt test <30 mmHg and those with an PETCO2 ≥ 30 mmHg at the end of the head-up tilt test. Significantly more female patients were present in the group with an PETCO2 < 30 mmHg compared to the group with an PETCO2 ≥ 30 mmHg (p = 0.008). By definition, the PETCO2 at the end-tilt phase was lower in the patients with an PETCO2 < 30 mmHg compared to the group with an PETCO2 ≥ 30 mmHg (p < 0.0001), but was also lower in the pre-tilt supine position and in the post-tilt supine recovery phase (both p < 0.0001). Absolute cerebral blood flow values pre-tilt, at end-tilt, and in the supine post-tilt recovery phase were lower in the patients with an PETCO2 < 30 mmHg compared to the group with an PETCO2 ≥ 30 mmHg (p ranging between 0.005 and 0.0006).
Table 2a.
Demographic data of ME/CFS patients with an end-tidal CO2 < 30 mmHg or ≥ 30 mmHg at the end of the tilt test.
| PETCO2 < 30 mmHg at end-tilt (n = 31) |
PETCO2 ≥ 30 mmHg at end-tilt (n = 29) |
p-value | |
|---|---|---|---|
| Demographic characteristics: | |||
| Age (years) | 41 (14) | 37 (11) | 0.30 |
| Male/Female | 1/30 | 8/21 | 0.0083 * |
| Mild/moderate/severe disease | 7/12/12 | 9/16/4 | 0.09 * |
| Height (cm) | 172 (7) | 171 (10) | 0.71 |
| Weight (kg) | 70 (14) | 74 (20) | 0.34 |
| BMI (kg/m2) | 23.8 (4.6) | 25.4 (6.8) | 0.27 |
| BSA (duBois; m2) | 1.82 (0.17) | 1.86 (0.24) | 0.51 |
| Disease duration (years) | 7 (3–12) | 8 (4–14) | 0.62 † |
BMI: body mass index; BSA: body surface area; formula of Dubois; mild/moderate/severe disease: according to the ICC criteria; normHRBP: normal heart rate and blood pressure response; POTS: postural orthostatic tachycardia syndrome; * Chi-square; † Mann-Whitney U test.
Table 2b.
Tilt-test data of ME/CFS patients with an end-tidal CO2 < 30 mmHg or ≥ 30 mmHg at the end of the tilt test.
| PETCO2 < 30 mmHg at end-tilt (n = 31) |
PETCO2 ≥ 30 mmHg at end-tilt (n = 29) |
p-value | |
|---|---|---|---|
| Tilt test results: | |||
| NormHRBP/dOH/POTS | 10/3/18 | 20/1/8 | 0.02 * |
| HR supine (bpm) | 79 (13) | 75 (12) | 0.22 |
| HR end-tilt (bpm) | 106 (20) | 97 (18) | 0.08 |
| HR post-tilt (bpm) | 74 (12) | 72 (10) | 0.38 |
| SBP supine (mmHg) | 142 (19) | 137 (18) | 0.24 |
| SBP end-tilt (mmHg) | 135 (24) | 134 (21) | 0.95 |
| SBP post-tilt (mmHg) | 138 (18) | 133 (15) | 0.26 |
| DBP supine (mmHg) | 86 (12) | 83 (10) | 0.30 |
| DBP end-tilt (mmHg) | 89 (15) | 92 (13) | 0.41 |
| DBP post-tilt (mmHg) | 85 (12) | 81 (9) | 0.20 |
| PETCO2 supine (mmHg) | 34 (3) | 39 (2) | <0.0001 |
| PETCO2 end-tilt (mmHg) | 25 (3) | 33 (3) | <0.0001 |
| PETCO2 post-tilt (mmHg) | 31 (4) | 36 (3) | <0.0001 |
| CI supine (L/min/m2) | 2.63 (0.55) | 2.59 (0.42) | 0.74 |
| CI end-tilt (L/min/m2) | 2.08 (0.47) | 1.96 (0.33) | 0.27 |
| CI post-tilt (L/min/m2) | 2.66 (0.52) | 2.56 (0.43) | 0.43 |
| CBF supine (ml/min) | 552 (101) | 620 (78) | 0.005 |
| CBF end-tilt (ml/min) | 383 (70) | 449 (70) | 0.0006 |
| CBF post-tilt (ml/min) | 454 (74) | 527 (83) | 0.0007 |
CBF: cerebral blood flow; CI: cardiac index; DBP: diastolic blood pressure; HR: Heart rate; normHRBP: normal heart rate and blood pressure response; PET: end-tidal pressure; POTS: postural orthostatic tachycardia syndrome; SBP: systolic blood pressure; * Chi-square.
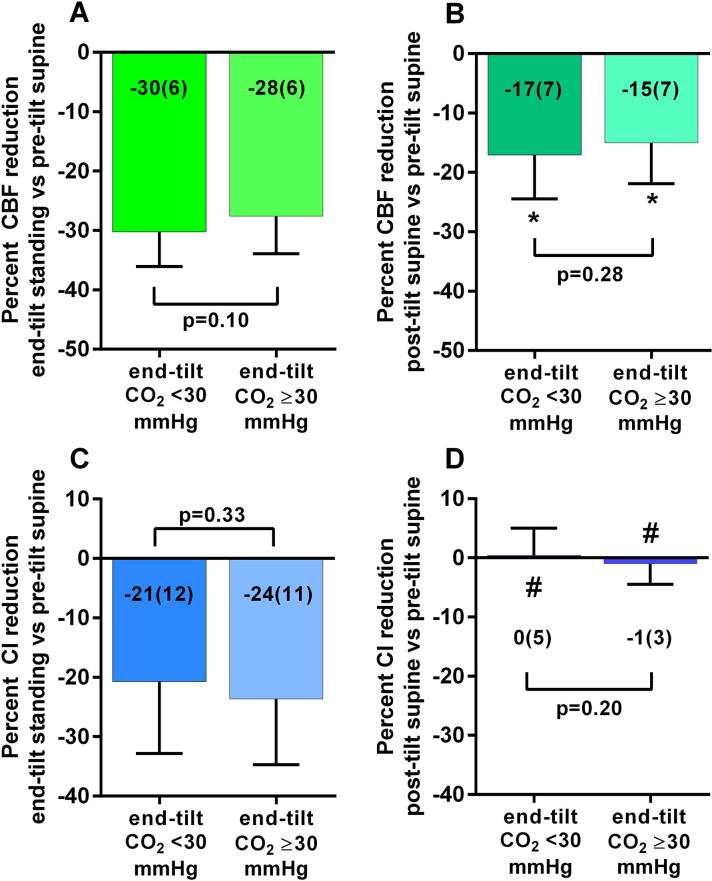
Fig. 3 shows the percent reduction in cerebral blood flow and cardiac index for the two PETCO2 groups. No significant differences between patients with and without an PETCO2 < 30 mmHg were found at end-tilt (Fig. 3a; p = 0.10) or post-tilt (Fig. 3b; p = 0.28) compared to supine values. In both groups the cerebral blood flow reduction in the recovery phase was significantly lower than zero (Fig. 3c; p < 0.0001). No significant differences in cardiac index changes between patients with and without an PETCO2 < 30 mmHg were found at end-tilt compared to supine pre-tilt (Fig. 3c; p = 0.33). No significant differences in cardiac index changes between patients with and without an PETCO2 < 30 mmHg were found in the supine post-tilt recovery phase compared to pre-tilt supine values (Fig. 3d; p = 0.20). The cardiac index reduction in the recovery phase was not different from zero in either group.
Fig. 3.
Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a reduced PETCO2 (<30 mmHg) vs patients with a normal PETCO2 (≥30 mmHg) at the end of the tilt phase. Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a reduced end-tidal PCO2 (<30 mmHg) vs patients with a normal end-tidal PCO2 (≥30 mmHg) at the end of the tilt phase. CBF: cerebral blood flow; CI: cardiac index; CO2: P end-tidal CO2; *: columns significantly different from zero (p < 0.0001); #: columns not significantly different from zero.
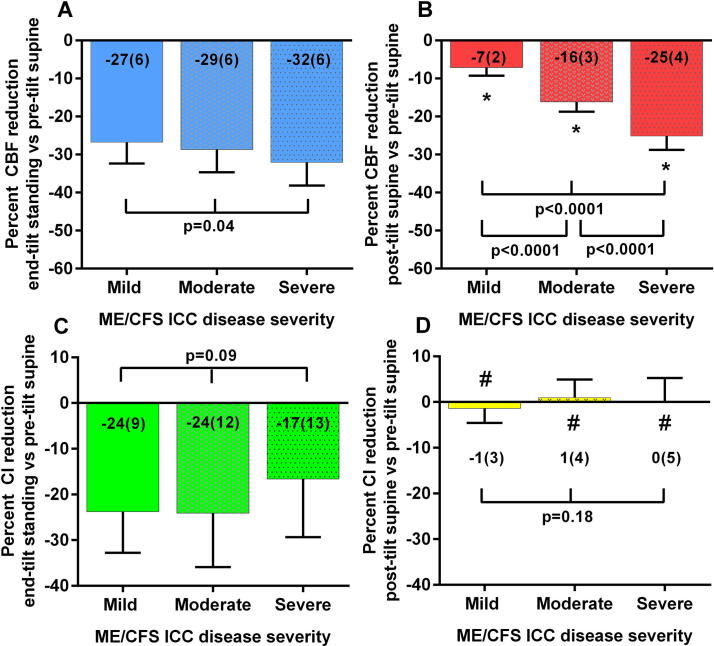
Table 3a, Table 3b shows the demographic (a) and tilt table test characteristics (b) for ME/CFS patients with a mild, moderate, or severe disease according to the International Concensus Criteria (Carruthers et al., 2011). No differences in baseline characteristics were found. During the tilt test a significantly higher heart rate was found in patients with severe versus mild disease (p = 0.005). Fig. 4 shows the percent cerebral blood flow and cardiac index results for the 3 disease severity groups. No significant differences between groups were found for the percent cerebral blood flow reduction at end-tilt compared to the pre-test supine values (Fig. 4a; p = 0.04). Supine post-tilt cerebral blood flow reduction was significantly lower than pre-test supine values for all three groups (all p < 0.0001). There were differences in the degree of post-tilt supine values between groups, with increasing cerebral blood flow reductions from mild to severe disease (Fig. 4b; all p < 0.0001). No significant differences were found for the percent reduction in cardiac index at end-tilt (Fig. 4c; p = 0.09). The percent cardiac index reduction did not differ between the pre-tilt and post-tilt supine phases (Fig. 4d; p = 0.18).
Table 3a.
Demographic data of ME/CFS patients subdivided according to the ICC severity grading: mild, moderate and severe ME/CFS.
| Group 1 Mild (n = 16) |
Group 2 Moderate (n = 28) |
Group 3 Severe (n = 16) |
Ordinary one-way ANOVA with post hoc Tukey’s test/Kruskal-Wallis with post hoc Dunn’s correction | |
|---|---|---|---|---|
| Baseline characteristics: | ||||
| Age (years) | 44 (15) | 38 (12) | 37 (10) | F (2, 57) = 1.74; p = 0.18 # |
| Male/Female | 5/11 | 4/24 | 0/16 | Chi-square (3x2) = 0.05 * |
| Height (cm) | 174 (10) | 171 (8) | 170 (8) | F (2, 57) = 0.69; p = 0.51 # |
| Weight (kg) | 78 (18) | 70 (16) | 70 (18) | F (2, 57) = 1.07; p = 0.35 # |
| BMI (kg/m2) | 25.8 (5.8) | 24.0 (5.0) | 24.4 (7.0) | F (2, 57) = 0.46; p = 0.64 # |
| BSA (duBois; m2) | 1.91 (0.23) | 1.81 (0.21) | 1.80 (0.18) | F (2, 57) = 1.46; p = 0.24 # |
| Disease duration (years) | 6 (4–12) | 8 (4–15) | 7 (3–12) | X = 0.92; p = 0.64 ǂ |
BMI: body mass index; BSA: body surface area; formula of Dubois; * Chi-square; # one-way ANOVA with post hoc Tukey’s test; ǂ Kruskal-Wallis with post hoc Dunn’s correction.
Table 3b.
Tilt-test data of ME/CFS patients subdivided according to the ICC severity grading: mild, moderate and severe ME/CFS.
| Group 1 Mild (n = 16) |
Group 2 Moderate (n = 28) |
Group 3 Severe (n = 16) |
Ordinary one-way ANOVA with post hoc Tukey’s test/Kruskal-Wallis with post hoc Dunn’s correction | |
|---|---|---|---|---|
| Tilt test results: | ||||
| normHRBP/dOH/POTS | 10/3/3 | 16/0/12 | 4/1/11 | Chi-square (3x3) = 0.01 * |
| HR supine (bpm) | 75 (15) | 74 (10) | 84 (11) | F (2, 57) = 3.73; p = 0.03 # |
| HR end-tilt (bpm) | 94 (16) | 99 (18) | 115 (20) | F (2, 57) = 5.83; p = 0.005; 1 vs 3; p = 0.005 # |
| HR post-tilt (bpm) | 71 (12) | 71(10) | 78 (11) | F (2, 57) = 2.43; p = 0.10 # |
| SBP supine (mmHg) | 144 (22) | 135 (16) | 143 (19) | F (2, 57) = 1.43; p = 0.25 # |
| SBP end-tilt (mmHg) | 133 (20) | 136 (26) | 134 (20) | F (2, 57) = 0.07; p = 0.93 # |
| SBP posttest (mmHg) | 136 (16) | 134 (18) | 138 (16) | F (2, 57) = 0.29; p = 0.75 # |
| DBP supine (mmHg) | 86 (10) | 83 (11) | 85 (12) | F (2, 57) = 0.32; p = 0.73 # |
| DBP end-tilt (mmHg) | 87 (11) | 92 (17) | 92 (12) | F (2, 57) = 0.85; p = 0.43 # |
| DBP post-tilt (mmHg) | 80 (8) | 84 (13) | 84 (9) | F (2, 57) = 0.75; p = 0.48 # |
| PETCO2 supine (mmHg) | 38 (4) | 36 (4) | 35 (3) | F (2, 57) = 2.65; p = 0.08 # |
| PETCO2 end-tilt (mmHg) | 31 (5) | 29 (5) | 26 (5) | F (2, 57) = 3.31; p = 0.04 # |
| PETCO2 post-tilt (mmHg) | 35 (5) | 34 (4) | 32 (4) | F (2, 57) = 2.08; p = 0.13 # |
| CI supine (L/min/m2) | 2.49 (0.29) | 2.60 (0.51) | 2.74 (0.58) | F (2, 57) = 1.07; p = 0.35 # |
| CI end-tilt (L/min/m2) | 1.89 (0.31) | 1.96 (0.39) | 2.26 (0.45) | F (2, 57) = 4.23; p = 0.02 # |
| CI post-tilt (L/min/m2) | 2.45 (0.29) | 2.63 (0.52) | 2.74 (0.55) | F (2, 57) = 1.50; p = 0.23 # |
| CBF supine (ml/min) | 572 (110) | 578 (92) | 610 (86) | F (2, 57) = 0.78; p = 0.46 # |
| CBF end-tilt (ml/min) | 419 (90) | 412 (71) | 416 (75) | F (2, 57) = 0.05; p = 0.95 # |
| CBF post-tilt (ml/min) | 532 (106) | 484 (75) | 457 (69) | F (2, 57) = 3.36; p = 0.04 # |
CBF: cerebral blood flow; CI: cardiac index; DBP: diastolic blood pressure; dOH: delayed orthostatic hypotension; HR: heart rate; normHRBP: normal heart rate and blood pressure; PET: end-tidal pressure; POTS: postural orthostatic tachycardia syndrome; SBP: systolic blood pressure; * Chi-square; # one-way ANOVA with post hoc Tukey’s test.
Fig. 4.
Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a mild, moderate and severe disease according to the ICC criteria (Carruthers et al., 2011). Percent cerebral blood flow reduction and percent cardiac index reduction, relative to the pre-tilt values, at end-tilt, and in the supine recovery phase post-tilt, in patients with a mild, moderate and severe disease according to the ICC criteria (Carruthers et al., 2011). CBF: cerebral blood flow; CI: cardiac index; *: columns significantly different from zero (p < 0.0001); #: columns not significantly different from zero.
The time interval at which the cardiac index by finger plethysmography in the post-tilt recovery phase had returned to values equal or above the supine baseline cardiac index values was a median of 36 s (Interquartile Range (IQR) 31–62 s).
The multiple regression analysis showed that only disease severity was linearly related with the percent cerebral blood flow reduction in the post-tilt recovery phase relative to the pre-tilt supine cerebral blood flow values: taking mild disease as the reference, both moderate and severe disease were significantly and linearly related to a higher degree of percent cerebral blood flow reduction in the post-recovery phase: both p < 0.0001. Hierarchal multiple regression analysis showed that disease severity was the most important predictor of the percent cerebral blood flow reduction in the post-tilt recovery phase (F change (2,57) = 148.730, p < 0.0001), the ratio: cardiac index in the recovery phase/cardiac index supine added marginally to the model 1: F change (3,56) = 4.135, p = 0.047. Other clinical and tilt test characteristics did not significantly add to the percent cerebral blood flow reduction post-tilt.
4. Discussion
The main and novel finding of this study is that recovery of cerebral blood flow after tilt testing in ME/CFS patients is incomplete when extracranial Doppler imaging is performed starting 5 min after returning to the supine position. Recovery was significantly slower in the more severely affected patients (see Fig. 4). In contrast, cardiac index had returned to the supine pre-test values in all three severity groups. Disease severity was the best predictor for the degree of the relative cerebral blood flow reduction in the recovery phase; none of the other measured clinical (age, gender, BMI, BSA, disease duration) and tilt test parameters (hemodynamic categories, heart rate, blood pressure, end-tidal PCO2 and cardiac index) affected the cerebral blood flow reduction post-tilt.
The mechanisms of the cerebral flow regulation are complex and have been studied in a variety of groups, including healthy and diseased individuals. Those mechanisms involve the cerebral perfusion pressure, PO2 and PCO2, flow-metabolism coupling, innervation of cerebral vessels, and blood viscosity: see for a recent review Castle-Kirszbaum et al. (2021). The mechanisms for the slow cerebral blood flow recovery and the dependence of the cerebral blood flow recovery on the severity of ME/CFS patients are unknown.
As systolic and diastolic pressures are similar between the mild, moderate and severe patient groups, the perfusion pressure is not likely responsible for the differences in cerebral flow recovery.
Moreover, the slower recovery of cerebral blood flow in severe ME/CFS patients may be related to a lower end-tidal PCO2 compared to the mild and moderately affected patients. ANOVA analysis showed that there is a trend towards lower end-tidal PCO2 levels at end-tilt from mild to moderate to severe patients. As previous studies have shown that a PCO2 reduction leads to cerebral artery constriction and a reduced cerebral blood flow (see for a review Hoiland et al.) (Hoiland et al., 2019), this mechanism may contribute to the slower recovery in these patients. Nevertheless, the multiple and hierarchical regression analysis showed that the end-tidal PCO2 did not contribute to the influence of disease severity on cerebral blood flow reduction at post-tilt. However, this subgroup analysis only included a limited number of patients per severity group. Therefore, a larger study is needed to more confidently determine the influence of PCO2 on the cerebral blood flow recovery rate.
Studies in hibernating mammals show that they experience extreme metabolic states and body temperature changes as they transition between euthermia, a state resembling typical warm blooded mammals, and prolonged torpor, a state of suspended animation where the brain receives as low as 10% of normal cerebral blood flow (Dave et al., 2012). The heart can show a hibernation response following flow reduction due to obstructive coronary artery disease (Kloner, 2020). In the setting of low blood flow (reduced oxygen supply), there is an adaptive downregulation of function (reduced oxygen demand) and metabolism to minimize ischemia and prevent myocardial necrosis. Whether this occurs in the brain and whether it is dependent on disease severity, and helps explain the delayed recovery of cerebral blood flow, would need to be examined.
Theoretically, the lower cerebral blood flow values post-tilt can be due to decreased arterial supply, and reduced venous return to the heart. The cardiac index in the recovery phase post-tilt had returned to pre-tilt values, making it unlikely that decreased arterial supply is the underlying mechanism of the reduced cerebral blood flow post-tilt. Venous flow restriction, as is present in venous thoracic outlet syndrome, may be hypothesized to cause lower cerebral blood flow (Moore and Wei Lum, 2015), however this is an unlikely cause in the present study as supine pre-tilt and supine post-tilt were measured in the same horizontal position.
Two studies have reported endothelial dysfunction in ME/CFS patients measuring post-occlusion flow-mediated dilation of the large brachial arteries and the post-occlusion microcirculatory response in the skin (Newton et al., 2012, Scherbakov et al., 2020). Although endothelial dysfunction in the arm needs to be coupled to a possible macro- and microvascular dysfunction in the brain, an interesting observation in the study of Scherbakov et al. is that the degree of endothelial dysfunction is positively related to the extent of symptoms: the larger the endothelial dysfunction, the higher the disease severity (Scherbakov et al., 2020). After a mild ischemic stress, like a tilt test with a short-lasting cerebral blood flow reduction, it is possible that a similar endothelial dysfunction is responsible for delayed recovery of cerebral blood flow.
Catecholamines and many other neurohormones increase during a tilt test and return to normal values post-test (Jardine et al., 1997). The rise and subsequent fall in catecholamines may induce a temporarily cerebral vasodilation and an increase in cerebral blood flow, followed by a reduction in cerebral flow (Bola and Kiyatkin, 2018). As we did not measure catecholamines, the effects on cerebral flow changes in the three different patient groups need to be studied in the future.
Finally, a decrease/increase in blood viscosity may increase/decrease cerebral flow. However, as shear stress is the driving force for vascular tone, a reduction of blood viscosity (with increased flow) is counterbalanced by the reduction of the shear stress, with arteriolar vasoconstriction. Therefore, blood viscosity may have limited influence on cerebral blood flow (Hoiland et al., 2019).
Cardiac index measurements were performed after the cerebral blood flow measurements, starting at mean 9 min in the post-test recovery phase. Because cerebral blood flow measurements started at 5 min in the post-test recovery phase, it could be argued that cardiac index had more time to return to the baseline supine measurements. To test this, we explored the recovery speed of the finger plethysmography derived cardiac index data; results showed that the cardiac index had returned to supine pre-tilt values within 1 min after the start of the tilt-back. We recently demonstrated that Nexfin cardiac index and stroke volume indices (SVI) underestimate the changes during tilt testing as compared to Doppler derived SVI and cardiac index measurements (van Campen et al., 2021b). Although this underestimation of cardiac index changes by Nexfin may result in a faster recovery compared to Doppler measurements, we can be reasonably certain that the cardiac index had returned to baseline values before the start of the cerebral blood flow measurements.
We found in this study that post-tilt cerebral blood flow was reduced in all ME/CFS patients irrespective of the disease severity. In studies using transcranial Doppler, little attention has been paid in different diseases to the recovery speed of the cerebral blood flow velocity after being tilted back to the horizontal position. One study in orthostatic intolerance patients showed that cerebral blood flow velocities returned to normal within the first 2 min post-tilt (Novak et al., 1998). Patient examples from the same investigators showed the same rapid recovery trends in POTS patients, hypocapnic cerebral hypoperfusion patients, and in orthostatic cerebral hypoperfusion syndrome patients (Novak, 2016, Novak, 2018). Whether the slow return to baseline supine values in ME/CFS patients is a unique feature of the disease remains to be determined.
Several clinical implications of this study warrant further emphasis. First, the finding of a greater decline in cerebral blood flow after the completion of an orthostatic stress test in those with severe ME/CFS is consistent with the clinical observation that more severely ill patients often need a longer recovery time after any activity than patients with a milder degree of ME/CFS. We have recently demonstrated that orthostatic stress can provoke prolonged post-exertional symptoms in ME/CFS patients (van Campen et al., 2021a). Whether the duration and severity of the post-exertional symptoms is related to the degree of cerebral blood flow recovery post testing will need to be assessed in future studies.
Second, our study shows that in response to head-up tilt, ME/CFS patients develop a clinically important cerebral blood flow reduction regardless of their hemodynamic response to tilt test. We found no significant differences in cerebral blood flow reductions between groups with a normal heart rate and blood pressure response, delayed orthostatic hypotension, or POTS. This suggests that the degree of cerebral blood flow reduction is a more important contributor to orthostatic intolerance symptoms than the type of hemodynamic of heart rate and blood pressure response. The fact that the hemodynamic results of the orthostatic stress test are not predictive of delayed cerebral blood flow recovery has been confirmed by other groups in different patient populations (Novak, 2018, Park et al., 2017, Shin et al., 2015) using transcranial Doppler. In our previous study, the cerebral blood flow reduction was less severe in patients with a normal heart rate and blood pressure response, compared to patients with delayed orthostatic hypotension and POTS (van Campen et al., 2020c). The differences can be explained by inclusion differences: in the former study, patients with a normal heart rate and blood pressure response and without orthostatic intolerance were included as part of the prospective study. In the present study patients without orthostatic intolerance were excluded. These patients without orthostatic intolerance almost all had a normal heart rate and blood pressure response and a normal or near-normal cerebral blood flow reduction during tilt testing, diluting the effect of cerebral blood flow reduction in patients with a normal heart rate and blood pressure response. The fact that the cerebral blood flow reduction at end-tilt had no effect on the cerebral blood flow reduction in the recovery phase post-tilt indicates that other mechanisms may play a role.
Third, a large number of studies using transcranial Doppler have shown that cerebral blood flow velocity is influenced by PETCO2 (Del Pozzi et al., 2014, Immink et al., 2014, Laffey and Kavanagh, 2002, Novak et al., 1998, Sato et al., 2012, Stewart et al., 2018a, Stewart et al., 2018b). Using extracranial Doppler, this finding was also confirmed in our previous study (van Campen et al., 2020c). Similarly, in the present study, the PETCO2 values at the end of the tilt phase and in the recovery phase were not predictive of the post-tilt cerebral blood flow reduction (Fig. 3 and multiple regression analysis). The exact mechanism of the persistent cerebral blood flow reduction needs further study.
Limitations: we cannot exclude the possibility that referral bias may have created a study sample that differs from the general population of those with ME/CFS. However, the cerebral blood flow reductions during tilt in this sample were similar to the cerebral blood flow reductions we had reported in our larger study of 429 adults with ME/CFS. We elected not to expose those with the most severe functional impairments to tilt testing, but inclusion of the most impaired patients would not have been expected to change the direction of the results. Whether disease severity differences lead to differences in cerebral blood flow reduction during daily-life stressors like sitting/standing or performing mental tasks will need to be studied in the future. Individuals with ME/CFS have been reported to have variable function from day to day and week to week. The influence of these variations also needs to be studied. Our focus was on the prevalence and risk factors for persistent reductions of cerebral blood flow after head-up tilt testing; investigations of cerebral autoregulation and regional cerebral blood flow were beyond the scope of this study, but these topics would be important to investigate in the future. Moreover, whether the findings of post-tilt testing cerebral blood flow reductions impact the severity and duration of post-exertional malaise needs to be investigated further. Also, we only measured cerebral blood flow at one time-point in the recovery phase, namely at 5 min in the recovery phase. Multiple time-point cerebral blood flow studies are needed to estimate when cerebral blood flow returns to normal post-tilt. Furthermore, a distinction is made e.g. in the American consensus statement of Shen et al (Shen et al., 2017) between POTS and postural tachycardia. POTS is a combination of the heart rate increase and symptomatology, whereas postural tachycardia is defined as a heart rate increase only without orthostatic symptoms. In theory, the orthostatic intolerance complaints of the studied POTS patients may not be due to POTS but due to another orthostatic intolerance mechanism like venous pooling. In this study we could not discriminate between these two and therefore ascribed the orthostatic intolerance symptomatology to the POTS. Further studies are needed to explore this potential difference. Finally, we did not study the post-tilt cerebral blood flow in healthy controls. However, in a previous study the reduction of cerebral blood flow during the tilt test in healthy controls was mean 6% (van Campen et al., 2020c), which is much less than the mean 29% reduction in the patients of the present study. Given this limited reduction in cerebral blood flow in healthy controls during the tilt test, and the data of Novak et al. showing a rapid return to baseline flow velocity values in healthy controls (Novak et al., 1998), we believe it is unlikely that healthy controls have a prolonged post-tilt recovery cerebral blood flow.
Clinical implications: the finding that orthostatic stress elicits a post-stress cerebral blood flow reduction and that disease severity greatly influences the cerebral blood flow reduction may have implications on the advice of energy management after a stressor and on the advice of lying down after a stressor in these ME/CFS patients.
Conclusions: in ME/CFS patients with demonstrable orthostatic intolerance during a tilt test, a significant reduction in cerebral blood flow compared to pre-tilt values was observed in the recovery phase after the tilt test in all groups, greater in those with increasing ME/CFS disease severity. The mechanisms for the delayed recovery of cerebral blood flow and its impact on post-exertional malaise need further study.
Acknowledgments
Acknowledgements
Dr Rowe is supported by the Sunshine Natural Wellbeing Foundation Professorship.
Funding
This study was not funded.
Ethics
The study was carried out in accordance with the Declaration of Helsinki. All ME/CFS participants gave informed, written consent. The use of descriptive clinical data of patients was approved by the medical ethics committee of the Slotervaart Hospital, Amsterdam, the Netherlands, P1450.
Author contributions
CMCVC, PCR, and FCV conceived the study, CMCVC and FCV collected the data, CMCVC performed the primary data analysis and FCV, and PCR performed secondary data analyses. All authors were involved in the drafting and review of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Barde P.B., Deepak K.K. Effect of postural challenges on non-invasive cardiac output measurement with impedance cardiography in young healthy adults using new horizontal electrode placement method. IJBAR. 2012;3(11):806–809. [Google Scholar]
- Bola R.A., Kiyatkin E.A. Inflow of oxygen and glucose in brain tissue induced by intravenous norepinephrine: relationships with central metabolic and peripheral vascular responses. J Neurophysiol. 2018;119(2):499–508. doi: 10.1152/jn.00692.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Holaigah I., Rowe P.C., Kan J., Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274(12):961–967. [PubMed] [Google Scholar]
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle-Kirszbaum M., Parkin W.G., Goldschlager T., Lewis P.M. Cardiac output and cerebral blood flow: a systematic review of cardio-cerebral coupling. J Neurosurg Anesthesiol. 2021 doi: 10.1097/ANA.0000000000000768. [DOI] [PubMed] [Google Scholar]
- Dave K.R., Christian S.L., Perez-Pinzon M.A., Drew K.L. Neuroprotection: lessons from hibernators. Comp Biochem Physiol B Biochem Mol Biol. 2012;162(1-3):1–9. doi: 10.1016/j.cbpb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo F., Hargreaves J., Kakkar V.V. Pathogenesis and management of delayed orthostatic hypotension in patients with chronic fatigue syndrome. Clin Auton Res. 1997;7(4):185–190. doi: 10.1007/BF02267980. [DOI] [PubMed] [Google Scholar]
- Del Pozzi A.T., Pandey A., Medow M.S., Messer Z.R., Stewart J.M. Blunted cerebral blood flow velocity in response to a nitric oxide donor in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2014;307(3):H397–H404. doi: 10.1152/ajpheart.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez, D.A., De Buyzere, M.L., Drieghe, B., Vanhaverbeke, F., Taes ,Y., Michielsen, W., et al. Long- and short-term blood pressure and RR-interval variability and psychosomatic distress in chronic fatigue syndrome. Clin Sci (Lond) 1998;94(1):57-63. [DOI] [PubMed]
- Fedorowski A., Burri P., Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27(5):976–982. doi: 10.1097/hjh.0b013e3283279860. [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161(1-2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Hoiland R.L., Fisher J.A., Ainslie P.N. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol. 2019;9(3):1101–1154. doi: 10.1002/cphy.c180021. [DOI] [PubMed] [Google Scholar]
- Hollingsworth K.G., Jones D.E., Taylor R., Blamire A.M., Newton J.L. Impaired cardiovascular response to standing in chronic fatigue syndrome. Eur J Clin Invest. 2010;40(7):608–615. doi: 10.1111/j.1365-2362.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- Immink R.V., Pott F.C., Secher N.H., van Lieshout J.J. Hyperventilation, cerebral perfusion, and syncope. J Appl Physiol. 2014;116(7):844–851. doi: 10.1152/japplphysiol.00637.2013. [DOI] [PubMed] [Google Scholar]
- Institute Of Medicine (IOM). Beyond mayalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington DC: The National Academies Press; 2015. p. 304. [PubMed]
- Jardine D.L., Melton I.C., Crozier I.G., Bennett S.I., Donald R.A., Ikram H. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am J Cardiol. 1997;79(9):1302–1306. doi: 10.1016/s0002-9149(9x)00084-9. [DOI] [PubMed] [Google Scholar]
- Jones J.F., Nicholson A., Nisenbaum R., Papanicolaou D.A., Solomon L., Boneva R. Orthostatic instability in a population-based study of chronic fatigue syndrome. Am J Med. 2005;118(12):1415.e19–1415.e28. doi: 10.1016/j.amjmed.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Kloner R.A. Stunned and hibernating myocardium: Where are we nearly 4 decades later? J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto F., Venet T., Schiller N.B., Sebastian A., Foster E. Measurement of aortic blood flow by Doppler echocardiography: temporal, technician, and reader variability in normal subjects and the application of generalizability theory in clinical research. J Am Soc Echocardiogr. 1995;8(5):647–653. doi: 10.1016/s0894-7317(05)80378-5. [DOI] [PubMed] [Google Scholar]
- Laffey J.G., Kavanagh B.P. Hypocapnia. N Engl J Med. 2002;347(1):43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- LaManca J.J., Peckerman A., Walker J., Kesil W., Cook S., Taylor A. Cardiovascular response during head-up tilt in chronic fatigue syndrome. Clin Physiol. 1999;19(2):111–120. doi: 10.1046/j.1365-2281.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Miyake T., Nakamura T., Kouda K., Uenishi H., Yamamoto Y., Kawasaki S. Carotid blood flow, cardiovascular and endocrine responses during head-up tilt in patients with acute cerebrovascular diseases. Springerplus. 2014;3(1):191. doi: 10.1186/2193-1801-3-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Lum Y.W. Venous thoracic outlet syndrome. Vasc Med. 2015;20(2):182–189. doi: 10.1177/1358863X14568704. [DOI] [PubMed] [Google Scholar]
- Naschitz J.E., Rosner I., Rozenbaum M., Gaitini L., Bistritzki I., Zuckerman E. The capnography head-up tilt test for evaluation of chronic fatigue syndrome. Semin Arthritis Rheum. 2000;30(2):79–86. doi: 10.1053/sarh.2000.9201. [DOI] [PubMed] [Google Scholar]
- Naschitz J.E., Sabo E., Naschitz S., Rosner I., Rozenbaum M., Madelain F. Hemodynamics instability score in chronic fatigue syndrome and in non-chronic fatigue syndrome. Semin Arthritis Rheum. 2002;32(3):141–148. doi: 10.1053/sarh.2002.34608. [DOI] [PubMed] [Google Scholar]
- Newton D.J., Kennedy G., Chan K.K.F., Lang C.C., Belch J.J.F., Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. 2012;154(3):335–336. doi: 10.1016/j.ijcard.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Novak P. Orthostatic cerebral hypoperfusion syndrome. Front Aging Neurosci. 2016;8:22. doi: 10.3389/fnagi.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Gallyas F. Hypocapnic cerebral hypoperfusion: a biomarker of orthostatic intolerance. PLoS One. 2018;13(9):e0204419. doi: 10.1371/journal.pone.0204419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V., Spies J.M., Novak P., McPhee B.R., Rummans T.A., Low P.A. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29(9):1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- Park J., Kim H.T., Park K.M., Ha S.Y., Kim S.E., Shin K.J. Orthostatic dizziness in Parkinson's disease is attributed to cerebral hypoperfusion: a transcranial doppler study. J Clin Ultrasound. 2017;45(6):337–342. doi: 10.1002/jcu.22452. [DOI] [PubMed] [Google Scholar]
- Poole J., Herrell R., Ashton S., Goldberg J., Buchwald D. Results of isoproterenol tilt table testing in monozygotic twins discordant for chronic fatigue syndrome. Arch Intern Med. 2000;160(22):3461–3468. doi: 10.1001/archinte.160.22.3461. [DOI] [PubMed] [Google Scholar]
- Razumovsky, A.Y., DeBusk, K., Calkins, H., Snader, S., Lucas, K.E., Vyas, P., et al. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J Neuroimaging 2003;13(1):57-67. [PubMed]
- Sato, K., Sadamoto, T., Hirasawa, A., Oue, A., Subudhi, A.W., Miyazawa, T., et al. Differential blood flow responses to CO(2) in human internal and external carotid and vertebral arteries. J Physiol 2012;590(14):3277-3290. [DOI] [PMC free article] [PubMed]
- Scherbakov N., Szklarski M., Hartwig J., Sotzny F., Lorenz S., Meyer A. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020;7(3):1064–1071. doi: 10.1002/ehf2.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondorf R., Benoit J., Wein T., Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75(2-3):192–201. doi: 10.1016/s0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- Sheldon R.S., Grubb B.P., 2nd, Olshansky B., Shen W.K., Calkins H., Brignole M. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.K., Sheldon R.S., Benditt D.G., Cohen M.I., Forman D.E., Goldberger Z.D. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):620–663. doi: 10.1016/j.jacc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Shin K.J., Kim S.E., Park K.M., Park J., Ha S.Y., Kim S.E. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol Scand. 2015;134(2):108–115. doi: 10.1111/ane.12516. [DOI] [PubMed] [Google Scholar]
- Statistics L. Leard Statistics. Multiple regression using SPSS Statistics. Statistical tutorials and software guides. Retrieved from https://statistics.laerd.com/. 2015.
- Stewart J.M., Pianosi P., Shaban M.A., Terilli C., Svistunova M., Visintainer P. Hemodynamic characteristics of postural hyperventilation: POTS with hyperventilation versus panic versus voluntary hyperventilation. J Appl Physiol. 2018;125(5):1396–1403. doi: 10.1152/japplphysiol.00377.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Pianosi P., Shaban M.A., Terilli C., Svistunova M., Visintainer P. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.118.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeten D.H.P., Thomas D., Bell D.S. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320(1):1–8. doi: 10.1097/00000441-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Timmers H.J.L.M., Wieling W., Soetekouw P.M.M.B., Lenders J.W.M., Bleijenberg G., van der Meer J.W.M. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin Auton Res. 2002;12(4):273–280. doi: 10.1007/s10286-002-0014-1. [DOI] [PubMed] [Google Scholar]
- Toska K., Walløe L. Dynamic time course of hemodynamic responses after passive head-up tilt and tilt back to supine position. J Appl Physiol. 2002;92(4):1671–1676. doi: 10.1152/japplphysiol.00465.2000. [DOI] [PubMed] [Google Scholar]
- van Campen C(L)MC, Rowe PC, Verheugt FWA, Visser FC. Numeric rating scales show prolonged post-exertional symptoms after orthostatic testing of adults with myalgic encephalomyelitis/chronic fatigue syndrome. Front Med 2021;7:10. [DOI] [PMC free article] [PubMed]
- van Campen C(L)MC, Rowe PC, Visser FC. Cerebral blood flow is reduced in severe myalgic encephalomyelitis/chronic fatigue syndrome patients during mild orthostatic stress testing: an exploratory study at 20 degrees of head-up tilt testing. Healthcare (Basel) 2020a;8(2):169. [DOI] [PMC free article] [PubMed]
- van Campen C(L)MC, Rowe PC, Visser FC. Reductions in cerebral blood flow can be provoked by sitting in severe myalgic encephalomyelitis/chronic fatigue syndrome patients. Healthcare 2020b;8:394. [DOI] [PMC free article] [PubMed]
- van Campen C.M.C., Verheugt F.W.A., Rowe P.C., Visser F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin Neurophysiol Pract. 2020;5:50–58. doi: 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.M.C., Verheugt F.W.A., Rowe P.C., Visser F.C. Comparison of the finger plethysmography derived stroke volumes by Nexfin CO Trek and suprasternal aortic Doppler derived stroke volume measurements in adults with myalgic encephalomyelitis/chronic fatigue syndrome and in healthy controls. Techn. Health Care. 2021;29(4):629–642. doi: 10.3233/THC-202669. [DOI] [PubMed] [Google Scholar]
- van Campen C.M.C., Verheugt F.W.A., Visser F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin Neurophysiol Pract. 2018;3:91–95. doi: 10.1016/j.cnp.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C(L)MC, Visser FC. The abnormal Cardiac Index and Stroke Volume Index changes during a normal Tilt Table Test in ME/CFS patients compared to healthy volunteers, are not related to deconditioning. J. Thromb. Circ. 2018;JTC -107:1-8.
- van Campen C(L)MC, Visser FC. Validation of Stroke volume measured with suprasternal aortic Doppler imaging: comparison to transthoracic Stroke Volume measurements. J Thromb Circ 2018;JTC -106:1-5.
- van Campen C.M.C., Visser F.C., de Cock C.C., Vos H.S., Kamp O., Visser C.A. Comparison of the haemodynamics of different pacing sites in patients undergoing resynchronisation treatment: need for individualisation of lead localisation. Heart. 2006;92(12):1795–1800. doi: 10.1136/hrt.2004.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]