Abstract
Purpose
This longitudinal study aimed to disentangle the impact of chemotherapy on fatigue and hypothetically associated functional brain network alterations.
Methods
In total, 34 breast cancer patients treated with chemotherapy (BCC +), 32 patients not treated with chemotherapy (BCC −), and 35 non-cancer controls (NC) were included. Fatigue was assessed using the EORTC QLQ-C30 fatigue subscale at two time points: baseline (T1) and six months after completion of chemotherapy or matched intervals (T2). Participants also underwent resting-state functional magnetic resonance imaging (rsfMRI). An atlas spanning 90 cortical and subcortical brain regions was used to extract time series, after which Pearson correlation coefficients were calculated to construct a brain network per participant per timepoint. Network measures of local segregation and global integration were compared between groups and timepoints and correlated with fatigue.
Results
As expected, fatigue increased over time in the BCC + group (p = 0.025) leading to higher fatigue compared to NC at T2 (p = 0.023). Meanwhile, fatigue decreased from T1 to T2 in the BCC − group (p = 0.013). The BCC + group had significantly lower local efficiency than NC at T2 (p = 0.033), while a negative correlation was seen between fatigue and local efficiency across timepoints and all participants (T1 rho = − 0.274, p = 0.006; T2 rho = − 0.207, p = 0.039).
Conclusion
Although greater fatigue and lower local functional network segregation co-occur in breast cancer patients after chemotherapy, the relationship between the two generalized across participant subgroups, suggesting that local efficiency is a general neural correlate of fatigue.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-021-06326-0.
Keywords: Breast cancer, Fatigue, Network neuroscience, Functional connectivity, Graph theory
Introduction
Breast cancer is the most prevalent cancer in women and the second most commonly occurring cancer overall [1]. Treatments like chemotherapy are associated with significant side effects, ranging from acute symptoms such as vomiting and diarrhea [2] to long-term systemic complications such as cardiac toxicity, fatigue and cognitive decline [3–5]. Fatigue is a subjective feeling of exhaustion or tiredness described in terms of mental capacity, perceived energy, and psychological function [6]. Studies indicate that one in four breast cancer patients suffer from severe fatigue [7]. A major identified risk factor for severe fatigue is chemotherapy, where more than 50% of patients continue to suffer from fatigue months or even years after completion of chemotherapy [8]. However, the pathogenesis of cancer-related fatigue is not well understood, and the roles of several biochemical, physiological and psychological factors have been hypothesized. Differences in brain metabolites between fatigued and non-fatigued breast cancer survivors indicate the neurobiological changes associated with fatigue [9]. Furthermore, the close association of fatigue with symptoms such as cognitive complaints, poor sleep and depression highlights the involvement of the brain in fatigue pathogenesis in breast cancer patients [10].
Several studies have described neurotoxicity associated with chemotherapy agents. Neuroimaging studies have characterized diffuse structural changes in the brain following chemotherapy, such as significant white matter integrity changes and widespread abnormalities in gray matter volume [11–16]. Task-based functional MRI (fMRI) has demonstrated functional abnormalities in brain areas associated with cognitive function [17, 18]. Over the past decade, resting-state fMRI (rsfMRI) has been providing a reliable, non-invasive method of measuring the spontaneous or intrinsic activity of the brain by measuring the blood oxygen level-dependent (BOLD) signal. Studies have described the widespread abnormalities in resting-state networks of breast cancer survivors, highlighting chemotherapy-related functional changes in the brain [19, 20]. An association between intrinsic brain connectivity and self-reported fatigue has been reported, where enhanced connectivity between the frontal gyrus and several brain regions involved in self-referential thinking was seen [21]. Furthermore, greater left inferior parietal lobule to superior frontal gyrus connectivity was observed in the fatigued subgroup, which was associated with greater physical fatigue and poorer sleep quality.
More recently, a graph theory-based approach towards analyzing functional connectivity has gained more attention. Graph theory represents the brain as a graph consisting of nodes and edges, where nodes indicate anatomical elements and edges indicate the connectivity between them. In contrast to previously available analytic methods, it allows the visualization of connectivity patterns and quantitative characterization of global organization of the brain [22]. Among others, graph theory employs topological parameters such as functional integration and segregation to describe the properties of brain networks [23]. Segregation describes the degree to which network elements form specialized communities while integration refers to the ability to combine distributed information. Measures for integration include characteristic path length and global efficiency, while segregation can be quantified using the clustering coefficient and local efficiency [24, 25]. Their relevance in investigating how brain networks relate to normal development [26, 27], aging [28] and a wide range of symptoms associated with brain disorders [26, 29, 30] has been well-documented.
A few studies have employed rsfMRI and graph theory to characterize the topological organization of the brain in patients with breast cancer. Bruno et al. [31] showed a disruption in both regional and global network properties, signaling reduced efficiency of information transfer observed in breast cancer patients following chemotherapy [31]. The breast cancer group demonstrated significantly decreased global clustering as well as marginally decreased path length compared to healthy controls. Disrupted regional network characteristics such as nodal degree (the number of connections of a region) and hub locations were also observed in frontal, striatal and temporal areas of the brain. Xuan et al. [32] demonstrated an abnormal organization of large-scale functional brain networks in chemotherapy-treated breast cancer patients with cognitive impairment [32]. The breast cancer group had lower global and local efficiency compared with healthy controls. Local alterations such as higher nodal degree and functional connectivity were seen in several prefrontal, occipital and parietal regions. They also showed that the alterations in the network were correlated to the memory deficits observed in these group of patients. However, all of the studies so far have not been able to disentangle whether breast cancer pathogenesis or the chemotherapy was responsible for such network changes. Several of the studies were cross-sectional by design and only used healthy controls for comparison. In addition, most of them lacked a baseline characterization of the brain network prior to chemotherapy. Finally, none of these studies explored whether network alterations form a neural correlate of fatigue in these patients.
This longitudinal study investigated fatigue and brain network topology related to chemotherapy. Changes in network topology and fatigue from pre-treatment to post-treatment time points were compared between breast cancer patients who received chemotherapy, breast cancer patients who did not, and non-cancer controls. Both graph measures of integration and segregation were used to allow a comprehensive overview and enable the comparison of our results with previous studies. We hypothesized that chemotherapy-treated breast cancer patients would be more fatigued than the other groups. Moreover, we expected lower segregation and integration in those patients, and these network differences and changes to correlate with greater and increasing fatigue.
Methods and materials
Participants
Participants of the study were breast cancer patients who had undergone surgery under general anesthesia (BC) and age-matched controls with no history of breast cancer or chemotherapy (NC). BC patients were either scheduled to receive anthracycline-based chemotherapy with or without endocrine treatment (BCC +) or did not require chemotherapy or endocrine treatment (BCC −). Participants were eligible if the following criteria were met: female, under 70 years of age, no previous history of malignancy, a formal diagnosis of primary breast cancer without distant metastases and good command of the Dutch language. Participants scheduled to receive trastuzumab following chemotherapy were excluded from the study due to the longer duration of treatment in this patient group. Controls were recruited via participants and advertisements in participating hospitals. Only those participants who attended both time points and were scanned with the same MRI scanner at each time point were included. Following surgery, all BCC + participants received anthracycline-based chemotherapeutic agents (see Supplementary Table 1 for specific treatment regimens).
The study was approved by the Institutional Review Board of the Netherlands Cancer Institute, serving as the central ethical committee for all participating institutes. Written informed consent was obtained and the study was conducted according to the principles expressed in the Declaration of Helsinki and following institutional guidelines. The scanning was performed at the Academic Medical Center of the University of Amsterdam and the Spinoza Centre for Neuroimaging.
Data collection
All patients with breast cancer had undergone surgery under general anesthesia before inclusion (wide local excision or mastectomy), and baseline data (T1) were collected after surgery but before the start of adjuvant treatment. Follow-up assessment (T2) was done 6 months after the last cycle of chemotherapy in the BCC + group and at matched intervals in the other two groups.
Data was collected using patient-reported outcome questionnaires (PROs), neuropsychological assessments, and an MRI protocol. Longitudinal analyses of the task-related fMRI, cognitive performance and the correlation of cognitive impairment with psychological and social factors have been reported before [33–35]. The current study focuses on the analysis of the rsfMRI data and fatigue.
Fatigue was assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C-30 (QLQ-C30) fatigue subscale [36]. The EORTC QLQ-C30 assesses symptoms and functioning in cancer patients. It is a self-administered questionnaire with 30 symptom-focused questions scored on a numeric scale of 4 points from 1 (“not at all”) to 4 (“very much”). Scores are computed into five functional scales and multi-item symptom scales including fatigue, nausea, vomiting and pain. Furthermore, the Hopkins Symptom Checklist-25 (HSCL-25) was used to asses anxiety and depression, while the perceived stress score (PSS) was used to assess stress. Intelligence Quotient (IQ) was estimated using the Dutch version of the National Adult Reading Test (NART) [37].
MRI acquisition and preprocessing
MRI data were acquired using a 3 Tesla Intera full-body MRI scanner (Academic Medical Center) and a 3 Tesla Achieva full-body MRI scanner (Spinoza Centre for Neuroimaging) (both Philips Medical Systems, Best, The Netherlands). A SENSE 8-channel receiver head coil was used at both locations. Functional MRI acquisition was based on T2 weighted gradient echo-planar imaging (EPI) of 38 axial slices (voxel size 2.3 × 2.3 × 2.3 mm, interslice gap 0 mm, matrix size 96 × 96, TR = 2.1 s, TE = 25 ms). 180 volumes of rsfMRI were obtained with participants lying in the scanner relaxed with their eyes open.
The FMRIB software library (FSL v5.0.9) [38] was used for pre-processing of the structural and functional images. The structural scan was segmented into gray and white matter as well as cerebrospinal fluid using BET [39], after which the Automated Anatomical Labeling Atlas (AAL atlas) [40], which contains 78 cortical regions, was co-registered to this native space. FSL-FIRST was additionally used to acquire 12 subcortical regions (excluding the right and left nuclei accumbens), together forming the 90 nodes of the functional brain network.
Preprocessing of the rsfMRI included (a) brain tissue extraction using BET [39], (b) motion correction using McFLIRT [41] (c) spatial smoothing at 5 mm, (d) a high pass filter above 0.01 Hz, (e) alignment (registration) of participants rsfMRI data to MNI space using FLIRT and FNIRT [42], (f). extracting and regressing out of cerebrospinal fluid and white matter signals from the total rsfMRI signal. Furthermore, ICA-AROMA (Independent Component Analysis-Automatic Removal of Motion Artifacts) [43] was applied to remove residual motion artifacts. Finally, the average time series across all voxels within each of the 90 atlas regions were extracted for each participant.
Connectivity and network analysis
Pearson correlations were performed on the time series, resulting in a 90 × 90 connectivity matrix per participant. Absolute values of correlations were used, since the differential meaning of negative correlations is unknown. Calculated graph measures were normalized by dividing them with the corresponding graph values derived from 100 random networks. Random networks with identical numbers of nodes, edges and weight distribution were generated for each participant [44]. Weighted matrices were used, in light of recent findings [45], which suggest that weighted networks have overall higher test–retest and overall reliability in regional nodal efficiency compared to binarized networks.
Four graph measures were calculated to characterize the brain network’s topological organization. The clustering coefficient reflects the prevalence of clustered connectivity around individual nodes. The average clustering coefficient (Cp) generalized for weighted networks can be defined as the geometric mean of link weights associated with a node [46]. The characteristic path length (Lp) is equal to the average of the weighted path lengths between nodes in a network [25]. Global efficiency (Eglob) is the average inverse weighted path length. Local Efficiency (Eloc) of a network is equal to average efficiency of the local subgraphs. A generalization of these measures for weighted networks is given in Rubinov and Sporns [25].
All computations of graph measures were performed using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net) [25] using MATLAB (R2019a, version 9.6.0, The Mathworks Inc., Natick, Massachusetts). The BrainNet Viewer (http://www.nitrc.org/projects/bnv/) [47] was used for visualization of networks.
Statistical analysis
Differences in demographic, clinical variables, PROs and graph measures were analyzed using SPSS 25 (IBM, Armonk, NY) by means of one-way ANOVAs, X2 tests or t-tests as appropriate. Changes in graph measures and fatigue scores across the two time points were analyzed by repeated measures ANOVAs. No correction was performed for testing of multiple network measures. The level of significance was set at p < 0.05.
Given that EORTC QLQ-C30 fatigue scores are ordinal data, associations with graph measures were computed using Spearman’s correlation coefficients. Correlations between fatigue scores and potential covariates were also calculated, since previous literature has described the overlap between the symptoms of fatigue with other psychological disorders such as stress, anxiety, depression [48] and estrogen deprivation [49] especially in postmenopausal women. Thus, HSCL scores of anxiety and depression, PSS scores, menopausal status and age at measurement were used as covariates when analyzing the fatigue scores across groups and over time. Only graph measures and fatigue scores that were significantly different between groups or over time were further explored.
Results
Participant characteristics
A total of 101 participants (34 BCC + , 32 BCC − and 35 NC) were included. The clinical and demographic characteristics of these participants are summarized in Table 1.
Table 1.
Demographic and clinical variables of participants
| Variable | BCC + (n = 34) | BCC − (n = 32) | NC (n = 35) | p |
|---|---|---|---|---|
| Age at T1 in years (M ± SD) | 49.7 ± 9.8 | 50.8 ± 7.1 | 49.6 ± 10.3 | 0.836 |
| Age at T2 in years (M ± SD) | 50.6 ± 9.8 | 52.0 ± 7.4 | 50.6 ± 10.2 | 0.777 |
| Estimated IQ | 101.3 ± 13.7 | 104.6 ± 12.8 | 107.4 ± 12.8 | 0.160 |
| Educational level [n (%)] | ||||
| Low | 3 (8.8) | 5 (15.6) | 0 (0) | 0.198 |
| Middle | 8 (23.5) | 8 (25) | 8 (22.8) | |
| High | 23 (67.6) | 19 (59.4) | 27 (77.1) | |
| Premenopausal [n (%)] | ||||
| T1 | 19 (55.9) | 16 (50) | 17 (48.6) | 0.835 |
| T2 | 1 (2.9) | 13 (40.6) | 17 (48.6) | < 0.001a, b |
| Time between T1 and T2 (days) | 332.9 ± 61.5 | 340.9 ± 43.4 | 365.3 ± 62.8 | 0.053 |
| Breast cancer gradingc [n (%)] | ||||
| 1 | 2 (5.9) | 11 (34.4) | 0.020b | |
| 2 | 17 (50) | 11 (34.4) | ||
| 3 | 11 (32.4) | 5 (15.6) | ||
| Side of breast affected [n (%)] | ||||
| Right | 12 (40) | 15 (46.9) | 0.300 | |
| Left | 18 (60) | 13 (40.6) | ||
| Histology [n (%)] | ||||
| In-situ carcinoma | 0 | 16 (57.1) | < 0.001b | |
| Invasive carcinoma | 30 (100) | 10 (35.7) | ||
| Isolated tumor cells | 0 | 2 (7.1) | ||
| Origin [n (%)] | ||||
| Ductal | 28(93.3) | 25(89.3) | 0.078 | |
| Lobular | 2(0.07) | 0 | ||
| Isolated tumor cells | 0 | 3(0.11) | ||
| Treatment [n (%)] | ||||
| Radiotherapy | 25 (73.5) | 19 (59.4) | 0.223 | |
| Tamoxifen | 22 (64.7) | NA | ||
| Type of surgery | ||||
| Wide local excision | 19 (55.9) | 20 (62.5) | 0.512 | |
| Mastectomy | 11 (32.2) | 8 (25) | ||
| Chemotherapy complications [n (%)] | ||||
| No | 20 (58.8) | NA | ||
| Yes | 11 (32.4) | NA | ||
| Time since chemotherapy(days) | 208.5 ± 72.0 | NA | ||
BCC + breast cancer patients receiving chemotherapy, BCC − breast cancer patients with no chemotherapy, NC non-cancer controls, IQ intelligence quotient, NART dutch version of the national adult reading test, NA not applicable
Values indicate mean ± SD unless indicated otherwise
Significance was declared by p < 0.05 and significant differences are highlighted in bold
aIndicates a significant difference between BCC + and NC
bIndicates a significant difference between BCC + and BCC −
cGrade 1 refers to well differentiated, 2 moderately differentiated and 3 poorly differentiated
No significant differences were seen between groups with regards to age, estimated IQ, or educational status. At T1, no significant group difference was found in menopausal status, however a significant difference was seen at T2, where post-hoc testing revealed more postmenopausal women in BCC + compared to BCC − and NC [X2(2, N = 100) = 19.3, p < 0.001], as expected in women undergoing chemotherapeutic treatment.
Fatigue and related variables
A significant difference in EORTC QLQ-C30 fatigue scores was found between the groups at both time points [F(2,98) = 7.343, p = 0.001 at T1 and F(2,97) = 3.936, p = 0.023 at T2]. Post-hoc analysis revealed that at T1, the BCC − group reported significantly higher fatigue scores compared to the NC group whereas at T2, the BCC + group reported significantly higher fatigue scores than NC (see Fig. 1 below).
Fig. 1.
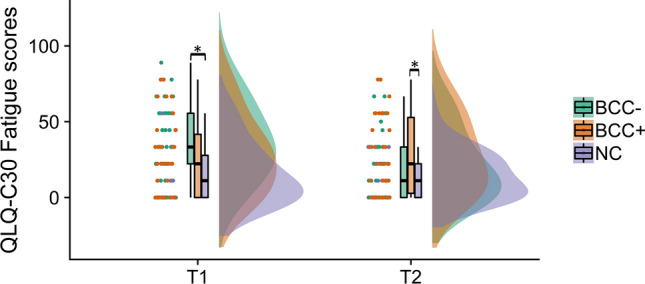
EORTC QLQ-C30 fatigue scores. BCC − breast cancer patients with no chemotherapy, BCC + breast cancer patients receiving chemotherapy; NC non-cancer controls; (See Supplementary Table 2 for more details). *Statistically significant difference defined as p < 0.005
There was also a main effect of time with regards to fatigue [F(2,97) = 7.384, p = 0.001]; post-hoc analysis group comparison revealed a significant increase in fatigue in the BCC + group (p = 0.025), while a significant decrease was seen in the BCC − group (p = 0.013) at T2. No significant change was observed in the NC group.
The observed significant difference between BCC − and NC in fatigue scores at T1 was still significant when adjusting for age at measurement, perceived stress score, menopausal status, and HSCL anxiety and depression subscales. However, at T2, the observed significant difference between BCC + and NC lost significance (p = 0.456) when adjusted for perceived stress score (p = 0.701), menopausal status (p = 0.054), HSCL depression score (p = 0.067) and HSCL anxiety score (p = 0.625). For scores on the PSS and HSCL-25 subscales, please refer to supplementary materials (Supplementary Table 2).
Network topology
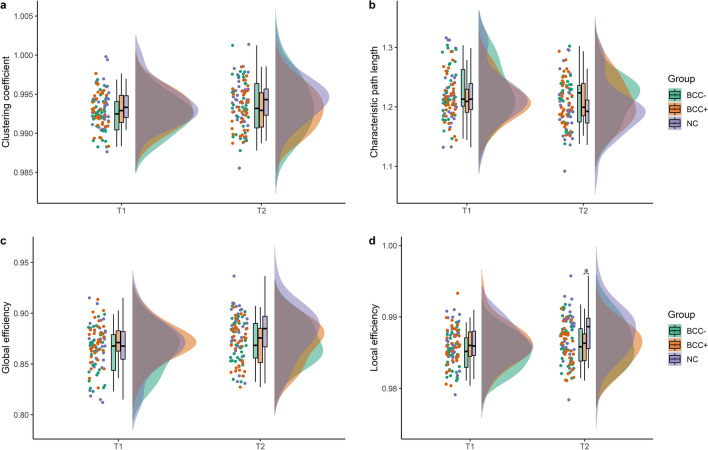
There was no group × time interaction for any of the graph measures Cp [F(1,98) = 3.428, p = 0.067], Lp [F(2,98) = 1.965, p = 0.146], Eglob [F(2,98) = 0.968, p = 0.384], Eloc [F(2,98) = 2.494, p = 0.088)]. Moreover, no significant main effects of group were seen except for Eloc [F(2,98) = 3.150, p = 0.047 and see Supplementary Table 4]. Therefore, post-hoc group differences at each time point were further explored for Eloc. There was no statistically significant difference among the three groups at T1, however, at T2 a significant difference was observed [F(2,98) = 3.612, p = 0.031] (Fig. 2d). Post-hoc analysis revealed that the BCC + group had significantly lower Eloc values compared to the NC group (p = 0.033). (See Table 2, Fig. 2).
Fig. 2.
Graph measure values of participants at T1 and T2. These values represent values after normalization by random networks. A Clustering coefficient (Cp) values of participants B Characteristic path length (Lp) of participants C Global efficiency (Eglob) values at T1 and T2 D Local efficiency (Eloc) of participants at T1 and T2. NC non-cancer control, BCC − breast cancer patients without chemotherapy, BCC + breast cancer patients with chemotherapy. *Statistically significant difference defined as p < 0.05
Table 2.
Normalized graph measures at time points T1 and T2
| Graph measure | NC (n = 35) | BCC − (n = 32) | BCC + (n = 34) | p | |
|---|---|---|---|---|---|
| Cp | T1 | 0.993 ± 0.002 | 0.992 ± 0.002 | 0.992 ± 0.002 | 0.996 |
| T2 | 0.994 ± 0.003 | 0.993 ± 0.003 | 0.993 ± 0.003 | 0.285 | |
| Lp | T1 | 1.218 ± 0.044 | 1.226 ± 0.043 | 1.213 ± 0.035 | 0.411 |
| T2 | 1.196 ± 0.044 | 1.211 ± 0.037 | 1.209 ± 0.038 | 0.207 | |
| Eloc | T1 | 0.986 ± 0.003 | 0.985 ± 0.002 | 0.986 ± 0.003 | 0.151 |
| T2 | 0.988 ± 0.003 | 0.984 ± 0.003 | 0.986 ± 0.003 | 0.031 | |
| Eglob | T1 | 0.867 ± 0.024 | 0.862 ± 0.021 | 0.871 ± 0.018 | 0.241 |
| T2 | 0.880 ± 0.025 | 0.871 ± 0.019 | 0.871 ± 0.020 | 0.477 | |
BCC − breast cancer patients with no chemotherapy, BCC + breast cancer patients receiving chemotherapy, NC non-cancer controls, Cp clustering coefficient, Lp characteristic path length, Eloc local efficiency, Eglob global efficiency
Values represent mean ± SD. Values were calculated after each participants’ network was normalized with random networks
Significance was declared by p < 0.05 and significant differences are highlighted in bold
Relationship between fatigue and network topology
For all participants as a whole, at both time points a significant negative correlation was observed between fatigue scores and Eloc (rho = − 0.274, p = 0.006 at T1 and rho = − 0.198, p = 0.047) (See Table 3). However, analysis on group level showed a significant negative correlation between Eloc and fatigue scores only in the NC (rho = − 0.34, p = 0.026) and BCC − group (rho = − 0.045, p = 0.024) at T1, and in the BCC − group (rho = − 0.5, p = 0.011) at T2 (See Supplementary Table 3).
Table 3.
Correlation between fatigue scores and graph measures for all participants
| Graph measures | EORTC QLQ-C30 fatigue score | |||
|---|---|---|---|---|
| T1 | T2 | |||
| rho | p | rho | p | |
| Cp | − 0.069 | 0.495 | 0.001 | 0.989 |
| Lp | 0.191 | 0.055 | 0.109 | 0.277 |
| Eglob | − 0.203 | 0.041 | − 0.069 | 0.492 |
| Eloc | − 0.274 | 0.006 | − 0.198 | 0.047 |
EORTC QLQ-C30 European organization for research and treatment of cancer health-related quality-of-life questionnaire, Cp clustering coefficient, Lp characteristic path length, Eloc local efficiency, Eglob global efficiency, rho Spearman’s rank correlation coefficient
Significance was declared by p < 0.05 and significant differences are highlighted in bold
Discussion
The present study examined fatigue and functional brain network topology of chemotherapy-treated breast cancer patients. The longitudinal design enabled comparisons between and within groups and two control groups were included: one of breast cancer patients who did not receive chemotherapy and one of age-matched non-cancer controls. As expected, patients in the BCC + group reported higher fatigue following chemotherapy than non-cancer controls. Moreover, the BCC + group experienced a significant increase in fatigue between the two time points while fatigue significantly decreased in the BCC- group. Following chemotherapy, differences in brain network topology were observed, with the BCC + group having lower local efficiency compared to the NC group. Finally, a negative association between local efficiency and fatigue scores was seen at both time points in several subgroups.
In line with previous findings [6, 50, 51], greater fatigue was reported in breast cancer patients compared to non-cancer controls. At T1, only BCC − patients reported a significantly higher fatigue score compared to NC. No similar pattern was found in the BCC + group. This finding could be attributed to other comorbidities or personal factors in the BCC − group that were not recorded in this study. Another explanation is that the BCC − patients were in a different emotional stage of cancer diagnosis and treatment compared to the BCC + patients receiving chemotherapy. Previous clinical and anecdotal evidence suggest that the immediate post-treatment phase is characterized by disruption, transition and increased stress which improves over time [52]. The significant decline in fatigue in the BCC- group over time may indicate the emotional progression of patients through the post-treatment stage and their adjustment to life. However, this explanation is speculative, particularly since the EORTC QLQ C-30 fatigue subscale mainly focuses on physical, not mental fatigue. At T2, greater fatigue was reported by the BCC + group compared to NC, where a statistically significant increase in fatigue was also seen between T1 and T2. This increasing level of fatigue can be attributed to chemotherapy, as several previous studies have reported the association between fatigue and chemotherapy in breast cancer patients [53, 54]. Furthermore, the change in menopausal status is a potential contributing factor as the role of premature menopause on prevalence of persistent fatigue in breast cancer patients has been described before [55]. However, cancer-related fatigue is a complex disorder whose pathogenesis is not well understood and the involvement of several psychological and biochemical systems requires further investigation. Our findings regarding comorbid differences between BCC + patients and controls regarding perceived stress, anxiety and depression further support a multidimensional view of fatigue in these patients.
We then investigated the functional brain network of the three different groups. No significant group differences in graph measures were seen at T1, supporting a previous study revealing no differences in global clustering prior to chemotherapeutic treatment [56]. At T2, only differences in Eloc were seen between the BCC + and NC group, such that the BCC + patients had lower local efficiency than the non-cancer controls, reflecting reduced network segregation. This finding is in line with previous studies showing lower clustering and local efficiency in chemotherapy-treated breast cancer patients as compared to healthy controls at different follow-up durations [31, 32]. However, we are unable to unequivocally conclude that these alterations in functional brain topology are caused by chemotherapy alone as the effect of time was non-significant. Moreover, given that no correction was done for testing of multiple network measures, interpretation of the changes requires careful consideration.
Furthermore, greater fatigue score was correlated with lower local efficiency at both time points, indicating that neural correlates of fatigue may be represented by changes in local information exchange and processing, in contrast to the other graph measures assessed in this study. This shows the significance of network efficiency for fatigue in general, as such correlations were present in both BCC + , BCC − and NC subgroups. Although lower local efficiency is thus not a chemotherapy-specific correlate of fatigue, the robustness of its association across subgroups does suggest that functional brain network topology is a relevant neural correlate of fatigue in any population.
This study has several advantages. The double-controlled, longitudinal design enabled us to determine the added effect of chemotherapy on the brain and fatigue symptoms experienced post-therapy and allowed us to disentangle both specific and general correlates of functional network topology. Extrapolation of brain networks using rsfMRI circumvents the bias associated with task-based fMRI and the use of graph measures allowed quantitative comparison of brain network topology among groups. However, the study also has limitations. Fatigue was measured using the EORTC QLQ-C30 scale, which mainly measures physical fatigue. Given its multidimensional nature, the use of domain-specific scales is important towards greater understanding of cancer-related fatigue [57, 58]. Also, baseline assessments were done after surgery under general anesthesia and the contribution of anesthetic agents to the observed change cannot be excluded. In addition, a subset of breast cancer patients underwent mastectomy; we did not investigate the effect of mastectomy on fatigue in this study. The generalization of our findings to all breast cancer patients should take the association between mastectomy and mental fatigue reported by some studies [59, 60] into consideration. Furthermore, it should be noted that 65% of the BCC + participants received tamoxifen therapy, whose effect on the brain is controversial and largely under investigated [61]. Due to the lack of adequate sample size we were not able to assess the specific effect of tamoxifen on brain networks in this study.
In summary, to the best of our knowledge, this is the first longitudinal study with two control groups investigating fatigue, resting-state functional brain networks and their association in breast cancer patients. The longitudinal design allowed for assessment of the potentially specific effects of chemotherapy on fatigue and functional brain network topology. The two control groups provided comparisons within breast cancer patients and with age-matched non-cancer controls. Our study shows the differences in network topology and fatigue among the groups following chemotherapy. Breast cancer patients exhibited increased fatigue and decreased local information processing and exchange following chemotherapy. However, a clear causal association between changes in brain topology and chemotherapy is yet to be established. Furthermore, a general correlation between network topology and fatigue across all groups was seen, for the first time indicating a general neural correlate in this population. Further studies using more sensitive fatigue measurement scales are required to better characterize fatigue experienced by breast cancer patients and its associations with brain functioning. Assessments further in time are also crucial in determining the persistence of changes and long-term effects of systemic cancer treatment on the brain.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- A
Doxorubicin
- AAL
Automated anatomical labeling
- BCC −
Breast cancer patients not treated with chemotherapy
- BCC +
Breast cancer patients treated with chemotherapy
- BOLD
Blood oxygen level dependent
- C
Cyclophosphamide
- Cp
Clustering coefficient
- DMN
Default mode network
- E
Epirubicin
- Eglob
Global efficiency
- Eloc
Local efficiency
- EORTC QLQ-C30
European organization for research and treatment of cancer quality of life questionnaire-C30
- EPI
Echo planar imaging
- F
5-Flurouracil
- fMRI
Functional magnetic resonance imaging
- HSCL-25
Hopkins symptoms checklist-25
- ICA-AROMA
Independent component analysis-automatic removal of motion artifacts
- Lp
Characteristic path length
- NART
Dutch adult reading test
- NC
Non-cancer control
- PROs
Patient reported outcomes
- PSS
Perceived stress scale
- rsfMRI
Resting-state functional magnetic resonance imaging
- SED
Standard error of measurement of the difference
- T
Docetaxel
Author contributions
BMB: conceptualization, methodology, software, formal analysis, writing-original draft, visualization. LD: conceptualization, methodology, writing-review and editing, visualization, supervision. MR: conceptualization, writing-review and editing, supervision. SS: writing-review and editing, supervision. ML: writing-review and editing.
Funding
This work is supported by funding from the Dutch Cancer Society, Grant Number (KWF 2009-4284).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Code for analysis is available upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Institutional Review Board of the Netherlands Cancer Institute and is in accordance with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golan S, Eggener S. GLOBOCAN 2012 v 1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. J Endourol. 2015;25:495–497. [Google Scholar]
- 2.Jiwa M, Long A, Shaw T, Pagey G, Halkett G, Pillai V, et al. The management of acute adverse effects of breast cancer treatment in general practice: A video-vignette study. J Med Internet Res. 2014;16:e204. doi: 10.2196/jmir.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbelen H, Gebruers N, Tjalma W. Late effects of cancer treatment in breast cancer survivors. South Asian J Cancer. 2015;04:182–182. doi: 10.4103/2278-330X.175956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson FME, Cross AJ, Baraniak AR. A new normal with chemobrain: experiences of the impact of chemotherapy-related cognitive deficits in long-term breast cancer survivors. Heal Psychol Open. 2019;6:2055102919832234. doi: 10.1177/2055102919832234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019;30:1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CA, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, et al. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manage. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Zick SM, Zwickey H, Wood L, Foerster B, Khabir T, Wright B, et al. Preliminary differences in peripheral immune markers and brain metabolites between fatigued and non-fatigued breast cancer survivors: a pilot study. Brain Imaging Behav. 2014;8:506–516. doi: 10.1007/s11682-013-9270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower JE. Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol. 2005;23:8280–8282. doi: 10.1200/JCO.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 12.De Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33:2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppelmans V, De Ruiter MB, Van Der Lijn F, Boogerd W, Seynaeve C, Van Der Lugt A, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 14.Stouten-Kemperman MM, de Ruiter MB, Caan MWA, Boogerd W, Kerst MJ, Reneman L, et al. Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp. 2015;36:4638–4647. doi: 10.1002/hbm.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amidi A, Wu LM. Structural brain alterations following adult non-CNS cancers: a systematic review of the neuroimaging literature. Acta Oncol (Madr) 2019;58:522–536. doi: 10.1080/0284186X.2018.1563716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mzayek Y, de Ruiter MB, Oldenburg HSA, Reneman L, Schagen SB. Measuring decline in white matter integrity after systemic treatment for breast cancer: omitting skeletonization enhances sensitivity. Brain Imaging Behav. 2020 doi: 10.1007/511682-020-00319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ruiter MB, Schagen SB. Functional MRI studies in non-CNS cancers. Brain Imaging Behav. 2013;7:388–408. doi: 10.1007/s11682-013-9249-9. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: a systematic review. Neurosci Biobehav Rev. 2018;92:304–317. doi: 10.1016/j.neubiorev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen BT, Jin T, Patel SK, Ye N, Ma H, Wong CW, et al. Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: a pilot longitudinal study. Breast Cancer Res Treat. 2019;176:181–189. doi: 10.1007/s10549-019-05230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Wang YF, Zheng LJ, Shi Z, Huang W, Zhang LJ. Network-level functional connectivity alterations in chemotherapy treated breast cancer patients: a longitudinal resting state functional MRI study. Cancer Imaging. 2020;20:73. doi: 10.1186/s40644-020-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. Altered resting brain connectivity in persistent cancer related fatigue. NeuroImage Clin. 2015;8:305–313. doi: 10.1016/j.nicl.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci. 2019;13:585. doi: 10.3389/fnins.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang DU. Complex networks: structure and dynamics. Phys Rep. 2006;424:175–308. doi: 10.1016/j.physrep.2005.10.009. [DOI] [Google Scholar]
- 25.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Li Y, Metzak P, He Y, Woodward TS. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 2010;50:862–872. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 30.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruno J, Hosseini SMH, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xuan H, Gan C, Li W, Huang Z, Wang L, Jia Q, et al. Altered network efficiency of functional brain networks in patients with breast cancer after chemotherapy. Oncotarget. 2017;8:105648–105661. doi: 10.18632/oncotarget.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menning S, De Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HSA, Reneman L, et al. Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS ONE. 2017;12:e0171724. doi: 10.1371/journal.pone.0171724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menning S, De Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. NeuroImage Clin. 2015;7:547–554. doi: 10.1016/j.nicl.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menning S, de Ruiter MB, Kieffer JM, Agelink van Rentergem J, Veltman DJ, Fruijtier A, et al. Cognitive impairment in a subset of breast cancer patients after systemic therapy—results from a longitudinal study. J Pain Symptom Manage. 2016;52:560–569. doi: 10.1016/j.jpainsymman.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 37.Schmand B, Bakker D, Saan R, Louman J. De Nederlandse Leestest voor Volwassenen: een maat voor het premorbide intelligentieniveau. Tijdschr Gerontol Geriatr. 1991;22:15–19. [PubMed] [Google Scholar]
- 38.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL—review. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 44.Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- 45.Xiang J, Xue J, Guo H, Li D, Cui X, Niu Y, et al. Graph-based network analysis of resting-state fMRI: test-retest reliability of binarized and weighted networks. Brain Imaging Behav. 2020;14:1361–1372. doi: 10.1007/s11682-019-00042-6. [DOI] [PubMed] [Google Scholar]
- 46.Onnela JP, Saramäki J, Kertész J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- 47.Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geiser F, Hahn C, Conrad R, Liedtke R, Sauerbruch T, Schmidt-Wolf I, et al. Interaction of psychological factors and the effect of epoetin alfa treatment in cancer patients on hemoglobin and fatigue. Support Care Cancer. 2007;15:273–278. doi: 10.1007/s00520-006-0132-8. [DOI] [PubMed] [Google Scholar]
- 49.Huang X, Zhang Q, Kang X, Song Y, Zhao W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: a cross-sectional study. BMC Cancer. 2010;10:453. doi: 10.1186/1471-2407-10-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16:1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 51.Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costanzo ES, Lutgendorf SK, Mattes ML, Trehan S, Robinson CB, Tewfik F, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sitzia J, Huggins L. Side effects of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998;6:13–21. doi: 10.1046/j.1523-5394.1998.1998006013.x. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/S0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 55.Gélinas C, Fillion L. Factors related to persistent fatigue following completion of breast cancer treatment. Oncol Nurs Forum. 2004;31:269–278. doi: 10.1188/04.ONF.269-278. [DOI] [PubMed] [Google Scholar]
- 56.Kesler SR, Adams M, Packer M, Rao V, Henneghan AM, Blayney DW, et al. Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav. 2017;7:e00643. doi: 10.1002/brb3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue. J Natl Compr Cancer Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedrich M, Nowe E, Hofmeister D, Kuhnt S, Leuteritz K, Sender A, et al. Psychometric properties of the fatigue questionnaire EORTC QLQ-FA12 and proposal of a cut-off value for young adults with cancer. Health Qual Life Outcomes. 2018;16:125. doi: 10.1186/s12955-018-0949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol Oxford University Press. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotonda C, Guillemin F, Bonnetain F, Velten M, Conroy T. Factors associated with fatigue after surgery in women with early-stage invasive breast cancer. Oncologist. 2013;18:467–475. doi: 10.1634/theoncologist.2012-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novick AM, Scott AT, Neill Epperson C, Schneck CD. Neuropsychiatric effects of tamoxifen: challenges and opportunities. Front Neuroendocrinol. 2020;59:100869. doi: 10.1016/j.yfrne.2020.100869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code for analysis is available upon reasonable request.